Abstract
Transcranial direct current stimulation has been shown to increase the efficiency of language therapy in chronic aphasia; however, to date, an optimal stimulation site has not been identified. We investigated whether neuromodulation of the right cerebellum can improve naming skills in chronic aphasia. Using a randomized, double-blind, sham-controlled, within-subject crossover study design, participants received anodal cerebellar stimulation (n = 12) or cathodal cerebellar stimulation (n = 12) + computerized aphasia therapy then sham + computerized aphasia therapy, or the opposite order. There was no significant effect of treatment (cerebellar stimulation versus sham) for trained naming. However, there was a significant order x treatment interaction, indicating that cerebellar stimulation was more effective than sham immediately post-treatment for participants who received cerebellar stimulation in the first phase. There was a significant effect of treatment (cerebellar stimulation versus sham) for untrained naming immediately post-treatment and the significant improvement in untrained naming was maintained at two months post-treatment. Greater gains in naming (relative to sham) were noted for participants receiving cathodal stimulation for both trained and untrained items. Thus, our study provides evidence that repetitive cerebellar transcranial direct stimulation combined with computerized aphasia treatment can improve picture naming in chronic post-stroke aphasia. These findings suggest that the right cerebellum might be an optimal stimulation site for aphasia rehabilitation and this could be an answer to handle heterogeneous participants who vary in their size and site of left hemisphere lesions.
Keywords: cerebellum, aphasia, language therapy, stroke, transcranial direct current stimulation
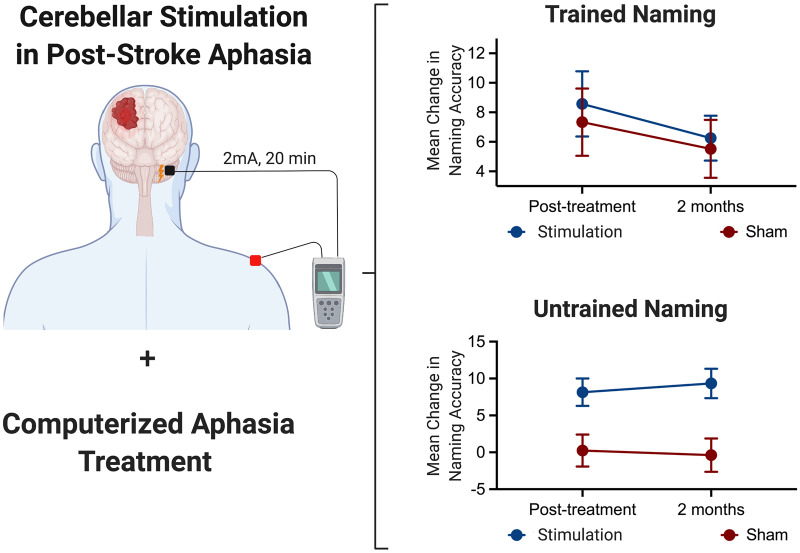
Using a randomized, double-blind, sham-controlled, within-subject crossover study design, Sebastian et al. investigated whether cerebellar transcranial direct current stimulation combined with computerized aphasia treatment can improve picture naming in chronic aphasia. Results indicate significant improvement in untrained naming and trained naming (only for participants who received stimulation first) compared to sham.
Graphical Abstract
Graphical Abstract.
Introduction
Anomia, or difficulty with naming, is the most common deficit in individuals with post-stroke aphasia, adversely impacting daily functioning and quality of life (Hilari et al., 2012). Transcranial direct current stimulation (tDCS) is a promising treatment modality that has been shown to increase the efficiency of anomia treatment in post-stroke aphasia (for reviews see Holland and Crinion, 2012; de Aguiar et al., 2015; Sandars et al., 2016; Bucur and Papagno, 2019; Elsner et al., 2019; Breining and Sebastian, 2020). A majority of the previous tDCS studies focusing on anomia treatment have targeted the intact perilesional left hemisphere regions. With respect to the stimulation locations, studies have targeted the left dorsolateral prefrontal cortex (e.g. Pestalozzi et al., 2018), left inferior frontal gyrus (e.g. Campana et al., 2015; Spielmann et al., 2018), left motor cortex (e.g. Meinzer et al., 2016; Darkow et al., 2017), left posterior perisylvian region (Wu et al., 2015), individualized optimal stimulation location using behavioural experiments prior to treatment (Shah-Basak et al., 2015) or individualized stimulation location on the basis of pre-treatment functional MRI (Baker et al., 2010; Fridriksson et al., 2011, 2018, 2019).
Individualizing stimulation location based on functional MRI can lead to substantial gains in language performance over that of treatment alone (sham) as demonstrated in a large randomized clinical trial (Fridriksson et al., 2018, 2019); however, it is cost-intensive and requires substantial technological expertise, thereby limiting the incorporation of tDCS in therapy clinics. Encephalomalacia at the lesion site also makes directly targeting the perilesional cortex difficult. The shunting of electrical current through the area of encephalomalacia may also result in unpredictable effects that vary from person to person (Turkeltaub et al., 2016). Targeting right hemisphere language homologs is an alternative approach, although the role of the right hemisphere in aphasia recovery is still hotly debated. It remains unclear whether excitation or inhibition is the preferred strategy for the right hemisphere neuromodulation (Anglade et al., 2014; Gainotti, 2015).
The right cerebellum could potentially be an optimal site for tDCS treatment in post-stroke aphasia. Multiple lines of evidence suggest that the right cerebellum is involved in a variety of cognitive and language functions, including word retrieval and generation, verbal working memory, language learning and semantic processing (for reviews see Desmond and Fiez, 1998; Murdoch, 2010; Stoodley, 2012; Keren-Happuch et al., 2014; Mariën et al., 2014; Mariën, 2017). The right cerebellum is distant enough from typical stroke locations associated with aphasia that electrical current flow patterns are unlikely to be affected by the encephalomalacia. Our group did a modelling study to understand the electric field distribution of the right cerebellar tDCS (Sebastian et al., 2017). The results indicated that the maximum electric field amplitude was generated in the right cerebellum with some spread to the left cerebellum but without spread to the adjacent occipital cortex or other cortical regions. Therefore, the right cerebellum might provide a structurally intact gateway to the affected neural networks of the cerebrum (Wessel and Hummel, 2018). Wessel and Hummel (2018) argue that cerebellar stimulation could be an answer to handle the heterogeneous features of stroke. By targeting the cerebellum, the same protocol can be used in different patient populations, leading to better patient stratification.
Cerebellar tDCS studies in healthy individuals provide evidence that tDCS to the right cerebellum can modulate language functions. Pope and Miall (2012) reported that right cerebellar cathodal tDCS improved performance on a verb generation task relative to anodal and sham tDCS. Turkeltaub et al. (2016) showed that both anodal and cathodal cerebellar tDCS improved verbal fluency performance, with a more robust effect for anodal tDCS. In addition, Turkeltaub et al. show that cerebellar tDCS can modulate connectivity between the right cerebellum and the left hemisphere language regions (Turkeltaub et al., 2016; D'Mello et al., 2017).
With respect to post-stroke aphasia and cerebellar tDCS, a case study by Sebastian et al. (2017) showed that both anodal cerebellar tDCS and sham tDCS coupled with language treatment resulted in improved spelling to dictation for trained and untrained words immediately after and 2 months post-treatment. However, the improvement was greater with anodal tDCS than with sham, especially for untrained items. Further, generalization to written picture naming was noted only with tDCS. In another study, Marangolo et al. (2018) investigated the effect of cerebellar tDCS coupled with language treatment in improving performance in a verb generation task in patients with aphasia. They used a randomized, double-blind, crossover study design. Each participant received cerebellar tDCS in four experimental conditions (right cathodal and sham stimulations during verb generation and verb naming tasks). Significant improvement was found only in the verb generation task following cathodal stimulation. The authors hypothesized that cerebellar tDCS is a viable tool for recovery from aphasia, particularly when the language task also demands the activation of nonlinguistic strategies, as in the case of the verb generation task, which requires executive and memory components.
