Abstract
Diagnostics, including those that work at point-of-care, are an essential part of successful public health responses to infectious diseases and pandemics. Yet, they are not always used or fit intended use settings. This paper reports on key insights from a qualitative study on how those engaged with developing and implementing new point-of-care (POC) diagnostics for tuberculosis (TB) and HIV ensure these technologies work at POC. Ethnographic fieldwork between 2015 and 2017 consisting of 53 semistructured interviews with global stakeholders and visits to workshops, companies, and conferences was combined with 15 semistructured interviews with stakeholders in India including providers, decision-makers, scientists and developers and visits to companies, clinics and laboratories. Our results show how developers and implementer of HIV and TB POC diagnostics aim to know and align their diagnostics to elements in more settings than just intended use, but also the setting of the developer, the global intermediaries, the bug/disease and the competitor. Actors and elements across these five settings define what a good diagnostic is, yet their needs might conflict or change and they are difficult to access. Aligning diagnostics to the POC requires continuous needs assessment throughout development and implementation phases as well as substantive, ongoing investment in relationships with users. The flexibility required for such continuous realigning and iteration clashes with established evaluation procedures and business models in global health and risks favouring certain products over others. The paper concludes with suggestions to strengthen this alignment work and applies this framework to research needs in the wake of COVID-19.
Keywords: diagnostics and tools, HIV, tuberculosis, qualitative study
Key questions.
What is already known?
Generating demand and supply for global health diagnostics is challenging and diagnostics that are cheaper, faster or involve less user steps do not automatically fit intended use settings or cut diagnostic delay.
What are the new findings?
This paper breaks new ground in that it offers a juxtaposition of different viewpoints of how diagnostics are made and implemented spanning both upstream and downstream innovation processes.
Fitting diagnostics to the point-of-care requires addressing variable, shifting and hard to access end-users and involves alignment work by developers and implementers across different settings that extend beyond the setting of intended use.
The development process required for these devices is iterative, which is in conflict with the traditional evaluation procedures in global health.
What do the new findings imply?
Creating more and new forms of funding for continuous needs assessment as well as welcoming spaces to safely engage with a variety of user needs and build relationships.
It is essential to think general infrastructure strengthening and innovation of affordable and available diagnostic technologies together to bridge the disconnect between developers and implementers.
Creating more flexibility in evaluation practices to respond to changes in the field and design iterations and to broaden the evidence-base for regulatory decisions to include considerations of access and user perspectives.
Introduction
Diagnostics are an essential part of successful public health responses to infectious diseases and pandemics.1 Experiences from recent outbreaks of yellow fever and Ebola show that ‘diagnostic tests in a format adapted for field use are essential for rapid containment of outbreaks, even in the presence of an effective vaccine’.1 Much hope is also put into the development of preferably low-cost point-of-care (POC) diagnostics for routine care of tuberculosis (TB) or sexually transmitted diseases.2 3 Yet, we also know that diagnostics that are cheaper, faster or involve less user steps are neither always used nor automatically fit intended use settings and cut diagnostic delay.4–7
How to develop diagnostics that fit the POC and various health systems? How to meet patient and provider needs in clinics, hospitals or primary care laboratories across different countries?
Many diagnostic companies that failed, did so because their product did not meet customer needs.8 Trust and demand by end-users are considered important for success of diagnostics. Yet, current design-processes happen top-down without much end-user involvement and often without a thorough assessment of the complex problems (POC) diagnostics are to solve (ie, dropping out of diagnostic pathways does not necessarily only happen because a test result is delayed).9
Common challenges to diagnostic development include fragmented funding pathways, limited access to specimens and reagents, inadequate national and community capacity for diagnostic testing and lack of incentives to develop and manufacture diagnostics during non-outbreak periods.1 Several authors have written about the challenges of generating demand and supply for global health diagnostics,9 of accessing finance and regulatory harmonisation,10 of retooling at country level amidst limited information, complex decision-making processes, inadequate business models and weak political commitment,7 11 of integrating diagnostics cost-effectively into existing diagnostics networks,12 of insufficient evaluations in settings of intended use and lack of resources for country adoption13; and of maintaining and implementing diagnostics in such a way that they reach beneficiaries.4 14 15 Others have, specifically for pandemic preparedness, mapped factors to speed up diagnostic development.16 But no study has examined in detail how POC diagnostics are being innovated for global health.
Early marketing and consumer research focused on how to fit products to cognitive and ergonomic properties of users. Since the 1970s, the design, management and innovation literature has recognised users as collaborators and innovators with valuable insights.17 18 Collaborative design approaches have involved users in design of products and services.19 Digital technologies enabled more interactions with users. Human centred-design approaches, mostly used for IT and internet products/services, investigate context of use before and during wider uptake.18 20 Science and technology studies approaches on the other hand, study how the innovation process unfolds, highlighting its contingent and interactive nature (eg,21–24). Design and use mutually constitute each other, meaning that users are influenced by and also shape technologies, not only once technologies are developed but also when assumptions about users are inscribed into material characteristics.18 25 26 In general, medical technologies do not evolve in a vacuum. How medical technologies look like and are used is shaped by business interest, scientific trends, regulatory environment, and social and political processes.
