Abstract
Introduction
Persistent hypercapnic failure in chronic obstructive pulmonary disease (COPD) is associated with poor prognosis. Long-term home non-invasive ventilation is recommended for patients with PaCO2 >7.0 kPa. Domiciliary high-flow nasal cannula (HFNC) reduces PaCO2 in short-term studies. This post-hoc analysis examines the effect of HFNC on PaCO2 levels, exacerbations and admissions in patients with COPD with persistent hypercapnic and hypoxic failures.
Methods
The original trial included 74 long-term oxygen-treated patients (31 HFNC treated/43 controls) with persistent hypercapnic failure (PaCO2 >6 kPa) who completed the 12-month study period. Baseline data included age, sex, blood gases, exacerbations and hospital admissions in the previous year. Data on blood gases were also recorded at 6 and 12 months for all patients. In addition, acute changes in blood gases after 30 min of HFNC use at site visits were examined, as were exacerbations and hospital admissions during study.
Results
Patients were comparable at baseline. After 12 months there was a 1.3% decrease in PaCO2 in patients using HFNC and a 7% increase in controls before HFNC use on site (p=0.003). After 30 min of HFNC at visits PaCO2 changed significantly, with comparable reductions, at 0, 6 and 12 months, including for controls who tried HFNC at study end (p<0.001). The exacerbation rate increased, compared with 12 months prestudy, by 2.2/year for controls (p<0.001) and 0.15/year for HFNC-treated patients (p=0.661). Hospital admission rates increased in the control group,+0.3/year from prestudy (p=0.180), And decreased by 0.67/year (p=0.013)for HFNC-treated patients.
Conclusion
This post-hoc analysis indicates that HFNC stabilises patients with COPD with persistent hypoxic and hypercapnic failures, in terms of PaCO2, exacerbations and number of hospitalisations, whereas those not receiving HFNC worsened. This suggests that HFNC is a possible treatment for patients with persistent hypercapnic COPD.
Keywords: COPD exacerbations, long-term oxygen therapy (LTOT)
Key messages.
Is high-flow nasal cannula (HFNC) beneficial for patients with chronic obstructive pulmonary disease (COPD) with chronic hypercapnic failure?
HFNC treatment stabilises patients with COPD with persistent hypercapnic failure, in terms of preventing progression in hypercapnia, number of exacerbations and hospitalisations.
This study suggests that HFNC is an effective treatment for patients with COPD with persistent hypercapnic failure.
Introduction
In patients with chronic obstructive pulmonary disease (COPD), persistent hypercapnic respiratory failure is associated with poor disease prognosis.1 In COPD, persistent hypercapnic failure is a predictor of more exacerbations,2 poor quality of life,3 impaired cognitive function, especially in combination with persistent hypoxic respiratory failure,4 and mortality.2 In acute hypercapnic respiratory failure, treatment with non-invasive ventilation (NIV) has been shown to improve prognosis,5 and NIV is an established treatment modality in respiratory acute care worldwide. The body of evidence for treatment with long-term home NIV (LTH-NIV) in persistent hypercapnic respiratory failure is growing6–9 and respiratory societies such as the European Respiratory Society have made guidelines for treatment with LTH-NIV in patients with COPD.10
Evidence for the benefit of high-flow nasal cannula (HFNC) in patients with COPD with persistent hypoxic failure is growing, with evidence most positive for its long-term use in hypoxemic, frequently exacerbating patients with COPD.11–13 In addition, studies have shown that PaCO2 levels are reduced with short-term and longer-term use of HFNC in COPD,12 14–16 both in chronic and stable conditions, but it has been argued that this is primarily due to a reduction of CO2 levels in the anatomical dead space.17 In the randomised controlled trial (RCT) by Storgaard et al,12 hereafter called the Aalborg Study, an inclusion criterion was persistent hypoxemic respiratory failure, although patients with concomitant hypercapnic respiratory failure were not excluded. The patients were comparable in terms of all blood gases at inclusion, but for PaCO2 there was a significant average treatment difference at 12 months in favour of HFNC treatment.
