Abstract
Objectives
To explore the relevance of T-follicular-helper (Tfh) and pathogenic peripheral-helper T-cells (Tph) in promoting ectopic lymphoid structures (ELS) and B-cell mucosa-associated lymphoid tissue (MALT) lymphomas (MALT-L) in Sjögren’s syndrome (SS) patients.
Methods
Salivary gland (SG) biopsies with matched peripheral blood were collected from four centres across the European Union. Transcriptomic (microarray and quantitative PCR) analysis, FACS T-cell immunophenotyping with intracellular cytokine detection, multicolor immune-fluorescence microscopy and in situ hybridisation were performed to characterise lesional and circulating Tfh and Tph-cells. SG-organ cultures were used to investigate functionally the blockade of T-cell costimulatory pathways on key proinflammatory cytokine production.
Results
Transcriptomic analysis in SG identified Tfh-signature, interleukin-21 (IL-21) and the inducible T-cell co-stimulator (ICOS) costimulatory pathway as the most upregulated genes in ELS+SS patients, with parotid MALT-L displaying a 400-folds increase in IL-21 mRNA. Peripheral CD4+CXC-motif chemokine receptor 5 (CXCR5)+programmed cell death protein 1 (PD1)+ICOS+ Tfh-like cells were significantly expanded in ELS+SS patients, were the main producers of IL-21, and closely correlated with circulating IgG and reduced complement C4. In the SG, lesional CD4+CD45RO+ICOS+PD1+ cells selectively infiltrated ELS+ tissues and were aberrantly expanded in parotid MALT-L. In ELS+SG and MALT-L parotids, conventional CXCR5+CD4+PD1+ICOS+Foxp3- Tfh-cells and a uniquely expanded population of CXCR5-CD4+PD1hiICOS+Foxp3- Tph-cells displayed frequent IL-21/interferon-γ double-production but poor IL-17 expression. Finally, ICOS blockade in ex vivo SG-organ cultures significantly reduced the production of IL-21 and inflammatory cytokines IL-6, IL-8 and tumour necrosis factor-α (TNF-α).
Conclusions
Overall, these findings highlight Tfh and Tph-cells, IL-21 and the ICOS costimulatory pathway as key pathogenic players in SS immunopathology and exploitable therapeutic targets in SS.
Keywords: sjogren's syndrome, t-lymphocyte subsets, cytokines, autoimmune diseases
Key messages.
What is already known about this subject?
In Sjogren’s syndrome (SS), germinal centres (GC) forming ectopically in salivary glands (SG) function as niches for autoreactive B-cells, which formation believed to results from the GC B and T-cell interaction. Ectopic GC are associated with evolution to B cell mucosa-associated lymphoid tissue (MALT) lymphoma in several studies.
T-follicular-helper (Tfh) and recently described pathogenic peripheral-helper T-cells (Tph) are key mediators in (autoreactive) B-cell differentiation through inducible T-cell costimulator (ICOS)-ICOS-L interaction and interleukin-21 (IL-21) production, but their relevance has not been investigated in SS ectopic GC reaction and MALT lymphoma.
What does this study add?
Tfh and Tph-cells are enriched in both SS peripheral blood and SG with GC, invariably express ICOS, represent the main source of IL-21 and frequently coexpress IL-21/interferon-γ, especially in parotid MALT-lymphoma.
ICOS blockade in ex vivo SG-organ cultures significantly reduced the production of IL-21 and inflammatory cytokines IL-6, IL-8 and tumour necrosis factor-α (TNF-α).
How might this impact on clinical practice or future developments?
Tfh and Tph-cells, IL-21 and the ICOS costimulatory pathway can be considered biomarkers of ectopic GC, may be used for patient stratification and represent exploitable therapeutic targets in patients with SS.
Introduction
Sjogren’s syndrome (SS) is characterised by lymphocytic infiltration of the exocrine glands, mainly the lacrimal and salivary glands (SG).1 The pathogenic role of B-cells in SS is a hallmark of the disease including the presence of circulating autoantibodies, alterations in peripheral B-cell subpopulations,2 B-cell predominance in advanced SG lesions3 and the increased risk of developing non-Hodgkin B-cell mucosa-associated lymphoid tissue (MALT)-lymphoma (MALT-L) in SS.4 In around 30%–40% of patients with SS, B-cell infiltrates forming in minor (labial) SG are organised in ectopic germinal centres (GC)5; follicles formed by aggregates of segregated B and T-cells endowed with a follicular dendritic cell (FDC) network. These structures, also known as ectopic lymphoid structures (ELS), function as niches for autoreactive B-cells.6
In physiological GC responses, efficient T-cell-dependent antigen-driven B-cell response in vivo depends on the development of functional GCs which require T-follicular helper (Tfh) cells, 7–9 where Tfh-secreted interleukin-21 (IL-21) is a critical factor for B-cell maturation.7 10–12 Tfh-cells are highly specialised CD4+ memory T-helper cells, characterised by high expression of the CXC-motif chemokine receptor 5 (CXCR5), the inducible T-cell costimulator (ICOS) molecule, the coinhibitory molecule programmed cell death protein 1 (PD-1) and the transcription factor Bcl6.13 Tfh-cells migrate to the B-cell follicle in response to the FDC- produced CXCR5 ligand, CXCL13.14 At the border with and inside the GC, Tfh-cells interact with B-cells through ICOS and its ligand, ICOSL, releasing high amounts of IL-21.15 16 Given their fundamental role as mediators of B-cell activation and antibody production, it is not surprising that Tfh-cells together with IL-21 have been linked to autoimmune diseases characterised by a B-cell hyperactivation and dysregulated GC response, including SS.17 18 Interestingly, recent work described alternative IL-21-producing Tfh-like cells (also designated ‘pathogenic T peripheral helper cells (Tph)’) as able to localise at inflammatory sites, such as RA synovium, in the absence of CXCR5 expression.19 Tph cells lack prototypic Tfh markers like CXCR5 and Bcl6 but expresses high levels of IL-21 and CD40L. Similarly to canonical Tfh, Tph-cells isolated from inflamed tissue19 20 can drive the differentiation of B-cells into antibody-secreting cells in vitro.19
To date, the relevance of Tfh and Tph-cells in the development of SG ELS and evolution to MALT-L in SS has not been clarified. To address this question, we performed a comprehensive investigation in matched SG histology, transcriptomic and lesional/peripheral T-cell immunophenotyping validated in samples from four different centres which identified a subset of Tfh and Tph-cells expressing high levels of IL-21 and/or IL-21/interferon-γ (IFN-γ) under the control of ICOS as a uniquely expanded population able to identify patients with SS with ELS and MALT-L.
Materials and methods
Patients sample collection
Blood and labial SG biopsies were collected after informed consent from patients with SS (n=83) fulfilling the 2002 revised criteria of the American-European Consensus Group,21 with non-specific chronic sialadenitis (NSCS) (n=65) and from healthy donors (HD) (n=12). SS parotids (n=15) with low-grade MALT-L and parotid adenocarcinoma (n=10) were obtained from the Guy’s Hospital Oral Pathology. MALT-L was diagnosed histologically by the presence of halos of monocytoid B-cells infiltrating epimyoepithelial islands and by variable, diversity and joining (VDJ) genes PCR for the heavy chain Ig genes22 for clonal populations.
Patient and public involvement
There were no funds or time allocated for patient and public involvement so we were unable to involve patients. Patients were invited to help us developing our dissemination strategy.
