Abstract
We performed a medaka bioassay for the carcinogenicity of methylazoxymethaol acetate (MAM-Ac) to examine the sequential histological changes in the liver from 3 days after exposure until tumor development. The medaka were exposed to MAM-Ac at a concentration of 2 ppm for 24 hours, and were necropsied at 3, 7, 10, 14, 21, 28, 35, 42, 49, 60, and 91 days after exposure. MAM-Ac induced four cases of hepatocellular adenoma and one case of hepatocellular carcinoma in 8 fish after 60 or 91 days of exposure. Histological changes in the liver until tumor development were divided into three phases. In the cytotoxic phase (1–10 days), MAM-Ac-exposed hepatocytes showed vacuolar degeneration and underwent necrosis and apoptosis, resulting in multiple foci of hepatocyte loss. In the repopulation phase (14–35 days), the areas of hepatocyte loss were filled with hepatic cysts and the remaining hepatocytes were surrounded by hepatic stellate-like cells (or spindle cells) and gradually disappeared. In the proliferation phase (42–91 days), the original hepatic parenchyma was regenerated and progressively replaced by regenerative hyperplastic nodules and/or liver neoplasms. The medaka retained a strong hepatocyte regenerative ability in response to liver injury. It is considered that this ability promotes the proliferation of initiated hepatocytes in multistep carcinogenesis and influences the development of liver tumor over a short period in medaka.
Keywords: carcinogenesis, liver, medaka, methylazoxymethaol acetate
Introduction
Fish models of carcinogenesis have been useful vertebrate models to screen potential carcinogens, as they are very sensitive, inexpensive and rapidly adopt as compared with rodent models1-3. Their usefulness for detecting carcinogenicity was confirmed by prior fish toxicity studies with powerful hepatocarcinogens such as diethylnitrosamine (DEN), dimethylnitrosamine (DMN), dimethylaminoazobenzene, N-methyl-N’-nitro-N-nitrosoguanidine, aflatoxin B1, polycyclic aromatic hydrocarbons, and methylazoxymethanol acetate (MAM-Ac) conducted in the 1980s and 1990s. However, fish models of carcinogenesis are thought to be less sensitive to the presence of carcinogens in organs other than the liver. Thus, there is a limit to detect the carcinogenicity of chemicals in these models4.
MAM-Ac, a neurotoxin and carcinogen, is a synthetic derivative of cycasin known to induce tumors in the liver, kidney, and small intestine of rats5, 6 and mice7. Among teleost fish, MAM-Ac has been known to induce medulloepithelioma in eyes of medaka8, pancreatic tumors in guppies9, nephroblastomas in guppies and medaka10, rhabdomyosarcomas, leiomyosarcomas, fibromas, and neuroblastomas in medaka10, and swimbladder tumors, kidney tumors, and glandular stomach tumors in rainbow trouts11. The liver is the most common target organ affected by MAM-Ac, and liver tumors have been induced in zebrafish, guppies, mosquitofish12, sheepshead minnow, fathead minnow10, and medaka13, 14. However, only a few reports have described the sequential histological changes in the liver of fish following MAM-Ac exposure13, 15. In the present study, we conducted a fish bioassay for the carcinogenicity of MAM-Ac exposure for 24 hours in medaka by examining the sequential histological changes in the liver from 3 days after exposure until tumor development.
Materials and Methods
Fish and maintenance
Japanese medaka (Oryzias latipes) were purchased from the National Institute of Informatics and were bred at the biological research laboratory, Nissan Chemical Corp. A breeding stock was used for the study and maintained in dechlorinated tap water at 25 ± 1°C under a 16:8 h light:dark photoperiod. The water quality parameters were as follows: pH, 7.0 to 7.5; oxygen concentration, 7.5 to 8.0 mg/L.
