Abstract
Soluble lectin-like oxidized low-density lipoprotein receptor-1 (sLOX-1), neutrophil gelatinase-associated lipocalin (NGAL), and matrix metalloproteinase-9 (MMP-9) are inflammatory biomarkers involved in plaque destabilization resulting in acute coronary syndrome (ACS). This study aimed to investigate the diagnostic value of a combination of biomarkers to discriminate plaque ruptures in the setting of ACS. Eighty-five ACS patients with optical coherence tomography (OCT) images of the culprit plaque were included and categorized into two groups: ACS with plaque rupture (Rupture group, n = 42) or without plaque rupture (Non-rupture group, n = 43) verified by OCT. A discriminative model of plaque rupture using several biomarkers was developed and validated. The Rupture group had higher white blood cell (WBC) counts and peak creatine kinase-myocardial band (CK-MB) levels (13.39 vs. 2.69 ng/mL, p = 0.0016). sLOX-1 (227.9 vs. 51.7 pg/mL, p < 0.0001) and MMP-9 (13.4 vs. 6.45 ng/mL, p = 0.0313) levels were significantly higher in the Rupture group, whereas NGAL showed a trend without statistical significance (59.03 vs. 53.80 ng/mL, p = 0.093). Receiver operating characteristic curves to differentiate Rupture group from Non-rupture group calculated the area under the curve for sLOX-1 (p < 0.001), MMP-9 (p = 0.0274), and NGAL (p = 0.0874) as 0.763, 0.645, and 0.609, respectively. A new combinatorial discriminative model including sLOX-1, MMP-9, WBC count, and the peak CK-MB level showed an area under the curve of 0.8431 (p < 0.001). With a cut-off point of 0.614, the sensitivity and specificity of plaque rupture were 62.2% and 97.6%, respectively. The new discriminative model using sLOX-1, MMP-9, WBC count, and peak CK-MB levels could better identify plaque rupture than each individual biomarker in ACS patients.
Subject terms: Cardiovascular biology, Biomarkers, Cardiology
Introduction
Acute coronary syndrome (ACS) is a major concern for morbidity and mortality in patients suffered by ischemic heart disease1, 2. A complex and diverse process, including endothelial dysfunction, vascular inflammation, and hypercoagulability, result in atherosclerosis and plaque destabilization leading to ACS3, 4. These pathophysiological components of coronary artery disease (CAD) may be detected by several biomarkers. Consequently, biomarker levels may be associated with the severity of CAD and hence may forecast the occurrence of adverse cardiovascular events or differentiate severe events from less severe ones in patients with CAD. Such associations have already been investigated for several biomarkers, such as C-reactive protein (CRP)5–7, soluble lectin-like oxidized low-density lipoprotein (LDL) receptor-1 (sLOX-1), matrix metalloproteinase-9 (MMP-9), and neutrophil gelatinase-associated lipocalin (NGAL).
sLOX-1 is implied to be linked with vulnerable plaque and subsequent plaque rupture, and a previous study has reported that it may discriminate between ACS with and without plaque rupture8. Other studies have shown that MMP-9 indicates atherosclerotic plaque rupture or vulnerability by elastin degradation, which advances breakdown of the thin fibrous caps of plaques9, 10. Thus, MMP-9 activity is thought to be the key to ACS development. On the other hand, NGAL may be associated in the progression of atherosclerosis via endothelial dysfunction, inflammatory processes, and matrix degradation, leading to atherosclerotic plaque instability by modulating the activity of MMP-911–13. In the presence of CAD, plasma NGAL level is significantly elevated and correlates with its severity and is notably higher in patients with acute myocardial infarction than in those with stable CAD13, 14.
While the most common cause of intra-coronary thrombosis is plaque rupture, which found in approximately 50% of patients with ACS15–17, other causes include plaque erosion, calcified plaque, tight stenosis, intramural hematoma, and spontaneous dissection. Recent studies revealed that ACS patients presenting with plaque rupture have worse prognosis18, 19. Therefore, differentiation between ruptured versus non-ruptured plaques in the culprit lesions is crucial for risk stratification and treatment strategy for patients with ACS.
Optical coherence tomography (OCT) has high resolution power and can discriminate several plaque characteristics of culprit lesions that result in ACS20–25. However, it is difficult to achieve timely risk stratification because OCT requires invasive and time-consuming coronary angiography.
Therefore, the identification of plaque ruptures without invasive testing in patients with ACS is necessary for quick risk assessment and appropriate management selection. Until now, data on the relationship between plaque rupture and serum biomarker levels are scarce.
In the present study, we sought to derive and validate a new discriminative model using several biomarkers for detecting plaque rupture in patients with ACS verified by OCT.
Methods
Study population and definitions
We screened 120 consecutive patients with ACS who arrived at the emergency room (ER) within 24 h after the onset of symptoms and underwent urgent percutaneous coronary intervention (PCI) between December 2014 and December 2015 at Korea University Anam Hospital. After the culprit lesions of ACS were identified based on coronary angiography findings, OCT examination of the culprit plaques was performed before PCI. Patients who did not undergo urgent PCI or those with cardiogenic shock, pulmonary congestion, fatal arrhythmia, or renal failure on regular hemodialysis were excluded from the study, as were those with inadequate OCT images due to massive residual thrombi or who required pre-dilation before OCT imaging8. As a result, 85 patients with appropriate OCT images were finally analyzed and categorized into two groups, namely, ACS with plaque rupture (Rupture group, n = 42) and ACS without plaque rupture (Non-rupture group, n = 43), verified by OCT8. The study protocol was approved by the Korea University Hospital Institute Review Board (2019AN0170), and written informed consent was obtained from all participants or their legal representatives. The study also complied with the Declaration of Helsinki.
ACS was defined as prolonged typical angina at rest (≥ 20 min) with significant coronary artery lesions confirmed on coronary angiography8. ST-segment elevation myocardial infarction (STEMI) was defined as ACS with new (or presumably new) ST-segment elevation (≥ 0.1 mV) in two or more contiguous leads on electrocardiography and elevated cardiac troponin26. Non-STEMI (NSTEMI) was defined as ACS with new (or presumably new) ST-segment deviation (≥ 0.05 mV) or T-wave inversion (≥ 0.2 mV) in two or more contiguous leads on electrocardiography and elevated cardiac troponin8.