The aim of this post-hoc analysis is therefore to examine further the changes primarily in PaCO2, but also in PaO2 in patients with persistent hypercapnic and hypoxemic respiratory failure over 12 months treatment with HFNC. Furthermore, changes in numbers of exacerbations and of hospital admission are compared 1 year prior to inclusion and during the study period within the two treatment groups.
Methods
In the Aalborg Study,12 200 patients with advanced COPD and persistent hypoxic failure as demonstrated by three consecutive arterial blood gases measured during stable conditions18 were randomised to usual care or usual care plus HFNC for 12 months. The inclusion process and follow-up is described in detail in the Aalborg Study12 and therefore is only summarised below.
Inclusion criteria were hypoxemic COPD with long-term oxygen therapy (LTOT), prescribed by a Pulmonary Medicine specialist, at least 3 months prior to study start. Exclusion criteria were malignant disease, terminal non-malignant disease other than COPD, unstable psychiatric disease or home-treatment with NIV. Persistent hypercapnic failure was not an inclusion or exclusion criteria. All patients received personalised inhaled medicine according to The Global Inititative for Chronic Obstructive Lung Disease (GOLD) recommendations,19 had previously undergone pulmonary rehabilitation, and were in specialised care in connection with LTOT treatment, according to the GOLD recommendations.20 Change of medication and attending rehabilitation were allowed, if recommended by the patient’s usual caregivers, but change of smoking habits during the study period would lead to exclusion.
Patients randomised to the HFNC group were treated with LTOT plus HFNC home treatment delivered by myAIRVO via Optiflow nasal cannula (both Fisher & Paykel Healthcare, Auckland, New Zealand) at a recommended flow rate of 20 L/min, 8 hours per day, preferably at night; however, there were no restrictions in the duration of use nor time of day. Blood gases including PaCO2 were measured using arterial blood gas analysis (ABL 800 Flex blood gas analyser, Radiometer, Copenhagen, Denmark) in both HFNC-treated patients and controls at baseline, and at 6-month and 12-month clinic visits. All patients allocated to HFNC used HFNC during the clinic visits and arterial blood gas analyses were repeated after 30 min use.
The 200 trial patients included 117 patients with concomitant persistent hypercapnic failure (PaCO2 >6 kPa). Of those 74, (31 treated with HFNC and 43 controls), completed the 12-month study period and were included in this post-hoc analysis. Following the initial blood gas analysis at the 12-month close-out visit, 21 control patients were also treated with HFNC for 30 min when repeat 30-min arterial blood gas analysis was performed, just as was done in the HFNC group at 0, 6 and 12-month visits. As well as blood gases, baseline data collected for all patients included age, sex, body mass index, modified Medical Research Council Score (mMRC), smoking status, forced expiratory volume in the first second (FEV1), percentage of predicted value (FEV1%), PaO2 and PaCO2, as well as exacerbations and hospital admissions in the previous year. Number of exacerbations, hospital admissions during study were included.
Baseline data on blood gases were compared statistically between groups. Change in PaCO2 over the 12 months observational period was investigated with multiple regression models. PaCO2 before and after 30 min were compared by paired t-tests. Number of exacerbations and hospital admissions pre and during study were compared with Poisson regression models.
The original RCT was approved by the North Jutland Ethical Committee (N-20110057), the Danish Data Protection Agency (2008-58-0028) and registered at ClinicalTrials.gov (NCT 02731872). Patients were informed according to the Helsinki Declaration and written consent was obtained prior to inclusion.
Results
In the HFNC group, the device was used on average 6.9 hours/day. Baseline data, demonstrated in table 1, were comparable, except for mMRC Score, which was significantly higher in the HFNC-treated patients.
Table 1.
