Abstract
Objective
The aim of the current study was to evaluate the 2-year cost-utility ratio between tapering conventional synthetic disease-modifying antirheumatic drugs (csDMARD) first followed by the tumour necrosis factor (TNF)-inhibitor, or vice versa, in patients with rheumatoid arthritis (RA).
Methods
Two-year data of the Tapering strategies in Rheumatoid Arthritis trial were used. Patients with RA, who used both a csDMARD and a TNF-inhibitor and had a well-controlled disease (disease activity score ≤2.4 and swollen joint count≤1) for at least 3 months, were randomised into gradual tapering the csDMARD first followed by the TNF-inhibitor, or vice versa. Quality-adjusted life years (QALYs) were derived from the European Quality of life questionnaire with 5 dimensions. Healthcare and productivity costs were calculated with data from patient records and questionnaires. The incremental cost-effectiveness ratio and the incremental net monetary benefit were used to assess cost effectiveness between both tapering strategies.
Results
94 patients started tapering their TNF-inhibitor first, while the other 95 tapered their csDMARD first. QALYs (SD) were, respectively, 1.64 (0.22) and 1.65 (0.22). Medication costs were significantly lower in the patients who tapered the TNF-inhibitor first, while indirect cost were higher due to more productivity loss (p=0.10). Therefore, total costs (SD) were €38 833 (€39 616) for tapering csDMARDs first, and €39 442 (€47 271) for tapering the TNF-inhibitor (p=0.88). For willingness-to-pay (WTP) levels <€83 800 tapering, the csDMARD first has the highest probability of being cost effective, while for WTP levels >€83 800 tapering the TNF-inhibitor first has the highest probability.
Conclusion
Our economic evaluation shows that costs are similar for both tapering strategies. Regardless of the WTP, tapering either the TNF-inhibitor or the csDMARD first is equally cost effective.
Trial registration number
NTR2754.
Keywords: rheumatoid arthritis, economics, tumour necrosis factor inhibitors, outcome assessment, health care, methotrexate
Key messages.
What is already known about this subject?
Savings on healthcare and societal costs could be obtained by tapering quickly and possibly stopping medication when patients with rheumatoid arthritis reach sustained remission.
Current European League Against Rheumatism guidelines recommend to taper medication when patients with rheumatoid arthritis are in sustained remission; however, evidence for an optimal tapering approach is currently sparse.
What does this study add?
The Tapering strategies in Rheumatoid Arthritis trial is the first randomised controlled trial which included a cost-utility analysis on two active, gradual tapering strategies.
How might this impact on clinical practice or future developments?
Both tapering strategies are cost effective and depending on the viewpoint (payer’s or societal perspective) and willingness-to-pay threshold, the tumour necrosis factor-inhibitor or conventional synthetic disease-modifying anti-rheumatic drugs can be tapered first.
Introduction
The optimal management for rheumatoid arthritis (RA) comprises an early, intensive and treat-to-target management approach, which has the highest chance of inducing remission and preventing joint damage.1 2 In case of sustained remission, tapering of treatment can be considered to reduce side-effects and save costs.3 In the Netherlands, more than €300 million are spent on the use of biological therapy for rheumatic diseases.4 On the other hand, treatment with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) generally costs only one-tenth of the cost of a biological.5 Although rheumatologists carefully consider initiation of biologicals, uniform tapering decisions are lacking, and therefore biological tapering is not always directly performed when sustained remission is achieved.6 Tapering of biologicals could reduce healthcare costs.
In the Tapering strategies in Rheumatoid Arthritis (TARA) trial, two tapering strategies were compared, namely tapering the tumour necrosis factor (TNF)-inhibitor first followed by the csDMARD, or vice versa. Within the first year, in which either the TNF-inhibitor or the csDMARD was gradually tapered within 6 months, there were no significant differences in flare rates, disease activity, functional ability and quality of life, although we did observe numerical differences (10% in flare rates), and less patients in clinical remission.7 From a clinical viewpoint, one could argue that the order of tapering is not relevant. On the other hand, TNF-inhibitors are far more expensive than csDMARDs; therefore, from a health economics perspective, it is more sensible to taper the TNF-inhibitor first. Previous studies already showed that tapering biologicals leads to a reduction of medication and medical consumption costs, also known as direct costs, but could also result in a decrease in quality of life.8–11 Tapering of medication might lead to an increase in disease activity and consequently to a disease flare. This could lead to more pain and disability, possibly resulting in more productivity loss and sick leave. However, not much is known about aforementioned possible effects. Furthermore, none of the aforementioned studies compared two active tapering strategies.11 12
Moreover, a previous study already showed that disease flares have a significant impact on patients’ lives, which among other things could lead to productivity loss.13 As mentioned earlier, the effect of a flare on societal costs is not known. Nor do we know whether the healthcare (direct) cost reduction due to tapering treatment outweigh the possible increase in productivity (indirect) costs.
