Abstract
Durvalumab is a selective, high-affinity human immunoglobulin monoclonal antibody in a class called check point inhibitors, that blocks PD-L1 on tumour cells. Despite clinical success in increasing progression-free survival rates in patients with stage III non-small-cell lung cancer, durvalumab has been associated with immune-related side effects such as pneumonitis and colitis. We present a case of an 84-year-old woman with acral vasculitis presenting as blue toe syndrome, associated with prolonged use of durvalumab. After 1 year of fortnightly durvalumab therapy postchemoradiation therapy, the patient came in with a left blue big toe, and later developed bilateral livedo racemosa. The diagnosis of durvalumab-associated vasculitis was made and treatment with prednisolone was started with clinical improvement.
Keywords: immunological products and vaccines, drugs and medicines, cancer intervention, vasculitis, respiratory system
Background
Durvalumab is an immune checkpoint inhibitor (ICI), which is approved in Australia for the treatment of uroepithelial carcinoma and stage III non-small-cell lung cancer (NSCLC) without progression following platinum-based chemoradiation therapy. It is a human engineered IgG1 monoclonal antibody inhibiting the PD1-ligand binding to the PD-1 receptor. It only binds to the PD1 ligand and not the PD-2 ligand reducing the risk of immune related toxicity.1 It has a reduced side effect profile when compared with the PD receptor inhibitors. The most common serious side effect is pneumonitis (12.6%) which can be fatal. Other side effects include fatigue, diarrhoea, pruritus rash, colitis and endocrinopathies.2 Outcomes of trials with durvalumab have shown a significant improvement in progression free survival compare to placebo of 16.8 months vs 5.6 months and an increased median time of death or distant metastases of 23.2 months vs 14.6 months with placebo.3 We report a case of vasculitis in an 84-year-old woman receiving durvalumab therapy for NSCLC to share the knowledge with our colleagues due to unusual presentation.
Case presentation
An 80-year-old woman was referred for admission by her general practitioner with a 3-day history of painless blue left great toe. There had been no preceding trauma or cold exposure. ‘She had noted the new onset of symptoms of painful Raynaud syndrome in her left hand’. Fourteen months prior, she was diagnosed with stage IIIa (T1N2M0) NSCLC of the right upper lobe on a CT arranged to investigate exertional dyspnoea. Medical history includes ex-smoker of 90 pack-years, ischaemic heart disease, hypertension, dyslipidaemia, aortic stenosis, deep venous thrombosis, chronic back pain with previous L3/4 laminectomy and right hip osteoarthritis.
Her long-term medications include amitriptyline 10 mg daily, aspirin 100 mg daily, frusemide 20 mg daily, methadone 5 mg daily, nifedipine 10 mg daily pregabalin 75 mg mane and 150 mg nocte, valsartan 40 mg.
She completed chemotherapy (carboplatin and paclitaxel) and radiotherapy (50 Gy over 30 sessions), followed by 21 cycles of fortnightly intravenous durvalumab for maintenance treatment. The final dose of durvalumab had been given 6 weeks prior to presentation. During the second month of durvalumab, she had an exacerbation of her chronic back, hip and leg pain. In the fifth month, she developed exertional dyspnoea and a CT scan showed pneumonitis. She was commenced on a dose of prednisolone commencing at 50 mg and together with the temporary withdrawal of durvalumab. Dyspnoea and arthralgias resolved completely in 6 weeks while prednisolone weaned off with the resumption of durvalumab treatment cycles. This was well tolerated until completion of the planned course 6 weeks prior to admission.