Blood sampling and measurement of biomarkers
Peripheral blood samples were collected from patients with ACS just before the PCI through the femoral or radial artery into a BD SST II Advance Tube (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Blood samples were allowed to clot for 30 min before 15 min of centrifugation at 1500×g. The serum was removed, aliquoted, and stored at − 80 °C prior to the determination of serum sLOX-1, NGAL, and MMP-9 levels using enzyme-linked immunosorbent assay (ELISA). Serum sLOX-1 levels were assessed using the Human LOX-1 ELISA Kit (Cell Biolabs, Inc., San Diego, CA, USA). The lower limit of detection for sLOX-1 was 40 pg/mL27. Total human MMP-9 level was determined using the Quantikine Human MMP-9 Immunoassay (R&D Systems, Inc., Minneapolis, MN, USA). The minimum detectable dose of MMP-9 was 0.156 ng/mL. Intra- and inter-assay coefficients of variation for the MMP-9 immunoassay were 1.9–2.9% and 6.9–7.9%, respectively. Serum NGAL levels were determined using the Quantikine Human Lipocalin-2/NGAL Immunoassay (R&D Systems, Inc.). The minimum detectable dose of NGAL was 0.012 ng/mL. The intra- and inter-assay coefficients of variation for the NGAL immunoassay were 3.1–4.4% and 5.6–7.9%, respectively. Serum high-sensitivity CRP levels were measured using latex-enhanced nephelometry (N-Latex CRP II; Siemens Healthcare Diagnostics, Tokyo, Japan). Serum high-sensitivity troponin I levels were measured using an electrochemiluminescence immunoassay (ECLusys Troponin hs; Roche Diagnostics, Tokyo, Japan). The assays were performed according to the manufacturer’s instructions. Each serum sample was evaluated twice. ELISA-based measurements were obtained using the Versa Max Microplate Reader (Molecular Devices Corporation, Sunnyvale, California, USA) at an optical density of 450 nm. Peripheral blood samples to measure serum creatine kinase-myocardial band (CK-MB) levels were serially obtained every 6 h after ER presentation until the CK-MB value began to decline, using an anti-human CK-MB monoclonal antibody (N-assay L CK-MB Nittobo; Nittobo Medical Co., Ltd., Fukushima, Japan).
OCT image acquisition and analysis
After aspiration of the thrombi using a thrombectomy catheter, whenever possible, the culprit lesion was examined using an OCT imaging catheter. OCT imaging of the culprit lesions (30 mm in length) was acquired with a frequency-domain OCT C7XR system and the Dragon Fly catheter (Lightlab Imaging/St. Jude Medical)8. In the C7XR system, a 2.7-F OCT imaging catheter was carefully advanced distal to the culprit lesion. The automated pullback was performed at 20 mm/s, while blood was displaced by a short injection of contrast media through the guiding catheter. The images were digitally stored for offline analysis. All OCT images were analyzed in the Korea University Anam Hospital core laboratory by two cardiologists (Jae-Young Cho and Hyung Joon Joo) who were blinded to the angiographic data and clinical presentations. When there was discordance between the observers, a consensus was obtained from an independent cardiologist.
Definition of plaque morphology by OCT
Plaque rupture was defined as the presence of a fibrous cap discontinuity and cavity formation in the plaque28. Ruptured plaques usually have an extensive lipid core and a thin fibrous cap. The fibrous cap is the thinnest part at the rupture site, and the plaque cavity indicates the loss of lipid core due to rupture. Plaque erosion was defined as the presence of an irregular luminal surface without plaque rupture in OCT images28. A calcified nodule was defined as the protrusion of a signal-poor or heterogeneous region with a sharply delineated border. A thrombus was defined as a protrusion inside the lumen of the artery with signal attenuation. A white thrombus, which consists mainly of platelets, was identified as signal-rich, low-backscattering protrusions in the OCT image, while red thrombus was identified as high-backscattering protrusions inside the lumen of the artery with signal-free shadowing in the OCT image. Lipid cores were defined as diffusely bordered, signal-poor regions.
Quantitative coronary angiography
Coronary angiography was performed by engaging a Judkins catheter after puncturing the radial artery or femoral artery. Coronary angiograms were analyzed using the Cardiovascular Angiography Analysis System (Pie Medical Imaging B.V., Maastricht, the Netherlands). The reference diameter, minimum lumen diameter, diameter stenosis, area stenosis, and lesion length were measured with a computerized quantitative analyzer using a caliper.
Statistical analysis
Continuous variables are presented as mean ± standard deviation and were compared using Student’s t-test or Wilcoxon test and categorical variables using the chi-square test. Because sLOX-1, high-sensitivity CRP, troponin I, and CK-MB levels were not normally distributed, these values are presented as median and interquartile range (IQR) and were compared using the Wilcoxon test. Correlations between each biomarker level and the peak CK-MB level were analyzed using Spearman’s rank order correlation test. The receiver operating characteristic (ROC) curve was used to assess whether the biomarkers (sLOX-1, NGAL, MMP-9) and white blood cell (WBC) count could differentiate between ruptured and non-ruptured plaques in patients with ACS. An area under the curve (AUC) of 1.0 indicated a test of perfect diagnostic value, whereas an AUC of 0.5 indicated no diagnostic value. The new discriminative model to detect plaque ruptures, which was built on multivariate logistic regression, was a score system ranging from 0 to 1 using a combination of sLOX-1, CK-MB, and MMP-9 levels and WBC count. The model equation formula is described below.
The best cut-off point was calculated using the Youden’s index. When the model ROC curve was statistically significant, the curve was compared with the WBC count and sLox-1, NAGL, and MMP-9 levels. A two-sided p-value of < 0.05 was considered statistically significant. Statistical analysis was performed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
OCT findings in patients with ACS
Plaque morphology findings confirmed by OCT in all 85 patients with ACS are shown in Fig. 1. Plaque rupture (49.4%) was the most prevalent finding, followed by plaque erosion (21.2%). The other findings included tight stenosis (22.4%), calcified nodules (3.5%), spontaneous dissection (2.4%), and intramural hematoma (1.2%).