Based on promising results of cerebellar tDCS in improving language skills in healthy controls and stroke participants with aphasia, we investigated whether tDCS to the right cerebellum coupled with computerized aphasia treatment can improve naming performance in individuals with chronic aphasia. Additionally, we investigated whether there are any differences in anodal versus cathodal cerebellar tDCS on naming performance as prior studies have shown beneficial language effects for anodal and cathodal cerebellar stimulation (Pope and Miall, 2012, Turkeltaub et al., 2016; Sebastian et al., 2017, Marangolo et al., 2018).
Materials and methods
Study design
A randomized, double-blind, sham-controlled, within-subject crossover study design was utilized. Participants who met eligibility criteria were randomly assigned using block randomization with a ratio of 1:1 to group anode or group cathode. Within each group, participants were randomly assigned to receive either ‘tDCS (tDCS first) then sham (sham second)’ or ‘sham (sham first) then tDCS (tDCS second)’. We used a crossover design to facilitate recruitment and reduce the effects of individual variability. The order of real and sham stimulation sessions was counterbalanced across participants.
Participants
Twenty-four right-handed participants with chronic aphasia participated in our study. The participant recruitment flowchart is depicted in Supplementary Fig. 1. This study is part of a larger study examining the behavioural and neural correlates of cerebellar tDCS in aphasia treatment [Clinical Trial registration NCT02901574]. Participants’ demographic information is summarized in Table 1. Most participants had large left middle cerebral artery strokes. Participant inclusion criteria were: left hemisphere stroke; longer than 6 months post-stroke; previously right-handed; aphasia as confirmed by using the short version of the Boston Diagnostic Aphasia Examination (Goodglass et al., 2001); and able to achieve at least 65% accuracy on a screening version of the aphasia treatment task (see details in the section titled Aphasia Treatment). Exclusion criteria were: lesion in the right cerebellum; a history of brain surgery; seizures during the previous 12 months; sensitive scalp (per patient report); and greater than 80% naming accuracy on the Philadelphia Naming Test (PNT; Roach et al., 1996). All participants provided written informed consent prior to participating in the study. The study was approved by the Institutional Review Board of the Johns Hopkins University School of Medicine, where all data collection occurred.
Table 1.
Demographic and Phase 1 baseline language scores for participants with aphasia
| Patient ID | Group | Age (years) | Gender | Education (years) | TPS | Lesion location | Stimulation order | Pre- treatment screening accuracy | BDAE aphasia severity | Baseline naming 80 scores | Baseline PNT scores |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | Anode | 53 | M | 18 | 12 | Left temporal, parietal | Sham first | 85 | 3 | 28 | 132 |
| P2 | Anode | 76 | M | 16 | 6.5 | Left temporal, insula | Sham first | 68 | 1 | 0 | 0 |
| P3 | Anode | 47 | M | 18 | 10 | Left frontal, temporal, parietal, subcortical | Sham first | 90 | 3 | 13 | 88 |
| P4 | Anode | 67 | M | 10 | 26.5 | Left frontal, temporal, parietal, insula | Sham first | 78 | 2 | 2 | 15 |
| P5 | Anode | 79 | M | 18 | 17 | Left subcortical, basal ganglia | Sham first | 70 | 2 | 0 | 21 |
| P6 | Anode | 68 | M | 14 | 72 | Left frontal, temporal, parietal | Sham first | 78 | 2 | 13 | 31 |
| P7 | Anode | 65 | M | 18 | 25 | Left frontal, temporal, parietal, insula, basal ganglia | tDCS first | 85 | 3 | 10 | 60 |
| P8 | Anode | 60 | M | 14 | 27 | Left frontal, temporal, parietal | tDCS first | 89 | 2 | 23 | 101 |
| P9 | Anode | 78 | F | 16 | 44 | Left temporal, parietal and occipital | tDCS first | 85 | 2 | 0 | 2 |
| P10 | Anode | 67 | M | 13 | 23 | Left frontal, temporal, parietal | tDCS first | 77.5 | 2 | 0 | 8 |
| P11 | Anode | 37 | F | 16 | 41.5 | Left frontal, temporal, parietal | tDCS first | 94 | 4 | 46 | 125 |
| P12 | Anode | 58 | M | 15 | 83 | Left frontal, temporal | tDCS first | 91.6 | 3 | 30 | 80 |
| P13 | Cathode | 64 | M | 16 | 11.5 | Left Basal ganglia, insula | Sham first | 78 | 4 | 40 | 113 |
| P14 | Cathode | 56 | M | 14 | 7.5 | Left frontal, temporal, parietal | Sham first | 87.5 | 3 | 24 | 87 |
| P15 | Cathode | 72 | M | 16 | 35 | Left frontal, parietal, subcortical | Sham first | 77.5 | 2 | 1 | 16 |
| P16 | Cathode | 44 | M | 16 | 26 | Left frontal, parietal | Sham first | 91 | 3 | 36 | 96 |
| P17 | Cathode | 74 | M | 16 | 6 | Left temporal, parietal, occipital | Sham first | 78 | 2 | 2 | 20 |
| P18 | Cathode | 69 | F | 12 | 24 | Left frontal, insula, subcortical | Sham first | 76 | 3 | 11 | 30 |
| P19 | Cathode | 59 | M | 13 | 118 | Left frontal, temporal, parietal, occipital | Sham first | 70 | 3 | 19 | 60 |
| P20 | Cathode | 50 | M | 18 | 63 | Left temporal, parietal, subcortical | tDCS first | 91 | 3 | 16 | 81 |
| P21 | Cathode | 67 | F | 23 | 12 | Left frontal, temporal, parietal | tDCS first | 68 | 1 | 0 | 0 |
| P22 | Cathode | 66 | M | 14 | 6 | Left frontal, parietal | tDCS first | 68 | 1 | 0 | 0 |
| P23 | Cathode | 65 | M | 17 | 53 | Left temporal, parietal, insula, basal ganglia | tDCS first | 66 | 4 | 38 | 102 |
| P24 | Cathode | 58 | M | 16 | 47 | Left frontal, temporal, parietal, occipital, insula | tDCS first | 81 | 3 | 21 | 86 |
BDAE: Boston Diagnostic Aphasia Examination; Baseline Naming 80: Correct scores for Naming 80 Test (trained naming) prior to starting Phase 1 treatment; Baseline PNT: Philadelphia Naming Test-Correct scores for PNT (untrained naming) prior to starting Phase 1 treatment; TPS: time post-stroke onset in months.
