Abstract
Pulmonary embolism (PE) is a potential life-threatening condition and risk-adapted diagnostic and therapeutic management conveys a favorable outcome. For patients at high risk for early complications and mortality, prompt exclusion or confirmation of PE by imaging is the key step to initiate and facilitate reperfusion treatment. Among patients with hemodynamic instability, systemic thrombolysis improves survival, whereas surgical embolectomy or percutaneous intervention are alternatives in experienced hands in scenarios where systemic thrombolysis is not the best preferred thromboreduction measure. For patients with suspected PE who are not at high risk for early complications and mortality, the organized approach using a structured evaluation system to assess the pretest probability, the age-adjusted D-dimer cut-offs, the appropriate selection of imaging tools, and proper interpretation of imaging results is important when deciding the allocation of treatment strategies. Patients with PE requires anticoagulation treatment. In patients with cancer and thrombosis, low-molecular-weight heparin (LMWH) used to be the standard regimen. Recently, three factor Xa inhibitors collectively show that non-vitamin K oral anticoagulants (NOACs) are as effective as LMWH in four randomized clinical trials. Therefore, NOACs are suitable and preferred in most conditions. Finally, chronic thromboembolic pulmonary hypertension is the most disabling long-term complication of PE. Because of its low incidence, the extra caution should be given when managing patients with PE.
Keywords: Anticoagulants, Diagnosis, Pulmonary embolism, Treatment
INTRODUCTION
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), represents a significant healthcare burden worldwide.1 Although DVT and PE occur at different vascular territories in the circulatory system, these two clinical conditions share a common pathogenesis centered on Virchow’s triad of blood flow stasis, vessel wall damage, and increased blood viscosity. The diagnostic work-up of both diseases requires high clinical awareness, an organized pathway including the use of a clinical decision rule and D-dimer testing, and the confirmatory imaging.2 After the timely verification of the diagnosis, DVT and PE are commonly managed with anticoagulants.3
We have previously reported the consensus on the diagnosis and treatment of DVT.4 In this document, experts reviewed the information of PE and made recommendations for clinical practices in diagnostic and therapeutic approaches pertinent to PE and updated our recommendations for pharmacological management of VTE.
EPIDEMIOLOGY
The incidence rate of VTE in the Western countries is 100 or greater per 100,000 person-years.5 Although Asian populations, compared with Western populations, are subject to the similar acquired risk factors for VTE and have a higher prevalence of strong thrombophilia,6 the incidence estimates in Asia are approximately 15 to 20% of the levels reported in Western countries.7 Nevertheless, the incidences of both DVT and PE continue to rise in Asia.8,9
Given the fact that the presentation of PE can range from a silent (asymptomatic) complication of DVT or an incident finding during diagnostic work-up for another disease(s) to syncope or sudden death, the true incidence of PE is difficult to determine. By estimation, the incidence of PE in East Asia is around 5 to 7 per 100,000 person-years.9-11 Even with anticoagulation treatment, the mortality rate related to PE remains high (12% at the first month and 24% at the first year).12,13
DIAGNOSIS
The clinical assessment and diagnostic testing for PE are the same in Asian populations as they are in Western populations. Because PE may be fatal in the acute phase or leads to chronic disability that impairs physical performance and quality of life,14,15 careful history taking, risk factor identification, and distinguishing presentations associated with suspected PE from other medical emergencies are important. However, common symptoms reported by patients as dyspnea or signs found by physicians as tachycardia and tachypnea are non-specific.16 The clinical suspicion of PE must be confirmed by objective testing, including invasive pulmonary angiography, computed tomographic pulmonary angiography, or lung ventilation/perfusion scintigraphy, among the majority of PE-likely patients, whereas PE can be safely excluded by a validated algorithm among PE-unlikely patients without further imaging. Therefore, the accurate and timely diagnosis of PE depends on the combined use of clinical assessment, plasma D-dimer measurement, and imaging tests.
Clinical presentations
Although common symptoms suggestive of PE including acute dyspnea or deteriorating dyspnea, chest pain, and/or hemoptysis are not challenging to identify,16,17 they are non-specific for PE. Syncope is infrequent but may occur without hemodynamic instability. The prevalence of PE in patients without an alternative explanation for syncope may reach up to a quarter.18 Furthermore, shock might be the central presentation of PE in around 5% of patients.14,19 Tachypnea is the most common sign followed by tachycardia.20,21 However, both are non-specific for PE. On contrary, about 70% of patients with symptomatic PE have concomitant DVT, in which up to a quarter is symptomatic.22 Therefore, symptoms suggestive of DVT, including leg edema, erythema, tenderness, and/or the palpable cord, may present.23 Physical examination may also identify signs of PE-associated pulmonary hypertension, such as elevated neck veins, a loud P2, a right-sided gallop, and a right ventricular lift.
Abnormalities on chest radiography, electrocardiography, or a blood gas analysis are neither specific for PE but useful in judging the differential diagnosis. Sinus tachycardia by electrocardiography is common.24 Tachycardia is associated with an increase in the risk of mortality even a cut-off of the heart rate from 86 beats/min is associated with an elevated risk of right ventricular dysfunction and the intermediate risk PE status.25 Other electrocardiographic markers that are useful in risk stratification for patients with PE include right bundle branch block and SIQIII-type patterns.26,27 But, the electrocardiogram is of limited diagnostic value in patients with suspected PE as those abnormalities found in suspected PE are equally prevalent in patients suspected of PE in whom PE is ultimately excluded.28 Hypoxia associated symptoms and signs are the cardinal presentation of PE. Hypoxemia by the blood gas analysis is a sensitive indicator of PE. Nevertheless, a normal alveolar-arterial oxygen gradient alone can not safely exclude the diagnosis of PE.29,30
Clinical assessment
As with the diagnosis of DVT, a key step for diagnosing PE is to assess a patient’s clinical probability of PE based on medical history and physical examination before any testing. Although there are structured clinical probability scoring systems developed to stratify patients,20,31-33 the Wells scoring system, which incorporates medical history and physical examination, is the most widely used pretest tool stratifying patients with suspected PE. This scoring system generally has a high specificity across the prevalence of PE in the studied populations.34 Given the original Wells scoring system is complexed by assigning each item with a different weight, a simplified version has been created and widely validated.35-37 Because both versions of the Wells scoring system have similar performance for exclusion of PE, the simplified Wells scoring system, listed in Table 1, is recommended, using the acronym MI-SHE-PO as a convenient pretest tool.