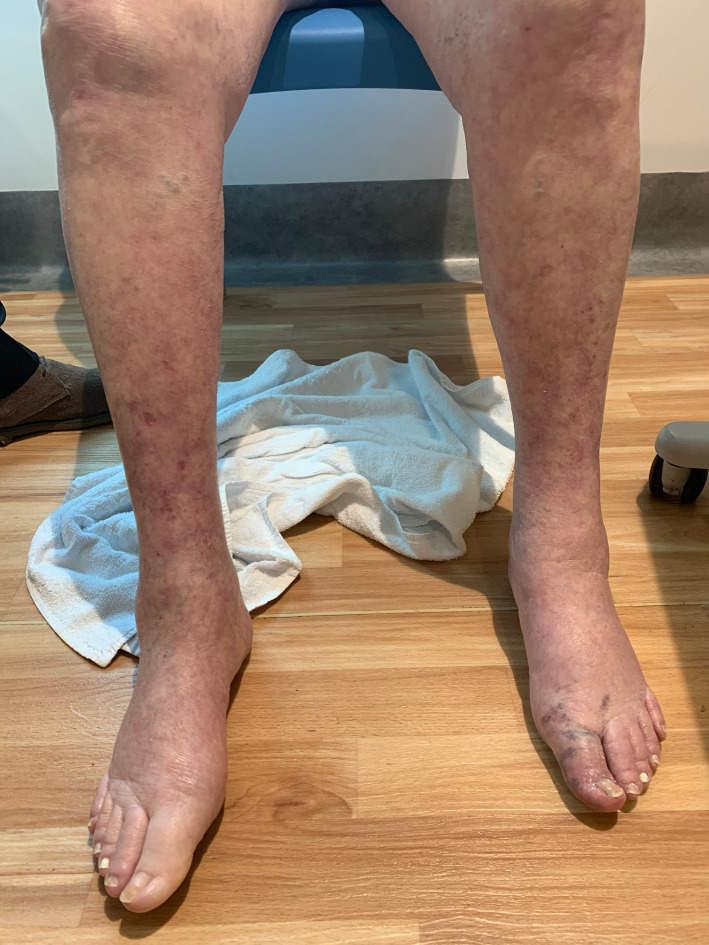
Physical examination showed that the patient was afebrile, normotensive and had normal oxygen saturation on pulse oximetry. An aortic stenotic murmur was audible. Chest auscultation revealed bilateral basal inspiratory crackles, most likely reflecting inflammation caused by lung cancer and pneumonitis. Peripheral pulses were palpable on both dorsalis pedis and posterior tibialis bilaterally. There was mild weakness of the proximal lower limbs. The left hallux distal to the metatarsal joint was cool and blue in colour (figure 1) with normal capillary refill.
Figure 1.
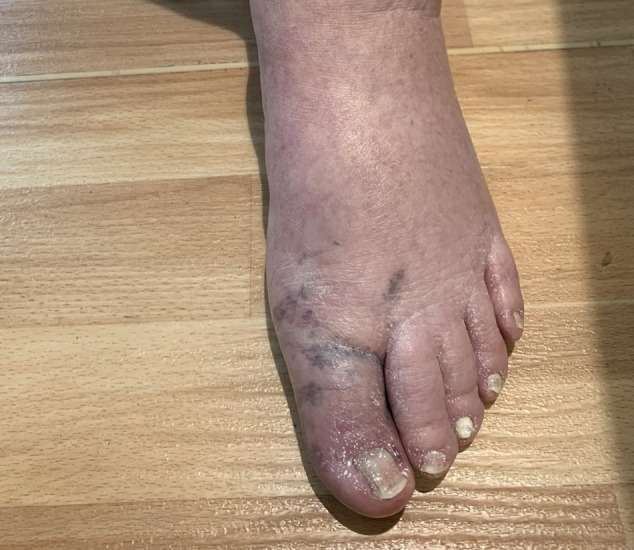
Blue toe at the left hallux distal to the metatarsal joint.