Therefore, our aim is to investigate which gradual tapering strategy has the best cost-utility ratio over a period of 2 years. Furthermore, we want to explore the effect of tapering on both medical and societal costs.
Patients and methods
Patients
For this study, data were used from the TARA trial (NTR2754). TARA, a multicentre, single-blinded trial was carried out in 12 rheumatology centres in the Netherlands between September 2011 and July 2016. All patients gave written informed consent before inclusion.
Primary aims of the TARA study were to assess effectiveness and cost effectiveness of two tapering strategies, from a societal perspective. An extended description of the TARA study and clinical effectiveness outcomes can be found elsewhere.7 14 Inclusion criteria for the TARA trial were: adult patients with RA, with a well-controlled disease, defined as a disease activity score (DAS44) ≤2.4 and a swollen joint count (SJC) ≤1 at two consecutive time points within a 3-month interval, who were using a combination of a csDMARD and a TNF-inhibitor.
Randomisation and masking
Patients were randomised using minimisation randomisation stratified for centre into tapering the csDMARD in the first year followed by tapering the TNF-inhibitor in the second year, or vice versa. No other factors were used for the minimisation randomisation. Trained research nurses, blinded to the allocated treatment arm throughout the study, examined the patients.
Design
The csDMARD and TNF-inhibitor were both gradually tapered in three steps. csDMARD tapering was realised by cutting the dosage into half, a quarter and thereafter it was stopped. The TNF-inhibitor was tapered by doubling the interval between gifts, followed by cutting the dosage into half, and thereafter it was stopped. If patients remained flare free, the first drug was completely tapered after 6 months.
Both tapering strategies had a treat-to-target approach with 3 monthly visits. At each visit, patients were assessed whether they maintained low disease activity (DAS≤2.4) while tapering their medication. If a disease flare occurred, defined as a DAS>2.4 and/or SJC>1, tapering was stopped and the last effective treatment, when the patient still had well-controlled disease, was restarted. No further attempts were taken to taper medication. Treatment was intensified at each visit until low disease activity was reached again.
Concurrent treatment with non-steroidal anti-inflammatory drugs and intra-articular glucocorticoid injections was allowed. In case of a flare, one intra-muscular glucocorticoid injection was allowed to be given as bridging therapy, in addition to switching to the last effective dosage of the csDMARD or TNF-inhibitor.
Effectiveness and cost assessment
The primary outcome of the TARA study was the number of disease flares. For the cost effectiveness, the main outcome was the incremental cost-effectiveness ratio (ICER). The ICER is the ratio of the difference in costs compared with the difference in quality-adjusted life years (QALYs) between both tapering strategies. Costs per QALY were calculated, since coverage of prescribed drugs by Dutch health insurance companies depends on this outcome. The required threshold per additional QALY gained to be funded for a new intervention in the Netherlands is €50 000.15–17 QALYs express the impact of the disease on patients’ health over time. Living in perfect health for 1 year corresponds to 1 QALY, living in perfect health for 2 years corresponds to 2 QALYs. Zero QALYs reflects death at baseline.18 QALYs were determined by calculating the area under the curve of the EuroQol questionnaire with 5 dimensions (EQ-5D) with three levels over a 2-year period.19
Total costs are divided into healthcare (direct) and productivity (indirect) costs. We analysed healthcare and productivity costs from a societal perspective. Healthcare costs are the costs of treatment and medical consumption, whereas productivity costs are costs due to presenteeism, that is, working while sick, and absenteeism, that is, sick leave and unemployment.20
Medication costs were calculated from doses reported in the patients’ case records, valued according to the Dutch college of health insurances (online supplementary table S1).5 Duration of hospitalisations and admission diagnosis was recorded every 3 months with the iMTA medical consumption questionnaire. Medical consumption, including hospital admissions, was valued at Dutch standard prices, except for costs of complementary and complementary medicine, which were based on American data, because no Dutch data are available (online supplementary table S2).21 22
annrheumdis-2020-217528supp001.pdf (224.4KB, pdf)
Productivity costs included absenteeism, such as sick leave and reduction in work time, and presenteeism, including working while sick. Every 3 months, patients filled out the iMTA productivity cost questionnaires.23 The friction cost method was used to calculate the productivity costs, which assumes replaceability of every employee in time.19 The friction cost period is the time between the start of long-term sick leave, and filling the position again. Costs due to sick leave are solely counted during this period, which encompasses 85 days in the Netherlands.24 Productivity losses were valued at age-dependent and sex-dependent standard hourly costs (online supplementary table S3).25 26 All prices were obtained for the year 2019. Costs were not discounted, because of only 2 years of follow-up.
