Abstract
Seven new species of the subfamily Neanurinae from north-western Iran are described and illustrated in detail. Endonura agnieskaesp. nov. differs from the most similar congener, E. reticulata (Axelson, 1905), in chaetotaxic details and the arrangement of tubercles on the dorsal side of the body. Endonura annaesp. nov. can be easily recognised by its wide labrum, the absence of chaetae C on the head and the presence of a toothed claw. Endonura schwendingerisp. nov. is especially distinctive due to the absence of chaetae A and Ocp on the head and the presence of the male ventral organ. Deutonura brevisetasp. nov. is related and most similar to D. persica Smolis, Shayanmehr & Yoosefi-Lafooraki, 2018, described recently and known from Mazandran Province in Iran. The new species can be easily distinguished by the following set of features: dark pigmented body, presence of chaetae C and Dl3 on the head, absence of microchaetae on the furca rudimentary, presence of thickened macrochaetae on dorsal side of body and absence of cryptopygy. The main characteristics of Deutonura sengletisp. nov. include a white body with dark pigmented eyes, the fusion of tubercles Di and De on the first thoracic segment and the presence of the male ventral organ. Deutonura iranicasp. nov. is superficially similar to D. gibbosa Porco, Bedos & Deharveng, 2010, a species known from the Alps and Jura in Europe, but it differs in the body colour and the number of labial chaetae and chaetae (L+So) on the head. Paravietnura rostratasp. nov., the first member of this enigmatic and intriguing genus known from Iran, is characterised by an unusually elongate ogival labrum and extreme reduction of dorsal chaetotaxy. Furthermore, new records of several other species of the subfamily: Cryptonura maxima Smolis, Falahati & Skarżyński, 2012; C. persica Smolis, Falahati & Skarżyński, 2012; Deutonura persica; Endonura longirostris Smolis, Shayanmehr, Kuznetsova & Yoosefi-Lafooraki, 2017; E. paracentaurea Smolis, Shayanmehr, Kuznetsova & Yoosefi-Lafooraki, 2017; Neanura deharvengi Smolis, Shayanmehr & Yoosefi-Lafooraki, 2018; N. muscorum (Templeton, 1835) and Protanura papillata Cassagnau & Delamare Deboutteville, 1955 are given. The present study is based on the rich material collected by Antoine Senglet and loaned by Peter J. Schwendinger.
Keywords: Asia, new records, springtails, taxonomy, western Palearctic
Introduction
Springtails, classified within the subfamily Neanurinae, differ significantly in terms of morphology and behaviour from other Collembola. First of all, they have completely lost the furcula and their movement may be defined as exceptionally slow compared to the majority of springtails. Another noticeable difference between them and the majority of other Collembola is the covering of the dorsal and lateral sides of the body by spherical structures naming tubercles, which make them resemble a mulberry. In addition, chaetae covering Neanurinae body are usually strongly developed, elongated and considerably widened, as well as covered with numerous teeth (Deharveng 1983; Smolis 2008). Paradoxically, although they do not have a furcula, i.e. structures enabling express escape from predators, Neanurinae are an example of an evolution success, demonstrated by its over 800 currently described taxa which constitutes nearly one tenth of all the known Collembola (Bellinger et al. 2020; Smolis and Greenslade 2020). Regarding the actual distribution of the subfamily, the largest species diversity is observed both in tropical and temperate forests on all continents, excluding Antarctica (i.e. Yosii 1976; Cassagnau and Deharveng 1984; Deharveng and Weiner 1984; Cassagnau 1988; Deharveng 1989; Deharveng and Bedos 1992; Cassagnau 1996; Deharveng and Suhardjono 2000; Palacios-Vargas and Simón Benito 2007; Zhi-Chun and Jian-Xiu 2008; Palacios-Vargas and Deharveng 2014; Smolis and Deharveng 2015; Luo and Palacios-Vargas 2016; Ji-Gang et al. 2018). Nevertheless, knowledge on global diversity of the subfamily is still insufficient and far from complete as many areas, i.e. the Middle East, North Africa, New Guinea or Central Asia, are poorly surveyed in this respect.
An examination of an exceptionally-rich material of Neanurinae from north-western Iran (Provinces: Gilan, Golestan, Kermanshah, Mazandaran, North Khorasan, Semnan and West Azerbaijan), collected in the early 1970s by Antoine Senglet and loaned for the presented studies by Peter J. Schwendinger (curator of the Muséum d’histoire naturelle in Geneva, Switzerland), has revealed seven unknown species of this subfamily. Their detailed and illustrated descriptions are provided with new records of several other known species classified to Neanurinae.
Materials and methods
The specimens were cleared in Nesbitt’s fluid, subsequently mounted on slides in Swan’s medium and studied using a Nikon Eclipse E600 phase contrast microscope. Figures were drawn with a camera lucida and prepared for publication using Adobe Photoshop CS3.
The whole material, types as well as the other material, is deposited in the Muséum d’histoire naturelle in Geneva, Switzerland.
Terminology
Terminology and layout of the tables used in the paper follow Deharveng (1983), Deharveng and Weiner (1984), Smolis and Deharveng (2006) and Smolis (2008).
Abbreviations
General morphology:
Abd. abdomen;
Ant. antenna;
AOIII sensory organ of antennal segment III;
Cx coxa;
Fe femur;
Scx2 subcoxa 2;
T tibiotarsus;
Th. thorax;
Tr trochanter;
VT ventral tube.
Groups of chaetae:
Ag antegenital;
An chaetae of anal lobes;
Ap apical;
Ca centroapical;
Cm centromedial;
Cp centroposterior;
D dorsal;
Fu furcal;
Vc ventrocentral;
Veorve ventroexternal;
Vea ventroexternoanterior;
Vem ventroexternomedial;
Vep ventroexternoposterior;
Vel ventroexternolateral;
Vec ventroexternocentral;
Vei ventroexternointernal;
Viorvi ventrointernal;
Vl ventrolateral.
Tubercles:
Af antenno-frontal;
Cl clypeal;
De dorsoexternal;
Di dorsointernal;
Dl dorsolateral;
L lateral;
Oc ocular;
So subocular.
Types of chaetae:
Ml long macrochaeta;
Mc short macrochaeta;
Mcc very short macrochaeta;
Me mesochaeta;
mi microchaeta;
ms s-microchaeta;
Sors chaeta s;
Bs s-chaeta on Ant. IV;
miA microchaetae on Ant. IV;
iv ordinary chaetae on ventral Ant. IV;
or organite of Ant. IV;
brs border s-chaeta on Ant. IV;
i ordinary chaeta on Ant. IV;
mou cylindrical s-chaetae on Ant. IV (“soies mousses”);
x labial papilla x;
L’ ordinary lateral chaeta on Abd. V;
B4, B5 ordinary chaetae on tibiotarsi.
Taxonomy
Endonura agnieskae sp. nov.
6A4AAFB9-E507-5ADE-9D56-17DB127BFCDC
http://zoobank.org/B8FE7E36-B1F9-4D2E-BA23-1588CCA1126D
Figures 1–13.
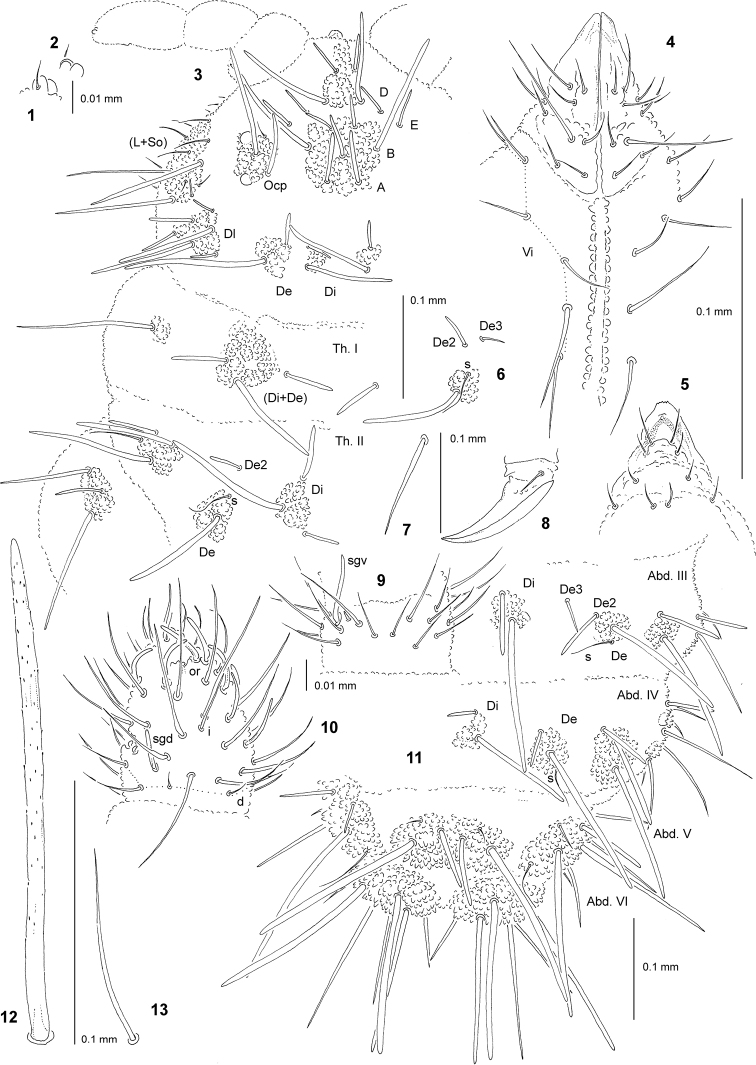
Endonura agnieskae sp. nov.: 1 apical bulb, dorsal view 2 apical bulb, ventral view 3 chaetotaxy of head and Th., dorsolateral view 4 chaetotaxy of labium and group Vi (holotype) 5 chaetotaxy and ventral sclerifications of labrum (holotype) 6 tubercle De of Th. III 7 chaeta B4 of leg III 8 claw of leg III, lateral view 9 ventral chaetotaxy of Ant. III 10 dorsal chaetotaxy of Ant. III–IV (holotype) 11 dorsal chaetotaxy of Abd. III–VI 12 chaeta Di1 of Abd. V 13 sensillum of Abd. V.
Table 1.
Chaetotaxy of Endonura agnieskae sp. nov.: Cephalic chaetotaxy–dorsal side.
| Tubercle | Number of chaetae | Types of chaetae | Names of chaetae |
|---|---|---|---|
| Cl | 4 | Ml | F |
| me | G | ||
| Af | 11 | Ml | B |
| Mc | A, O, C, D, E | ||
| Oc | 3 | Ml | Ocm |
| Mc | Ocp | ||
| mi | Oca | ||
| Di | 2 | Ml | Di1 |
| Mcc | Di2 | ||
| De | 2 | Ml | De1 |
| Mcc | De2 | ||
| Dl | 6 | Ml | Dl5, Dl1 |
| Mc | Dl3 | ||
| Mcc | Dl2, Dl4, Dl6 | ||
| (L+So) | 10 | Ml | L1, L4, So1 |
| Mcc | L2 | ||
| mi | L3, So2 | ||
| me | So3–6 |
Table 2.
Chaetotaxy of Endonura agnieskae sp. nov.: Chaetotaxy of antennae.
| Segment, Group | Number of chaetae | Segment, Group | Number of chaetae adult |
|---|---|---|---|
| I | 7 | IV | or, 8 S, i, 12 mou, 6 brs, 2 iv |
| II | 12 | ||
| III | 5 sensilla AO III | ||
| ve | 5 | ap | 8 bs, 5 miA |
| vc | 4 | ca | 2 bs, 3 miA |
| vi | 4 | cm | 3 bs, 1 miA |
| d | 5 | cp | 8 miA, 1 brs |
Table 3.
