Abstract
The use of polyhexamethylene guanidine hydrochloride (PHMG·HCl) as a humidifier disinfectant caused an outbreak of pulmonary disease, leading to the deaths of pregnant women and children in South Korea. However, limited information is available on the inhalation toxicity of PHMG·HCl. Therefore, this study aimed to characterize the subacute inhalation toxicity of PHMG·HCl by whole-body exposure in rats. F344 rats were exposed to 0 mg/m3, 1 mg/m3, 5 mg/m3, or 25 mg/m3 of PHMG·HCl for 6 h/day, 5 days/week for two weeks via whole-body inhalation. Emaciation and rale were observed in rats in the 25 mg/m3 PHMG·HCl group. Significant changes in body weight, hematology, serum chemistry and organ weight were observed in all PHMG·HCl-exposed groups. Gross lesions showed ballooning or red focus in the lungs of rats in the PHMG·HCl-exposed groups. In histopathological examination, most of histological lesions (including degeneration, atrophy, ulcer, inflammatory cell infiltration, inflammation, and fibrosis in nasal cavity, larynx, trachea, and lungs) indicated tissue damage by PHMG·HCl in all PHMG·HCl-exposed groups. Additionally, atrophy of the spleen, thymus, and reproductive organs; immaturity of the testes; and cell debris in the epididymides were affected by the reduction in body weight in PHMG·HCl-exposed groups. In conclusion, two-week repeated whole-body inhalation exposure of rats to PHMG·HCl reveled toxic effects on the respiratory system and secondary effects on other organs. The results of this study indicate that the no observable adverse effect level (NOAEL) for PHMG·HCl is below 1 mg/m3.
Keywords: polyhexamethylene guanidine hydrochloride (PHMG·HCl), subacute inhalation toxicity, humidifier disinfectants
Introduction
Polyhexamethylene guanidine (PHMG) is a derivative of the polymeric guanidine family and known to be a potent bactericide, virucide, and fungicide1, 2, 3. It is colorless, odorless, and non-corrosive4, and highly soluble in water5. PHMG has been widely used in fabric softeners, paints, detergents, and swimming pools and especially, as household humidifier disinfectants in South Korea 3, 5, 6, 7. In 2011, mist from humidifier disinfectants caused an outbreak of pulmonary disease, which leads to the deaths of pregnant women and children in South Korea7. The pulmonary injury of the patients was confirmed as acute interstitial pneumonia and fibrosis8, reported to be mainly associated with users of humidifier disinfectants containing PHMG·phosphate (PHMG·P), oligo (2-(2-ethoxy) ethoxyethyl guanidinium(PGH), a mixture of chloromethylisothiazolinone and methylisothiazolinone(CMIT/MIT)7. However, to a lesser extent, another derivative of PHMG, PHMG hydrochloride (PHMG·HCl) was also involved in the outbreak8.
The toxicity of PHMG·HCl has been rarely documented in humans or in in vitro and in vivo tests. PHMG·HCl has been reported to have low toxicity to humans9, 10. However, more than 12,500 patients in Russia who drank illegally manufactured vodka with 0.10–0.14% PHMG·HCl were reported to have suffered from acute cholestatic hepatitis11, 12. PHMG·HCl induces cellular toxicity through the production of intracellular reactive oxygen species (ROS) and gene expression profile alteration resulting in the progression to cell death and the down-regulation of antioxidants and detoxifying enzymes in human alveolar epithelial A549 cells13. Acute oral toxicity studies have shown that the median lethal dose (LD50) of 600 mg/kg is accompanied by signs of neurotoxicity, but a dose of 0.036 mg/kg is not associated with mortality or clinical signs of toxicity even though mid hepatocyte degeneration and tubular hydropic change may be observed14.
Only a few studies so far have reported so far on the oral toxicity of PHMG·HCl and the inhalation toxicity of PHMG·HCl has not yet been confirmed in the outbreak of humidifier disinfectant, Therefore, this study aimed to characterize the subacute inhalation toxicity of PHMG·HCl by whole-body exposure in rats.
Materials and Methods
Generation, Analysis, and Inhalation Chamber Monitoring
PHMG·HCl was obtained from Beyond Industry Co., Ltd. (Shanghai, China) through the Ministry of Environment. Filtered tap water was used as vehicle. PHMG·HCl was dissolved in water as 0.2 and 0.5% (w/v).
The PHMG·HCl aerosol was generated using an ultrasonic mist-generator in a whole body chamber (Chamber volume: 1 m3, SIS-20RG, Shibata, Saitama, Japan). The phase of PHMG·HCl aerosol was produced as mist in the inhalation chamber. The concentration of PHMG·HCl was measured using a personal sampler (Airchek XR 5000, SKC Inc., Eighty Four, PA, USA) with 25-mm micro glass fiber filters (Pallfex Membrane Filters, Pall Co., Charlotte, NC, USA). The mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD) were measured using a cascade impactor (nanoMOUDI Impactor, MSP Co., Shoreview, MN, USA). Samples were collected from the middle part of the port at a flow rate of 1 L/min. During the exposure period, the mass concentrations of the aerosols in the chamber were measured at least three times daily. The total airflow for each chamber was set at 20 L/min to achieve 1 L/min flow/rat. Chamber conditions including temperature, relative humidity, pressure, and air flow rate were automatically measured (ICS-20RG).
Animal husbandry and maintenance
Six-week-old specific-pathogen-free F344 rats of both sexes were purchased from Japan SLC, Inc. (Shizuoka, Japan) and acclimated for one week. The room was maintained at a temperature of 22 ± 3°C, relative humidity of 50 ± 20%, 12:12 h light:dark cycle, and fresh air ventilation (10–15 changes per hour). Rats were housed singly in stainless steel wire mesh cages (W 220 mm × L 750 mm × H 180 mm) and had free access to UV-irradiated rodent pellet diet (Teklad Global 18% Protein Rodent Diet, Harlan Laboratories, Inc., Indianapolis, IN, USA) and filtered tap water. The animal protocol was approved by the Institutional Animal Care and Use Committee at Occupational Safety and Health Research Institute (IACUC-1718).