Experimental design
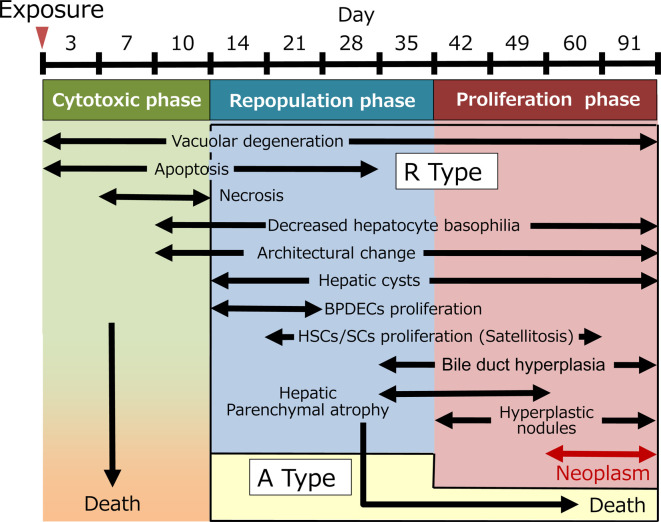
Six-month-old medaka were acclimatized for 2 weeks under test conditions. For the experiment, 160 female fish were divided into a control group (n = 45) and MAM-Ac exposure group (n = 115). In the MAM-Ac exposure group, individual batches of 10 or 11 fish were exposed to MAM-Ac (FUJIFILM Wako Pure Chemical Corp., Tokyo, Japan) at a concentration of 2 ppm for 24 hours in a static system using 3-L glass vessels containing 2 L of dechlorinated tap water. It is known from prior research that liver tumors are induced in medaka exposed to MAM-Ac at 2 ppm for 24 hours14. In the control group, individual batches of five fish were maintained for 24 hours using dechlorinated tap water without MAM-Ac under same conditions. The fish in both groups were transferred to 10 L of fresh water in 12-L glass vessels after the exposure period and were bred for 91 days in a semi-static system. Their survival was daily recorded. The water was changed twice every week, and daily feeding was performed with 0.5 mL of brine shrimp per fish. Three to 10 fish were necropsied at 3, 7, 10, 14, 21, 28, 35, 42, 49, 60, and 91 days after exposure (Fig. 1). This study was conducted according to the Guidelines for Animal Experimentation, Biological Research Laboratory, Nissan Chemical Corporation.
Fig. 1.
Experimental design.
Histopathological examination
The fish were sacrificed by overexposure to CO2 gas and fixed in Bouin’s solution for overnight before being refixed in 10% neutral-buffered formalin. The samples were embedded in paraffin, sectioned at a thickness of 4 µm, and stained routinely with hematoxylin and eosin for histopathological examination that was performed only for the liver samples obtained from the surviving fish at each sampling point but not those from the dead fish.
Results
The mortality rate was 0% (0/45) in the control group and 35.7% (41/115) in the MAM-Ac exposure group. The number of dead fish in the MAM-Ac exposure group increased at around 1 and 6 weeks after exposure (Fig. 2). Histopathological examination of the liver was performed in the surviving 119 fish (45 fish from the control group and 74 fish from MAM-Ac exposure group).
Fig. 2.
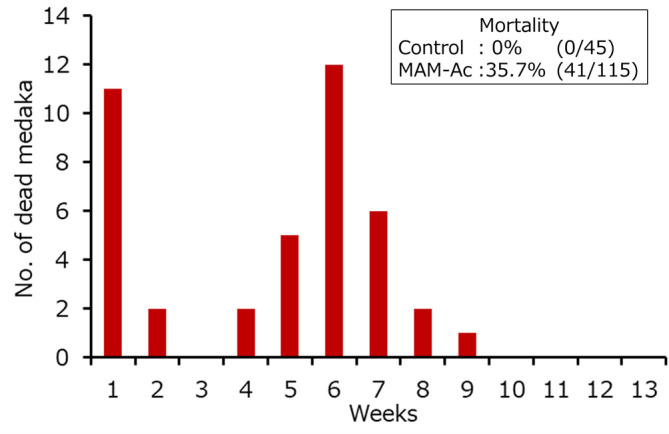
Number of dead medaka following exposure to methylazoxymethanol acetate.