Chaetotaxy of Endonura agnieskae sp. nov.: Postcephalic chaetotaxy.
| Terga | Legs | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Di | De | Dl | L | Scx2 | Cx | Tr | Fe | T | |
| Th. I | 3 | 1 | – | 0 | 3 | 6 | 13 | 19 | |
| Th. II | 3 | 2+s | 3+s+ms | 3 | 2 | 7 | 6 | 12 | 19 |
| Th. III | 3 | 3+s | 3+s | 3 | 2 | 8 | 6 | 11 | 18 |
| Sterna | |||||||||
| Abd. I | 2 | 3+s | 2 | 3 | VT: 4 | ||||
| Abd. II | 2 | 3+s | 2 | 3 | Ve: 5; chaeta Ve1 present | ||||
| Abd. III | 2 | 3+s | 2 | 3 | Vel:5–6; Fu: 5 me, 0 mi | ||||
| Abd. IV | 2 | 2+s | 3 | 5–6 | Vel: 4; Vec: 2; Vei: 2; Vl: 4 | ||||
| Abd. V | (3+3) | 7–8+s | Ag: 3; Vl: 1 | ||||||
| Abd. VI | 7 | Ve: 14; An: 2 mi | |||||||
Type material.
Holotype: adult female on slide, Iran, Mazandaran Province, Nashtarud, forest reserve, sifting, 10.VII.1973, leg. A. Senglet, sample 7318. Paratypes: 4 females, 2 males and 2 juveniles on slide, same data as holotype.
Other material.
Female on slide, Iran, Mazandaran Province, Kiasar (36°16'N, 53°25'E), 10.VII.1975, leg. A. Senglet, 7546; 9 females, 2 males and juvenile on slide, Gilan Province, Limir, large trees in marsh, sifting, 28.VI.1973, leg. A. Senglet, 7306; female on slide, Iran, Gilan Province, Paresar, tree holes, leaves, sifting, 2.VII.1973, leg. A. Senglet, 7310; female on slide, Gilan Province, road to Jirandeh, 1000 m a.s.l., forest, 9.VIII.1974, leg. A. Senglet, 7486; female on slide, Semnan Province, near Loveh (37°19'N, 55°46'E / 1300 m a.s.l.), 22.VIII.1975, leg. A. Senglet, 7574.
Etymology.
The new species is dedicated to Agnieszka, wife of the first author.
Diagnosis.
Habitus typical of the genus Endonura. Dorsal tubercles present and well developed. 2+2 large pigmented eyes. Buccal relatively short, labrum nonogival. Central area of head with complete chaetotaxy. Tubercles Cl and Af separate. Tubercles Dl and (L+So) on head with 6 and 10 chaetae, respectively. Tubercles Di on Th. I present and fused with tubercle De. Tubercles De on Th. II and III with 3 and 4 chaetae, respectively. Tubercles L on Abd. III and IV with 3–4 and 7 chaetae, respectively. Abd. IV and V with 8 and 3 tubercles, respectively. Furcal rest without mi. Claw without inner tooth. Tibiotarsi with chaetae B4 and B5 rather short.
Description.
General. Body length (without antennae): 0.8 (juvenile) to 1.7 mm (holotype: 1.5 mm). Colour of the body bluish-grey. 2+2 large black eyes, in a typical arrangement for the genus (one anterior and one posterior eye, Fig. 3).
Chaetal morphology. Dorsal ordinary chaetae of five types: long macrochaetae (Ml), short macrochaetae (Mc), very short macrochaetae (Mcc), mesochaetae and microchaetae. Long macrochaetae thick, slightly arc-like or straight, narrowly sheathed, feebly serrated, apically rounded (Figs 3, 6, 11, 12). Macrochaetae Mc and Mcc morphologically similar to long macrochaetae, but much shorter. Mesochaetae similar to ventral chaetae, thin, smooth and pointed. Microchaetae similar to mesochaetae, but clearly shorter. S-chaetae of terga thin, smooth and short, distinctly shorter than nearby macrochaetae (Figs 3, 6, 11, 13).
Antennae. Typical of the genus. Dorsal chaetotaxy of Ant. III–IV as Fig. 10 and Table 2. S-chaetae of Ant. IV of medium length and moderately thickened (Fig. 10). Apical vesicle distinct, trilobate (Figs 1 and 2). Ventral chaetotaxy of Ant. III–IV as Fig. 9 and Table 2, sensillum sgv long and slightly s-shaped.
Mouthparts. Buccal cone rather short with labral sclerifications nonogival. Labrum chaetotaxy: 4/2, 4 (Fig. 5). Labium with four basal, three distal and three lateral chaetae, papillae x absent (Fig. 4). Maxilla styliform, mandible thin and tridentate.
Dorsal chaetotaxy and tubercles. Chaetotaxy of head complete (Fig. 3). Tubercles Di on head present, on Th. I differentiated and fused with De. Th. III and Abd. I–III with chaetae De3 free (Figs 6 and 11). On Abd. I–III, the line of chaetae De1–chaeta s parallel to the dorsomedian line (Fig. 11). On Abd. IV chaetae Di1 short. Cryptopygy absent, Abd. VI well visible from above. Chaeta Di2 on Abd. V as Mc, Mccormi.
Ventral chaetotaxy. On head, groups Vea, Vem and Vep with 3, 3–4, 4 chaetae, respectively. Group Vi on head with 6 chaetae (Fig. 4). On Abd. IV, furca rudimentary without microchaetae. On Abd. IV, tubercle L without free chaeta.
Legs. Chaetotaxy of legs as in Table 3. Claw without internal tooth (Fig. 8). On tibiotarsi, chaeta M present and chaetae B4 and B5 rather short and pointed (Fig. 7).
Remarks.
Due to the general appearance, dorsal and ventral chaetotaxy, E. agnieskae sp. nov. strongly resembles E. reticulata (Axelson, 1905), Holarctic and circumboreal species occurring in tundra, boreal and temperate biotopes of northern Europe (Scandinavian Peninsula), north-eastern Asia and North America (Smolis et al. 2011). Nevertheless, these species can be easily distinguished from each other by the set of characters: size of the eyes (expressed by the ratio of anterior eye diameter and diameter of base of chaeta Ocm, in agnieskae 2:1, in reticulata 1:1 or 5:4), the number of lateral labial chaetae (in agnieskae three, in reticulata four), the length of chaetae Ocp and A on the head (in agnieskae, equal in length, in reticulata chaeta Ocp, longer than chaeta A), the presence of tubercle Di on Th. I (in agnieskae, present and fused with De, in reticulata, absent), the location of chaeta De2 on Abd. I–III (in agnieskae, connected with tubercle De, in reticulata, free), the location of chaeta s on Abd. I–III (in agnieskae, the line of chaetae De1–chaeta s parallel to the dorsomedian line, in reticulata, not parallel) and the length of chaeta Di1 on Abd. IV (in agnieskae, distinctly shorter than chaeta Di1 on Abd. III, in reticulata, longer or equal to chaeta Di1 on Abd. III).
Endonura annae sp. nov.
1D2FEFC0-529D-5E0B-853C-606294388B27
http://zoobank.org/A26E5348-95D7-4E84-BB9A-C50383757D11
Figures 14–27.
Endonura annae sp. nov.: 14 chaetotaxy of head and Th. (holotype), dorsolateral view 15 chaetotaxy and ventral sclerifications of labrum 16 Mandible 17 Maxilla 18 chaetotaxy of labium and group Vi19 apical bulb, dorsal view 20 apical bulb, ventral view 21 sensillum sgv and microsensillum of Ant. III 22 dorsal chaetotaxy of Ant. III–IV 23 dorsal chaetotaxy of Abd. III–VI (holotype) 24 sensillum of Abd. V 25 chaeta Di1 of Abd. V 26 tibiotarsus and claw of leg III, lateral view 27 tubercle L of Abd. IV.
Table 4.
Chaetotaxy of Endonura annae sp. nov.: Cephalic chaetotaxy–dorsal side.
| Tubercle | Number of chaetae | Types of chaetae | Names of chaetae |
|---|---|---|---|
| Cl | 4 | Ml | F |
| me | G | ||
| Af | 8 | Ml | B |
| Mc | A, E | ||
| Mcc | D | ||
| Oc | 3 | Ml | Ocm |
| Mc | Ocp | ||
| Mcc | Oca | ||
| Di | 2 | Ml | Di1 |
| Mc | Di2 | ||
| De | 2 | Ml | De1 |
| Mcc | De2 | ||
| Dl | 6 | Ml | Dl5, Dl1 |
| Mc | Dl4 | ||
| Mcc | Dl2, Dl3, Dl6 | ||
| (L+So) | 8 | Ml | L1, L4, So1 |
| Mcc | L2 | ||
| me | So3–6 |
Table 5.
Chaetotaxy of Endonura annae sp. nov.: Chaetotaxy of antennae.
| Segment, Group | Number of chaetae | Segment, Group | Number of chaetae adult |
|---|---|---|---|
| I | 7 | IV | or, 8 S, i, 12 mou, 6 brs, 2 iv |
| II | 12 | ||
| III | 5 sensilla AO III | ||
| ve | 5 | ap | 8 bs, 5 miA |
| vc | 4 | ca | 2 bs, 3 miA |
| vi | 4 | cm | 3 bs, 1 miA |
| d | 2 | cp | 8 miA, 1 brs |
Table 6.
Chaetotaxy of Endonura annae sp. nov.: Postcephalic chaetotaxy.
| Terga | Legs | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Di | De | Dl | L | Scx2 | Cx | Tr | Fe | T | |
| Th. I | 1 | 2 | 1 | – | 0 | 3 | 6 | 13 | 19 |
| Th. II | 3 | 2+s | 3+s+ms | 3 | 2 | 7 | 6 | 12 | 19 |
| Th. III | 3 | 3+s | 3+s | 3 | 2 | 8 | 6 | 11 | 18 |
| Sterna | |||||||||
| Abd. I | 2 | 3+s | 2 | 3 | VT: 4 | ||||
| Abd. II | 2 | 3+s | 2 | 3 | Ve: 5; chaeta Ve1 present | ||||
| Abd. III | 2 | 3+s | 2 | 3 | Vel: 5; Fu: 4–5 me, 0 mi | ||||
| Abd. IV | 2 | 2+s | 3 | 6 | Vel: 4; Vec: 2; Vei: 2; Vl: 4 | ||||
| Abd. V | (2+2) | 5+s | Ag: 3; Vl: 1, L‘: 1 | ||||||
| Abd. VI | 7 | Ve: 13–14; An: 2 mi | |||||||
Type material.
Holotype: adult female on slide, Iran, Gilan Province, road to Dyavaherdeh, 1100–1300 m a.s.l., 7.VIII. 1974, leg. A. Senglet, sample 7484. Paratypes: 2 females, male and juvenile on slide, same data as holotype.
Other material.
Iran, 7 females and male on slide, Gilan Province, near Asalem, 300–600 m a.s.l., large beeches, sifting, 30.VI.1973, leg. A. Senglet, 7308; 3 females on slide, Gilan Province, Shahrbijar, tree hole, humus, sifting, 6.IX.1973, leg. A. Senglet, 7366; 4 females and juvenile on slide, Gilan Province, Asalem (37°45'N, 48°57'E), leaves and tree holes, sifting, 11.VI.1975, leg. A. Senglet, 7519; juvenile on slide, Mazandaran Province, Pol-e Zanguleh, 2300 m a.s.l., 12.VII.1973, leg. A. Senglet, 7320.
Etymology.
The new species is dedicated to Anna, wife of the second author.
Diagnosis.
Habitus typical of the genus Endonura. Dorsal tubercles present and well developed. 2+2 large pigmented eyes. Buccal cone short, labrum nonogival. Head with chaetae A, B, D and E. Chaetae O and C absent. Tubercles Cl and Af separate. Tubercles Dl and (L+So) on head with 6 and 8 chaetae, respectively. Tubercles Di on Th. I present. Tubercles De on Th. II and III with 3 and 4 chaetae, respectively. Tubercles L on Abd. III and IV with 3 and 6 chaetae, respectively. Abd. IV and V with 8 and 3 tubercles, respectively. Furcal rest without mi. Claw with inner tooth. Tibiotarsi with chaetae B4 and B5 rather short.
Description.