Procedure
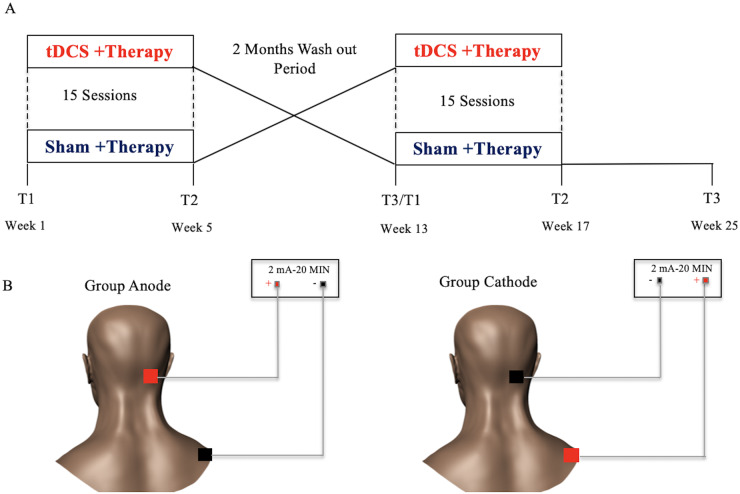
The initial screening visit occurred over 2 days. Participants underwent a detailed medical history, neurologic examination and language assessment using the following tests: Boston Diagnostic Aphasia Examination-short form (Goodglass et al., 2001), Boston Naming Test-short version (Mack et al., 1992), Pyramids and Palm Trees-short Version (Breining et al., 2015a), Hopkins Action Naming Test (Breining et al., 2015b) and PNT (Roach et al., 1996). Supplementary Table 1 shows language test scores for participants. Participants also completed an MRI session, which included T1- and T2-weighted structural MRI scan to rule out a lesion in the right cerebellum (stimulation site). For participants with contraindication for MRI (e.g. pacemaker, claustrophobia and ferromagnetic implants), we used their previously available MRI/CT scan. Participants took part in 2 intervention phases of 15 treatment sessions (3–5 sessions per week) with tDCS + computerized aphasia therapy and sham + computerized aphasia therapy, or the opposite order. Each intervention phase was separated by a washout period of 2 months. Before the start of phase 1 treatment, each participant received language assessments including outcome variables (T1, pre-treatment, phase 1). The same assessments were carried out at the end of 15 treatment sessions of either real or sham tDCS (T2, post-treatment, phase 1) and at 2 months post-treatment (T3, 2 months post-treatment, phase 1). The T3 assessment also served as a second baseline assessment (T1, pre-treatment, phase 2) for participants as they crossed over into the next phase of the study. After the second baseline assessment, participants began the second round of 15 treatment sessions of either real or sham tDCS. If they had received real stimulation first, they crossed over into the sham condition; if they received sham first, they crossed over into the real condition. Assessments were administered post-treatment (T2, post-treatment, phase 2), and at 2 months post-treatment (T3, 2 months post-treatment, phase 2) (Fig. 1A).
Figure 1.
Study design and stimulation. (A) A randomized, double-blind, sham-controlled, within-subject crossover study design was utilized. Participants took part in two intervention phases, separated by a wash out period of 2 months. (B) A 2 mA of anodal or cathodal stimulation was generated between two 5 cm × 5 cm saline soaked sponges, where one active electrode (anode in ‘group anode’ or cathode in ‘group cathode’) was placed on the right cerebellum, and the reference electrode (cathode in ‘group anode’ or anode in ‘group cathode’) was placed on the right shoulder.
Transcranial direct current stimulation
Brain stimulation was delivered for 20 min using a constant current stimulator (ActivaDose II tDCS Device or Soterix Medical 1 × 1 clinical trials device). Consistent with other studies on cerebellar tDCS (Pope and Miall, 2012; Ferrucci et al., 2016; Turkeltaub et al., 2016; Sebastian et al., 2017), the current study utilized 2 mA of anodal tDCS or cathodal tDCS generated between two 5 cm × 5 cm saline-soaked sponges. The active electrode (anode in ‘group anode’ or cathode in ‘group cathode’) was placed on the right cerebellar cortex, 1 cm under, and 4 cm lateral to the inion (approximately comparable to the projection of cerebellar lobule VII onto the scalp; Pope and Miall, 2012). The reference electrode (cathode in ‘group anode’ or anode in ‘group cathode’) was placed on the right shoulder (Fig. 1B).
Randomization and blinding
All participants and all members of the study team who administered the assessments and treatments were blinded to the order in which the participants received tDCS and sham stimulations. To blind participants as to whether they were receiving real or sham tDCS, the same scalp sensation was induced during the start of the sham tDCS sessions, in which the tDCS stimulation was applied to the scalp for 30 s in a ramp-up fashion, and then the current was gradually decreased over 15 s (Gandiga et al., 2006). Stimulation (for both conditions) started at the same time as the computerized aphasia treatment. Aphasia treatment continued for another 25 min after the completion of 20 min of real tDCS or sham tDCS. For the Soterix device, blinding was achieved by inputting a 6-digit blinded code for initiation of stimulation. For the Activa Dose Device, blinding was achieved by a custom hardware device developed by Julius Fridriksson’s group (see Fridriksson et al., 2018 for details). This hardware device was attached to the tDCS device. New codes or hardware boxes were provided to the treating clinician for each patient prior to the start of each intervention period. The senior authors of the study (A.E.H. and D.C.T.) performed the randomization and blinding. Blinding integrity was assessed at the end of each treatment phase.
Aphasia treatment
We utilized a treatment program developed by Fridriksson et al. (2009, 2011, 2018). The aphasia treatment was performed through a computerized task that involved matching pictures depicting common objects with words that were heard and seen (the face of the speaker below the nose is shown on the computer screen). The treatment involves all aspects of lexical-semantic processing and has been shown to improve naming in stroke patients with different underlying causes of naming deficits (Fridriksson et al., 2009). The treatment program consisted of 160 colour pictures depicting low-, medium- and high-frequency nouns and was randomly presented four times during the treatment with a semantic foil, phonological foil, unrelated word or target word. Half of the pairs represented a correct match. During treatment, a picture appeared on the computer screen for 2–5 s. Then, a video of the speaker was presented on the screen saying a word that either matched or did not match the preceding picture. The participant was instructed to press a green response button if the picture and spoken word matched and a red response button if they did not match. Immediate feedback was provided following each response in the form of a ‘smiley face’ for correct responses and a ‘frowny face’ for incorrect responses (Fig. 2). The computer program did not proceed to the next item until a response was recorded for the previous item. The duration of the stimulus presentation and time to respond was adjusted to match the speed of the participant. To ensure that participants with aphasia understand the treatment task, a pre-treatment screen, identical to the treatment task, was administered. Screening involved 40 high-frequency words: participants were given three chances to achieve 65% accuracy, a level of accuracy demonstrating that he or she understands the task requirements.
Figure 2.
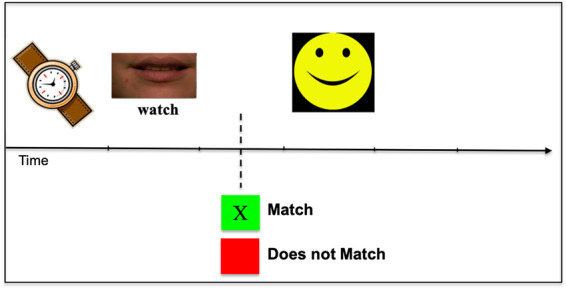
Computerized treatment task. During treatment, a picture appeared on the computer screen followed by a video of the speaker saying a word that either matched or did not match the preceding picture. The participant was instructed to press a green response button if the picture and spoken word matched and a red response button if they did not match. Immediate feedback was provided following each response in the form of a ‘smiley face’ for correct responses and a ‘frowny face’ for incorrect responses.
Outcome measures
Two outcome measures were used in this study: change from baseline in number of the correctly named items on the Naming 80 Test (trained naming) and change from baseline in the number of correctly named items on the PNT (untrained naming). Naming 80 Test consists of a subset of 80 pictures utilized in the treatment program. It should be noted that the treatment program consisted of 160 pictures; however, only half of the treatment items were selected to decrease assessment time at each time. All outcome measures were assessed before and after the end of the treatment, and at 2 months post-treatment completion for the tDCS and sham conditions. Naming accuracy was scored based on PNT scoring guidelines (Roach et al., 1996).
Adverse effects
Participants were assessed at the end of each treatment session for pain and discomfort. We used the Wong-Baker FACES Pain Rating Scale (Wong and Baker, 1988) to assess pain associated with tDCS. In addition, we also asked the participants if they had any discomforts such as itching, irritation, tingling or burning at the end of each treatment session.