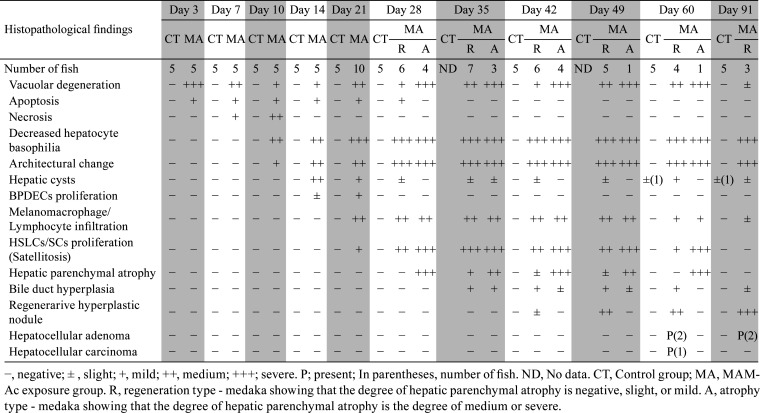
Histopathological findings of the liver are presented in Table 1. In the control group, the hepatocytes were concentrically arranged in tubules around the sinusoid, showing basophilic cytoplasm, and containing small vacuolar structures, which were glycogen (Fig. 3a). Hepatic cysts (spongiosis hepatis) were observed as spontaneous lesions in a few fish at 60 and 91 days after exposure. In the MAM-Ac exposure group, hepatocytes showed diffused vacuolar degeneration with apoptotic bodies at 3 days after exposure (Fig. 3b). At 7 days after exposure, small necrotic foci appeared scattered. The hepatocytes around them showed marked vacuolar degeneration, although the number of vacuoles in the hepatocytes at another site generally decreased relative to that observed at 3 days after exposure (Fig. 3c). Some hepatocytes contained apoptotic bodies. At 10 days after exposure, the hepatic parenchyma showed structural disorder of the hepatic cords and collapse of the sinusoids. The multifocal necrotic foci appeared enlarged, resulting in progressive hepatocyte loss. The remaining hepatocytes exhibited a decrease in cytoplasmic basophilia with apoptotic bodies (Fig. 3d). At 14 days after exposure, the areas of hepatocyte loss were filled with small multiloculated or large uniloculated hepatic cysts, comprising a meshwork of hepatic stellate-like cells or spindle cells (HSLCs/SCs) (considered to be Ito cells or perisinusoidal cells) (Fig. 3e). These cyst spaces contained eosinophilic flocculent material. At this stage, bile preductular epithelial cells (BPDECs) had proliferated into the small spaces between hepatocytes. The normal tubulosinusoidal pattern of these cells was disorganized (Fig. 3f). At 21 and 28 days after exposure, the HSLCs/SCs had proliferated into the regions of the hepatic cysts, infiltrated in between hepatocytes, and surrounded the individual and/or several hepatocytes (satellitosis). These hepatocytes showed swelling and vacuolar degeneration (Fig. 3g). Further, the melanomacrophages and lymphocytes infiltrated into the hepatic parenchyma. In addition to these findings, some fish showed remarkable hepatic parenchymal atrophy with more extensive satellitosis at 28 days after exposure. Consequently, the fish were stratified as either “regeneration type” or “atrophy type” from 28 days onward after exposure depending on the degree of hepatic parenchymal atrophy. The fish with mild or less hepatic parenchymal atrophy were classified as “regeneration type”, while those with medium or severe hepatic parenchymal atrophy were classified as “atrophy type”.
Table 1. Liver Histopathological Findings in Methylazoxymetanol Acetate-exposed Medaka.
Fig. 3.
Gross appearance and histological changes in the liver. a) Normal liver. Control group. 3 days. Hematoxylin and eosin (HE) stain. Bar = 200 µm (left) and 50 µm (right). b) Vacuolar degeneration with apoptotic bodies (➡) in hepatocytes. Methylazoxymethanol acetate (MAM-Ac) exposure group. 3 days. HE stain. Bar = 200 µm (left) and 50 µm (right). c) Small necrotic foci and marked vacuolated hepatocytes around them. Apoptotic bodies (➡) in hepatocytes. MAM-Ac exposure group. 7 days. HE stain. Bar = 200 µm (left) and 50 µm (right). d) Enlarged focal necrosis with architectural changes of hepatic cords and sinusoids. Apoptotic bodies (➡) and decreased cytoplasmic basophilia in hepatocytes. MAM-Ac exposure group. 10 days. HE stain. Bar = 200 µm (left) and 50 µm (right). e) Large areas of hepatocyte loss filled with hepatic cysts. MAM-Ac exposure group. 14 days. HE stain. Bar = 200 µm (left) and 50 µm (right). f) Proliferation of bile preductular epithelial cells in small spaces between hepatocytes. MAM-Ac exposure group. 14 days. HE stain. Bar = 200 µm (left) and 50 µm (right). g) Proliferation and infiltration of hepatic stellate-like cells or spindle cells. Formation of satellized hepatocytes. MAM-Ac exposure group. 