Willingness to pay
To help decide which tapering strategy has the highest chance of being cost effective, two indicators were used. First, the cost-effectiveness acceptability curves (CEAC) were derived to show the probability of each tapering strategy being cost effective at different levels of willingness-to-pay (WTP) thresholds in comparison with each other.27 Second, the incremental net monetary benefit (iNMB) was used to express the incremental value of the tapering strategies in monetary terms at different levels of WTP per QALY. This results in an alternate measure which reports on cost-effectiveness without using the ICER. The iNMB was calculated as the incremental benefit times different levels of WTP, minus the incremental costs. A positive iNMB indicates that the tapering the TNF-inhibitor first is cost effective compared with tapering the csDMARD first.27
Statistical analysis
The cost-effectiveness analysis follows a superiority design. Sample size calculation was based on the number of disease flares after 1 year, which was described previously.7 All analysis were performed following an intention-to-treat approach.
After 2 years of follow-up, 13/94 (13.8%) in the tapering the csDMARD first group had dropped out, versus 9/95 (9.5%) in the tapering the TNF-inhibitor first group. Furthermore, 7.6% of patients who completed the trial did not completely fill out the questionnaires. Multiple imputations with chained equations, with 40 imputations, were used to handle missing data in baseline variables as well as in the follow-up data.28 An imputation regression model was constructed to impute EQ-5D, unemployment, loss of productivity due to sick leave (absenteeism) and not fully functioning (presenteeism) and the (decrease in) number of working hours.
For EQ-5D, presenteeism and the amount of working hours, linear regression was used. The percentages of missingness for these variables were, respectively, 14.9%, 6.7% and 18.6%. For presenteeism, we log transformed the variable and used linear regression to impute values. For unemployment (13.6% missing values), we used logistic regression, and for sick leave (7.9% missing values) we used a Poisson regression model. The choice of imputation models was based on the distribution of the individual variables. In the regression models, we used age, gender, baseline values and the tapering strategy as independent variables. Differences between imputed data, created with aforementioned models, and complete cases were minimal and showed that our imputation models are reliable (online supplemental table S4).
The main outcome was the ICER. A probabilistic sensitivity analysis for the estimation of the ICER was performed by bootstrapping with 1000 iterations using a Monte Carlo simulation. Results were plotted in a cost-effectiveness plane and were used to estimate the 95% CI of the ICER.
Differences in outcomes between groups were analysed with linear regression models, and to account for stratified randomisation by centre, intercepts for each centre were included.
All data were analysed using STATA V.15, using a value of p≤0.05 as the level of statistical significance.
Results
Patients
A total of 189 patients were randomly assigned to taper the csDMARD (n=94) or TNF-inhibitor (n=95) first. Over 2 years, 22 patients (11.6%) withdrew from the study, resulting in 167 patients with a complete follow-up. At baseline, patients had an average symptom duration of 6.8 years and were predominantly women (66.1%) with an average age of 56.6 years (table 1). The majority of patients (55%) used etanercept as their TNF-inhibitor. At baseline, 47 (25%) of patients were aged above 65, which was the average age of retirement in the Netherlands in 2018.29 Of the 142 patients under 65, 99 patients (70%) had paid work at baseline (table 1).
Table 1.
Baseline characteristics of both tapering groups
| Tapering csDMARDs first (n=94) |
Tapering TNF-inhibitor first (n=95) |
|
| Demographic | ||
| Age (years), mean (SD) | 55.9 (14) | 57.2 (11) |
| Aged above 65, n (%) | 22 (23) | 25 (26) |
| Gender, female, n (%) | 67 (71) | 58 (61) |
| Quality of life | ||
| EQ-5D index, mean (SD) | 0.86 (0.12) | 0.87 (0.11) |
| Disease characteristics | ||
| Symptom duration (years), median (IQR) | 6.0 (4.3–8.5) | 6.3 (4.1–8.9) |
| RF positive, n (%) | 49 (57) | 56 (64) |
| ACPA positive, n (%) | 61 (72) | 65 (75) |
| DAS, mean (SD) | 1.1 (0.6) | 1.0 (0.5) |
| Use of csDMARDs * | ||
| MTX, n (%) | 89 (95) | 84 (88) |
| SASP, n (%) | 10 (11) | 12 (13) |
| HCQ, n (%) | 24 (26) | 37 (39) |
| Leflunomide, n (%) | 2 (2) | 4 (4) |
| Use of TNF-inhibitors | ||
| Etanercept, n (%) | 52 (55) | 52 (55) |
| Adalimumab, n (%) | 36 (39) | 40 (43) |
| Other, n (%) † | 6 (7) | 3 (3) |
| Worker-related outcomes | ||
| Paid work, n (%) ‡ | 47 (61) | 52 (68) |
| Working hours per week, mean (SD) | 28 (8) | 29 (11) |
*Some patients used a combination of conventional synthetic disease modifying antirheumatic drugs (csDMARDs).
†Certolizumab or golimumab.
‡Number of patients with paid work and aged under 65.
ACPA, anticitrullinated protein antibody; DAS, disease activity score based on 44 joints; EQ-5D, European Quality of life questionnaire with 5 dimensions; HCQ, hydroxychloroquine; MTX, methotrexate; RF, rheumatoid factor; SASP, salazopyrine; TNF, tumour necrosis factor.