Figure 1.
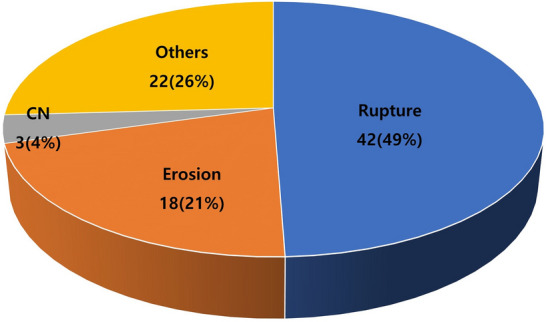
Composition of plaque morphology findings confirmed by OCT (n = 85). CN calcified nodule.
Baseline characteristics
Comparisons of baseline characteristics between the Rupture group and Non-rupture group are summarized in Table 1. Patients in the Rupture group were more likely to be men (88.1 vs. 69.8%, p = 0.0387) and have a clinical diagnosis of myocardial infarction (45.2 vs. 23.3%, p = 0.033) than those in the Non-rupture group. Ages were similar between the groups. Among the risk factors, body mass index (24.0, [22.9–26.7] vs. 23.4, [21.6–26.1] kg/m2, p = 0.0787) and incidence of diabetes mellitus (28.6 vs. 14.0%, p = 0.0991) showed a trend to be higher in the Rupture group but not hypertension, dyslipidemia, and smoking history. WBC count (8.37, [7.04–11.46] vs. 6.82, [5.79–8.8] × 103/μL, p = 0.0098) was higher in the Rupture group, whereas lipid profiles showed no statistical differences between the groups.
Table 1.
Baseline characteristics.
| Rupture group (n = 42) | Non-rupture group (n = 43) | p-value | |
|---|---|---|---|
| Age, years | 60.2 ± 11.5 | 62.7 ± 11.6 | 0.3253 |
| Male sex | 37 (88.1) | 30 (69.8) | 0.0387 |
| BMI, kg/m2 | 24.0 (22.9–26.7) | 23.4 (21.6–26.1) | 0.0787 |
| Clinical diagnosis | 0.0825 | ||
| Unstable angina | 23 (54.8) | 33 (76.7) | |
| NSTEMI | 7 (16.7) | 5 (11.6) | |
| STEMI | 12 (28.6) | 5 (11.6) | |
| Diabetes mellitus | 12 (28.6) | 6 (14.0) | 0.0991 |
| Hypertension | 25 (59.6) | 22 (51.2) | 0.4382 |
| Dyslipidemia | 41 (97.6) | 43 (100.0) | 0.3088 |
| Smoking | 0.1135 | ||
| Smoker | 23 (54.8) | 14 (32.6) | |
| Ex-smoker | 1 (2.4) | 1 (2.3) | |
| Never smoker | 18 (42.9) | 28 (65.1) | |
| Previous PCI | 8 (19.1) | 7 (16.3) | 0.7378 |
| Previous CVA | 1 (2.4) | 3 (4.0) | 0.3171 |
| Creatinine, mg/dL | 1.05 (0.82–1.2) | 0.95 (0.83–1.06) | 0.1112 |
| Pro BNP, pg/mL | 158.5 (44.1–709.6) | 81.3 (19.5–610.3) | 0.4824 |
| WBC, × 103/μL | 8.37 (7.04–11.46) | 6.82 (5.79–8.8) | 0.0098 |
| Ejection fraction, % | 60 (50–60) | 60 (57–60) | 0.1222 |
| Total cholesterol, mg/dL | 172 (59–213) | 168 (130.5–201) | 0.8109 |
| LDL-cholesterol, mg/dL | 113 (101–159) | 111.5 (96–151) | 0.5698 |
| HDL-cholesterol, mg/dL | 44.5 (35.5–83) | 49 (41–62) | 0.4223 |
| Triglyceride, mg/dL | 116 (89–169) | 97 (66–138) | 0.1403 |
Data are presented as mean ± SD or median (interquartile range) or n (percentage).
BMI body mass index, NSTEMI non-ST-segment elevation myocardial infarction, STEMI ST-segment elevation myocardial infarction, PCI percutaneous coronary intervention, CVA cerebrovascular accidents, BNP B-type natriuretic peptide, WBC white blood cell, LDL low-density lipoprotein, HDL high-density lipoprotein.
Comparison of biomarker levels between Rupture group and Non-rupture group
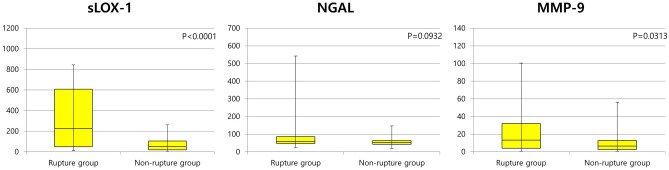
Different levels of various biomarkers within each group are shown in Table 2. Median values of the peak CK-MB (13.39 [2.23–182.8] vs. 2.69 [1.65–5.19] ng/mL, p = 0.0016), sLOX-1 (227.87 [49.45–607.3] vs. 51.7 [19.6–104.3] pg/mL, p < 0.0001), and MMP-9 (13.36 [4.12–32.06] vs. 6.45 [2.56–12.94] ng/mL, p = 0.0313) levels were significantly higher in the Rupture group (Fig. 2). Scattered plot graph of peak CK-MB between two groups are presented in Supplementary Fig. S1. NGAL level (59.0 [47.2–85.9] vs. 53.1 [43.0–63.7] ng/mL, p = 0.0932) was numerically higher in the Rupture group without statistical significance. Troponin I and high-sensitivity CRP levels were not different between the groups. This trend was maintained even when comparing Non-rupture group and Rupture group only in MI patients. Comparing biomarker levels between Non-rupture group and Rupture group in patients with MI are presented in Supplementary Table S1.
Table 2.
