Abstract
Nitric oxide (NO.) is a radical molecule produced by mammalian phagocytic cells as part of the innate immune response to bacterial pathogens. It exerts its antimicrobial activity in part by impairing the function of metalloproteins, particularly those containing iron and zinc cofactors. The pathogenic Gram-negative bacterium Salmonella enterica serovar Typhimurium undergoes dynamic changes in its cellular content of the four most common metal cofactors following exposure to NO. stress. Zinc, iron and magnesium all decrease in response to NO. while cellular manganese increases significantly. Manganese acquisition is driven primarily by increased expression of the mntH and sitABCD transporters following derepression of MntR and Fur. ZupT also contributes to manganese acquisition in response to nitrosative stress. S. Typhimurium mutants lacking manganese importers are more sensitive to NO., indicating that manganese is important for resistance to nitrosative stress.
Graphical Abstract
In response to nitric oxide, Salmonella Typhimurium acquires manganese and exports zinc, magnesium and iron. Uptake is driven by expression of transporters MntH and SitABCD as well as by ZupT.
Introduction
Phagocytic cells of the mammalian innate immune system produce both superoxide and nitric oxide (NO.) as a defense against bacterial pathogens. Mice lacking the inducible nitric oxide synthase (iNOS) are significantly more susceptible to infection than those capable of producing NO. 1. The bacterial targets of NO. are less well understood than those of reactive oxygen species (ROS), therefore the mechanisms of NO.-mediated bacteriostasis and bacterial resistance to nitrosative stress are topics of significant biological interest.
Primary cellular targets of reactive nitrogen species (RNS) include metal centers and cysteine residues. NO. covalently modifies protein thiols which can disrupt redox-based signaling, inhibit enzymatic reactions, and mobilize metals, such as zinc, that rely on cysteine ligands 2–5. NO. also interacts with iron to form dinitrosyl-iron complexes (DNICs), which can impair the activity of a variety of regulatory proteins and enzymes 6–11.
A microarray to identify NO.-responsive genes in Salmonella enterica serovar Typhimurium (S. Typhimurium) identified manganese transporters mntH and sitABCD among the most highly up-regulated genes, suggesting that manganese may play a role in cellular resistance to nitrosative stress 5. Manganese exists primarily as the divalent cation Mn2+ in biological systems and is the fourth most common metal cofactor after magnesium, zinc and iron 12. MntH is a manganese-proton symporter in the natural resistance-associated macrophage protein (NRAMP) family 13,14. While MntH function is not required under standard laboratory growth conditions in rich medium, expression is induced when cells are limited for manganese or iron as well as by oxidative stress 14–17. SitABCD is an ATP-binding cassette (ABC) manganese importer that is expressed under conditions similar to those regulating MntH 18,19. Manganese transport by MntH- and SitABCD-type systems promotes survival and virulence of pathogenic bacteria such as Escherichia coli, Staphylococcus aureus and S. Typhimurium in the face of nutritional immunity and oxidative stress 13,20–25.
The requirement for manganese in response to oxidative stress is well characterized, but a role in resistance to nitrosative stress has not previously been shown in bacteria. Under conditions of oxidative stress, manganese activates the manganese-dependent superoxide dismutase SodA and catalase KatN 26–28. Manganese has also been shown to prevent mismetallation by zinc and to preserve the activity of some mononuclear iron enzymes upon loss of the native iron ligand 29–32. Given the potential for NO. to disrupt cellular metal homeostasis, manganese might play similar roles in preserving enzyme function and preventing zinc mismetallation under conditions of nitrosative stress. Here we show that NO. disrupts homeostasis of iron, magnesium and manganese in addition to zinc 2. Manganese acquisition by MntH, SitABCD and a third transporter, ZupT, is required for the nitrosative stress response in S. Typhimurium.
Materials and methods
Growth conditions
Unless otherwise specified, Salmonella enterica serovar Typhimurium (ATCC 14028s) and Escherichia coli (TB1) were grown in Luria-Bertani (LB, Fisher) medium at 37°C with shaking at 250 rpm. Antibiotic selection was used for strain construction only at the following concentrations: 100 μg ml−1 ampicillin (Amp), 50 μg ml−1 kanamycin (Kan), and 20 μg ml−1 chloramphenicol (Cm). The minimal medium employed in these studies was M9: 1x M9 salts (Difco), 0.1 mM CaCl2, 2 mM MgSO4, 0.4% glucose.
Strain and plasmid construction
All strains and plasmids are listed in Supplementary Table 1, and all primers are listed in Supplementary Table 2. S. Typhimurium ATCC 14028s (JK237) was used as the wild-type strain and the genetic background for all S. Typhimurium mutant strains. Deletion mutant strains were constructed using the λ-RED mediated recombination method and, where applicable, pCP20 was used to remove antibiotic resistance cassettes by Flp-FRT recombination 33. The mntH11:kan and sit100::MudCm mutations were published previously 13,34. P22HT105/int bacteriophage was used for mutant strain construction by phage transduction 35. All mutant strains were verified by PCR.
E. coli (TB1) was used as the host strain for all cloning. Plasmids pEF97 and pEF98 were generated by amplifying the upstream promoter region plus coding sequence for mntH using primers EFP307 and EFP308 and sitABCD using primers EFP309 and EFP310. Amplification products were digested with BamHI and HindIII for mntH and KpnI and HindIII for sitABCD. Digested fragments were ligated into pRB3–273C digested with the same enzyme pair in reverse orientation to the multiple cloning site 36. Constructs were confirmed by sequencing using JKP227 and JKP244. Additional internal primers EFP321 and EFP322 were used to fully sequence the insert for pEF98. Plasmids pEF97, pEF98 and pJK671 were electroporated into JK831 to create strains EF674, EF675 and EF676 37.
Gene expression analysis
Primer sequences for assessing transcript levels of mntH, sitA and zupT are listed in Supplementary Table 2. S. Typhimurium was grown overnight in LB, diluted 1:1000 into 60 mL fresh LB and grown to an optical density at 600 nm (OD600) ~1. The culture was then subdivided into 5 duplicate pairs of 5 mL cultures in 18×150 mm glass tubes. One set was treated with 2 mM diethylamine NONOate (DEANO) and one set left untreated. Cultures were returned to shaking at 37°C. At various times post-treatment (0, 5, 15, 30, 45, 60 min), 1.5 mL of treated and untreated culture were pelleted by centrifugation and resuspended in 800 μL Trizol for RNA isolation. RNA and cDNA were prepared as described previously 38. Quantitative PCR (qPCR) was carried out using SYBR Green on a BioRad CFX96 real-time system with rpoD as the internal control. The rpoD primer sequences have been published previously 39. Statistical significance was determined by one-sample t-test compared to a hypothetical mean of 1 using GraphPad Prism.