Investigation revealed normocytic anaemia (haemoglobin of 94 g/L (normal 115–165 g/L)), acute renal impairment (urea of 11.9 mmol/L (normal 3.5–11.0 mmol/L) and creatinine 151 umol/L (normal 45–90 umol/L)) compared with patient’s baseline creatinine of 110 umol/L. C reactive protein was 54 mg/L (normal <3.0 mg/L). Iron studies showed ferritin of 81 µg/L (normal 30–300) with transferrin saturation of 7% (normal 14%–45%). Urine culture and three sets of blood cultures had no bacterial growth. There was no proteinuria and urinary sediment was inactive. Venous Doppler ultrasound revealed no deep vein thrombosis. Abdominal ultrasound showed normal calibre aorta of 17 mm with mild atherosclerotic plaque. MRI of the spine and hips showed multilevel discogenic and facet joint degenerative changes and severe left hip osteoarthritis. A screen for autoimmune vasculitis revealed a positive perinuclear anti-neutrophil cytoplasmic antibodies (p-ANCA). Enzyme-Linked-Immunosorbent-Assay (ELISA) for antimyeloperoxidase (MPO) and antiproteinase 3 (PR3) were negative. Antinuclear, extractable nuclear antibodies (ANAs) and cold agglutinins were not detected. Antiphospholipid body antibodies to IgG and IgM were negative.
Treatment
Her initial management was commenced with intravenous ceftriaxone and vancomycin with the clinical concern of infective endocarditis. The patient developed daily fevers >38.5°C and a rash consistent with livedo racemosa on her bilateral lower limbs distally (figure 2), resembling blue toe syndrome. A transthoracic echocardiogram was arranged which showed known aortic stenosis with no vegetations. Five days later, she developed dyspnoea and dry cough without hypoxia. A CT chest demonstrated residual lung cancer and adjacent areas of ground glass infiltrate, consistent with pneumonitis. A procalcitonin level was normal. On day 6 of admission, prednisolone was commenced and antibiotics were ceased. Her fever abated and dyspnoea improved as well as the blue discolouration of the left hallux and bilateral lower limb rash also resolved. Her renal function returned to baseline and 1 g iron carboxymaltose infusion was administered.
Figure 2.
Livedo racemosa on bilateral lower limbs distally.
Outcome
Her discharge diagnosis was durvalumab-associated vasculitis and would be followed up in the outpatient clinic. By the Naranjo Algorithm, we estimate that the Adverse Drug Reaction Probability Score for durvalumab-associated vasculitis would be 6–7.
Discussion
Blue toe syndrome was first described as a sudden onset of acute pain and cyanosis in one or more toes4 Blue toe syndrome is characterised by a blue violaceous discolouration of one or more digits in the absence of obvious trauma, serious cold-induced injury or disorders producing generalised cyanosis.5 It is seen most commonly with cholesterol emboli following intravascular procedures or the commencement of anticoagulation. There are other causes including vasculitis (systemic sclerosis, scleroderma, polyarteritis nodosa, Behcet’s), prothrombotic conditions (antiphospholipid syndrome, disseminated intravascular coagulation), bacterial endocarditis, drugs and vascular dissection.6 7 Investigations of the non-vascular causes for the blue toe syndrome were negative. Although our patient had significant past history of vascular diseases, all her vascular conditions were quiescent clinically and she had no recent intravascular procedures. Taking together in the context of negative blood culture and unremarkable echocardiogram, the development of fever, pneumonitis and livedo vasculitis pointed towards the differential diagnosis of paraneoplastic vasculitis, namely paraneoplastic blue toe syndrome in our patient. Our next question is what caused the paraneoplastic vasculitis/blue toe syndrome as her non-operable NSCLC had responded well to chemotherapy and immunotherapy according to the post-therapy clinical assessment. If cancer itself is not likely to be the cause, treatment-related causes may play the causative role. Durvalumab-associated vasculitis/blue toe syndrome will certainly be the most likely diagnosis in the context of the clinical management history of improved blue toe syndrome on withdrawal of durvalumab. Paraneoplastic blue toe syndrome has been reported in one previous case report with immunotherapy named sunitinib (a tyrosine kinase inhibitor) in a patient for treatment with metastatic renal cell carcinoma.7 In resemblance to blue toe syndrome, another case8 reported a 66-year-old patient presented with paraneoplastic ‘blue finger syndrome’, namely paraneoplastic Raynaud’s phenomenon or the so-called ‘paraneoplastic acral vascular syndrome’. The ‘blue finger syndrome’ was associated with occult malignancy of early adenocarcinoma of the colon.8