Comparison of biomarker levels between Non-rupture group and Rupture group in patients with ACS.
| Rupture group (n = 42) | Non-rupture group (n = 43) | p-value | |
|---|---|---|---|
| Troponin I (ng/mL) | 0.21 (0.12–5.82) | 0.19 (0.1–0.92) | 0.7244 |
| Peak CK-MB (ng/mL) | 13.39 (2.23–182.8) | 2.69 (1.65–5.19) | 0.0016 |
| High-sensitivity CRP (mg/L) | 1.35 (0.62–3.94) | 1.08 (0.63–4.13) | 0.6635 |
| sLOX-1 (pg/mL) | 227.87 (49.45–607.3) | 51.7 (19.6–104.3) | < .0001 |
| NGAL (ng/mL) | 59.0 (47.2–85.9) | 53.1 (43.0–63.7) | 0.0932 |
| MMP-9 (ng/mL) | 13.36 (4.12–32.06) | 6.45 (2.56–12.94) | 0.0313 |
Data are presented as median (interquartile range).
ACS acute coronary syndrome, CK-MB creatine kinase-muscle/brain, CRP C-reactive protein, sLOX-1 soluble lectin-like oxidized low-density lipoprotein receptor-1, NGAL neutrophil gelatinase-associated lipocalin, MMP-9 matrix metalloproteinase-9.
Figure 2.
Comparison of sLOX-1, NGAL, and MMP-9 levels between the Rupture group and Non-rupture group. sLOX-1 soluble lectin-like oxidized low-density lipoprotein receptor-1, NGAL neutrophil gelatinase-associated lipocalin, MMP-9 matrix metalloproteinase-9.
Correlation between peak CK-MB and biomarker levels
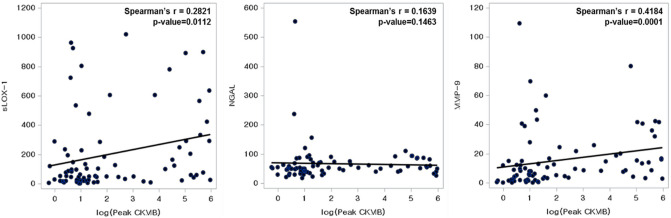
A correlation between the peak CK-MB and biomarker levels was observed (Fig. 3). sLOX-1 (Spearman ρ = 0.2821, p = 0.011) and MMP-9 (Spearman ρ = 0.418, p = 0.0001) levels, but not NGAL (Spearman ρ = 0.164, p = 0.146) level, were significantly and proportionally correlated with the peak CK-MB level.
Figure 3.
Correlation between the peak CK-MB level and biomarker levels. CK-MB creatine kinase-muscle/brain, sLOX-1 soluble lectin-like oxidized low-density lipoprotein receptor-1, NGAL neutrophil gelatinase-associated lipocalin, MMP-9 matrix metalloproteinase-9.
A new discriminative model using sLOX-1, MMP-9, and peak CK-MB and WBC to differentiate Rupture group from Non-rupture group
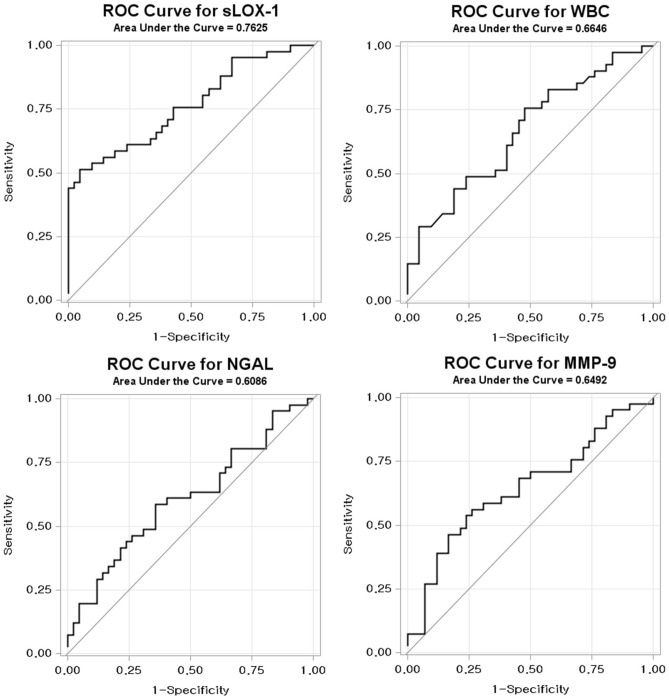
The ROC curve analysis for sLOX-1, NGAL, MMP-9, and WBC to detect Rupture group using Non-rupture group as a negative reference is shown in Fig. 4. The AUCs were 0.763 (p < 0.0001), 0.645 (p = 0.0274), 0.609 (p = 0.0874), 0.665 (p = 0.0016), and 0.701 (p = 0.0007) for sLOX-1, MMP-9, NGAL, WBC, and the peak CK-MB level, respectively. The optimal cut-off values were 236.4 pg/mL (50% sensitivity and 92.9% specificity), 12.7 ng/mL (54.8% sensitivity and 74.4% specificity), 56.0 ng/mL (57.1% sensitivity and 65.1 specificity), and 6.900/µL (75.6% sensitivity and 52.4% specificity) for sLOX-1, MMP-9, NGAL, and WBC count, respectively. The AUC value of the new discriminative model using LOX-1, MMP-9, WBC count, and the peak CK-MB level in combination was 0.843 (p < 0.0001), and the optimal cut-off value was 0.614, which was calculated using the Youden’s index (62.2% sensitivity and 97.6% specificity; Fig. 5). The ROC curve of the new predictive model to detect Rupture group in patients with ACS was superior to sLOX-1 (p = 0.0346), MMP-9 (p = 0.0002), NAGL (p = 0.0004), WBC count (p = 0.0016), and peak CK-MB level alone (p = 0.0199; Table 3).
Figure 4.
Receiver operating characteristic (ROC) curves for differentiating Rupture group from Non-rupture group. sLOX-1 soluble lectin-like oxidized low-density lipoprotein receptor-1, WBC white blood cell; NGAL neutrophil gelatinase-associated lipocalin, MMP-9 matrix metalloproteinase-9.
Figure 5.