General. Body length (without antennae): 0.8 to 1.45 mm (holotype: 1.25 mm). Colour of the body white. 2+2 large black eyes, in a typical arrangement for the genus (Fig. 14).
Chaetal morphology. Dorsal ordinary chaetae of four types: long macrochaetae (Ml), short macrochaetae (Mc), very short macrochaetae (Mcc) and mesochaetae. Long macrochaetae thick, slightly arc-like, narrowly sheathed, feebly serrated, apically rounded (Figs 14, 23, 25). Macrochaetae Mc and Mcc morphologically similar to long macrochaetae, but shorter. Mesochaetae similar to ventral chaetae, thin, smooth and pointed. S–chaetae of terga thin, smooth and short, notably shorter than nearby macrochaetae (Figs 14, 23, 24).
Antennae. Typical of the genus. Dorsal chaetotaxy of Ant. III–IV as Fig. 22 and Table 5. S-chaetae of Ant. IV of medium length and moderately thickened, sensillum sgd notably short (Fig. 22). Ant. III with two chaetae d. Apical vesicle distinct, trilobate (Figs 19, 20). Ventral chaetotaxy of Ant. III as in Table 5, sensillum sgv long and slightly s-shaped (Fig. 21).
Mouthparts. Buccal short and wide with labral sclerifications nonogival (Fig. 15). Labrum chaetotaxy: 4/2, 4 (Fig. 15). Labium with four basal, three distal and four lateral chaetae, papillae x absent (Fig. 18). Maxilla styliform (Fig. 17), mandible with four teeth and relatively thin (Fig. 16).
Dorsal chaetotaxy and tubercles. Head without chaetae O, C, So2 and L3 (Fig. 14). Chaetae D free and not connected with tubercle. Tubercles Di on Th. I differentiated, not fused with tubercles De (Fig. 14). Th. III and Abd. I–III without free chaetae De2 and De3 (Figs 14, 23). On Abd. I–III, the line of chaetae De1–chaeta s perpendicular to the dorsomedian line. On Abd. III–IV, chaetae Di1 notably longer than chaetae Di1 of Abd. V (Fig. 23). On Abd. V, tubercle (Di+Di) with 2+2 chaetae. Cryptopygy strongly developed, Abd. VI practically not visible from above (Fig. 23).
Ventral chaetotaxy. On head, groups Vea, Vem and Vep with 3, 4, 4 chaetae, respectively. Group Vi on head with 6 chaetae (Fig. 18). On Abd. IV, furca rudimentary without macrochaetae, tubercle L with 6 chaetae (Fig. 27). On Abd. V, chaetae Vl and L’ present.
Legs. Chaetotaxy of legs as in Table 6. Claw with internal tooth. On tibiotarsi, chaeta M present and chaetae B4 and B5 relatively short and pointed (Fig. 26).
Remarks.
Morphologically, E. annae sp. nov. is strongly reminiscent of E. persica Smolis, Kahrarian, Piwnik & Skarżyński, 2016, taxon described from Kermanshah Province in northern Iran (Smolis et al. 2016a). Nevertheless, the new species can be easily recognised by several characters, including: the absence of chaeta C on the head (in persica present), the presence of 6 chaetae Dl on the head (in persica 5), wide and short buccal cone (in persica narrow and long), chaetae E on the head connected with tubercle Af (in persica free), chaetae De2 and De3 on Th. II–III, connected with tubercle De (in persica free), 2+2 chaetae Di on Abd. V (in persica 3+3) and strong cryptopygy (in persica, slightly developed).
E. annae sp. nov. is also similar to two species with toothed claw: E. dentifera Smolis, Skarżyński, Pomorski & Kaprus’, 2007 and E. dobrolyubovae Smolis & Kuznetsova, 2018, described from the Crimea and the Caucasus, respectively (Smolis et al. 2007; Smolis and Kuznetsova 2018). These species differ, however, in a number of details: the shape of the buccal cone (in annae, wide and short, in dentifera and dobrolyubovae, narrow and relatively long), the presence of chaeta C on the head (in annae, absent, in dentifera and dobrolyubovae, present), the presence and location of chaeta E on the head (in annae, present and connected with tubercle Af, in dentifera, present and free, in dobrolyubovae, absent), the number of chaetae (L+So) on the head (in dentifera, 10 chaetae, in annae and dobrolyubovae, 8 chaetae), the presence of tubercle Di on Th. I (in annae, present, in dentifera and dobrolyubovae, absent), the location of chaetae De3 on Th. III and Abd. I–III (in annae, connected with tubercle De, in dentifera and dobrolyubovae, free), the presence of male ventral organ (in annae and dentifera, absent, in dobrolyubovae, present) and the presence of cryptopygy (in annae, present, in dentifera and dobrolyubovae, absent).
Endonura schwendingeri sp. nov.
CF02EA0E-092F-5015-A6E6-BF2206B3C027
http://zoobank.org/19378FF6-D560-4C9E-A729-B7681C695986
Figures 28–41.
Endonura schwendingeri sp. nov.: 28 chaetotaxy of labium 29 chaetotaxy of head and Th. (holotype), dorsolateral view 30 apical part of labrum 31 Mandible 32 Maxilla 33 tibiotarsus and claw of leg III, lateral view 34 apical bulb, ventral view 35 apical bulb, dorsal view 36 dorsal chaetotaxy of Ant. III–IV 37 sensillum sgv and microsensillum of Ant. III 38 ventral chaetotaxy of Abd. II–VI (adult male) 39 dorsal chaetotaxy of Abd. III–VI 40 chaeta Di1 of Abd. V 41 sensillum of Abd. V.
Table 7.
Chaetotaxy of Endonura schwendingeri sp. nov.: Cephalic chaetotaxy–dorsal side.
| Tubercle | Number of chaetae | Types of chaetae | Names of chaetae |
| Cl | 4 | Ml | F |
| me | G | ||
| Af | 6 | Ml | B |
| mi | C, D | ||
| Oc | 2 | Ml | Ocm |
| mi | Oca | ||
| Di | 2 | Mc | Di1 |
| mi | Di2 | ||
| De | 2 | Ml | De1 |
| Mcc | De2 | ||
| Dl | 5 | Ml | Dl5, Dl1 |
| Mcc | Dl4 | ||
| mi | Dl2, Dl6 | ||
| (L+So) | 7 | Ml | L1, L4, So1 |
| me | So3–6 |
Table 8.
Chaetotaxy of Endonura schwendingeri sp. nov.: Chaetotaxy of antennae.
| Segment, Group | Number of chaetae | Segment, Group | Number of chaetae adult |
| I | 7 | IV | or, 8 S, i, 12 mou, 6 brs, 2 iv |
| II | 12 | ||
| III | 5 sensilla AO III | ||
| ve | 5 | ap | 8 bs, 5 miA |
| vc | 4 | ca | 2 bs, 3 miA |
| vi | 4 | cm | 3 bs, 1 miA |
| d | 5 | cp | 8 miA, 1 brs |
Table 9.
Chaetotaxy of Endonura schwendingeri sp. nov.: Postcephalic chaetotaxy.
| Terga | Legs | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Di | De | Dl | L | Scx2 | Cx | Tr | Fe | T | |
| Th. I | 1 | 2 | 1 | – | 0 | 3 | 6 | 13 | 19 |
| Th. II | 3 | 2+s | 3+s+ms | 3 | 2 | 7 | 6 | 12 | 19 |
| Th. III | 3 | 3+s | 3+s | 3 | 2 | 8 | 6 | 11 | 18 |
| Sterna | |||||||||
| Abd. I | 2 | 3+s | 2 | 2 | VT: 4 | ||||
| Abd. II | 2 | 3+s | 2 | 2 | Ve: 4–5; chaeta Ve1 present | ||||
| Abd. III | 2 | 3+s | 2 | 2 | Vel: 3–4; Fu: 5 me, 0 mi | ||||
| Abd. IV | 2 | 2+s | 3 | 4 | Vel: 4; Vec: 2; Vei: 2; Vl: 4 | ||||
| Abd. V | (3+3) | 5+s | Ag: 3; Vl: 1 | ||||||
| Abd. VI | 7 | Ve: 11–12; An: 2 mi | |||||||
Type material.
Holotype: female on slide, Iran, Gilan Province, Paresar, tree holes, leaves, sifting, 2.VII.1973, leg. A. Senglet, sample 7310. Paratypes: 3 females and male on slide, same data as holotype.
Other material.
Iran, 3 females and male on slide, Gilan Province, Lunak, 600 m a.s.l., forest, leaves, trunk, sifting, 6.VII.1973, leg. A. Senglet, 7313.
Etymology.
The new species is dedicated to Peter J. Schwendinger, curator of the Muséum d’histoire naturelle in Geneva and prominent Austrian Arachnologist.
Diagnosis.
Habitus typical of the genus Endonura. Dorsal tubercles present. 2+2 large pigmented eyes. Buccal cone relatively long, labrum nonogival. Head with chaetae B, C and D. Chaeta O absent. Tubercles Cl and Af separate. Tubercles Dl and (L+So) on head with 5 and 7 chaetae, respectively. Tubercles Di on Th. I absent. Tubercles De on Th. II and III with 3 and 4 chaetae, respectively. Tubercles L on Abd. III and IV with 2 and 4 chaetae, respectively. Abd. IV and V with 8 and 3 tubercles, respectively. Furcal rest without mi. Claw with inner tooth. Tibiotarsi with chaetae B4 and B5 long.
Description.
General. Body length (without antennae): 0.5 (juvenile) to 1.15 mm (holotype: 1.1 mm). Colour of the body bluish-grey. 2+2 large black eyes, in a typical arrangement for the genus (Fig. 29).
Chaetal morphology. Dorsal ordinary chaetae of five types: long macrochaetae (Ml), short macrochaetae (Mc), very short macrochaetae (Mcc), mesochaetae and microchaetae. Long macrochaetae relatively thin, straight or slightly arc-like, narrowly sheathed, feebly serrated, apically rounded (Figs 29, 39, 40). Macrochaetae Mc and Mcc morphologically similar to long macrochaetae, but much shorter (Figs 29, 39). Mesochaetae similar to ventral chaetae, thin, smooth and pointed. Microchaetae similar to mesochaetae, but clearly shorter (Figs 29, 39). S–chaetae of terga thin, smooth and short, notably shorter than nearby macrochaetae (Figs 29, 39, 41).
Antennae. Typical of the genus. Dorsal chaetotaxy of Ant. III–IV as Fig. 36 and Table 8. S–chaetae of Ant. IV of medium length and thickened, sensillum sgd short and straight (Fig. 36). Apical vesicle distinct, trilobate (Figs 34, 35). Ventral chaetotaxy of Ant. III–IV Table 8, sensillum sgv as Fig. 37.
Mouthparts. Buccal cone relatively short with labral sclerifications nonogival (Fig. 30). Labrum chaetotaxy: 4/2, 4. Labium with four basal, three distal and three lateral chaetae, papillae x absent (Fig. 28). Maxilla styliform (Fig. 32), mandible relatively thin with two basal and two apical teeth (Fig. 31).
Dorsal chaetotaxy and tubercles. Head without chaetae A, E, Ocp, Dl3, So2, L2 and L3 absent (Fig. 29), chaeta D free. Tubercles Di on Th. I not differentiated (Fig. 29). On Th. III chaetae De2 and De3 free, on Abd. I–III chaetae De3 free (Figs 29, 39). On Abd. I–III, the line of chaetae De1–chaeta s non perpendicular to the dorsomedian line. Cryptopygy present, but weakly developed, Abd. VI partially visible from above (Fig. 39).
Ventral chaetotaxy. On head, groups Vea, Vem and Vep with 3, 4 and 4 chaetae, respectively. Group Vi on head with 6 chaetae. On Abd. IV, furca rudimentary without microchaetae (Fig. 38). On Abd. IV, group L without free chaeta. On Abd. V, chaetae Vl present, chaetae L’ absent (Fig. 38). Male with thick and forked chaetae (male ventral organ) on anal plates (Abd. VI) and in groups: Ag (Abd. V); Vei, Vec and Vel (Abd. IV) and Fu (Abd. III) (Fig. 38).