Table 1. MI-SHE-PO acronym for Wells prediction rules for diagnosing pulmonary embolism171-173.
| Clinical characteristics | Original version* | Simplified version# |
| Points | Points | |
| Malignancy, active | +1.0 | +1 |
| Immobilization or surgery within the past 4 weeks | +1.5 | +1 |
| Signs of DVT | +3.0 | +1 |
| Hemoptysis | +1.0 | +1 |
| Elevated heart rate (≥ 100 beats per minutes) | +1.5 | +1 |
| Prior VTE | +1.5 | +1 |
| Other diagnoses are less likely than PE | +3.0 | +1 |
* Two-level clinical probability: unlikely, ≤ 4; likely, > 4. # Two-level clinical probability: unlikely, < 2; likely, ≥ 2.
DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
When an extensive clinical history of the patient has been obtained, the physician can get confirmation of whether the presence of active malignancy, any recent instances leading to immobilization or surgery (within 4 weeks), or prior VTE. Those are factors predisposing patients to both DVT and PE. Upon physical examination, leg swelling, either confined to the calf or the entire leg, visible collateral venous circulation, and leg pain while palpating should alert physicians to DVT. Hemoptysis and tachycardia are common symptoms and signs other than findings on legs.23 If there is no other tentative diagnosis at least as likely as PE, the likelihood of PE is increased. Nonetheless, it should be noted that the item, any alternative diagnosis less likely than PE, is subjective and may reduce the inter-observer reproducibility of this clinical probability scoring system.
Regardless of the scoring system used, the proportion of patients with confirmed PE can be expected to be 6% in the low-probability category, 23% in the moderate-probability category, and 49% in the high probability category.38 The proportion of patients with confirmed PE is 8% in the PE-unlikely category and 34% in the PE-likely category when using the two-level classification.38 But, the final caveat is that there has been no prospective data on evaluating the diagnostic performance of any clinical probability scoring systems in the East Asian populations.39,40
D-dimer
Fibrin D-dimer is the degradation product of cross-linked fibrin. D-dimer levels are typically elevated in patients with VTE because of simultaneous activation of coagulation and fibrinolysis. However, it may be positive in patients with inflammatory states such as cancer and infection and in the elderly patients. Therefore, a positive D-dimer test is not confirmatory enough for the diagnosis of PE.
A number of D-dimer assays are available. The meta-analysis of 111 D-dimer test evaluations for suspected PE showed that the enzyme-linked immunofluorescence assay, microplate enzyme-linked immunosorbent assay, and latex quantitative assay had a greater sensitivity than did the whole-blood D-dimer assay, latex semiquantitative assay, and latex qualitative assay (97%, 95%, and 95% versus 87%, 88%, and 75%).41
Given its fair sensitivity and poor specificity, D-dimer testing is best considered together with the clinical probability. Overall, the assays with a higher sensitivity yield a higher negative predictive value and are preferable. The highly sensitive D-dimer assays (the quantitative enzyme-linked immunosorbent assay and its derived assays and immunoturbidimetric tests) are best used to exclude PE patients at a lower likelihood,41-43 whereas a negative D-dimer test by any less sensitive assays may not be safe to exclude the diagnosis.44-46 Finally, using the point-of-care D-dimer assays in the hospital setting for exclusion of PE requires extra cautions, especially for those qualitative assays that have a lower sensitivity.47
Computed tomographic pulmonary angiography
Multidetector computed tomographic pulmonary angiography has been widely used for the diagnosis of PE. It has the advantage of making rapid diagnosis, visualizing thrombosis down to the segmental to subsegmental level of the pulmonary arteries, and providing alternative diagnoses for acute dyspnea and/or chest pain. Overall, the sensitivity and specificity of adequate computed tomographic pulmonary angiography is 83% and 96%, respectively, in symptomatic patients.48 However, the predictive value of the computed tomographic pulmonary angiography is modified by the clinical probability where physicians are required to utilize their clinical judgement and/or more tests when discordance between the clinical probability and the result of computed tomographic pulmonary angiography occurs.
In most patients with PE, routine imaging of the leg (either by ultrasound or computed tomography) does not substantially increase the diagnostic yield.49,50 Furthermore, the diagnostic validity of isolated subsegmental PE by multidetector computed tomographic pulmonary angiography is under debate,51 partially being contributed to beam-hardening attenuation, the partial volume effect, and poor contrast opacification.52
Pulmonary angiography
Invasive pulmonary angiography has been the gold standard for the diagnosis or exclusion of PE. The prior reported procedure-related mortality rate and the rate of major non-fatal complications were 0.5 and 1%, respectively.53 Nevertheless, the interobserver agreement for isolated subsegment PE was achieved in only two third of patients.54 With the advancement of computed tomographic pulmonary angiography, nowadays, it is often used when non-invasive imaging modalities fail to confirm the diagnosis of PE or when the diagnosis of chronic thromboembolic pulmonary hypertension needs to be validated.55
Lung ventilation/perfusion scintigraphy
The lung ventilation/perfusion scan is an alternative imaging modality to (computed tomographic) pulmonary angiography to verify the diagnosis of PE. However, its accessibility may be limited to time and institutes. The purpose of the ventilation scan is to increase specificity. Therefore, perfusion only scanning might be acceptable in patients with a normal chest x-ray. The reporting usually has three tiers where a normal scan excludes PE and a high probability scan is considered to be diagnostic.56 A nondiagnostic scan requires further testing. The clinical utility of the lung ventilation/perfusion scan is limited by the high frequency of nondiagnostic scans.56,57 Currently, the lung ventilation/perfusion scan still serves as an important early step in the diagnostic work-up of patients with suspected chronic thromboembolic pulmonary hypertension.55
Other imaging modalities
Magnetic resonance angiography has been evaluated for the application in the diagnosis of PE for several years. Magnetic resonance angiography with or without enhancement or using various sequences has a similar sensitivity and specificity comparing with early multidetector computed tomographic scanners.58-60 Although being promising as being radiation free and less restrained by the renal requirement, the proportion of technically inadequate images or inconclusive results was high and the availability and accessibility was low in the emergency settings. Those drawbacks prevent its wide clinical application when managing patients with suspected PE.