Baseline characteristics on HFNC-treated patients and controls
| HFNC (n=31) |
Controls (n=43) |
P value | |
| Sex (% female) | 68 | 70 | 0.90 |
| Age (years) | 67 () | 68 () | 0.88 |
| Active smokers, % | 13 | 14 | 0.80 |
| BMI | 26.6 (8.8) | 26.0 (6.2) | 0.51 |
| mMRC Score* | 3.2 (0.9) | 3.7 (0.9) | 0.04 |
| Exacerbations 12 months before baseline, n | 2.8 (1.9) | 2.6 (1.7) | 0.42 |
| PaO2, kPa (on LTOT) | 9.8 (1.5) | 10.0 (1.6) | 0.38 |
| Oxygen saturation (%) | 95 (2.2) | 95 (2.5) | 0.57 |
| pH | 7.39 (0.03) | 7.40 (0.03) | 0.76 |
| Standard bicarbonate (mmol/L) | 30.1 (2.6) | 30.3 (2.3) | 0.51 |
| Base excess | 7.5 (3.6) | 7.4 (2.7) | 0.81 |
| PaCO2, kPa (on LTOT) | 7.3 (1.2) | 7.2 (0.6) | 0.45 |
| FEV1% | 24.5 (8.8) | 26.1 (6.2) | 0.18 |
Mean (SD) unless otherwise indicated.
BMI, body mass index; FEV1%, forced expiratory volume in the first second, as percentage of expected value; kPa, kilo Pascal; LTOT, long-term oxygen therapy; mMRC, modified Medical Research Council Score; PaCO2, Partial arterial pressure of carbon dioxide; PaO2, Partial arterial pressure of oxygen.
Figure 1 demonstrates the treatment effect at 12 months in HFNC-treated compared with controls, showing that the treatment effect was greater with increased baseline PaCO2 (p=0.038). By comparison of means, for patients with hypercapnia with baseline PaCO2 >6 kPa, the differential effect was a 1.3% decrease using HFNC (p=0.624), compared with an increase of 7% for controls, a difference significant at p=0.003.
Figure 1.
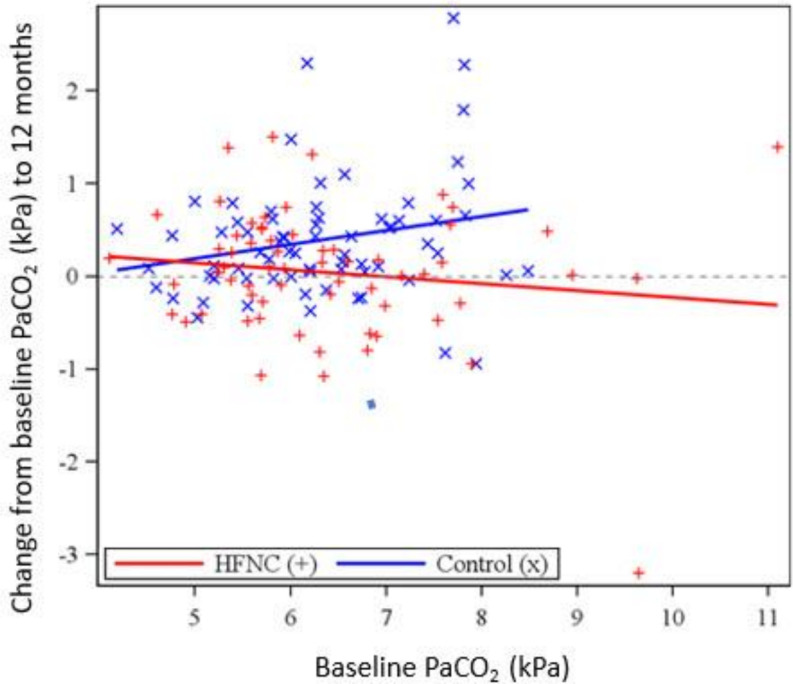
The relationship between change to PaCO2 at 12 months and baseline values by treatment group.
