Abstract
Traditionally, safety evaluation at the early stage of drug discovery research has been done using in silico, in vitro, and in vivo systems in this order because of limitations on the amount of compounds available and the throughput ability of the assay systems. While these in vitro assays are very effective tools for detecting particular tissue-specific toxicity phenotypes, it is difficult to detect toxicity based on complex mechanisms involving multiple organs and tissues. Therefore, the development of novel high throughput in vivo evaluation systems has been expected for a long time. The zebrafish (Danio rerio) is a vertebrate with many attractive characteristics for use in drug discovery, such as a small size, transparency, gene and protein similarity with mammals (80% or more), and ease of genetic modification to establish human disease models. Actually, in recent years, the zebrafish has attracted interest as a novel experimental animal. In this article, the author summarized the features of zebrafish that make it a suitable laboratory animal, and introduced and discussed the applications of zebrafish to preclinical toxicity testing, including evaluations of teratogenicity, hepatotoxicity, and nephrotoxicity based on morphological findings, evaluation of cardiotoxicity using functional endpoints, and assessment of seizure and drug abuse liability.
Keywords: developmental toxicity, hepatotoxicity, nephrotoxicity, cardiotoxicity, abuse, seizures
Introduction
From the viewpoint of a wide delivery of drugs to many patients, safety is of paramount importance. Thus, the development of highly safe drugs is desirable. Therefore, in recent years, it has become more and more important to select compounds with the desirable safety and effectiveness profile during the early stages of drug discovery. Simplified cell-based assay systems using monolayer culture have been used for toxicological drug screening due to the limited quantity of the compounds and the throughput ability of the systems. While the monolayer culture system is a very effective tool for detecting particular tissue-specific toxicity phenotypes, it is difficult to detect toxicity based on complex mechanisms involving multiple organs and tissues. Several new in vitro assay tools, e.g., spheroid/organoid culture, organ-on-a-chip, or tissue slice, are being developed to improve the detectability of a monolayer culture. Most of these new in vitro assay tools, however, do not have sufficient throughput capability and are expensive on a cost per assay basis, compared to conventional tools. Novel high throughput in vivo systems, therefore, are needed for early stage drug screening.
As an aquatic species, the zebrafish has been used for a long time for environmental toxicity assessment of chemicals, and its application to drug discovery research has been expanding in the last decades. Many technologies, including live imaging, genome editing, among others, have accelerated the use of zebrafish by pharmaceutical industries. Also, because their larvae are amenable to microplate-based application, the use of zebrafish for safety assessment or toxicology research is increasing year by year.
In this article, the author focuses on zebrafish-based assay systems of teratogenicity, seizure, and cardiotoxicity, which are already in practical use. The author also recognizes assay systems of hepatotoxicity and nephrotoxicity that are expected to be in practical use in the near future. Furthermore, the author also mentions the possibility of a zebrafish abuse liability model that is thought to be useful not only for drug development, but also for addressing the social problems of drug abuse.
Zebrafish as an Experimental Species
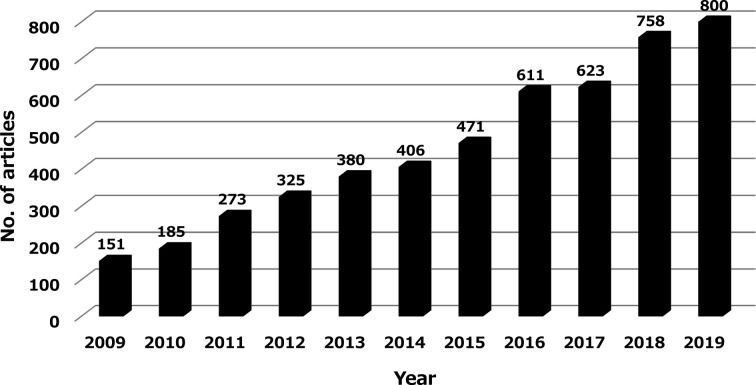
The zebrafish is a tropical freshwater fish of Indian origin, whose adult body length is about 4–5 cm1. It has been used as a disease model in basic research since the 1970s and became widely used in embryology research in the 1990s when gene intervention technology became readily available. Since the zebrafish in vivo assay system can have a high throughput, its use in recent years has been expanded to drug discovery research, especially in the toxicology research areas, such as those described later on. Indeed, the number of research articles that can be searched with “zebrafish” and “toxicity or toxicology” in Pubmed has been growing steadily and has increased more than 5-fold over the past decade (Fig. 1).
Fig. 1.
Number of research articles found by searching “zebrafish” AND “toxicity” OR “toxicology” in Pubmed, over the past decade.
The reasons why zebrafish is now drawing attention as an experimental species useful in toxicological research include:
1. It is a vertebrate whose major tissues and organs are comparable to those of humans2, 3.
2. It has more than 70% of genomic homology to humans, and about 84% of known human disease-causing genes4.
3. A comprehensive analysis of all transcripts has been completed and an Expressed Sequence Tag (EST) database has been developed5.
4. Genetic manipulation, such as genome editing, is easy to apply6.
5. Its lifetime is short, making it easy to create a disease model6.
6. The embryos and larvae are small enough to keep in 384-well microplates (50 μL of water)6.
7. Breeding is easy and a large number of animals can be kept in a small space with low maintenance costs (which, for example, is one dollar per mouse and one cent per zebrafish)6.
8. It is highly fertile (about 200 eggs per spawn every week) and it is easy to prepare the required number of animals7.
9. Zebrafish can absorb test compounds through the mouth, gills, or skin, especially through the skin of larvae8. Thus, test article administration can be done just by adding the compound to breeding water.
10. Water insoluble compounds, macromolecular compounds, or proteins can be also administered by direct injection into the yolk sac, vein sinus, or circulating blood9. In addition, compounds can be administered to adult zebrafish via an oral or intraperitoneal route10, 11.
11. As it uses external fertilization, live embryos are easily accessible for manipulation.
12. Its development is extremely rapid, making it easy to conduct a developmental study in a short period (organogenesis is almost completed within 24 hours after fertilization, and hatching occurs within 3 to 4 days)2.
13. Embryos and larvae are transparent throughout the development period and morphological monitoring can be done throughout all developmental stages without dissection9.
On the other hand, some limitations of the zebrafish as an experimental species have been pointed out:
1. Some tissues/organs of mammals are missing (lungs, prostate, mammary glands, among others)12.
2. Some tissues/organs of zebrafish are embryologically different from those of mammals (kidneys [single nephron {pronephron}in larvae, free of Henle loops]13, 14, heart [one atrium /ventricle]15, among others).
3. Some tissues/organs of zebrafish quickly regenerate, unlike those of mammals (retinas, spinal cord, kidneys, heart, and liver, among others)16.
4. Adult fish are still too large for use in high throughput screening.
5. Because of the limited amount of material in its biological samples (blood, tissues, among others), multi-endpoint assays or pharmacokinetics/pharmacodynamics (PK/PD) in a single animal are difficult to conduct/evaluate.
6. Because of its small size, technical skills are required for experimental operations (administration and blood/tissue sampling in adult zebrafish, among others).
Understanding before Application of Zebrafish to Toxicology Research
Developmental stages of zebrafish
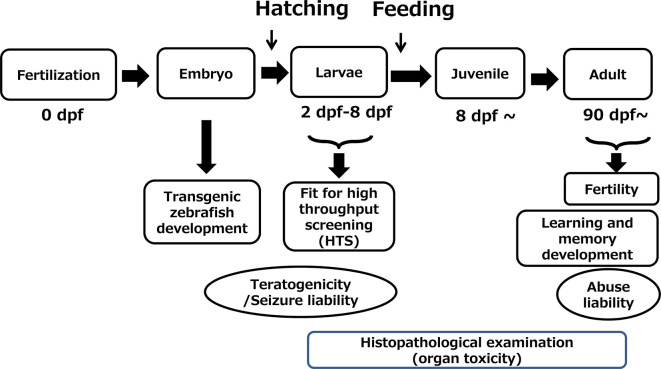
Examples of zebrafish applications in toxicology research have been reported, but the developmental stages used are different depending on the research purpose (Fig. 2). Zebrafish larvae up to 8 days of post fertilization (dpf) are suitable for high throughput screening because they can grow without nutrient supply. When larvae are over 8 dpf, they need a nutrient supply from the external environment. At this stage, only animals with a sufficient ability to access food can survive. Since the transparency of individuals rapidly decreases as they transition from juveniles to adults, microscopic assessment requiring transparency, such as teratogenicity evaluation, become more difficult. On the other hand, tissues and organs develop greatly as a juvenile ages into an adult, making it easy to take out various tissues or organs and conduct histopathologic examinations. Thus, the adult stage can be used for evaluation of organ toxicity. Furthermore, because the brain also develops with age, some behavioral tasks that require higher brain functions, such as memory and learning, can be evaluated in the adult stage. Thus, each developmental stage of zebrafish (embryo, larvae, adult) has advantages and disadvantages and a proper stage should be selected depending on the purpose of the study.