When visualizing right-heart thrombi detected by either transthoracic or transesophageal echocardiography, PE is essentially confirmed. However, only a minority of patients with PE (≈ 4%) had intracardiac thrombus, mostly in the right atrium.19 PE may cause right ventricular strain by acute pressure overload. Proposed echocardiographic parameters signaling right ventricular pressure overload are listed in Table 2. Although being defined, they have not been widely validated in any prospective clinical trials for exclusion or confirmation of the diagnosis of PE.61 In addition, right ventricular strain can be frequently seen in patients with cardiac or pulmonary conditions other than PE. The modest sensitivity and the moderate to high specificity of right ventricular dysfunction and dilatation or pulmonary hypertension on echocardiography preclude the clinical utility of echocardiography in the unselected populations.62-64 Overall, using echocardiography in the diagnosis of PE has a poor sensitivity and a fair specificity at ≈ 80%.65 Nevertheless, it is useful in the differential diagnosis of acute dyspnea in patients with hemodynamic instability.
Table 2. Echocardiographic parameters suggestive of right ventricular pressure overload61,65.
| Positive predictive value | Negative predictive value | |
| RV dilatation | 76% | 85% |
| RV/LV ratio | 66% | 79% |
| McConnell sign | 81% | 72% |
| Flattened interventricular septum | 77% | 66% |
| 60/60 sign | 72% | 37% |
| Decreased TAPSE | 61% | 84% |
| Right-heart thrombus | 100% | 62% |
LV, left ventricle; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion.
It has been expected that thromboemboli in the pulmonary arteries originate from DVT in a lower limb.22 In the early days when invasive angiography and venography were routinely performed for diagnosing VTE, DVT was present in 70% of patients with PE.66 It had been suggested to use leg compressional ultrasound to confirm the diagnosis of PE in patients with inconclusive lung imaging or in those with clinical DVT.67 Data from compressional ultrasound of the proximal legs, the most commonly used protocol, suggested DVT presented in only 30% of patients with PE.50,68 Both the positive and negative predictive values of leg compressional ultrasound for symptomatic proximal DVT were 100%. However, it should be noted that the values decreased to 71% and 94%, respectively, in asymptomatic patients.4 Currently when diagnosing PE, leg compressional ultrasound should be reserved for patients with a contraindication for computed tomographic pulmonary angiography or it may be the frontline option in those with signs and symptoms suggestive of proximal DVT and greater concerns for excessive radiation exposure.
Diagnostic algorithm
VTE is a serious condition that is potentially fatal when being left untreated,69 whereas anticoagulation treatment is usually effective but carries the risk for major bleeding of ≈ 2%, of which ≈ 10% is fatal.70 Therefore, diagnostic certainty needs to be high enough to start or to withhold anticoagulation treatment. Given that currently there is no reliable stand-alone test that is non-invasive, beginning with the assessment of the clinical probability in the absence of hemodynamic instability at presentation as for the diagnostic work-up before allocating further imaging tests is the mainstream. In patients with hemodynamic instability in whom acute PE is suspected, right ventricular dysfunction shown by the echocardiography warrants further computed tomographic pulmonary angiography to search for PE.
The use of D-dimer testing in patients with the low clinical probability safely excludes PE without further imaging tests in ≈ 40% and the 3-month diagnostic failure rate is 0.5%.43,71 The application of the age-adjusted cut-offs (age × 10 ug/L above 50 years) has improved the specificity of D-dimer testing (high sensitivity) in the elderly patients without compromising patient safety.72 Since 2014, the European guidelines have adopted the age-adjusted D-dimer cut-offs for the exclusion of PE.61,73 The original and simplified Wells scoring systems combined with age-adjusted D-dimer testing have similar performances in exclusion of PE. Given its convenience in clinical practice, the combination of the simplified Wells score and the age-adjusted D-dimer cut-offs is preferred.74
In other patients who are more likely to have PE by the clinical probability or have an elevated D-dimer level, the computed tomographic pulmonary angiography-based strategy is favored (Figure 1). In patients in whom computed tomographic pulmonary angiography is contraindicated or who are more susceptible to radiation and are managed in the hospital where timely scintigraphy is available, lung ventilation/perfusion scintigraphy plus leg compression ultrasound is a valid option.
Figure 1.

Diagnosis flowchart for patients with stable hemodynamic and suspected pulmonary embolism. * 500 ug/L if age is 50 years and less and age × 10 ug/L if age is above 50 years with D-dimer testing (high sensitivity). † Pulmonary embolism is confirmed if thrombosis visualized above the subsegmental level. ‡ Clinical judgement and/or more tests (e.g. lung ventilation/perfusion scintigraphy or leg compression ultrasound) are required in patients with a Wells score ≥ 2. PE, pulmonary embolism.
Other algorithms
The pulmonary embolism rule-out criteria has been developed for emergency department patients with the purpose of selecting patients in whom diagnostic workup should not be initiated. The criteria include: 1. an arterial oxygen saturation ≤ 94%; 2. a pulse rate ≥ 100 beats per minute; 3. patient aged ≥ 50 years; 4. unilateral leg swelling; 5. hemoptysis; 6. recent trauma or surgery; 7. prior VTE; and 8. exogenous estrogen use. In patients who meet none of the above criteria, it appears safe to exclude PE without any further testing.75 It is estimated that computed tomographic pulmonary angiography could be avoided in 10% of patients. However, without D-dimer testing, the pulmonary embolism rule-out criteria might fail to capture subsegmental PE in those who are negative for all criteria when the prevalence of PE is expected to be > 3%.75
The other diagnostic algorithm intended to simplify PE diagnosis and to minimize the use of computed tomographic pulmonary angiography is the YEARS rule, which consists of three clinical items of the Wells scoring system and the D-dimer level. The items from the Wells scoring system are signs of DVT, hemoptysis, and PE more likely than an alternative diagnosis. In addition, the D-dimer cut-off is set at < 1000 ng/mL in patients without any clinical items and < 500 ng/mL in patients with one or more clinical items. All other patients undergo computed tomographic pulmonary angiography. Compared with the current standard algorithm with the age-adjusted D-dimer threshold, the advantage of the YEARS rule is an absolute reduction of 14% of computed tomographic pulmonary angiography in those aged < 50 years with the 3-month diagnostic failure rate of 0.1%.33 The pregnancy-adapted YEARS rule using leg compression ultrasound for women with signs of DVT as the initial step has high efficacy and potentially avoid computed tomographic pulmonary angiography in 39% of pregnancy women.76 Therefore, using the YEARS rule in the pregnant patients appears safe (Figure 2).