Building on the previous cited concerns in the diagnostic community and adopting this particular analytical lens, this paper reports key lessons from a qualitative study on diagnostic innovation for TB and HIV. This study asked: how do those engaged with developing and implementing new POC diagnostics for TB and HIV ensure these technologies work in a POC setting? The theoretical implications for alignment theory and user studies have been published.27 Here, we have distilled key messages for the diagnostic community, including end-users, researchers, developers, implementers, donors, regulators, policymakers, non-governmental organisations (NGOs) and consultants in the field. Ethnographic research of how diagnostics are made and implemented, spanning both upstream and downstream innovation processes, allows a juxtaposition of viewpoints. Analysing the bigger picture in which these innovation processes occur is important, yet difficult to oversee if engaged in the daily tasks of solving R&D puzzles, designing an evaluation trial, raising funds or implementing a diagnostic in a clinic workflow.
Methods
This study was conducted between July 2015 and January 2017. We used purposeful and convenience-based sampling and included end-users and those involved in designing, deploying, funding and evaluating diagnostics for TB and HIV. In recent years, a few new TB diagnostics have been introduced. Among them is the Xpert MTB/RIF by Cepheid, a molecular test of TB and resistance to rifampicin (one of the main anti-TB drugs) that provides results in 90 min. Many of the TB developers are smaller start-ups based in the USA/European Union with some exceptions in India and China. Few are established diagnostic companies with an existing portfolio of rapid tests.
The field of HIV has a long history of testing at POC with disposable rapid tests and many companies that produce these tests. Most of these kits use lateral flow technique, a fingerprick blood sample and take up to 20 min to perform. Instrument-based testing for HIV to monitor CD4 and viral load at POC is less common with some (near-)POC diagnostics in development or on the market.28 We interviewed developers of HIV and TB POC diagnostics in different stages of development, including some with diagnostics that are in use and others that failed (exited the market or not completed). In this paper we draw on examples from across the development stages and diseases which allows covering different parts of the innovation process.
Data collection happened in two sets of ethnographic fieldwork. The first set of material consists of 53 semi-structured interviews with diagnostic developers, donors, members of civil society, industry consultants, international organisations, policy makers, regulators and researchers who are distributed geographically and act globally. These interviews were conducted in person and via Skype or telephone and combined with visits to workshops, companies and conferences in Europe and North America. A second set of material is based on fieldwork in Bangalore, India, including 15 interviews with diagnostic developers, decision-makers, NGO programme officers, scientists, TB and HIV programme officers, laboratory managers, technicians and nurses using TB and HIV diagnostics as well as visits to companies, clinics and laboratories (see online supplemental appendix 1 for participant profiles).
bmjgh-2020-003457supp001.pdf (57.8KB, pdf)
The interviews were guided by a topic list which covered the participant’s involvement with diagnostic development; development processes; handling of challenges; evaluation and regulatory practices; understanding of the POC; and practices of making the diagnostic work. Analysis and data collection emerged iteratively. The fieldwork notes, interview notes and transcripts, for those that were electronically recorded (all but five), were analysed with Nvivo using a constructionist thematic analysis.29 The coding scheme (online supplemental appendix 2) was developed based on the research question, notes and fieldwork reports and codes identified from reading the material. Fieldwork reports allowed initial data analysis of innovation and alignment practices and dimensions and collation of codes into potential themes. This analysis was further deepened once coding was completed by going back and forth between the coded material and the initial analysis (looking for examples, reordering, sorting, summarising30), by writing memos on selected themes and by reviewing theoretical approaches to make sense of the data.
bmjgh-2020-003457supp002.pdf (76.7KB, pdf)
Patient and public involvement
Because this paper focused on diagnostic test developers and implementers, there was no direct patient and public engagement on the paper.
Results
We ordered the results along four broad development phases and summarised key lessons for each in box 1.
Box 1. Take away lessons per development phase.
Design and concept
Getting to know the users of point-of-care (POC) diagnostics requires:
Substantive, ongoing investment in relationships because users vary, shift and are hard to access: spend time and challenge users.
Developers need to know and align to more settings than the intended use but also global intermediaries, diseases/bugs, competitors.
Needs assessment is a core business: needs to be continuously done in all phases, not only once or twice before research and development.
A certain amount of uncertainty about the elements and users at POC will remain.
Research and development
Knowing the POC does not stop but remains core business.
-
Close cooperation with evaluators, different users and better understanding of changing settings helps:
When deciding on continuous trade-offs and going through design iterations.
When contextualising isolated, shifting, variable user wishes.
Evaluation and launch readiness
Temporality challenges: the reality of getting things done (regulation, design lock, launch etc) clashes with the ongoing alignment and iterations.
Greater flexibility in evaluation practices to respond to changes in the field, to allow iterations and to assess technical and implementation aspects separately.
Regulatory approval processes should ask for evidence to inform trade-off between accuracy and access.
Implementation and maintenance
Continuous (re-)alignment by implementers requires staff and other resources.
Impact on access, utilisation and equity of earlier alignment choices becomes clear.
Knowing the POC never stops, investing in ways to get feedback from users and managing multiplicity.