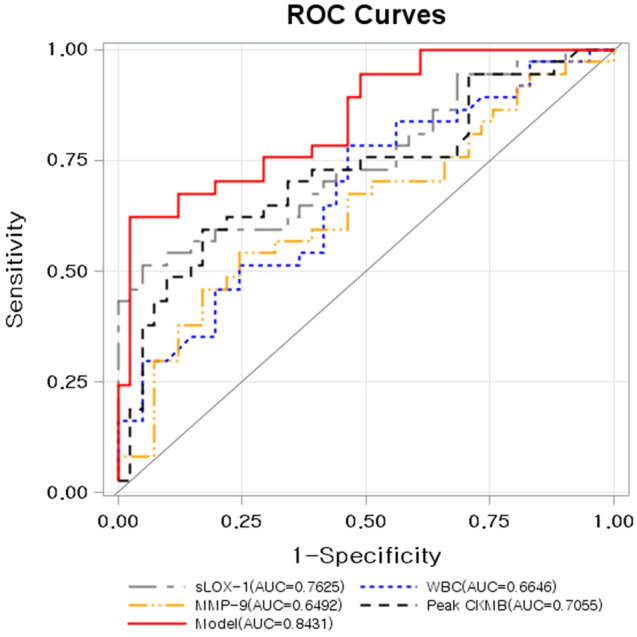
Receiver operating characteristics (ROC) curves displaying the additional discriminatory ability of the new predictive model using sLOX-1, MMP-9, and peak CK-MB levels and WBC count in combination for differentiating Rupture group from Non-rupture group. AUC area under the curve, sLOX-1 soluble lectin-like oxidized low-density lipoprotein receptor-1, MMP-9 matrix metalloproteinase-9, CK-MB creatine kinase-muscle/brain; and WBC white blood cell.
Table 3.
Comparison between the new predictive model and each biomarker in terms of their power in differentiating Rupture group from Non-rupture group in patients with ACS.
| AUC (95% CI) | Cut-off point | Sensitivity | Specificity | p-value | |
|---|---|---|---|---|---|
| Model | 0.843 (0.757, 0.929) | 0.61 | 62.2 | 97.6 | – |
| sLOX-1 | 0.763 (0.660, 0.865) | 236.4 | 50 | 95.35 | 0.0346 |
| WBC | 0.665 (0.548, 0.781) | 6.9 | 75.61 | 52.38 | 0.0016 |
| NGAL | 0.609 (0.486, 0.731) | 56.0 | 57.14 | 65.12 | 0.0004 |
| MMP-9 | 0.645 (0.524, 0.766) | 12.7 | 54.76 | 74.42 | 0.0002 |
| Peak CK-MB | 0.706 (0.587, 0.824) | 7.3 | 57.9 | 83.3 | 0.0199 |
The cut-off point is calculated using the Youden’s index.
ACS acute coronary syndrome, AUC area under the curve, sLOX-1 soluble lectin-like oxidized low-density lipoprotein receptor-1, WBC white blood cell, NGAL neutrophil gelatinase-associated lipocalin, MMP-9 matrix metalloproteinase-9, CK-MB creatine kinase-muscle/brain.
p-values are the comparison results between new predictive model and each biomarker.
Discussion
Study findings
This study investigated the association between circulating plasma biomarkers and plaque characteristics defined by OCT in patients with ACS. Consequently, we demonstrated that plasma sLOX-1 and MMP-9 levels, but not NGAL levels, were higher in Rupture group than Non-rupture group. In addition, we evaluated a new discriminative model comprising several biomarkers, cardiac enzymes, and WBC count to differentiate Rupture group from Non-rupture group, which displayed an AUC of 0.843 (p < 0.0001). When this model was compared with each biomarker, it showed an improved discriminatory ability to differentiate Rupture group from Non-rupture group.
The major findings of this study were as follows: (1) conventional cardiac biomarkers, including troponin I and high-sensitivity CRP, cannot differentiate between ACS with and without plaque ruptures, which is in line with previous findings8; (2) sLOX-1, MMP-9, and the peak CK-MB levels and WBC count have a potential to differentiate Rupture group from Non-rupture group, and among them, sLOX-1 has the greatest discriminatory ability; (3) a new discriminative model, developed by combining sLOX-1, MMP-9, and peak CK-MB levels with WBC count, could better discriminate plaque ruptures than individual biomarkers; (4) higher sLOX-1 and MMP-9 levels were well correlated with a higher peak CK-MB level and WBC count, which may reflect increased myocardial damage and subsequent inflammatory reaction in Rupture group than Non-rupture group; and (5) NGAL had no discriminatory power between Rupture group and Non-rupture group, although it is believed to be associated with poor clinical outcomes in ACS29.
Biomarkers and ACS
The peak CK-MB level represents the amount of myocardial damage in ACS, which is associated with worsened long-term prognosis. In the present study, sLOX-1 and MMP-9 were significantly correlated with the peak CK-MB level, whereas NGAL was not well correlated.
MMPs, a family of zinc-containing endoproteinases, degrade the extracellular matrix components30. Vascular smooth muscle cells, macrophages, and endothelial cells express MMPs in accordance with inflammatory stimuli and oxidative stress. MMP-9 advances breakdown of the thin fibrous caps of plaques by elastic degradation, which indicates atherosclerotic plaque rupture or vulnerability9, 10. A previous study has shown that patients with polymorphisms of the MMP-9 promotor have elevated MMP-9 expression, which was associated with acute myocardial infarction31. Many other studies have revealed that patients with ACS have significantly higher MMP-9 levels, with the highest in patients with STEMI, than in those with stable angina or healthy controls9, 32–35.
However, Nishiguchi et al. reported that systemic MMP-9 level in patients with plaque rupture was equivalent to the level without plaque rupture36. In their study, local MMP-9 was higher in the plaque rupture group. Since the concentration of systemic MMP-9 was much dilute and lower than that of local MMP-9, more samples might have been needed to show statistical differences at lower concentrations. Also, there was a difference in the time taken for sampling between the present study and one by Nishiguchi et al., which may have influenced the results.