Figure 2.
Diagnosis flowchart for pregnant patients with stable hemodynamic and suspected pulmonary embolism. DVT, deep vein thrombosis; PE, pulmonary embolism.
To use those new algorithms requires extra cautions as their purpose is to avoid "unnecessary" computed tomographic pulmonary angiography. The prevalence of PE in the management studies ranged between 3% and 14%. Therefore, whether the generalizability of their findings to a more prevalent cohort (e.g. the elderly population) and/or for patients in whom computed tomographic pulmonary angiography is contraindicated requires further studies.
Recommendations
• To diagnose PE requires a systematic assessment.
• An acronym of MI-SHE-PO for the Well score is the comprehensive initial to stratify patients.
• The combination of the Wells score and a D-dimer test, using the age-adjusted cut-offs, is validated in allocating further imaging studies.
• In patients who requires further imaging studies, the computed tomographic pulmonary angiography-based strategy is favored.
• For patients in whom computed tomographic pulmonary angiography is deemed unfeasible, lung ventilation/perfusion scintigraphy plus leg compression ultrasound can be an alternative, provided local experience does exist.
PROGNOSIS ASSESSMENT
The prognosis of patients with PE varies substantially and is determined by patient hemodynamics, concomitant disease conditions, and right ventricular function.77 The clinical classification by PE severity based on the early mortality rate facilitates the proper allocation of the initial therapeutic approach.
A number of models have been derived and validated in PE over the past two decades.78-82 Nevertheless, the hemodynamic at the presentation when PE is suspected is one of the strongest determinants of early death.14,83,84 Consequently, both diagnostic and therapeutic strategies should be prioritized in those with pulselessness or sustained hypotension (systolic blood pressure < 90 mmHg for at least 15 minutes or requiring inotropic support).
PE severity index
The PE severity index which incorporates patient characteristics, comorbidities, and clinical conditions has been developed and extensively validated to assess the risk of early death (30-day mortality).85,86 The original version contains 11 differently weighted variables and its simplified version retains 6 fixed value items (Table 3). The performance of those two versions is similar and both have good sensitivity and poor specificity for early mortality.81,87As the majority of patients with PE are hemodynamically stable at presentation,88 both versions have a high negative predictive value. Despite the development and application of the PE severity index, there was only one randomized trial that compared outpatient versus inpatient treatment of patients with PE who were at lower risk for early mortality by this model.89
Table 3. Risk stratification models for patients with pulmonary embolism by the risk of 30-day mortality81,174.
| PESI | Simplified PESI | ||||
| Variable | Point | Variable | Point | ||
| Patient characteristics | Patient characteristics | ||||
| Age | Age in years | Age | 1 if age > 80 years | ||
| Male sex | 10 | ||||
| Comorbidity | Comorbidity | ||||
| Cancer | 30 | Cancer | 1 | ||
| Heart failure | 10 | ||||
| Chronic pulmonary disease | 10 | ||||
| Chronic cardiopulmonary disease | 1 | ||||
| Clinical finding | Clinical finding | ||||
| SBP < 100 mmHg | 30 | SBP < 100 mmHg | 1 | ||
| Heart rate ≥ 110 beats per minute | 20 | Heart rate ≥ 110 beats per minute | 1 | ||
| Respiratory rate ≥ 30 breaths per minute | 20 | ||||
| Arterial oxygen saturation < 90%* | 20 | Arterial oxygen saturation < 90%* | 1 | ||
| Temperature < 36 °C | 20 | ||||
| Altered mental status# | 60 | ||||
| Risk category | Point | Mortality† | Risk category | Point | Mortality† |
| Class I, very low | ≤ 65 | 1.1% | Low | 0 | 1.0% |
| Class II, low | 66-85 | 3.1% | High | ≥ 1 | 10.9% |
| Class III, intermediate | 86-105 | 6.5% | |||
| Class IV, high | 106-125 | 10.4% | |||
| Class V, very high | > 125 | 24.5% |
* With and without the supplemental oxygen. # Defined as disorientation, lethargy, stupor, or coma. † Estimates in the derivation cohorts.
PESI, pulmonary embolism severity index; SBP, systolic blood pressure.
Imaging markers
Right ventricular failure due to acute pressure overload is considered to be the primary cause of early death in patients with PE. Among patients with PE, 40% reported to have right ventricular hypokinesis, which marked a higher mortality within 3 months.19 In addition to right ventricular dilatation (without hypertrophy), paradox septal systolic motion and/or pulmonary hypertension identifies normotensive patients who develops latent hemodynamic impairment and/or in-hospital mortality.90 Nevertheless, the predictive values of echocardiographic parameters are generally poor, reflecting the requirement for the standardized assessment.91 Currently, measuring right ventricular and left ventricular end-diastolic (defined by the electrocardiogram R-wave) diameters at the valvular level during late diastole and tricuspid annular plane systolic excursion in the M-mode presentation at peak systole are recommended.
Beyond the diagnostic role, computed tomographic pulmonary angiography provides additional prognostic information by showing right ventricular dysfunction. The ratio of right ventricular end-diastolic diameter to left ventricular end-diastolic diameter at the valvular plane in the transverse images is used as an indicator of right ventricular dysfunction. Although the ideal cut-off for such the ratio is debatable,92,93 the increased ratio appears to rise prognostic specificity.