Design and concept phase
In the design and concept phase, developers try to understand the POC and how the new diagnostic should look like. In doing so, they face different challenges than developers of laboratory-based diagnostics. One of the specific challenges identified in our data is that there are many different users that engage with a TB or HIV POC diagnostics. They include technicians, patients, different types of clinic staff (nurses, clinicians, community health workers), laboratory managers, ministries of health, NGOs, regulators and funders who deploy, conduct, maintain, evaluate, monitor or purchase these diagnostics. According to a programme officer at the Clinton Health Access Initiative (CHAI) who supports developers of global health diagnostics, it is essential to understand all the different aspects of POC use:
Understand clinical use, not just operational design, engage clinicians, nurses etcetera; Understand markets, business case, market entry and the flexibilities you have in addressing them (programme officer CHAI 1)
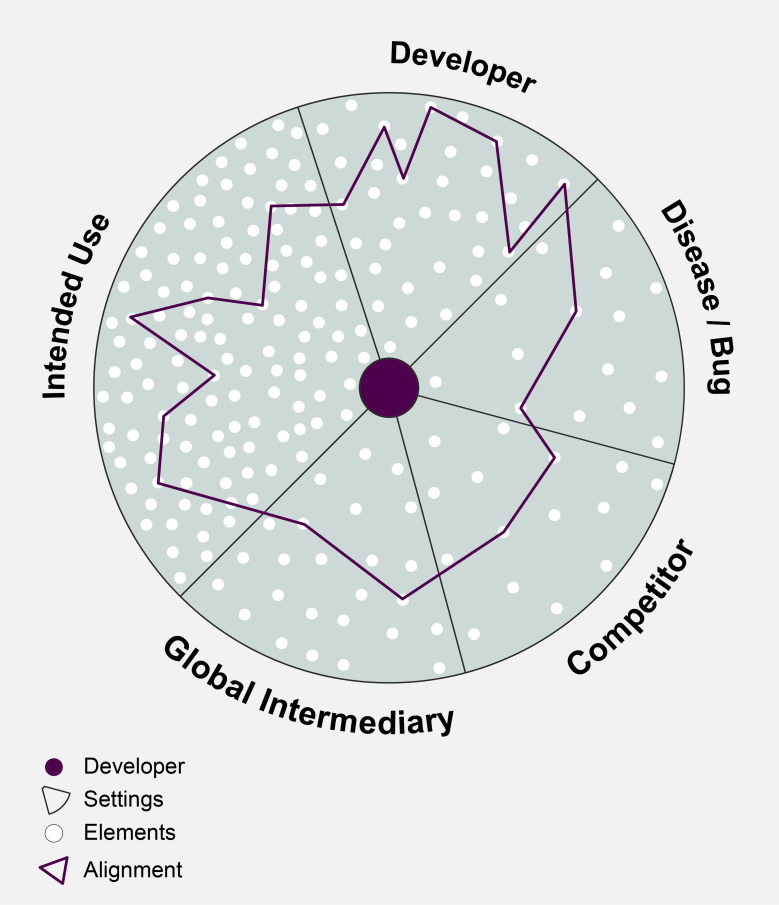
Given the global health nature of this endeavour, the multiple users are spread out across vastly different laboratories, clinics and communities. The developers we spoke to try to understand the many different elements that matter to these users, for instance, patient pathways and numbers, local epidemiology, cost to the clinic and to patients, available staff, equipment, environmental factors, infrastructure, timing requirements, local protocols, alternative testing options, maintenance requirements, quality control systems, market dynamics and so on. Developers are trying to connect the dots (as illustrated in a schematic way in figure 1) and align elements that matter to users in intended use settings with elements they have available ‘in house’, in the developer setting, resulting in a particular alignment pattern. This could include the financial, human and technical resources, techniques and manufacturing capacities the developer can rely on, but also stakeholder buy-in, management support and political will within the company.
Figure 1.
Aligning elements across five settings.
In doing so, developers of POC diagnostics face multiple challenges: according to a donor many TB test developers do not sufficiently account for how decentralising diagnostics complicates logistics, servicing and maintenance in resource-constrained settings: ‘… a lot of the companies that are innovating, they haven’t really thought [it] through. They may have a diagnostic innovation but they don’t have a business model to succeed’ (donor 1). An officer at PATH highlights how some of these challenges are brushed over during grant writing: ‘… you can’t be negative writing grants. You can’t go (…) we’re going to do this, but it is probably going to fail because …—all this is broken’ (programme officer PATH 1). Also, with such diverse and variable users at POC, the developer is bound to get different, potentially conflicting answers about how the diagnostic should look. What is more, developers we interviewed outline how clinicians and nurses are not in the same way dedicated to the diagnostic as a laboratory technician would be. Instead, their focus is on patients and routine care and multiple healthcare workers share responsibility for the same diagnostic device (test developer 9, 11). A developer with an HIV diagnostic on the market outlines how they rarely have direct contact with end-users and instead interact with the supervisors of those who supervise and train end-users (test developer 9). Further, the traditional customers of diagnostic companies are individual hospitals (chains) and not ministries of health, donor groups or NGOs. Some of these new customers might not be accustomed with handling new technologies and interacting with diagnostic companies (industry consultant 2). These challenges are compounded by the fact that it is hard to access or generate data on these variables, multiple and shifting users of POC diagnostics, as a programme officer at CHAI highlights:
… it is also difficult to get good data on what is happening in clinics, how tests are used, for instance, not just how many tests are run, but data on patient important outcomes, time to result, time of treatment initiation, etc. (programme officer CHAI 2)
According to an industry consultant, developers find it difficult to get permission to visit public clinics or programmes, use staff time to answer questions or try out a prototype. Also, there are many factors that influence that data including institutional structures, procurement systems, epidemiological context, clinician behaviour and workflow changes (industry consultant 2).
A key lesson in this phase is that it takes work and time to generate good user feedback, to continuously interact and establish relationships that last years and generate political interest and access to collaborators (industry consultant 2, test developer 6). A developer of a HIV POC diagnostic currently in use highlights that these relationships help to achieve a better fit of the technology with its users, but also with the later stages of organising evaluation studies and market launch (test developer 6).