Fig. 2.
Developmental stages that are suitable for each research purpose. dpf: days post-fertilization.
Endpoints in zebrafish systems for toxicology research
There are various endpoints that can be used for zebrafish applications in toxicology research. Although embryonic death is a simple endpoint to use for evaluating general toxicity, more complex endpoints, that take advantage of the zebrafish as a whole-body system, can be used. Endpoints of whole-body assay systems are largely divided into three categories: (1) morphological changes, (2) functional evaluation, and (3) behavior.
Examples of Applications
Embryo and fetal developmental toxicity
Mammals, such as rats and rabbits, are commonly used to assess a drug’s embryo and fetal developmental toxicity (EFD). However, these EFD studies using mammals are not suitable for early-stage drug development screening because of limited throughput, high cost, and long duration. In addition, alternatives to animal testing have been actively sought from the viewpoint of the 3Rs principle for a more ethical use of animals in testing. To date, whole embryo culture (WEC)17 and embryonic stem cell test (EST)18 have been established as in vitro assay systems with modest throughput. However, these assay systems are not commonly used in drug discovery, because the culture period (of up to 3 days) prevents the use of WEC for evaluating the entire organogenesis process and EST has only been used to evaluate differentiation into cardiomyocytes. Thus, EFD assays using zebrafish have attracted attention as new alternative in vivo assays to overcome these problems, because they can cover the entire organogenesis process and can be used to evaluate not only mesodermal, but also ectodermal and endodermal derived organs. In addition, regarding the selection of the animal species for EFD assay, the zebrafish has an advantage over mammals in its rapidity of organogenesis and ease of visceral observation due to the transparency of its embryo. Actually, many reports have shown that teratogenicity in mammals could be detected in zebrafish19, 20. Figure 3 shows typical morphological changes, suggesting that malformation occurs in zebrafish treated with retinoic acid. As such, pharmaceutical companies began using this assay for compound screening to find drug candidates or to evaluate the risk of drug candidates to humans as part of a safety study to support the regulatory applications.
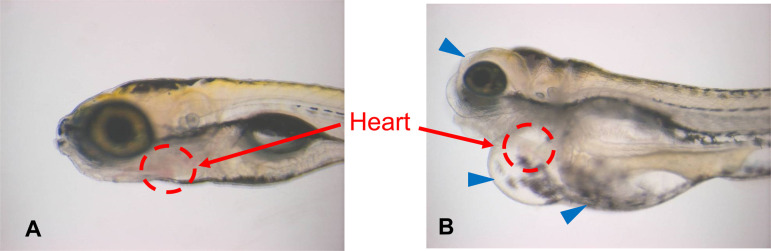
Fig. 3.
Typical morphological changes in zebrafish larvae treated with vehicle (A) or retinoic acid (B). Edema in face, heart, and abdomen (arrowhead) was observed in the larvae (6 dpf) treated with retinoic acid (0.013 μM).
The study concept is basically similar to that in EFD studies using mammals. Namely, the zebrafish is exposed to test compound during the organogenesis period, and after the exposure, morphology of the external surface, skeleton, and viscera of the body are examined. Generally, in assay systems using zebrafish, it is common to collect the fertilized eggs and to examine the morphology of the structures, such as the body shape, somites, notochord, tail, fins, heart, face, neural tube, pharyngeal arches, and jaw, after exposure to the compound during the organogenesis period (at least 5–72 h post fertilization [hpf])21. However, in the protocol, visual observations of morphology have to be manually conducted for each individual and their many and complicated endpoints (items). Consequently, some of the throughput of the assay is sacrificed. To address the problem, some researchers have proposed more simplified methods. Yamashita et al. reported a high-throughput and high-sensitivity assay method22. To increase the throughput, they limited the examination to the heart, face, body shape, and blood circulation, and scored them individually according to the extent of the morphological abnormalities and functional endpoints. Teratogenicity of each test compound was judged based on the respective score with a cut-off value set by analysis of the background data. Another way to increase the throughput of the assay is to automate morphological observation. Actually, some imaging technology (high-speed confocal imaging and laser manipulation for superficial and deep organ imaging) has been applied23. When this technology becomes available, the throughput of the zebrafish EFD assay will be greatly improved.
On the other hand, discussions about the scope of the regulatory use of the assay are actively progressing in the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use(ICH)S5(R3) Expert Working Group24. At present, the guideline does not directly mention the use of zebrafish, but their use for EFD assays is permissible as “non-mammalian in vivo assays (alternative assays).” Although the alternative assay methods are not described in the guideline, data accuracy and credibility obtained from robust assays are especially required. For that, creating a standard protocol that harmonizes those in Japan, U.S., and Europe is the most important issue to be addressed. Regarding the EFD assay protocol, the methodological concept is overall the same, but there are a lot of variations in details (zebrafish line, exposure period, observation endpoints, criteria of evaluation, solvents, among others). There are concerns that such variations could lead to inter-laboratory differences and poor data reproducibility. Actually, as for differences in the zebrafish line, the EK line is known to be especially sensitive to ethanol, a typical teratogen, when compared with other lines25. As part of increasing the credibility of the zebrafish EFD assay system, large-scale verification has been conducted by a consortium that aimed to confirm inter-facility differences and harmonize methods. In the consortium by American and European pharmaceutical companies, an optimum assay protocol was achieved that had an 85% concordance with the data of mammalians26. Regarding the use of this zebrafish EFD assay for regulatory purposes in pharmaceutical companies, some problems remain unsolved, other than the above, such as: to evaluate the effect of metabolites, to confirm drug exposure levels, and to ensure the reliability of the data (GLP or not).
Central nervous system toxicity
There are various neurotoxicity evaluation systems that use zebrafish. Parng et al. reported that tissue damage of the central nervous system, including the optic nerve, motor nerve, dopaminergic neurons, and myelin sheath could be observed histopathologically in zebrafish27. In addition, neural cell apoptosis, proliferation, and oxidation can be monitored externally in zebrafish because of its transparency. Pathological changes in zebrafish treated with neurotoxic substances, such as ethanol, 6-OHDA, taxol, acrylamide, 2,3,7,8-Tetrachlorodibenzodioxin (TCDD), neomycin, and retinoic acid have been reported to be similar to those in mammals27. However, to date, neurotoxicity evaluation by morphologic endpoints is not common, and behavioral endpoints are mainly used in drug discovery research.
Hereafter in this article, the author focuses on the seizure liability and abuse liability assays applications.
Seizure liability
Seizure is a critical toxicity in drug development, and some drugs have been withdrawn from the market due to their association with the risk of seizures28. As seizure induction is often seen in non-clinical toxicity studies of drugs (especially the ones that act on the central nervous system [CNS]), it is important to screen out drugs associated with seizure risks as early as possible in the drug discovery process.
The use of rodent models for evaluation of seizure risks is unsuitable for early stage drug screening. To improve the throughput ability, several in vitro assay systems, such as brain slice cultures29, primary culture of neurons30, and nematodes (C. elegans)31 have been proposed. However, the use of these assay systems is limited by inaccuracy due to pharmacokinetic differences caused by drug metabolism, penetration of the blood brain barrier (BBB), or lack of the anatomical structure of the CNS.
In the zebrafish brain, the existence of GABAergic neurons32 and H1 receptor33, which play an important role in the occurrence of seizures, and BBB34, 35 has been confirmed. Regarding the BBB, although expression of tight junction proteins, such as ZO-1 and Claudin-5, has already been observed at 3 dpf, the results of transcytosis experiments have shown that BBB functional maturity is reached at 5 dpf36. Thus, the zebrafish is thought to have the minimal functions required for occurrence of seizures, and is expected to be a new model for assessing drug-induced seizure risk.
For evaluation of the seizure-inducing potential using zebrafish, electrophysiological field potential recordings electroencephalogram (EEG) and behavioral assays have been introduced. For recording the brain electrophysiological field potential, a method involving the insertion of a single glass electrode into the intracranial space or on the top of the cranium of an adult paralyzed fish was initially used37. Recently, a multi-electrode array method which can non-invasively record electrical activity of the heads of intact larvae using a MED64 system has been reported38. Actually, the use of zebrafish as a screening system for assessment of seizure risk is now already in practice, and several facilities have utilized these systems39.
A behavioral assay to assess seizure risk is focused on larval zebrafish locomotor activity using a video tracking system. In this assay, high speed movements were defined as seizure-like behavior and were quantified40, 41. This type of assay is more practical than brain electrophysiological field potential recording; however, its prediction ability for seizure in mammals has not been sufficient, because some typical seizure-inducing drugs (e.g. antibiotics, antihistamines, and antidepressants) are not detected. To improve the prediction ability of the previously reported method, the author focused on the rapid motion (locomotion and circling movement) of zebrafish after exposure to pentylenetetrazole (PTZ), and extended the exposure time and combined it with light stimulation (Fig. 4). As a result, the author succeeded in improving the sensitivity of the method42.