Statistical analysis
Descriptive statistics and t-tests were used to compare participant characteristics and adverse effects. To assess the effect of tDCS treatment on change in naming accuracy, we performed separate linear mixed-effects models for the two outcome measures: Naming 80 test (trained naming) and PNT (untrained naming). Linear mixed-effects regression models with a random intercept for participants were fit via the maximum likelihood method using the MIXED command in Stata. Change in correct naming immediately post-treatment minus pre-treatment and 2 months post-treatment minus pre-treatment for the sham and tDCS conditions was the dependent variable. Fixed effects including the following: treatment (two levels: tDCS versus sham), time (two levels: 2 months post-treatment versus post-treatment), order of treatment (two levels: tDCS first then sham versus the reverse) and their interactions. An additional model was fit separated by group (anode versus cathode). The main effects and interactions of the variables in the models were evaluated by the Wald tests. LINCOM command in Stata was used to estimate the difference between the coefficients of tDCS and sham conditions for significant main effects and their interactions. Statistical analyses were done with Stata, version 16 (Statacorp, College Station, TX).
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Results
Of the 24 enrolled participants, 21 completed the study; 3 dropped out during phase 1 and were not considered in the present analyses. P16 dropped out because of personal reasons, and P6 and P11 dropped out due to an illness unrelated to the study.
Safety and tolerability
All 21 participants tolerated the stimulation well without any adverse effects. With respect to pain, no participants reported pain during the tDCS stimulation sessions. One participant reported very mild pain (Wong and Baker pain rating scale score = 1) during one of the sham sessions. For the other four symptoms (irritation, itching, burning or tingling sensation) we scored yes = 1 or no = 0 for each session. We compared irritation, itching, tingling and burning from 315 sessions for tDCS and sham conditions. Transient irritation was reported for 3% (SD = 0.18) of the tDCS sessions and 0.6% (SD = 0.05) for the sham sessions; transient itching was reported for 5.7% (SD = 0.23) of the tDCS sessions and 4.7% (SD = 0.21) for the sham sessions, tingling was not reported for any of the tDCS sessions and 0.6% (SD = 0.05) was reported for the sham sessions, and burning was reported by 1 participant during one tDCS session. No significant differences were evident between the tDCS and sham sessions with the exception of ‘irritation’ which was more pronounced during tDCS [t (314) =2.92, P = 0.004].
Integrity of blinding
To ensure proper blinding, each participant and clinician was asked to guess the stimulation type at the end of each treatment phase. Stroke participants’ guessing accuracy was 42.8% and the clinician’s guessing accuracy was 47.6%. This indicates that each group’s accuracy was essentially at chance.
Participant treatment accuracy
All participants improved on the aphasia treatment task as indicated by greater task accuracy on the last treatment session compared with the first treatment session. The mean change in accuracy between the last and the first treatment session was significant for both phase 1 and phase 2. Phase 1: mean change in accuracy= 12.93%, SD = 8.7 [t (20) =6.63, P = 0.000], phase 2: mean change in accuracy = 7.67, SD = 8.26 [t (20) =4.15, P = 0.001].
Outcome variables
At baseline before the start of phase 1 treatment, there was no statistically significant difference between the groups. Naming 80: group anode (M = 10.60, SE = 3.89) and group cathode (M = 15.64, SE = 4.38), [t (19) = 0.85, P = 0.40] and PNT: group anode (M = 50.70 SE = 15.05) and group Cathode (M = 54.09, SE = 12.67), [t (19) = 0.17, P = 0.86]. In addition, there was no statistically significant difference between the groups for months post-onset since stroke: group anode: M = 27.40, SE = 7.05) and group cathode (M = 34.81, SE = 10.34), [t (19) = 0.58, P = 0.56].
Naming 80 test (trained naming)
In the initial model (combining both groups), there was no significant effect for treatment, time, or order. However, there was a significant order × treatment interaction immediately post-treatment (P = 0.004). The interaction was such that for participants who received tDCS first (order: tDCS first then sham), there was a significant improvement in naming immediately post-tDCS compared to sham (difference in mean change in naming accuracy between tDCS and sham was 5.8, 95% CI from 1.5 to 10.1, P = 0.008) versus no significant improvement in naming immediately post-tDCS compared to sham if sham came first (the difference in mean change in naming accuracy between tDCS and sham −2.9, 95% CI from −6.98 to 1.16, P = 0.16 for participants who received sham first). There was no statistically significant difference between tDCS and sham at the 2 months post-treatment for either order of treatment (b = −0.9; 95% CI: −5.16 to 3.37; P = 0.68 if tDCS was received first and b = 1.2; 95% CI −2.96 to 5.44; P = 0.56 if sham was received first). Figure 3 shows the mean change from baseline in Naming 80 Test accuracy for tDCS and sham conditions. Supplementary Table 2 and Supplementary Fig. 2 show the mean Naming 80 Test accuracy separated by phase, condition, and time. Supplementary Fig. 4 shows the individual data points across the three time points for Naming 80 Test.
Figure 3.
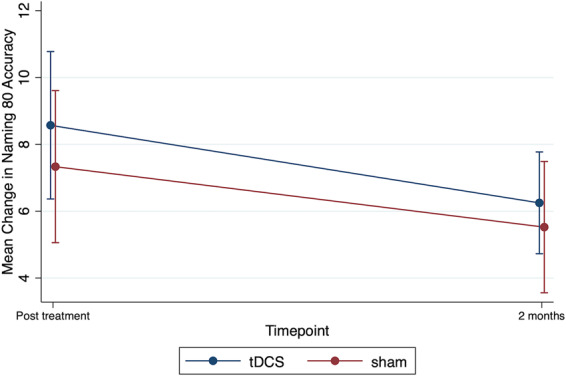
Trained naming accuracy. Mean change from baseline in Naming 80 test accuracy for sham and tDCS conditions immediately post-treatment and 2 months post-treatment. Error bars show standard errors.
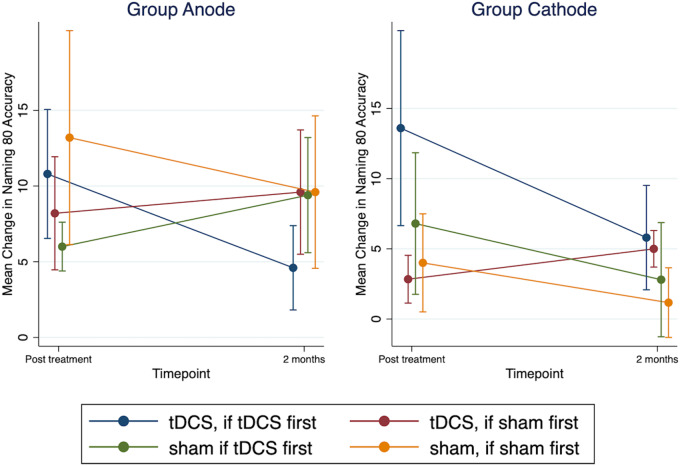
In the second model (separated by group), there was no significant main effect for treatment, time, or order for group anode and group cathode. No interaction effects were significant for group anode. However, similar to the initial model, there was a significant order × treatment interaction for group cathode (P = 0.022). The interaction was such that for participants in group cathode who received tDCS first (order: ‘tDCS first then sham’), there was a significant improvement in naming immediately post-tDCS compared to sham (difference in mean change in naming accuracy for tDCS and sham was 6.8, 95% CI from 1.76 to 11.84, P-value = 0.008), versus no significant improvement in naming immediately post-tDCS compared to sham if sham came first (the difference in mean change in naming accuracy between tDCS and sham −1.17, 95% CI from −5.77 to 3.44, P = 0.62 for participants who received ‘sham first’). The treatment effect at the two months post-treatment did not reach statistical significance regardless of order: for participants who received tDCS first (b = 3.0; 95% CI: −2.04 to 8.04; P = 0.24) or for participants who received sham first (b = 2.3; 95% CI: −2.61 to 7.17; P = 0.36). Figure 4 shows the mean change in Naming 80 Test accuracy separated by group (anode, cathode) and order (‘tDCS first then sham’, ‘sham first then tDCS’).