Inductively Coupled Plasma-Mass Spectrometry (ICP-MS)
All ICP-MS analyses were conducted using strain variants that were also flagellar mutants (JK377, EF622, EF623, EF624, EF650, EF651, EF653) to increase pelleting efficiency. S. Typhimurium overnight cultures were subcultured 1:1000 into 100 mL fresh LB and grown to OD600 ~1. Prior to treatment, the culture was subdivided into 10 individual 5 mL aliquots in 18×150 mm glass tubes. Five aliquots were treated with 2 mM DEANO and 5 remained untreated. Cultures were returned to shaking at 37°C. At 0, 5, 15, 30, 45 and 60 min post-treatment, 4.5 mL of treated and untreated culture were pelleted by centrifugation then washed twice with ultrapure water. Pellets were resuspended in analytical grade nitric acid, boiled, then diluted 1:10 with ultrapure water for final analysis. Samples were analyzed on an Agilent 8900x QQQ ICP-MS. CFU were also enumerated at each time point so that total metal concentrations for each sample could be normalized according to cell number. Statistical significance was determined by one sample t-test compared to a hypothetical mean of 100 in GraphPad Prism.
Nitric oxide sensitivity assays and CFU enumeration
Wild-type S. Typhimurium (JK237) and mutant strains lacking manganese efflux pumps (JK601, JK603, JK605, JK715, JK719, JK720 and JK831) were grown overnight in LB medium then normalized to OD600 1 and diluted 1:1000 into a final volume of 300 μL in a flat-bottom 96-well non-treated tissue culture microtiter plate (Midwest Scientific). Cultures were treated with final concentrations of 5 mM SperNO (LB), 1 mM SperNO (M9) or 1.5 mM SperNO (M9). Cultures were grown aerobically with shaking at 567 cpm (3 mm) in a Biotek Synergy HTX multi-mode 96-well plate reader at 37°C. Growth was monitored by recording OD600 every 15 min for 40 hr. Statistical significance was determined by comparing the time required to reach 50% maximum OD600 by unpaired two-tailed t-test. Complementation of the JK831 growth defect was assessed as described above using JK895 (wild-type S. Typhimurium containing empty plasmid) and strains EF674, EF675 and EF676. Manganese supplementation was carried out as above in M9 medium supplemented with 0.1 μM MnSO4. For enumeration of CFU during lag phase, overnight cultures were diluted 1:1000 in 3 mL fresh LB with 5 mM SperNO in a 18×150 mm glass culture tube and grown at 37°C with shaking at 250 rpm. Samples (20 μL) were taken at the time of inoculation and every hour thereafter until growth resumed, diluted in phosphate-buffered saline (PBS) and plated onto LB agar in triplicate. Plates were grown overnight at 37°C before counting. Statistical significance was determined by unpaired two-tailed t-test.
Results
Nitrosative stress alters cellular metal abundances.
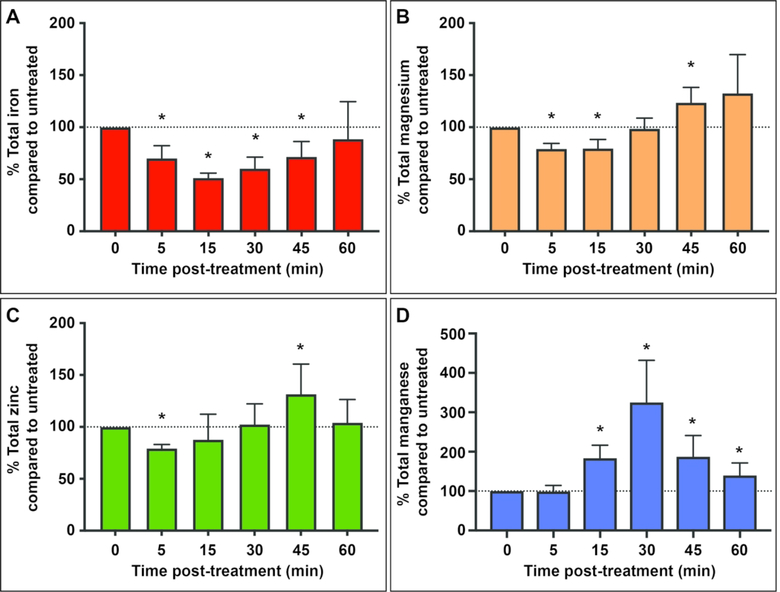
To obtain a comprehensive understanding of how metal flux changes in response to nitrosative stress, S. Typhimurium cultures were grown in LB, treated with 2 mM diethylamine NONOate (DEANO) and monitored using inductively coupled plasma mass spectrometry (ICP-MS). DEANO is a fast-release NO. donor that allows the response to, and subsequent recovery from, nitrosative stress to be observed over the course of 60 min 2. Between 5 and 15 min post-treatment, cellular iron and magnesium levels decreased in response to NO. before recovering to near-baseline levels by 60 min post-treatment (Figure 1 A&B). These patterns are similar to what had previously been observed for zinc (Figure 1C) 2. Levels of calcium, a metal that binds carboxylate ligands like magnesium though with a greater variety of bond angles and a smaller hydration radius, did not vary significantly in response to NO. (Supplementary Figure 1). In contrast to the response for other metals, manganese levels rose following treatment with NO., peaked at 30 min, then decreased back to near untreated levels by 60 min (Figure 1D).
Figure 1. Total cellular iron and magnesium decrease in response to NO. while intracellular manganese increases.
Total cellular iron (A), magnesium (B), zinc (C) and manganese (D) contents were determined by inductively-coupled plasma mass spectrometry (ICP-MS) following addition of 2 mM diethylamine NONOate (DEANO) to OD600 ≈ 1 S. Typhimurium cultures and compared to that of untreated cells. (A) Total cellular iron initially decreased beginning 5 min post-treatment before increasing again starting at 30 min. By 60 min post-treatment, iron levels were no longer significantly different from pre-treatment levels. (B) Magnesium levels also decreased at 5 and 15 min post-treatment before increasing and briefly exceeding pre-treatment levels at 45 min post-treatment. (C) Cellular zinc levels analyzed and published previously are reproduced here for comparison. 2 Zinc levels decreased at 5 min post-treatment then recovered to pre-treatment levels after briefly exceeding them at 45 min post-treatment. (D) Cellular manganese levels rose beginning at 15 min post-treatment, peaked at 30 min and then proceed to decrease and approach pre-treatment levels by 60 min. Statistical significance (*) compared to pre-treatment metal level was determined by one sample t-test to a hypothetical mean of 100. For iron at 5, 15 and 30 min post-treatment, p<0.001. At 45 min, p=0.0021. For magnesium, at 5 min post-treatment, p<0.001, at 15 min, p=0.006, and at 45 min, p=0.0213. For manganese at 15 min post-treatment, p<0.001, at 30 min, p=0.0014, at 45 min, p=0.0047 and at 60 min post-treatment, p=0.0146. Error bars represent standard deviation.
The expression of manganese transporters mntH and sitABCD is upregulated in response to nitrosative stress via MntR and Fur derepression.