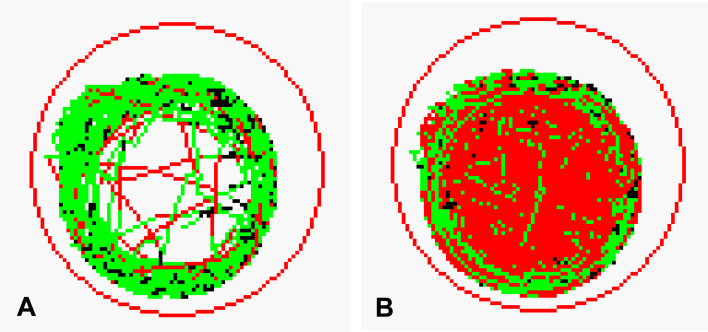
Fig. 4.
Track of movement of larval zebrafish treated with vehicle (A) or Pentylenetetrazole (B). The low, medium, and high speeds are indicated by the black lines (<5 mm/s), green lines (5–20 mm/s), and red lines (>20 mm/s), respectively. Lines in each figure are tracks of movement for one zebrafish at each speed.
Abuse liability
Drug abuse has social, physical, and economic impacts, and has been a worldwide issue for a long time. Thus, regulatory authorities, such as the FDA and EMA, require preclinical and clinical assessment of abuse potential of any compound with central nervous system activity. Drugs with abuse potential are subject to restrictions in manufacturing, distribution, use, and handling, from the regulatory authorities at the time of approval or after the approval. Once such drugs are on the market, pharmaceutical companies must take responsibility for the various social problems associated with the drugs. Therefore, it is important to identify the drugs with abuse potential early in the process. However, currently available nonclinical methods are used to evaluate abuse potential in either rodents or monkeys, and these methods are not practical to use at an early stage of drug discovery due to their time- and cost-intensive nature. Under such circumstances, the zebrafish is now attracting attention as an animal model for detecting abuse potential in the drug discovery process.
Although the brain of the zebrafish is neuroanatomically simple when compared with that of mammals, the basic structure is similar, and it has a distinct forebrain, mesencephalon, and hindbrain43. Though the zebrafish brain does not have a nerve projecting to the nucleus accumbens from the ventral tegmental cortex of the middle cerebrum, which is known as the reward system in mammals, the dopamine nerve projecting to the forebrain, which corresponds to the midbrain border of mammals, has been confirmed27, 44. Furthermore, information on neurotransmitters in zebrafish has been accumulating; the presence of GABA, dopamine, histamine, glutamate, norepinephrine, serotonin, acetylcholine, and glycine in the central nervous system is confirmed in zebrafish, as well as in mammals45. In addition to these neurotransmitters, the zebrafish brain also expresses opioid μ receptors that are deeply implicated in drug abuse in mammals, and the biological function of these neurotransmitters and receptors is thought to be the same as those of mammals46. In a microarray analysis of the brain of zebrafish treated with ethanol or nicotine, changes in gene pathways that are related to abuse liability in humans were confirmed 47.
As for learning and memory, the zebrafish does not have a hippocampus, which is thought to be important for spatial learning in mammals. Nonetheless, its learning behavior based on instincts, such as seeking food and avoiding danger, is already observed in larvae. From this, it is assumed that the zebrafish has memory and learning systems that are different from those of mammals, and it is believed that the cerebral cortex plays a role in memory and learning48. Actually, memory and learning have been actively studied in zebrafish. It has been reported that zebrafish can behave similarly to mammals in experimental tasks such as a T-maze49, radial maze task50, fear conditioning memory learning51, and alternating reaction learning52. Thus, the zebrafish can perform associative learning tasks that indicate the presence of higher brain functions, even if its brain structure is quite simple. For this reason, the zebrafish is thought to be applicable to abuse liability assays.
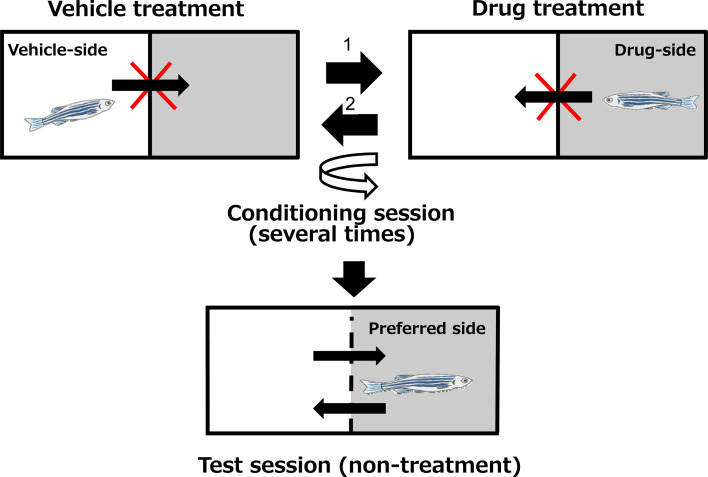
As for the evaluation of drug abuse liability, conditioned place preference (CPP) tests in rodents are generally used as a simple method. To date, some rodent CPP-based methods using zebrafish to assess the abuse potential of drugs have been reported (Fig. 5). In principle, animals memorize the drug intake sensation in association with some spatial cues in the experimental device (box), and consciously move to the preferable space. The reward effect is evaluated quantitatively by calculating the difference between the lengths of time animals spend in the drug box and in the non-drug box. It has been reported that the reward effects of major drugs with abuse potential, such as amphetamine53, morphine54, cocaine55, nicotine50, and ethanol56, can be detected successfully in zebrafish using the CPP method.
Fig. 5.
Paradigm of the Conditioned Place Preference (CPP) method using zebrafish.
The above evaluations were conducted using adult zebrafish, and there has been no report of abuse liability studies using larvae. From the viewpoint of cost, the amount of test substance and ease of penetration into the brain, larvae are preferable to adults for drug screening in CNS evaluation during the early stages of drug development. Considering that some reports of memory and learning tests use larvae of 6 to 8 dpf, it can be assumed that larvae at this stage are already equipped with the basic functions of memory and learning. However, to detect the reward effect using the CPP method, considerably higher brain functions are necessary. To be used in the CPP method, the brain has to do a series of work including: (1) perception and recognition of sensory stimulations by drugs, (2) processing of the sensation information with spatial information in the brain, (3) fixing them as memory, (4) recalling the memory based on spatial information, (5) invoking action based on the memory. Though it is unknown so far whether larvae have such sophisticated brain functions, in the author’s experience, it seems difficult to detect the reward effect of drugs in larvae using this CPP method. However, since the sensitivity could be increased by another approach, such as genetic manipulation, future progress can be expected.
Hepatotoxicity
Drug-induced liver injury (DILI) is one of the most common causes of developmental termination or withdrawal from the market57. As in vivo evaluation using mammals is time consuming and labor intensive, various in vitro assay systems have been reported, and cryopreserved human hepatocytes have been used as a gold standard for DILI screening so far. Monolayer culture systems, however, do not have sufficient potential for DILI evaluation, and many novel methods, e.g., co-culture with non-parenchymal cells58, 3D spheroids59, organ-on-a-chip models60, and liver slice models61, have been proposed. Although these methods are useful for accurate in vitro evaluation, their assay systems are complicated and hard to use in early-stage screening. Therefore, simple, miniaturized, high-throughput in vivo evaluation systems are required for drug screening, and zebrafish are at the forefront of candidate systems. Some reports suggest that the zebrafish can be a versatile preclinical model for DILI screening62. In addition, the combined use of zebrafish and an in vitro whole cell imaging assay enhances the detection of DILI63.
The livers of zebrafish have bile ducts, portal veins, and hepatic arteries that are randomly placed in the hepatic parenchyma64. Despite the anatomical differences between the species, cells constituting the liver are the same as in mammals. Pathological changes, such as cholestasis65, steatosis66, necrosis67, and hepatic tumors68 have also been reported in zebrafish.
The hepatic tissue of zebrafish is also known to perform fundamental functions such as lipid metabolism, vitamin metabolism, protein synthesis, or growth factor synthesis. In addition, 94 isoforms of cytochrome P450 (CYPs) have been identified69, and 18 CYP gene families of zebrafish are homologues to those of humans or other mammals. However, whether expression of these CYPs is accompanied by sufficient activity remains controversial70, and it is also pointed out that the CYP2 family has low homology with the mammalian CYP2 family69. Therefore, it may be necessary to transfer a CYP gene into zebrafish to detect DILI via metabolic activation.
As for the defense mechanism against xenobiotic-induced injury, it has been reported that mechanisms against oxidative stress, such as the Keap1-Nrf2 system, exist in zebrafish as well as in mammals71. The author has reported the usefulness of Nrf2a-deficient zebrafish, created using the CRISPR-Cas9 technique, for evaluating oxidative stress-related liver toxicity in drug discovery research72. Although such genome editing technique can improve the detection power and simplify the phenotypic assay visually73, it requires some skills and time.