Arterial blood gas values before and after use of 30 min of HFNC in HFNC-treated and for controls at each visit, baseline, 6 months and 12 months, are shown in online supplemental tables S1 and S2). Significant or near-significant changes over 12 months in PaO2 were similar between groups: controls (ΔPaO2=−0.45 kPa, p=0.014); HFNC-treated, (ΔPaO2=−0.31 kPa, p=0.062). Over 12 months there was a Δ-standard bicarbonate of 0.74 mmol/L (p=0.68) in controls and −0.06 mmol/L (p=0.73) in HFNC-treated patients.
bmjresp-2020-000712supp001.pdf (75.2KB, pdf)
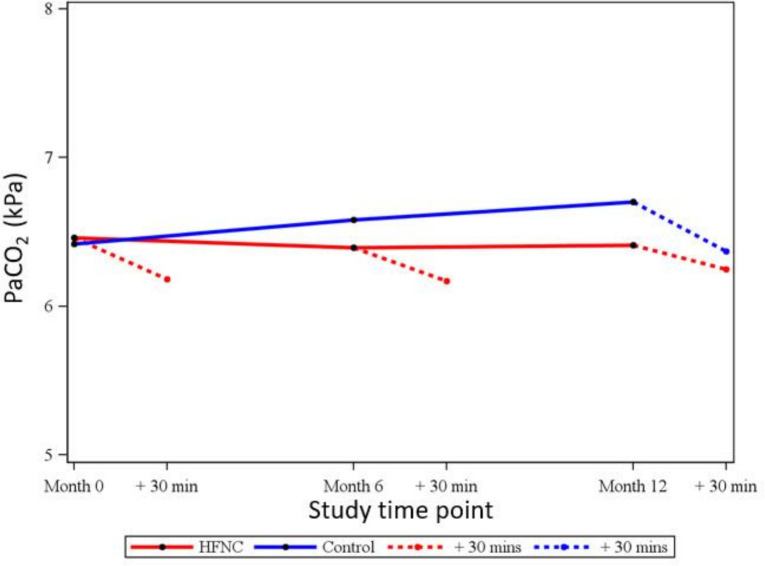
Figure 2 shows the relative magnitudes of the immediate and long-term changes for PaCO2 which changed significantly at all assessments (all p<0.001) after 30 min use of HFNC. The changes were consistent in size whether at 0, 6 or 12 months, including for control patients who tried HFNC at study end, and averaged −0.28 kPa or −4.4%.
Figure 2.
Development of mean PaCO2over 12 months in HFNC and control groups, with change after 30 minutes of HFNC treatment also shown at each clinic visit and for control patients trying HFNC at study end.
The exacerbation rate increased, compared with 12 months prestudy, by 2.2/year for controls (p<0.001) and remained stable for HFNC-treated patients (increase of 0.15/year, p=0.661). There was no significant change in hospital admission rates in the control group, +0.3/year from prestudy (p=0.180), while for HFNC-treated patients, the modelled difference between 0 and 12 months use showed a decrease in hospital admission rate of 0.67/year (p=0.013).
Discussion
This post-hoc analysis showed a preventive effect of HFNC on progressive hypercapnic respiratory failure, stabilising in patients with COPD compared with a PaCO2 increase in controls. The magnitude of this difference increased for the patients who were more hypercapnic. In addition, stability was seen in exacerbation rates and decrease in hospital admission rates in HFNC-treated patients, whereas an increase in exacerbations rates and stability in admission rates were seen in controls on usual care.
This is the first analysis to demonstrate the effect of long-term use of domiciliary HFNC on arterial blood gas CO2 values in patients with persistent hypercapnic failure. Previous short-term studies have shown PaCO2 to decrease.13–15 21 However, it has been argued that this was merely due to a CO2 reduction in the anatomical dead space, which has also been simulated in in vitro studies, for example by Onodera et al.22 In this current analysis a slight long-term reduction in PaCO2 for patients with HFNC can be described as stabilisation, in opposition to a significant increase in PaCO2 for control patients. This contrasts to two LTH-NIV long-term studies, where a reduction in mean PaCO2 of 5%–11% was seen, while the control group remained stable in the study by Murphy et al and actually reduced PaCO2 in the study by Köhnlein et al.6 9 However, although comparable in many ways, only two-thirds of the patients in both studies suffered from persistent hypoxemic failure at inclusion.6 9 Hypercapnia has previously been recognised as a prognostic marker of progressed disease.23–25 The patients in the Aalborg Study may therefore have suffered from more progressive disease as they had PaCO2 levels approximately 1 kPa higher than control patients in the Murphy et al study. In fact, the patients in the Murphy study6 actually had PaCO2 levels lower than the current recommended threshold for initiating LTH-NIV. The increase in PaCO2 in the control group in the Aalborg Study is consistent with changes previously observed in persistent hypercapnic failure which, by nature, is progressive.26 27
In patients with persistent hypercapnic failure the central chemoreceptors are resistant to carbon dioxide levels in the blood and patients become dependent on hypoxic drive.28 Therefore, changes in PaO2 may influence PaCO2 levels. However, in this analysis no significant changes were seen in PaO2 over the 12 months period in HFNC-treated patients and in controls a reduction in PaO2 was seen, which theoretically should stimulate the respiratory drive and reduce PaCO2. There is inconsistency between the two groups, where both reduce O2 levels, although not significantly in HFNC treated, who stabilise PaCO2. On the other hand PaCO2 increase in controls, indicating that the effect of the hypoxic drive is of minor importance in this matter. In addition, this further supports that increased PaCO2 levels in controls is a sign of progressive disease and that HFNC treatment has a stabilising effect on COPD.