Experimental design
A total of 40 rats (20 males and 20 females) were assigned randomly to one of four groups (5 per sex per group; 0 mg/m3, 1 mg/m3, 5 mg/m3, or 25 mg/m3) and exposed for 6 h/day, 5 days/week for two weeks. The PHMG·HCl concentrations used were selected on the basis of an acute toxicity study performed previously (data not shown) using a scale factor of three. Exposures were conducted in accordance with test No. 412 (Subacute Inhalation Toxicity, 2009) by the Organization for Economic Co-operation and Development (OECD)15. Inhalation exposures were conducted from 10:00 to 16:00. All rats were euthanized after two weeks of inhalation.
Clinical observations and body weight
All animals were examined twice daily for mortality and clinical signs, and weighed individually on day 1, 3, 6, and 13 of inhalation exposure.
Hematology and serum biochemistry
In hematology, all animals were fasted overnight before necropsy and blood collection. Blood samples were taken from the abdominal aorta using a syringe with a 24-gauge needle under isoflurane anesthesia (Hana Pharm, Kyonggi-Do, Korea) and collected into vacutainers containing EDTA-2K (Becton Dickinson, Franklin Lakes, NJ, USA). The absolute or relative number in the following parameters were determined in this study: total erythrocyte (RBC), hemoglobin concentration (HGB), hematocrit (HCT), mean cell volume (MCV), mean cell hemoglobin (MCH), mean cell hemoglobin concentration (MCHC), reticulocytes (RET), platelets (PLT), whole leukocytes (WBC), neutrophils (NEU), eosinophils (EOS), basophils (BAS), lymphocytes (LYM) and monocytes (MON). In serum chemistry, blood samples were centrifuged at 1,811×g at 4°C for 10 minutes within 90 minute of collection. The following serum chemistry parameters were evaluated using an automated analyzer (TBA-120FR, Toshiba Medical Systems, Tochigi, Japan): total protein (TP), albumin (ALB), blood urea nitrogen (BUN), creatinine (CREA), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), glucose (GLU), total cholesterol (T-CHO), and triglycerides (TG).
Gross exanimation and histopathology
Gross examinations of organs in the cranial, thoracic, and abdominal cavities of the rats were conducted. The absolute and relative (organ-to-body) weights of the brain, lungs, heart, liver, spleen, and kidneys were measured. The following tissues were removed from each animal at necropsy: liver, kidneys, heart, brain, spleen, trachea, tracheobronchial lymph node, larynx, lungs and nasal cavity; in males, seminal vesicle, prostate, testes, and epididymides; in female, ovaries, uterus, and vagina. The nasal cavity was sectioned at four levels: 1, posterior to the upper incisors; 2, incisive papilla; 3, second palatine crest; and 4, first molar teeth. The organs were preserved in 10% neutral buffered formalin. All organs were embedded in paraffin, sectioned at 3–4 μm, stained with hematoxylin and eosin (H&E), and examined microscopically at low and high power fields.
Data analyses
Differences among groups in the various parameters were determined using SPSS (ver. 18.0, IBM, Chicago, IL, USA) software. The homogeneity of variance was analyzed by Levene’s test, followed by either one-way analysis of variance for samples with homogenous variance or the Kruskal-Wallis test for samples with heterogeneous variance. Scheffe or Dunnett’s multiple range test was used to compare the result of each experimental group with that of the control group if the first statistical result was significant.
Results
Chamber monitoring
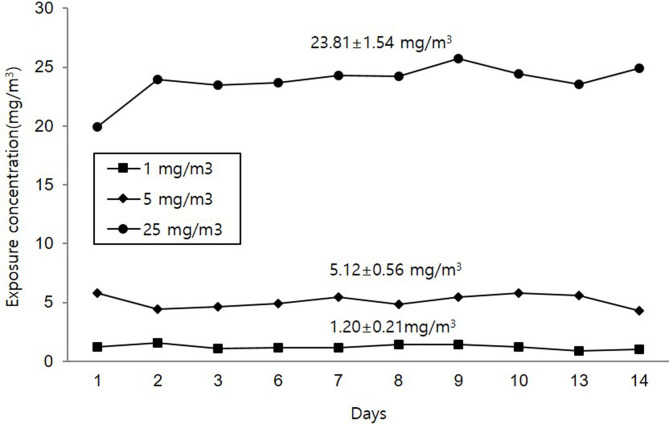
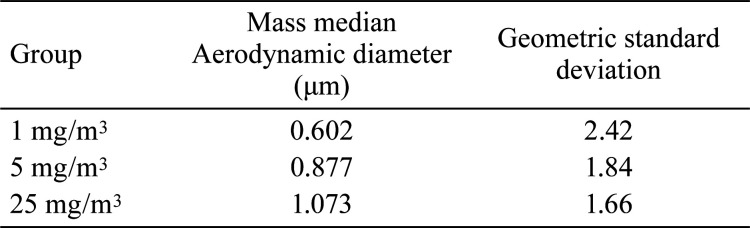
The ranges of chamber conditions were 19.6–21.9°C, 49.5–74.8% relative humidity, −66.8–−58.1 mmH2O pressure, and 220.1–278.2 L/min flow rate. The average concentrations of PHMG·HCl during the study were 1.20.1 ± 0.21 mg/m3, 5.12 ± 0.56 mg/m3, and 23.81 ± 1.54 mg/m3 for 1, 5, and 25 mg/m3 PHMG·HCl groups, respectively (Fig. 1). The MMAD was 0.602, 0.877, and 1.073 μm, and the GSD 2.42, 1.84, and 1.66 mg/m3 for 1, 5, and 25 mg/m3 PHMG·HCl groups, respectively (Table 1).
Fig. 1.
PHMG·HCl concentrations in the inhalation chamber during the study.
Table 1. Particle Size Distribution.
Clinical signs
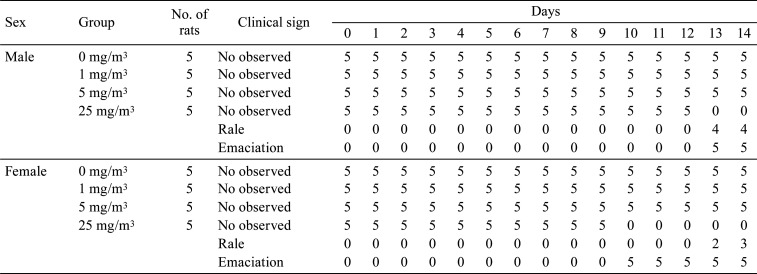
No deaths were observed in any groups. Emaciation and rale were observed in males and females exposed to 25 mg/m3 (Table 2).