On the other hand, representative hepatotoxic drugs in mammals were reported to induce hepatotoxicity in wild-type larvae without genome editing74. With regards to the experimental design, usually 3–5 dpf larvae are treated with drugs for 48–72 h. The endpoints used include: histopathology (degeneration, necrosis, vacuolation, among others), liver size or yolk retention measured using live images, classical liver enzymes (aspartate transaminase [AST] or alanine aminotransferase [ALT]), and novel biomarkers in the liver, which will be described later. Among these, liver size reduction results from liver inflammation, degeneration or necrosis, and yolk absorption can be delayed if liver functions are impaired. Thus, it is suggested that liver size and yolk sac size in larvae are both good markers of hepatotoxicity from a quantitative point of view74.
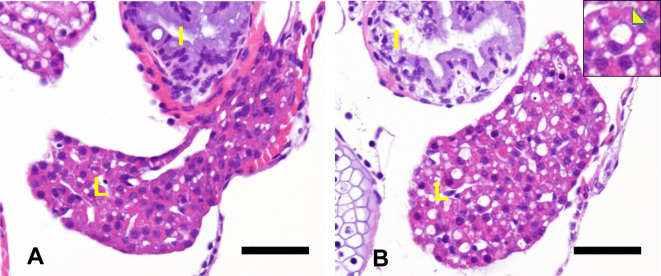
An example of morphological changes induced by acetaminophen in 3 dpf zebrafish is shown in Figure 6. Increased vacuolation of hepatocytes can be seen, which is thought to be a degenerative change. In the author’s experience, vacuolization is often histopathologically observed when juvenile zebrafish are briefly exposed to a hepatotoxic substance, and there are many similar reports of morphologic effects by hepatotoxic substances (tamoxifen, among others).
Fig. 6.
Histological images of the liver of zebrafish larvae (4 dpf) treated with vehicle (A) or 8 mM acetaminophen for 24 h (B) (hematoxylin and eosin [H&E] stain). Vacuolation of hepatocytes can be seen in the acetaminophen-treated fish. Scale bar = 40 μm. Inset shows the higher magnification of vacuolated hepatocytes (arrowhead). L: liver, I: intestine.
As a potential biomarker of hepatotoxicity, gene expression in the liver was investigated using in situ hybridization (ISH) or reverse transcription polymerase chain reaction (RT-PCR). Verstraelen et al. detected already known candidate genes and estimated the predictability of hepatotoxicity of tested drugs in mammals. As a result, they concluded that in adult zebrafish, the same hepatotoxicant specifically alters the expression of genes encoding albumin-like protein, ceruloplasmin, liver fatty acid binding protein (L-Fabp10a), and transferrin, all of them regarded as biomarkers of hepatotoxicity75. In addition, liver expression of miR-122, a liver-specific toxicity biomarker in mammals, was also reported to be a possible biomarker of hepatotoxicity in zebrafish76.
Nephrotoxicity
Kidney is an anatomically complex organ that has multiple physiological roles, and in addition to the liver, it is one of the most common toxicity targets. Thus, methods to evaluate nephrotoxicity are very important; however, there are no available in vitro screening methods that have a good correlation with nephrotoxicity in mammals. As for cell culture systems, 2D monolayer culture has been used at the drug discovery stage because of its simplicity and low cost. However, problems exist, such as the fact that epithelial cells in monolayer culture are not well differentiated, and that there is a lack of three dimensional interactions of renal tubules and blood vessels, as well as other tissues and cells that play diverse roles, such as metabolic waste excretion, balancing acid-base levels, maintenance of fluid homeostasis, and hormone secretion. Thus, the applicability of in vitro assay systems to test nephrotoxicity are limited since a single type of cell cannot perform all the functions of the kidney. In recent years, many researchers have tried to create “kidney-like tissue” structures in vitro and use them for toxicology77, 78, 79. Although these stem cell-derived organoid tissues might have the potential to mimic in vivo toxicological phenotypes, these systems have been associated with several drawbacks linked to a lack of robustness of differentiation from stem cells, difficulty determining the quantitative endpoints for toxicity, and a lack of sufficient throughput ability. The zebrafish is now expected to serve as a new model system that can overcome the limitations of in vitro nephrotoxicity assay systems.
In mammals, three progressively complex kidney structures develop during embryogenesis (the pronephros, mesonephros, and metanephros). On the other hand, only the pronephric and mesonephric kidneys develop in zebrafish80. Regarding larvae, the functional pronephros contains only two segmented nephrons, which fuse with a glomerulus located on the ventral side of the dorsal aorta, and contain two proximal and two distal tubule segments81. On the other hand, in adult zebrafish, the mesonephros begins to form between about 12 and 14 dpf, with the progressive addition of nephrons to the existing pronephric pair attached to the dorsal body wall and maturation into a functional “permanent” kidney82. This mesonephros has segments similar to those found in the pronephros, but they are more numerous (approximately 450 nephrons at 6 months of age) and grouped in branched arrangements81. Despite the phylogenetic differences between zebrafish and mammals, the composition of the nephron in zebrafish is similar to that in humans at the cellular and molecular levels83.
In toxicity studies using zebrafish, renal injury can be evaluated by examining both the morphology and function, similar to general toxicity studies using mammals. Considering the use of larvae in the assessment of nephrotoxicity, its biggest advantage is simplicity. The most notable indicator of nephrotoxicity is “edema,” which can be grossly evaluated84. While the gills in larvae, which are known to be dominant sites of ammonia excretion and osmoregulation, remain immature up to one week after fertilization, glomerular filtration of the kidney is complete by 48 hours after fertilization. As the kidney is responsible for waste discharge from the body at the early developmental stage, edema often results from a collapse in body fluid homeostasis when the larvae are exposed to nephrotoxic substances. Gorgulho reported the usefulness of larvae for the evaluation of nephrotoxicity, focusing on the morphological and functional changes, and showed that all three different nephrotoxicants commonly induced mitochondrial abnormalities, including dysmorphic shapes, mitochondrial swelling, cristae disruption, and/or loss of matrix granules, which suggested that these mitochondrial alterations may be a good indicator of nephrotoxicity85.
As for renal functions in zebrafish, the glomerular filtration rate can be determined from the clearance of high molecular weight fluorescein isothiocyanate or dextran from the circulating blood86. These substances, however, have to be manually injected into the larval venous sinus of the heart. Considering the technical difficulties of carrier injection into the larval venous sinus, these methods of functional analysis might not be applicable for routine assays. Alternatively, abdominal injection can be easily done in adult fish87.
Other than the above, in contrast to mammals, zebrafish can generate new nephrons throughout their life span, and neo-nephrogenesis is reported to be increased after renal injury88. Thus, generation of new nephrons (nephrogenesis) is a possible indicator of renal damage in both larvae and adult fish.
So far, specific biomarkers of renal function, equivalent to blood urea nitrogen (BUN) and creatinine in mammals, have not been identified in zebrafish. However, proteinuria in incubation medium has recently been introduced as an indicator of a glomerular filtration barrier function in larvae89. To conduct a biochemical examination in larvae, the biggest problem is collecting a sufficient volume of sample (blood, urine, among others) due to the small size of the animal. Thus, homogenates of whole body are often used instead of body fluids. Some researchers have tried to measure the creatinine content in the whole body to evaluate renal toxicity90. Also, whole-body transcriptional changes in certain genes, such as the Nephrin gene, have been reported as biomarkers of renal toxicity in larvae89.
As examples of drug-induced kidney injury (DIKI) in zebrafish, the effects of gentamicin and cisplatin on the kidney have been well investigated84. Nephrotoxicity induced by gentamicin in larvae is histopathologically characterized by lysosomal phospholipidosis, flattening of brush borders, accumulation of debris within the tubular lumen, and tubular and glomerular dilatation, which is similar to the manifestations of aminoglycoside-induced nephrotoxicity seen in mammals. In addition, intraperitoneal accumulation of leucocytes with occasional infiltration into the glomeruli, a typical feature of DIKI in humans in aminoglycoside treatment, was also reported84. Regarding the cisplatin-induced nephrotoxicity in larvae, cellular vacuolation, flattening and loss of brush borders, tubular dilatation, and a marked decrease in cell height in the proximal pronephric tubules were noted84, very similar to findings in mammals.