Immunohistochemistry and immunofluorescence) on SG tissue
After deparaffinisation, formalin-fixed paraffin-embedded sections were incubated with antigen retrieval solution pH6 (Dako) in pressure cooker (for CD20, CD138, CD3, CD68, CD4, CD45RO, PD1, ICOS, BCL6) or with proteinase-K (Dako) (for CD21). After appropriate blocking steps and primary antibody (online supplementary table S1) staining, slides were incubated with HRP-conjugated secondary antibody and developed with DAB (Dako). For immunofluorescence (IF), slides were incubated with the relevant secondary antibodies (online supplementary table S1) prior to 4′,6-diamidino-2-phenylindole (DAPI) nuclear counterstaining. Images were captured using Olympus BX61 Motorised Microscope or confocal microscope (Leica DM5500Q).
annrheumdis-2020-217646supp001.pdf (3.4MB, pdf)
Flow cytometry
Flow-cytometry analysis was performed on 52 frozen peripheral blood mononuclear cells (PBMCs) prior thawing. Egressed cells from one parotid MALT-L and PBMCs were stimulated with PMA (50 ng/mL), ionomycyn (750 ng/mL), Brefeldin-A (10 µg/mL) in RPMI complete (RPMI +10% fetal bovine serum) medium, for 3 hours. Cells were stained using Zombie Aqua Live/Dead kit (Biolegend) for 15 min, washed and incubated for 10 min with human Fc TruStain FcX (Biolegend). Cells were stained for surface antigens, fixed, permeabilised (fixation-permeabilisation buffer; eBioscience) and stained for intracellular cytokines. Antibodies used are listed in online supplementary table S2. Cells were acquired using a LSR Fortessa II (BD Biosciences) flow cytometer and analysed with FlowJo V.10 software.
Whole-genome microarray analysis and quantitative gene expression profiling in human SG tissue
For whole-genome microarray, biotin-labelled amplified complementary RNA (cRNA) from total SG RNA was amplified according to the MessageAmp Premier Protocol (ThermoFisher). cRNA (20 µg) was fragmented for hybridisation on Affymetrix Human Genome U133 Plus 2.0 GeneChip arrays. Data capture and quality assessments were performed with the GeneChip Operating Software tool and data analysis with R using affy, frma and dependent packages to generate a scaled gene expression matrix. eBayes R package was used for statistical analysis of differential transcript expression and gene signature analysis was performed using the GSVA package (Gene Set Variation Analysis)23 and a collection of established gene signatures. A list of genes defining the gene signatures are listed in online supplementary table S3. Targeted quantitative TaqMan RT-PCR was performed as previously described24; primers and probes are listed in online supplementary table S4.
RNAScope fluorescence in situ hybridisation for IL-21 RNA
In situ hybridisation for IL-21 RNA (NM_021803.3) was performed following manufacturer’s instructions (Advanced Cell Diagnostics) on optimally prepared paraffin SG tissue. RNAscope probe Hs-IL21 (code 401251) was applied for 2 hours at 40°C and the signal was detected using Alexa Fluor 488 Tyramide Reagent (ThermoFisher, code B40953). Slides were then washed in PBS and stained for ICOS (Abcam, code Ab105227) and CD4 (Dako, code M7310) overnight. After incubation with secondary antibodies, slides were DAPI counterstained and mounted in ProLong Gold (ThermoFisher, code P36930). Slides were digitised using NanoZoomer S60 slide scanner (Hamamatsu Photonics). DapB (code 310043) and Hs-POLR2A (code 310451) were used as negative and positive control probes, respectively.
ICOS blockade in vitro assay
SG-organ cultures were performed in complete RPMI medium. Each SG lobule was cut in half and each half incubated with either anti-ICOS blocking antibody (clone JTA-009)25 26 or its isotype control (10 µg/mL), for 3 days and MALT-L for 24 hours, due to the different time for cell egression. Multiple samples from one parotid MALT-L parotidectomy and 3 SS patient labial SG (2–8 lobules per patient) were tested. All supernatants were collected and frozen before analysis.
Protein detection in sera and supernatants
IL-21 levels in serum was quantified using an IL-21 ELISA (Biolegend, code 433804) following manufacturer’s instructions. In the organ culture supernatant, cytokines levels were screened with Proteome profiler human XL cytokine array kit (R&D, code ARY022B) in line with manufacturer’s instruction. Cytokines were quantified using customised multiplex liquid phase immunoassay (Biolegend, LegendPlex) and/or with specific ELISA assays (IL-6 and IL-8, Biolegend, 430 504 and 431 504, respectively).
Statistical analysis
Differences in quantitative variables were analysed by the Mann-Whitney U-test when comparing two groups and by Kruskal-Wallis with Dunn’s post-test correction when comparing multiple groups. χ2 test with Yates’ correction when required or Fisher’s exact test when appropriate were used to evaluate associations of qualitative variables in the different groups. Spearman’s rank analysis was performed for non-parametric variable correlations. All the statistical analyses were performed using GraphPad Prism V.7 for Windows (GraphPad Software, USA).
Results
Expansion of circulating IL-21 producing Tfh cells identifies patients with SS with systemic immune activation and higher SG infiltration
We first analysed circulating IL-21 level and the frequency of IL-21 production in conventional Tfh (CXCR5+PD1+ICOS+) and the recently described Tph (CXCR5-PD1hi ICOS+) in patients with SS and their correlation with clinical, immunological and histopathological activity. Patient characteristics are summarised in table 1.
Table 1.
Summary of the patient characteristics
| Complete cohort | Sjögren’s syndrome n=83 | NSCS n=65 |
Healthy donors n=12 |
| Gender, F/M | 75/8 | 57/8 | 11/1 |
| Age in years mean | 54 [55] (14.0) | 55 [56] (13.3) | 43 [44] (7.4) |
| [median] (SD) | |||
| Primary/ Secondary Sjogren’s syndrome |
74/9 | ||
| Disease duration in years | 5.9 [3] (6.0) | 5.1 [3] (5.6) | |
| Mean [median] (SD) | |||
| ESSDAI | 5.5 [5] (4.9) | n/a | |
| Mean [median] (SD) | |||
| Anti-Ro | 59% | 0% | |
| Positive of total | |||
| Anti-La | 35% | 0% | |
| Positive of total | |||
| Rheumatoid factor | 51% | 11% | |
| Positive of total | |||
| Serum IgG (g/L) | 15.4 [14.0] (5.8) | 11.0 [11.1] (3.7) | |
| Mean [median] (SD) | |||
| Serum IgA (g/L) | 2.9 [2.3] (1.8) | 2.8 [2.5] (2.4) | |
| Mean [median] (SD) | |||
| Serum IgM (g/L) | 1.5 [1.1] (2.0) | 1.3 [1.1] (0.7) | |
| Mean [median] (SD) | |||
| Serum C3 (g/L) | 1.2 [1.2] (0.3) | 1.5 [1.3] (1.3) | |
| Mean [median] (SD) | |||
| Serum C4 (g/L) | 0.2 [0.2] (0.1) | 0.3 [0.3] (0.1) | |
| Mean [median] (SD) | |||
| Lymphocytes count (x109/L) | 1.6 [1.5] (0.6) | 2.2 [2.1] (0.8) | |
| Mean [median] (SD) | |||
| Treatments | |||
| Hydroxychloroquine csDMARDs* |
35/83 8/83 |
||
| Steroids | 5/83 |
*csDMARDs other than hydroxychloroquine.
csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; ESSDAI, EULAR Sjogren's syndrome disease activity index; NSCA, non-specific chronic sialadenitis.
IL-21 serum levels were increased in patients with SS in comparison to NSCS patients and HD (figure 1A), but showed no correlation with ESSDAI or immunological abnormalities (figure 1B). Also, IL-21 levels did not differ between patients with SS stratified for extraglandular involvement (figure 1C) or ELS (online supplementary figure S1) in the SG (online supplementary figure S2A).
Figure 1.