Table 2. Summary of Clinical Signs.
Body weight
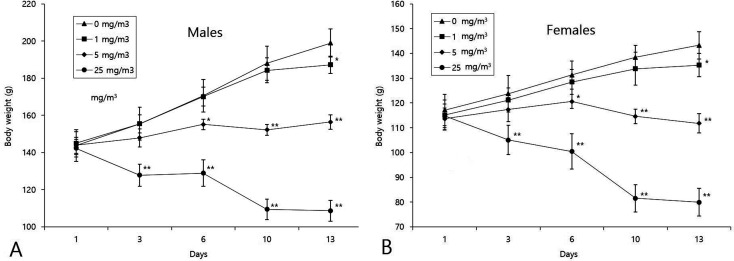
Body weights decreased significantly in males and female exposed to 25, 5, and 1 mg/m3 from day 3, 6, and 13 onward, respectively (Fig. 2).
Fig. 2.
Changes in body weight during the study. Significant differences compared with the control: *p<0.05, **p<0.01.
Hematology
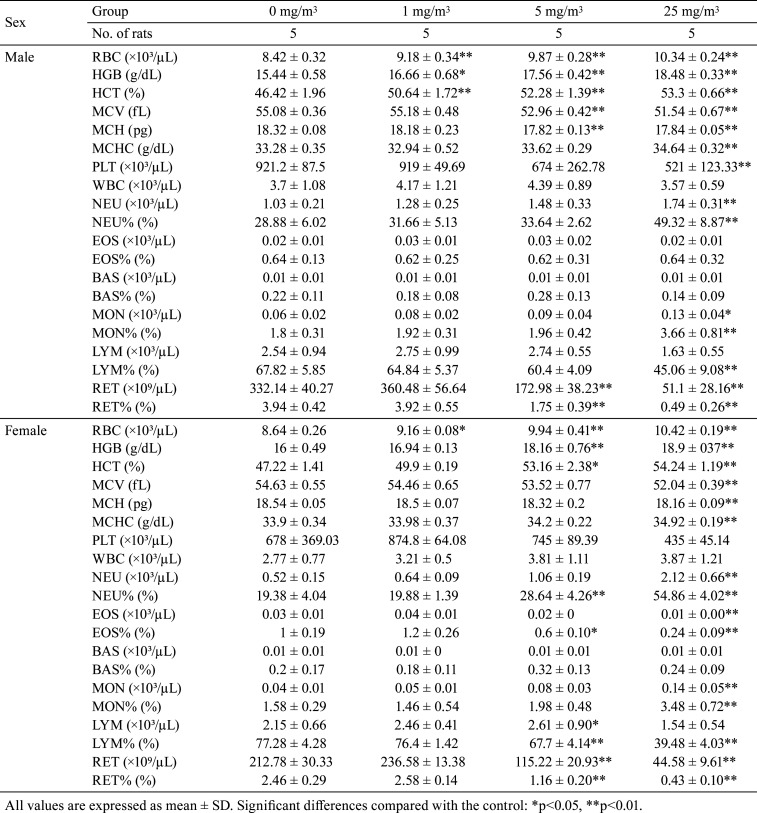
RBC, HCT, and HGB showed increasing trend or significant increases in males and females exposed to 1, 5, and 25 mg/m3. MCHC increased significantly in males and female exposed to 25 mg/m3. In contrast, MCH and MCV decreased significantly in males exposed to 5 and 25 mg/m3 and females exposed to 25 mg/m3. Additionally, RET and RET% decreased significantly in males and females exposed to 5 and 25 mg/m3. PLT showed decreasing trends or significant decrease in males and females exposed to 25 mg/m3. MON, MON%, and NEU increased significantly in males and females exposed to 25 mg/m3. NEU% increased significantly in males exposed to 25 mg/m3 and females exposed to 5 and 25 mg/m3. However, LYM% showed decreasing trend or significant decrease in males exposed to 25 mg/m3 and females exposed to 5 and 25 mg/m3. EOS and EOS% decreased significantly in females exposed to 25 mg/m3 (Table 3).
Table 3. Summary of Hematological Parameters.
Serum biochemistry
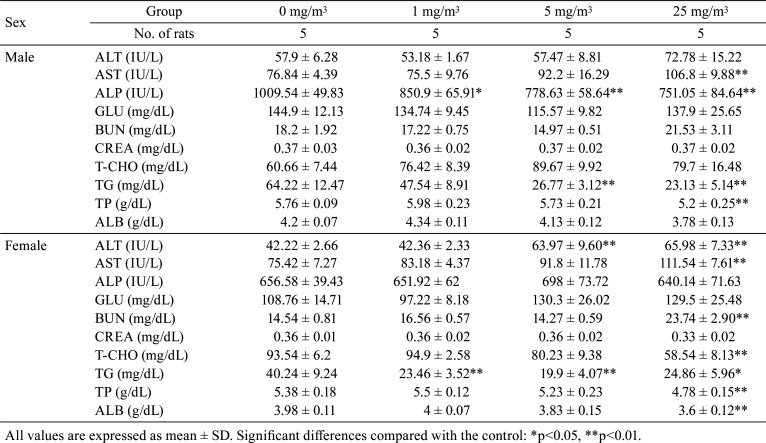
ALT showed increasing trends or significant increase in males exposed to 25 mg/m3 and females exposed to 5 and 25 mg/m3. Additionally, AST increased significantly in males and females exposed to 25 mg/m3. BUN showed increasing trends or significant increases in males and females exposed to 25 mg/m3. In contrast, ALP decreased significantly in males exposed to 1, 5, and 25 mg/m3. Moreover, TG decreased significantly in males exposed to 5 and 25 mg/m3 and females exposed to 1, 5, and 25 mg/m3. TP and ALB decreased significantly in males and females exposed to 25 mg/m3. T-CHO decreased significantly in females exposed to 25 mg/m3 (Table 4).