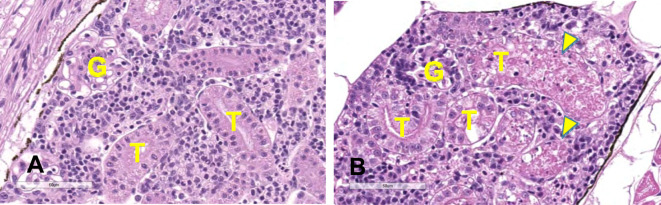
On the other hand, the use of adult zebrafish for the evaluation of nephrotoxicity has also attracted attention. As described above, unlike larvae, adult zebrafish have kidneys with numerous nephrons, a higher degree of structural complexity, and functional maturity91, 92, 93. Therefore, the response of adult zebrafish to nephrotoxic agents can be expected to be more similar to that of mammals. A large-scale study on the reactivity of adult zebrafish to existing nephrotoxicants, with a focus on histopathological examination, reported94 that adult zebrafish not only exhibit pathological changes similar to those of mammals, but are also very sensitive to drugs that cause proximal tubular injury, such as cisplatin, gentamicin, among others. Even in the author’s experience, tubular necrosis, which is unlikely to occur in larvae, was histopathologically noted in adults treated with cisplatin (Fig. 7). Further, adults are technically easier to handle on an individual basis than larvae, and the functional evaluation of the kidney can be incorporated routinely into the toxicity evaluation, as described above. Additionally, it may also be possible to detect kidney-specific biomarkers by collecting the kidney itself from adult zebrafish. Although further detailed studies are needed in the future, several nephrotoxic gene marker candidates have been proposed in the adult zebrafish. While the sensitivity to toxic substances is often higher in embryos and larvae than in adults, it seems that embryos and larvae have an immature mechanism of defense against toxic substances. In addition, the adult zebrafish kidney has a high regenerative capacity, including nephron epithelial regeneration and neonephrogenesis87, which may be one of the causes.
Fig. 7.
Histological images of the kidney of adult zebrafish (3 months of age) treated with vehicle (A) or 100 μM cisplatin for 72 h (B) (hematoxylin and eosin [H&E] stain). Normal glomerulus (G) and tubules (T) can be seen in the vehicle-treated fish. Necrosis of tubules (arrowheads) can be seen in the cisplatin-treated fish.
The evaluation using larvae or adult fish each has its pros and cons. Therefore, when assessing nephrotoxicity in zebrafish, it is desirable to select a test system according to the purpose and then to give due consideration to their specific features.
Cardiotoxicity
Cardiotoxicity, such as proarrhythmia or cardiac contraction abnormality, among others, is a major cause of termination of drug development in the early and late stages. Thus, the appropriate evaluation of the cardiotoxicity in the early stage is very important for drug development.
Cardiac function can be affected, even if not directly, by effects on the central nervous system, the autonomic nervous system, and the endocrine system. Some cell-based assays, such as the human ether-à-go-go-related gene (hERG) and Cav/Nav assays used for proarrhythmia evaluation and an ex-vivo assay, such as the Langendorff assay used for cardiac contraction abnormality, are both general tools in the early stage of drug development.
On the other hand, whole body cardiac function assays (proarrhythmia and cardiac contraction abnormality) using animals (e.g., non-rodent telemetry) are considered to be more important for decision making in drug development. However, conducting in vivo studies in mammals at the discovery stage of drug development is unrealistic because of the throughput issue. Therefore, expectations are high for the success of more concise cardiotoxicity evaluation systems, like the zebrafish model, which is one of the smallest experimental vertebrate animals.
As for zebrafish embryology, the heart of the zebrafish is derived from the mesoderm during the developmental period, with cardiac tubes being formed by 26 hpf and the cardiac loop being formed during symmetrical extension. Blood flow begins at 24 hpf, and then angiogenic vessels that perfuse the trunk of embryo (intersegmental vessels) sprout from the vasculogenic vessels. At 48 hpf, the basic structure of the heart is complete after formation of the valves and endocardial endothelial cells of the AV junction (atrioventricular node). In this process, initiation of blood flow plays an important role in advancing heart development. The process of heart development is thought to be generally the same in zebrafish and in mammals95. Also, major molecular pathways regulating angiogenesis have been reported to be conserved between zebrafish and mammals.
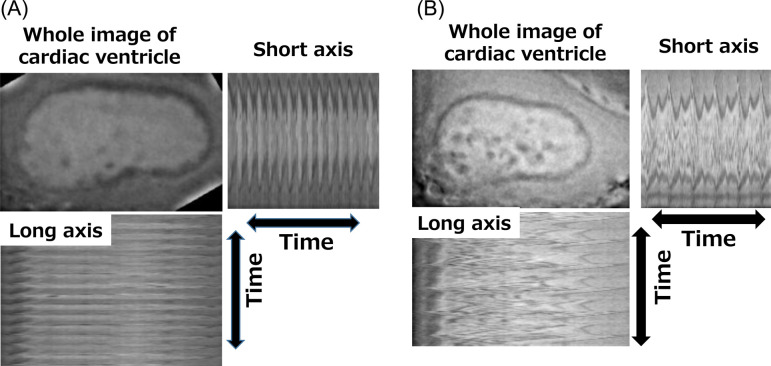
The heart anatomically consists of one atrial chamber and one ventricle in zebrafish and two atrial chambers and two ventricles in mammals. The hearts of zebrafish have an advantage over the hearts of mammals in they can be observed using live imaging techniques, making it simpler and easier to capture a dynamic change of contraction. On the other hand, the zebrafish has no circulatory route equivalent to pulmonary circulation, and the effect of this on the evaluation of the cardiac function is unknown. As examples of drug-induced cardiotoxicity, Milan et al. reported that electrocardiographic monitoring in zebrafish could detect QT prolongation induced by drugs known to cause QT prolongation in humans (astemizole, haloperidol, pimozide, and terfenadine)96. They also demonstrated that it was possible to measure the heart rate in larvae by observing the movement of the heart with a CCD camera under a microscope, and assessed the validity of using the heart rate as an indicator of cardiotoxicity (arrhythmia) using about 100 compounds that are known to cause QT prolongation in humans. As a result, a good correlation was shown between bradycardia (heart rate reduction) in zebrafish and QT prolongation in humans97, 98. On the other hand, Umemoto et al. adapted the method of fluorescent cardiac imaging method to zebrafish99. Applying the fluorescent cardiac imaging method, Honda et al. were able to evaluate heart contraction in zebrafish first by measuring the end diastolic diameter (VDd) and the end systolic diameter (VDs), and then by serially analyzing the change in the lumen size of the ventricle (Fig. 8)100. They also demonstrated a good concordance between contractility in zebrafish and contractility in mammals using various known cardiotoxic compounds, such as myocardial ion channel inhibitors, β agonists, and so on. Interestingly, by monitoring the movement of the heart simultaneously with the above contractility evaluation, atrioventricular block, characterized by unsynchronized beating between the atria and ventricles, was detected in the presence of compounds that are known to cause TdP/QT prolongation in mammals.
Fig. 8.
Typical cardiac imaging using fluorescence probes in a control zebrafish larvae (A) and zebrafish larvae with drug-induced arrhythmia (B).
The transgenic (Tg) zebrafish is useful when evaluating functional changes of the heart. Most strains of genetically modified zebrafish were originally created to examine the effects of substances on cardiogenesis, but they can also be used for cardiotoxicity assessment, depending on the purpose.
Wen et al. evaluated the heart rate by using Tg zebrafish, which expresses green fluorescent protein (GFP) in the heart, and by capturing the movement of the atrium and ventricle using fluorescence imaging101. They concluded that the change of heart morphology in the Tg zebrafish can be observed more easily, making the evaluation of cardiac function more accurate than that in wildtype zebrafish.
Yozzo KL established a high throughput cardiotoxicity assay system using transgenic zebrafish (fli1:egfp) that stably express eGFP102. The system was a high-content screening system with morphological endpoints (body length, pericardial area, and intersegmental vessel area) and functional endpoints (heart rate and circulation). In addition to the above functional changes, it was suggested that structural changes could be detected by examining the progression of heart failure morphologically (damage, cardiac arrest) and changes in specific molecular markers of cardiac defects, such as cardiac troponin T and atrial natriuretic peptide103.
On the other hand, zebrafish use in cardiovascular research has some limitations:
1. Usual study designs including zebrafish embryos are unsuitable for studying many of the chronic cardiovascular disease processes, such as chronic heart failure, atherosclerosis, and aortic aneurysm seen in mammals because of the short treatment period104.
2. Cardiac muscles can regenerate in zebrafish, unlike in mammals105.
Conclusion
Zebrafish have only been used recently for safety testing in drug discovery. However, the use of zebrafish is rapidly spreading due to their convenience and applicability. To date, few toxicities can be practically assessed using zebrafish. However, the number of evaluable target organs or toxicities is expected to increase in the future. Especially, this miniaturized in vivo assay system has the power to identify toxicities that simplified cell-based assay systems cannot detect. For toxicity that cannot be detected in wildtype zebrafish, the use of genetically modified or mutant zebrafish can be implemented and will be put to practical use by pharmaceutical companies in the future.
It is also desirable to establish a method that can obtain essential data, such as general toxicity, from one animal. Although it is necessary to quantify the effects of exposure, technical problems remain, such as insufficient ADME data in zebrafish, small blood sample size, measurement of drug concentrations in small samples, among others.