As a result, and to navigate these challenges of multiple, shifting and difficult to access users, many developers rely on what we term global intermediaries to mediate between developers and users of POC diagnostics. These intermediaries include individual industry consultants and those employed by international organisations, donors and NGOs with a focus on technology (including CHAI, UNITAID, Stop TB Partnership, Medicines Sans Frontieres (MSF), Foundation for Innovative New Diagnostics (FIND), PATH, the Gates Foundation, Wellcome Trust and WHO working groups). Their support includes providing access to important networks in global health, key opinion leaders, distributors and decision-makers in countries, but also involves organising access to end-users, clinics and samples (programme officer CHAI 3, test developer 1, 3). They map out different country characteristics, markets and needs, their TB or HIV programmes, epidemiology and regulatory requirements (programme officers WHO 1, 3). They publish target product profiles (TPPs) (eg, WHO, 2014), market landscapes and cost-effectiveness modelling. They aide developers in setting up independent evaluation studies to acquire WHO approval, and provide contacts during acute disease outbreaks (test developer 6). This means global intermediaries are important mediators and translators but also have a gatekeeping function. Examples of the latter include direct engineering advice that might contradict user feedback (test developer 527); prioritising cost at the expense of other advantages of improved diagnostics (test developer 12); and not supporting competitors or those that do not fit the TPPs because of limited capacities (test developer 4, officer PATH 1; officer FIND 2). Global intermediaries with their agendas and standards are therefore important to take into account when developing a diagnostic for global health.
Beyond users and global intermediaries, the dynamics of the particular disease and competitors define what a good test is.31 Developing a diagnostic for TB, HIV, malaria, Ebola or COVID-19 poses different requirements with regards to patient profiles, funding availability, detections and evolution of strains, sample characteristics, technical and regulatory requirements that a developer needs to align to. HIV and TB histories of disease control efforts, for instance, differ markedly with potentially different roles envisioned for diagnostics in decentralisation and counselling efforts, patient pathways, and workflows. Diagnostic developers are measured against gold standards and competitors with similar diagnostic technologies in terms of accuracy but also price. A TB test developer voiced concerns over the high accuracy of Xpert MTB/Rif in combination with a US$10 market price (facilitated by a donor supported buy-down). For a competitor, this seems impossible to reach without compromising either accuracy or low cost (test developer 20).
Our results show how developers aim to know and continuously align their diagnostic to many different elements across five settings across five different settings (see box 2 and figure 1).
Box 2. Developers align to five different settings and their elements.
Developer setting: techniques, resources, size, different types of companies vary in user involvement, starting points and ambitions.
Intended use setting: with its varied and shifting users, infrastructure, skills, capacities, routines, throughput, patient profiles and markets.
Global intermediaries setting: donors, groups such as Foundation for Innovative New Diagnostics, Clinton Health Access Initiative, Medicines Sans Frontieres, Stop TB Partnership, industry consultants and so on; with its global policies (WHO), markets, standards, regulations and scientific data requirements.
Disease/bug setting: with its dynamics of microbiological changes, patient profiles, funding availability, technical challenges, sample and regulatory requirements.
Competitor setting: companies with emerging/new tests, gold standards, including their ability to generate scientific data, funding, support and political will.
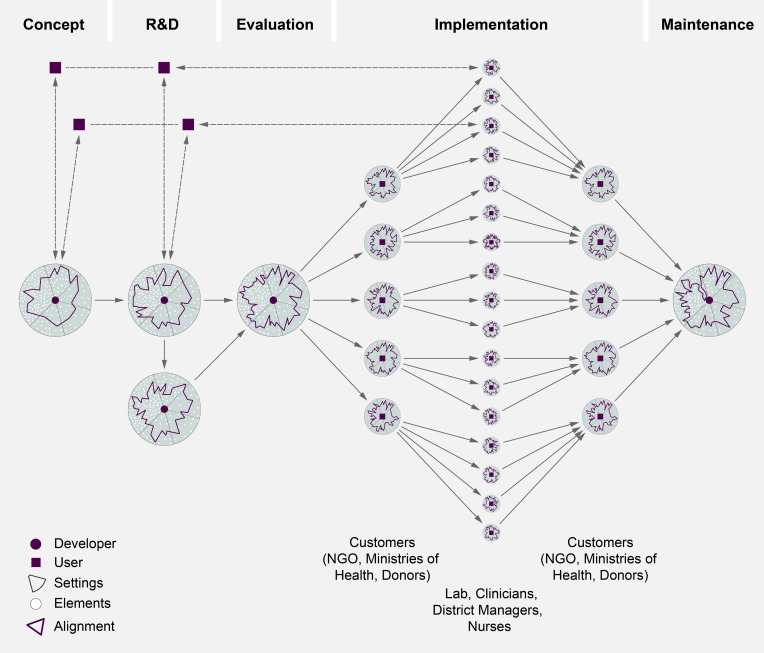
Importantly, a certain amount of uncertainty will remain as no one can ever know or foresee all these elements. Yet according to our interviewees, throughout development pathways, developers have to continuously decide what and how to align with each other: which user needs to consider, what global agendas to accept or ignore, how to decide on a particular trade-off during R&D. The resulting alignment patterns differ and can change throughout the project because there are so many options (see figure 2).
Figure 2.
Alignment throughout development phases. NGO, non-governmental organisation.
The key lesson is that users of POC diagnostics vary, shift and are hard to access which increases the importance of intermediaries who support but also have gatekeeping functions. Developers need to know and align to more settings than the intended use but also global intermediaries, diseases/bugs, competitors.