Table 4. Summary of Serum Chemical Parameters.
Organ weight
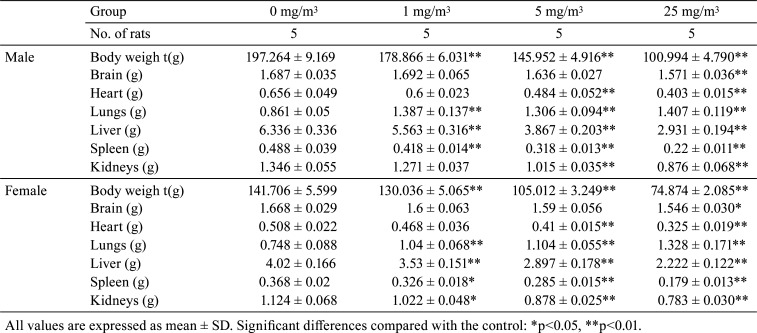
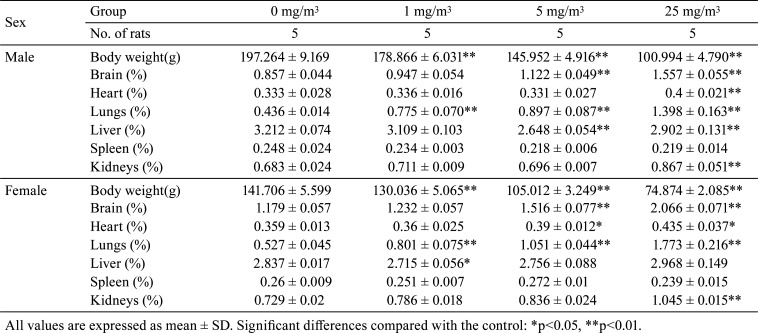
The absolute and relative organ weights of the lungs increased significantly in males and females exposed to 1, 5, and 25 mg/m3. The absolute organ weight of the liver decreased significantly in males and females exposed to 1, 5, and 25 mg/m3, as did the relative organ weight of the liver in males exposed to 5 and 25 mg/m3 and females exposed to 1 mg/m3. The absolute organ weight of the spleen decreased significantly in males and females exposed to 1, 5, and 25 mg/m3. The absolute organ weight of the kidneys decreased significantly in males exposed to 5 and 25 mg/m3 and females exposed to 1, 5, and 25 mg/m3. However, the relative organ weight of the kidneys increased significantly in males and females exposed to 25 mg/m3. The absolute organ weight of the heart decreased significantly in males and females exposed to 5 and 25 mg/m3, and the relative organ weight of the heart increased significantly in males and females exposed to 25 mg/m3. The absolute organ weight of the brain decreased significantly in males and females exposed to 25 mg/m3, and the relative organ weight of the brain increased significantly in males and females exposed to 5 and 25 mg/m3 (Table 5 and 6).
Table 5. Summary of Absolute Organ Weight.
Table 6. Summary of Relative Organ Weight.
Gross lesion evaluations
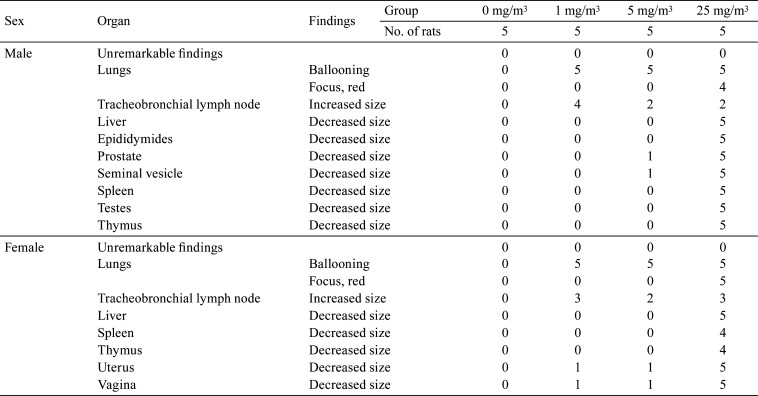
Ballooning was observed in the lungs of males and females exposed to 1, 5, and 25 mg/m3, and red focus was observed in the lungs of males and females exposed to 25 mg/m3. Increased size in the tracheobronchial lymph node was observed in males and females exposed to 1, 5, and 25 mg/m3, and red focus and decreased size in the liver, spleen, and thymus were observed in males and females exposed to 25 mg/m3. Additionally, decreased size was observed in the testes and epididymides of males exposed to 25 mg/m3, in the seminal vesicle and prostate of males exposed to 5 and 25 mg/m3, and in the uterus and vagina of females exposed to 1, 5, and 25 mg/m3 (Table 7).
Table 7. Summary of Gross Findings.