Healthcare costs
Mean healthcare costs (SD) were €22 484 (€8069) for tapering the csDMARD first and €13 616 (€9162) for tapering the TNF-inhibitor first (p<0.001; table 2). Respectively, 86% and 71% of healthcare costs were medication costs. The faster savings due to less TNF-inhibitor use within the group that tapered the TNF-inhibitor first was the main driver of the difference in direct costs. Within the group who tapered the csDMARDs first, 81 (86%) were using full-dose TNF-inhibitor after 12 months, and 32 (34%) patients after 24 months. In the TNF-inhibitor tapering first group, this was 16 (17%) after 12 months, and 25 (26%) after 24 months.
Table 2.
Healthcare costs over 2 years of follow-up in the Tapering strategies in Rheumatoid Arthritis study according to intention to treat
| Tapering csDMARDs first (n=94) | Tapering TNF-inhibitor first (n=95) | |||
| Number of visits, mean (SD) | Mean costs (SD) | Number of visits, mean (SD) | Mean costs (SD) | |
| Medication* | ||||
| csDMARDs* | €436 (€87) | €972 (€123) | ||
| TNF-inhibitor* | €19 417 (€738) | €9673 (€863) | ||
| Prednisone | €2.46 (€0.54) | €2.84 (€0.59) | ||
| Medical consumption | ||||
| Hospitalisation | 13 ‡ | €326 (€1313) | 15 ‡ | €558 (€2271) |
| Standard healthcare | ||||
| Primary care physician | 7.7 (9) | €260 (€302) | 8.9 (9) | €303 (€318) |
| Specialist | 12.0 (6) | €1153 (€647) | 12 (6) | €1203 (€738) |
| Psychologist | 0.5 (2) | €18 (€83) | 1.2 (8) | €40 (€266) |
| Paramedical care | ||||
| Physical therapy | 14.4 (32) | €506 (€1110) | 15.9 (31) | €554 (€1063) |
| Dietitian | 0.46 (2) | €14 (€62) | 0.040 (0.3) | €1.31 (€8.95) |
| Social worker | 0.14 (0.6) | €9.40 (€41) | 0.20 (0.8) | €14 (€52) |
| Speech therapist | 0.04 (0.3) | €1.32 (€10) | 0.02 (0.2) | €0.65 (€6.36) |
| Complementary medicine | ||||
| Homeopathy | 0.83 (3) | €26 (€97) | 0.44 (2) | €14 (€67) |
| Total healthcare costs, mean (SD) | €22 484 (€8069) | €13 616 (€9162) | ||
*p<0.001 (linear regression adjusted for stratified randomisation).
†Number reflects the number of patients who got hospitalised within the 2 years of follow-up.
csDMARDs, conventional synthetic disease modifying antirheumatic drugs; TNF, tumour necrosis factor.
Productivity costs
Average productivity costs (SD) for tapering csDMARDs first and TNF-inhibitor first were, respectively, €16 349 (€38 277) and €25 826 (€46 289; p=0.10; tables 3 and 4). Within the 2 years of follow-up, 20 (43%) patients with paid work called in sick with an average duration of 9 days in the initial csDMARD tapering group versus 26 patients (50%) with an average duration of 12 days within the initial TNF-inhibitor tapering group. Of those patients, respectively, 2 and 1 had long-term sickness (>3 months). Two patients who tapered the csDMARD first became unemployed, versus six in the group who tapered the TNF-inhibitor first. The working population had an average workweek of 32 hours after 24 months of follow-up. A decrease in working hours was seen in 8 and 11 patients in, respectively, the csDMARD and TNF-inhibitor tapering first group. Their average workweek decreased with 15 hours in the csDMARD tapering first group and 19 hours in the TNF-inhibitor tapering first group. Within the working population, 34 patients in the csDMARD tapering first group and 41 patients in the TNF-inhibitor tapering first group indicated that they had days on which they were less productive. On average, this were 5 and 6 days per month, with a mean productivity loss on these days of 28% and 26%, respectively (table 3). Subanalyses of men and women did not result in differences in productivity costs (data not shown).
Table 3.
Productivity costs over 2 years of follow-up.
| Tapering csDMARDs first (n=94) | Tapering TNF-inhibitor first (n=95) | |
| Absenteeism | ||
| Unemployment | ||
| Became unemployed, n (%) | 2 (4) | 6 (11) |
| Sick leave (during 2-year follow-up) | ||
| Occurrence, n (%) | 20 (21) | 26 (27) |
| Long-term sickness, n (%) | 2 (2) | 1 (1) |
| Days absent, mean (SD)* | 9.0 (23) | 12.3 (22) |
| Contract hours† | ||
| Working hours per week after 2 years, mean (SD) | 32 (8.9) | 33 (12) |
| Reduction of working hours per week, n (%) | 8 (8) | 11 (11) |
| Amount of reduction, hours, mean (SD)‡ | 15 (11) | 19 (17) |
| Presenteeism | ||
| Number of patients, n (%) | 34 (36) | 41 (43) |
| Number of days per month, mean (SD)§ | 5.3 (0.9) | 6.1 (1.1) |
| Average productivity loss, proportion (SD)¶ | 27.9% (13%) | 26.4% (15%) |
*Only indicated when patients reported sick leave.