21 days. HE stain. Bar = 200 µm (left) and 50 µm (right). h) Decrease in satellized hepatocytes and replacement with adipose tissue. Regeneration type (R type). MAM-Ac exposure group. 35 days. HE stain. Bar = 200 µm (left) and 50 µm (right). i) Formation of small-sized regenerative hyperplastic nodules (➡) comprising marked eosinophilic hepatocytes with clear nuclei. R type. MAM-Ac exposure group. 42 days. HE stain. Bar = 200 µm (left) and 50 µm (right). j) Multiple, small- to medium-sized regenerative hyperplastic nodules. Atypia in some hyperplastic hepatocytes. R type. MAM-Ac exposure group. 60 days. HE stain. Bar = 500 µm (center) and 60 µm (right/left). k) Replacement of hepatic parenchyma by regenerative hyperplastic nodules. R type. MAM-Ac exposure group. 49 days (left) and 91 days (right). HE stain. Bar = 600 µm. l) Gross appearance of multiple pale masses in liver. Hepatocellular adenoma (⇨) (right/lower). 91 days. Hepatocellular carcinoma (➡) (left/lower). 60 days. m) Regenerative hyperplastic nodules (right/higher) and hepatocellular adenoma (right/lower). Irregular cords of tumor cells showing minor cellular pleomorphism. Proliferation of spindle-shaped cells in interstitium. R type. MAM-Ac exposure group. 91 days. HE stain. Bar = 750 µm (left) and 50 µm (right). n) Hepatocellular carcinoma (right/lower) and regenerative hyperplastic nodules (right/higher). Solid growth of tumor cells showing pleomorphism and anaplasia. R type. MAM-Ac exposure group. 60 days. HE stain. Bar = 900 µm (left) and 50 µm (right). o) Marked hepatic parenchymal atrophy. Replacement of hepatic parenchyma by adipose tissue. (left, control; right, MAM-Ac). Atrophy type. 42 days. HE stain. Bar = 300 µm.
Regeneration type
In this fish population, most of the remaining hepatocytes were satellized at 35 days after exposure (Fig. 3h) and tended to decrease owing to their replacement with the adipose tissue, resulting in mild hepatic parenchymal atrophy. Further, the bile duct proliferation was scattered. At 42 and 49 days after exposure, small foci comprising more eosinophilic hepatocytes with clear nuclei appeared scattered (Fig. 3i). These foci subsequently developed multiple small- to medium-sized regenerative hyperplastic nodules, and some hyperplastic hepatocytes in them showed atypia (Fig. 3j). At this stage, the satellized hepatocytes gradually disappeared and were replaced by regenerative hyperplastic nodules. The major portion of the hepatic parenchyma was occupied by these nodules (Fig. 3k). At 60 and 91 days after exposure, multiple pale masses were macroscopically observed in the liver (Fig. 3l). Histologically, these masses comprised regenerative hyperplastic nodules and/or liver tumors, and the original hepatic parenchyma was replaced by these masses. As the liver tumors, hepatocellular adenoma was detected in two cases at 60 days after exposure and in two cases at 91 days after exposure, while hepatocellular carcinoma was detected in one case at 60 days after exposure. The cases of hepatocellular adenoma showed the architecture of irregular cellular cords that were thicker than the normal ones (Fig. 3m). The tumor cells comprised a monomorphic population with limited cellular pleomorphism and round, pleomorphic, and large nuclei. The interstitium of the tumor contained a focally extensive or diffused proliferation of spindle-shaped cells (Fig. 3m). The case of hepatocellular carcinoma showed a solid growth pattern with densely cellular and thickened cords and had invaded into the adjacent regenerative hyperplastic nodules (Fig. 3n). The tumor was composed of pleomorphic and anaplastic cells, which varied from spindle to stellate and/or polygonal with nuclear atypia and multiple nuclei.
Atrophic type
In this fish population, the satellized hepatocytes disappeared from 35 days onward after exposure and the melanomacrophages and lymphocytes infiltrated with time. Most hepatic parenchyma was replaced by the adipose tissue without the formation of regenerative hyperplastic nodules (Fig. 3o). At 91 days after exposure, there were no fish of this type.