The short-term, 30 min change in PaCO2 with HFNC is persistent over the 12-month study and consistent with previous findings of reduced hypercapnia as a result of a clearing of CO2 from the anatomical dead space.29 This PaCO2 reduction is comparable to those seen in the previously mentioned studies by Bräunlich and Pisani et al which also only demonstrated a short-term reduction in PaCO2 levels.14 15 21 Although none of the short-term studies have included clinical measures. It has been suggested that it may be of clinical importance to patients with hypercapnia with tachypnoea;29 however, more studies are needed to support this theory.
Persistent hypercapnic failure has been shown to affect number of exacerbations negatively2 and exacerbations have been shown to be more prevalent and more severe in progressed COPD.30 31 In this study, a reduction in exacerbation rate was seen in HFNC-treated patients with stable PaCO2 levels, although these patients suffered from late-stage COPD. Previously, PaCO2 reduction by LTH-NIV long-term treatment has been shown to lead to reduction in number of exacerbations and a significant increase in time to readmission.32 A previous long-term study with high-flow treatment of patients with mixed obstructive lung diseases showed prolonged time to first exacerbation; however, whether patients had persistent hypercapnic failure is not described in the paper.33 The only other longer study to investigate PaCO2 development over a period of HFNC treatment, 12 weeks to be exact, reports improvement of health-related quality of life, whereas no other clinical significant changes were seen.13 Indeed, this post-hoc analysis also shows a decrease in hospitalisation rates in HFNC-treated patients. A recent meta-analysis by Wilson et al found that the effect of LTH-NIV on hospital admissions is sparse; however, the number of patients in need of admissions reduced.8 The findings of this analysis support the conclusion from the work by Bräunlich et al14 16 21 that HFNC may be considered for patients with COPD with persistent hypercapnic failure, especially if LTH-NIV cannot be used. To conclude, this post-hoc analysis of the data from the Aalborg 12-month RCT is the first to indicate that not only does HFNC have a stabilising effect on the number of exacerbations in patients with COPD with combined, persistent hypercapnic and hypoxic failure, but, in addition, that HFNC is an alternative add-on to LTH-NIV in terms of preventing severe exacerbations, which for patients with COPD inevitably is crucial for reducing both morbidity and mortality.
This analysis has limitations. It is a post-hoc study, and therefore not originally powered for these analyses. In addition, it is worth noticing that the patients in this study were treated with a flow of 20 L/min. A higher flow may have been more beneficial if lowering PaCO2 had been the primary target of the study. This is, however, the only long-term RCT data available at the moment, and therefore important as an indicator and a hypothesis generator for future studies.
In conclusion, this study indicates that HFNC stabilises patients with persistent hypoxemic and hypercapnic COPD by preventing progression of hypercapnia, and also stabilises exacerbations and reduces number of hospitalisations, whereas those not receiving HFNC worsened over time. This suggests that HFNC is an effective treatment for patients with persistent hypercapnic COPD. As this is a post-hoc analysis this should of course be confirmed in future studies.