IL-21 and T follicular helper (Tfh) cells in peripheral blood of SS and NSCS patients. (A) ELISA quantification of IL-21 serum level (pg/mL) from HD (n=12), NSCS (n=37) and SS (n=37). (B) Correlations of IL-21 serum level (pg/mL) with serum level of IgG, IgM, IgA, C3, C4 and ESSDAI in NSCS (n=37) and SS (n=37) patients. Spearman and p value are shown for the significant correlation (IgG, in red). (C) IL-21 serum level (pg/mL) in SS (n=31) according to ESSDAI domains. Box and whiskers plot show median and 5–95 percentile. Statistical analysis by Kruskal-Wallis-test with Dunn’s post-test correction for multiple comparison (A). (D) Average frequencies of Tfh-cell subsets, identified on the basis of ICOS and PD-1 expression and their frequencies distribution (E) in NSCS (white dots, n=10) and SS (dark grey dots, n=42). (F) Frequency of IFN-γ+, IL-21+ and IFN-γ+-IL-21+ double producing cells as percentage of PD-1+ICOS+, gated on CXCR5+CD4+ cells, detected by flow-cytometry on PBMC stimulation with PMA and ionomycin. Frequency of IL-21+ cells segregating the SS cohort for Ro (G) (Ro- (n=17) and Ro+ (n=20)) and La presence (H) (La- (n=27) and La+ (n=10)). Spearman correlation of IL-21+ cell (I, J, O), IFN-γ+ (K, L, P) or IL-21+-IFN-γ+ cell frequency (M, N, Q) with IgG (g/L) and C4 (g/L) serum levels and SG focus score in NSCS (n=10) and SS (n=42) patients. (R) Frequency of Tfh-cells subsets identified on the basis of ICOS and PD-1 expression in SS cohort segregated for ELS presence (ELS-, light grey dots (n=17), ELS+, dark grey dots (n=25)). (S) Frequency of CXCR5-PD-1hi cells, as percentage of CD4+ cells, in NSCS (n=10) and SS (n=42), and segregating the SS cohort for the presence of ELS. Statistical analysis by Mann-Whitney U t-test in (C), (E), (F), (G), (H), (R), (S). All graphs represent mean±SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. ELS, ectopic lymphoid structure; HD, healthy donor; ICOS, inducible T-cell costimulator; IFN-γ, interferon-γ; IL-21, interleukin-21; NSCS, non-specific chronic sialoadenitis; PD-1, programmed cell death protein 1; PNS, peripheral nervous system; SS, Sjogren’s syndrome.
Because of the heterogeneity of markers used so far to define Tfh-cells,18 we performed a comprehensive analysis of Tfh-cell subsets, by using PD-1 and ICOS expression to identify bona-fide Tfh-cells as CD4+CD25-Foxp3-CXCR5+ICOS+PD-1+,27 assessing also their functional capacity to produce IFN-γ, IL-17 and IL-21 (online supplementary figure S2B).
Circulating CXCR5+CD4+ T-cells were higher in patients with SS compared with NSCS (online supplementary figure S2C), with no differences in the general CD4+ frequency (online supplementary figure S2D). More specifically, patients with SS had an expansion of circulating CXCR5+ICOS+PD-1+ activated Tfh-cells (figure 1D, E, online supplementary figure S2E, O) which displayed significantly increased IFN-γ, IL-21 and double IL-21/IFN-γ production in patients with SS compared with NSCS (figure 1F). Circulating IL-21-producing CXCR5+ICOS+PD-1+ (figure 1G, H) but not total CXCR5+ICOS+PD-1+ or IFN-γ producing Tfh-cells (online supplementary figure S2F–H) were higher in patients with SS with anti-Ro/SSA and anti-La/SSB autoantibodies. Moreover, circulating IFN-γ+ and IL-21+CXCR5+ICOS+PD-1+ Tfh-cells positively correlated with serum IgG levels and inversely with complement C4 (figure 1I–L) unlike IL-21/IFN-γ double producers (figure 1M, N) and the total CXCR5+ICOS+PD-1+ Tfh subset (online supplementary figure S2I–J).
Next, we investigated whether circulating Tfh-cells could identify patients with SS with more extensive SG infiltration and the presence of SG ELS (online supplementary figure S1). IL-21, IFN-γ and IL-21/IFN-γ-producing CXCR5+ICOS+PD-1+ Tfh-cells (figure 1O–Q), but also total CXCR5+ICOS+PD-1+ Tfh-cells (online supplementary figure S2K) closely correlated with the SG focus score while circulating CXCR5+CD4+ T-cells double expressing ICOS+PD-1+ were higher in patients with SS with ELS (figure 1R and online supplementary figure 2L, M, P).
We next analysed the recently described population of Tph cells19 defined as CXCR5-PD1hiCD4+ T-cells. Although circulating Tph were increased in the blood of patients with SS compared with NSCS, as previously described,28 we did not observe a selective expansion of Tph in the ELS+ subgroup (online supplementary figure 1S).
Transcriptomic analysis of Tfh-cell signature and an increased IL-21 and IL-21R mRNA expression identify SG tissue with ELS
We next performed transcriptomic analysis of SG tissue comparing ELS+ SS, ELS- SS and NSCS patients. Unsupervised clustering showed that ELS+ patients clustered differently from ELS- and NSCS (figure 2A). A supervised analysis, by pre-classifying patients into ELS+, ELS- and NSCS, gave similar results, showing a clear association between B-cells, plasma cells and Tfh signatures with the presence of ELS in SG (figure 2B). GSVA score confirmed that B-cell, Tfh-cell and plasma-cell signatures all segregate ELS+ from ELS- and NCSC patients (figure 2C–E). On analysing the Tfh signature, we identified 4 Tfh-cell associated genes: CXCR5, ICOS, PDCD1, SH2D1A as most upregulated in ELS+SS patients (figure 2F–I). SH2D1A gene encodes for SLAM-associated protein that stabilises B and T-cell interaction and is essential for the differentiation of functional Tfh-cells.29
Figure 2.
Tfh-cell signature and IL-21 expression correlate with lymphocytic infiltration, ELS formation and GC B-cell genes in SS SGs. (A) Unsupervised whole-genome microarray analysis from SG RNA of NSCS (n=15) and SS (n=30), segregated for the absence (ELS-, n=15) and the presence of ELS (ELS+, n=15). (B) Supervised whole-genome microarray analysis of cohort described in (A), with the signature pathways rank-ordered for expression intensity. (C–E) GSVA for indicated signatures and (F–I) gene expression intensity comparison of Tfh-cell signature pathway genes (encoding respectively for CXCR5, ICOS, SAP and PD-1) in NSCS (n=15), ELS- (n=15) and ELS+ (n=15) SG. Linear regression model statistics. All graphs represent mean±SEM. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Real-time PCR expression for IL-21 (J) and IL-21 receptor (IL21R) (K) on total RNA from SG biopsies from NSCS (n=37) and SS (n=29) patients, segregated on the basis of absence (ELS-, n=7) and presence of ELS (ELS+, n=22). Quantification of IL-21 mRNA (L–O) and IL-21R expression (P–S) in SG tissue biopsies, according to histological semi-quantitative score (0 to 3) of B (CD20), T (CD3), plasma cells (CD138) and macrophages (CD68). Statistical analysis by Kruskal-Wallis-test with Dunn’s post-test correction for multiple comparison. (T–Y) Spearman correlation analysis between IL-21 mRNA in SG and indicated lymphoid and GC B-cell genes. All graphs represent mean±SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. CXCR5, CXC-motif chemokine receptor 5; ELS, ectopic lymphoid structure; GC, germinal centres; GSVA, Gene Set Variation Analysis; ICOS, inducible T-cell co-stimulator; IL-21, interleukin-21; NSCS, non-specific chronic sialoadenitis; PD-1, programmed cell death protein 1; SAP, SLAM-associated protein; SG, Salivary gland; SS, Sjogren’s syndrome; Tfh, T-follicular-helper.
Although the IL-21/IL-21R pathway has been implicated in the pathogenesis of SS,30 there is no available evidence of its implication in ELS development. Thus, we next performed a targeted gene expression profiling of IL-21 and IL-21R together with other ELS and GC B-cell-related genes and showed that IL-21 and IL-21R mRNA were significantly increased in the SG of ELS+SS compared with ELS- SS and NSCS patients (figure 2J, K). Additionally, IL-21/IL-21R gene expression levels increased in parallel with the infiltrating CD20+ B-cells, CD3+ T-cells and CD138+ plasma cells, but not CD68+ macrophages as assessed by immunohistochemistry (figure 2L–S). Furthermore, IL-21 mRNA also correlated with genes associated with ELS (LTB, CXCL13) and functional B-cell activation (BAFF, AICDA, PAX5) (figure 2T–Y). Overall, these data suggest a role for the IL-21/IL-21R pathway in driving local B-cell activation within ELS in SG.