Several other studies have reported robust associations between MMP-9 level and the subsequent risk of cardiovascular complications, such as cardiovascular mortality and nonfatal myocardial infarction. Therefore, MMP-9 may act as not only a causative agent on plaque destabilization but also a circulating marker reflecting a proinflammatory state37, 38.
Plasma oxidized LDL cholesterol levels are related to the thrombogenicity of coronary lesions in patients with unstable angina39. LOX-1, a scavenger receptor for the uptake of atherogenic oxidized LDL in the arterial wall, is plentifully expressed in advanced atherosclerotic plaque and plays an important role in the development of oxidative stress and inflammation40, 41. When LOX-1 is cleaved by proteases at its proximal membrane extracellular domain following acute myocardial ischemia, sLOX-1 is released into the circulation42, 43. sLOX-1 may arise from activated platelets, atheroma, or endothelial cells40. Therefore, circulating sLOX-1 levels are increased in patients with ACS. As a result, plasma sLOX-1 levels are a useful biomarker of ACS44.
Taken together, MMP-9 is a causal proteinase that may contribute to plaque rupture by eroding or weakening a plaque cap and sLOX-1 is a consequent product of plaque rupture; thus, the two biomarkers directly reflect plaque rupture, and of the two, sLOX-1 has a stronger correlation with plaque rupture than MMP-9 in our study.
NGAL, a 25 kDa glycoprotein isolated from the granule of mature neutrophils, covalently binds to MMP-9 and increases MMP-9 activity45, 46. NGAL is expressed in smooth muscle cells, macrophages, and endothelial cells in atherosclerotic plaques11, 47. Several studies have shown increased NGAL levels in CAD, which imply the involvement of NGAL in the atherosclerotic process14, 48. In addition, a study has shown that NGAL levels were positively correlated with lesion complexity and the severity of CAD in patients with ACS49. However, NGAL binding to MMP-9 increases the rate of MMP-9/tissue inhibitor of metalloproteinase46. This stromal factor, the tissue inhibitor of metalloproteinase, inhibits the activity of MMP-9. Thus, NGAL activation plays as a modulator and leads to increased MMP-9 activity and plaque instability rather than plaque rupture itself. One study reported that NGAL is associated with long-term prognosis in patients with acute myocardial infarction because it has no direct relevance with plaque rupture but with plaque destabilization29.
Clinical implications of the identification of plaque rupture in patients with ACS
Several studies have proved that plaque rupture is the main cause of ACS and worsens the prognosis compared to Non-rupture group19, 50, 51. Plaque rupture is indeed the most common cause of coronary thrombosis, found in nearly 50% of patients with ACS, while plaque erosion is found in up to one-third of patients with ACS15–17. The other underlying mechanisms of ACS include calcified plaque, tight stenosis, intramural hematoma, and spontaneous dissection. Plaque rupture is often associated with positive remodeling, large plaque burden, and red thrombus52. Large plaque burdens in patients with plaque rupture may lead to residual plaque after PCI, which is a known predictor of stent failure19. Also, a previous study reported that patients with plaque rupture may have diffuse vulnerable features throughout the whole coronary tree18. These mechanisms together may reflect the worsened prognoses in patients with plaque rupture. On the other hand, plaque erosion is associated with negative remodeling, modest plaque burden, white thrombus, uncommon features of a fibroatheroma, and proximal distribution52. Given these findings, it may be reasonable to treat patients with plaque erosion by effective antithrombotic treatment without early invasive stent implantation53.
However, no single biomarker can successfully differentiate plaque rupture from non-plaque rupture in patients with ACS. Intravascular imaging tools such as OCT have advantages in detecting plaque rupture in the catheterization laboratory setting but have limitations in their usage due to the requirement of invasive procedures, such as coronary angiogram, low accessibility, high cost, possible complication risks, and time consumption. Therefore, discriminating ruptured and non-ruptured plaques using novel models comprising biomarkers without invasive procedures would be valuable for rapid risk stratification and predicting the prognoses of patients with ACS. At present, since new biomarkers including sLOX-1, MMP-9 are not established as routine laboratory tests, it may take considerable time to calculate this novel discriminative model and apply it to the treatment strategy of ACS patients. In our study, as described in the Methods section, it took about an hour to obtain each biomarker value after blood sampling. Calculating model and decision-making process would be faster if biomarker values are obtained though vein sampling as soon as patients with suspected ACS arrives at the emergency room. However, assumption of that values of biomarkers are similar between arterial sampling and venous sampling should be confirmed in future studies. With future technical advances to make inflammatory marker testing easier, more accurate and faster than it is today, we expect that the model developed in this study can be useful for identifying plaque ruptures quickly in bed-side setting and establishing treatment strategy accordingly. By quickly suspecting plaque rupture in an ACS setting using this discriminative model, the decision-making process for early invasive treatment strategies might be initiated earlier and more aggressive approach to use intravascular imaging tool to get stent optimization would be recommended, thereby improving the likelihood of better clinical outcomes. We believe that the present study is meaningful as it shows the possibility to create a more powerful model that can identify plaque rupture in patients with ACS using the existing biomarkers and laboratory data without invasive imaging tools.
Limitations
This study has several limitations. First, this study has all inherent limitations of a small-sized observational study. Second, we excluded patients requiring pre-dilation before OCT imaging. Third, because OCT signals may be interfered with by residual microthrombi, patients in the Non-rupture group may have had minor plaque rupture that was undetectable by OCT. Fourth, risk factors including sex, diabetes mellitus, and body mass index, which were different in both groups, may have affected the results of biomarkers. Fifth, although most sampling was performed just before the PCI procedure, the exact timing of blood sampling was not specified. Therefore, the effect of ischemic time on blood concentrations of biomarker could not be corrected because the time from symptom onset to sampling could not be accurately determined. However, because this study was conducted at a single institution with similar practices in treating patients with ACS and there was no difference in the ischemic time between the groups, the time from ischemic onset to sampling was likely to be similar in both groups. Finally, because blood sampling was performed through the femoral or radial artery rather than the coronary artery, blood concentrations of these biomarkers may have been affected by vascular beds other than the coronary artery. Therefore, sampling through the coronary artery is necessary to avoid this confounding factor in future research.