Biomarkers
The abrupt increase in pulmonary vascular resistance results in right ventricular dilatation, which is followed by ischemia and then dysfunction.73 Biomarkers of increased myocardial stretch and/or myocardial injury have been investigated for their prognostic values in patients with PE. An elevated cardiac troponin level (above the normal thresholds dependent on the assay used) was associated with an increased risk of mortality, both in overall patients and in those who were hemodynamically stable at presentation.94 Similarly, an increased brain-type natriuretic peptide or N-terminal-pro-brain-type natriuretic peptide level at baseline was associated with right ventricular dysfunction and adverse clinical outcomes.95
Other surrogate markers indicating low cardiac output and its complications, such as renal dysfunction or kidney injury, have been reported to be useful for risk stratification in patients with PE, including plasma lactate, copeptin, neutrophil gelatinase-associated lipocalin, and cystatin C in patients with normotensive.96,97
Combined risk assessment model
Among patients with PE at low risk by the risk prediction models, 34% had evidence of right ventricular dysfunction by imaging and 26% had an elevated cardiac troponin level.98 Both were associated with an increased mortality in patients with PE who were classified as being at low risk by the risk prediction models. Since the prognostic information from the clinical, imaging, and laboratory findings is complementary,99-101 their integration provides better calibration in prediction of early death in patients with PE who are at the non-high risk categories.98 The accuracy for early mortality is better with the integrated model than with the clinical model, particularly in patients at intermediate risk by the PE severity index.102
A 3-tier risk stratification model is recommended, in which patients at intermediate risk are further classified into two subcategories. For patients who are at high risk, prompt pulmonary artery reperfusion and intensive care are needed, whereas early discharge and outpatient or home treatment may be feasible in patients who are at low risk.103,104
Recommendations
• The assessment of prognosis is essential after the diagnosis of PE is confirmed.
• Hemodynamic stability is the major determination for early outcomes in patients with PE.
• Right ventricular dysfunction by imaging and/or myocardial strain by biomarkers are markers signaling worse clinical outcomes.
• The PE severity index, a structured assessment combing patient characteristics, comorbidities, and clinical conditions, is a useful tool to evaluate the risk of early death in patients with stable hemodynamics.
• The clinical risk and imaging and/or biomarker levels are complimentary when assessing early outcomes.
• The therapeutic strategy should be prioritized in patients with pulselessness or sustained hypotension.
TREATMENT FOR PE
Thrombolysis and embolectomy
Thromboembolic obstruction of pulmonary arterial vasculature and subsequent elevation of right ventricular afterload hallmark the initiation of the vicious pathophysiologic cycle leading to the hemodynamic compromise and fatality.105 Either pharmacological or surgical removal of thrombus leads to faster improvements in pulmonary circulation and reductions in pulmonary hypertension and right ventricular dilatation.106-109 The early evidence suggested that systemic thrombolysis improved survival in patients with increased thrombus burden.110 In patients who are at intermediate risk for early mortality, systemic thrombolysis has been compared with heparin (Table 4).111-114 Although there was no difference in mortality, systemic thrombolysis reduced the need for emergent escalation of treatment due to hemodynamic decompensation within the first week of hospitalization when compared to anticoagulation alone in this patient population.111,113,114 In the PEITHO study, the largest trial of thrombolysis in pulmonary embolism to date, the risk of major bleeding and of hemorrhagic stroke was substantially higher with systemic thrombolysis.113 The catheter-directed pharmacological reperfusion could have been a promise for a balance between improvement in right ventricular function and the excessive risk of major bleeding.109 The early experience suggested that the local delivery of one tenth of the dose used for systemic thrombolysis over 15 hours improved early hemodynamic parameters and right ventricular function.115 Using a lower dose and shorter infusion duration may be feasible in selected patients with intermediate risk.116 Nevertheless, the catheter-directed treatment requires local expertise and a high institutional volume to ensure satisfactory outcomes. There is certainly a need for a randomized trial comparing the catheter-directed treatment with systemic thrombolysis and with anticoagulation that is powered to evaluate clinical outcomes before the widely accepted utility of the catheter-directed pharmacological reperfusion strategy (Table 5). Therefore, in most patients with right ventricular dysfunction and the elevated level of cardiac biomarkers, a watchful waiting strategy is preferred over routine thrombolysis.117
Table 4. Randomized trials of systemic thrombolysis in patients with intermediate risk of pulmonary embolism111-114.
| MAPPET-3 | MOPETT | TOPCOAT | PEITHO | |
| Publication year | 2002 | 2013 | 2014 | 2014 |
| Patient no. | 256 | 121 | 83 | 1006 |
| PE severity | RV dysfunction by echocardiograph, right heart catheterization, or elec-trocardiogram | ≥ 2 lobar involvement by computed tomographic pulmonary angiography or lung ventilation/perfusion scintigraphy | RV strain by echocardiography or an elevated troponin or brain natriuretic peptide level | RV dysfunction by echocardiography or spiral compute tomography and an elevated troponin |
| Thrombolysis regimen | Alteplase 100 mg over 2 hours (a 10-mg bolus, followed by a 90-mg intravenous infusion) | Alteplase 50 mg over 2 hours (a 10-mg bolus, followed by a 40-mg intravenous infusion) if weight ≥ 50 kg or 0.5 mg/kg over 2 hours (a 10-mg bolus, followed by the reminder intravenous infusion) if weight < 50 kg | A single weight-based intravenous bolus of tenecteplase | A single weight-based intravenous bolus of tenecteplase |
| Initial anticoagulation regimen | UFH adjusted to maintain the activated partial thromboplastin time at 2.0 to 2.5 times the upper limit of normal | UFH or enoxaparin | Low-molecular-weight heparin | UFH adjusted to maintain the activated partial thromboplastin time at 2.0 to 2.5 times the upper limit of normal |
| Primary endpoint | In-hospital death or clinical deterioration that required an escalation of treatment | The development of pulmonary hypertension at intermediate-term follow up | Death, circulatory shock, intubation or major bleeding within 5 days or recurrent PE, poor functional capacity or an SF-36 Physical Component Summary < 30 at 90-day follow-up | Death or hemodynamic decompensation within 7 days |
| Efficacy | 14% absolute reduction in the primary endpoint | 41% absolute reduction in the primary endpoint | No difference | 3% absolute reduction in the primary endpoint |
| Major bleeding | No difference | No difference | Not reported | 9% absolute increase |
PE, pulmonary embolism; RV, right ventricle; UFH, unfractionated heparin.