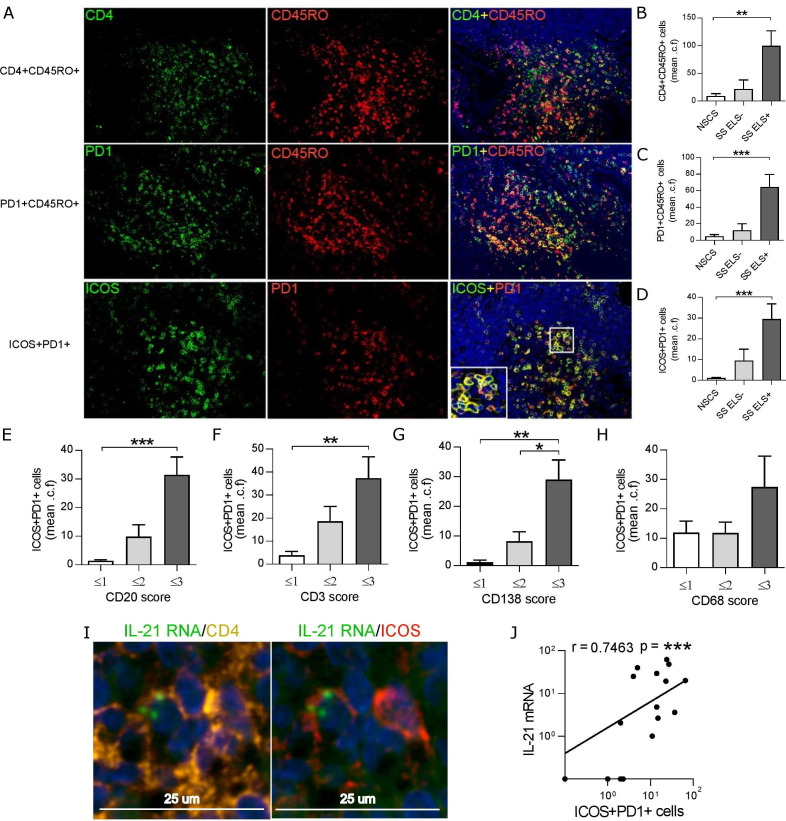
PD1+ICOS+CD4+ T-cells infiltrate ELS+ SG, express IL-21 and co-localise with ectopic B-cell follicles
Sequential double IF stainings for memory T-cells (CD4+CD45RO+), activated-memory T-cells (CD45RO+PD1+) and Tfh-like cells (PD1+ICOS+) in SS SGs demonstrated a progressive increase in all cell types, but particularly an enrichment in Tfh-like cells within B-cell follicles in ELS+ (figure 3A–D). Additionally, semiquantitative assessment of cell infiltration demonstrated higher levels of PD1+ICOS+ cells in patients with high infiltration of B-cells (CD20+), T-cells (CD3+) and plasma cells (CD138+), but not macrophages (CD68+) (figure 3E–H). In situ hybridisation for IL-21 mRNA in combination with CD4/ICOS immunostaining confirmed that IL-21 expression was confined to ICOS+CD4+ T-cells within ELS (figure 3I and online supplementary figure S4). In keeping with this data, infiltrating PD1+ICOS+ T-cell number positively correlated with IL-21 mRNA expression in SS SG (figure 3J).
Figure 3.
PD-1+ICOS+CD45RO+CD4+ cells are increased within SG with ELS in SS and produce IL-21. (A) Representative immunofluorescence detection of CD4+CD45RO+ (top row), PD1+CD45RO+ (middle row) and ICOS+PD1+ cells (bottom row) in SG biopsy tissues with ELS. (B–D) Quantification (mean counts per field, a minimum of 5 random fields) of the double positive cells for each double immunofluorescence combination in SG biopsy tissues from NSCS (n=8) and SS (n=12) patients. Images displayed at x20 magnification. (E–H) ICOS+PD1+ cell count was segregated according to histological semi-quantitative score (0 to 3) of B (CD20), T (CD3) plasma cells (CD138) and macrophages (CD68). Statistical analysis by Kruskal-Wallis-test with Dunn’s post-test correction for multiple comparison (B–H). (I) Representative fluorescent in situ hybridisation (FISH) detection of IL-21 RNA, costained with CD4 and ICOS in SG biopsy tissues with ELS. (J) Spearman correlation analysis between SG real-time PCR IL-21 mRNA expression with ICOS+PD1+ cell count. All graphs represent mean±SEM. *p<0.05, **p<0.01, ***p<0.001. ELS, ectopic lymphoid structure; ICOS, inducible T-cell co-stimulator; NSCS, non-specific chronic sialoadenitis; PD-1, programmed cell death protein 1; SG, salivary gland; SS, Sjogren’s syndrome.
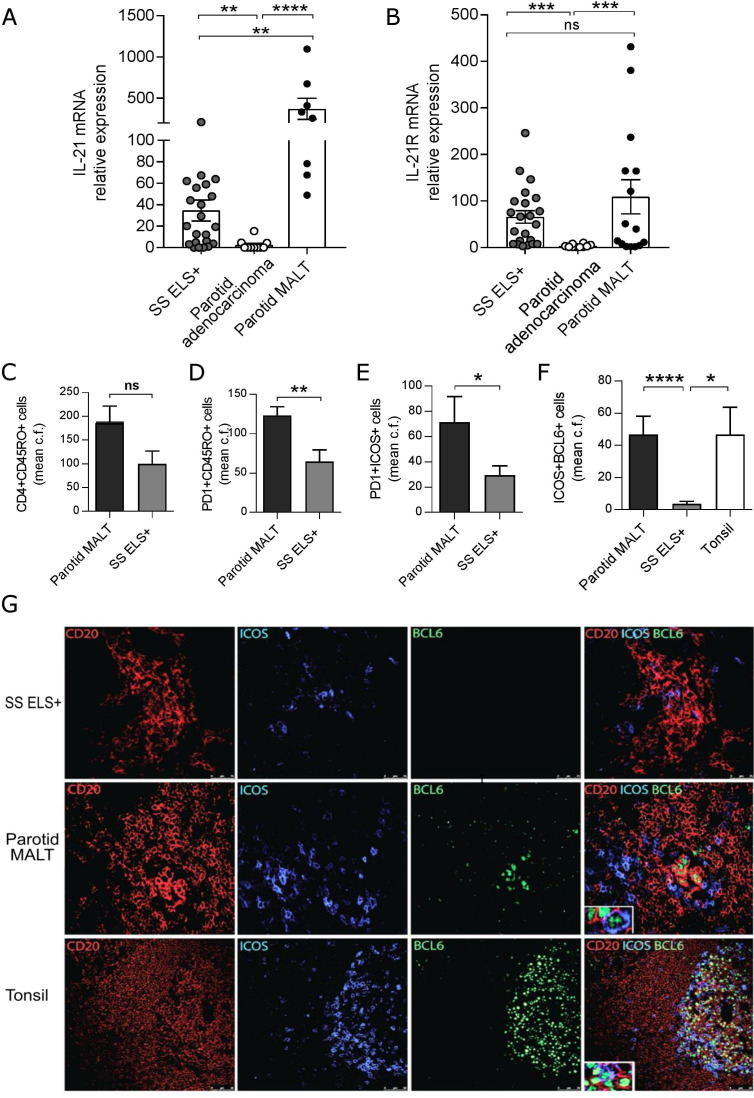
Aberrant expansion of IL-21 and ICOS+PD1+BCL6+ infiltrating T-cells in the evolution to parotid MALT B-cell lymphomas
Uncontrolled B-cell activation and aberrant somatic hypermutation22 within SS SG can lead to genetic instability and progression to parotid B-cell MALT-L in around 5% of patients with SS,4 suggesting that lymphomagenesis in SS is partially sustained by T-cell-driven responses, similarly to gastric MALT-L,31 32 although also extra follicular and T cell-independent mechanisms are key in neoplastic B cell expansion.4 Therefore, we assessed IL-21 production and Tfh-cells infiltration in SS parotids with MALT-L. First, IL-21 mRNA expression displayed ~400-fold increase in parotid MALT-L compared with control parotids and ~20-fold increase compared with ELS+ minor SG biopsies (figure 4A, B). MALT-L also showed an expanded infiltration of activated-memory (CD45RO+PD1+) and Tfh-like T-cells (PD1+ICOS+) to levels significantly higher compared with ELS+SS SGs (figure 4C–E).