Legs. Chaetotaxy of legs as in Table 9. Claw with internal tooth. On tibiotarsi, chaeta M present and chaetae B4 and B5 relatively long and pointed (Fig. 33).
Remarks.
Since E. schwendingeri sp. nov. is characterised by chaetotaxic features unknown in other members of the genus, for example, the absence of chaetae A and Ocp on the head, its closer affinities with other Endonura species are currently uncertain and hard to assess. However, taking into account the weak development of tuberculation, delicate buccal cone and the presence of well-developed male ventral organ, the new species seems to be most similar to E. quadriseta Cassagnau & Péja, 1979, a form shortly described from Greece (Cassagnau and Péja 1979), but recently re-described, based on types and a new material from the Crimea (Smolis et al. 2007). Nevertheless, besides characters mentioned above, these taxa differ in numerous features: the number of lateral labial chaetae (in schwendingeri, three, in quadriseta, four), the presence of chaetae C and O on the head (in schwendingeri, absent, in quadriseta, present), the number of chaetae (L+So) on the head (in schwendingeri, 7, in quadriseta, 9), the number of chaetae Dl on the head (in schwendingeri, 5, in quadriseta, 6), the number of chaetae L on Abd. III and IV (in schwendingeri, 2 and 4, in quadriseta, 4 and 7) and the presence of an internal tooth on claws (in schwendingeri, present, in quadriseta, absent).
Deutonura breviseta sp. nov.
D04DCB32-1579-58B7-BF3B-BC723607FC14
http://zoobank.org/98297969-EFC7-42C8-9597-14ED4612CD03
Figs 42–52 , Tables 10 , 11 , 12
Figures 42–52.
Deutonura breviseta sp. nov.: 42 chaetotaxy of head, Th. and Abd. I (holotype), dorsolateral view 43 chaetotaxy of tubercles Dl and (L+So), lateral view 44 apical bulb, dorsal view 45 apical bulb, ventral view 46 dorsal chaetotaxy of Ant. III–IV 47 ventral chaetotaxy of Ant. III 48 ventral chaetotaxy of Abd. IV–V (adult male) 49 claw of leg III, lateral view 50 dorsal chaetotaxy of Abd. IV–VI (holotype) 51 sensillum of Abd. V 52 chaeta Di1 of Abd. V.
Table 10.
Chaetotaxy of Deutonura breviseta sp. nov.: Cephalic chaetotaxy–dorsal side.
| Tubercle | Number of chaetae | Types of chaetae | Names of chaetae |
|---|---|---|---|
| Cl | 4 | Ml | F |
| Mc | G | ||
| Af | 8 | Ml | B |
| Mc | A | ||
| mi | C | ||
| mior me | D | ||
| Oc | 3 | Ml | Ocm, Ocp |
| mi | Oca | ||
| (Di+De) | 4 | Ml | Di1, De1 |
| Mc | Di2 | ||
| Mcc or mi | De2 | ||
| Dl | 6 | Ml | Dl5, Dl1 |
| Mc | Dl3, Dl4 | ||
| Mcc or mi | Dl6 | ||
| mi | Dl2 | ||
| (L+So) | 9 | Ml | L1, L4, So1 |
| me | So3–6 | ||
| mi | L2, So2 |
Table 11.
Chaetotaxy of Deutonura breviseta sp. nov.: Chaetotaxy of antennae.
| Segment, Group | Number of chaetae | Segment, Group | Number of chaetae adult |
|---|---|---|---|
| I | 7 | IV | or, 8 S, i, 12 mou, 6 brs, 2 iv |
| II | 12 | ||
| III | 5 sensilla AO III | ||
| ve | 5 | ap | 8 bs, 5 miA |
| vc | 4 | ca | 2 bs, 3 miA |
| vi | 4 | cm | 3 bs, 1 miA |
| d | 5 | cp | 8 miA, 1 brs |
Table 12.
Chaetotaxy of Deutonura breviseta sp. nov.: Postcephalic chaetotaxy.
| Terga | Legs | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Di | De | Dl | L | Scx2 | Cx | Tr | Fe | T | |
| Th. I | 1 | 2 | 1 | – | 0 | 3 | 6 | 13 | 19 |
| Th. II | 3 | 2+s | 3+s+ms | 3 | 2 | 7 | 6 | 12 | 19 |
| Th. III | 3 | 3+s | 3+s | 3 | 2 | 8 | 6 | 11 | 18 |
| Sterna | |||||||||
| Abd. I | 2 | 3+s | 2 | 3 | VT: 4 | ||||
| Abd. II | 2 | 3+s | 2 | 3 | Ve: 5; chaeta Ve1 present | ||||
| Abd. III | 2 | 3+s | 2 | 3 | Vel: 5; Fu: 5 me, 0 mi | ||||
| Abd. IV | 2 | 2+s | 3 | 6 | Vel: 4; Vec: 2; Vei: 2; Vl: 4 | ||||
| Abd. V | (3+3) | 5+s | Ag: 3; Vl: 1, L‘: 1 | ||||||
| Abd. VI | 7 | Ve: 14; An: 2 mi | |||||||
Type material.
Holotype: male on slide, Iran, Gilan Province, near Asalem, 300–600 m a.s.l., large beeches, sifting, 30.VI.1973, leg. A. Senglet, sample 7308. Paratypes: 3 females and 2 males on slide, same data as holotype.
Other material.
Iran, female on slide, Gilan Province, Asalem (37°45'N, 48°57'E), leaves and tree holes, sifting, 11.VI.1975, leg. A. Senglet, 7519; female, male and 2 juveniles on slide, Gilan Province, Paresar, tree holes, leaves, sifting, 2.VII.1973, leg. A. Senglet, 7310; male on slide, Mazandaran Province, Nashtarud, forest, reserve, sifting, 10.VII.1973, leg. A. Senglet, 7318; female and 3 males on slide, Mazandaran Province, near Amol, forest, sifting, 18.VII.1973, leg. A. Senglet, 7329b; 4 females, 3 males and juvenile on slide, Mazandaran Province, Aliabad, 30.VII.1974, leg. A. Senglet, 7475; female on slide, Gilan Province, road to Dyavaherdeh, 1100–1300 m a.s.l., 7.VIII. 1974, leg. A. Senglet, 7484.
Etymology.
The name of the new species is referring to its exceptionally short macrochaetae Ml.
Diagnosis.
Habitus typical of the genus Deutonura. Dorsal tubercles present and well developed. 2+2 large pigmented eyes. Buccal cone relatively long and wide, labrum without ogival sclerifications. Head without chaetae E, O and L3. Tubercles Cl and Af separate. No granular area between chaetae A and B on head. Tubercles De on Th. II and III with 3 and 4 chaetae, respectively. Tubercles Di on Abd. V not bilobed. Cryptopygy not developed. Male ventral organ present.
Description.
General. Body length (without antennae): 0.7 (juvenile) to 1.7 mm (holotype: 0.85 mm). Colour of the body white. 2+2 large black eyes, in a typical arrangement for the genus (Figs 42, 43).
Chaetal morphology. Dorsal ordinary chaetae of five types: long macrochaetae (Ml), short macrochaetae (Mc), very short macrochaetae (Mcc), mesochaetae and microchaetae. Long macrochaetae thickened, slightly arc-like or straight, narrowly sheathed, serrated, apically rounded and extended at apex (Figs 42, 43, 50, 52). Macrochaetae Mc and Mcc morphologically similar to long macrochaetae, but much shorter (Figs 42, 43, 50). Mesochaetae similar to ventral chaetae, thin, smooth and pointed. Microchaetae similar to mesochaetae, but clearly shorter (Figs 42, 43). S-chaetae of terga thin, smooth and short, notably shorter than nearby macrochaetae (Figs 42, 50, 51).
Antennae. Typical of the genus. Dorsal chaetotaxy of Ant. III–IV as in Fig. 46 and Table 11. S-chaetae of Ant. IV of medium length and relatively thin, sensillum sgd short and straight (Fig. 46). Apical vesicle distinct, trilobate (Figs 44, 45). Ventral chaetotaxy of Ant. III as in Fig. 47 and Table 11, ventral chaetotaxy of Ant. IV as Table 11.
Mouthparts. Buccal cone relatively short and wide, labral sclerifications nonogival (Fig. 42). Labrum chaetotaxy: 2/2, 4. Labium with four basal, three distal and four lateral chaetae, papillae x absent. Maxilla styliform mandible thin and tridentate.
Dorsal chaetotaxy and tubercles. Head without granular area between chaetae A and B. Elementary tubercles DE and EE on head absent (Fig. 42). Head without chaetae E, O and L3, chaeta D free (Figs 42, 43). Chaetae Ocm and Ocp of nearly equal length. Chaetae De2 on head usually as Mcc, rarely as mi (Fig. 42). Chaeta Dl6 on head as Mccormi. Th. I with tubercles Di and De not fused. Chaetae Di3 on Th. II–III free. On Th. III, chaetae De2 slightly shorter than De3 (Fig. 42). On Abd. I–III, chaetae De2 distinctly shorter than De3 (Fig. 50). Cryptopygy absent, Abd. VI well visible from above.
Ventral chaetotaxy. On head, groups Vea, Vem and Vep with 3, 4 and 4 chaetae, respectively. Group Vi on head with 6 chaetae. On Abd. IV, furca rudimentary without microchaetae. Male with thick and forked chaetae (male ventral organ) around genital aperture (Abd. V). On Abd. V, chaetae Vl and L’ present (Fig. 48).
Legs. Chaetotaxy of legs as in Table 12. Claw without internal tooth (Fig. 49). On tibiotarsi, chaeta M present and chaetae B4 and B5 relatively long and pointed.
Remarks.
Deutonura breviseta sp. nov. seems to be closest to D. persica Smolis, Shayanmehr & Yoosefi-Lafooraki, 2018 recently described from the northern part of Iran (Mazandaran Province, Smolis et al. 2018). However, these species differ in numerous characters, including the number of lateral labial chaetae (in breviseta, four, in persica, three), the presence of chaetae C on the head (in breviseta, present, in persica, absent), the number of chaetae (L+So) on the head (in breviseta, 9, in persica 8), the presence of chaetae Dl3 on the head (in breviseta, present, in persica, absent), the presence of microchaetae on furca rudimentary (in breviseta, absent, in persica, present) and the presence of cryptopygy (in breviseta, present, in persica, absent). Additionally, male ventral organ in D. breviseta sp. nov. is built of thickened and forked chaetae on Abd. V only (in persica, also on Abd. III, IV and VI).
Deutonura sengleti sp. nov.
148B3247-C0DD-503B-B4E1-92EF3AB43783
http://zoobank.org/15B48E2F-B8EF-4C46-8A2F-CC09CEDDD62A
Figs 53–61 , Tables 13 , 14 , 15
Figures 53–61.
Deutonura sengleti sp. nov. 53 chaetotaxy of head, Th. and Abd. I (holotype), dorsolateral view 54 dorsal chaetotaxy of Ant. III–IV 55 ventral chaetotaxy of Ant. III 56 chaeta Di1 of Abd. V 57 sensillum of Abd. V 58 apical part of labrum 59 chaetotaxy and ventral sclerifications of labrum 60 dorsal chaetotaxy of Abd. V–VI (holotype) 61 ventral chaetotaxy of Abd. III–IV (adult male).
Table 13.
Chaetotaxy of Deutonura sengleti sp. nov.: Cephalic chaetotaxy–dorsal side.
| Tubercle | Number of chaetae | Types of chaetae | Names of chaetae |
|---|---|---|---|
| Cl | 4 | Ml | F |
| Mc | G | ||
| Af | 10 | Ml | B |
| Mc | A | ||
| Mcc or mi | C | ||
| mi | D, E | ||
| Oc | 3 | Ml | Ocm, Ocp |
| mi | Oca | ||
| (Di+De) | 4 | Ml | Di1, De1 |
| Mc | Di2 | ||
| mi or Mcc | De2 | ||
| Dl | 6 | Ml | Dl5, Dl1 |
| Mc | Dl3, Dl4 | ||
| mi or Mcc | Dl2 | ||
| mi | Dl6 | ||
| (L+So) | 8 | Ml | L1, L4, So1 |
| me | So3–6 | ||
| mi or Mcc | L2 |
Table 14.