Footnotes
Contributors: LHS has contributed to data gathering, data management and writing the paper. UMW has contributed to design, statistics and writing of the paper. H-UH has contributed to data management and statistics. All authors have contributed to the review of the paper.
Funding: The study was funded by Fisher & Paykel Healthcare.
Patient and public involvement statement: Patients or the public were not involved in the design, or conduct, or reporting or dissemination plans of our research.
Patient consent for publication: LHS and H-UH have been contracted to Fisher & Paykel Healthcare. LHS and UMW have received speaker’s fee and travel grants from Fisher & Paykel Healthcare.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Aida A, Miyamoto K, Nishimura M, et al. Prognostic value of hypercapnia in patients with chronic respiratory failure during long-term oxygen therapy. Am J Respir Crit Care Med 1998;158:188–93. 10.1164/ajrccm.158.1.9703092 [DOI] [PubMed] [Google Scholar]
- 2.Vitacca M. Exacerbations of COPD: predictive factors, treatment and outcome. Monaldi Arch Chest Dis 2001;56:137–43. [PubMed] [Google Scholar]
- 3.Duiverman ML, Wempe JB, Bladder G, et al. Health-Related quality of life in COPD patients with chronic respiratory failure. Eur Respir J 2008;32:379–86. 10.1183/09031936.00163607 [DOI] [PubMed] [Google Scholar]
- 4.Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Respir J 2010;35:913–22. 10.1183/09031936.00125109 [DOI] [PubMed] [Google Scholar]
- 5.Plant PK, Owen JL, Elliott MW. Noninvasive ventilation in acute exacerbations of chronic obstructive pulmonary disease. Acute Exacerbations Chronic Obstr Pulm Dis 2003:405–24. [Google Scholar]
- 6.Murphy PB, Rehal S, Arbane G, et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized clinical trial. JAMA 2017;317:2177. 10.1001/jama.2017.4451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gantzhorn EK, Prior TS, Hilberg O. Long-Term non-invasive ventilation for stable chronic hypercapnic COPD. Eur Clin Respir J 2019;6:1644893. 10.1080/20018525.2019.1644893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson ME, Dobler CC, Morrow AS, et al. Association of home noninvasive positive pressure ventilation with clinical outcomes in chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA 2020;323:455. 10.1001/jama.2019.22343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Köhnlein T, Windisch W, Köhler D, et al. Non-Invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med 2014;2:698–705. 10.1016/S2213-2600(14)70153-5 [DOI] [PubMed] [Google Scholar]
- 10.Ergan B, Oczkowski S, Rochwerg B, et al. European respiratory Society guidelines on long-term home non-invasive ventilation for management of COPD. Eur Respir J 2019;54. 10.1183/13993003.01003-2019. [Epub ahead of print: 28 Sep 2019]. [DOI] [PubMed] [Google Scholar]
- 11.Elshof J, Duiverman ML. Clinical evidence of nasal high-flow therapy in chronic obstructive pulmonary disease patients. Respiration 2020;99:140–53. 10.1159/000505583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Storgaard LH, Hockey H-U, Laursen BS, et al. Long-Term effects of oxygen-enriched high-flow nasal cannula treatment in COPD patients with chronic hypoxemic respiratory failure. Int J Chron Obstruct Pulmon Dis 2018;13:1195–205. 10.2147/COPD.S159666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagata K, Kikuchi T, Horie T, et al. Domiciliary high-flow nasal cannula oxygen therapy for patients with stable hypercapnic chronic obstructive pulmonary disease. A multicenter randomized crossover trial. Ann Am Thorac Soc 2018;15:432–9. 10.1513/AnnalsATS.201706-425OC [DOI] [PubMed] [Google Scholar]
- 14.Bräunlich J, Mauersberger F, Wirtz H. Effectiveness of nasal highflow in hypercapnic COPD patients is flow and leakage dependent. BMC Pulm Med 2018;18:14. 10.1186/s12890-018-0576-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pisani L, Betti S, Biglia C, et al. Effects of high-flow nasal cannula in patients with persistent hypercapnia after an acute COPD exacerbation: a prospective pilot study. BMC Pulm Med 2020;20:1–9. 10.1186/s12890-020-1048-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bräunlich J, Dellweg D, Bastian A, et al. Nasal high-flow versus noninvasive ventilation in patients with chronic hypercapnic COPD]]>. Int J COPD 2019;14:1411–21. 10.2147/COPD.S206111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biselli PJC, Kirkness JP, Grote L, et al. Nasal high-flow therapy reduces work of breathing compared with oxygen during sleep in COPD and smoking controls: a prospective observational study. J Appl Physiol 2017;122:82–8. 10.1152/japplphysiol.00279.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardinge M, Annandale J, Bourne S, et al. British thoracic Society guidelines for home oxygen use in adults. Thorax 2015;70 Suppl 1:i1–43. 10.1136/thoraxjnl-2015-206865 [DOI] [PubMed] [Google Scholar]
- 19.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: gold executive summary. Am J Respir Crit Care Med 2013;187:347–65. 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 20.GOLD GOLD 2017 Global Strategy for the Diagnosis, Management and Prevention of COPD - Global Initiative for Chronic Obstructive Lung Disease - GOLD 2017.