Figure 4.
Development of parotid malt lymphomas is associated with elevated SG IL-21 and IL-21R expression. real-time PCR expression for IL-21 (A) and IL21R (B) on RNA extracted from SS labial SG biopsies with ELS (ELS+, n=22), SS parotid SG MALT-lymphoma (n=15) and parotid adenocarcinoma (n=10). Quantification (mean counts per field) of (C) CD4+CD45RO+, (D) PD1+CD45RO+, (E) PD1+ICOS+ cells in SS labial SG biopsies with ELS (ELS+, n=10) and SS parotid SG MALT-lymphoma (n=7). Mann-Whitney U t-test statistics. (F) Mean counts per field of ICOS+BCL6+ cells between SS parotid SG MALT-lymphoma (n=23), SS labial SG biopsies with ELS (ELS+, n=21) and tonsils (n=3). Statistical analysis by Kruskal-Wallis-test with Dunn’s post-test correction for multiple comparisons (A, B, F). (G) Representative immunofluorescence detection of ICOS+BCL6+ cells relative to B (CD20+) cell aggregates in SG biopsy tissues with ELS, parotid SG MALT-lymphoma and tonsil. All graphs represent mean±SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. ELS, ectopic lymphoid structures; ICOS, inducible T-cell costimulator; IL-21, interleukin-21; MALT, mucosa-associated lymphoid tissue; SG, Salivary gland; SS, Sjögren’s syndrome.
Of relevance, within parotid MALT-L, ICOS+PD1+ T-cells acquired BCL6, a transcription factor essential for Tfh-cell differentiation more frequently than in ELS+ minor SG biopsies (figure 4F) and resided in close proximity to CD20+BCL6+ GC B-cells (figure 4G).
Unique SG expansion of Tfh and Tph double IL-21/IFN-γ producers marks the evolution to ELS and parotid B-cell MALT-L
Fluorescence-activated cell sorting (FACS) profiling of T-cell subsets in parotid MALT-L show how IL-21-producing CD4+ Th cells represented the most expanded CD4+ population accounting for ~30% of the whole lesional CD4+ T-cells (figure 5A, B); in comparison Th17 cells, known to be involved in ELS formation,33 34 made up of less than 1% of cytokine-producing CD4+ T-cells within MALT-L. Interestingly, IL-21+ CD4+ T-cells frequently displayed concomitant production of IFN-γ but not IL-17, IL-10 or Granzyme-B in both labial SG and parotid MALT-L which clearly distinguish these cells from CD4+ T-cells infiltrating inflamed human tonsils (online supplementary figure S3).
Figure 5.
SG inflammation and development of parotid MALT lymphomas is associated with increased Tfh-cell numbers with increased production of IL-21 and IFN-γ. (A) Pie chart showing cytokines production by T helper (CD4+) cells isolated from parotid SG MALT-lymphoma (n=1), on stimulation with PMA and ionomycin and flow-cytometry analysis. (B) Representative flow-cytometry dot plots for cytokines production by T helper (CD4+) cells and (C) tSNE plots for indicated markers in T helper (CD4+) cells from parotid SG MALT-lymphoma. Flow-cytometry gating strategy for the identification of (D) IL-21 and IFN-γ producing Tfh-cells (identified as CD4+CD25-Foxp3- CXCR5+ICOS+PD-1+) and (E) pathogenic T peripheral helper cells (identified as CD4+CD25-Foxp3-CXCR5-ICOS+PD-1+) in parotid SG MALT-lymphoma (n=1). ELS, ectopic lymphoid structure; ICOS, inducible T-cell costimulator; IFN-γ, interferon-γ; IL-21, interleukin-21; MALT, mucosa-associated lymphoid tissue; NSCS, non-specific chronic sialoadenitis; PD-1, programmed cell death protein 1; SG, Salivary gland; SS, Sjogren’s syndrome; Tfh, T-follicular-helper.
Dimensionality reduction analysis (t-Distributed Stochastic Neighbor Embedding, t-SNE), revealed that IL-21 and IFN-γ-producing lesional T-cells in MALT-L fell within both ICOS+ and PD1+ CD4+ T-cells but displayed variable expression of CXCR5 (figure 5C). Accordingly, both ICOS (from ~70% to ~90%) and CXCR5 (from ~30% to ~60%) expression were enriched in IL-21-single producers compared to single IFN-γ or IL-21/IFN-γ-double producers (figure 5D), suggesting that bona fide CD4+CD25-Foxp3-CXCR5+ICOS+PD-1+ Tfh-cells account for most of lesional IL-21 production in parotid MALT-L. Of interest, the CXCR5- population of CD4+PD-1hi T-cells displayed a phenotype highly compatible with Tph-cells, with >95% ICOS expression and enriched in both IL-21 and IFN-γ production (figure 5E). Overall, these data support the hypothesis that the aberrant expansion of IL-21 and IL-21/IFN-γ-producing ICOS+PD-1+CD4+ T-cells, independently from CXCR5 expression, represents an important step in ELS maintenance and MALT-L development in the SG of patients with SS.
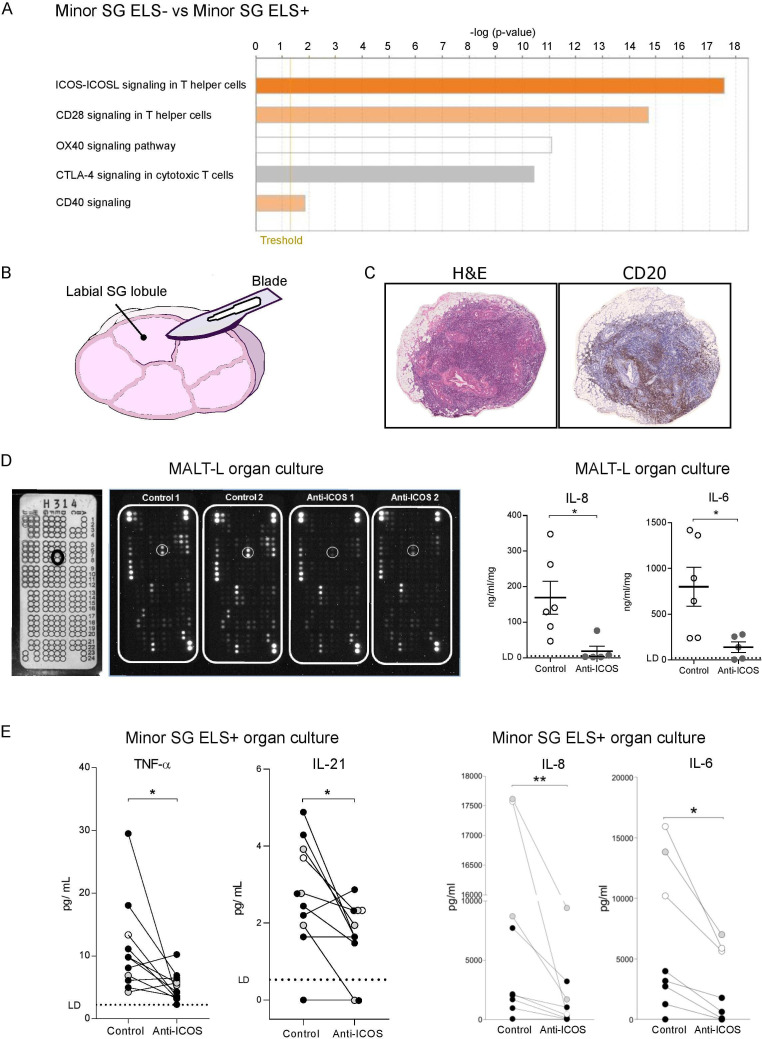
Functional ICOS-blockade controls the release of IL-21 and other proinflammatory cytokines in SG organ cultures
Taken together, our data clearly indicate an expansion of ICOS+CD4+ T-cell subsets (both Tfh-like and Tph-like) in patients with SS with ELS and MALT-L. Additionally, hierarchical analysis of costimulatory pathways of microarray data identified ICOS-ICOS-L as the most upregulated pathway in SG with ELS (figure 6A), supporting a critical role for ICOS. In order to functionally investigate the importance of this pathway we set up organ cultures using either ELS+ labial SG (figure 6B) or parotid MALT-L tissue (figure 6C) incubated with a blocking, non-depleting anti-ICOS monoclonal antibody (mAb) or its isotype control. In parotid MALT-L tissue treated with the anti-ICOS mAb, a protein array screening assay on the organ culture supernatant showed down-modulation of several proteins including IL-8 and IL-6 as also confirmed by ELISA (figure 6D). Likewise, analysis of minor SG-organ culture supernatants showed that incubation with the anti-ICOS mAb reduced the levels of IL-21, TNF-α, IL-6 and IL-8 (figure 6E) in comparison to isotype control-treated glands.