†Only indicated when patients had paid work.
‡Only indicated for those with a reduction in working hours.
§Average productivity score was only obtained for patients indicating that they had loss of productivity.
¶Productivity loss was indicated only for the days with productivity loss for those who reported to suffer from loss of productivity.
csDMARDs, conventional synthetic disease modifying antirheumatic drugs; TNF, tumour necrosis factor.
Table 4.
Total costs and quality-adjusted life years (QALYs) over the 2-year follow-up period
| Tapering csDMARD first | Tapering TNF-inhibitor first | |
| Total costs | €38 833 (€39 616) | €39 442 (€47 271) |
| Total healthcare costs* | €22 484 (€8069) | €13 616 (€9162) |
| Medication* | €19 858 (€7343) | €10 648 (€8642) |
| Medical consumption | €2297 (€1684) | €2393 (€1775) |
| Hospitalisation | €330 (€1319) | €575 (€2305) |
| Total productivity costs | €16 349 (€38 277) | €25 826 (€46 289) |
| Absenteeism | €17 581 (€39 576) | €23 577 (€45 382) |
| Presenteeism | €3290 (€9952) | €4777 (€14 620) |
| QALYs (EQ-5D, AUC), mean (SD) | 1.64 (0.22) | 1.65 (0.22) |
All values are indicated as mean (SD).
*p<0.0001 (linear regression adjusted for stratified randomisation).
AUC, area under the curve; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; EQ-5D, EuroQol questionnaire with 5 dimensions; TNF, tumour necrosis factor.
Cost-effectiveness analysis
The mean EQ-5D index (SD) after 24 months of follow-up was 0.81 (0.13) for tapering the csDMARD first, and 0.83 (0.16) for tapering the TNF-inhibitor first. Average QALYs (SD) over 2 years for tapering csDMARDs first or TNF-inhibitor first were, respectively, 1.64 (0.22) and 1.65 (0.22; table 4). Total costs (SD) were €38 833 (€39 616) for tapering csDMARDs first, and €39 442 (€47 271) for tapering the TNF-inhibitor (p=0.88; table 4).
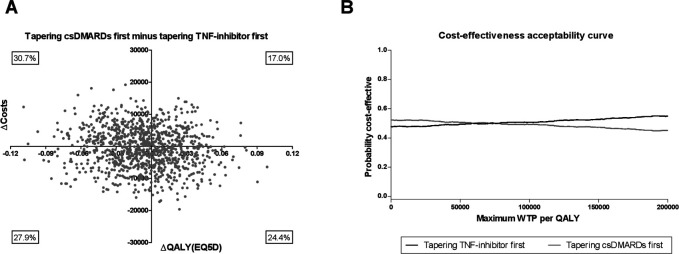
The ICER (95% CI) between tapering csDMARDs first minus the TNF-inhibitor first was €60 919 per QALY (−€90 638 per QALY to €212 475 per QALY), indicating that tapering TNF-inhibitor first was on average €60 919 less expensive per QALY compared with tapering the csDMARD first. However, the CI is very wide due to a minimal difference in QALYs and costs between the two tapering strategies. To illustrate this, the analysis of uncertainty in the estimation of the ICER was visualised with the cost-effectiveness planes for the two tapering strategies compared with each other (figure 1A). The iNMB was €1134 (95% CI €761 to €1507) in favour of tapering TNF-inhibitor first for a WTP level of €50 000, which is the current level of WTP in the Netherlands for treatment of RA (online supplemental figure S1).15–17 Our CEAC shows similar results (figure 1B). For WTP levels <€83 800 tapering, the csDMARD first has the highest probability of being cost effective, while for WTP levels >€83 800 tapering the TNF-inhibitor first has the highest probability. In between WTP levels of €53 800 and €83 800, both strategies were evenly cost effective (probability 50%). This indicates that depending on the WTP threshold either tapering the TNF-inhibitor or csDMARD first is more cost effective. Moreover, the CEAC shows that both lines are almost horizontal after the crossing and that the difference is small, which is due to the small differences in QALYs and costs.
Figure 1.