Chaetotaxy of Deutonura sengleti sp. nov.: Chaetotaxy of antennae.
| Segment, Group | Number of chaetae | Segment, Group | Number of chaetae adult |
|---|---|---|---|
| I | 7 | IV | or, 8 S, i, 12 mou, 6 brs, 2 iv |
| II | 12 | ||
| III | 5 sensilla AO III | ||
| ve | 5 | ap | 8 bs, 5 miA |
| vc | 4 | ca | 2 bs, 3 miA |
| vi | 4 | cm | 3 bs, 1 miA |
| d | 5 | cp | 8 miA, 1 brs |
Table 15.
Chaetotaxy of Deutonura sengleti sp. nov.: Postcephalic chaetotaxy.
| Terga | Legs | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Di | De | Dl | L | Scx2 | Cx | Tr | Fe | T | |
| Th. I | 3 | 1 | – | 0 | 3 | 6 | 13 | 19 | |
| Th. II | 3 | 2+s | 3+s+ms | 3 | 2 | 7 | 6 | 12 | 19 |
| Th. III | 3 | 3+s | 3+s | 3 | 2 | 8 | 6 | 11 | 18 |
| Sterna | |||||||||
| Abd. I | 2 | 3+s | 2 | 3 | VT: 4 | ||||
| Abd. II | 2 | 3+s | 2 | 3 | Ve: 5; chaeta Ve1 present | ||||
| Abd. III | 2 | 3+s | 2 | 3 | Vel: 4–5; Fu: 5 me, 6 mi | ||||
| Abd. IV | 2 | 2+s | 3 | 6 | Vel: 4; Vec: 2; Vei: 2; Vl: 4 | ||||
| Abd. V | (3+3) | 5+s | Ag: 3; Vl: 1, L‘: 1 | ||||||
| Abd. VI | 7 | Ve: 14; An: 2 mi | |||||||
Type material.
Holotype: female on slide, Iran, Gilan Province, Shahrbijar, tree hole, humus, sifting, 6.IX.1973, leg. A. Senglet, sample 7366. Paratypes: 2 males on slide, same data as holotype.
Other material.
Iran, 2 males on slide, Gilan Province, Limir, large trees in marsh, sifting, 28.VI.1973, leg. A. Senglet, 7306; female, 2 males and juvenile on slide, Gilan Province, road to Jirandeh, 1000 m a.s.l., forest, 9.VIII.1974, leg. A. Senglet, 7486; female, male and juvenile on slide, Gilan Province, near Asalem (37°38'N, 48°48'E), 1800 m a.s.l., tree holes, sifting, 10.VI.1975, leg. A. Senglet, 7516; 2 males and juvenile on slide, Gilan Province, near Asalem (37°40'N, 48°52'E), 1200 m a.s.l., tree holes, sifting, 10.VI.1975, leg. A. Senglet, 7517; female on slide Gilan Province, Asalem (37°45'N, 48°57'E), leaves and tree holes, sifting, 11.VI.1975, leg. A. Senglet, 7519; male on slide, Mazandaran Province, near Amol, forest, sifting, 18.VII.1973, leg. A. Senglet, 7329b; male on slide, Mazandaran Province, road to Tchorteh, 800 m a.s.l., tree and leaves, sifting, 5.VIII.1974, leg. A. Senglet, 7482.
Etymology.
The new species is dedicated to Antoine Senglet, collector of the Iranian material studied and prominent Swiss Arachnologist.
Diagnosis.
Habitus typical of the genus Deutonura. Dorsal tubercles present and well developed. 2+2 large pigmented eyes. Buccal cone relatively long and narrow, labrum without ogival sclerifications. Head without chaetae O, So2 and L3. Tubercles Cl and Af separate. No granular area between chaetae A and B on head. Tubercles De on Th. II and III with 3 and 4 chaetae, respectively. Tubercles Di on Abd. V not bilobed. Cryptopygy not developed. Male ventral organ present.
Description.
General. Body length (without antennae): 0.85 (juvenile) to 1.55 mm (holotype: 1.45 mm). Colour of the body bluish-grey. 2+2 large black eyes, in a typical arrangement for the genus (Fig. 53).
Chaetal morphology. Dorsal ordinary chaetae of five types: long macrochaetae (Ml), short macrochaetae (Mc), very short macrochaetae (Mcc), mesochaetae and microchaetae. Long macrochaetae thickened, slightly arc-like or straight, narrowly sheathed, serrated, cylindrical, apically rounded (Figs 53, 56, 60). Macrochaetae Mc and Mcc morphologically similar to long macrochaetae, but much shorter (Figs 53, 60). Mesochaetae similar to ventral chaetae, thin, smooth and pointed. Microchaetae similar to mesochaetae, but clearly shorter (Figs 53, 60). S-chaetae of terga thin, smooth and short, notably shorter than nearby macrochaetae (Figs 53, 57, 60).
Antennae. Typical of the genus. Dorsal chaetotaxy of Ant. III–IV as in Fig. 54 and Table 14. S–chaetae of Ant. IV long and relatively thin, S3 notably longer than others, sensillum sgd of medium size and straight (Fig. 54). Apical vesicle distinct, trilobate. Ventral chaetotaxy of Ant. III as in Fig. 55 and Table 14.
Mouthparts. Buccal cone relatively long and narrow, labral sclerifications nonogival (Figs 58, 59). Labrum chaetotaxy: 4/2, 4 (Fig. 59). Labium with four basal, three distal and four lateral chaetae, papillae x absent. Maxilla styliform mandible thin and tridentate.
Dorsal chaetotaxy and tubercles. Head without granular area between chaetae A and B. Elementary tubercles DE and EE on head absent (Fig. 53). Head without chaetae O, L3 and So2, chaeta D free. Chaetae C as Mccormi (Fig. 53). Chaetae Ocm and Ocp of nearly equal length. Chaetae De2 on head as mior rarely Mcc (Fig. 53). Th. I with tubercles Di and De fused (Fig. 53). Chaetae Di3 on Th. II–III free. On Th. III, chaetae De2 slightly longer than De3 (Fig. 53). On Abd. I–III, chaetae De2 shorter than De3. Cryptopygy absent, Abd. VI well visible from above.
Ventral chaetotaxy. On head, groups Vea, Vem and Vep with 3, 4 and 4 chaetae, respectively. Group Vi on head with 6 chaetae. On Abd. IV, furca rudimentary with 6 minute microchaetae without visible chaetopores (Fig. 61). Male with thick and forked chaetae (male ventral organ) on furca rudimentary (Abd. IV, Fig. 61) and around genital aperture (Abd. V). On Abd. V, chaetae Vl and L’ present.
Legs. Chaetotaxy of legs as in Table 15. Claw without internal tooth. On tibiotarsi, chaeta M present and chaetae B4 and B5 of medium size and pointed.
Remarks.
The new species runs in the most recent key to Deutonura species (Deharveng et al. 2015) to D. caerulescens Deharveng, 1982 from France (Deharveng 1982). However, these species differ in the number of chaetae (L+So) on the head (in sengleti, 8, in caerulescens, 9–10), the presence of microchaetae on furca rudimentary (in sengleti, present, in caerulescens, absent), the number of chaetae L on Abd. III and IV (in sengleti, 3 and 6 chaetae, in caerulescens, 4 and 8 chaetae), the number of chaetae on tubercle (De+Dl+L) of Abd. V (in sengleti, 5+s, in caerulescens, 7+s) and ratio of chaetae Di1:Di2:Di3 on Abd. V (in sengleti, 1:4:16, in caerulescens, 1:2:4 or 1:3:7).
Deutonura iranica sp. nov.
0B499BA7-1D6A-5DAB-B3F3-228F3DEF02B8
http://zoobank.org/A3E5E3DA-122E-4C11-888D-CF1265288184
Figs 62–71 , Table 16 , 17 , 18
Figures 62–71.
Deutonura iranica sp. nov.: 62 chaetotaxy and ventral sclerifications of labrum 63 chaetotaxy of head and Th. (holotype), dorsolateral view 64 dorsal chaetotaxy of Ant. III–IV 65 ventral chaetotaxy of Ant. III–IV 66 chaetotaxy of tubercles L of Abd. III–IV, ventral view 67 chaetotaxy of labium and group Vi68 dorsal chaetotaxy of Abd. III–VI (holotype) 69 chaeta Di1 of Abd. V 70 chaeta Di2 of Abd. V 71 sensillum of Abd. V.
Table 16.
Chaetotaxy of Deutonura iranica sp. nov.: Cephalic chaetotaxy–dorsal side.
| Tubercle | Number of chaetae | Types of chaetae | Names of chaetae |
| Cl | 4 | M | F |
| Mc | G | ||
| Af | 10 | Ml | B |
| Mc | A, E | ||
| Mcc | C, D | ||
| Oc | 3 | Ml | Ocm |
| Mc | Ocp | ||
| mi | Oca | ||
| (Di+De) | 4 | Ml | Di1, De1 |
| Mcc | Di2, De2 | ||
| Dl | 6 | Ml | Dl5, Dl1 |
| Mc | Dl3, Dl4 | ||
| Mcc | Dl2, Dl6 | ||
| (L+So) | 7 | Ml | L1, L4, So1 |
| me | So3–6 |
Table 17.
Chaetotaxy of Deutonura iranica sp. nov.: Chaetotaxy of antennae.
| Segment, Group | Number of chaetae | Segment, Group | Number of chaetae II instar |
|---|---|---|---|
| I | 7 | IV | or, 8 S, i, 10 mou, 4 brs, 2 iv |
| II | 12 | ||
| III | 5 sensilla AO III | ||
| ve | 5 | ap | 8 bs, 5 miA |
| vc | 4 | ca | 2 bs, 3 miA |
| vi | 4 | cm | 3 bs, 1 miA |
| d | 5 | cp | 8 miA, 1 brs |
Table 18.
Chaetotaxy of Deutonura iranica sp. nov.: Postcephalic chaetotaxy.
| Terga | Legs | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Di | De | Dl | L | Scx2 | Cx | Tr | Fe | T | |
| Th. I | 1 | 2 | 1 | - | 0 | 3 | 6 | 13 | 19 |
| Th. II | 3 | 2+s | 3+s+ms | 3 | 2 | 7 | 6 | 12 | 19 |
| Th. III | 3 | 3+s | 3+s | 3 | 2 | 8 | 6 | 11 | 18 |
| Sterna | |||||||||
| Abd. I | 2 | 3+s | 2 | 3 | VT: 4 | ||||
| Abd. II | 2 | 3+s | 2 | 3 | Ve: 5; chaeta Ve1 present | ||||
| Abd. III | 2 | 3+s | 2 | 4 | Vel: 5; Fu: 4 me, 0 mi | ||||
| Abd. IV | 2 | 2+s | 3 | 8 | Vel: 4; Vec: 2; Vei: 2; Vl: 4 | ||||
| Abd. V | (3+3) | 7+s | Ag: 3; Vl: 1, L‘: 1 | ||||||
| Abd. VI | 7 | Ve: 14; An: 2 mi | |||||||
Type material.
Holotype: juvenile (second instar) on slide, Iran, West Azerbaijan Province, Choj (38°37'N, 45°02'E), 1.VI.1975, leg. A. Senglet, sample 7503.
Etymology.
The species name refers to the country of its collecting.
Diagnosis.
Habitus typical of the genus Deutonura. Dorsal tubercles present and well developed. 2+2 large pigmented eyes. Buccal cone relatively long and narrow, labrum without ogival sclerifications. Head without chaetae O, So2, L2 and L3. Tubercles Cl and Af separate. No granular area between chaetae A and B on head. Tubercles De on Th. II and III with 3 and 4 chaetae, respectively. Tubercles Di on Abd. V bilobed. Cryptopygy strongly developed.