Research and development
In the research and development phase developers we interviewed outlined how they would go through design iterations, testing of prototypes and trade-off decisions (between aspects such as enhancing the throughput of the device at the expense of processing time, or choosing a better material but risking a higher price or patent rights). Test developers of an HIV POC diagnostic highlight how design iterations such as tightening the cartridge cap to prevent reopening; incorporating a battery to deal with power fluctuation, or redesigning the screen interface happen when developers give out their prototypes to end-users they have established a relationship with in earlier phases (test developer 6, 7; illustrated in figure 2). A developer of a TB diagnostic is frustrated that the turn-around time of their prototype is still 40 min instead of the 15 min he would like. But how quick would a diagnostic really have to be in order to be used? What trade-offs in design, cost, accuracy and delays to market entry should the developer enter for shortening the turn-around time (test developer 3)? There are no easy answers to these questions. In order to decide on continuous trade-off decisions, go through design iterations and contextualise isolated user needs, it helps if developers have close relationships with evaluators and different users and have a good understanding of these five settings. Knowing the POC does not stop after design and concept phase. It remains core business as aligning to the many elements across the five settings is ongoing and resulting alignment patterns change through development phases (see figure 2).
Evaluation and launch readiness
During evaluation studies, ongoing design iterations and alignment have to stop, because the core of the technology should not change. Yet, this so-called design lock can clash with ongoing alignment and iterations. According to the developers we interviewed, it would help to have more flexibilities during evaluation phases, either to respond to changes in the field, such as the epidemiology, or in the pathogens (eg, to respond to discovery of new variants or new mechanisms of antimicrobial resistance) and to allow more iterations and possibly assess technical and implementation aspects separately.32 Currently, diagnostics are evaluated in demonstration studies at research sites which also run the gold standard comparator test. According to an officer at the Stop TB Partnership, which has overseen many projects implementing Xpert MTB/Rif, assessing implementation in routine care situations in intended sites of use rather than research sites, would allow tackling some of the implementation challenges, such as power, languages, workflows, error reporting, supply chain issues, earlier:
… when FIND does the demonstration studies, you probably want to do some other work (…) to involve groups that can do it, but are not producing the quality of evidence, (…) not in the same types of laboratories, in the same way you need to have that quality assured data and use. (…) you won’t get those things that you will find later on without giving them [the diagnostics] to a number of different places and asking them to implement it. (Officer Stop TB Partnership 1)
What is more, evaluation studies assess diagnostics mainly for their sensitivity and specificity and were designed for laboratory-based diagnostics. Donors and global intermediaries assume that POC diagnostics can be simple, rapid and cheap, function without relying on a laboratory or highly skilled technicians, while also reaching high levels of accuracy. Country stakeholders of 14 countries ranked sensitivity the most important element in the TPPs for TB diagnostics.33 Yet, a TB test developer argues that in order to optimise accessibility at POC, a developer might have to compromise accuracy (test developer 20). Regulatory approval processes should be cognizant of this trade-off and require evidence accordingly.11
Implementation and maintenance
During implementation and maintenance phase, the alignment work is moving from the developer to the implementer. In the case of POC diagnostics, these are large NGOs, ministries of health or donors that are purchasing, distributing and deploying diagnostics to the different clinics where clinicians, laboratory technicians, nurses or community health workers continue to (re-)align the diagnostics to the specific aspects of their situation. Figure 2 shows how the alignment situation multiplies.
Implementers might have different priorities than developers and this has consequences for the utilisation of diagnostics and what features implementers prefer. For instance, in a HIV clinic in Bangalore, the nurse compromises rapidity and expands the turn-around time by two or 3 days to explain a test result. She explains how she slowly introduces a positive HIV test result to not scare the patient away (nurse 1):
Some of them [HIV patients] don’t accept, they say no and are adamant, they say there is no chance of me getting it at all. So we have to do a lot of counseling for them. (…) means for two days we try to relieve them a bit saying it is there but it is slight or we have to see, have to do another test, so like that.(…) we delay it, we delay a little in telling them the report. They should be strong in the mind (nurse 1)
The common practice of batching HIV testing and Xpert MTB/Rif twice a day, increases patient wait times but also alters the preference of the laboratory technician in this clinic for bulk packaging of test strips (laboratory technician 3).
This continued (re-)alignment work shapes diagnostics and requires considerable time and resources. A HIV programme officer characterises the management effort when implementing rapid HIV tests in Bangalore as navigating an ocean of different providers (public hospitals, clinics, private doctors, blood banks and nursing homes), testing sites and referral practices (implementer 2). Testing, training and awareness efforts require orchestrating networks and schedules of public and private actors, piggybacking on trainings for other disease control programmes, and training staff at various sites. Yet, the implementer’s alignment work falls short of reaching individual private practitioners. Collecting data from these clinics would outstrip the capacities of her office (implementer 2). Similarly, a medical officer in a public district hospital required time, resources and negotiation skills to organise free care in the isolation ward at a nearby medical college for patients diagnosed with MDR-TB by Xpert MTB/Rif at his site (implementer 5). This work of organising linkage to care is not part of the diagnostic per se. Yet, it is essential because otherwise patients would not be able to access care within a feasible distance, the numbers of lost-to-follow up would increase and the diagnostic test would have been rendered useless.
According to a programme officer at MSF, necessary infrastructure and human resources to operate POC diagnostics need to be added onto systems, otherwise POC testing is overburdening already stretched out capacities.