Summary of economic evaluation of tapering conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) first minus tapering tumour necrosis factor (TNF)-inhibitor first. (A) Results of 1000 bootstrapped replications, presented in a cost-effectiveness plane which represents uncertainty of the cost-effectiveness ratio. (B) Cost-effectiveness acceptability curve for tapering csDMARDs first versus tapering TNF-inhibitor first. Results of 1000 bootstrapped replication, presented for several levels of willingness to pay (WTP), indicated per quality-adjusted life year (QALY).
annrheumdis-2020-217528supp002.pdf (45.5KB, pdf)
Discussion
In this study, we showed that healthcare costs were significantly lower in patients who tapered the TNF-inhibitor first, but productivity costs in this group were higher due to more absenteeism and presenteeism compared with the patients who tapered the csDMARD first. The ICER (95% CI) between tapering csDMARDs first minus the TNF-inhibitor first was €60 919 per QALY (−€90 638 to €212 475). Total costs (SD) were €38 833 (€39 616) for tapering csDMARDs first, and €39 442 (€47 271) for tapering the TNF-inhibitor first (p=0.88). Depending on the WTP threshold either tapering the TNF-inhibitor or csDMARD first has the highest probability of being cost effective.
Previous studies showed that savings on healthcare and societal costs could be obtained by treating to target within newly diagnosed patients with RA.30 More savings could be obtained by tapering quickly, and possibly stopping the medication when patients with RA reach sustained remission. Currently, several trials have reported on the feasibility of tapering; however, cost-effectiveness analyses are scarce. A systematic review on tapering and stopping treatment in patients with RA reported that only 2 out of 14 included studies performed a cost-effectiveness analysis, although costs are nowadays an important reason why tapering or stopping treatment is considered by treating rheumatologists.31
Previous studies reported on the cost effectiveness of tapering or stopping medication versus a continuation group. The Dose REduction Strategy of Subcutaneous TNF inhibitors (DRESS) study for example showed a significant cost-saving after tapering of adalimumab or etanercept, without a clinically meaningful loss in QALYs.12 The Spacing ofTNF-blocker injections in Rheumatoid ArthritiS Study (STRASS) also reported on cost effectiveness. Within this trial, the interval between TNF-inhibitor injections was extended and compared with a control group that continued their medication. Healthcare costs were significantly lower in the tapering group, but this was accompanied with a significant loss in QALYs.11 Although both studies also reported on productivity costs, they did not take presenteeism into account. In our study, the QALYs did not differ between both tapering strategies and were comparable to the QALYs of the control groups in previous mentioned trials (DRESS 1.67 and STRASS 1.68).11 12
The strengths of the current study include the randomised design. Although originally the TARA trial was powered to find a 20% difference in disease flares, cost effectiveness was a parallel primary outcome. Also, validated outcome measures were used for the QALY calculation. Furthermore, we used real data to calculate healthcare and productivity costs, instead of using a model. Moreover, for calculating productivity costs, we included absenteeism as well as presenteeism, thereby taking into account all costs due to productivity loss. Finally, the TARA trial is the first randomised controlled trial reporting on the cost utility between two gradual tapering strategies.
Some limitations should also be noted. First of all, the targeted sample size was not reached. This was due to difficulties with inclusion, and the start of another trial using the same pool of eligible patients. For the primary outcome (disease flares), we performed a sensitivity analysis, which showed similar outcomes.7 Furthermore, the follow-up duration was only 2 years. Ideally, long-term effects of tapering and stopping treatment should be taken into account as well. In our current design, patients completely tapered their medication after 18 months, if no flare occurred. This means that we only have 6 months of follow-up when patients are in DMARD-free remission. Late flares were, therefore, not considered in our study and might change current outcomes by an increase in healthcare costs on the long term, but might also influence productivity costs and quality of life.
Generalisability of the current study might be difficult, since every country has its own social security and healthcare system. Also, treatment prices differ. Costs of labour vary between countries, and more importantly, rules and regulations for social security regulation differ across countries. The possibility to stay at home when not feeling well is very different across countries within Europe.32 In the Netherlands, people can call in sick without consulting a doctor, while this is obligatory in some other countries. This could cause a shift between presenteeism and absenteeism when comparing the Netherlands to other countries. Fortunately, in our current analysis, we do take into account both. Since we found that the group that tapered the TNF-inhibitor first encountered more costs due to both presenteeism and absenteeism (table 3, p=0.39 and p=0.20, respectively), we believe that our indirect costs are generalisable to all countries.
In the current study, we found a significant difference in medication costs between both tapering groups. The difference in healthcare costs could change due to price variations of csDMARD and especially biologicals between countries. To investigate this, we performed a sensitivity analysis with varying levels of biological prices of 30%, 50% and 200% of the prices we currently used (online supplemental figure S2). Lowering biological prices was in favour of tapering the csDMARD first, while higher biological prices showed the opposite. However, biological costs are consistently higher than csDMARD costs in any country, meaning that the direction of the medication cost difference could be generalisable to other countries. For the current analysis, we used 2019 prices to make our results as relevant as possible, since the prices for biologicals have decreased dramatically.
annrheumdis-2020-217528supp003.pdf (349.7KB, pdf)
In conclusion, medication costs are lower when the TNF-inhibitor is tapered first, but this is counterbalanced with a higher loss of productivity and, therefore, cost savings are similar for both tapering strategies. Regardless of the WTP threshold, tapering the TNF-inhibitor or csDMARD first is equally cost effective.