Discussion
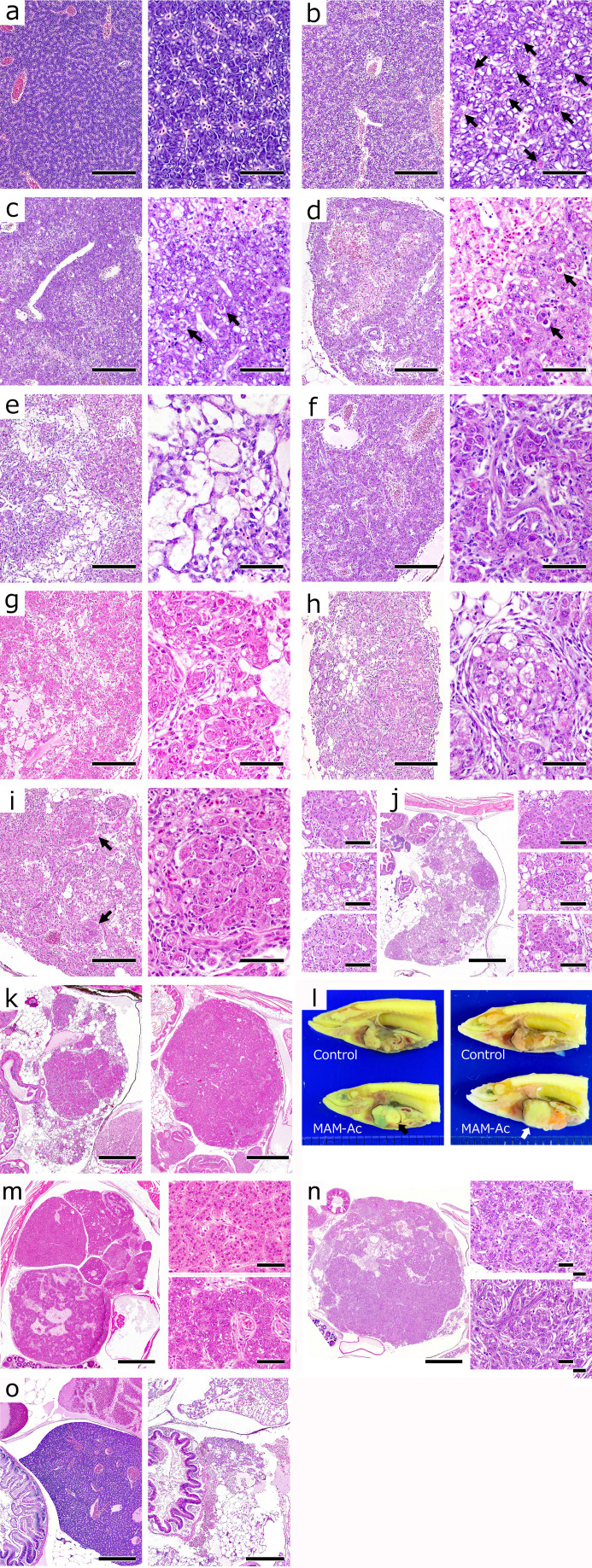
In the present study, MAM-Ac exposure at 2 ppm for 24 hours in a population of 115 medaka induced four cases of hepatocellular adenoma and one case of hepatocellular carcinoma from 60 days onward after the exposure. The sequential histological changes of the liver from the time of exposure until tumor development were divided into three phases16 (Fig. 4). In the cytotoxic phase (1–10 days), the MAM-Ac-exposed hepatocytes exhibited vacuolar degeneration and underwent necrosis and apoptosis, resulting in the multiple foci of hepatocyte loss. In the repopulation phase (14–35 days), the areas of hepatocyte loss were filled with hepatic cysts derived from the HSLCs/SCs, while the remaining hepatocytes were surrounded by these HSLCs/SCs and gradually decreased. In the proliferation phase (42–91 days), regenerative hyperplastic nodules multifocally developed and replaced the hepatic parenchyma. In the present study, it was difficult to differentiate between these small hyperplastic nodules and foci of cellular alteration17, 18. However, some of these small nodules, including atypical hepatocytes, were thought to be the foci of cellular alteration that could progress to liver tumors. From these results, it is concluded that the medaka retain a strong hepatocyte regenerative ability in response to liver injury, resulting in the rapid development of regenerative hyperplastic nodules. This ability may have promoted the proliferation of initiated hepatocytes in multistep carcinogenesis and influenced the development of liver tumor over a short period in medaka. On the other hand, the number of dead fish suggested that the bimodal peaks occurred at around 1 and 6 weeks after MAM-Ac exposure. Although we did not conduct histopathological examinations of these fish, the early-stage death may have resulted from the direct acute toxicity of MAM-Ac. In the case of late-stage death, the change in the number of dead fish tended to coincide with the incidence of atrophic type fish. Hence, the cause of late-stage death is probably associated with the relationship with the hepatic failure in response to severe hepatic parenchymal atrophy.
Fig. 4.
Progression of hepatic lesions in methylazoxymethaol acetate-exposed medaka. Regeneration type, R Type; Atrophic type, A Type.