In conclusion, the new discriminative model using sLOX-1, MMP-9, and peak CK-MB levels and WBC count may identify plaque ruptures in an ACS setting. Further larger-sized prospective studies to externally validate the findings of this study are warranted.
Supplementary information
Acknowledgements
The authors also would like to thank Yeunjin Park for her excellent assistance for English editing.
Author contributions
H.K. contributed to data analysis and interpretation and drafting the manuscript. D.H.J., J-Y.C., H.J.J., J.H.P. and S.J.H. contributed to data collection, interpretation and discussion of the study. J-H.K. contributed to the design concept. S.-A.C. contributed to the statistical analysis. D.-S.L. and C.W.Y. equally contributed to the study conception and design, data collection and interpretation, and revising and finalizing approval of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cheol Woong Yu, Email: ycw717@naver.com.
Do-Sun Lim, Email: dslmd@kumc.or.kr.
Supplementary information
is available for this paper at 10.1038/s41598-020-77413-3.
References
- 1.Mozaffarian D, et al. Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Mandelzweig L, et al. The second Euro Heart Survey on acute coronary syndromes: characteristics, treatment, and outcome of patients with ACS in Europe and the Mediterranean Basin in 2004. Eur. Heart J. 2006;27:2285–2293. doi: 10.1093/eurheartj/ehl196. [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 4.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 5.James SK, et al. Troponin and C-reactive protein have different relations to subsequent mortality and myocardial infarction after acute coronary syndrome: a GUSTO-IV substudy. J. Am. Coll. Cardiol. 2003;41:916–924. doi: 10.1016/S0735-1097(02)02969-8. [DOI] [PubMed] [Google Scholar]
- 6.Makrygiannis SS, et al. Prognostic usefulness of serial C-reactive protein measurements in ST-elevation acute myocardial infarction. Am. J. Cardiol. 2013;111:26–30. doi: 10.1016/j.amjcard.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 7.Sabatine MS, et al. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 2007;115:1528–1536. doi: 10.1161/CIRCULATIONAHA.106.649939. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi N, et al. Soluble lectin-like oxidized LDL receptor-1 (sLOX-1) as a valuable diagnostic marker for rupture of thin-cap fibroatheroma: verification by optical coherence tomography. Int. J. Cardiol. 2013;168:3217–3223. doi: 10.1016/j.ijcard.2013.04.110. [DOI] [PubMed] [Google Scholar]
- 9.Kai H, et al. Peripheral blood levels of matrix metalloproteases-2 and -9 are elevated in patients with acute coronary syndromes. J. Am. Coll. Cardiol. 1998;32:368–372. doi: 10.1016/S0735-1097(98)00250-2. [DOI] [PubMed] [Google Scholar]
- 10.Loftus IM, et al. Increased matrix metalloproteinase-9 activity in unstable carotid plaques. A potential role in acute plaque disruption. Stroke. 2000;31:40–47. doi: 10.1161/01.STR.31.1.40. [DOI] [PubMed] [Google Scholar]
- 11.Hemdahl AL, et al. Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2006;26:136–142. doi: 10.1161/01.ATV.0000193567.88685.f4. [DOI] [PubMed] [Google Scholar]
- 12.Bu DX, et al. Induction of neutrophil gelatinase-associated lipocalin in vascular injury via activation of nuclear factor-kappaB. Am. J. Pathol. 2006;169:2245–2253. doi: 10.2353/ajpath.2006.050706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta K, Shukla M, Cowland JB, Malemud CJ, Haqqi TM. Neutrophil gelatinase-associated lipocalin is expressed in osteoarthritis and forms a complex with matrix metalloproteinase 9. Arthritis Rheum. 2007;56:3326–3335. doi: 10.1002/art.22879. [DOI] [PubMed] [Google Scholar]
- 14.Zografos T, et al. Association of neutrophil gelatinase-associated lipocalin with the severity of coronary artery disease. Am. J. Cardiol. 2009;104:917–920. doi: 10.1016/j.amjcard.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 15.Cheruvu PK, et al. Frequency and distribution of thin-cap fibroatheroma and ruptured plaques in human coronary arteries: a pathologic study. J. Am. Coll. Cardiol. 2007;50:940–949. doi: 10.1016/j.jacc.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 16.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000;20:1262–1275. doi: 10.1161/01.ATV.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 17.Arbustini E, et al. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart. 1999;82:269–272. doi: 10.1136/hrt.82.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vergallo R, et al. Pancoronary plaque vulnerability in patients with acute coronary syndrome and ruptured culprit plaque: a 3-vessel optical coherence tomography study. Am. Heart J. 2014;167:59–67. doi: 10.1016/j.ahj.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Niccoli G, et al. Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur. Heart J. 2015;36:1377–1384. doi: 10.1093/eurheartj/ehv029. [DOI] [PubMed] [Google Scholar]
- 20.Jang IK, et al. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation. 2005;111:1551–1555. doi: 10.1161/01.CIR.0000159354.43778.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubo T, et al. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J. Am. Coll. Cardiol. 2007;50:933–939. doi: 10.1016/j.jacc.2007.04.082. [DOI] [PubMed] [Google Scholar]
- 22.Kubo T, Akasaka T. Optical coherence tomography imaging: current status and future perspectives: current and future developments in OCT. Cardiovasc. Interv. Ther. 2010;25:2–10. doi: 10.1007/s12928-009-0006-3. [DOI] [PubMed] [Google Scholar]
- 23.Bezerra HG, Costa MA, Guagliumi G, Rollins AM, Simon DI. Intracoronary optical coherence tomography: a comprehensive review clinical and research applications. JACC Cardiovasc. Interv. 2009;2:1035–1046. doi: 10.1016/j.jcin.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka A, et al. Morphology of exertion-triggered plaque rupture in patients with acute coronary syndrome: an optical coherence tomography study. Circulation. 2008;118:2368–2373. doi: 10.1161/CIRCULATIONAHA.108.782540. [DOI] [PubMed] [Google Scholar]