… so what has been suggested by some is POC workforce who can really be responsible, trained, who can do the quality control, make sure the machine is kept in the right space, in the right room, without dust. Just be responsible for that! No one else is gonna do that if they are not hired to do that. Especially if 50 random people … perform the test (programme officer MSF 1)
Increased funding and support for health system and infrastructure strengthening is essential to support diagnostic innovation. Box 1 provides an overview of the key lessons per development phase.
Discussion
When developing and implementing a POC diagnostic for global health, developers are not only designing an artefact but a usage. Understood in this way, a range of different actors and elements need to be considered. Compared with laboratory-based diagnostics, the users of POC diagnostics are multiple, variable, shift and are difficult to access. The implication of this difference means the developer is further removed from the users and it takes more work to involve and get to know users. In this context then, the role of intermediaries becomes important to translate and bridge between developers and the many users. We showed how fitting a diagnostic to the POC, involves more than just aligning it to the intended setting of use but also to the settings of developer, global intermediaries, diseases/bugs and competitors. Actors and elements across these five settings define what a good diagnostic is. Their needs might conflict and change over time. Consequently, understanding these user needs is crucial and requires continuous effort and substantive investment to access and manage the multiplicity of needs and settings. Currently, according to our interviewees, it is insufficiently done.
When developing diagnostics for centralised, relatively well-equipped laboratories, we can expect it to be easier to understand the intended use setting, as users are better defined, stable and less variable. The role of global intermediaries might be limited to funding, global guideline-making and evaluation. Meeting technical standards of competitors and regulatory requirements might be easier as these diagnostics can rely on more resources, equipment and user skills, the absence of which means POC developers have to make trade-offs affecting accuracy. The choices that developers and later implementers make in aligning the diagnostic to the different elements across the five settings have implications for access, utilisation and equity of diagnostics. It is crucial to work through design iterations, trade-off decisions and alignment work in close relation with the different actors and users across these five settings.
In fitting diagnostics to the POC, a certain amount of flexibility is required to continuously (re)-align in a continuous iterative process of testing change ideas.34 Yet, this goes against established evaluation procedures and business models in global health which risk favouring certain products over others.31 It also shows how difficult it is to back up from promises, bench marks or gold standards. This not only has to do with donor discourses about cheap, rapid and simple yet accurate tests, but also with the way global evidence is made.
Also, aligning to these various elements continues through implementation and maintenance phases,4 35 and it is resource-intensive. Ethnographies of diagnostics in use similarly show the work and resources required for diagnosing at POC.6 15 36 37 The promise to overcome absent laboratory infrastructure and skilled human resources with POC diagnostics is misleading. POC testing still requires those ingredients, although in slightly different forms.9 This means infrastructure strengthening and innovation of affordable and available diagnostic technologies needs to happen jointly.1 How to bridge between designers and implementers and improve alignment work? Based on our data and above analysis we outline suggestions in box 3.
Box 3. Suggestions to strengthen alignment work.
-
Create safer, more welcoming spaces to engage with a variety of user needs and build relationships:
In clinics: explore possibilities of setting up welcoming learning environment, engage healthcare workers early to understand clinical use.
In guideline development meetings: improve representation of different user needs with more diverse evidence (including operational and qualitative research), create safer spaces for community and patient group members to speak up.
In Ministries of Health: coordinate country needs, create a formal mechanisms for ministries to get feedback from their users before and during implementation.
In global target product profile (TPP) consultations: more flexible TPPs and stronger consultative writing process, clarify role of global intermediaries and involve early on.
Clarify understanding of point-of-care (POC) as part of a comprehensive outlook on health.
Increased and new forms of funding for continuous needs assessment.
Increased funding and attention to alignment work of implementers.
Plan for technology design, health system and infrastructure strengthening in parallel, they are inseparable and they influence each other.
Improved coordination among intermediaries to avoid duplicating efforts.
Be realistic/honest about timelines, funding, team, in country support feasibility.
-
Harmonised, simplified regulatory process:
Type of data: it might not always be necessary to go through randomized controlled trial (RCTs), more evaluations in less controlled sites than demonstration studies.
Timing: more flexibility to respond to changes in the field, to allow iterations and to assess technical and implementation aspects separately.
Rethink gold standards: possibly apply some of the POC diagnostics criteria to laboratory-based tests (improve turn-around time for getting results back).
Different indicators/measurements are needed for clinical utility of tests to supplement accuracy measurements.
Improved business models: for instance, not for profit; collaborative instead of exclusive; global consortium; different supply/deployment model via central labs instead of individual suppliers; different incentives and new kind of funding mechanisms for next generation followers.
Local development of POC diagnostics requires additional/different support.
Our framework offers a way to develop an agenda for future research needs on COVID-19 diagnostics: how is needs assessment in innovation processes done during a pandemic? Does the speed require limit inclusivity and thoroughness? During a pandemic, the regulatory and guideline making processes are fast forwarded. What type of data and evidence is used? How do innovators handle the considerable uncertainty around the setting of the bug/disease? Which strains should the diagnostic target? How will the virus evolve? How to ensure commercial viability in non-outbreak periods? Experiences during the Ebola outbreak showed that more funding for diagnostic development is made available, but risks fragmentation and duplication.1 The reality and speed of the pandemic clashes even more with regulation, design locks, current procurement and business models for diagnostics and will possibly force greater flexibility.