Histopathological examination
Nasal cavity
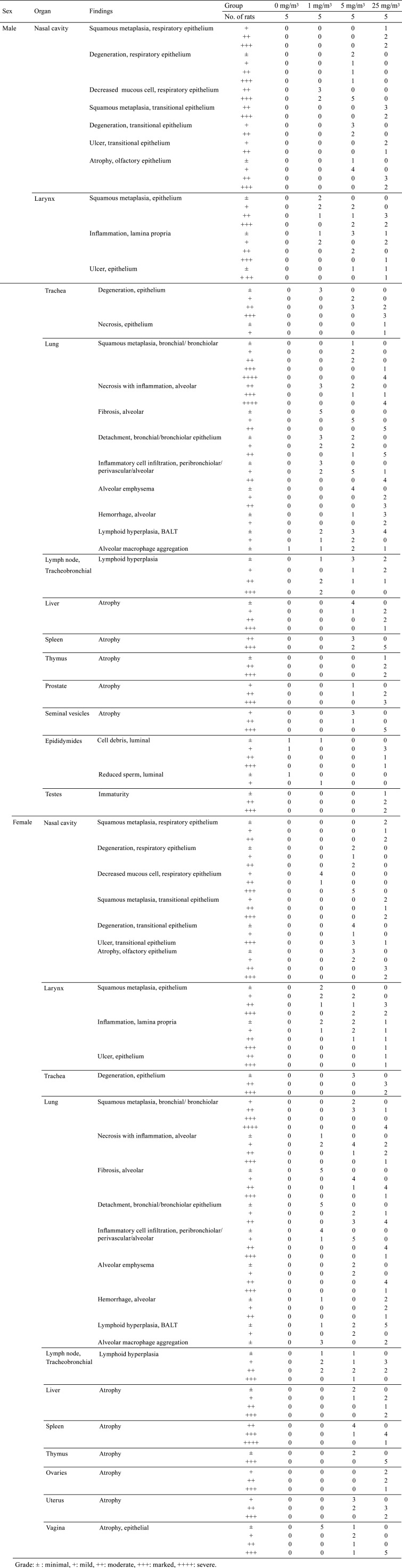
Squamous metaplasia of respiratory and transitional epithelium were observed in males and females exposed to 25 mg/m3. Degeneration of respiratory and transitional epithelium were observed in males and females exposed to 5 mg/m3. A decrease in number of mucous cells in the respiratory epithelium was observed in males and females exposed to 1 and 5 mg/m3. Atrophy of olfactory epithelium was observed in males and females exposed to 5 and 25 mg/m3. Ulcer of transitional epithelium was observed in males exposed to 25 mg/m3 and females exposed to 5 and 25 mg/m3. The severity of the nasal cavity lesion increased in a dose-related manner (Table 8 and Fig. 3A–D).
Table 8. Summary of Histopathology.
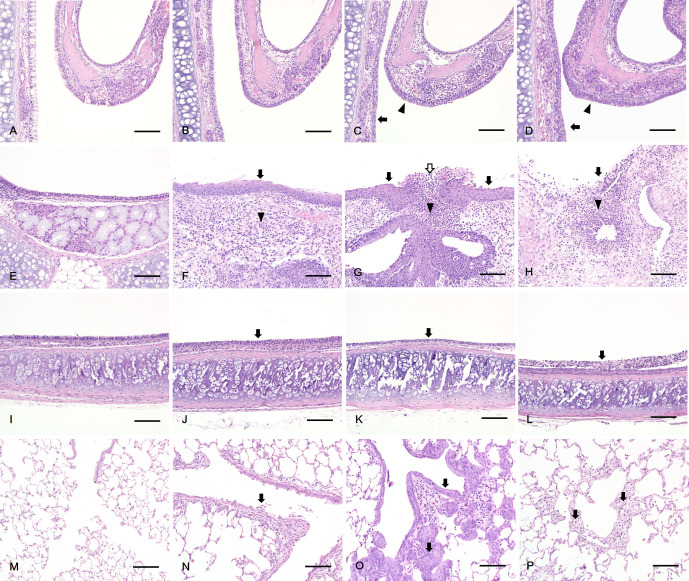
Fig. 3.
Histopathology of rats exposed to PHMG·HCl. In the nasal cavity, (A, B) No abnormal lesion was observed in the control (A) and 1 mg/m3 PHMG·HCl (B) groups. (C) Degeneration of respiratory epithelium (arrow) and transitional epithelium (arrowhead) in the 5 mg/m3 PHMG·HCl group. (D) Squamous metaplasia of respiratory epithelium (arrow) and transitional epithelium (arrowhead) in the 25 mg/m3 PHMG·HCl group. In the larynx, (E) No abnormal lesion was observed in the control group. (F, G) Squamous metaplasia of the epithelium (arrow), inflammation of the lamina propria (arrowhead) in 1 (F) and 5 mg/m3 (G) PHMG·HCl groups and ulcer of the epithelium (white arrow) in 5 mg/m3 (G) PHMG·HCl group. (H) Ulcer of the epithelium (arrow) and inflammation of the lamina propria (arrowhead) in the 25 mg/m3 PHMG·HCl groups. In the trachea, (I) No abnormal lesion was observed in the control group. (J, K) Degeneration of the epithelium (arrow) in 1(J) and 5 mg/m3 (K) PHMG·HCl groups. (L) Necrosis of the epithelium (arrow) in the 25 mg/m3 PHMG·HCl group. In the lung, (M) No abnormal lesion was observed in the control group. (N) Detachment of the bronchiolar epithelium (arrow) in the 1 mg/m3 PHMG·HCl group. (O) Squamous metaplasia of bronchiolar epithelium (arrow) in the 5 mg/m3 PHMG·HCl group. (P) Alveolar fibrosis (arrow) in the 25 mg/m3 PHMG·HCl group. Scale bars=100 μm, Magnification: ×200, H&E staining.
Larynx
Squamous metaplasia of epithelium and inflammation of the lamina propria were observed in males and females exposed to 1, 5, and 25 mg/m3. Ulcer of the epithelium was observed in males exposed to 5 and 25 mg/m3 and females exposed to 25 mg/m3. The severity of the larynx lesion increased in a dose-related manner (Table 8 and Fig. 3E–H).
Trachea
Degeneratoin of the epithelium was observed in males exposed to 1, 5, and 25 mg/m3 and females exposed to 5 and 25 mg/m3. Necrosis of the epithelium was observed in males exposed to 25 mg/m3. The severity of the trachea lesion increased in a dose-related manner (Table 8 and Fig. 3I–L).
Lung
Squamous metaplasia of bronchial and bronchiolar epithelium and alveolar emphysema were observed in males and females exposed to 5 and 25 mg/m3. Addtionally, necrosis with inflammation; alveolar fibrosis; detachment of bronchial and bronchiolar epithelium; and peribronchiolar, perivascular, and alveolar inflammatory cell infiltration were observed in males and females exposed to 1, 5, and 25 mg/m3. Alveolar hemorrhage was observed in males exposed to 5 and 25 mg/m3 and females exposed to 1 and 25 mg/m3. Lymphoid hyperplasia of bronchus-associated lymphoid tissue (BALT) was observed in males and females exposed to 1, 5, and 25 mg/m3. Alveolar macrophage aggregation was observed in males exposed to 0, 1, 5, and 25 mg/m3 and females exposed to 1 and 25 mg/m3. The severity of the lung lesion increased in a dose-related manner (Table 8 and Fig. 3M–P).