- 25.Kubo T, et al. Comparison of vascular response after sirolimus-eluting stent implantation between patients with unstable and stable angina pectoris: a serial optical coherence tomography study. JACC Cardiovasc. Imaging. 2008;1:475–484. doi: 10.1016/j.jcmg.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Ibanez B, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2017 doi: 10.1093/eurheartj/ehx393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura M, et al. Generation of monoclonal antibodies against a soluble form of lectin-like oxidized low-density lipoprotein receptor-1 and development of a sensitive chemiluminescent enzyme immunoassay. J. Pharm. Biomed. Anal. 2010;51:158–163. doi: 10.1016/j.jpba.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 28.Tearney GJ, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for intravascular optical coherence tomography standardization and validation. J. Am. Coll. Cardiol. 2012;59:1058–1072. doi: 10.1016/j.jacc.2011.09.079. [DOI] [PubMed] [Google Scholar]
- 29.Lindberg S, et al. Prognostic utility of neutrophil gelatinase-associated lipocalin in predicting mortality and cardiovascular events in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J. Am. Coll. Cardiol. 2012;60:339–345. doi: 10.1016/j.jacc.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Dollery CM, McEwan JR, Henney AM. Matrix metalloproteinases and cardiovascular disease. Circ. Res. 1995;77:863–868. doi: 10.1161/01.RES.77.5.863. [DOI] [PubMed] [Google Scholar]
- 31.Kim PJ, et al. Functional polymorphism in the promoter region of matrix metalloproteinase-9 is strongly associated with acute myocardial infarction. Korean Circ. J. 2005;35:192–196. doi: 10.4070/kcj.2005.35.2.192. [DOI] [Google Scholar]
- 32.Hamed GM, Fattah MF. Clinical relevance of matrix metalloproteinase 9 in patients with acute coronary syndrome. Clin. Appl. Thromb. Hemost. 2015;21:705–711. doi: 10.1177/1076029614567309. [DOI] [PubMed] [Google Scholar]
- 33.Tanindi A, Sahinarslan A, Elbeg S, Cemri M. Relationship between MMP-1, MMP-9, TIMP-1, IL-6 and risk factors, clinical presentation, extent and severity of atherosclerotic coronary artery disease. Open Cardiovasc. Med. J. 2011;5:110–116. doi: 10.2174/1874192401105010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo C, et al. Correlation between the severity of coronary artery lesions and levels of estrogen, hs-CRP and MMP-9. Exp. Ther. Med. 2014;7:1177–1180. doi: 10.3892/etm.2014.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nurkic J, Ljuca F, Nurkic M, Jahic E, Jahic M. Biomarkers of plaque instability in acute coronary syndrome patients. Med. Arh. 2010;64:103–106. [PubMed] [Google Scholar]
- 36.Nishiguchi T, et al. Local matrix metalloproteinase 9 level determines early clinical presentation of ST-segment-elevation myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2016;36:2460–2467. doi: 10.1161/ATVBAHA.116.308099. [DOI] [PubMed] [Google Scholar]
- 37.Blankenberg S, et al. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107:1579–1585. doi: 10.1161/01.CIR.0000058700.41738.12. [DOI] [PubMed] [Google Scholar]
- 38.Jonsson S, et al. Increased levels of leukocyte-derived MMP-9 in patients with stable angina pectoris. PLoS ONE. 2011;6:e19340. doi: 10.1371/journal.pone.0019340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamashita H, et al. Elevated plasma levels of oxidized low-density lipoprotein relate to the presence of angiographically detected complex and thrombotic coronary artery lesion morphology in patients with unstable angina. Circ. J. 2007;71:681–687. doi: 10.1253/circj.71.681. [DOI] [PubMed] [Google Scholar]
- 40.Pothineni NVK, et al. LOX-1 in atherosclerosis and myocardial ischemia: biology, genetics, and modulation. J. Am. Coll. Cardiol. 2017;69:2759–2768. doi: 10.1016/j.jacc.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 41.Sawamura T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 42.Murase T, et al. Identification of soluble forms of lectin-like oxidized LDL receptor-1. Arterioscler. Thromb. Vasc. Biol. 2000;20:715–720. doi: 10.1161/01.ATV.20.3.715. [DOI] [PubMed] [Google Scholar]
- 43.Mitsuoka H, et al. Interleukin 18 stimulates release of soluble lectin-like oxidized LDL receptor-1 (sLOX-1) Atherosclerosis. 2009;202:176–182. doi: 10.1016/j.atherosclerosis.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Hayashida K, et al. Serum soluble lectin-like oxidized low-density lipoprotein receptor-1 levels are elevated in acute coronary syndrome: a novel marker for early diagnosis. Circulation. 2005;112:812–818. doi: 10.1161/CIRCULATIONAHA.104.468397. [DOI] [PubMed] [Google Scholar]
- 45.Kjeldsen L, Bainton DF, Sengelov H, Borregaard N. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood. 1994;83:799–807. doi: 10.1182/blood.V83.3.799.799. [DOI] [PubMed] [Google Scholar]
- 46.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J. Biol. Chem. 2001;276:37258–37265. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- 47.Cruz DN, Gaiao S, Maisel A, Ronco C, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of cardiovascular disease: a systematic review. Clin. Chem. Lab. Med. 2012;50:1533–1545. doi: 10.1515/cclm-2012-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi KM, et al. Implication of lipocalin-2 and visfatin levels in patients with coronary heart disease. Eur. J. Endocrinol. 2008;158:203–207. doi: 10.1530/EJE-07-0633. [DOI] [PubMed] [Google Scholar]
- 49.Soylu K, et al. Serum neutrophil gelatinase-associated lipocalin levels are correlated with the complexity and the severity of atherosclerosis in acute coronary syndrome. Anatol. J. Cardiol. 2015;15:450–455. doi: 10.5152/akd.2014.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davies MJ. Anatomic features in victims of sudden coronary death. Coronary artery pathology. Circulation. 1992;85:19–24. [PubMed] [Google Scholar]
- 51.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 52.Kwon JE, et al. Multimodality intravascular imaging assessment of plaque erosion versus plaque rupture in patients with acute coronary syndrome. Korean Circ. J. 2016;46:499–506. doi: 10.4070/kcj.2016.46.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jia H, et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J. Am. Coll. Cardiol. 2013;62:1748–1758. doi: 10.1016/j.jacc.2013.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.