Figure 6.
The blockade of ICOS-ICOS ligand signalling pathway reduces pro-inflammatory cytokines level in SG with ELS and MALT-lymphoma organ culture in SS. (A) Ingenuity Pathway Analysis (IPA) of microarray data obtained from the analysis of RNA extracted from ELS- and ELS+ SG. The orange-coloured bars (ICOS-ICOSL, CD28, CD40) show predicted pathway activation (with positive z-score), while the white bar (OX40 signalling pathway) indicates a z-score at or very close to 0 and the grey bar (CTLA4) pathways where no prediction can be made. (B) Schematic representation of a labial minor SG lobule cut longitudinally in half for the organ culture experiment. (C) representative histological images (H&E and IHC for CD20) of inflammatory infiltration in a parotid SG with B-cell non-Hodgkin MALT-lymphoma from a patient with SS. (D) Multiplex antibody array for cytokine analysis in the supernatant of a parotid MALT-lymphoma (n=1) organ culture, treated with an anti-ICOS blockade or its isotype control. The array template (white panel on the left) shows the coordinate reference of analytes with IL-6 spots highlighted (black circle). Black panels show the arrays incubated with organ culture supernatants treated with isotype control or anti-ICOS blockade with white circles around IL-6 spots. ELISA quantification of IL-8 and IL-6 in the supernatants of MALT-lymphoma organ culture, treated with an anti-ICOS blockade (grey dots) or its isotype control (white dots). Each dot represents a technical replicate. (E) Levels of TNF-α, IL-21, IL-8 and IL-6 detected in the supernatant of minor SG lobule organ culture, treated with an anti-ICOS blockade or its isotype control. The same colour dots represent minor SG lobules (from 2 to 8 lobules) from the same patient with SS (patients with SS, n=3). The lines link the two halves of the same lobules treated with the anti-ICOS blockade or its isotype control. Limit of detection (LD) for each cytokine is highlighted with a dashed line. ELISA quantification of IL-8 and IL-6 in the supernatants of minor SG lobules organ culture, treated with an anti-ICOS blockade or its isotype control, for cytokine levels that reach the Legendplex’ upper detection limit. Statistical analysis by Wilcoxon t-test. *p<0.05, **p<0.01. (F) All graphs represent mean±SEM. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. ELS, ectopic lymphoid structure; ICOS, inducible T-cell costimulator; IFN-γ, interferon-γ; IHC, immunohistochemistry; IL-21, interleukin-21; MALT, mucosa-associated lymphoid tissue; NSCS, non-specific chronic sialoadenitis; PD-1, programmed cell death protein 1; SG, Salivary gland; SS, Sjogren’s syndrome; Tfh, T-follicular-helper.
Overall, these data confirm that the ICOS-ICOSL pathway is critical for the activation of important T-cell related proinflammatory pathways in both labial and parotid glands.
Discussion
Here, we present a comprehensive analysis of peripheral and lesional CD4+ T-cells at different stages of SG immunopathology in a large cohort of patients with SS, a disease in which ectopic GC have been linked to the progression towards severe extraglandular manifestations and B-cell MALT-L.4 To start with, we reported the first high throughput transcriptomic profiling of ELS+ and ELS- SG tissues. Microarray analysis identified, using both unsupervised and supervised cluster analysis, a Tfh-cell signature, IL-21 and the ICOS-costimulatory pathway as the most upregulated gene clusters in the ELS+ vs ELS- subset of patients with SS. Using quantitative PCR and in situ hybridisation on SG tissue we confirmed that IL-21 mRNA was highly upregulated in SG with ectopic GCs and lesional Tfh-like cells. Strikingly, an aberrant expansion of infiltrating Tfh-like cells acquiring expression of the key transcription factor BCL6 was observed in parotid MALT-L, together with a disproportionate upregulation in IL-21 transcripts. Characterisation of T cell infiltration in control parotid was limited by the lack of matched histology samples with total RNA. These results are of great interest as MALT-L in SS arises from post-GC B cells with a marginal zone phenotype often bearing a RF+ autoreactive B cell receptor.35 Although extra follicular mechanisms are also likely to be involved in neoplastic B cell expansion, including B cell receptor cross-linking36 and aberrant NFkB activation37 via engagement of BAFF and Toll-like receptors4, the evidence of ongoing intratumour clonal diversification and aberrant somatic hypermutation suggest that ectopic GCs play an active role in supporting B cell lymphomagenesis.22 38 Deep phenotyping of lesional CD4+ T-cells in parotid MALT-L by flow cytometry revealed unexpected and highly novel results whereby we identified two main CD4+ T-cell subsets responsible for IL-21 production. One subset represented the bona fide Tfh-cells (Foxp3-CXCR5+ ICOS+PD1+) while the other, which accounted for around 50% of total IL-21 production in parotid MALT-L, shared striking similarities with the recently described CXCR5-PD1hiICOS+ pathogenic Tph-cells.19 Although these results should be interpreted with caution, as FACS analysis was performed on one MALT-L sample, together with transcriptomic and histology data on a larger cohort, our results provide the first evidence that this subset may be directly implicated in MALT B-cell-lymphomagenesis being uniquely able to provide proliferative B-cell signals at extrafollicular sites. Additionally, both Tfh and Tph-like cells in parotid MALT-L and ELS+ SG displayed heterogeneity in cytokine production with expanded populations of single IL-21, single IFN-γ and double IL-21/IFN-γ producers; it is unclear whether these represent distinct or evolutionary subsets driven by the local inflammatory milieu but our work prompts further investigation into our understanding of the heterogeneity and pathogenic properties of the expanding family of Tfh-like cells.39
Due to the heterogeneity in marker combinations used to identify peripheral Tfh-cells in patients with SS in previous works,28 40–44 we performed a comprehensive analysis of Tfh-like cell subsets in peripheral blood matched with SG on the basis of the expression of CXCR5, ICOS and PD-1 coupled with intracellular cytokine analysis. Our work indicated that circulating CXCR5+ICOS+PD-1+ Tfh-cells, which represent a partially differentiated state compared with Tfh in secondary lymphoid organs, but are able to maintain B-cell differentiating functions45 are significantly expanded in ELS+ patients. Notably, the expansion of IL-21 producing CXCR5+ICOS+PD-1+ Tfh subsets positively correlated with SG focus score and these Tfh-cells were selectively enriched in patients with ELS in the SG biopsies suggesting that IL-21+ Tfh-cells may represent a useful blood biomarker of SG immunopathology. Additionally, we further expanded on previous works by showing how circulating CXCR5+ICOS+PD-1+ Tfh subsets producing high levels of both IL-21 and IFN-γ (including the detection of double IL-21/IFN-γ producers) are selectively enriched in patients with SS with anti-Ro/SSA and anti-La/SSB autoantibodies, positively correlated with IgG levels and negatively with complement C4. Overall these data support the notion that circulating Tfh in patients with SS play an active role in the autoimmune B-cell dysregulation typical of patients with SS as previously suggested.41 42 These data also link well with previous work showing that an over expansion of Tfh-cells is associated with a dysregulation of B-cell dynamics44 and the production of autoantibodies46 and that an expansion of Tfh-cells might lower the selection pressure on GC B-cells, leading to the emergence of autoreactive B-cells.47
Finally, in addition to identifying IL-21 and ICOS as key pro-inflammatory and co-stimulatory signals promoting autoreactive B-cell activation and lymphoma progression in patients with SS, we also provide proof-of-concept functional evidence in SG organ cultures that candidates blocking the ICOS-pathway as a novel therapeutic option in SS. Here we report that an anti-ICOS non-depleting blocking antibody incubated with either ELS+ SG biopsies or parotid MALT-L induced a drastic downregulation of IL-21 and other key proinflammatory cytokines. These data are in line with the evidence that ICOS is required for the reciprocal activation of T-cell to B-cells48 and that IL-21 expression by Tfh requires the interaction with ICOS-L (which can also be expressed by SG epithelial cells).49 Our results are highly relevant to the recently completed phase IIa clinical trial with an anti-ICOSL mAb in primary SS (NCT02334306) and will help interpreting the clinical efficacy of targeting the ICOS/ICOSL costimulatory pathway in SS by prompting the use of patient stratification on the basis of lesional and/or peripheral Tfh-cell signatures to achieve maximum clinical efficacy.