The geo-politics involved mean the setting of global intermediaries’ changes. What are the implications for innovation processes and access to diagnostics? How is innovation incentivised and governed? With COVID-19, countries are racing for testing equipment. Catharina Boehme, CEO of FIND, speaks in a webinar of an ongoing supply chain war with countries pressuring test producers to move production capacities away from TB, malaria and HIV towards COVID-19.38 Global initiatives aim to coordinate innovation efforts and ensure equitable access to new tools.39 Future research needs to analyse the impact of such mechanisms alongside country-led responses.
Future research also needs to monitor deployment of COVID-19 diagnostics and analyse questions of distribution and rationing. Which channels and algorithms are used? How is testing for COVID-19 integrated with diagnostic services, especially if diagnostic platforms are shared? Severe disruptions of routine health services are predicted to increase global TB, malaria and HIV incidence and death considerably.40 41 Manufacturers deprioritise production of TB, malaria and HIV diagnostics in the face of COVID-19.42 Who will be tested (first)? Who has access? How and based on what forms of knowledge are decision made when designing testing programmes? Public health responses including testing and tracing in pandemic situations require next to diagnostics, investment in infrastructure and staff to ensure continuous realignment to specific situations and settings.
Acknowledgments
We are grateful to all the participants for granting their insights and valuable time, and for Vijayashree Yellappa and the Institute of Public Health in Bangalore, India for supporting with part of the data collection. Anja Krumeich, Klasien Horstman and Harro van Lente provided important support throughout this project.
Footnotes
Handling editor: Seye Abimbola
Contributors: NE conceived and designed the study, collected and analysed the data and wrote the first draft. PFGW supported data analysis and revision of the paper.
Funding: This work was supported by a VENI grant from the Dutch Science Foundation NWO (grant number 16.158.004).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study was approved by UMREC, the ethical review board of Maastricht University. Prior to the interview participants signed information sheets and informed consent forms. In this paper, the companies and technologies are anonymised for those technologies that are in use.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon request. Public deposition of the data would compromise interviewee privacy. De-identified data are available upon individual request with the necessary editing to preserve anonymity of the study participants. please contact Nora Engel https://orcid.org/0000-0002-1823-3908.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Kelly-Cirino CD, Nkengasong J, Kettler H, et al. . Importance of diagnostics in epidemic and pandemic preparedness. BMJ Glob Health 2019;4:e001179. 10.1136/bmjgh-2018-001179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toskin I, Peeling RW, Mabey D, et al. . Point-Of-Care tests for STIs: the way forward. Sex Transm Infect 2017;93:S1–2. 10.1136/sextrans-2016-053074 [DOI] [PubMed] [Google Scholar]
- 3.Denkinger CM, Pai M. Point-Of-Care tuberculosis diagnosis: are we there yet? Lancet Infect Dis 2012;12:169–70. 10.1016/S1473-3099(11)70257-2 [DOI] [PubMed] [Google Scholar]
- 4.Albert H, Nathavitharana RR, Isaacs C, et al. . Development, roll-out and impact of Xpert MTB/RIF for tuberculosis: what lessons have we learnt and how can we do better? Eur Respir J 2016;48:516–25. 10.1183/13993003.00543-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engel N, Ganesh G, Patil M, et al. . Point-Of-Care testing in India: missed opportunities to realize the true potential of point-of-care testing programs. BMC Health Serv Res 2015;15:550. 10.1186/s12913-015-1223-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beisel U, Umlauf R, Hutchinson E, et al. . The complexities of simple technologies: re-imagining the role of rapid diagnostic tests in malaria control efforts. Malar J 2016;15:64. 10.1186/s12936-016-1083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pai M, Palamountain KM. New tuberculosis technologies: challenges for retooling and scale-up. Int J Tuberc Lung Dis 2012;16:1281–90. 10.5588/ijtld.12.0391 [DOI] [PubMed] [Google Scholar]
- 8.Urdea M, Thayer R. Halteres diagnostics report 2018. Emeryville, CA: Halteres, 2018. [Google Scholar]
- 9.Engel N, Wachter K, Pai M, et al. . Addressing the challenges of diagnostics demand and supply: insights from an online global health discussion platform. BMJ Glob Health 2016;1:e000132. 10.1136/bmjgh-2016-000132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNerney R. Diagnostics for developing countries. Diagnostics 2015;5:200–9. 10.3390/diagnostics5020200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palamountain KM, Baker J, Cowan EP, et al. . Perspectives on introduction and implementation of new point-of-care diagnostic tests. J Infect Dis 2012;205 Suppl 2:S181–90. 10.1093/infdis/jis203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schito M, Peter TF, Cavanaugh S, et al. . Opportunities and challenges for cost-efficient implementation of new point-of-care diagnostics for HIV and tuberculosis. J Infect Dis 2012;205 Suppl 2:S169–80. 10.1093/infdis/jis044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ditiu L, Boehme C. Crossing the valleys of death in TB: from development to roll-out, 2017. Available: http://gbchealth.org/crossing-the-valleys-of-death-in-tb-from-development-to-roll-out/#_ftnref2