Table 5. Recent trials of ultrasound-assisted catheter-directed thrombolysis in patients with pulmonary embolism109,115,116.
| ULTIMA | SEATTLE II | OPTALYSE PE | |
| Publication year | 2014 | 2015 | 2018 |
| Patient no. | 59 | 150 | 101 |
| Design | Two arms comparing thrombolysis and no thrombolysis | Single arm | Four arms comparing four thrombolytic regimens |
| No control group | |||
| PE severity | Intermediate-risk PE defined by RV/LV diameter ratio ≥ 1.0 on imaging | Massive or submassive PE | Intermediate-risk PE defined by RV/LV diameter ratio ≥ 0.9 on imaging |
| Thrombolysis regimen | Alteplase 1 mg/h per catheter for 5 hours then 0.5 mg/h per catheter for 10 hours (the maximum dose was 20 ± 1 mg for patients with bilateral device placement and 10 ± 0.5 mg for patients with unilateral device placement) | Alteplase 1 mg/h for 24 hours if unilateral device placement and for 12 hours if bilateral device placement | Either one of the following: Arm 1: alteplase 2 mg/h per catheter for 2 hours. Arm 2: alteplase 1 mg/h per catheter for 4 hours. Arm 3: alteplase 1 mg/h per catheter for 6 hours. Arm 4: alteplase 2 mg/h per catheter for 6 hours. |
| Primary endpoint | The difference in RV/LV diameter ratio from baseline to 24 hours | The change in RV/LV diameter ratio from baseline to 48 hours | The change in RV/LV diameter ratio from baseline to 48 hours |
LV, left ventricle; PE, pulmonary embolism; RV, right ventricle.
It has been reported that ≈ 8% of patients who underwent systemic thrombolysis failed to achieve clinical stability and restore right ventricular function.118 Although there is no randomized trials comparing surgical embolectomy with pharmacological thrombolysis, surgical embolectomy for patients with PE is as effective and as safe as pharmacological thrombolysis.119,120 According to local expertise, surgical embolectomy should be considered when thrombolysis is contraindicated or when thrombolysis fails to achieve hemodynamic improvement.
Non-vitamin K oral anticoagulants (NOACs)
Oral anticoagulants are the main stay for VTE treatment and the duration of treatment should include at least 3 months. The development of four NOACs has changed the treatment pattern observed over the recent years.121
There were six pivotal trials of four NOACs in the therapeutic area of VTE over the past decade.122-127 Although there was only one study specifically examining the efficacy of one of the NOACs in patients with PE,124 the general information of all four NOACs applied to patients with DVT and to patients with PE.128 With either well-managed vitamin K antagonists (VKAs) or NOACs, the overall recurrent rate of VTE was around 2% within 6 to 12 months. However, treatment with a NOAC significantly reduced the risk of major bleeding by 39%. Therefore, NOACs as a therapeutic class for VTE are as effective as but safer than well-managed VKAs (with the targeted international normalized ratio between 2.0 and 3.0).128
These NOACs are classified into two therapeutic strategies — one has a heparin lead-in phase and the other uses a single-drug approach with escalated doses in the first phase.4 The strategy of using no up-front heparin has been tested in the EINSTEIN program and in the AMPLIFY study.123-125 In the predefined safety analysis of the EINSTEIN-PE study, serial imaging suggested that there was no difference in clot resolution between the conventional heparin lead-in management and rivaroxaban after 3 weeks.129 As expected,130,131 most patients had complete (41%) or partial (47%) resolution after treatment. Therefore, the single-drug approach strategy with an initial escalation of therapeutic doses may be considered in selected patients in whom inpatient care can be shorten. Another aspect in PE treatment with NOACs is that there might be concerns of using NOACs in patients with more extensive PE or those with intermediate risk as the anatomical extent of PE is associated with the recurrent rate of PE regardless of treatment.132 Nevertheless, in the subanalysis from the Hokusai-VTE study, the efficacy and safety of edoxaban compared with well-managed VKAs were maintained in patients with evidence of right ventricular dysfunction.133
After the first 3 to 6 months, extending anticoagulation treatment is considered in patients without a transient reversible risk factor.4 Aspirin appears to reduce the risk of recurrence by 32% when compared with placebo,134 where either standard or low dose rivaroxaban further reduce the risk by 48%.135 Compared with standard-intensity VKAs, dabigatran and edoxaban appear to be as effective as but safer.136,137 Overall, current evidence from multiple clinical trials comparing different regimens suggests that standard-intensity VKAs and NOACs are preferred over aspirin when considering long-term secondary prevention of VTE with a higher risk of major bleeding observed with standard-intensity VKAs.138
Despite that NOACs are widely used in most of patients with PE, VKAs with a targeted international normalized ratio between 2.0 and 3.0 are used in patients when they have any contraindications to NOACs (e.g. those with renal dysfunction, rheumatic mitral stenosis, and/or a mechanical heart valve).
Cancer-associated thrombosis
By estimation, ≈ 20% of VTE is cancer-associated thrombosis.139 VTE is more common in patients with cancer as they have a several-fold increased risk of VTE compared with the general population.140 The occurrence of VTE in patients with cancer usually signals an advanced disease stage and poor prognosis.141 The pathogenesis is not limited to cancer biology per se but multifactorial, including patient characteristics and treatment side effects.142
Treating cancer-associated thrombosis is challenging as the risk of recurrence and the risk of bleeding are both higher in patients with cancer than in those without cancer.143 Low-molecular-weight heparin (LMWH) has been the mainstay for the treatment of VTE in patients with cancer over the past decade.144,145 LMWH was more effective on treatment and secondary prevention of cancer-associated thrombosis than VKAs (with a targeted international normalized ratio between 2.0 and 3.0).146,147 The meta-analysis of six clinical trials in patients with cancer-associated thrombosis further showed that LMWH reduced the risk of recurrent VTE by 44% while sharing the similar rate of major bleeding when compared to VKAs.148