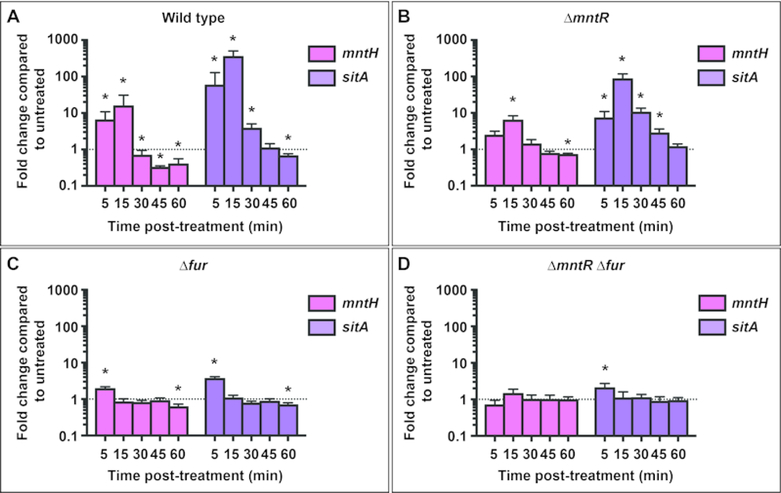
Previous microarray data showed that mntH and sitABCD are expressed in response to nitrosative stress, but the regulatory mechanism was not determined 5. The rise and subsequent fall of cellular manganese observed by ICP-MS suggested that transporter expression is also likely to exhibit a dynamic response to NO. stress. S. Typhimurium cultures were treated with 2 mM DEANO, sampled for RNA isolation over the course of 60 min, and then transporter gene expression was determined by qPCR. Increased expression of mntH (pink) and sitA (purple) was observed within 5 min of 2 mM DEANO treatment (Figure 2A). Expression peaked at 15 min post-treatment before exhibiting dramatic repression by 30 min post-treatment. This repression corresponds with the peak in manganese level (Figure 1C). The 15–30 min post-treatment interval is the same period during which S. Typhimurium has previously been shown to have detoxified the majority of the NO. released from DEANO 2.
Figure 2. Expression of mntH and sitA increases in response to NO. and is primarily regulated by Fur.
Total RNA was isolated from untreated OD600 ≈ 1 S. Typhimurium cultures and cultures treated with 2 mM DEANO. Expression of mntH and sitA was determined by qPCR. Data are presented as positive mean fold-change compared to untreated cells of the same genetic background. Error bars represent standard deviation. Statistical significance (*) was determined by a one-sample t-test to a hypothetical mean of 1, indicating no difference in expression between treated and untreated cultures. (A) Expression of mntH (pink) and sitA (purple) increased at 5 and 15 min post-treatment before rapidly decreasing to levels below those of untreated cultures. For mntH expression at 5 min, p=0.0114, at 15 min, p=0.0463, at 30 min, p=0.0499, and at 45 and 60 min, p<0.001. For sitA expression at 5 min, p=0.0449, at 15 min, p<0.001, at 30 min, p=0.0041 and at 60 min, p<0.001. (B) In the absence of mntR, the overall patterns of NO. induced expression for mntH and sitA were similar to wild-type though the magnitude of expression changes was smaller. Expression of mntH showed significant upregulation at 15 min post-treatment (p=0.0331) and downregulation at 60 min (p=0.006). Expression of sitA was significantly increased at 5 min (p=0.013), 15 min (p=0.0342), 30 min (p=0.0263) and 45 min (p=0.0456) post-treatment. (C) In the absence of fur, a modest increase in expression was observed in response to NO. 5 min post-treatment for both mntH (p=0.012) and sitA (0.004) before returning to baseline levels. A small decrease in expression was observed for both genes 60 min post-treatment (mntH p=0.0221, sitA p=0.0236). (D) In the absence of both mntR and fur, there was no further change in expression of mntH in response to NO.. Expression of sitA increased approximately 2-fold at 5 min post-treatment (p=0.0311) but was no different than untreated cultures at all other times.
Two different regulators have been implicated in control of mntH and sitABCD expression: the iron-binding repressor Fur and the manganese-binding repressor MntR 18,15,16. OxyR, an activator that responds to oxidative stress, has also been shown to regulate mntH expression 15. The sitABCD promoter of S. Typhimurium does not contain an OxyR binding site and thus does not respond to OxyR regulation 18. A deletion mutant was constructed for each regulator to determine its contribution to manganese transporter expression following nitrosative stress. Deletion of the mntR and fur repressors leads to derepression of mntH and sitA in the absence of NO. (Supplementary Figure 2). To examine expression specifically resulting from NO.-mediated regulation, rather than repressor deletion, data are shown as fold-change compared to expression in untreated cells of the same genetic background, thus normalizing for basal derepression. Expression in the ΔmntR strain (Figure 2B) followed a similar pattern to that in the wild-type strain (Figure 2A) but, the magnitude of NO.-mediated induction was lower for both mntH and sitA. Repression between 30 and 60 min was not as dramatic in the ΔmntR strain compared to wild-type. In the Δfur strain (Figure 2C), a modest increase in expression (~2–4 fold) was observed 5 min post-treatment but by 15 min expression returned to the same level as in the untreated culture, suggesting that repression by MntR was relieved in response to NO. but then quickly restored. No additional activation of mntH expression was observed in response to NO. in the ΔmntR Δfur strain (Figure 2D), demonstrating that OxyR does not regulate mntH expression in response to NO..
ZupT contributes to dynamic manganese transport following NO. stress.
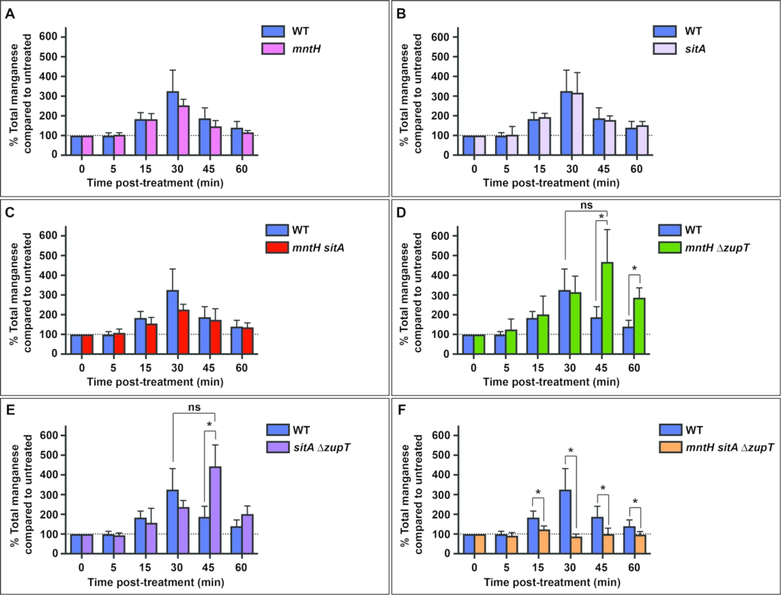
Since mntH and sitA exhibited a transcriptional response to NO., mutants specifically lacking each of these transport systems were investigated for their ability to acquire manganese in response to nitrosative stress. Neither an mntH mutant (Figure 3A, pink) nor a sitA mutant (Figure 3B, violet) varied significantly from wild-type (blue) with regard to manganese acquisition in response to NO.. A double mntH sitA mutant (Figure 3C, red) appeared to acquire less manganese than the wild-type strain, but the difference failed to achieve statistical significance. This result suggested that an additional transporter must contribute to the observed phenotype. The sequence encoding zupT, a transporter with broad cation specificity that had previously been implicated in manganese transport as well as zinc transport, was therefore deleted in combination with mntH and sitA 37,40. Both an mntH ΔzupT mutant (Figure 3D, green) and a sitA ΔzupT mutant (Figure 3E, purple) were delayed for manganese acquisition compared to wild-type, reaching peak levels 45 min post-treatment, but the maximum amounts of manganese acquired by these mutants were not significantly different from wild-type. Only an mntH sitA ΔzupT mutant (Figure 3F, orange) completely failed to acquire manganese in response to NO., thus confirming a role for NO.-responsive acquisition of manganese via ZupT. Actual values are shown in Supplementary Table 3. Expression of zupT does not change in response to NO. nor is it expressed at greater levels in an mntH sitA mutant, thus regulation of manganese acquisition in this genetic background must occur via a different mechanism (Supplementary Figure 3).