Vasculitis has rarely been reported with the use of ICI’s which is surprising considering vasculitis is common in immunological conditions. A study showed that the deletion of the Pd receptor in mice caused a lupus like syndrome with a positive ANA.9–12 Most cases of systemic lupus erythematosus with ICI’s have had the disease prior to therapy. A review of 20 cases of ICI’s associated vasculitis found that the majority had large vessel vasculitis; that is, giant cell arteritis and cerebral angiitis. Giant cell arteritis is an inflammatory disorder that commonly commences in the adventitia as the media is considered a immunologically privileged area. It has been shown that the dendritic cells in the adventitia have reduced PD1 expression which can lead to cytotoxic T cells invading the tunica media. Only three case of acral vasculitis have been described with ICI’s (ipilimumab, durvalumab and tremelimumab) which present with painful necrotic lesion involving the fingers and are negative for autoantibodies.13 Mechanisms of immunological damage associated with ICI’s use include, cytotoxic T cell attack, autoantibodies, NK cell activation, Treg cell dysregulation and immune complex disease perhaps with the monoclonal antibody.14 With any inflammatory response, PD1 is upregulated on the vascular endothelium and if this is inhibited experimentally, it can lead to a severe neutrophilic vasculitis as seen in a mouse model of myocarditis.15
The lesions seen in our patient of livedo racemosa and blue toe syndrome are reminiscent of cutaneous polyarteritis nodosa. Unfortunately, no skin biopsy was done to confirm this but the lesions rapidly responded to steroids. Her antibody profile with the ELISA was negative for MPO and PR3 but the immunofluorescence was positive for a perinuclear ANCA. Although this can be a non-specific finding, patients with cutaneous polyarteritis nodosa are negative for MPO and PR3 but have a positive immunofluorescence of a perinuclear pattern. Perinuclear staining on immunofluorescence has been found with antibodies to lactoferrin, elastase and cathepsin G in conditions such as drug induced vasculitis, inflammatory bowel disease and HIV.16
In a series of patient with cutaneous polyarteritis, the antibody was found to be antilysosomal-associated membrane protein 2 (anti-Lamp2).17 Lamp 2 maybe a biomarker for lung cancer and antibodies have an association with pauci-immune glomerulonephritis.18 19 In conclusion, we report the first case of blue toe syndrome associated with the prolonged use of durvalumab.
Patient’s perspective.
I was worried about my ‘blue big left side toe’. I went to my GP who had no idea what she was looking at. She decided I didn’t look well and took my blood pressure which was very low. She sent me to hospital by ambulance, where everyone came to look at my blue toe, to my surprise. I was very pleased when doctors finally got rid of my ‘blue toe’ and was feeling much better. I was glad to go home after 9 days in the hospital.
As for Durvalumab, I will leave up to the doctors to decide. It has left me with a lot of inflammation which I never experienced before the treatment
Learning points.
Blue toe syndrome can be associated with serious vasculitis caused by immunotherapy.
Immune checkpoint inhibitors can lead to vasculitis with a possible genetic pathogenesis.
A comprehensive medical history including exploring the use of various medications is essential in the investigative workup of blue toe syndrome.
Acknowledgments
Staff in the Department of Internal Medicine, St John of God, Midland Hospital, Perth, Western Australia, AustraliaStaff at Curtin Medical School, Faculty of Health Sciences, Curtin University, Perth, Western Australia, Australia.