Among alkylating agents, DEN19 and DMN20 are known to induce hepatic lesions similar to those associated with MAM-Ac, including vacuolar degeneration, hepatocellular necrosis, hepatic cyst (spongiosis), HSLCs/SCs proliferation, nodular regeneration, and liver tumors in medaka. In particular, BPDECs proliferation and fibrosis are known to be the characteristic histological changes in the medaka exposed to DMN at 100 ppm for 2 weeks21. On the contrary, the characteristic hepatic lesions in the present study were the result of HSLCs/SCs proliferation and regenerative hyperplastic nodule formation, contradictory to that observed for the above mentioned two lesions. The histopathological examination reported herein show that BPDECs and HSLCs/SCs proliferation was a compensatory reaction to fill the area of hepatocyte loss in the repopulation phase. In addition, BPDECs proliferation occurred in response to a small area of hepatocyte loss, while HSLCs/SCs proliferation was evident in response to greater hepatocyte loss. Thus, it is speculated that HSLCs/SCs proliferation takes the role of a prominent lesion, because MAM-Ac exposure induces relatively large necrotic foci, leading to a large area of hepatocyte loss. Fibrosis is associated with HSLCs/SCs activation in the late stage22, 23. In the present study, although HSLCs/SCs proliferated with the formation of satellized hepatocytes, the regenerative hyperplastic nodules were thought to undergo rapid proliferation and replace the liver parenchyma before fibrosis development. Therefore, these differences in the histological changes between DMN and MAM-Ac may be related to the severity of hepatic damage and the hepatocyte loss in the cytotoxic phase. Nevertheless, it is speculated that these histological changes in the medaka are a common process of hepatocarcinogenesis following liver injury induced by alkylating agents.
Small fish species are suitable for the detection of some potential hepatocarcinogens24, 25 and are considered as excellent research models to delineate the mechanism underlying toxicities, including carcinogenicity4. In particular, the medaka is known to be highly sensitive to hepatocarcinogens as compared to other small fish species such as sheepshead minnow, Gulf killifish, fathead minnow, Rivulus, and inland silverside10. However, one must consider the anatomical, pathological, and physiological differences in the liver of medaka and rodents before extrapolating the findings of the medaka model of hepatocarcinogenesis to rodent models26, 27. We compared the difference in the sensitivity of hepatocarcinogenesis using alkylating agents between rats and medaka. In the present study, the exposure dose of MAM-Ac would be assumed to be 2 mg/kg/day, if MAM-Ac exposure levels in medaka were considered to be the same as MAM-Ac concentration in water. In contrast, a single intraperitoneal administration of MAM-Ac at 35 mg/kg/day in rats is reported to induce liver tumors as early as 10 months after treatment28. Furthermore, the mutant frequencies to methylated DNA adducts in the medaka exposed twice weekly for 2 weeks to DMN are shown to be up to 20 times higher than those in rats exposed via drinking water for 14 days29. Therefore, the sensitivity of medaka to alkylating agent-induced hepatocarcinogenesis was higher than that of rats, considering the time of tumor formation and DNA mutant frequency. On the other hand, alkylating agents induce the formation of O6-methylguanine DNA, which plays an important role in mutagenesis30. In DEN-exposed medaka, methylated DNA adducts increases in the liver after 24 hours of exposure31. In contrast, O6-methylguanine DNA methyltransferase, known to play an important role in the repair of methylated DNA adducts, level markedly reduces from 1 to 7 days after exposure and thereafter slightly increases in MAM-Ac-exposed medaka32. Therefore, it is supposed that the limited ability to repair DNA adducts in the liver is one of the important factors that contributed to the higher sensitivity of medaka to MAM-Ac-induced hepatocarcinogenicity than that of rats.
Considering these results, MAM-Ac induced liver tumors within a short period in medaka owing to the rapid regeneration ability of hepatocytes following liver injury and the limited repair ability of DNA adducts. The medaka model of carcinogenesis may easily be applied to detect sequential histopathological events in hepatocarcinogenesis, as liver tumors rapidly develop in medaka after a short exposure period. Thus, the medaka model of carcinogenesis is useful for elucidating the mechanisms and carcinogenic process of liver tumors and is an important in vivo alternative method to detect potential hepatocarcinogenesis in response to environmental contaminants and pollutants.
Disclosure of Potential Conflicts of Interest
The authors declare that there is no conflict of interest.
Acknowledgments
The authors would like to thank Ms. Kaori Maejima, Ms. Hiromi Asako, Mr. Atsushi Funakoshi, Ms. Yukiko Sudo, Mr. Makoto Tsuchiya, and Mr. Yoshinori Tanaka for their excellent technical assistance.