Figure 3. Manganese acquisition by S. Typhimurium in response to NO. depends on ZupT as well as MntH and SitABCD.
S. Typhimurium cultures were treated with 2 mM DEANO at OD600 ≈ 1, and total cellular manganese was monitored by ICP-MS. Data are the mean % total manganese compared to untreated culture for each mutant strain shown adjacent to the wild-type data. Error bars represent standard deviation. (A&B) Single mutants in mntH (pink, A) and sitA (grey, B) were not altered for manganese acquisition compared to wild-type (blue). (C) An mntH sitA mutant (red) did not acquire significantly lower levels of manganese than wild-type. (D&E) An mntH ΔzupT mutant (green) and a sitA ΔzupT mutant (purple) each acquired manganese in response to NO., but the response was delayed compared to wild-type, peaking at 45 min post-treatment before falling. The total amount of manganese acquired did not differ significantly from wild-type (ns). (F) An mntH sitA ΔzupT mutant (orange) did not acquire manganese in response to NO.. Statistically significant differences between mutant and wild-type (*) were determined by unpaired two-tailed t-test. For mntH ΔzupT at 45 min, p=0.0025 and at 60 min, p<0.001. For sitA ΔzupT at 45 min, p<0.001. For mntH sitA ΔzupT at 15 min, p=0.0084, at 30 min, p<0.001, at 45 min, p=0.0078 and at 60 min, p=0.0186.
Manganese transport mutants are more sensitive to nitrosative stress.
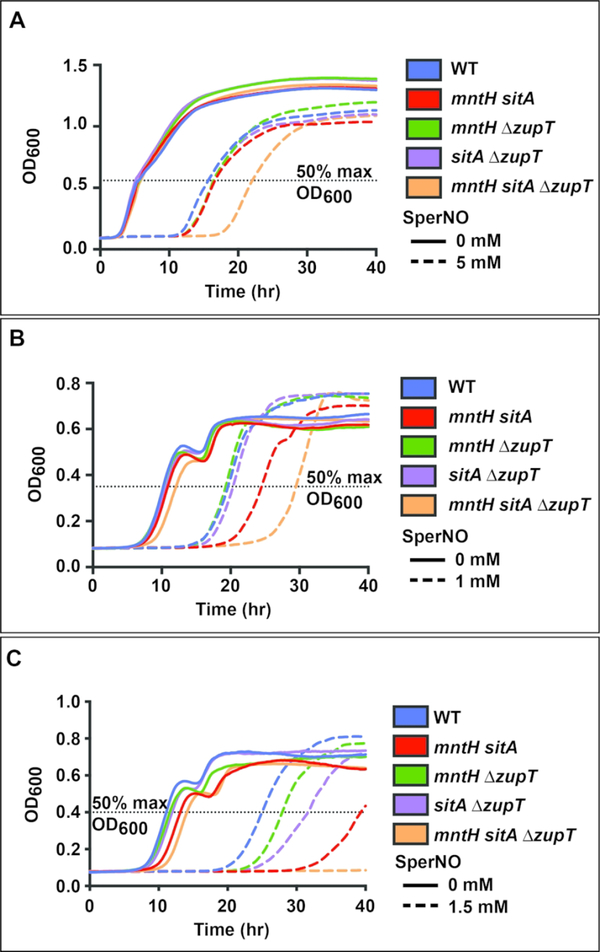
To determine whether manganese is required for the nitrosative stress response, S. Typhimurium mutants were grown in the presence of the sustained-release NO. donor spermine NONOate (SperNO). The mntH and sitA mutants that achieved wild-type levels of manganese acquisition were not impaired for growth in response to NO., nor was a ΔzupT mutant (Supplementary Figure 4). In LB medium, which contains manganese 41, only the mntH sitA ΔzupT mutant that was impaired for additional manganese acquisition displayed a delayed exit from lag phase (Figure 4A, orange dashed line) compared to wild-type (blue dashed line). Plasmid-based expression of any single transporter (MntH, SitABCD or ZupT) from its native promoter was sufficient to complement the recovery defect (Supplementary Figure 5). In minimal medium (M9) that does not include transition metals, both the mntH sitA ΔzupT strain (Figure 4B, orange dashed line) and the mntH sitA strain (Figure 4B, red dashed line) displayed increased sensitivity to 1 mM SperNO compared to wild-type (Figure 4B, blue dashed line). When the concentration of SperNO was increased to 1.5 mM (Figure 4C), all four mutants displayed increased sensitivity compared to wild-type (blue dashed line). The mntH ΔzupT strain (green dashed line) displayed the smallest stress phenotype followed by the sitA ΔzupT strain (purple dashed line) and the mntH sitA strain (red dashed line). Supplementation with 0.1 μM MnSO4 was sufficient to complement the recovery defects of the mntH sitA, sitA ΔzupT and mntH ΔzupT strains but not that of the mntH sitA ΔzupT triple mutant (Supplementary Figure 6).
Figure 4. Manganese transport mutants are more sensitive to nitrosative stress.
Growth of wild-type and mutant S. Typhimurium was monitored in rich medium (A) or minimal medium (B&C) following treatment with SperNO (dashed lines). (A) In LB medium with 5 mM SperNO, only the mntH sitA ΔzupT strain (orange dashed line, p<0.001) showed a delayed exit from lag phase and increased time to reach 50% maximum optical density compared to wild-type (blue dashed line). (B) In M9 glucose medium with 1 mM SperNO, both an mntH sitA mutant (red dashed line) and the mntH sitA ΔzupT mutant (orange dashed line) showed delayed growth (p<0.001). (C) In M9 glucose with 1.5 mM SperNO, the delays were more pronounced with all mutants showing delayed growth relative to wild-type (mntH sitA p<0.001, mntH ΔzupT p=0.002, sitA ΔzupT p<0.001, mntH sitA ΔzupT not determined). Statistical significance between wild-type and mutant growth was determined using time to reach 50% maximum OD600 by unpaired two-tailed t-test.
Manganese transport mutants recover more slowly from nitrosative stress but do not experience NO.-mediated death or undergo selection for resistance.