Although the analysis of the whole zebrafish genome has been completed, there is still missing information and background data, such as differences between species. Problems to be solved in the future include building up of fundamental knowledge about the zebrafish’s behavioral characteristics, anatomy, or physiological aspects, and improving breeding, quality maintenance methods, handling/experimental techniques, and so on. To solve these problems for practical application, collaboration between industry and academia is desired.
Disclosure of Potential Conflicts of Interest
The author has no conflicts of interest to be disclosed regarding this paper.
Acknowledgments
I wish to thank Tomoaki Tochitani, Akihito Yamashita, and Yayoi Honda for their expert advice and suggestions.
References
- 1.Spence R, Gerlach G, Lawrence C, and Smith C. The behaviour and ecology of the zebrafish, Danio rerio. Biol Rev Camb Philos Soc. 83: 13–34. 2008. [DOI] [PubMed] [Google Scholar]
- 2.Goldsmith JR, and Jobin C. Think small: zebrafish as a model system of human pathology. J Biomed Biotechnol. 2012: 817341 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams CH, and Hong CC. Multi-step usage of in vivo models during rational drug design and discovery. Int J Mol Sci. 12: 2262–2274. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 496: 498–503. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SHIGEN/ National Institute of Genetics (NIG). from Worldwide Genetic Resources website: https://s higen.nig.ac.jp/wgr/top/top.jsp.
- 6.Delvecchio C, Tiefenbach J, and Krause HM. The zebrafish: a powerful platform for in vivo, HTS drug discovery. Assay Drug Dev Technol. 9: 354–361. 2011. [DOI] [PubMed] [Google Scholar]
- 7.Hoo JY, Kumari Y, Shaikh MF, Hue SM, and Goh BH. Zebrafish: A versatile animal model for fertility research. BioMed Res Int. 2016: 9732780 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakravarthy S, Sadagopan S, Nair A, and Sukumaran SK. Zebrafish as an in vivo high-throughput model for genotoxicity. Zebrafish. 11: 154–166. 2014. [DOI] [PubMed] [Google Scholar]
- 9.Berens EB, Sharif GM, Wellstein A, and Glasgow E. Testing the Vascular Invasive Ability of Cancer Cells in Zebrafish (Danio Rerio). J Vis Exp. 117: 55007 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zang L, Morikane D, Shimada Y, Tanaka T, and Nishimura N. A novel protocol for the oral administration of test chemicals to adult zebrafish. Zebrafish. 8: 203–210. 2011. [DOI] [PubMed] [Google Scholar]
- 11.Drummond IA, and Davidson AJ. Zebrafish kidney development. Methods Cell Biol. 100: 233–260. 2010. [DOI] [PubMed] [Google Scholar]
- 12.Wolf JC, Baumgartner WA, Blazer VS, Camus AC, Engelhardt JA, Fournie JW, Frasca S, Jr , Groman DB, Kent ML, Khoo LH, et al. Nonlesions, misdiagnoses, missed diagnoses, and other interpretive challenges in fish histopathology studies: a guide for investigators, authors, reviewers, and readers. Toxicol Pathol. 43: 297–325. 2015. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura H, and Imai M. Control of renal function in freshwater and marine teleosts. Fed Proc. 41: 2355–2360. 1982. [PubMed] [Google Scholar]
- 14.McKee RA, and Wingert RA. Zebrafish renal pathology: emerging models of acute kidney injury. Curr Pathobiol Rep. 3: 171–181. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hove JR, and Craig MP. High-speed confocal imaging of zebrafish heart development. Methods Mol Biol. 843: 309–328. 2012. [DOI] [PubMed] [Google Scholar]
- 16.Marques IJ, Lupi E, and Mercader N. Model systems for regeneration: zebrafish. Development. 146: dev167692 2019. [DOI] [PubMed] [Google Scholar]
- 17.Webster WS, Brown-Woodman PD, and Ritchie HE. A review of the contribution of whole embryo culture to the determination of hazard and risk in teratogenicity testing. Int J Dev Biol. 41: 329–335. 1997. [PubMed] [Google Scholar]
- 18.Koseki N, Deguchi J, Yamada T, Funabashi H, and Seki T. Improvement of the embryonic stem cell test endpoint analysis by use of field potential detection. J Toxicol Sci. 35: 619–629. 2010. [DOI] [PubMed] [Google Scholar]
- 19.Panzica-Kelly JM, Zhang CX, Danberry TL, Flood A, DeLan JW, Brannen KC, and Augustine-Rauch KA. Morphological score assignment guidelines for the dechorionated zebrafish teratogenicity assay. Birth Defects Res B Dev Reprod Toxicol. 89: 382–395. 2010. [DOI] [PubMed] [Google Scholar]
- 20.Beedie SL, Rore HM, Barnett S, Chau CH, Luo W, Greig NH, Figg WD, and Vargesson N. In vivo screening and discovery of novel candidate thalidomide analogs in the zebrafish embryo and chicken embryo model systems. Oncotarget. 7: 33237–33245. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brannen KC, Panzica-Kelly JM, Danberry TL, and Augustine-Rauch KA. Development of a zebrafish embryo teratogenicity assay and quantitative prediction model. Birth Defects Res B Dev Reprod Toxicol. 89: 66–77. 2010. [DOI] [PubMed] [Google Scholar]
- 22.Yamashita A, Inada H, Chihara K, Yamada T, Deguchi J, and Funabashi H. Improvement of the evaluation method for teratogenicity using zebrafish embryos. J Toxicol Sci. 39: 453–464. 2014. [DOI] [PubMed] [Google Scholar]
- 23.Pardo-Martin C, Chang TY, Koo BK, Gilleland CL, Wasserman SC, and Yanik MF. High-throughput in vivo vertebrate screening. Nat Methods. 7: 634–636. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Draft ICH harmonized guideline: Detection of toxicity to reproduction for human pharmaceuticals S5(R3). 2017, from Pharmaceuticals and Medical Devices Agency (PMDA) website: https://www.pmda.go.jp/files/000220226.pdf.
- 25.Arenzana FJ, Carvan MJ, 3rd , Aijón J, Sánchez-González R, Arévalo R, and Porteros A. Teratogenic effects of ethanol exposure on zebrafish visual system development. Neurotoxicol Teratol. 28: 342–348. 2006. [DOI] [PubMed] [Google Scholar]
- 26.Gustafson AL, Stedman DB, Ball J, Hillegass JM, Flood A, Zhang CX, Panzica-Kelly J, Cao J, Coburn A, Enright BP, Tornesi MB, Hetheridge M, and Augustine-Rauch KA. Inter-laboratory assessment of a harmonized zebrafish developmental toxicology assay - progress report on phase I. Reprod Toxicol. 33: 155–164. 2012. [DOI] [PubMed] [Google Scholar]
- 27.Parng C, Roy NM, Ton C, Lin Y, and McGrath P. Neurotoxicity assessment using zebrafish. J Pharmacol Toxicol Methods. 55: 103–112. 2007. [DOI] [PubMed] [Google Scholar]
- 28.Tsutani K. Various aspects of drug withdrawal. Jpn J Clin Pharmacol Ther. 40: 7–16. 2009. [Google Scholar]
- 29.Easter A, Sharp TH, Valentin JP, and Pollard CE. Pharmacological validation of a semi-automated in vitro hippocampal brain slice assay for assessment of seizure liability. J Pharmacol Toxicol Methods. 56: 223–233. 2007. [DOI] [PubMed] [Google Scholar]
- 30.Deshpande LS, Lou JK, Mian A, Blair RE, Sombati S, and DeLorenzo RJ. In vitro status epilepticus but not spontaneous recurrent seizures cause cell death in cultured hippocampal neurons. Epilepsy Res. 75: 171–179. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandey R, Gupta S, Tandon S, Wolkenhauer O, Vera J, and Gupta SK. Baccoside A suppresses epileptic-like seizure/convulsion in Caenorhabditis elegans. Seizure. 19: 439–442. 2010. [DOI] [PubMed] [Google Scholar]
- 32.Kim YJ, Nam RH, Yoo YM, and Lee CJ. Identification and functional evidence of GABAergic neurons in parts of the brain of adult zebrafish (Danio rerio). Neurosci Lett. 355: 29–32. 2004. [DOI] [PubMed] [Google Scholar]
- 33.Peitsaro N, Sundvik M, Anichtchik OV, Kaslin J, and Panula P. Identification of zebrafish histamine H1, H2 and H3 receptors and effects of histaminergic ligands on behavior. Biochem Pharmacol. 73: 1205–1214. 2007. [DOI] [PubMed] [Google Scholar]
- 34.Jeong JY, Kwon HB, Ahn JC, Kang D, Kwon SH, Park JA, and Kim KW. Functional and developmental analysis of the blood-brain barrier in zebrafish. Brain Res Bull. 75: 619–628. 2008. [DOI] [PubMed] [Google Scholar]