Description.
General. Body length (without antennae): holotype: 1.05 mm. Colour of the body white. 2+2 large black eyes, in a typical arrangement for the genus (Fig. 63).
Chaetal morphology. Dorsal ordinary chaetae of five types: long macrochaetae (Ml), short macrochaetae (Mc), very short macrochaetae (Mcc), mesochaetae and microchaetae. Long macrochaetae relatively thin, arc-like or straight, narrowly sheathed, feebly serrated, apically sharply pointed (Figs 63, 68–70). Macrochaetae Mc and Mcc morphologically similar to long macrochaetae, but much shorter (Figs 63, 68). Mesochaetae similar to ventral chaetae, thin, smooth and pointed. Microchaetae similar to mesochaetae, but clearly shorter. S-chaetae of terga thin, smooth and short, shorter than nearby macrochaetae (Figs 63, 68, 71).
Antennae. Typical of the genus. Dorsal chaetotaxy of Ant. III–IV as in Fig. 64 and Table 17. S-chaetae of Ant. IV long and relatively thin, S3 notably longer than others, sensillum sgd of medium size and straight (Fig. 64). Apical vesicle distinct, trilobate. Ventral chaetotaxy of Ant. III–IV as in Fig. 65 and Table 17.
Mouthparts. Buccal cone relatively long and narrow, labral sclerifications nonogival (Figs 62, 67). Labrum chaetotaxy: 4/2, 4 (Fig. 62). Labium with four basal, three distal and four lateral chaetae, papillae x absent (Fig. 67). Maxilla styliform mandible thin and tridentate.
Dorsal chaetotaxy and tubercles. Head without granular area between chaetae A and B. Elementary tubercles DE and EE on head present (Fig. 63). Head without chaetae O, L2, L3 and So2. Chaetae C as Mcc. Chaetae Ocp notably shorter than Ocm. Chaetae De2 on head as Mcc (Fig. 63). Th. I with tubercles Di and De not fused. Chaetae Di3 on Th. II–III connected with tubercle Di. On Th. III, chaetae De2 slightly longer than De3 (Fig. 63). On Abd. I–III, chaetae De2 longer than De3 (Fig. 68). Cryptopygy present and strongly developed, Abd. VI invisible from above (Fig. 68).
Ventral chaetotaxy. On head, groups Vea, Vem and Vep with 4, 3 and 4 chaetae, respectively. Group Vi on head with 6 chaetae (Fig. 67). Tubercles L on Abd. III and IV with 4 and 6 chaetae, respectively (Fig. 66). On Abd. IV, furca rudimentary without microchaetae. On Abd. V, chaetae Vl and L’ present.
Legs. Chaetotaxy of legs as in Table 18. Claw without internal tooth. On tibiotarsi, chaeta M present and chaetae B4 and B5 of medium size and pointed.
Remarks.
Since juveniles (beginning from the first instar) of the subfamily Neanurinae are characterised by the complete chaetotaxy of the head, thorax and abdomen, we decided to describe the new species despite having only one specimen of the second instar. D. iranica sp. nov. runs in the most recent key to Deutonura species (Deharveng et al. 2015) to D. gibbosa Porco, Bedos & Deharveng, 2010, a form common and widespread in southern France (the Alps and Jura), Switzerland, Italy and Slovenia (Porco et al. 2010). Both species are readily distinguished from most members of the genus by the presence of very prominent and conspicuously bilobed tubercle (Di+Di) on the penultimate abdominal segment. This unique character is additionally associated with the specific chaetotaxic arrangement of chaetae Di, with their shift backwards. D. iranica sp. nov. can be easily separated from D. gibbosa by the presence of white body colour (in gibbosa deep to light blue), the presence of 7 chaetae on cephalic tubercle (L+So) (in gibbosa, 8–9 chaetae), the presence of cephalic chaetae Ocp equal chaetae A (in gibbosa, chaetae Ocp distinctly longer than A) and the presence of 4 lateral labial chaetae (in gibbosa, 3 chaetae).
Paravietnura rostrata sp. nov.
FBFBEB25-68F5-52EE-982B-F10D766B149F
http://zoobank.org/E4B57858-235D-4FCC-99D6-A7AFBE6848A7
Figs 72–82 , Tables 19 , 20 , 21
Figures 72–82.
Paravietnura rostrata sp. nov.: 72 apical part of Ant. IV, dorsal view 73 sensillum sgd and microsensilla of AOIII, dorsolateral view 74 sensillum sgv and microsensillum of Ant. III 75 chaetotaxy of head and Th. (holotype), dorsolateral view 76 chaetotaxy and ventral sclerifications of labrum 77 chaetotaxy of labium and group Vi78 furca rudimentary 79 dorsal chaetotaxy of Abd. III–VI (holotype) 80 claw of leg III, lateral view 81 chaeta Di1 of Abd. III 82 sensillum of Abd. IV.
Table 19.
Chaetotaxy of Paravietnura rostrata sp. nov.: Cephalic chaetotaxy–dorsal side.
| Tubercle | Number of chaetae | Types of chaetae | Names of chaetae |
|---|---|---|---|
| Cl | 2 | Ml | F |
| (Af+2Oc) | 4 | Ml | B, Ocp |
| (Di+De) | 2 | Ml | Di1, De1 |
| (Dl+L+So) | 9 | impossible to recognise |
Table 20.
Chaetotaxy of Paravietnura rostrata sp. nov.: Chaetotaxy of antennae.
| Segment, Group | Number of chaetae | Segment, Group | Number of chaetae II instar |
|---|---|---|---|
| I | 7 | IV | or, 8 S, i, 10 mou, 4 brs, 2 iv |
| II | 11 | ||
| III | 5 sensilla AO III | ||
| ve | 5 | ap | 8 bs, 5 miA |
| vc | 4 | ca | 2 bs, 3 miA |
| vi | 4 | cm | 3 bs, 1 miA |
| d | 4 | cp | 8 miA, 1 brs |
Table 21.
Chaetotaxy of Paravietnura rostrata sp. nov.: Postcephalic chaetotaxy.
| Terga | Legs | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Di | De | Dl | L | Scx2 | Cx | Tr | Fe | T | |
| Th. I | 1 | 1 | 1 | – | 0 | 3 | 6 | 13 | 19 |
| Th. II | 2 | 1+s | 2+s+ms | 3 | 2 | 7 | 6 | 12 | 19 |
| Th. III | 2 | 1+s | 2+s | 3 | 2 | 8 | 6 | 11 | 18 |
| Sterna | |||||||||
| Abd. I | 2 | 2+s | 2 | 2 | VT: 4 | ||||
| Abd. II | 2 | 2+s | 2 | 2 | Ve: 3; chaeta Ve1 present | ||||
| Abd. III | 2 | 2+s | 2 | 2 | Vel: 3; Fu: 4 me, 4 mi | ||||
| Abd. IV | (1+1) | 1+s | 3 | 3 | Vel: 2; Vec: 2; Vei: 2; Vl: 4 | ||||
| Abd. V | 4+s | Ag: 2; Vl: 1 | |||||||
| Abd. VI | 7 | Ve: 11; An: 1mi | |||||||
Type material.
Holotype: juvenile (second instar) on slide, Iran, Gilan Province, Shahrbijar, tree hole, humus, sifting, 6.IX.1973, leg. A. Senglet, sample 7366.
Etymology.
The name of the new species referring to its exceptionally-long buccal cone.
Diagnosis.
Habitus typical of the genus Paravietnura with stumpy and short body. Macrochaetae long thick and widely sheathed. 2+2 large pigmented eyes. Buccal cone extremely long and narrow, labrum with ogival sclerifications. Tubercle (Af + 2Oc) with chaetae B and Ocm, chaetae A and Ocp absent. Tubercle Cl without chaetae G. Tubercle (Dl+L+So) with 9 chaetae. Furca rudimentary with minute and difficult microchaetae, without chaetopores.
Description.
General. Body length (without antennae): holotype: 0.45 mm. Colour of the body bluish. 2+2 large black eyes, in a typical arrangement for the genus (Fig. 75).
Chaetal morphology. Dorsal ordinary chaetae of five types: long macrochaetae (Ml), short macrochaetae (Mc), very short macrochaetae (Mcc), mesochaetae and microchaetae. Long macrochaetae thickened, arc-like, widely sheathed, strongly serrated, apically rounded (Figs 75, 79, 81). Macrochaetae Mc and Mcc morphologically similar to long macrochaetae, but much shorter (Figs 75, 79). Mesochaetae similar to ventral chaetae, thin, smooth and pointed. Microchaetae similar to mesochaetae, but clearly shorter (Figs 75, 79). S–chaetae of terga thin, smooth and short, shorter than nearby macrochaetae (Figs 75, 79, 82).
Antennae. Typical of the genus. Dorsal and ventral chaetotaxy of Ant. III–IV as in Figs 72–74 and Table 20. S-chaetae of Ant. IV relatively short and thin (Fig. 72), sensillum sgd of medium size and straight (Fig. 73), sensillum sgv relatively long and slightly s-shaped (Fig. 74). Apical vesicle distinct, bilobate (Fig. 72).
Mouthparts. Buccal cone extremely elongated with labral sclerifications ogival (Figs 76, 77). Labrum chaetotaxy: 0/2, 4, without prelabral chaetae (Fig. 76). Labium with three basal, three distal and two lateral chaetae, papillae x absent (Fig. 77). Maxilla styliform mandible thin and tridentate.
Dorsal chaetotaxy and tubercles. Chaetotaxy of head as in Fig. 75 and Table 19. Chaetotaxy of Th. and Abd. As in Figs 75, 79 and Table 21. On Th. I, tubercle De with one chaeta (Fig. 75). On Th. II and III, chaetae Di 3 absent. Th. II and III with two chaetae De (Fig. 75). On Abd. IV, chaetae Di1 distinctly longer than Abd. V (Fig. 79). On Abd. V, chaetae Di2 and Di3 absent. Tubercle Di of Abd. IV partially fused (Fig. 79). Cryptopygy present and strongly developed, Abd. VI invisible from above (Fig. 79).
Ventral chaetotaxy. On head, groups Vea, Vem and Vep with 3, 2 and 4 chaetae, respectively. Group Vi on head with 5 chaetae (Fig. 77). On Abd. IV, furca rudimentary with 4 minute microchaetae and 4 mesochaetae (Fig. 78). On Abd. V, chaetae Vl present and L’ absent.
Legs. Chaetotaxy of legs as in Table 21. Claw without internal tooth (Fig. 80). On tibiotarsi, chaeta M present and chaetae B4 and B5 of medium size and pointed.
Remarks.
No doubt, the new species is the third member of the remarkable Neanurinae genus Paravietnura Smolis & Kuznetsova, 2018 described recently from the Caucasus (Smolis and Kuznetsova 2018). Paravietnura rostrata sp. nov. seems to be the closest to P. notabilis Smolis & Kuznetsova, 2018; however, it can be easily separated from the mentioned species because of the reduction of its cephalic chaetotaxy (in rostrata, chaetae G and Ocp absent, in notabilis, present), extremely elongated labrum, which is well visible from above (in notabilis, feebly elongated and practically invisible from above), absence of prelabral chaetae (in notabilis, 2 chaetae present), the presence of 1+1 chaetae De on Th. I (in notabilis, 2+2 chaetae present), the absence of chaetae Di3 on Th. (in notabilis, present), reduction of the number of chaetae De on Th. II and III (in rostrata, 1+s chaetae, in notabilis, 2+s and 3+s chaetae, respectively), the absence of chaetae De2 and De3 on Abd. I–III (in notabilis, present), the fusion of tubercles Di on Abd. IV (in notabilis, not fused) and the presence of 1 chaeta Di on Abd. V (in notabilis, 3 chaetae Di present).
New Records
Cryptonura maxima
Smolis, Falahati & Skarżyński, 2012
60F1401A-46E8-548B-A8CC-EC165051640C
Material.