Footnotes
Contributors: SG is the first author designing, initially drafting and reviewing the manuscript. DX is the corresponding author editing and reviewing the manuscript. JH is the senior author designing, editing and critically reviewing the manuscript. DP is the senior and cocorresponding author designing, editing and critically reviewing the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Mezquita L, Planchard D. Durvalumab for the treatment of non-small cell lung cancer. Expert Rev Respir Med 2018;12:627–39. 10.1080/17476348.2018.1494575 [DOI] [PubMed] [Google Scholar]
- 2.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017;377:1919–29. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 3.Yang H, Shen K, Zhu C, et al. Safety and efficacy of durvalumab (MEDI4736) in various solid tumors. Drug Des Devel Ther 2018;12:2085–96. 10.2147/DDDT.S162214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karmody AM, Powers SR, Monaco VJ, et al. "Blue toe" syndrome. An indication for limb salvage surgery. Arch Surg 1976;111:1263–8. 10.1001/archsurg.1976.01360290097015 [DOI] [PubMed] [Google Scholar]
- 5.Hirschmann JV, Raugi GJ. Blue (or purple) toe syndrome. J Am Acad Dermatol 2009;60:1–20. 10.1016/j.jaad.2008.09.038 [DOI] [PubMed] [Google Scholar]
- 6.Neuman R, Wabbijn M, Guillen S, et al. Blue toe syndrome as a first sign of systemic sclerosis. BMJ Case Rep 2018;2018. 10.1136/bcr-2017-221613. [Epub ahead of print: 05 Jan 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Postema PG, Regeer MV, van der Valk PR, et al. Blue toe syndrome and sunitinib. Int J Clin Oncol 2011;16:574–6. 10.1007/s10147-010-0153-7 [DOI] [PubMed] [Google Scholar]
- 8.Schattner A. Out of the blue finger ischaemia and occult colorectal cancer. BMJ Case Rep 2017;2017:bcr2016218779. 10.1136/bcr-2016-218779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999;11:141–51. 10.1016/S1074-7613(00)80089-8 [DOI] [PubMed] [Google Scholar]
- 10.Weinmann SC, Pisetsky DS. Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology 2019;58:vii59–67. 10.1093/rheumatology/kez308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comont T, Sibaud V, Mourey L, et al. Immune checkpoint inhibitor-related acral vasculitis. J Immunother Cancer 2018;6:120. 10.1186/s40425-018-0443-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daxini A, Cronin K, Sreih AG. Vasculitis associated with immune checkpoint inhibitors-a systematic review. Clin Rheumatol 2018;37:2579–84. 10.1007/s10067-018-4177-0 [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Watanabe R, Berry GJ, et al. Immunoinhibitory checkpoint deficiency in medium and large vessel vasculitis. Proc Natl Acad Sci U S A 2017;114:E970–9. 10.1073/pnas.1616848114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grabie N, Gotsman I, DaCosta R, et al. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation 2007;116:2062–71. 10.1161/CIRCULATIONAHA.107.709360 [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi S, Kimura S, Soma Y, et al. Lysosomal-Associated membrane protein-2 plays an important role in the pathogenesis of primary cutaneous vasculitis. Rheumatology 2013;52:1592–8. 10.1093/rheumatology/ket159 [DOI] [PubMed] [Google Scholar]
- 16.Kallenberg CGM. Usefulness of antineutrophil cytoplasmic autoantibodies in diagnosing and managing systemic vasculitis. Curr Opin Rheumatol 2016;28:8–14. 10.1097/BOR.0000000000000233 [DOI] [PubMed] [Google Scholar]
- 17.Kawakami T, Ishizu A, Arimura Y, et al. Serum anti-lysosomal-associated membrane protein-2 antibody levels in cutaneous polyarteritis nodosa. Acta Derm Venereol 2013;93:70–3. 10.2340/00015555-1418 [DOI] [PubMed] [Google Scholar]
- 18.Soltermann A, Ossola R, Kilgus-Hawelski S, et al. N-glycoprotein profiling of lung adenocarcinoma pleural effusions by shotgun proteomics. Cancer 2008;114:124–33. 10.1002/cncr.23349 [DOI] [PubMed] [Google Scholar]
- 19.Kain R, Exner M, Brandes R, et al. Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat Med 2008;14:1088–96. 10.1038/nm.1874 [DOI] [PMC free article] [PubMed] [Google Scholar]