Despite supportive evidence from multiple clinical trials and guidelines recommendations, the use of LMWH in patients with cancer and VTE is limited, whereas VKAs remain frequently adopted.149,150 In the pivotal trials of NOACs, there were only a small number of patients with cancer (5%) were enrolled. It appeared that the efficacy and safety of NOACs are comparable to those of VKAs in patients with cancer and VTE.151
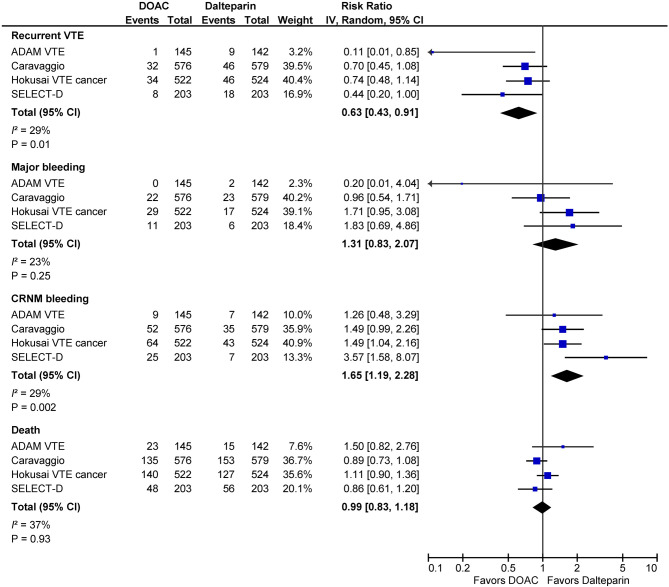
In the area of cancer-associated thrombosis, first and foremost, four open-label randomized clinical trials comparing NOACs with LMWH have completed and reported their results (Table 6). The first report came from the Hokusai VTE Cancer study,152 in which 1050 patients with active cancer and symptomatic or incidentally detected VTE were randomly allocated to either edoxaban or LMWH. For the primary composite endpoint of recurrent VTE or major bleeding, treatment with edoxaban (with a heparin lead-in phase) was noninferior to treatment with LMWH during 12 months after randomization regardless of treatment duration. When compared with LMWH, edoxaban marginally improved the rate of recurrent VTE [hazard ratio (HR), 0.71; 95% confidence interval (CI), 0.48-1.06; p = 0.09]. However, there was an increased risk of major bleeding (HR, 1.77; 95% CI, 1.03-3.04; p = 0.04). The excess of major bleeding was contributed to patients with gastrointestinal cancer (including upper and lower gastrointestinal tract and the pancreatic or hepatobiliary system).153 The second report came from the SELECT-D study, in which 406 patients with cancer and VTE were randomized to the a single-drug approach with the escalated dose of rivaroxaban in the first 3 weeks followed by the standard dose or to LMWH.154 When compared with LMWH, the rate of recurrent VTE was lower with rivaroxaban (HR, 0.43; 95% CI, 0.19 to 0.99) at the cost of an increase in bleeding, occurring excessively in patients with gastrointestinal cancer. The evidence of the third NOAC also suggested that there was a trend of a lower rate of recurrent VTE favoring apixaban over LMWH.155,156 In the Caravaggio study,156 the rate of recurrent VTE was slightly lower with apixaban than with LMWH (HR, 0.63; 95% CI, 0.37 to 1.07; p = 0.09). Meanwhile, the rate of major or clinically relevant nonmajor bleeding was similar between two treatments. Being distinct from edoxaban and rivaroxaban, there was no excess of major gastrointestinal bleeding with apixaban when compared with LMWH. Collectively, NOACs have better efficacy than LMWH and balanced net clinical benefits (Figure 3). Furthermore, compared with subcutaneous LMWH injection, NOACs had better treatment adherence and overall satisfaction.152,155 Therefore, NOACs are effective for cancer patients with acute VTE, although caution is needed in patients at high risk of bleeding.157-159
Table 6. Randomized trial of non-vitamin K oral anticoagulants for patients with cancer associated thrombosis152,154-156.
| Hokusai VTE Cancer | SELECT-D | ADAM VTE | Caravaggio | |
| NCT02073682 | ISRCTN86712308 | NCT02585713 | NCT03045406 | |
| Publication year | 2018 | 2018 | 2020 | 2020 |
| Patient no. | 1050 | 406 | 300 | 1170 |
| Study arm treatment | Edoxaban 60 mg (30 mg in patients with CrCl of 30 to 50 mL/min or weight of 60 kg or less or in those receiving concomitant treatment with potent P-glycoprotein inhibitors) once daily after 5-day low-molecular-weight heparin lead-in | Rivaroxaban, 15 mg twice daily for the first 3 weeks followed by 20 mg once daily | Apixaban 10 mg twice daily for 7 days followed by 5 mg twice daily | Apixaban 10 mg twice daily for 7 days followed by 5 mg twice daily |
| Control arm treatment | Dalteparin 200 IU/kg once daily for 30 days then 150 IU/kg once daily | Dalteparin 200 IU/kg once daily for 30 days then 150 IU/kg once daily | Dalteparin 200 IU/kg once daily for the first month followed by 150 IU/kg | Dalteparin 200 IU/kg once daily for the first month followed by 150 IU/kg |
| Study duration | 12 months | 6 months | 6 months | 6 months |
| Specific exclusion | ECOG performance status > 2 | Weight < 40 kg | CrCl < 30 mL/min | ECOG performance status > 2 |
| CrCl < 30 mL/min | ECOG performance status > 2 | Concomitant use of strong CYP3A4 inducers | CrCl < 30 mL/min | |
| Concomitant use of P-glycoprotein inhibitors | Concomitant use of strong CYP3A4 inhibitors or inducers or P-glycoprotein inhibitors or inducers | Concomitant use of strong CYP3A4 inhibitors or inducers or P-glycoprotein inhibitors or inducers | ||
| Thrombocytopenia (< 50000/μL) | Primary esophageal or gastro-esophageal cancer | Thrombocytopenia (< 75000/μL) | ||
| Primary endpoint | Recurrent VTE or major bleeding | Recurrent VTE | Major bleeding | Recurrent VTE |
CrCl, creatinine clearance; CYP, cytochrome P-450; ECOG, Eastern Cooperative Oncology Group; VTE, venous thromboembolism.
Figure 3.
Pooled efficacy and safety of factor Xa inhibitors compared with low-molecular-weight heparin in patients with cancer associated thrombosis. CI, confidence interval; CRNM, clinically relevant nonmajor; VTE, venous thromboembolism.
Multidisciplinary management
Treatment options for patients with PE, especially for patients who are at high risk of early complications and/or mortality, have been expanded and sometimes have controversy between medical practice. With limited comparable data, deciding which strategy is more appropriate than the others for each patient become challenging. The pulmonary embolism response team (PERT) consisting of local expertise in the field of diagnosis and treatment of PE can be set up to improve patient care. This particularly holds true for patients with PE and cardiac arrest, in whom immediate extracorporeal membrane oxygenation support in addition to cardiopulmonary resuscitation is often required.160,161 After cardiopulmonary resuscitation, thrombolysis, surgical embolectomy, and mechanical embolectomy are reasonable emergency treatment options.162 The standard contraindications to thrombolysis should be weighed against potentially lifesaving benefits.