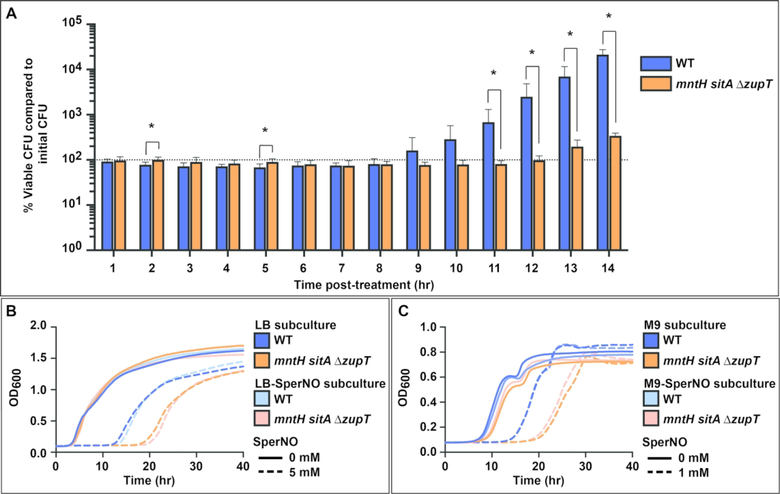
To understand the reason for the difference in lag-phase exit time of the mntH sitA ΔzupT mutant, cells were grown in LB with 5 mM SperNO then samples were obtained for CFU enumeration and compared to initial CFU (Figure 5A). During the prolonged lag phase, the number of viable CFU remained consistent between wild-type (blue bars) and mntH sitA ΔzupT (orange bars) demonstrating that inability to acquire manganese does not result in death of the transport mutants. To determine whether outgrowth is due to selection for mutants that are less NO.-sensitive, cells were treated with NO. in a standard LB assay for 40 hrs, subcultured, and subjected to subsequent challenge. The subcultured cells were no more resistant than S. Typhimurium strains that had not previously experienced NO. stress when grown in either rich (Figure 5B) or minimal (Figure 5C) medium. Taken together, these data show that manganese enhances the rate of recovery rather than promoting cell survival. Outgrowth represents recovery of the starting population, not growth of a small population containing secondary mutations that confer enhanced resistance.
Figure 5. Viable CFU and sensitivity to NO. stress do not change following NO.-challenge.
(A) Viable CFU, shown as a percentage of initial CFU, remained consistent between wild-type (blue) and mntH sitA ΔzupT (orange) S. Typhimurium during the prolonged lag phase resulting from NO. stress. There was no significant difference in viable CFU between the two strains except at 2 hr and 5 hr, when the mutant displayed a small but statistically significant increased percentage of viable CFU (p=0.011 and p=0.013, respectively). Following recovery from the stress, normal growth kinetics resumed, and CFU began to increase with wild-type showing significantly greater viable CFU than mntH sitA ΔzupT from 11–14 hr (p=0.038, p=0.032, p=0.025, p=0.004). Statistical significance was determined by unpaired two-tailed t-test. (B &C) Wild-type and mntH sitA ΔzupT S. Typhimurium from NO. sensitivity assays that had been grown either in LB or LB with 5 mM SperNO were subcultured and used as the starting inocula for subsequent sensitivity assays. The population of mntH sitA ΔzupT cells arising from the initial NO. challenge (pale orange dashed lines) was not more resistant to NO. than mntH sitA ΔzupT cells that had not previously been exposed to NO. (dark orange dashed lines) in either LB (B) or M9 (C) medium.
Discussion
Nitric oxide is a key chemical defense of the mammalian innate immune response, but its cellular targets and mechanisms of antimicrobial activity are incompletely characterized. NO. has previously been shown to mobilize zinc from metalloproteins by nitrosylating cysteine residues and zinc efflux was shown to be an important part of the response to nitrosative stress in S. Typhimurium 2,4. The effect of NO. on homeostasis of other biologically relevant metals, however, was not determined. Here we show that total cellular iron and magnesium levels also decrease in response to nitrosative stress, while manganese levels increase (Figure 1). Changes in metal availability and relative abundance have the potential to alter the function of proteins that depend on a specific metal cofactor. To promote correct metallation in vivo, bacterial cells must hold metals with more favorable free energies to lower concentrations 42. When free metal concentrations increase intracellularly, efflux is necessary to avoid metal intoxication and iron-catalyzed Fenton chemistry 2,30,43,44.
The role that efflux of magnesium and iron plays in resistance to nitrosative stress has not previously been characterized and the transporters responsible for efflux of these metals are not known. Prior work has shown that magnesium supplementation is required to protect ΔphoQ, ΔmgtA and ΔmgtBC S. Typhimurium that are deficient for magnesium import from cell death caused specifically by nitroxidative species 53. Metal flux in response to NO. has not been determined in these mutant backgrounds and may differ from that of wild-type observed here. It is also possible that since magnesium levels initially decrease in response to NO. but then recover to pre-stress levels, both efflux and acquisition are required during different phases of the stress response. The iron-citrate efflux transporter IceT is induced in response to NO., and a mutant was found to be more sensitive to nitrosative stress 44. IceT is therefore one possible mediator of the observed iron efflux that can be investigated further but may not be the only iron transport system involved. In general, the biological conditions and transport proteins that promote efflux of iron and magnesium are poorly understood.
Nitric oxide is known to disrupt iron binding but direct interaction of NO. with the carboxylate ligands preferred by magnesium or with magnesium ions has not been demonstrated. Nitration of tyrosine, which is found in some magnesium binding sites, can occur under aerobic conditions 45,46. Metalloproteins, however, are not the only source of intracellular magnesium. A significant percentage of cellular magnesium associates with ribosomal RNAs to reinforce ribosome structure, and cellular magnesium levels are correlated with ribosome number 47,48. Ribosomal proteins were found to be targets of S-nitrosylation in S. Typhimurium, which may disrupt ribosome structure 2. Nucleoside triphosphates within cells exist primarily bound to magnesium and chemical disruption of ATP production results in magnesium efflux from some eukaryotic cell types 49. Since nitrosative stress has been shown to reduce nucleoside triphosphate levels in S. Typhimurium, a similar mechanism may drive the magnesium efflux observed here 50. Under standard growth conditions, S. Typhimurium has been reported to maintain free magnesium at a concentration of approximately 1 mM 51,52. Our data show that cellular iron and magnesium levels decrease in response to nitrosative stress but determining the intracellular sources of these displaced ions and whether their efflux is required for resistance to nitrosative stress is beyond the scope of this work.
While the need to activate MnSOD in response to oxidative stress is well characterized, a demand for manganese under conditions of nitrosative stress has not been explored 17,22,27. Manganese is acquired in response to NO., driven primarily by increased expression of SitABCD and MntH, which are typically repressed by both Fur and MntR (Supplementary Figure 2, Figure 2). NO. has previously been shown to relieve repression by Fur through nitrosylation of iron, resulting in a structure that no longer binds DNA efficiently 8. The mechanism by which NO. is also able to relieve repression by MntR, a manganese-binding regulator lacking cysteines, is not currently known. A third transporter, ZupT, is required for acquisition of manganese in the absence of MntH and SitABCD, despite not exhibiting a change in expression (Supplementary Figure 3). Work in E. coli has suggested that expression of zupT is constitutive 40,54. A non-transcriptional mechanism of regulating manganese transport by ZupT or concurrent regulation of one or more manganese efflux transporters, such as a homologue of MntP, may therefore contribute to the mntH sitA phenotype 55–57.
Manganese is involved in the adaptive response of S. Typhimurium to nitrosative stress, as evidenced by the prolonged lag phases of transporter mutants impaired for manganese acquisition (Figure 4). In rich medium, only an mntH sitA ΔzupT mutant displays increased sensitivity to NO., but in minimal medium a clear hierarchy of phenotypes is evident. This suggests that, at least in vitro, SitABCD, which also exhibits the greatest expression increase, is the primary Mn transporter, with MntH playing a secondary role. ZupT appears to be the least effective transporter of the three under manganese-limited conditions, but offers some protection against the effects of nitrosative stress.