Figure 4.
Trained naming accuracy separated by group and order. Mean change from baseline in Naming 80 test accuracy for group anode and group cathode. Within each group, participants were assigned to receive ‘tDCS first then sham’ or ‘sham first then tDCS’. ‘Blue’ and ‘Green’ colours show participants who received tDCS first followed by sham. ‘Brown’ and ‘Orange’ colours show participants who received sham first followed by tDCS. Error bars show standard errors.
PNT (untrained naming)
In the initial model (combining both groups), there was a significant effect of treatment (P = 0.015). However, there were no statistically significant order or time interactions, which indicated that the effect for treatment did not significantly vary by time post-treatment or the order in which the treatment was received. For order ‘tDCS first then sham’, the mean difference in change in naming accuracy between tDCS and sham was 9.57 (95% CI from 1.7 to 17.38, P = 0.016) immediately post-treatment. Likewise, at 2 months post-treatment, the mean difference in change in naming accuracy between tDCS and sham was 10.22 (95% CI from 2.22 to 18.22, P = 0.012). For order ‘sham first then tDCS’, the mean difference in change in naming accuracy between tDCS and sham was 6.22 (95% CI from −0.87 to 13.31, P = 0.086) immediately post-treatment. Similarly, at the 2 months post-treatment, the mean difference in change in naming accuracy between tDCS and sham was 11.39 (95% CI from 4.30 to 18.48, P = 0.002). No other interactions (2- or 3-way) reached significance. Figure 5 shows the mean change from baseline in PNT accuracy for tDCS and sham conditions. Supplementary Table 2 and Supplementary Fig. 3 show the mean PNT accuracy separated by phase, condition, and time. Supplementary Fig. 5 shows the individual data points across the three time points for PNT.
Figure 5.
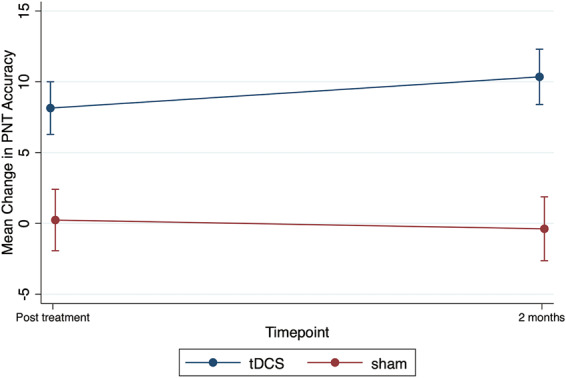
Untrained naming accuracy. Mean change from baseline in PNT accuracy for sham and tDCS conditions immediately post-treatment and 2 months post-treatment. Error bars show standard errors.
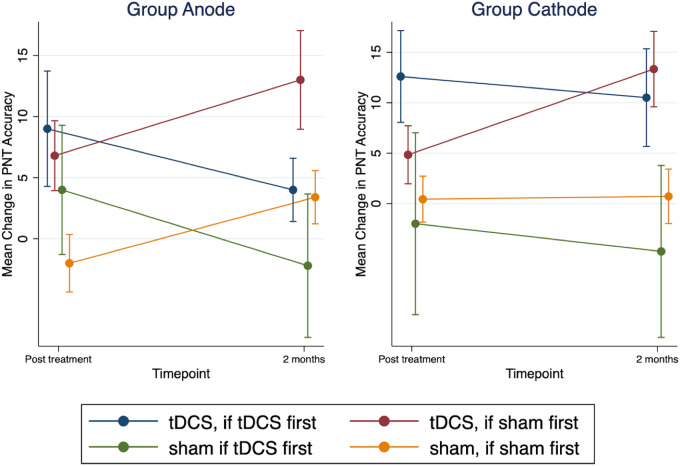
In the second model (separated by group), no statistically significant treatment effects were observed in group anode. In the model for group cathode, similar to the combined models, treatment effects did not significantly vary by time point or order, i.e., no statistically significant interactions were observed. For group cathode, for order ‘tDCS first then sham’, the mean difference in change in naming accuracy between tDCS and sham was 15. 24 (95% CI from 3.81 to 26.68, P = 0.009) immediately post-treatment and 15.25 (95% CI from 3.2 to 27.23, P = 0.013) 2 months post-treatment. For order ‘sham first then tDCS’, the mean difference in change in naming accuracy between tDCS and sham was 3. 97 (95% CI from −5.48 to 13.43, P = 0.41) immediately post-treatment and 12.86 (95% CI from 3.39 to 22.32, P = 0.008) 2 months post-treatment. Figure 6 shows the mean change in PNT accuracy separated by group (anode, cathode) and order (tDCS first then sham, sham first then tDCS).
Figure 6.
Untrained naming accuracy separated by group and order. Mean change from baseline in PNT accuracy for group anode and group cathode. Within each group, participants were assigned to receive ‘tDCS first then sham’ or ‘sham first then tDCS’. ‘Blue’ and ‘Green’ colours show participants who received tDCS first followed by sham. ‘Brown’ and ‘Orange’ colours show participants who received sham first followed by tDCS. Error bars show standard errors.
Discussion
Neuromodulation is a promising adjunct to speech and language treatment of post-stroke aphasia; however, to date, an optimal stimulation site has not been identified. In this study, we show that stimulation of the right posterolateral cerebellum combined with computerized aphasia treatment can improve picture naming in chronic post-stroke aphasia. There are three main results of this study: (i) for trained items, the effect for cerebellar tDCS was not significant. The order x treatment interaction was however significant, indicating that cerebellar tDCS was more effective than sham immediately post-treatment for participants who received stimulation in phase 1 (order ‘tDCS first’); (ii) for untrained items, the effect of cerebellar tDCS was significant immediately post-treatment and at 2 months post-treatment; and (iii) greater gains in naming (relative to sham) were noted for participants receiving cathodal stimulation for both trained and untrained items.
Potential mechanism of action of cerebellar tDCS
The increased behavioural gains observed for untrained and (less striking in) trained naming could be due to the relatively high duration and intensity of stimulation (15 sessions, 3–5 times per week of tDCS). At the cortical level, repeated tDCS sessions administered concurrently with behavioural training are thought to act via mechanisms similar to long-term potentiation, which is critical for neuroplasticity and memory consolidation (Reis et al., 2009, 2015; Fritsch et al., 2010). However, it’s unknown whether these mechanisms also underlie the improvement in behaviour observed with cerebellar tDCS.
It has been proposed that cerebellar tDCS is most likely to produce its effects by polarizing Purkinje cells and changing the levels/pattern of activity in the deep cerebellar output nuclei (Galea et al., 2009; Grimaldi et al., 2016). Evidence from animal studies suggests that Purkinje cells respond to cerebellar tDCS (Grimaldi et al., 2016). Thus, it is likely that cerebellar tDCS could influence long-term depression (LTD) in Purkinje cells. LTD of Purkinje cells plays a role not only in motor function but also in cognitive tasks (Vigot, 2003). Animal studies have also shown that learning is mediated in part by LTD in Purkinje cells (Ito, 1982). Based on this, we speculate that the improvement observed in naming skills could be related to the role of the cerebellum in learning. Previous studies in humans have shown that cerebellar tDCS facilitates motor skill learning (Cantarero et al., 2015; Wessel et al., 2016) and procedural learning (Ferrucci et al., 2013). Cortical tDCS studies have reported beneficial effects of stimulation on language learning for familiar and unfamiliar objects (e.g. Meinzer et al., 2014; Fiori et al., 2018). In addition, multisession cortical tDCS studies report transfer effects to untrained materials (Cappelletti et al., 2013; Park et al., 2014). Therefore, multisession cerebellar tDCS combined with aphasia treatment may enhance the learning of compensatory strategies and re-learning of language during aphasia treatment, resulting in improvement in trained and untrained naming.