Acknowledgments
The authors especially thank the participating patients in the TARA trial for their willingness to contribute to the study and for their cooperation. They thank all rheumatologists, research nurses, coinvestigators, data managers and laboratory personnel from the following participating centres: Erasmus MC, Rotterdam; Maasstad hospital, Rotterdam; Sint Fransiscus Gasthuis & Vlietland hospital, Rotterdam & Schiedam; Amphia hospital, Breda; Reinier de Graaf hospital, Delft; Antonius hospital, Utrecht; Bravis hospital, Bergen op Zoom & Roosendaal; Groene hart hospital, Gouda; Albert Schweitzer hospital, Dordrecht; Haga hospital, Den Haag; and Beatrix hospital, Gorinchem.
Footnotes
Handling editor: Josef S Smolen
PHPdJ and JJL contributed equally.
Contributors: PHPdJ and EvM were responsible for acquisition of data, statistically analysing the data and performing the health economics analyses. JJL was responsible for study conception and obtaining grant funding. TMK was responsible for acquisition of data and statistical analysis. NHAMD, AHG, MHdJ, AEW, JMH and W-KL were responsible for acquisition of data. All authors were responsible for interpretation of the data and for drafting, revising and approving the final submitted manuscript.
Funding: This work was supported by ZonMw (grant number 171102014).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Patient consent for publication: Not required.
Ethics approval: Medical ethics committees at each participating center approved the study protocol.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request by contacting the corresponding author.
References
- 1. Klarenbeek NB, Allaart CF, Kerstens PJSM, et al. . The best story: on strategy trials in rheumatoid arthritis. Curr Opin Rheumatol 2009;21:291–8. 10.1097/BOR.0b013e32832a2f1c [DOI] [PubMed] [Google Scholar]
- 2. de Jong PH, Hazes JM, Han HK, et al. . Randomised comparison of initial triple DMARD therapy with methotrexate monotherapy in combination with low-dose glucocorticoid bridging therapy; 1-year data of the tREACH trial. Ann Rheum Dis 2014;73:1331–9. 10.1136/annrheumdis-2013-204788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smolen JS, Landewé R, Bijlsma J, et al. . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. 10.1136/annrheumdis-2016-210715 [DOI] [PubMed] [Google Scholar]
- 4. GIPdatabank Biologicals, 2014-2018, 2019. Available: https://www.gipdatabank.nl/ [Accessed 17 Jun 2020].
- 5. Medicijnkosten Zorginstituut Nederland, 2019. Available: http://www.medicijnkosten.nl [Accessed 18 Aug 2019].
- 6. Kuijper TM, Folmer R, Stolk EA, et al. . Doctors' preferences in de-escalating DMARDs in rheumatoid arthritis: a discrete choice experiment. Arthritis Res Ther 2017;19:78. 10.1186/s13075-017-1287-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Mulligen E, de Jong PHP, Kuijper TM, et al. . Gradual tapering TNF inhibitors versus conventional synthetic DMARDs after achieving controlled disease in patients with rheumatoid arthritis: first-year results of the randomised controlled tara study. Ann Rheum Dis 2019;78:746–53. 10.1136/annrheumdis-2018-214970 [DOI] [PubMed] [Google Scholar]
- 8. Birkner B, Rech J, Stargardt T. Cost-Utility analysis of de-escalating biological disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis. PLoS One 2020;15:e0226754. 10.1371/journal.pone.0226754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kobelt G. Treating to target with etanercept in rheumatoid arthritis: cost-effectiveness of dose reductions when remission is achieved. Value Health 2014;17:537–44. 10.1016/j.jval.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 10. Tran-Duy A, Ghiti Moghadam M, Oude Voshaar MAH, et al. . An economic evaluation of stopping versus continuing tumor necrosis factor inhibitor treatment in rheumatoid arthritis patients with disease remission or low disease activity: results from a pragmatic open-label trial. Arthritis Rheumatol 2018;70:1557–64. 10.1002/art.40546 [DOI] [PubMed] [Google Scholar]
- 11. Vanier A, Mariette X, Tubach F, et al. . Cost-Effectiveness of TNF-Blocker injection spacing for patients with established rheumatoid arthritis in remission: an economic evaluation from the spacing of TNF-Blocker injections in rheumatoid arthritis trial. Value Health 2017;20:577–85. 10.1016/j.jval.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 12. Kievit W, van Herwaarden N, van den Hoogen FH, et al. . Disease activity-guided dose optimisation of adalimumab and etanercept is a cost-effective strategy compared with non-tapering tight control rheumatoid arthritis care: analyses of the dress study. Ann Rheum Dis 2016;75:1939–44. 10.1136/annrheumdis-2015-208317 [DOI] [PubMed] [Google Scholar]