References
- 1.Hatanaka J, Doke N, Harada T, Aikawa T, and Enomoto M. Usefulness and rapidity of screening for the toxicity and carcinogenicity of chemicals in medaka, Oryzias latipes. Jpn J Exp Med. 52: 243–253. 1982. [PubMed] [Google Scholar]
- 2.Bunton TE. Experimental chemical carcinogenesis in fish. Toxicol Pathol. 24: 603–618. 1996. [DOI] [PubMed] [Google Scholar]
- 3.Hawkins WE, Walker WW, Fournie JW, Manning CS, and Krol RM. Use of the Japanese medaka (Oryzias latipes) and guppy (Poecilia reticulata) in carcinogenesis testing under national toxicology program protocols. Toxicol Pathol. 31(Suppl): 88–91. 2003. [DOI] [PubMed] [Google Scholar]
- 4.Kissling GE, Bernheim NJ, Hawkins WE, Wolfe MJ, Jokinen MP, Smith CS, Herbert RA, and Boorman GA. The utility of the guppy (Poecilia reticulata) and medaka (Oryzias latipes) in evaluation of chemicals for carcinogenicity. Toxicol Sci. 92: 143–156. 2006. [DOI] [PubMed] [Google Scholar]
- 5.Hirono I, Laqueur GL, and Spatz M. Tumor induction in Fischer and Osborne-Mendel rats by a single administration of cycasin. J Natl Cancer Inst. 40: 1003–1010. 1968. [PubMed] [Google Scholar]
- 6.Laqueur GL, McDaniel EG, and Matsumoto H. Tumor induction in germfree rats with methylazoxymethanol (MAM) and synthetic MAM acetate. J Natl Cancer Inst. 39: 355–371. 1967. [PubMed] [Google Scholar]
- 7.O’Gara RW, Brown JM, and Whiting MG. Induction of hepatic and renal tumors by topical application of aqueous extract of cycad nut to artificial skin ulcers in mice. Fed Proc. 23: 1382–1383. 1964. [PubMed] [Google Scholar]
- 8.Hawkins WE, Fournie JW, Overstreet RM, and Walker WW. Intraocular neoplasms induced by methylazoxymethanol acetate in Japanese medaka (Oryzias latipes). J Natl Cancer Inst. 76: 453–465. 1986. [PubMed] [Google Scholar]
- 9.Fournie JW, Hawkins WE, Overstreet RM, and Walker WW. Exocrine pancreatic neoplasms induced by methylazoxymethanol acetate in the guppy Poecilia reticulata. J Natl Cancer Inst. 78: 715–725. 1987. [PubMed] [Google Scholar]
- 10.Hawkins WE, Overstreet RM, Fournie JW, and Walker WW. Development of aquarium fish models for environmental carcinogenesis: tumor induction in seven species. J Appl Toxicol. 5: 261–264. 1985. [DOI] [PubMed] [Google Scholar]
- 11.Bailey GS, Williams DE, and Hendricks JD. Fish models for environmental carcinogenesis: the rainbow trout. Environ Health Perspect. 104(Suppl 1): 5–21. 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Law JM, Hawkins WE, Overstreet RM, and Walker WW. Hepatocarcinogenesis in western mosquitofish (Gambusia affinis) exposed to methylazoxymethanol acetate. J Comp Pathol. 110: 117–127. 1994. [DOI] [PubMed] [Google Scholar]
- 13.Harada T, Hatanaka J, and Enomoto M. Liver cell carcinomas in the medaka (Oryzias latipes) induced by methylazoxymethanol-acetate. J Comp Pathol. 98: 441–452. 1988. [DOI] [PubMed] [Google Scholar]
- 14.Aoki K, and Matsudaira H. Factors influencing tumorigenesis in the liver after treatment with methylazoxymethanol acetate in a teleost, Oryzias latipes. In: Phyletic Approaches to Cancer, 1st ed. Dawe CJ (ed). Jpn Sci Soc Press, Tokyo. 215–216. 1981. [Google Scholar]
- 15.Hinton DE, Lantz RC, and Hampton JA. Effect of age and exposure to a carcinogen on the structure of the medaka liver: a morphometric study. Natl Cancer Inst Monogr. 65: 239–249. 1984. [PubMed] [Google Scholar]
- 16.Moore MJ, and Myers MS. Pathobiology of chemimcal-associated neoplasa in fish. In: Aquatic Toxicilogy, 1st ed. Malins DC, and Ostrander GK (ed). CRC Press, Boca Raton. 327–386. 1994. [Google Scholar]