Tracheobronchial lymph node
Lymphoid hyperplasia was observed in males and females exposed to 1, 5, and 25 mg/m3. The severity of the tracheobronchial lymph node lesion increased in a dose-related manner (Table 8).
Other organs
Atrophy of the liver was observed in males and females exposed to 5 and 25 mg/m3. Atrophy of the spleen was observed in males and females exposed to 5 and 25 mg/m3. Atrophy of the thymus was observed in males exposed to 25 mg/m3 and females exposed to 5 and 25 mg/m3. Atrophy of the prostate and seminal vesicle was observed in males exposed to 5 and 25 mg/m3. Cell debris was observed in the epididymides of males exposed to 0, 1, and 25 mg/m3, and reduced sperm was observed in males exposed to 0 and 1 mg/m3. Immaturity was observed in the testes of males exposed to 25 mg/m3. Atrophy of the ovary, uterus, and vagina was observed in females exposed to 5 mg/m3; 5 and 25 mg/m3; and 1, 5, and 25 mg/m3, respectively (Table 8).
Discussion
In the present study, the treatment-related effects of PHMG·HCl in rats were observed in clinical signs; body weight gain; hematology and serum biochemistry; organ weight; gross lesions and histopathological lesions in the nasal cavity, larynx, trachea, lungs, tracheobronchial lymph node, liver, spleen, thymus, seminal vesicle, prostate, testes, epididymides, ovary, uterus, and vagina.
Body weights decreased significantly in males and female exposed to 1, 5 and 25 mg/m3 until termination of the study. In particular, body weight decreased far less than that in the start of the study in the male and female exposed to 5 and 25 mg/m3, which corresponds to aggravated clinical signs including emaciation and rale observed in the males and females exposed to 25 mg/m3. This may be attributed to lung inflammation associated with cytokines and stress-induced anorexia even though we did not evaluate food consumption in this study. Inflammatory cytokines and stress are reported to act on the hypothalamus and induce anorexia16, 17.
RBC, HCT, HGB, MCHC, MCH, MCV, RET, RET %, and PLT changed significantly in males and females exposed to 1, 5, and 25 mg/m3. This may be attributed to decreased hematopoiesis caused by anorexia18, 19. In addition, MON%, NEU, NEU%, and LYM% changed significantly in males and females exposed to 5 and 25 mg/m3. This may be associated with inflammation lesions including necrosis, inflammatory cell infiltration, and fibrosis20. Moreover, decrease in LYM% may be associated with stress responses21.
Increase or increasing trends in ALT and AST was observed in males and females exposed to 5 and 25 mg/m3. This may be attributed to atrophy of the liver 18, because integrity of hepatocyte membranes might be disrupted by a decrease in size of the hepatocytes, which causes leakage of enzymes. Increases or increasing trends in BUN was observed in males and females exposed to 25 mg/m3. This may be attributed to dehydration or catabolism of protein, because increases or increasing trends in RBC, HCT, HGB, MCH, and MCHC were observed 17, and clinical signs included emaciation22, 23.
Changes in ALP, TG, TP, ALB and T-CHO were observed in males and females exposed to 1, 5, and 25 mg/m3. This may be attributed to decreased food consumption19, which we speculate from decreased body weights even though we did not measure food consumption. Changes in the absolute and relative organ weights of the brain, heart, liver, spleen, and kidneys were observed in females exposed to 1, 5, and 25 mg/m3. These were associated with the decreased body weights of rats. Increases in the absolute and relative organ weights of the lungs were observed in males and females exposed to 1, 5, and 25 mg/m3. These may have been affected by lung inflammation24, 25.
Most of histological lesions (including degeneration, atrophy, ulcer, inflammatory cell infiltration, inflammation, and fibrosis in nasal cavity, larynx, trachea, and lungs) indicated tissue damage by test substance26. In particular, lesions in the lungs were observed mainly at the junction of terminal bronchioles and alveolar ducts (the centriacinar region) where the velocity of air flow is decreased and aerosol particulates can be easily deposited and induce damage to cells25, 26. Similarly, a centrilobular pattern of lesions was also observed in human patients exposed to humidifier disinfectants27. Many studies have reported that the toxicity of PHMG is related to oxidative stress. PHMG·P produces ROS in human alveolar A549 cells, mouse macrophage RAW264.7 cells, or in vitro air-liquid interface (ALI) co-culture models and causes fibrosis and inflammation via cellular signals, such as cytokines13, 28, 29. In particular, 4-hydroxynonenal (4-HNE), an oxidative stress marker, was confirmed by immunohistochemistry in the macrophages of the fibrotic tissue and the bronchiolar epithelium, mainly in Clara cells in 13-week inhalation study of PHMG·HCl. This indicated that these cells plays a critical role in damaging the lung30.
Squamous metaplasia is considered to be an adaptive or protective response to irritation than a precursor to neoplastic lesions31. Notably, it has been reported that the larynx of rodents is more sensitively affected by inhaled xenobiotics than those of non-rodents. However, these findings lacks relevance for humans because of differences in anatomical structures32. Even though squamous metaplasia is an adaptive or protective change, it should be considered an adverse effect because of the severity of the lesion and concomitant degenerative/necrotic and/or hyperplastic changes33. Interestingly, the grade of squamous metaplasia of lung in our study is found to be more severe than that in 13-week inhalation study of PHMG·HCl (Inhalation concentration: 0 mg/m3, 0.13 mg/m3, 0.4 mg/m3, and 1.20 mg/m3)30. This indicate that the epithelium of lung, mainly at the junction of terminal bronchioles and alveolar ducts (the centriacinar region) is more severely damaged by PHMG·HCl and rapidly progressed to squamous metaplasia because of concentration of PHMG·HCl much higher than that in 13-week inhalation study period even though squamous metaplasia is generally found in long term study. These lesions of the respiratory system were also observed in a PHMG·P inhalation toxicity study 34.