- 14.Pai NP, Vadnais C, Denkinger C, et al. . Point-Of-Care testing for infectious diseases: diversity, complexity, and barriers in low- and middle-income countries. PLoS Med 2012;9:e1001306. 10.1371/journal.pmed.1001306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engel N, Ganesh G, Patil M, et al. . Barriers to point-of-care testing in India: results from qualitative research across different settings, users and major diseases. PLoS One 2015;10:e0135112. 10.1371/journal.pone.0135112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perkins MD, Dye C, Balasegaram M, et al. . Diagnostic preparedness for infectious disease outbreaks. The Lancet 2017;390:2211–4. 10.1016/S0140-6736(17)31224-2 [DOI] [PubMed] [Google Scholar]
- 17.van Hippel E. Democratizing innovation. Cambridge: MIT Press, 2005. [Google Scholar]
- 18.Hyysalo S, Jensen TE, Oudshoorn N, The New Production of Users: Changing innovation collectives and involvement strategies. Oxon, New York: Routledge, 2016. [Google Scholar]
- 19.Schuler D, Namioka A. Participatory design: principles and practices. Hillsdale, NJ, US: Lawrence Erlbaum Associates, Inc, 1993: xiii, 319–xiii, p. [Google Scholar]
- 20.Norman DA, Draper SW. User centered system design; new perspectives on Human-Computer interaction. L. Erlbaum Associates Inc, 1986. [Google Scholar]
- 21.Prasad A. Imperial Technoscience: transnational histories of MRI in the United States, Britain and India. MIT Press, 2014. [Google Scholar]
- 22.Engel N, Bijker W. Innovating tuberculosis control in India. Economic and Political Weekly 2012;47:111–8. [Google Scholar]
- 23.Timmermans S, Berg M. The gold standard. The challenge of evidence-based medicine and standardization in health care. Philadelphia: Temple University Press, 2003. [Google Scholar]
- 24.Timmermans S. Trust in standards: transitioning clinical exome sequencing from bench to bedside. Soc Stud Sci 2015;45:77–99. 10.1177/0306312714559323 [DOI] [PubMed] [Google Scholar]
- 25.Akrich M. The De-Scription of Technical Objects : Bijker WE, Law J, Shaping technology / building Society: studies in Sociotechnical change. Cambridge, London: The MIT Press, 1992: 205–24. [Google Scholar]
- 26.Oudshoorn N, Pinch TJ. Users and Non-Users Matter : Oudshoorn N, Pinch TJ, How users matter: the Co-Construction of users and technology. London, Cambridge MA: MIT Press, 2003: 1–25. [Google Scholar]
- 27.Engel N. Aligning in the dark: variable and shifting (user-) settings in developing point-of-care diagnostics for tuberculosis and HIV. Soc Stud Sci 2020;50:50–75. 10.1177/0306312719900545 [DOI] [PubMed] [Google Scholar]
- 28.Medicins sans Frontieres Putting HIV and HCV to the test: a product guide for point-of-care CD4 tests and laboratory-based and point-of-care HIV and HCV viral load tests. Geneva: MSF access campaign, 2017. [Google Scholar]
- 29.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77–101. 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- 30.Rubin HJ, Rubin IS. Qualitative interviewing: the art of hearing data. 2nd ed Thousands Oaks, London, New Delhi: Sage Publications, 2005. [Google Scholar]
- 31.Engel N, Krumeich A. Valuing simplicity: developing a good point of care diagnostic. Frontiers in Sociology 2020;5 10.3389/fsoc.2020.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cobelens F, van den Hof S, Pai M, et al. . Which new diagnostics for tuberculosis, and when? J Infect Dis 2012;205 Suppl 2:S191–8. 10.1093/infdis/jis188 [DOI] [PubMed] [Google Scholar]
- 33.Adepoyibi T, Lilis L, Greb H, et al. . Which attributes within target product profiles for tuberculosis diagnostics are the most important to focus on? Int J Tuberc Lung Dis 2018;22:425–8. 10.5588/ijtld.17.0312 [DOI] [PubMed] [Google Scholar]
- 34.Backhouse A, Ogunlayi F. Quality improvement into practice. BMJ 2020;35:m865 10.1136/bmj.m865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.England K, Masini T, Fajardo E. Detecting tuberculosis: rapid tools but slow progress. Public Health Action 2019;9:80–3. 10.5588/pha.19.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yellapa V, Devadasan N, Krumeich A, et al. . How patients navigate the diagnostic ecosystem in a fragmented health system: a qualitative study from India. Glob Health Action 2017;10:1350452 10.1080/16549716.2017.1350452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engel N, Davids M, Blankvoort N, et al. . Making HIV testing work at the point of care in South Africa: a qualitative study of diagnostic practices. BMC Health Serv Res 2017;17:408 10.1186/s12913-017-2353-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The Union Panel discussion: how to prevent 1.4 million deaths: advancing TB care and prevention in the time of COVID-19: the Union, 2020. Available: https://coursesonline.theunion.org/theunion/2020/covid-19-outbreak/301150/catharina.boehme.cheri.vincent.marijke.wijnroks.tereza.kasaeva.lucica.ditiu.html?f=menu%3D8%2Abrowseby%3D8%2Asortby%3D2%2Alabel%3D19867
- 39.World Health Oganization Access to COVID-19 tools (act) accelerator; 2020.
- 40.Partnership S. The potential impact of the COVID-19 Reponse on tuberculosis in high-burden countries: a modeling analysis. Geneva: StopTB Partnership, 2020. [Google Scholar]
- 41.Pai M. forbescom, 2020. Available: https://www.forbes.com/sites/madhukarpai/2020/05/11/aids-tb-and-malaria-set-to-get-deadlier-due-to-coronavirus/#791b8b21d2fc
- 42.Bosley S. Demand for coronavirus tests raises concerns over HIV and malaria. The Guardian 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2020-003457supp001.pdf (57.8KB, pdf)
bmjgh-2020-003457supp002.pdf (76.7KB, pdf)