Acknowledgments
We thank the BCI/WHRI Flow Cytometry Core Facility for their technical assistance. Thanks to Dr. Claudio Mauro for critical reading of the manuscript.
Footnotes
Handling editor: Josef S Smolen
Twitter: @Elena Pontarini, @FeliceRivellese, @BaroneLab
EP, WJM-B and CC contributed equally.
Presented at: An abstract (OP0279) of present work has been presented as selected oral presentation at the Annual European Congress of Rheumatology EULAR 2019.
Contributors: EP, WJM-B, CC and MB designed the study EP, WJM-B, CC, DL, RC, EP, EC, FRD, L-FJ, EG, AB, EG, JC and GC performed the experiments. EP, WJM-B, CC, EC, L-FJ, EP, JC and GC analysed the data. AT, NS, CB, IP, PR, RG, FB, BF, SJB, SC, RP and SC provided help with and facilitated the collection and characterisation of human samples. MB and CP provided scientific insight and provided resources for this study. EP and MB wrote the manuscript. All authors edited and reviewed the manuscript.
Funding: This work was supported by project grants from the Medical Research Council (MR/N003063/1 to MB), versus Arthritis UK (grant 20 089 to MB and grant 21 753 to EP) and the William Harvey Research Foundation (to MB). FR is funded by an NIHR Transitional Research Fellowship (TRF-2018–11-ST2-002). BF and SJB have received support from the NIHR Birmingham Biomedical Research Centre and the National Institute for Health Research (NIHR)/Wellcome Trust Birmingham Clinical Research Facility.
Competing interests: BF: Consultancy for Novartis, Roche, and BMS. SJB: Consultancy for Astrazeneca, Biogen, BMS, Celgene, Medimmune, MTPharma, Novartis, Ono, UCB, xtlbio. MB: consultancy and/or unrestricted grant support from Medimmune, GSK, Janssen, UCB. GC and JC are AstraZeneca employees and own company stocks.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: Sample collection from four European cohorts was approved by ethics committees: REC 05/Q0702/1-Rheumatology/Oral medicine clinic-QMUL, 13/WA/0392-University Hospitals Birmingham National Health Service Foundation Trust, Harmonics 4683 (14/9/2017)-Sapienza University of Rome, MEMORAT-University of Pisa.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplementary information. The data that support the findings of this study are available from the corresponding authors on reasonable request.
References
- 1. Mariette X, Criswell LA. Primary Sjögren's syndrome. N Engl J Med 2018;378:931–9. 10.1056/NEJMcp1702514 [DOI] [PubMed] [Google Scholar]
- 2. Hansen A, Odendahl M, Reiter K, et al. . Diminished peripheral blood memory B cells and accumulation of memory B cells in the salivary glands of patients with Sjögren's syndrome. Arthritis Rheum 2002;46:2160–71. 10.1002/art.10445 [DOI] [PubMed] [Google Scholar]
- 3. Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM. Characteristics of the minor salivary gland infiltrates in Sjögren's syndrome. J Autoimmun 2010;34:400–7. 10.1016/j.jaut.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 4. Nocturne G, Pontarini E, Bombardieri M, et al. . Lymphomas complicating primary Sjö gren’s syndrome: from autoimmunity to lymphoma. Rheumatology 2019. 10.1093/rheumatology/kez052. [Epub ahead of print: 05 Mar 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barone F, Bombardieri M, Manzo A, et al. . Association of CXCL13 and CCL21 expression with the progressive organization of lymphoid-like structures in Sjögren's syndrome. Arthritis Rheum 2005;52:1773–84. 10.1002/art.21062 [DOI] [PubMed] [Google Scholar]
- 6. Bombardieri M, Lewis M, Pitzalis C. Ectopic lymphoid neogenesis in rheumatic autoimmune diseases. Nat Rev Rheumatol 2017;13:141–54. 10.1038/nrrheum.2016.217 [DOI] [PubMed] [Google Scholar]
- 7. Nurieva RI, Chung Y, Hwang D, et al. . Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 2008;29:138–49. 10.1016/j.immuni.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu D, Rao S, Tsai LM, et al. . The transcriptional repressor BCL-6 directs T follicular helper cell lineage commitment. Immunity 2009;31:457–68. 10.1016/j.immuni.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 9. Johnston RJ, Poholek AC, DiToro D, et al. . Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 2009;325:1006–10. 10.1126/science.1175870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Avery DT, Deenick EK, Ma CS, et al. . B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J Exp Med 2010;207:155–71. 10.1084/jem.20091706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zotos D, Coquet JM, Zhang Y, et al. . Il-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med 2010;207:365–78. 10.1084/jem.20091777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Linterman MA, Beaton L, Yu D, et al. . Il-21 acts directly on B cells to regulate BCL-6 expression and germinal center responses. J Exp Med 2010;207:353–63. 10.1084/jem.20091738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crotty S. Follicular helper CD4 T cells (Tfh). Annu Rev Immunol 2011;29:621–63. 10.1146/annurev-immunol-031210-101400 [DOI] [PubMed] [Google Scholar]
- 14. Breitfeld D, Ohl L, Kremmer E, et al. . Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med 2000;192:1545–52. 10.1084/jem.192.11.1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weinstein JS, Bertino SA, Hernandez SG, et al. . B cells in T follicular helper cell development and function: separable roles in delivery of ICOS ligand and antigen. J Immunol 2014;192:3166–79. 10.4049/jimmunol.1302617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Choi YS, Kageyama R, Eto D, et al. . Icos receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor BCL6. Immunity 2011;34:932–46. 10.1016/j.immuni.2011.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crotty S. T follicular helper cell biology: a decade of discovery and diseases. Immunity 2019;50:1132–48. 10.1016/j.immuni.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gensous N, Charrier M, Duluc D, et al. . T follicular helper cells in autoimmune disorders. Front Immunol 2018;9:1637. 10.3389/fimmu.2018.01637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rao DA, Gurish MF, Marshall JL, et al. . Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 2017;542:110–4. 10.1038/nature20810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manzo A, Vitolo B, Humby F, et al. . Mature antigen-experienced T helper cells synthesize and secrete the B cell chemoattractant CXCL13 in the inflammatory environment of the rheumatoid joint. Arthritis Rheum 2008;58:3377–87. 10.1002/art.23966 [DOI] [PubMed] [Google Scholar]