Lymphoid hyperplasia of the tracheobronchial lymph node and BALT of the lung were observed in males and females exposed to 1, 5, and 25 mg/m3. These are considered to be immune responses to PHMG·HCl35. Atrophy of the liver and spleen are considered to be effects of decreased body weight in males and females exposed to 5 and 25 mg/m336, 37. Atrophy of the thymus is considered to be a stress response affected by aggravated clinical signs including decreased body weight in males and females exposed to 5 and 25 mg/m338. Atrophy of reproductive organs in both male and female rats indicate alteration of sexual hormones affected by aggravated clinical signs including decreases in body weight39, 40.
In conclusion, two-week repeated whole-body inhalation exposure of rats to three different concentrations of PHMG·HCl reveled toxic effects on the respiratory system and secondary effect on other organs. The results of this study indicate that the no observable adverse effect level (NOAEL) for PHMG·HCl is below 1 mg/m3. The present study provides useful information regarding inhalation toxicity of PHMG·HCl.
Disclosure of Potential Conflict of Interest
The authors declare that there are no conflicts of interest in connection with this paper.
Acknowledgments
This work was supported by the Korea Occupational Safety and Health Agency, Ministry of Labor, Republic of Korea, and a Grant-in-Aid for chemical hazard assessment, 2020.
References
- 1.Krebs FC, Miller SR, Ferguson ML, Labib M, Rando RF, and Wigdahl B. Polybiguanides, particularly polyethylene hexamethylene biguanide, have activity against human immunodeficiency virus type 1. Biomed Pharmacother. 59: 438–445. 2005. [DOI] [PubMed] [Google Scholar]
- 2.Müller G, and Kramer A. Effect of selected wound antiseptics on adult articular cartilage (bovine sesamoid bone) in the presence of Escherichia coli and Staphylococcus aureus. J Orthop Res. 23: 127–133. 2005. [DOI] [PubMed] [Google Scholar]
- 3.Oulé MK, Quinn K, Dickman M, Bernier AM, Rondeau S, De Moissac D, Boisvert A, and Diop L. Akwaton, polyhexamethylene-guanidine hydrochloride-based sporicidal disinfectant: a novel tool to fight bacterial spores and nosocomial infections. J Med Microbiol. 61: 1421–1427. 2012. [DOI] [PubMed] [Google Scholar]
- 4.Kuznetsov YI. Physicochemical aspects of metal corrosion inhibition in aqueous solutions. Russ Chem Rev. 73: 75–87. 2004. [Google Scholar]
- 5.Ohta S, Misawa Y, Miyamoto H, Makino M, Nagai K, Shiraishi T, Nakagawa Y, Yamato S, Tachikawa E, and Zenda H. A comparative study of characteristics of current-type and conventional-type cationic bactericides. Biol Pharm Bull. 24: 1093–1096. 2001. [DOI] [PubMed] [Google Scholar]
- 6.Vitt A, Sofrata A, Slizen V, Sugars RV, Gustafsson A, Gudkova EI, Kazeko LA, Ramberg P, and Buhlin K. Antimicrobial activity of polyhexamethylene guanidine phosphate in comparison to chlorhexidine using the quantitative suspension method. Ann Clin Microbiol Antimicrob. 14: 36 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park DU, Ryu SH, Lim HK, Kim SK, Choi YY, Ahn JJ, Lee E, Hong SB, Do KH, Cho JL, Bae MJ, Shin DC, Paek DM, and Hong SJ. Types of household humidifier disinfectant and associated risk of lung injury (HDLI) in South Korea. Sci Total Environ. 596-597: 53–60. 2017. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Kim YH, and Kwon JH. Fatal misuse of humidifier disinfectants in Korea: importance of screening risk assessment and implications for management of chemicals in consumer products. Environ Sci Technol. 46: 2498–2500. 2012. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda T, Tazuke S, and Watanabe M. Interaction of biologically active molecules with phospholipid membranes. I. Fluorescence depolarization studies on the effect of polymeric biocide bearing biguanide groups in the main chain. Biochim Biophys Acta. 735: 380–386. 1983. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda T, Ledwith A, Bamford CH, and Hann RA. Interaction of a polymeric biguanide biocide with phospholipid membranes. Biochim Biophys Acta. 769: 57–66. 1984. [DOI] [PubMed] [Google Scholar]
- 11.Lachenmeier DW, Monakhova YB, and Rehm J. Influence of unrecorded alcohol consumption on liver cirrhosis mortality. World J Gastroenterol. 20: 7217–7222. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostapenko YN, Brusin KM, Zobnin YV, Shchupak AY, Vishnevetskiy MK, Sentsov VG, Novikova OV, Alekseenko SA, Lebed’ko OA, and Puchkov YB. Acute cholestatic liver injury caused by polyhexamethyleneguanidine hydrochloride admixed to ethyl alcohol. Clin Toxicol (Phila). 49: 471–477. 2011. [DOI] [PubMed] [Google Scholar]
- 13.Jung HN, Zerin T, Podder B, Song HY, and Kim YS. Cytotoxicity and gene expression profiling of polyhexamethylene guanidine hydrochloride in human alveolar A549 cells. Toxicol In Vitro. 28: 684–692. 2014. [DOI] [PubMed] [Google Scholar]
- 14.Asiedu-Gyekye IJ, Mahmood SA, Awortwe C, and Nyarko AK. A preliminary safety evaluation of polyhexamethylene guanidine hydrochloride. Int J Toxicol. 33: 523–531. 2014. [DOI] [PubMed] [Google Scholar]
- 15.Organisation for Economic Co-operation and Development (OECD) Test No 412: Subacute Inhalation Toxicity: 28-day study. 2009, from OECD Guidelines for the Testing of Chemicals, Section 4 website: https://www.oecd.org/env/ehs/testing/test-no-412-subacute-inhalation-toxicity-28-day-study-9789264070783-en.htm.