Manganese acquisition is required to speed recovery from nitrosative stress but has no impact on survival (Figure 5A) or response to subsequent NO. challenge (Figures 5B&5C). While elucidating the exact mechanisms by which manganese protects cells from NO· will require further investigation, there are several possibilities. Zinc is of particular concern during nitrosative stress, since zinc is mobilized by NO. and is highly stable as a metal cofactor under non-stress conditions 2,58,59. Manganese may be required to buffer against increased free zinc and protect against mismetallation. Manganese is capable of substituting structurally and enzymatically for magnesium and iron, which decrease in response to nitrosative stress. Unlike iron, manganese does not catalyze Fenton chemistry in vivo and could help to maintain limited function of certain iron-dependent enzymes if they are targets of NO. or are indirectly affected by iron efflux 30,60–62. Exchanging magnesium cofactors for manganese has the potential to temporarily restructure carbon flux, directing resources away from precursor pools and toward energy-generating pathways 62. It is not possible to conclude from the presented data whether the observed shift in magnesium to manganese ratio would be sufficient to drive cofactor exchange in S. Typhimurium. However, data from other bacterial species show that even brief disruptions of the cellular ratio of magnesium to manganese can shift the ion content and function of some proteins 63,64. Glycolysis has been shown to be important for S. Typhimurium pathogenesis, and in Staphylococcus aureus, nitrosative stress drives the pathogen toward glucose consumption 65,66. Glycolysis is known to be disrupted by manganese limitation, and thus acquisition in response to nitrosative stress may be required to mediate this shift 67. Finally, ribonucleotide reductase activity is required for DNA synthesis. While eukaryotes only have one form of this enzyme, equivalent to the iron-binding NrdAB complex, S. Typhimurium has several forms 68. Both the eukaryotic ribonucleotide reductase complex and NrdA of S. Typhimurium have been shown to be modified by nitrosative species 2,69,70. The genes comprising the alternative manganese-binding version of the enzyme complex (nrdEFHI) are upregulated in S. Typhimurium in response to NO. 5. Thus, manganese acquisition may be required to sustain ribonucleotide reductase activity following nitrosative stress.
While we have shown that manganese acquisition is required for the response of S. Typhimurium to NO. in vitro, the role of manganese in the response to NO. during infection of a mammalian host is not presently known. Given the importance of manganese for both oxidative and nitrosative stress responses, and the temporal separation of these responses in vivo, it may prove challenging to distinguish the specific roles of manganese in response to the individual mediators 71,72. In mice lacking a functional NADPH oxidase, mntH sitA ΔzupT S. Typhimurium still exhibits a growth defect compared to wild-type and also displays a defect relative to a ΔsodA mutant, suggesting that manganese acquisition is important in vivo for reasons in addition to oxidative stress resistance 22. Determining the role of manganese in resisting the effects of nitrosative stress during infection will be an important avenue of future investigation.
Oxidative stress resistance remains the primary function of manganese in bacterial systems, but here we have shown that manganese also plays an important role in resistance to nitrosative stress. The fluxes in manganese, magnesium, iron and zinc driven by NO. reveal a global remodeling of intracellular metal contents and suggest that a comprehensive understanding of the bacterial response to nitrosative stress will require the consideration of how metals change, not just as isolated species, but in relation to one another. Identifying the transporters and regulators involved as well as the protein targets affected by these metal shifts will reveal additional novel aspects of the antimicrobial effects of NO..
Supplementary Material
Significance to Metallomics.
Manganese is a cofactor for bacterial enzymes involved in metabolism and oxidative stress resistance. Mammalian immune cells also produce nitric oxide, which we show leads to efflux of iron, magnesium and zinc and acquisition of manganese by Salmonella Typhimurium. Manganese promotes recovery from nitrosative stress, possibly by preventing mismetallation by more toxic metals or activating alternative versions of enzymes. Since control of intracellular metal concentrations is required to ensure correct metal binding and protein function, our data suggest that future studies should consider global changes to metal homeostasis and examine expanded roles for manganese in bacterial pathogenesis.
Acknowledgements
We thank Ms. Sarah Hasty for technical assistance with ICP-MS sample preparation. S.Y and E.R.F were supported by startup funding from Rhodes College. J.E.K., S.J.L. and F.C.F were supported by NIH AI112640 and AI118962. S.L.N and C.A.M were supported by NHMRC 1140554 and 1180826. S.L.N. was supported by NHMRC Early Career Research Fellowship 1142695. C.A.M. was supported by Australian Research Council Future Fellowship FT170100006.
Footnotes
Conflict of interest statement
The authors declare no conflicts of interest.
References
- 1.MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K and Hutchinson N, Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase, Cell, 1995, 81, 641–650. [DOI] [PubMed] [Google Scholar]
- 2.Frawley ER, Karlinsey JE, Singhal A, Libby SJ, Doulias P-T, Ischiropoulos H and Fang FC, Nitric Oxide Disrupts Zinc Homeostasis in Salmonella enterica Serovar Typhimurium, mBio,, DOI: 10.1128/mBio.01040-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urbano R, Karlinsey JE, Libby SJ, Doulias P-T, Ischiropoulos H, Warheit-Niemi HI, Liggitt DH, Horswill AR and Fang FC, Host Nitric Oxide Disrupts Microbial Cell-to-Cell Communication to Inhibit Staphylococcal Virulence, Cell Host Microbe, 2018, 23, 594–606.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schapiro JM, Libby SJ and Fang FC, Inhibition of bacterial DNA replication by zinc mobilization during nitrosative stress, Proc. Natl. Acad. Sci. U. S. A, 2003, 100, 8496–8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson AR, Payne EC, Younger N, Karlinsey JE, Thomas VC, Becker LA, Navarre WW, Castor ME, Libby SJ and Fang FC, Multiple Targets of Nitric Oxide in the Tricarboxylic Acid (TCA) Cycle of Salmonella enterica Serovar Typhimurium, Cell Host Microbe, 2011, 10, 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duan X, Yang J, Ren B, Tan G and Ding H, Reactivity of Nitric Oxide with the [4Fe-4S] Cluster of Dihydroxyacid Dehydratase from Escherichia coli, Biochem. J, 2009, 417, 783–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner PR, Costantino G, Szabó C and Salzman AL, Nitric oxide sensitivity of the aconitases, J. Biol. Chem, 1997, 272, 25071–25076. [DOI] [PubMed] [Google Scholar]