- 21. Vitali C, Bombardieri S, Jonsson R, et al. . Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European consensus group. Ann Rheum Dis 2002;61:554–8. 10.1136/ard.61.6.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bombardieri M, Barone F, Humby F, et al. . Activation-induced cytidine deaminase expression in follicular dendritic cell networks and interfollicular large B cells supports functionality of ectopic lymphoid neogenesis in autoimmune sialoadenitis and malt lymphoma in Sjögren's syndrome. J Immunol 2007;179:4929–38. 10.4049/jimmunol.179.7.4929 [DOI] [PubMed] [Google Scholar]
- 23. Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-Seq data. BMC Bioinformatics 2013;14:7. 10.1186/1471-2105-14-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Croia C, Astorri E, Murray-Brown W, et al. . Implication of Epstein-Barr virus infection in disease-specific autoreactive B cell activation in ectopic lymphoid structures of Sjögren's syndrome. Arthritis Rheumatol 2014;66:2545–57. 10.1002/art.38726 [DOI] [PubMed] [Google Scholar]
- 25. Tajima N, Tezuka K, Tanaka M, et al. . Critical role of activation-inducible lymphocyte immunomediatory molecule/inducible costimulator in the effector function of human T cells: a comparative in vitro study of effects of its blockade and CD28 blockade in human beings and monkeys. Hum Immunol 2008;69:399–408. 10.1016/j.humimm.2008.05.002 [DOI] [PubMed] [Google Scholar]
- 26. Kawamoto M, Harigai M, Hara M, et al. . Expression and function of inducible co-stimulator in patients with systemic lupus erythematosus: possible involvement in excessive interferon-gamma and anti-double-stranded DNA antibody production. Arthritis Res Ther 2006;8:R62. 10.1186/ar1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deng J, Wei Y, Fonseca VR, et al. . T follicular helper cells and T follicular regulatory cells in rheumatic diseases. Nat Rev Rheumatol 2019;15:475–90. 10.1038/s41584-019-0254-2 [DOI] [PubMed] [Google Scholar]
- 28. Verstappen GM, Meiners PM, Corneth OBJ, et al. . Attenuation of follicular helper T cell-dependent B cell hyperactivity by abatacept treatment in primary Sjögren's syndrome. Arthritis Rheumatol 2017;69:1850–61. 10.1002/art.40165 [DOI] [PubMed] [Google Scholar]
- 29. McCausland MM, Yusuf I, Tran H, et al. . Sap regulation of follicular helper CD4 T cell development and humoral immunity is independent of SLAM and Fyn kinase. J Immunol 2007;178:817–28. 10.4049/jimmunol.178.2.817 [DOI] [PubMed] [Google Scholar]
- 30. Kwok S-K, Lee J, Yu D, et al. . A pathogenetic role for IL-21 in primary Sjögren syndrome. Nat Rev Rheumatol 2015;11:368–74. 10.1038/nrrheum.2014.225 [DOI] [PubMed] [Google Scholar]
- 31. Greiner A, Knörr C, Qin Y, et al. . Low-grade B cell lymphomas of mucosa-associated lymphoid tissue (MALT-type) require CD40-mediated signaling and Th2-type cytokines for in vitro growth and differentiation. Am J Pathol 1997;150:1583–93. [PMC free article] [PubMed] [Google Scholar]
- 32. Craig VJ, Cogliatti SB, Arnold I, et al. . B-cell receptor signaling and CD40 ligand-independent T cell help cooperate in Helicobacter-induced malt lymphomagenesis. Leukemia 2010;24:1186–96. 10.1038/leu.2010.76 [DOI] [PubMed] [Google Scholar]
- 33. Peters A, Pitcher LA, Sullivan JM, et al. . Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity 2011;35:986–96. 10.1016/j.immuni.2011.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, et al. . The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol 2011;12:639–46. 10.1038/ni.2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nocturne G, Virone A, Ng W-F, et al. . Rheumatoid factor and disease activity are independent predictors of lymphoma in primary Sjögren's syndrome. Arthritis Rheumatol 2016;68:977–85. 10.1002/art.39518 [DOI] [PubMed] [Google Scholar]
- 36. Glauzy S, Boccitto M, Bannock JM, et al. . Antigen-driven lymphoproliferations in Sjögren’s Syndrome patients accumulate in complement receptor 2/CD21−/low B cells. Arthritis Rheumatol 2018;70:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nocturne G, Boudaoud S, Miceli-Richard C, et al. . Germline and somatic genetic variations of TNFAIP3 in lymphoma complicating primary Sjogren's syndrome. Blood 2013;122:4068–76. 10.1182/blood-2013-05-503383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Deutsch AJA, Aigelsreiter A, Staber PB, et al. . Malt lymphoma and extranodal diffuse large B-cell lymphoma are targeted by aberrant somatic hypermutation. Blood 2007;109:3500–4. 10.1182/blood-2006-06-030494 [DOI] [PubMed] [Google Scholar]
- 39. Hutloff A. T follicular helper-like cells in inflamed non-lymphoid tissues. Front Immunol 2018;9:1707. 10.3389/fimmu.2018.01707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jin L, Yu D, Li X, et al. . CD4+CXCR5+ follicular helper T cells in salivary gland promote B cells maturation in patients with primary Sjogren's syndrome. Int J Clin Exp Pathol 2014;7:1988–96. [PMC free article] [PubMed] [Google Scholar]
- 41. Verstappen GM, Nakshbandi U, Mossel E, et al. . Is the T follicular regulatory:follicular helper T cell ratio in blood a biomarker for ectopic lymphoid structure formation in sjögren's syndrome? comment on the article by fonseca et al. Arthritis Rheumatol 2018;70:1354–5. 10.1002/art.40488 [DOI] [PubMed] [Google Scholar]
- 42. Szabó K, Papp G, Szántó A, et al. . A comprehensive investigation on the distribution of circulating follicular T helper cells and B cell subsets in primary Sjögren's syndrome and systemic lupus erythematosus. Clin Exp Immunol 2016;183:76–89. 10.1111/cei.12703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Szabo K, Papp G, Barath S, et al. . Follicular helper T cells may play an important role in the severity of primary Sjögren's syndrome. Clin Immunol 2013;147:95–104. 10.1016/j.clim.2013.02.024 [DOI] [PubMed] [Google Scholar]
- 44. Brokstad KA, Fredriksen M, Zhou F, et al. . T follicular-like helper cells in the peripheral blood of patients with primary Sjögren's syndrome. Scand J Immunol 2018;88:e12679 10.1111/sji.12679 [DOI] [PubMed] [Google Scholar]
- 45. He J, Tsai LM, Leong YA, et al. . Circulating precursor CCR7(lo)PD-1(hi) CXCR5⁺ CD4⁺ T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity 2013;39:770–81. 10.1016/j.immuni.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 46. Ma CS, Deenick EK. Human T follicular helper (Tfh) cells and disease. Immunol Cell Biol 2014;92:64–71. 10.1038/icb.2013.55 [DOI] [PubMed] [Google Scholar]
- 47. Vinuesa CG. Hiv and T follicular helper cells: a dangerous relationship. J Clin Invest 2012;122:3059–62. 10.1172/JCI65175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wikenheiser DJ, Stumhofer JS. ICOS co-stimulation: friend or foe? Front Immunol 2016;7:304. 10.3389/fimmu.2016.00304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gong Y-Z, Nititham J, Taylor K, et al. . Differentiation of follicular helper T cells by salivary gland epithelial cells in primary Sjögren's syndrome. J Autoimmun 2014;51:57–66. 10.1016/j.jaut.2013.11.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2020-217646supp001.pdf (3.4MB, pdf)