- 17.Boorman GA, Botts S, Bunton TE, Fournie JW, Harshbarger JC, Hawkins WE, Hinton DE, Jokinen MP, Okihiro MS, and Wolfe MJ. Diagnostic criteria for degenerative, inflammatory, proliferative nonneoplastic and neoplastic liver lesions in medaka (Oryzias latipes): consensus of a National Toxicology Program Pathology Working Group. Toxicol Pathol. 25: 202–210. 1997. [DOI] [PubMed] [Google Scholar]
- 18.Thoolen B, Maronpot RR, Harada T, Nyska A, Rousseaux C, Nolte T, Malarkey DE, Kaufmann W, Küttler K, Deschl U, Nakae D, Gregson R, Vinlove MP, Brix AE, Singh B, Belpoggi F, and Ward JM. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol Pathol. 38(Suppl): 5S–81S. 2010. [DOI] [PubMed] [Google Scholar]
- 19.Bunton TE. Diethylnitrosamine (DEN)-induced degenerative, proliferative, and neoplastic lesions in the liver of the medaka Oatipes (ryzias l) following short-term exposure. Mar Environ Res. 28: 369–374. 1989. [Google Scholar]
- 20.Hobbie KR, DeAngelo AB, George MH, and Law JM. Neoplastic and nonneoplastic liver lesions induced by dimethylnitrosamine in Japanese medaka fish. Vet Pathol. 49: 372–385. 2012. [DOI] [PubMed] [Google Scholar]
- 21.Van Wettere AJ, Law JM, Hinton DE, and Kullman SW. Anchoring hepatic gene expression with development of fibrosis and neoplasia in a toxicant-induced fish model of liver injury. Toxicol Pathol. 41: 744–760. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shrivastav AB, and Pandey G. Fish liver cancer by chemical pollutants. Int J Animal Vet. Fishery Allied Sci. 1: 17–23. 2014. [Google Scholar]
- 23.Hernandez-Gea V, and Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 6: 425–456. 2011. [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa T, and Takayama S. Importance of hepatic neoplasms in lower vertebrate animals as a tool in cancer research. J Toxicol Environ Health. 5: 537–550. 1979. [DOI] [PubMed] [Google Scholar]
- 25.Law JM. Mechanistic considerations in small fish carcinogenicity testing. ILAR J. 42: 274–284. 2001. [DOI] [PubMed] [Google Scholar]
- 26.Hardman RC, Volz DC, Kullman SW, and Hinton DE. An in vivo look at vertebrate liver architecture: three-dimensional reconstructions from medaka (Oryzias latipes). Anat Rec (Hoboken). 290: 770–782. 2007. [DOI] [PubMed] [Google Scholar]
- 27.Wolf JC, and Wheeler JR. A critical review of histopathological findings associated with endocrine and non-endocrine hepatic toxicity in fish models. Aquat Toxicol. 197: 60–78. 2018. [DOI] [PubMed] [Google Scholar]
- 28.Zedeck MS, and Sternberg SS. Tumor induction in intact and regenerating liver of adult rats by a single treatment with methylazoxymethanol acetate. Chem Biol Interact. 17: 291–296. 1977. [DOI] [PubMed] [Google Scholar]
- 29.Hobbie KR, Deangelo AB, King LC, Winn RN, and Law JM. Toward a molecular equivalent dose: use of the medaka model in comparative risk assessment. Comp Biochem Physiol C Toxicol Pharmacol. 149: 141–151. 2009. [DOI] [PubMed] [Google Scholar]
- 30.Verbeek B, Southgate TD, Gilham DE, and Margison GP. O6-Methylguanine-DNA methyltransferase inactivation and chemotherapy. Br Med Bull. 85: 17–33. 2008. [DOI] [PubMed] [Google Scholar]
- 31.Law JM, Bull M, Nakamura J, and Swenberg JA. Molecular dosimetry of DNA adducts in the medaka small fish model. Carcinogenesis. 19: 515–518. 1998. [DOI] [PubMed] [Google Scholar]
- 32.Aoki K, Nakatsuru Y, Sakurai J, Sato A, Masahito P, and Ishikawa T. Age dependence of O6-methylguanine-DNA methyltransferase activity and its depletion after carcinogen treatment in the teleost medaka (Oryzias latipes). Mutat Res. 293: 225–231. 1993. [DOI] [PubMed] [Google Scholar]