Iran, Mazandaran Province, Baladeh, 2200 m a.s.l., 12.VII.1974, leg. A. Senglet, sample 7459; numerous specimens on slide, Iran Mazandaran Province, Aliabad, 30.VII.1974, leg. A. Senglet, 7475.
Note.
Up to date, the species was known from the Elburz Mts. in Golestan Province (Smolis et al. 2012).
Cryptonura persica
Smolis, Falahati & Skarżyński, 2012
AFDC7E5C-B0D9-55D2-A37F-397E76621E71
Material.
Iran, Mazandaran Province, near Gorgan, forest, mosses, sifting, 20.VII.1973, leg. A. Senglet, sample 7332; Mazandaran Province, near Shahpasand, leaves, sifting, 29.VII.1974, leg. A. Senglet, 7473; West Azerbaijan Province, Choj (38°37'N, 45°02'E), 1.VI.1975, leg. A. Senglet, 7503; Golestan Province, near Tangrah (37°23'N, 55°50'E), 16.VII.1975, leg. A. Senglet, 7552; North Khorasan Province, near Tangrah (37°20'N, 56°01'E), 16.VII.1975, leg. A Senglet, 7553; Golestan Province, near Loveh (37°20'N, 55°44'E / 700 m a.s.l.), 21.VIII.1975, leg. A. Senglet, 7572; Golestan Province, near Loveh (37°18'N, 55°43'E / 1200 m a.s.l.), 21.VIII.1975, leg. A Senglet, 7573; Semnan Province, near Loveh (37°19'N, 55°46'E / 1300 m a.s.l.), 22.VIII.1975, leg. A. Senglet, 7574.
Note.
Similarly to the previous species, C. persica was known exclusively from the Elburz Mts. in Golestan Province (Smolis et al. 2012). The outlined records, from provinces West Azerbaijan, Mazandaran, Semnan and North Khorasan, shows that this form seems to be quite common and widespread in north-western Iran.
Deutonura persica
Smolis, Shayanmehr & Yoosefi-Lafooraki, 2018
D09450A7-F45E-5FEA-B42F-6CB08643DF14
Material.
Iran, Gilan Province, near Asalem (37°42'N, 48°53'E), 450 m a.s.l., tree holes, sifting, 10.VI.1975, leg. A. Senglet, sample 7518; Iran, Mazandaran Province, Ivel (36°14'N, 53°37'E / 1500 m a.s.l.), under stones, 11.VII.1975, leg. A Senglet, 7547A.
Note.
Until now, the species was known from its type locality only: Hezarjarib Forest in region Neka in Mazandaran Province (Smolis et al. 2018).
Endonura longirostris
Smolis, Shayanmehr, Kuznetsova & Yoosefi-Lafooraki, 2017
80C7150E-9F51-56D0-8917-C932DDA54A04
Material.
Iran, Mazandaran Province, Nashtarud, forest, reserve, sifting, 10.VII.1973, leg. A. Senglet, sample 7318; Iran, Mazandaran Province, near Delaam, forest, 4.VIII.1974, leg. A. Senglet, 7478; Golestan Province, near Loveh (37°20'N, 55°44'E / 700 m a.s.l.), 21.VIII.1975, leg. A. Senglet, 7572.
Note.
Up to now, this very characteristic member of the genus Endonura was known from two localities in Mazandaran Province (Smolis et al. 2017).
Endonura paracentaurea
Smolis, Shayanmehr, Kuznetsova & Yoosefi-Lafooraki, 2017
2FD81BE4-C854-52F8-9258-4FADF32EB08D
Material.
Iran, Gilan Province, Limir, ;large trees in marsh, sifting, 28.VI.1973, leg. A. Senglet, sample 7306; Gilan Province, Shahrbijar, tree hole, humus, sifting, 6.IX.1973, leg. A. Senglet, 7366; Mazandaran Province, road to Tchorteh, 800 m a.s.l., tree and leaves, sifting, 5.VIII.1974, leg. A. Senglet, 7482.
Note.
Until now, Endonura paracentaurea was recorded exclusively from Mazandaran Province (Smolis et al. 2017).
Neanura deharvengi
Smolis, Shayanmehr & Yoosefi-Lafooraki, 2018
02BC902A-14A3-591A-8CC1-F5284DDACBA1
Material.
Iran, Gilan Province, Limir, big trees in marsh, sifting, 28.VI.1973, leg. A. Senglet, sample 7306; Mazandaran Province, Nashtarud, forest, reserve, sifting, 10.VII.1973, leg. A. Senglet, 7318; Mazandaran Province, Kiasar, very dry forest, sifting, 22.VII.1973, leg. A. Senglet, 7334.
Note.
To date, this unique member of the genus Neanura MacGillivray, 1893 characterised by strong reduction of cephalic chaetotaxy, was recorded from two localities in Mazandaran Province only (Smolis et al. 2018).
Neanura muscorum
(Templeton, 1835)
4071A517-C817-551F-ACE4-744E97A5AF78
Material.
Iran, Gilan Province, Zandżan (36°43'N, 48°21'E), 15.IX.1973, leg. A. Senglet, sample 7372.
Note.
Up to now, this cosmopolitan and the most widespread member of the subfamily Neanurinae was recorded from three Iranian provinces: Zanjan, Gilan and Mazandaran (Cox 1982, Yahyapour 2012).
Protanura papillata
Cassagnau & Delamare Deboutteville, 1955
DE62A109-DC0F-5945-8EE4-87F3CF6B6449
Material.
Iran, Kermanshah Province, Geravand, 5.VIII.1973, leg. A. Senglet, sample 7344.
Note.
This species is known from Lebanon, Israel and Iran (Smolis et al. 2016b). The present record is the third from Kermanshah Province.
Discussion
Until recently, the whole knowledge on richness and diversity of Iranian Neanurinae was based solely on a Cox’s (1982) paper, in which four European and rather common taxa, i.e. Neanura muscorum and Bilobella aurantiaca (Caroli, 1912) were mentioned. However, the last decade has resulted in a real explosion of research on Iranian Collembola. Taking into account all recent data, one can conclude that Neanurinae fauna of Iran contains 21 species of the following genera: Bilobella Caroli, 1912 – 1; Cryptonura Cassagnau, 1979 – 2; Deutonura Cassagnau, 1979 – 5; Endonura Cassagnau, 1979 – 8; Neanura MacGilliwray, 1893 – 2; Paravietnura Smolis & Kuznetsova, 2018 – 1; Persanura Mayvan, Smolis & Skarżyński, 2015 – 1; Protanura Börner, 1906 and Thaumanura Börner, 1932 – 1 (Cox 1982; Smolis et al. 2012; Mayvan et al. 2015b; Smolis et al. 2016a, b, 2017; Smolis and Kuznetsova 2018; Smolis et al. 2018). Despite the fact that the image of diversity and richness of Iranian Neanurinae is still incomplete, some general comments can be made.
Firstly, the Iranian fauna is characterised by a remarkable percentage of endemites, since seventeen species are known exclusively from this country. This number is probably underestimated as earlier records of some taxa, i.e. Bilobella aurantiaca, Thaumanura echinata (Kos, 1940) and Deutonura decolorata (Gama & Gisin, 1964 in: Gisin 1964) are rather unlikely and should be revised. Such a high number of endemites is certainly noteworthy; nevertheless, it is a known and rather general phenomenon for this group of springtails. Research conducted, both in tropical and temperate forests, indicated that Neanurinae have a strong tendency to speciation and their fauna on a larger geographical scale is often characterised by a high degree of endemism (e.g. Deharveng 1979; Cassagnau and Palacios-Vargas 1983; Deharveng and Weiner 1984; Cassagnau and Deharveng 1984; Cassagnau 1988, 1996; Greenslade 1994; Palacios-Vargas and Simón Benito 2007; Janion et al. 2011; Queiroz and Deharveng 2015; Smolis 2017).
Secondly, in terms of species richness, this fauna should be treated even today as very rich. Especially, the Hyrcanian forest, where sixteen species of the subfamily were noted, seems to be not only a national but also a regional hot spot. The observed situation, however, may not be especially surprising as this huge and diversified area covers almost one million hectares and ranges from west to east through five Iranian Provinces: Ardabil, Gilan, Mazandaran, Golestan and North Khorasan. In addition, this forest is a worldwide and commonly-known refuge for many iconic and spectacular mammals, i.e. the Persian leopard Panthera pardus ciscaucasica, trees, i.e. the Persian ironwood Parrotia persica, the Caspian locust tree Gleditsia capsica and insects, i.e. the longhorn beetle Parandra caspia, the red flat beetle Cucujus muelleri (e.g. Sagheb-Talebi et al. 2014; Mayvan et al. 2015a; Müller et al. 2015; Bussler 2017).
Finally, current and especially future knowledge (many regions of Iran still remain unexplored, see Shayanmehr et al. 2013, Fig. 2) of the Iranian Neanurinae fauna could shed light on key issues such as its origin and relationship with fauna of neighbouring regions. For example, the similarity of Iranian fauna to that of the Caucasus (presence of genera Paravietnura and Persanura) and the east Mediterranean region (presence of Protanura papillata and genus Cryptonura) should already be underlined.
Supplementary Material
Acknowledgements
The work was financially supported by the Institute of Environmental Biology, Faculty of Biological Science, University of Wrocław, Poland (project no. 1076/Ś/IBŚ/2020). We are grateful to Prof. Louis Deharveng and Prof. Javier Arbea who reviewed the manuscript. We are also much indebted to the Editor Prof. Wanda Maria Weiner for helpful remarks.
Citation
Smolis A, Skarżyński D (2020) Contribution to the knowledge of Neanurinae of north-western Iran with description of seven new species (Collembola, Neanuridae). ZooKeys 992: 105–138. https://doi.org/10.3897/zookeys.992.56921
References
- Axelson WM. (1905) Einige neue Collembolen aus Finland. Zoologisher Anzeiger 28: 788–794. [Google Scholar]
- Bellinger PF, Christiansen KA, Janssens F. (2020) Checklist of the Collembola of the world. http://www.collembola.org [Accessed 13 July 2020]
- Börner C. (1906) Das System der Collembolen nebst Beschreibung neuer Collembolen des Hamburger Naturhistorischen Museums. Mitteilungen aus den Naturhistorischen Museum in Hamburg, XXIII. Jahrgang, 2. Beiheft zum Jahrbuch der Hamburgischen Wissenschaftlichen Austalten, XXIII, 1905, Hamburg 1906, 147–188.
- Börner C. (1932) Apterygota. In: Brohmer P. (Ed.) Fauna von Deutschland, 4th ed.Auflage 4, Leipzig, 136–143.
- Bussler H. (2017) Cucujus muelleri sp. nov. aus den kaspischen Gebirgswäldern des Iran (Coleoptera: Cucujidae). Nachrichtenblatt der Bayerischen Entomologen 66(3/4): 54–58.