- 13. Ghiti Moghadam M, Ten Klooster PM, Vonkeman HE, et al. . Impact of stopping tumor necrosis factor inhibitors on rheumatoid arthritis patients' burden of disease. Arthritis Care Res 2018;70:516–24. 10.1002/acr.23315 [DOI] [PubMed] [Google Scholar]
- 14. van Mulligen E, Weel AE, Hazes JM, et al. . Tapering towards DMARD-free remission in established rheumatoid arthritis: 2-year results of the tara trial. Ann Rheum Dis 2020;1181. 10.1136/annrheumdis-2020-217485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zwaap J, Knies S, van der Meijden C, et al. . Kosteneffectiviteit in de praktijk : Minister van Volksgezondheid WeS. Nederland: Zorginstituut Nederland, 2015. [Google Scholar]
- 16. Cross M, Smith E, Hoy D, et al. . The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73:1316–22. 10.1136/annrheumdis-2013-204627 [DOI] [PubMed] [Google Scholar]
- 17. Ubel PA, Hirth RA, Chernew ME, et al. . What is the price of life and why doesn't it increase at the rate of inflation? Arch Intern Med 2003;163:1637–41. 10.1001/archinte.163.14.1637 [DOI] [PubMed] [Google Scholar]
- 18. Torrance GW. Measurement of health state utilities for economic appraisal. J Health Econ 1986;5:1–30. 10.1016/0167-6296(86)90020-2 [DOI] [PubMed] [Google Scholar]
- 19. Hurst NP, Kind P, Ruta D, et al. . Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ-5D). Br J Rheumatol 1997;36:551–9. 10.1093/rheumatology/36.5.551 [DOI] [PubMed] [Google Scholar]
- 20. Huscher D, Merkesdal S, Thiele K, et al. . Cost of illness in rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and systemic lupus erythematosus in Germany. Ann Rheum Dis 2006;65:1175–83. 10.1136/ard.2005.046367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nahin RL, Barnes PM, Stussman BJ, et al. . Costs of complementary and alternative medicine (cam) and frequency of visits to CAM practitioners: United States, 2007. Natl Health Stat Report 2009:1–14. [PubMed] [Google Scholar]
- 22. Hakkaart-van Roijen L, Van der Linden N, Bouwmans CAM, et al. . Kostenhandleiding. Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg, 2015. [Google Scholar]
- 23. Bouwmans C, Krol M, Brouwer W, et al. . IMTA productivity cost questionnaire (IPCQ). Value Health 2014;17:A550. 10.1016/j.jval.2014.08.1791 [DOI] [PubMed] [Google Scholar]
- 24. Koopmanschap MA, Rutten FF, van Ineveld BM, et al. . The friction cost method for measuring indirect costs of disease. J Health Econ 1995;14:171–89. 10.1016/0167-6296(94)00044-5 [DOI] [PubMed] [Google Scholar]
- 25. Statline C. Beloning en arbeidsvolume van werknemers, 2019. Available: https://opendata.cbs.nl/statline/#/CBS/nl/dataset/84165NED/table?dl=217EE&ts=1566303305202 [Accessed 01 Jun 2019].
- 26. Statline C. Beloningsverschillen tussen mannen en vrouwen, 2019. Available: https://opendata.cbs.nl/statline/#/CBS/nl/dataset/81901NED/table?dl=217EC [Accessed 01 Jun 2019].
- 27. Drummond MF, Sculpher MJ, Torrance GW, et al. . Methods for the economic evaluation of health care programme. Third edition Oxford: Oxford University Press, 2005. [Google Scholar]
- 28. Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? some practical clarifications of multiple imputation theory. Prev Sci 2007;8:206–13. 10.1007/s11121-007-0070-9 [DOI] [PubMed] [Google Scholar]
- 29. CBS Pensioenleeftijd werknemers in 2018, 2019. Available: https://www.cbs.nl/nl-nl/maatwerk/2019/32/pensioenleeftijd-werknemers-in-2018 [Accessed 12 Feb 2020].
- 30. de Jong PHP, Hazes JM, Buisman LR, et al. . Best cost-effectiveness and worker productivity with initial triple DMARD therapy compared with methotrexate monotherapy in early rheumatoid arthritis: cost-utility analysis of the tREACH trial. Rheumatology 2016;55:2138–47. 10.1093/rheumatology/kew321 [DOI] [PubMed] [Google Scholar]
- 31. van Herwaarden N, den Broeder AA, Jacobs W, et al. . Down-titration and discontinuation strategies of tumor necrosis factor-blocking agents for rheumatoid arthritis in patients with low disease activity. Cochrane Database Syst Rev 2014:CD010455. 10.1002/14651858.CD010455.pub2 [DOI] [PubMed] [Google Scholar]
- 32. Boonen A, van der Heijde D, Landewé R, et al. . Work status and productivity costs due to ankylosing spondylitis: comparison of three European countries. Ann Rheum Dis 2002;61:429–37. 10.1136/ard.61.5.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2020-217528supp001.pdf (224.4KB, pdf)
annrheumdis-2020-217528supp002.pdf (45.5KB, pdf)
annrheumdis-2020-217528supp003.pdf (349.7KB, pdf)