- 16.Braun TP, and Marks DL. Pathophysiology and treatment of inflammatory anorexia in chronic disease. J Cachexia Sarcopenia Muscle. 1: 135–145. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bazhan N, and Zelena D. Food-intake regulation during stress by the hypothalamo-pituitary-adrenal axis. Brain Res Bull. 95: 46–53. 2013. [DOI] [PubMed] [Google Scholar]
- 18.Haschek WM, Rousseaux CG, and Wallig MA. Clinical pathology. In: Fundamentals of Toxicologic Pathology, 2nd ed. Academic press, London, UK, 43–65. 2009. [Google Scholar]
- 19.Asanuma F, Miyata H, Iwaki Y, and Kimura M. Feature on erythropoiesis in dietary restricted rats. J Vet Med Sci. 73: 89–96. 2011. [DOI] [PubMed] [Google Scholar]
- 20.Evans GO. Leukocytes. In: Animal Hematotoxicology: A Practical Handbook for Toxicologists and Biomedical Researchers. 2nd ed. CRC Press, New York, 65–83. 2009. [Google Scholar]
- 21.York MJ. Clinical pathology. In: A Comprehensive Guide to Toxicology in Preclinical Drug Development, 1st ed., Faqi AS (ed). Academic Press, London, 167–210. 2013. [Google Scholar]
- 22.Evans GO. Assessment of nephrotoxicity. In: Animal Clinical Chemistry: A Practical Handbook for Toxicologists and Biomedical Researchers, 2nd ed. CRC Press, New York, 67–86. 2009. [Google Scholar]
- 23.Durham WJ, Dillon EL, and Sheffield-Moore M. Inflammatory burden and amino acid metabolism in cancer cachexia. Curr Opin Clin Nutr Metab Care. 12: 72–77. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wahlström E, Ollerstam A, Sundius L, and Zhang H. Use of lung weight as biomarker for assessment of lung toxicity in rat inhalation studies. Toxicol Pathol. 41: 902–912. 2013. [DOI] [PubMed] [Google Scholar]
- 25.Leikauf GD. Toxic responses of the respiratory system. In: Casarett and Doull’s Toxicology: The Basic Science of Poisons. 8th ed. Curtis D(eds). McGraw-Hill Professional, New York. 691–731. 2013. [Google Scholar]
- 26.Renne R, Brix A, Harkema J, Herbert R, Kittel B, Lewis D, March T, Nagano K, Pino M, Rittinghausen S, Rosenbruch M, Tellier P, and Wohrmann T. Proliferative and nonproliferative lesions of the rat and mouse respiratory tract. Toxicol Pathol. 37(Suppl): 5S–73S. 2009. [DOI] [PubMed] [Google Scholar]
- 27.Huh JW, Hong SB, Do KH, Koo HJ, Jang SJ, Lee MS, Paek D, Park DU, Lim CM, and Koh Y. Inhalation lung injury associated with humidifier disinfectants in adults. J Korean Med Sci. 31: 1857–1862. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim HR, Lee K, Park CW, Song JA, Shin DY, Park YJ, and Chung KH. Polyhexamethylene guanidine phosphate aerosol particles induce pulmonary inflammatory and fibrotic responses. Arch Toxicol. 90: 617–632. 2016. [DOI] [PubMed] [Google Scholar]
- 29.Kim HR, Shin DY, and Chung KH. The role of NF-κB signaling pathway in polyhexamethylene guanidine phosphate induced inflammatory response in mouse macrophage RAW264.7 cells. Toxicol Lett. 233: 148–155. 2015. [DOI] [PubMed] [Google Scholar]
- 30.Lee YH, Seo DS, Lee MJ, and Cha HG. Immunohistochemical characterization of oxidative stress in the lungs of rats exposed to the humidifier disinfectant polyhexamethylene guanidine hydrochloride. J Toxicol Pathol. 32: 311–317. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herbert RA, Janardhan KS, Pandri AR, Cesta MA, and Miller RA. Nose, larynx and trachea. In: Boorman’s Pathology of the Rat: Reference and Atlas, 2nd ed. Suttie AW (ed). Academic Press, San Diego. 3911–3435. 2018. [Google Scholar]
- 32.Mowat V, Alexander DJ, and Pilling AM. A comparison of rodent and nonrodent laryngeal and tracheal bifurcation sensitivities in inhalation toxicity studies and their relevance for human exposure. Toxicol Pathol. 45: 216–222. 2017. [DOI] [PubMed] [Google Scholar]
- 33.Pandiri AR, Kerlin RL, Mann PC, Everds NE, Sharma AK, Myers LP, and Steinbach TJ. Is it adverse, nonadverse, adaptive, or artifact? Toxicol Pathol. 45: 238–247. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee K, Heo Y, Kim YJ, Yeom DH, Kim S, Kim YJ, Lee MY, and Lee SH. A safety study for management of the existing chemicals (I). National Institute of Environmental Research. 26–79. 2013. (in Korean).
- 35.Agarwal DK, Dogra RKS, and Shanker R. Pathobiochemical response of tracheobronchial lymph nodes following intratracheal instillation of polyvinylchloride dust in rats. Arch Toxicol. 65: 510–517. 1991. [DOI] [PubMed] [Google Scholar]
- 36.Thoolen B, Maronpot RR, Harada T, Nyska A, Rousseaux C, Nolte T, Malarkey DE, Kaufmann W, Küttler K, Deschl U, Nakae D, Gregson R, Vinlove MP, Brix AE, Singh B, Belpoggi F, and Ward JM. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol Pathol. 38(Suppl): 5S–81S. 2010. [DOI] [PubMed] [Google Scholar]
- 37.Suttie AW. Histopathology of the spleen. Toxicol Pathol. 34: 466–503. 2006. [DOI] [PubMed] [Google Scholar]
- 38.Pearse G. Histopathology of the thymus. Toxicol Pathol. 34: 515–547. 2006. [DOI] [PubMed] [Google Scholar]
- 39.Creasy D, Bube A, de Rijk E, Kandori H, Kuwahara M, Masson R, Nolte T, Reams R, Regan K, Rehm S, Rogerson P, and Whitney K. Proliferative and nonproliferative lesions of the rat and mouse male reproductive system. Toxicol Pathol. 40(Suppl): 40S–121S. 2012. [DOI] [PubMed] [Google Scholar]
- 40.Dixon D, Alison R, Bach U, Colman K, Foley GL, Harleman JH, Haworth R, Herbert R, Heuser A, Long G, Mirsky M, Regan K, Van Esch E, Westwood FR, Vidal J, and Yoshida M. Nonproliferative and proliferative lesions of the rat and mouse female reproductive system. J Toxicol Pathol. 27(Suppl): 1S–107S. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]