- Caroli E. (1912) Contribuzioni alla conosceza dei Collemboli italiani. I. La tribu degli Achorutini. Archivio Zoologico Italiano 6: 349–374. [Google Scholar]
- Cassagnau P. (1979) Les Collemboles Neanuridae des Pays Dinaro-Balkaniques: leur interêt phylogénétique et biogéographique. Biologia Gallo – Hellenica 8: 185–203. [Google Scholar]
- Cassagnau P. (1988) Les Collemboles Neanurinae des Masif du sud de l’Inde et de Ceylan. Travaux du Laboratoire d’écobiologie des Arthropodes Edaphiques, Toulouse 5(4): 21–51. [Google Scholar]
- Cassagnau P. (1996) Collemboles Neanurini primitifs d’Afrique et de Madagascar. Annales de la Société entomologique de France 32(2): 121–161. [Google Scholar]
- Cassagnau P, Deharveng L. (1984) Collemboles des Philippines. 1. Les lobelliens multicolores de montagnes de Luzon. Travaux du Laboratoire d’écobiologie des Arthropodes Edaphiques, Toulouse 5(1): 1–11. [Google Scholar]
- Cassagnau P, Delamare Deboutteville C. (1955) Mission Henri Coiffait (1951). 3. Collemboles. Archives de Zoologie Expérimentale et Génerale 91: 365–395. [Google Scholar]
- Cassagnau P, Palacios-Vargas JG. (1983) Contribution a l’étude des Collemboles Neanurinae d’Amerique Latine. Travaux de Laboratoire d’Écobiologie des Arthropodes Édaphiques, Toulouse 4(1): 1–16. [Google Scholar]
- Cassagnau P, Péja N. (1979) Diagnoses préliminaries de quelques Neanuridae de Grèce et d’Albanie. Biologia Gallo – Hellenica 8: 205–222. [Google Scholar]
- Cox P. (1982) The Collembola fauna of north and north western Iran. Entomologist’s Monthly Magazine 118: 39–43. [Google Scholar]
- Deharveng L. (1979) Contribution à la connaissance des Collemboles Neanurinae de France et de la Peninsule Iberique. Travaux du Laboratoire ďÉcobiologie des Arthropodes Edaphiques, Toulouse 1: 1–61. [Google Scholar]
- Deharveng L. (1982) Contribution à l’étude des Deutonura du groupe phlegraea. Travaux de Laboratoire d’Écobiologie des A rthropodes Édaphiques, Toulouse 3(2): 1–20. [Google Scholar]
- Deharveng L. (1983) Morphologie évolutive des Collemboles Neanurinae en particulier de la lignée néanurienne. Travaux de Laboratoire d’Écobiologie des Arthropodes Édaphiques, Toulouse 4: 1–63. [Google Scholar]
- Deharveng L. (1989) The genus Paranura Axelson, 1902 in Thailand (CollembolaNeanurinae). Tropical Zoology 2: 103–121. 10.1080/03946975.1989.10539432 [DOI] [Google Scholar]
- Deharveng L, Bedos A. (1992) Blasconurella, a new genus of Neanurinae from Thailand, with five new species. Tropical Zoology 5: 299–311. 10.1080/03946975.1992.10539201 [DOI] [Google Scholar]
- Deharveng L, Weiner WM. (1984) Collemboles de Corée du Nord III–Morulinae et Neanurinae. Travaux de Laboratoire d’Écobiologie des Arthropodes Édaphiques, Toulouse 4: 1–61. [Google Scholar]
- Deharveng L, Suhardjono Y. (2000) Sulobella yoshii, a New Genus New Species of Lobellini (Collembola: Neanurinae) from South Sulawesi, with Comments on the Tribe Lobellini. Contributions from the Biological Laboratory Kyoto University 29(2): 83–87. [Google Scholar]
- Deharveng L, Mouloud SA, Bedos A. (2015) A new species of Deutonura (Collembola: Neanuridae: Neanurinae) from Algeria, with revised diagnosis of the genus and key to the western Palearctic species. Zootaxa 4000(4): 464–472. 10.11646/zootaxa.4000.4.5 [DOI] [PubMed] [Google Scholar]
- Gisin H. (1964) Collemboles d’Europe. VI. Revue suisse de Zoologie 71(20): 383–391. 10.5962/bhl.part.75615 [DOI] [Google Scholar]
- Greenslade P. (1994) Collembola. In: Houston WWK. (Ed.) Zoological catalogue of Australia.Volume 22. Protura, Collembola, Diplura. CSIRO, Melbourne, 19–138.
- Janion C, Bedos A, Deharveng L. (2011) The genus Ectonura Cassagnau, 1980 in South Africa (Collembola: Neanuridae: Neanurinae), with a key to South African Neanurinae. ZooKeys 136: 31–45. 10.3897/zookeys.136.1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji-Gang J, Cheng-Wang H, Yun-Xia L. (2018) A new species of Lobellina and first record of Vietnura from China (Collembola: Neanuridae: Neanurinae). ZooKeys 807: 13–28. 10.3897/zookeys.807.24941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos F. (1940) Terricole Collembolen aus Slovenien. Glasnik, Bulletin de la Société Scientifique de Skoplje 22: 136–168. [Google Scholar]
- Luo Y, Palacios-Vargas JG. (2016) On the genus Paralobella (Collembola: Neanurinae: Lobellini) with description of a new Chinese species. Zootaxa 4066(3): 343–350. 10.11646/zootaxa.4066.3.10 [DOI] [PubMed] [Google Scholar]
- MacGillivray AD. (1893) North American Thysanura – IV. Canadian Entomologist 25: 313–318. 10.4039/Ent25313-12 [DOI] [Google Scholar]
- Mayvan MM, Shayanmehr M, Scheu S. (2015a) Depth distribution and inter-annual fluctuations in density and diversity of Collembola in an Iranian Hyrcanian Forest. Soil Organisms 87(3): 239–247. [Google Scholar]
- Mayvan MM, Smolis A, Skarżyński D. (2015b) Persanura hyrcanica, a new genus and species of Neanurinae (Collembola: Neanuridae) from Iran, with a key to genera of the tribe Neanurini. Zootaxa 3918: 552–558. 10.11646/zootaxa.3918.4.4 [DOI] [PubMed] [Google Scholar]
- Müller J, Thorn S, Baier R, Sagheb-Talebi K, Barimani HV, Seibold S, Ulyshen MD, Gossner MM. (2016) Protecting the forests while allowing removal of damaged trees may imperil saproxylic insects biodiversity in the Hyrcanian Beech Forests of Iran. Conservation Letters 9(2): 106–113. 10.1111/conl.12187 [DOI] [Google Scholar]
- Palacios-Vargas JG, Deharveng L. (2014) First record of the genus Australonura Cassagnau 1980 (Collembola: Neanuridae) in the New World, with description of new species from Paraguay. Zootaxa 3779(1): 33–47. 10.11646/zootaxa.3779.1.6 [DOI] [PubMed] [Google Scholar]
- Palacios-Vargas JG, Simón Benito JC. (2007) A new genus and three new species of Neanuridae (Collembola) from North America. Journal of Cave and Karst Studies 69(3): 318–325. 10.3958/0147-1724-32.3.169 [DOI] [Google Scholar]
- Porco D, Bedos A, Deharveng L. (2010) Description and DNA barcoding assessment of the new species Deutonura gibbosa (Collembola: Neanuridae: Neanurinae), a common springtail of Alps and Jura. Zootaxa 2639: 59–68. 10.11646/zootaxa.2639.1.6 [DOI] [Google Scholar]
- Queiroz GC, Deharveng L. (2015) New genus, new species and new record of Neanurinae (Collembola, Neanuridae) for the Neotropics. Zootaxa 4020(1): 134–152. 10.11646/zootaxa.4020.1.5 [DOI] [PubMed] [Google Scholar]
- Sagheb-Talebi K, Sajedi T, Pourhashemi M. (2014) Forests of Iran – a treasure from the past, a hope for the future. Plant and Vegetation vol. 10, 152 pp. 10.1007/978-94-007-7371-4 [DOI]
- Shayanmehr M, Yahyapour E, Kahrarian M, Yoosefi-Lafooraki E. (2013) An Introduction to Iranian Collembola (Hexapoda): an update to the species list. ZooKeys 335, 69–83. 10.3897/zookeys.335.5491 [DOI] [PMC free article] [PubMed]
- Smolis A. (2008) Redescription of four Polish Endonura Cassagnau, 1979 (Collembola, Neanuridae, Neanurinae), with a nomenclature of the ventral chaetae of antennae. Zootaxa 1858: 9–36. 10.11646/zootaxa.1858.1.2 [DOI] [Google Scholar]
- Smolis A. (2017) Contribution to the knowledge of Neanurinae of Vietnam with description of three new species (Collembola, Neanuridae). ZooKeys 688: 15–23. 10.3897/zookeys.688.12307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolis A, Deharveng L. (2006) Vitronura mascula, a new species of Neanurinae (Collembola: Neanuridae) from northern Vietnam, with a key to the species of the genus. Revue suisse de Zoologie 113: 263–268. 10.5962/bhl.part.80349 [DOI] [Google Scholar]
- Smolis A, Deharveng L. (2015) Diversity of Paranura Axelson, 1902 (Collembola: Neanuridae: Neanurinae) in Pacific Region of Russia and United States. Zootaxa 4033(2): 203–236. 10.11646/zootaxa.4033.2.2 [DOI] [PubMed] [Google Scholar]
- Smolis A, Deharveng L, Kaprus’ IJ. (2011) Studies on the non-European Endonura Cassagnau, 1979 (Collembola, Neanuridae, Neanurinae). Zootaxa 3004: 45–56. 10.11646/zootaxa.3004.1.4 [DOI] [Google Scholar]
- Smolis A, Falahati A, Skarżyński D. (2012) The genus Cryptonura Cassagnau, 1979 (Collembola: Neanuridae: Neanurinae) in Iran. Zootaxa 3530: 51–58. 10.11646/zootaxa.3530.1.5 [DOI] [Google Scholar]
- Smolis A, Greenslade P. (2020) New Lobellini (Collembola: Neanuridae) from Queensland contribute to understanding distribution and ecology of Australian fauna. Austral Entomology 59: 253–264. 10.1111/aen.12460 [DOI] [Google Scholar]
- Smolis A, Kuznetsova N. (2018) Paravietnura gen. n., an intriguing genus of Neanurini from the Caucasus (Collembola, Neanuridae, Neanurinae). Zookeys 739: 41–54. 10.3897/zookeys.739.22041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolis A, Shayanmehr M, Yoosefi-Lafooraki E. (2018) New members of the genera Neanura MacGilliwray, 1893 and Deutonura Cassagnau, 1979 (Collembola: Neanuridae) from the Middle East. European Journal of Taxonomy 406: 1–16. 10.5852/ejt.2018.406 [DOI] [Google Scholar]
- Smolis A, Kahrarian M, Piwnik A, Skarżyński D. (2016a) Endonura Cassagnau in Iran, with a key of the genus (Collembola, Neanuridae, Neanurinae). Zookeys 553: 53–71. 10.3897/zookeys.553.6009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolis A, Shayanmehr M, Kuznetsova N, Yoosefi-Lafooraki E. (2017) Three new remarkable species of the genus Endonura Cassagnau, 1979 from the Middle East and Central Asia (Collembola, Neanuridae, Neanurinae, Neanurini). Zookeys 673: 135–151. 10.3897/zookeys.673.12084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolis A, Skarżyński D, Kahrarian M, Kaprus’ IJ. (2016b) Redescription of Protanura papillata Cassagnau & Delamare Deboutteville, 1955 (Collembola, Neanuridae, Neanurinae), with new records from Middle East, and with supplemented diagnosis and key to the genus. Zootaxa 4092(2): 293–300. 10.11646/zootaxa.4092.2.11 [DOI] [PubMed] [Google Scholar]
- Smolis A, Skarżyński D, Pomorski RJ, Kaprus’ IJ. (2007) Redescription of Endonura taurica (Stach, 1951) and E. quadriseta Cassagnau & Péja, 1979, and description of two new species of the genus Endonura Cassagnau, 1979 (Collembola: Neanuridae: Neanurinae) from the Crimea (Ukraine). Zootaxa 1442: 19–35. 10.11646/zootaxa.1442.1.2 [DOI] [Google Scholar]
- Templeton R. (1835) Thysanura Hiberbnicae, or descriptions of such species of spring-tailed insects (Podura and Lepisma, Lin.) as have been observed in Ireland. Transactions of the Entomological Society of London 1(2): 89–98. 10.1111/j.1365-2311.1838.tb00147.x [DOI] [Google Scholar]
- Yahyapour E. (2012) Faunistic Study on Collembola (Insecta: Apterygota) in Sari Regions. MSc thesis, Sari, Vol. 1. Sari Agricaltural Science and Natural Resources University, Iran, 96 pp. [in Persian with English abstract] [Google Scholar]
- Yossi R. (1976) On some Neanurid Collembola of Southeast Asia. Nature and life in Southeast Asia 7: 257–299. [Google Scholar]
- Zhi-Chun M, Jian-Xiu C. (2008) A new species of Lobellina (Collembola: Neanuridae) from China. Zootaxa 4033(2): 203–236. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.