Polarity-independent effect on naming
The performance of participants receiving cathodal stimulation (relative to sham) was better compared to participants receiving anodal stimulation (relative to sham) for order ‘tDCS first’ for both trained and untrained items. Significantly greater gains in naming were noted for group cathode immediately post-treatment and at 2 months post-treatment for order ‘tDCS first’. However, the overall mean changes in naming (combining both phases) were similar in group anode and group cathode immediately post-treatment. At 2 months post-treatment, the overall mean change in naming was higher for group cathode compared to group anode. Our results suggest that cathodal stimulation might be more favourable than anodal stimulation but the fairly small difference does not allow strong conclusions regarding polarity specific effects.
Thus, these findings are consistent with the as-yet unclear directionality of the effects of anodal versus cathodal cerebellar tDCS on cognitive task performance (Grimaldi et al., 2016). The results of the present study are not unusual since anodal and cathodal cerebellar tDCS have been reported to have polarity-independent effects on working memory (e.g. Ferrucci et al., 2013), motor learning (e.g. Galea et al., 2009), and language processing (e.g. Pope and Miall, 2012). For example, in a study of verb generation in healthy individuals, cathodal cerebellar tDCS applied over the right cerebellum facilitated performance on the verb generation task, as compared to anodal tDCS and sham tDCS (Pope and Miall, 2012). In contrast, Turkeltaub et al. (2016) found that both anodal and cathodal cerebellar tDCS enhanced the performance on a phonemic fluency task compared to sham; however, the anodal effect was found to be more robust.
The lack of polarity specific effects could be due to the complexity of gyral folding of the cerebellar cortex, which in turn can causes hyperpolarization in some neurons while others may be depolarized simultaneously, leading to different global effects of cerebellar tDCS (van Dun et al., 2016b; Woods et al., 2016). In addition, it is also unclear whether cerebellar tDCS affects only the Purkinje cells or whether it affects other entities, such as parallel fibres, climbing fibres, mossy fibres and basket cells. Furthermore, substantial individual variability in anatomy as well as the neurophysiological constitution, plays a critical role in the efficacy of cerebellar tDCS (Oldrati and Schutter, 2018). Thus, the difference seen between-group anode and group cathode could also be related to individual variability.
Differential effect of cerebellar tDCS in the crossover phase
An important finding was that tDCS effects compared to sham were more pronounced for order ‘tDCS first’ for both trained and untrained naming. Although, the order effect was not significant, the mean gain in naming was higher for order ‘tDCS first then sham’ compared to ‘sham first then tDCS’. One possible explanation for this finding could be related to the role that the cerebellum plays in language processing. Prior studies have shown that the role cerebellum plays in language processing depends on task demands (Stoodley et al., 2010, 2012; Pope and Miall, 2012, 2014; Boehringer et al., 2013; Marangolo et al., 2018). Both tDCS and sham conditions were paired with a computerized aphasia treatment task that involved matching pictures depicting common objects with words that were heard and seen. Colour pictures depicting low-, medium- and high-frequency nouns were randomly presented four times during the treatment with a semantic foil, phonological foil, unrelated word or target word. Participants had to press two different buttons to indicate whether the picture and spoken word matched or did not match. Participants also received immediate feedback on whether their response was correct or incorrect. Our working hypothesis is that the right cerebellum showed increased preferential activity during learning in phase 1, which then decreased as the task was repeated and became more efficient. It should be noted that even though participants showed a significant improvement on the aphasia treatment task (indicated by greater task accuracy on the last treatment session compared with the first treatment session) for both phase 1 and 2, the magnitude of improvement was lower in phase 2 compared to phase 1. Thus, lower tDCS treatment gains in phase 2 could be due to the treatment task becoming more automatic and easier to perform (with some participants being at or near ceiling at the beginning of phase 2).
Another explanation for lower treatment gains in phase 2 could be due to tDCS carry-over effects. We took several steps to minimize the potential for carryover effects. We used a 2-month break in between phase 1 and phase 2 with the assumption that the tDCS effect will have washed out by that time. In addition, we also adopted an analysis approach utilized by our colleagues (Tsapkini et al., 2018; de Aguiar et al., 2020) to allow for the case that the tDCS effect does not wash out in 2 months. In our analysis, we used the ‘change in naming’ as the main outcome, i.e., the score at each post-treatment/follow-up minus the score at baseline of the respective phase. This means that any effect of tDCS versus sham found in phase 2 will not reflect the improvement itself at the level of performance in naming, i.e., the fact that the participants who got tDCS first are naming more accurately than those who got sham. Therefore, any effect of tDCS versus sham that carries over from 2 months of phase 1 to only the level of performance in phase 2 will cancel in the tDCS versus sham comparisons of ‘change in naming’ at phase 2. In this way, we eliminated one of the possible ‘carryover’ effects of tDCS: the improvement at the mere level of performance (Tsapkini et al., 2018). Indeed, it is possible that tDCS effects carry over into the subsequent sham condition for some participants, resulting in an inflated sham performance in the second phase.
Individual variability in cerebellar tDCS response
The results of this study indicate that there is marked individual variability in the cerebellar tDCS response. Based on the spaghetti plots shown in Supplementary Figs 4 and 5, it is evident that some participants showed large gains, some showed small gains, and some did not show any gains at all. In general, a majority of the participants showed improvement with tDCS despite severe aphasia; however, participants with profound naming deficits showed no change or very minimal change (participants with naming score of 0). It is possible that participants with some residual naming ability could derive greater benefit from combining cerebellar tDCS with training compared to participants who have profound naming deficits.
Cerebellar tDCS and computerized aphasia treatment
The results of our study add to the growing number of studies that indicate that repetitive sessions of tDCS combined with language therapy can enhance the naming outcomes in chronic aphasia. However, closer inspection of the results of the published studies reveals substantial heterogeneity of the tDCS response. One reason could be that different types of therapy may have differential effects on the nature and extent of neuroplasticity that occurs within the language networks and may differentially engage left-hemisphere versus right-hemisphere networks (Crosson et al., 2019), resulting in a variable treatment outcome. One can reduce the treatment-induced variability by using a therapy protocol that has been successfully paired with tDCS. We utilized a computerized treatment task that has been shown to improve naming in participants with aphasia when combined with tDCS (Baker et al., 2010; Fridriksson et al., 2011, 2018, 2019). Similar to the Fridriksson et al. (2018, 2019) studies, we found greater improvement in naming with tDCS compared to sham. However, there are several differences in study design: parallel sham-controlled study versus within-subject cross over study; left hemisphere anodal stimulation versus right cerebellar anodal/cathodal stimulation; 1 mA versus 2 mA current strength. Please see Supplementary Table 3 for a comparison of Cohen's d's between our study versus Fridriksson et al. (2018, 2019). We chose a computerized aphasia treatment program because the treatment time and intensity were the same for the tDCS and sham conditions. There are other types of aphasia treatments that are probably equally or more effective for improving naming; however, the purpose of the current study was not to evaluate the effectiveness of aphasia treatment but to determine the adjuvant benefit of cerebellar tDCS when combined with a proven form of computerized aphasia treatment.