- 35.Fleming A, Diekmann H, and Goldsmith P. Functional characterisation of the maturation of the blood-brain barrier in larval zebrafish. PLoS One. 8: e77548 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Brown NM, Megason SG, and Gu C. Suppression of transcytosis regulates zebrafish blood-brain barrier function. eLife. 8: e47326 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pineda R, Beattie CE, and Hall CW. Recording the adult zebrafish cerebral field potential during pentylenetetrazole seizures. J Neurosci Methods. 200: 20–28. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer M, Dhamne SC, LaCoursiere CM, Tambunan D, Poduri A, and Rotenberg A. Microarray noninvasive neuronal seizure recordings from intact larval zebrafish. PLoS One. 11: e0156498 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winter MJ, Redfern WS, Hayfield AJ, Owen SF, Valentin JP, and Hutchinson TH. Validation of a larval zebrafish locomotor assay for assessing the seizure liability of early-stage development drugs. J Pharmacol Toxicol Methods. 57: 176–187. 2008. [DOI] [PubMed] [Google Scholar]
- 40.Baraban SC, Taylor MR, Castro PA, and Baier H. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience. 131: 759–768. 2005. [DOI] [PubMed] [Google Scholar]
- 41.Wong K, Stewart A, Gilder T, Wu N, Frank K, Gaikwad S, Suciu C, Dileo J, Utterback E, Chang K, Grossman L, Cachat J, and Kalueff AV. Modeling seizure-related behavioral and endocrine phenotypes in adult zebrafish. Brain Res. 1348: 209–215. 2010. [DOI] [PubMed] [Google Scholar]
- 42.Koseki N, Deguchi J, Yamashita A, Miyawaki I, and Funabashi H. Establishment of a novel experimental protocol for drug-induced seizure liability screening based on a locomotor activity assay in zebrafish. J Toxicol Sci. 39: 579–600. 2014. [DOI] [PubMed] [Google Scholar]
- 43.Wullimann MF. The brain of the zebrafish Danio rerio: a neuroanatomical atlas. In: Neuroanatomy of the Zebrafish Brain: A Topological Atlas. 1st ed. Wullimann MF, Rupp B, and Reichert H (eds). Birkhauser, Basel. 19–87. 1996. [Google Scholar]
- 44.Rink E, and Wullimann MF. Connections of the ventral telencephalon and tyrosine hydroxylase distribution in the zebrafish brain (Danio rerio) lead to identification of an ascending dopaminergic system in a teleost. Brain Res Bull. 57: 385–387. 2002. [DOI] [PubMed] [Google Scholar]
- 45.Rico EP, Rosemberg DB, Seibt KJ, Capiotti KM, Da Silva RS, and Bonan CD. Zebrafish neurotransmitter systems as potential pharmacological and toxicological targets. Neurotoxicol Teratol. 33: 608–617. 2011. [DOI] [PubMed] [Google Scholar]
- 46.Sanchez-Simon FM, and Rodriguez RE. Developmental expression and distribution of opioid receptors in zebrafish. Neuroscience. 151: 129–137. 2008. [DOI] [PubMed] [Google Scholar]
- 47.Kily LJ, Cowe YC, Hussain O, Patel S, McElwaine S, Cotter FE, and Brennan CH. Gene expression changes in a zebrafish model of drug dependency suggest conservation of neuro-adaptation pathways. J Exp Biol. 211: 1623–1634. 2008. [DOI] [PubMed] [Google Scholar]
- 48.Tropepe V, and Sive HL. Can zebrafish be used as a model to study the neurodevelopmental causes of autism? Genes Brain Behav. 2: 268–281. 2003. [DOI] [PubMed] [Google Scholar]
- 49.Muthuraman A, Nafisa K, Sowmya MS, Arpitha BM, Choedon N, Sandy CD, Rishitha N, and Johurul I. Role of ambrisentan (selective endothelin-A receptor antagonist) on cigarette smoke exposure induced cognitive impairment in Danio rerio. Life Sci. 222: 133–139. 2019. [DOI] [PubMed] [Google Scholar]
- 50.Faillace MP, Pisera-Fuster A, and Bernabeu R. Evaluation of the rewarding properties of nicotine and caffeine by implementation of a five-choice conditioned place preference task in zebrafish. Prog Neuropsychopharmacol Biol Psychiatry. 84(Pt A): 160–172. 2018. [DOI] [PubMed] [Google Scholar]
- 51.Kenney JW, Scott IC, Josselyn SA, and Frankland PW. Contextual fear conditioning in zebrafish. Learn Mem. 24: 516–523. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parker MO, Brock AJ, Sudwarts A, and Brennan CH. Atomoxetine reduces anticipatory responding in a 5-choice serial reaction time task for adult zebrafish. Psychopharmacology (Berl). 231: 2671–2679. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ninkovic J, and Bally-Cuif L. The zebrafish as a model system for assessing the reinforcing properties of drugs of abuse. Methods. 39: 262–274. 2006. [DOI] [PubMed] [Google Scholar]
- 54.Mathur P, Lau B, and Guo S. Conditioned place preference behavior in zebrafish. Nat Protoc. 6: 338–345. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Darland T, and Dowling JE. Behavioral screening for cocaine sensitivity in mutagenized zebrafish. Proc Natl Acad Sci USA. 98: 11691–11696. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mathur P, Berberoglu MA, and Guo S. Preference for ethanol in zebrafish following a single exposure. Behav Brain Res. 217: 128–133. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weaver RJ, Blomme EA, Chadwick AE, Copple IM, Gerets HHJ, Goldring CE, Guillouzo A, Hewitt PG, Ingelman-Sundberg M, Jensen KG, Juhila S, Klingmüller U, Labbe G, Liguori MJ, Lovatt CA, Morgan P, Naisbitt DJ, Pieters RHH, Snoeys J, van de Water B, Williams DP, and Park BK. Managing the challenge of drug-induced liver injury: a roadmap for the development and deployment of preclinical predictive models. Nat Rev Drug Discov. 19: 131–148. 2020. [DOI] [PubMed] [Google Scholar]
- 58.Granitzny A, Knebel J, Müller M, Braun A, Steinberg P, Dasenbrock C, and Hansen T. Evaluation of a human in vitro hepatocyte-NPC co-culture model for the prediction of idiosyncratic drug-induced liver injury: A pilot study. Toxicol Rep. 4: 89–103. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Proctor WR, Foster AJ, Vogt J, Summers C, Middleton B, Pilling MA, Shienson D, Kijanska M, Ströbel S, Kelm JM, Morgan P, Messner S, and Williams D. Utility of spherical human liver microtissues for prediction of clinical drug-induced liver injury. Arch Toxicol. 91: 2849–2863. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin C, and Khetani SR. Advances in engineered liver models for investigating drug-induced liver injury. BioMed Res Int. 2016: 1829148 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Usta OB, McCarty WJ, Bale S, Hegde M, Jindal R, Bhushan A, Golberg I, and Yarmush ML. Microengineered cell and tissue systems for drug screening and toxicology applications: Evolution of in-vitro liver technologies. Technology (Singap World Sci). 3: 1–26. 2015; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McGrath P, and Li CQ. Zebrafish: a predictive model for assessing drug-induced toxicity. Drug Discov Today. 13: 394–401. 2008. [DOI] [PubMed] [Google Scholar]
- 63.Hill A, Mesens N, Steemans M, Xu JJ, and Aleo MD. Comparisons between in vitro whole cell imaging and in vivo zebrafish-based approaches for identifying potential human hepatotoxicants earlier in pharmaceutical development. Drug Metab Rev. 44: 127–140. 2012. [DOI] [PubMed] [Google Scholar]
- 64.Lorent K, Yeo SY, Oda T, Chandrasekharappa S, Chitnis A, Matthews RP, and Pack M. Inhibition of Jagged-mediated Notch signaling disrupts zebrafish biliary development and generates multi-organ defects compatible with an Alagille syndrome phenocopy. Development. 131: 5753–5766. 2004. [DOI] [PubMed] [Google Scholar]
- 65.Pham DH, and Yin C. Zebrafish as a model to study cholestatic liver diseases. Methods Mol Biol. 1981: 273–289. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schlegel A. Studying non-alcoholic fatty liver disease with zebrafish: a confluence of optics, genetics, and physiology. Cell Mol Life Sci. 69: 3953–3961. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vliegenthart AD, Tucker CS, Del Pozo J, and Dear JW. Zebrafish as model organisms for studying drug-induced liver injury. Br J Clin Pharmacol. 78: 1217–1227. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu JW, Ho YJ, Yang YJ, Liao HA, Ciou SC, Lin LI, and Ou DL. Zebrafish as a disease model for studying human hepatocellular carcinoma. World J Gastroenterol. 21: 12042–12058. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goldstone JV, McArthur AG, Kubota A, Zanette J, Parente T, Jönsson ME, Nelson DR, and Stegeman JJ. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genomics. 11: 643–663. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alderton W, Berghmans S, Butler P, Chassaing H, Fleming A, Golder Z, Richards F, and Gardner I. Accumulation and metabolism of drugs and CYP probe substrates in zebrafish larvae. Xenobiotica. 40: 547–557. 2010. [DOI] [PubMed] [Google Scholar]