- 8.D’Autréaux B, Touati D, Bersch B, Latour J-M and Michaud-Soret I, Direct inhibition by nitric oxide of the transcriptional ferric uptake regulation protein via nitrosylation of the iron, Proc. Natl. Acad. Sci. U. S. A, 2002, 99, 16619–16624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren B, Zhang N, Yang J and Ding H, Nitric Oxide-Induced Bacteriostasis and Modification of Iron-Sulfur Proteins in Escherichia coli, Mol. Microbiol, 2008, 70, 953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crack JC and Le Brun NE, Mass Spectrometric Identification of [4Fe-4S](NO)x Intermediates of Nitric Oxide Sensing by Regulatory Iron-Sulfur Cluster Proteins, Chem. Weinh. Bergstr. Ger, 2019, 25, 3675–3684. [DOI] [PubMed] [Google Scholar]
- 11.Fleischhacker AS and Kiley PJ, Iron-containing transcription factors and their roles as sensors, Curr. Opin. Chem. Biol, 2011, 15, 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andreini C, Bertini I, Cavallaro G, Holliday GL and Thornton JM, Metal ions in biological catalysis: from enzyme databases to general principles, J. Biol. Inorg. Chem. JBIC Publ. Soc. Biol. Inorg. Chem, 2008, 13, 1205–1218. [DOI] [PubMed] [Google Scholar]
- 13.Kehres DG, Zaharik ML, Finlay BB and Maguire ME, The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen, Mol. Microbiol, 2000, 36, 1085–1100. [DOI] [PubMed] [Google Scholar]
- 14.Makui H, Roig E, Cole ST, Helmann JD, Gros P and Cellier MF, Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter, Mol. Microbiol, 2000, 35, 1065–1078. [DOI] [PubMed] [Google Scholar]
- 15.Kehres DG, Janakiraman A, Slauch JM and Maguire ME, Regulation of Salmonella enterica Serovar Typhimurium mntH Transcription by H2O2, Fe2+, and Mn2+, J. Bacteriol, 2002, 184, 3151–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patzer SI and Hantke K, Dual Repression by Fe2+-Fur and Mn2+-MntR of the mntH Gene, Encoding an NRAMP-Like Mn2+ Transporter in Escherichia coli, J. Bacteriol, 2001, 183, 4806–4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anjem A, Varghese S and Imlay JA, Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli, Mol. Microbiol, 2009, 72, 844–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda JS, Janakiraman A, Kehres DG, Maguire ME and Slauch JM, Transcriptional Regulation of sitABCD of Salmonella enterica Serovar Typhimurium by MntR and Fur, J. Bacteriol, 2005, 187, 912–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kehres DG, Janakiraman A, Slauch JM and Maguire ME, SitABCD Is the Alkaline Mn2+ Transporter of Salmonella enterica Serovar Typhimurium, J. Bacteriol, 2002, 184, 3159–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson ES, Paulley JT, Gaines JM, Valderas MW, Martin DW, Menscher E, Brown TD, Burns CS and Roop RM, The Manganese Transporter MntH Is a Critical Virulence Determinant for Brucella abortus 2308 in Experimentally Infected Mice, Infect. Immun, 2009, 77, 3466–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyer E, Bergevin I, Malo D, Gros P and Cellier MFM, Acquisition of Mn(II) in Addition to Fe(II) Is Required for Full Virulence of Salmonella enterica Serovar Typhimurium, Infect. Immun, 2002, 70, 6032–6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz-Ochoa VE, Lam D, Lee CS, Klaus S, Behnsen J, Liu JZ, Chim N, Nuccio S-P, Rathi SG, Mastroianni JR, Edwards RA, Jacobo CM, Cerasi M, Battistoni A, Ouellette AJ, Goulding CW, Chazin WJ, Skaar EP and Raffatellu M, Salmonella mitigates oxidative stress and thrives in the inflamed gut by evading calprotectin-mediated manganese sequestration, Cell Host Microbe, 2016, 19, 814–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kehl-Fie TE, Zhang Y, Moore JL, Farrand AJ, Hood MI, Rathi S, Chazin WJ, Caprioli RM and Skaar EP, MntABC and MntH contribute to systemic Staphylococcus aureus infection by competing with calprotectin for nutrient manganese, Infect. Immun, 2013, 81, 3395–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabri M, Caza M, Proulx J, Lymberopoulos MH, Brée A, Moulin-Schouleur M, Curtiss R and Dozois CM, Contribution of the SitABCD, MntH, and FeoB Metal Transporters to the Virulence of Avian Pathogenic Escherichia coli O78 Strain χ7122, Infect. Immun, 2008, 76, 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaharik ML, Cullen VL, Fung AM, Libby SJ, Kujat Choy SL, Coburn B, Kehres DG, Maguire ME, Fang FC and Finlay BB, The Salmonella enterica Serovar Typhimurium Divalent Cation Transport Systems MntH and SitABCD Are Essential for Virulence in an Nramp1G169 Murine Typhoid Model, Infect. Immun, 2004, 72, 5522–5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imlay JA, The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium, Nat. Rev. Microbiol, 2013, 11, 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keele BB, McCord JM and Fridovich I, Superoxide dismutase from Escherichia coli B. A new manganese-containing enzyme, J. Biol. Chem, 1970, 245, 6176–6181. [PubMed] [Google Scholar]
- 28.Robbe-Saule V, Coynault C, Ibanez-Ruiz M, Hermant D and Norel F, Identification of a non-haem catalase in Salmonella and its regulation by RpoS (sigmaS), Mol. Microbiol, 2001, 39, 1533–1545. [DOI] [PubMed] [Google Scholar]
- 29.Anjem A and Imlay JA, Mononuclear iron enzymes are primary targets of hydrogen peroxide stress, J. Biol. Chem, 2012, 287, 15544–15556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imlay JA, The Mismetallation of Enzymes during Oxidative Stress, J. Biol. Chem, 2014, 289, 28121–28128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sobota JM and Imlay JA, Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese, Proc. Natl. Acad. Sci. U. S. A, 2011, 108, 5402–5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu M and Imlay JA, Superoxide poisons mononuclear iron enzymes by causing mismetallation, Mol. Microbiol, 2013, 89, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Datsenko KA and Wanner BL, One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products, Proc. Natl. Acad. Sci. U. S. A, 2000, 97, 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janakiraman A and Slauch JM, The putative iron transport system SitABCD encoded on SPI1 is required for full virulence of Salmonella typhimurium, Mol. Microbiol, 2000, 35, 1146–1155. [DOI] [PubMed] [Google Scholar]
- 35.Davis RW, Botstein D, Roth JR and Cold Spring Harbor Laboratory, Advanced bacterial genetics: a manual for genetic engineering, Cold Spring Harbor Laboratory, Cold Spring Harbor (NY), 1982. [Google Scholar]
- 36.Berggren RE, Wunderlich A, Ziegler E, Schleicher M, Duke RC, Looney D and Fang FC, HIV gp120-specific cell-mediated immune responses in mice after oral immunization with recombinant Salmonella, J. Acquir. Immune Defic. Syndr. Hum. Retrovirology Off. Publ. Int. Retrovirology Assoc, 1995, 10, 489–495. [PubMed] [Google Scholar]
- 37.Karlinsey JE, Maguire ME, Becker LA, Crouch M-LV and Fang FC, The phage shock protein PspA facilitates divalent metal transport and is required for virulence of Salmonella enterica sv. Typhimurium, Mol. Microbiol, 2010, 78, 669–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Will WR, Bale DH, Reid PJ, Libby SJ and Fang FC, Evolutionary expansion of a regulatory network by counter-silencing, Nat. Commun, 2014, 5, 5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karlinsey JE, Bang I-S, Becker LA, Frawley ER, Porwollik S, Robbins HF, Thomas VC, Urbano R, McClelland M and Fang FC, The NsrR regulon in nitrosative stress resistance of Salmonella enterica serovar Typhimurium, Mol. Microbiol, 2012, 85, 1179–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grass G, Franke S, Taudte N, Nies DH, Kucharski LM, Maguire ME and Rensing C, The Metal Permease ZupT from Escherichia coli Is a Transporter with a Broad Substrate Spectrum, J. Bacteriol, 2005, 187, 1604–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Outten CE and O’Halloran TV, Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis, Science, 2001, 292, 2488–2492. [DOI] [PubMed] [Google Scholar]