- 21.Bräunlich J, Seyfarth H-J, Wirtz H. Nasal high-flow versus non-invasive ventilation in stable hypercapnic COPD: a preliminary report. Multidiscip Respir Med 2015;10:1–3. 10.1186/s40248-015-0019-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onodera Y, Akimoto R, Suzuki H, et al. Corrigendum: A high-flow nasal cannula system set at relatively low flow effectively washes out CO2 from the anatomical dead space of a respiratory-system model. Korean J Anesthesiol 2018;71:75. 10.4097/kjae.2018.71.1.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang H, Xiang P, Zhang E, et al. Is hypercapnia associated with poor prognosis in chronic obstructive pulmonary disease? a long-term follow-up cohort study. BMJ Open 2015;5:e008909–8. 10.1136/bmjopen-2015-008909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan GP, McArdle N, Dhaliwal SS, et al. Patterns of use, survival and prognostic factors in patients receiving home mechanical ventilation in Western Australia: a single centre historical cohort study. Chron Respir Dis 2018;15:356–64. 10.1177/1479972318755723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brat K, Plutinsky M, Hejduk K, et al. Respiratory parameters predict poor outcome in COPD patients, category gold 2017 B. Int J Chron Obstruct Pulmon Dis 2018;13:1037–52. 10.2147/COPD.S147262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliven A, Kelsen SG, Deal EC, et al. Mechanisms underlying CO2 retention during flow-resistive loading in patients with chronic obstructive pulmonary disease. J Clin Invest 1983;71:1442–9. 10.1172/JCI110897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.West JB. Causes of carbon dioxide retention in lung disease. N Engl J Med Overseas Ed 1971;284:1232–6. 10.1056/NEJM197106032842202 [DOI] [PubMed] [Google Scholar]
- 28.Brinkman JE, Toro F, Sharma S. Physiology, Respiratory Drive [Internet]. StatPearls, 2020. Available: http://www.ncbi.nlm.nih.gov/pubmed/29494021 [PubMed]
- 29.Fricke K, Tatkov S, Domanski U, et al. Nasal high flow reduces hypercapnia by clearance of anatomical dead space in a COPD patient. Respir Med Case Rep 2016;19:115–7. 10.1016/j.rmcr.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363:1128–38. 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 31.Hurst JR, Skolnik N, Hansen GJ, et al. Understanding the impact of chronic obstructive pulmonary disease exacerbations on patient health and quality of life. Eur J Intern Med 2019;2020:1–6. [DOI] [PubMed] [Google Scholar]
- 32.Dreher M, Neuzeret P-C, Windisch W, et al. Prevalence of chronic hypercapnia in severe chronic obstructive pulmonary disease: data from the homevent registry. Int J Chron Obstruct Pulmon Dis 2019;14:2377–84. 10.2147/COPD.S222803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rea H, McAuley S, Jayaram L, et al. The clinical utility of long-term Humidification therapy in chronic airway disease. Respir Med 2010;104:525–33. 10.1016/j.rmed.2009.12.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2020-000712supp001.pdf (75.2KB, pdf)