- 71.Ma J, Li M, Kalavagunta PK, Li J, He Q, Zhang Y, Ahmad O, Yin H, Wang T, and Shang J. Protective effects of cichoric acid on H2O2-induced oxidative injury in hepatocytes and larval zebrafish models. Biomed Pharmacother. 104: 679–685. 2018. [DOI] [PubMed] [Google Scholar]
- 72.Yamashita A, Deguchi J, Honda Y, Yamada T, Miyawaki I, Nishimura Y, and Tanaka T. Increased susceptibility to oxidative stress-induced toxicological evaluation by genetically modified nrf2a-deficient zebrafish. J Pharmacol Toxicol Methods. 96: 34–45. 2019. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, Han L, He Q, Chen W, Sun C, Wang X, Chen X, Wang R, Hsiao CD, and Liu K. A rapid assessment for predicting drug-induced hepatotoxicity using zebrafish. J Pharmacol Toxicol Methods. 84: 102–110. 2017. [DOI] [PubMed] [Google Scholar]
- 74.He JH, Guo SY, Zhu F, Zhu JJ, Chen YX, Huang CJ, Gao JM, Dong QX, Xuan YX, and Li CQ. A zebrafish phenotypic assay for assessing drug-induced hepatotoxicity. J Pharmacol Toxicol Methods. 67: 25–32. 2013. [DOI] [PubMed] [Google Scholar]
- 75.Verstraelen S, Peers B, Maho W, Hollanders K, Remy S, Berckmans P, Covaci A, and Witters H. Phenotypic and biomarker evaluation of zebrafish larvae as an alternative model to predict mammalian hepatotoxicity. J Appl Toxicol. 36: 1194–1206. 2016. [DOI] [PubMed] [Google Scholar]
- 76.Nam HS, Hwang KS, Jeong YM, Ryu JI, Choi TY, Bae MA, Son WC, You KH, Son HY, and Kim CH. Expression of miRNA-122 induced by liver toxicants in zebrafish. BioMed Res Int. 2016: 1473578 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davies JA. Biological techniques: Kidney tissue grown from induced stem cells. Nature. 526: 512–513. 2015. [DOI] [PubMed] [Google Scholar]
- 78.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, and Little MH. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 526: 564–568. 2015. [DOI] [PubMed] [Google Scholar]
- 79.Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, and Bonventre JV. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol. 33: 1193–1200. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Swanhart LM, Cosentino CC, Diep CQ, Davidson AJ, de Caestecker M, and Hukriede NA. Zebrafish kidney development: basic science to translational research. Birth Defects Res C Embryo Today. 93: 141–156. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McCampbell KK, Springer KN, and Wingert RA. Atlas of cellular dynamics during zebrafish adult kidney regeneration. Stem Cells Int. 2015. 547636. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hostetter CL, Sullivan-Brown JL, and Burdine RD. Zebrafish pronephros: a model for understanding cystic kidney disease. Dev Dyn. 228: 514–522. 2003. [DOI] [PubMed] [Google Scholar]
- 83.Wingert RA, and Davidson AJ. The zebrafish pronephros: a model to study nephron segmentation. Kidney Int. 73: 1120–1127. 2008. [DOI] [PubMed] [Google Scholar]
- 84.Hentschel DM, Park KM, Cilenti L, Zervos AS, Drummond I, and Bonventre JV. Acute renal failure in zebrafish: a novel system to study a complex disease. Am J Physiol Renal Physiol. 288: F923–F929. 2005. [DOI] [PubMed] [Google Scholar]
- 85.Gorgulho R, Jacinto R, Lopes SS, Pereira SA, Tranfield EM, Martins GG, Gualda EJ, Derks RJE, Correia AC, Steenvoorden E, Pintado P, Mayboroda OA, Monteiro EC, and Morello J. Usefulness of zebrafish larvae to evaluate drug-induced functional and morphological renal tubular alterations. Arch Toxicol. 92: 411–423. 2018. [DOI] [PubMed] [Google Scholar]
- 86.Kotb AM, Müller T, Xie J, Anand-Apte B, Endlich K, and Endlich N. Simultaneous assessment of glomerular filtration and barrier function in live zebrafish. Am J Physiol Renal Physiol. 307: F1427–F1434. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kinkel MD, Eames SC, Philipson LH, and Prince VE. Intraperitoneal injection into adult zebrafish. J Vis Exp. 42: e2126–e2129. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gerlach GF, and Wingert RA. Kidney organogenesis in the zebrafish: insights into vertebrate nephrogenesis and regeneration. Wiley Interdiscip Rev Dev Biol. 2: 559–585. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Müller-Deile J, Schenk H, Schroder P, Schulze K, Bolaños-Palmieri P, Siegerist F, Endlich N, Haller H, and Schiffer M. Circulating factors cause proteinuria in parabiotic zebrafish. Kidney Int. 96: 342–349. 2019. [DOI] [PubMed] [Google Scholar]
- 90.Wang X, Liu KC, Sun GJ, Han LW, Wang RC, Peng WB, Sun C, Hsiao CD, Zhang Y, and Hou HR. Evaluation of nephrotoxic effects of aristolochic acid on zebrafish (Danio rerio) larvae. Hum Exp Toxicol. 35: 974–982. 2016. [DOI] [PubMed] [Google Scholar]
- 91.Diep CQ, Ma D, Deo RC, Holm TM, Naylor RW, Arora N, Wingert RA, Bollig F, Djordjevic G, Lichman B, Zhu H, Ikenaga T, Ono F, Englert C, Cowan CA, Hukriede NA, Handin RI, and Davidson AJ. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature. 470: 95–100. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kroeger PT, Jr , and Wingert RA. Using zebrafish to study podocyte genesis during kidney development and regeneration. Genesis. 52: 771–792. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou W, Boucher RC, Bollig F, Englert C, and Hildebrandt F. Characterization of mesonephric development and regeneration using transgenic zebrafish. Am J Physiol Renal Physiol. 299: F1040–F1047. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kato Y, Tonomura Y, Hanafusa H, Nishimura K, Fukushima T, and Ueno M. Adult zebrafish model for screening drug-induced kidney injury. Toxicol Sci. 174: 241–253. 2020. [DOI] [PubMed] [Google Scholar]
- 95.Nguyen CT, Lu Q, Wang Y, and Chen JN. Zebrafish as a model for cardiovascular development and disease. Drug Discov Today Dis Models. 5: 135–140. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Milan DJ, Jones IL, Ellinor PT, and MacRae CA. In vivo recording of adult zebrafish electrocardiogram and assessment of drug-induced QT prolongation. Am J Physiol Heart Circ Physiol. 291: H269–H273. 2006. [DOI] [PubMed] [Google Scholar]
- 97.Milan DJ, Peterson TA, Ruskin JN, Peterson RT, and MacRae CA. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation. 107: 1355–1358. 2003. [DOI] [PubMed] [Google Scholar]
- 98.Burns CG, Milan DJ, Grande EJ, Rottbauer W, MacRae CA, and Fishman MC. High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nat Chem Biol. 1: 263–264. 2005. [DOI] [PubMed] [Google Scholar]
- 99.Umemoto N, Nishimura Y, Shimada Y, Yamanaka Y, Kishi S, Ito S, Okamori K, Nakamura Y, Kuroyanagi J, Zhang Z, Zang L, Wang Z, Nishimura N, and Tanaka T. Fluorescent-based methods for gene knockdown and functional cardiac imaging in zebrafish. Mol Biotechnol. 55: 131–142. 2013. 23674069 [Google Scholar]
- 100.Yamashita A, and Deguchi J. Usefulness of zebrafish for safety evaluation in drug discovery . Sumitomo Chemical Paper. 2015: 53–56. 2015. [Google Scholar]
- 101.Wen D, Liu A, Chen F, Yang J, and Dai R. Validation of visualized transgenic zebrafish as a high throughput model to assay bradycardia related cardio toxicity risk candidates. J Appl Toxicol. 32: 834–842. 2012. [DOI] [PubMed] [Google Scholar]
- 102.Yozzo KL, Isales GM, Raftery TD, and Volz DC. High-content screening assay for identification of chemicals impacting cardiovascular function in zebrafish embryos. Environ Sci Technol. 47: 11302–11310. 2013. [DOI] [PubMed] [Google Scholar]
- 103.Huang CC, Monte A, Cook JM, Kabir MS, and Peterson KP. Zebrafish heart failure models for the evaluation of chemical probes and drugs. Assay Drug Dev Technol. 11: 561–572. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chico TJ, Ingham PW, and Crossman DC. Modeling cardiovascular disease in the zebrafish. Trends Cardiovasc Med. 18: 150–155. 2008. [DOI] [PubMed] [Google Scholar]
- 105.Chablais F, and Jazwinska A. The regenerative capacity of the zebrafish heart is dependent on TGFβ signaling. Development. 139: 1921–1930. 2012. [DOI] [PubMed] [Google Scholar]