- 42.Osman D, Martini MA, Foster AW, Chen J, Scott AJP, Morton RJ, Steed JW, Lurie-Luke E, Huggins TG, Lawrence AD, Deery E, Warren MJ, Chivers PT and Robinson NJ, Bacterial sensors define intracellular free energies for correct enzyme metalation, Nat. Chem. Biol, 2019, 15, 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frawley ER and Fang FC, The ins and outs of bacterial iron metabolism, Mol. Microbiol, 2014, 93, 609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frawley ER, Crouch M-LV, Bingham-Ramos LK, Robbins HF, Wang W, Wright GD and Fang FC, Iron and citrate export by a major facilitator superfamily pump regulates metabolism and stress resistance in Salmonella Typhimurium, Proc. Natl. Acad. Sci. U. S. A, 2013, 110, 12054–12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrer-Sueta G, Campolo N, Trujillo M, Bartesaghi S, Carballal S, Romero N, Alvarez B and Radi R, Biochemistry of Peroxynitrite and Protein Tyrosine Nitration, Chem. Rev, 2018, 118, 1338–1408. [DOI] [PubMed] [Google Scholar]
- 46.Dudev T, Cowan JA and Lim C, Competitive Binding in Magnesium Coordination Chemistry: Water versus Ligands of Biological Interest, J. Am. Chem. Soc, 1999, 121, 7665–7673. [Google Scholar]
- 47.Nierhaus KH, Mg2+, K+, and the Ribosome, J. Bacteriol, 2014, 196, 3817–3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akanuma G, Kobayashi A, Suzuki S, Kawamura F, Shiwa Y, Watanabe S, Yoshikawa H, Hanai R and Ishizuka M, Defect in the Formation of 70S Ribosomes Caused by Lack of Ribosomal Protein L34 Can Be Suppressed by Magnesium, J. Bacteriol, 2014, 196, 3820–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romani AMP, CELLULAR MAGNESIUM HOMEOSTASIS, Arch. Biochem. Biophys, 2011, 512, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fitzsimmons LF, Liu L, Kim J-S, Jones-Carson J and Vázquez-Torres A, Salmonella Reprograms Nucleotide Metabolism in Its Adaptation to Nitrosative Stress, mBio,, DOI: 10.1128/mBio.00211-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Froschauer EM, Kolisek M, Dieterich F, Schweigel M and Schweyen RJ, Fluorescence measurements of free [Mg2+] by use of mag-fura 2 in Salmonella enterica, FEMS Microbiol. Lett, 2004, 237, 49–55. [DOI] [PubMed] [Google Scholar]
- 52.Alatossava T, Jütte H, Kuhn A and Kellenberger E, Manipulation of intracellular magnesium content in polymyxin B nonapeptide-sensitized Escherichia coli by ionophore A23187., J. Bacteriol, 1985, 162, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bourret TJ, Liu L, Shaw JA, Husain M and Vázquez-Torres A, Magnesium homeostasis protects Salmonella against nitrooxidative stress, Sci. Rep,, DOI: 10.1038/s41598-017-15445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang D, Hosteen O and Fierke CA, ZntR-mediated transcription of zntA responds to nanomolar intracellular free zinc, J. Inorg. Biochem, 2012, 111, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dambach M, Sandoval M, Updegrove TB, Anantharaman V, Aravind L, Waters LS and Storz G, The ubiquitous yybP-ykoY riboswitch is a manganese-responsive regulatory element, Mol. Cell, 2015, 57, 1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin JE, Waters LS, Storz G and Imlay JA, The Escherichia coli small protein MntS and exporter MntP optimize the intracellular concentration of manganese, PLoS Genet, 2015, 11, e1004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waters LS, Sandoval M and Storz G, The Escherichia coli MntR Miniregulon Includes Genes Encoding a Small Protein and an Efflux Pump Required for Manganese Homeostasis, J. Bacteriol, 2011, 193, 5887–5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Irving H and Williams RJP, Order of Stability of Metal Complexes, Nature, 1948, 162, 746–747. [Google Scholar]
- 59.Schapiro JM, Libby SJ and Fang FC, Inhibition of bacterial DNA replication by zinc mobilization during nitrosative stress, Proc. Natl. Acad. Sci. U. S. A, 2003, 100, 8496–8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aguirre JD and Culotta VC, Battles with Iron: Manganese in Oxidative Stress Protection, J. Biol. Chem, 2012, 287, 13541–13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheton PL-B and Archibald FS, Manganese complexes and the generation and scavenging of hydroxyl free radicals, Free Radic. Biol. Med, 1988, 5, 325–333. [DOI] [PubMed] [Google Scholar]
- 62.Kehres DG and Maguire ME, Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria, FEMS Microbiol. Rev, 2003, 27, 263–290. [DOI] [PubMed] [Google Scholar]
- 63.Hohle TH and O’Brian MR, Magnesium-dependent processes are targets of bacterial manganese toxicity, Mol. Microbiol, 2014, 93, 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pi H, Wendel BM and Helmann JD, Dysregulation of magnesium transport protects Bacillus subtilis against manganese and cobalt intoxication, J. Bacteriol,, DOI: 10.1128/JB.00711-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bowden SD, Rowley G, Hinton JCD and Thompson A, Glucose and glycolysis are required for the successful infection of macrophages and mice by Salmonella enterica serovar typhimurium, Infect. Immun, 2009, 77, 3117–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vitko NP, Spahich NA and Richardson AR, Glycolytic dependency of high-level nitric oxide resistance and virulence in Staphylococcus aureus, mBio,, DOI: 10.1128/mBio.00045-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Radin JN, Kelliher JL, Párraga Solórzano PK and Kehl-Fie TE, The Two-Component System ArlRS and Alterations in Metabolism Enable Staphylococcus aureus to Resist Calprotectin-Induced Manganese Starvation, PLoS Pathog, 2016, 12, e1006040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Torrents E, Ribonucleotide reductases: essential enzymes for bacterial life, Front. Cell. Infect. Microbiol,, DOI: 10.3389/fcimb.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lepoivre M, Fieschi F, Coves J, Thelander L and Fontecave M, Inactivation of ribonucleotide reductase by nitric oxide, Biochem. Biophys. Res. Commun, 1991, 179, 442–448. [DOI] [PubMed] [Google Scholar]
- 70.Roy B, Lepoivre M, Henry Y and Fontecave M, Inhibition of Ribonucleotide Reductase by Nitric Oxide Derived from Thionitrites: Reversible Modifications of Both Subunits, Biochemistry, 1995, 34, 5411–5418. [DOI] [PubMed] [Google Scholar]
- 71.Mastroeni P, Vazquez-Torres A, Fang FC, Xu Y, Khan S, Hormaeche CE and Dougan G, Antimicrobial Actions of the Nadph Phagocyte Oxidase and Inducible Nitric Oxide Synthase in Experimental Salmonellosis. II. Effects on Microbial Proliferation and Host Survival in Vivo, J. Exp. Med, 2000, 192, 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H and Fang FC, Antimicrobial Actions of the NADPH Phagocyte Oxidase and Inducible Nitric Oxide Synthase in Experimental Salmonellosis. I. Effects on Microbial Killing by Activated Peritoneal Macrophages in Vitro, J. Exp. Med, 2000, 192, 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.