Limitations
There are several limitations to our study. Our sample size is small; therefore, these findings need to be confirmed in a larger trial. A second limitation is that we assessed pain and discomfort at the end of each 45-min treatment session. It is possible that any pain due to tDCS would have subsided by the time the pain scale was administered. However, due to the nature of the treatment task (computerized treatment task), it was not possible to administer the pain scale right after the completion of the stimulation at 20 min. A third limitation is that we cannot determine if improvement in naming translates to improvements in functional communication or quality of life for participants with aphasia. We are currently analysing the American Speech-Language-Hearing Association Functional Assessment of Communication Skills for Adults (ASHA FACS) scores in a subset of our participants to determine whether improvement in naming translates to improvements in functional communication. A fourth limitation is that the individual variability observed in this study could be related to the medications the participants were taking. A recent review indicates that many medications may impact the efficacy of tDCS (McLaren et al., 2018). For example, selective serotonin reuptake inhibitors (SSRIs) have been shown to influence the after effects of tDCS (Nitsche et al., 2009). Seven participants in this study were taking SSRI. However, given this was a crossover study, and participants were likely taking the same medications in both phases, it is unclear to what extent medications (including SSRIs) might have interacted with tDCS to influence the results of the study. Finally, the performance of participants receiving anodal was not significantly different from sham for trained and untrained items. This could be due to small sample size and/or individual variability. Future investigations will need to address the polarity specific mechanism of action of cerebellar tDCS to refine its application in aphasia rehabilitation. Resting-state functional connectivity might provide key insights into the neural mechanisms underlying polarity-specific changes in the network dynamics induced by cerebellar tDCS. Such imaging may aid in determining predictors of treatment outcome for anodal and cathodal tDCS, in order to provide more effective, targeted treatment for people with aphasia.
Conclusions
This study provides novel evidence that repeated stimulation of the right cerebellum along with aphasia treatment can improve naming in chronic post-stroke aphasia. Cerebellar tDCS is easily delivered, is well tolerated and has not shown serious adverse effects. In addition, cerebellar tDCS montage can be easily implemented in clinical practice. Targeting the intact right cerebellum allows for the possibility of identifying a single target that can be used across groups of people with aphasia with varying lesion sites and size in the left hemisphere.
Supplementary material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgements
We thank Drs. Gayane Yenokyan, Richard Thompson and Brian Caffo for their assistance with statistical analyses. We thank Lynsey Keator and Dr. Shannon Sheppard for their assistance with data collection, and Drs. Julius Fridriksson, Chris Rorden and Taylor Hayanik for providing the treatment materials and tDCS devices.
Funding
The research reported in this paper was supported by the National Institutes of Health (National Institute on Deafness and Other Communication Disorders) through awards K99/R00 DC015554 and P50 DC014664.
Competing interests
The authors report no competing interests.
Glossary
- mA =
milliamps
- PNT =
Philadelphia Naming Test
- tDCS =
transcranial direct current stimulation
Contributor Information
Rajani Sebastian, Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Ji Hyun Kim, Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Rachel Brenowitz, Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Donna C Tippett, Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Department of Otolaryngology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
John E Desmond, Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Pablo A Celnik, Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Argye E Hillis, Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Department of Cognitive Science, Johns Hopkins University, Baltimore, MD, USA.
References
- Anglade C, Thiel A, Ansaldo AI. The complementary role of the cerebral hemispheres in recovery from aphasia after stroke: a critical review of literature [Review]. Brain Injury 2014; 28: 138–45. [DOI] [PubMed] [Google Scholar]
- Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke 2010; 41: 1229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehringer A, Macher K, Dukart J, Villringer A, Pleger B. Cerebellar transcranial direct current stimulation modulates verbal working memory. Brain Stim 2013; 6: 649–53. [DOI] [PubMed] [Google Scholar]
- Breining BL, Lala T, Martínez Cuitiño M, Manes F, Peristeri E, Tsapkini K, et al. A brief assessment of object semantics in primary progressive aphasia. Aphasiology 2015. a; 29: 488–505. [Google Scholar]
- Breining BL, Tippett DC, Davis C, Posner J, Sebastian R, Oishie K, et al. Assessing dissociations of object and action naming in acute stroke. 2015. b. Paper presented at the Clinical Aphasiology Conference, Monterey, CA.
- Breining BL, Sebastian R. Neuromodulation in post-stroke aphasia treatment [Review]. Curr Phys Med Rehab Rep 2020; 22: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucur M, Papagno C. Are transcranial brain stimulation effects long-lasting in post-stroke aphasia? A comparative systematic review and meta-analysis on naming performance [Review]. Neurosci Biobehav Rev 2019; 102: 264–89. [DOI] [PubMed] [Google Scholar]
- Campana S, Caltagirone C, Marangolo P. Combining voxel-based lesion-symptom mapping (VLSM) with A-tDCS language treatment: predicting outcome of recovery in nonfluent chronic aphasia. Brain Stim 2015; 8: 769–76. [DOI] [PubMed] [Google Scholar]
- Cantarero G, Spampinato D, Reis J, Ajagbe L, Thompson T, Kulkarni K, et al. Cerebellar direct current stimulation enhances on-line motor skill acquisition through an effect on accuracy. J Neurosci 2015; 35: 3285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelletti M, Gessaroli E, Hithersay R, Mitolo M, Didino D, Kanai R, et al. Transfer of cognitive training across magnitude dimensions achieved with concurrent brain stimulation of the parietal lobe. J Neurosci 2013; 33: 14899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B, Rodriguez AD, Copland D, Fridriksson J, Krishnamurthy LC, Meinzer M, et al. Neuroplasticity and aphasia treatments: new approaches for an old problem. J Neurol Neurosurg Psychiatry 2019; 90: 1147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Mello AM, Turkeltaub PE, Stoodley CJ. Cerebellar tDCS modulates neural circuits during semantic prediction: a combined tDCS-fMRI study. J Neurosci 2017; 37: 1604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond JE, Fiez JA. Neuroimaging studies of the cerebellum: language, learning and memory [Review]. Trends Cogn Sci 1998; 2: 355–62. [DOI] [PubMed] [Google Scholar]
- Darkow R, Martin A, Würtz A, Flöel A, Meinzer M. Transcranial direct current stimulation effects on neural processing in post‐stroke aphasia. Hum Brain Map 2017; 38: 1518–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Aguiar V, Paolazzi CL, Miceli G. tDCS in post-stroke aphasia: the role of stimulation parameters, behavioral treatment and patient characteristics [Review]. Cortex 2015; 63: 296–316. [DOI] [PubMed] [Google Scholar]
- de Aguiar V, Zhao Y, Faria A, Ficek B, Webster KT, Wendt H, et al. Brain volumes as predictors of tDCS effects in primary progressive aphasia. Brain Lang 2020; 200: 104707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation for improving aphasia after stroke: what’s the current evidence? [Review]. Stroke 2019; 50: e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci R, Brunoni AR, Parazzini M, Vergari M, Rossi E, Fumagalli M, et al. Modulating human procedural learning by cerebellar transcranial direct current stimulation. Cerebellum 2013; 12: 485–92. [DOI] [PubMed] [Google Scholar]
- Ferrucci R, Bocci T, Cortese F, Ruggiero F, Priori A. Cerebellar transcranial direct current stimulation in neurological disease. Cereb Ataxias 2016; 3: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori V, Kunz L, Kuhnke P, Marangolo P, Hartwigsen G. Transcranial direct current stimulation (tDCS) facilitates verb learning by altering effective connectivity in the healthy brain. NeuroImage 2018; 181: 550–9. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Baker JM, Whiteside J, Eoute D, Jr, Moser D, Vesselinov R, et al. Treating visual speech perception to improve speech production in nonfluent aphasia. Stroke 2009; 40: 853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Richardson JD, Baker JM, Rorden C. Transcranial direct current stimulation improves naming reaction time in fluent aphasia: a double-blind, sham-controlled study. Stroke 2011; 42: 819–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Rorden C, Elm J, Sen S, George MS, Bonilha L. Transcranial direct current stimulation vs sham stimulation to treat aphasia after stroke: a randomized clinical trial. JAMA Neurol 2018; 75: 1470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Basilakos A, Stark BC, Rorden C, Elm J, Gottfried M, et al. Transcranial direct current stimulation to treat aphasia: longitudinal analysis of a randomized controlled trial. Brain Stim 2019; 12: 190–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 2010; 66: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci 2009; 29: 9115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 2006; 117: 845–50. [DOI] [PubMed] [Google Scholar]
- Gainotti G. Contrasting opinions on the role of the right hemisphere in the recovery of language. A critical survey [Review]. Aphasiology 2015; 29: 1020–37. [Google Scholar]
- Goodglass H, Kaplan E, Barresi B. BDAE-3: Boston Diagnostic Aphasia Examination. 3rd edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Grimaldi G, Argyropoulos GP, Bastian A, Cortes M, Davis NJ, Edwards DJ, et al. Cerebellar transcranial direct current stimulation (ctDCS) a novel approach to understanding cerebellar function in health and disease [Review]. Neuroscientist 2016; 22: 83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilari K, Needle JJ, Harrison KL. What are the important factors in health-related quality of life for people with aphasia? A systematic review [Review]. Arch Phy Med Rehab 2012; 93: S86–95. [DOI] [PubMed] [Google Scholar]
- Holland R, Crinion J. Can tDCS enhance treatment of aphasia after stroke?[Review]. Aphasiology 2012; 26: 1169–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Cerebellar control of the vestibulo-ocular reflex: around the flocculus hypothesis. Annu Rev Neurosci 1982; 5: 275–97. [DOI] [PubMed] [Google Scholar]
- Keren-Happuch E, Chen SH, Ho MH, Desmond JE. A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Hum Brain Mapp 2014; 35: 593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack WJ, Freed DM, Williams BW, Henderson VW. Boston naming test: shortened versions for use in Alzheimer's disease. J Gerontol 1992; 47: P154–8. [DOI] [PubMed] [Google Scholar]
- Marangolo P, Fiori V, Caltagirone C, Pisano F, Priori A. Transcranial cerebellar direct current stimulation enhances verb generation but not verb naming in poststroke aphasia. J Cogn Neurosci 2018; 30: 188–99. [DOI] [PubMed] [Google Scholar]
- Mariën P, Ackermann H, Adamaszek M, Barwood CH, Beaton A, Desmond J, et al. Consensus paper: language and the cerebellum: an ongoing enigma [Review]. Cerebellum 2014; 13: 386–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariën P. A role for the cerebellum in language and related cognitive and affective functions In: Neural mechanisms of language. Boston, MA: Springer; 2017. p. 175–198. [Google Scholar]
- McLaren ME, Nissim NR, Woods AJ. The effects of medication use in transcranial direct current stimulation: a brief review. Brain Stim 2018; 11: 52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Jähnigen S, Copland DA, Darkow R, Grittner U, Avirame K, et al. Transcranial direct current stimulation over multiple days improves learning and maintenance of a novel vocabulary. Cortex 2014; 50: 137–47. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Darkow R, Lindenberg R, Flöel A. Electrical stimulation of the motor cortex enhances treatment outcome in post-stroke aphasia. Brain 2016; 139: 1152–63. [DOI] [PubMed] [Google Scholar]
- Murdoch BE. The cerebellum and language: historical perspective and review [Review]. Cortex 2010; 46: 858–68. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Kuo MF, Karrasch R, Wächter B, Liebetanz D, Paulus W. Serotonin affects transcranial direct current–induced neuroplasticity in humans. Biol Psychiatry 2009; 66: 503–8. [DOI] [PubMed] [Google Scholar]
- Oldrati V, Schutter DJ. Targeting the human cerebellum with transcranial direct current stimulation to modulate behavior: a meta-analysis. Cerebellum 2018; 17: 228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Seo JH, Kim YH, Ko MH. Long-term effects of transcranial direct current stimulation combined with computer-assisted cognitive training in healthy older adults. Neuroreport 2014; 25: 122–6. [DOI] [PubMed] [Google Scholar]
- Pestalozzi MI, Di Pietro M, Gaytanidis CM, Spierer L, Schnider A, Chouiter L, et al. Effects of prefrontal transcranial direct current stimulation on lexical access in chronic poststroke aphasia. Neurorehabil Neural Repair 2018; 32: 913–23. [DOI] [PubMed] [Google Scholar]
- Pope PA, Miall RC. Task-specific facilitation of cognition by cathodal transcranial direct current stimulation of the cerebellum. Brain Stim 2012; 5: 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope PA, Miall RC. Restoring cognitive functions using non-invasive brain stimulation techniques in patients with cerebellar disorders. Front Psychiatry 2014; 5: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA 2009; 106: 1590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Fischer JT, Prichard G, Weiller C, Cohen LG, Fritsch B. Time-but not sleep-dependent consolidation of tDCS-enhanced visuomotor skills. Cerebral Cortex 2015; 25: 109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach A, Schwartz MF, Martin N, Grewal RS, Brecher A. The Philadelphia naming test: scoring and rationale. Clin Aphasio 1996; 24: 121–33. [Google Scholar]
- Sandars M, Cloutman L, Woollams AM. Taking sides: an integrative review of the impact of laterality and polarity on efficacy of therapeutic transcranial direct current stimulation for anomia in chronic poststroke aphasia [Review]. Neural Plast 2016; 2016: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian R, Saxena S, Tsapkini K, Faria AV, Long C, Wright A, et al. Cerebellar tDCS: a novel approach to augment language treatment post-stroke. Front Hum Neurosci 2017; 10: 695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah-Basak PP, Norise C, Garcia G, Torres J, Faseyitan O, Hamilton RH. Individualized treatment with transcranial direct current stimulation in patients with chronic non-fluent aphasia due to stroke. Front Hum Neurosci 2015; 9: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielmann K, van de Sandt-Koenderman WM, Heijenbrok-Kal MH, Ribbers GM. Transcranial direct current stimulation does not improve language outcome in subacute poststroke aphasia. Stroke 2018; 49: 1018–20. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ. The cerebellum and cognition: evidence from functional imaging studies [Review]. Cerebellum 2012; 11: 352–65. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. An fMRI study of intra-individual functional topography in the human cerebellum. Behav Neuro 2010; 23: 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage 2012; 59: 1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K, Webster KT, Ficek BN, Desmond JE, Onyike CU, Rapp B, et al. Electrical brain stimulation in different variants of primary progressive aphasia: a randomized clinical trial. Alzheimer's & Dementia. Trans Res Clin Interven 2018; 4: 461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Swears MK, D’Mello AM, Stoodley CJ. Cerebellar tDCS as a novel treatment for aphasia? Evidence from behavioral and resting-state functional connectivity data in healthy adults. Restor Neurol Neurosci 2016; 34: 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dun K, Bodranghien FC, Mariën P, Manto MU. tDCS of the cerebellum: where do we stand in 2016? Technical issues and critical review of the literature. Front Hum Neurosci 2016; 11: 10–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigot R. Cerebellar long-term depression: a mechanism for learning and memory. Med Sci 2003; 19: 437–41. [DOI] [PubMed] [Google Scholar]
- Wessel MJ, Zimerman M, Timmermann JE, Heise KF, Gerloff C, Hummel FC. Enhancing consolidation of a new temporal motor skill by cerebellar noninvasive stimulation. Cereb Cortex 2016; 26: 1660–7. [DOI] [PubMed] [Google Scholar]
- Wessel MJ, Hummel FC. Non-invasive cerebellar stimulation: a promising approach for stroke recovery? Cerebellum 2018; 17: 359–71. [DOI] [PubMed] [Google Scholar]
- Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools [Review]. Clin Neurophysiol 2016; 127: 1031–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs 1988; 14: 9–17. [PubMed] [Google Scholar]
- Wu D, Wang J, Yuan Y. Effects of transcranial direct current stimulation on naming and cortical excitability in stroke patients with aphasia. Neurosci Lett 2015; 589: 115–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.