The structure of a PERT can vary and often include members from emergency medicine, radiology, cardiology, vascular surgery, critical care medicine, and/or pulmonary medicine. The introduction of the PERT has facilitated the decision making, favoring more advanced and aggressive management for patients who are at intermediate risk.163,164 Although the scope of a PERT was largely intended to provide recommendations to patients who are or will be in need of thromboreduction strategies, the initial experience showed that the PERT was often consulted for patients who had low-risk PE and complex comorbidities.165 It is encouraging to form multidisciplinary dialogues and collaborations within the institution for PE management. Nevertheless, whether a PERT can improve patient outcomes requests more research.
Recommendations
• Systemic thrombolysis or its alternatives, surgical or percutaneous thromboreduction measures, is life-saving in patients with hemodynamic instability.
• Timely administration of anticoagulation is essential in treatment of PE.
• The single-drug approach using rivaroxaban or apixaban and the 2-phase approach using parenteral heparin bridged to dabigatran or edoxaban are preferred over VKAs in most of patients with acute VTE.
• When considering to extend anticoagulation treatment, NOACs are the preferable agents.
• In patients with cancer-associated thrombosis, three factor Xa inhibitors and LMWH have similar efficacy for secondary VTE prevention. However, cautions are required in patients at higher risk of bleeding (e.g. those with thrombocytopenia or unresectable gastrointestinal cancer) or at potential for drug-drug interactions.
LONG-TERM COMPLICATIONS OF PE
After acute PE, complete PE resolution occurs in ≈ 40% after the first month treatment and in ≈ 80% after six months.129,166 However, residual thrombi can be persistent and organized and later lead to the life-threatening complication known as chronic thromboembolic pulmonary hypertension (CTEPH) in a small proportion of patients.
Functional deterioration, chronic dyspnea, and/or impaired quality of life are reported in ≈ 50% of patients who have received effective anticoagulation for PE for more than 3 months.15 Clinical determinants for the exercise limitation include the female gender, younger age, a larger body mass index, and smoking history.167 However, there is no association between exercise impairment and right ventricular dysfunction or residual pulmonary vascular obstruction.167,168
On the other hand, CTEPH occurs in ≈ 3% of patients who survive from a first episode of PE. CTEPH is characterized by symptoms ranging from persistent dyspnea to evidence suggestive of right heart failure, elevated pulmonary artery pressure with normal or low wedge pressure, and imaging showing residual pulmonary vascular obstruction.169 In other patients who have characteristic symptoms and perfusion defects of CTEPH but normal pulmonary artery pressure at rest, chronic thromboembolic disease occurs.
The concept of post-PE syndrome is growing but its spectrum and the diagnosis criteria are still controversial at the present time.170 With regard to CTEPH and chronic thromboembolic disease, the relevant information has been updated in the 2018 Taiwan Society of Cardiology focused update on diagnosis and treatment of pulmonary arterial hypertension.55
SUMMARY
PE is part of VTE continuum. It could bring fatal consequences within few days or even hours without appropriate management. Therefore, rapidly identifying patients at risk and implanting the comprehensive diagnostic algorithm facilitates the timely therapeutics in the appropriate patients. The diagnosis of PE requires a sophisticated evaluation from pretest probability assessment till the confirmation by the imaging, whereas the treatment focuses on the use of anticoagulation. In patients at high risk of early complications and mortality, immediate thromboreduction treatment, either with pharmacological, surgical, or percutaneous measures, can be life-saving. In the rest of patients, either a parenteral heparin lead-in versus the single-drug approach or once daily versus twice daily NOACs are preferred over warfarin in most of the scenarios. Now, in patients with cancer and VTE, three factor Xa inhibitors are collectively as effective as, if not better than, the standard regimen, LMWH. Finally, a minority of patients with PE will develop long-term disturbing complications that requires further surveys and management by pulmonary hypertension specialists.
DISCLOSURE
Kang-Ling Wang reports honoraria from Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Novartis, and Pfizer.
Other authors have no relevant conflict of interest.
FUNDING SOURCES
This study was supported, in part, by the grant from the Ministry of Health and Welfare (MOHW109-TDU-B-211-114001).
REFERENCES
- 1.The Lancet Haematology. Thromboembolism:an under appreciated cause of death. Lancet Haematol. 2015;2:e393. doi: 10.1016/S2352-3026(15)00202-1. [DOI] [PubMed] [Google Scholar]
- 2.Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388:3060–3073. doi: 10.1016/S0140-6736(16)30514-1. [DOI] [PubMed] [Google Scholar]
- 3.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Wang KL, Chu PH, Lee CH, et al. Management of venous thromboembolisms: part I. The consensus for deep vein thrombosis. Acta Cardiol Sin. 2016;32:1–22. doi: 10.6515/ACS20151228A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raskob GE, Angchaisuksiri P, Blanco AN, et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. 2014;34:2363–2371. doi: 10.1161/ATVBAHA.114.304488. [DOI] [PubMed] [Google Scholar]
- 6.Wang KL, Yap ES, Goto S, et al. The diagnosis and treatment of venous thromboembolism in Asian patients. Thromb J. 2018;16:4. doi: 10.1186/s12959-017-0155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee LH, Gallus A, Jindal R, et al. Incidence of venous thromboembolism in Asian populations: a systematic review. Thromb Haemost. 2017;117:2243–2260. doi: 10.1160/TH17-02-0134. [DOI] [PubMed] [Google Scholar]
- 8.Sakuma M, Konno Y, Shirato K. Increasing mortality from pulmonary embolism in Japan, 1951-2000. Circ J. 2002;66:1144–1149. doi: 10.1253/circj.66.1144. [DOI] [PubMed] [Google Scholar]
- 9.Jang MJ, Bang SM, Oh D. Incidence of venous thromboembolism in Korea: from the Health Insurance Review and Assessment Service database. J Thromb Haemost. 2011;9:85–91. doi: 10.1111/j.1538-7836.2010.04108.x. [DOI] [PubMed] [Google Scholar]
- 10.Sakuma M, Nakamura M, Yamada N, et al. Venous thromboembolism: deep vein thrombosis with pulmonary embolism, deep vein thrombosis alone, and pulmonary embolism alone. Circ J. 2009;73:305–309. doi: 10.1253/circj.cj-08-0372. [DOI] [PubMed] [Google Scholar]
- 11.Lee CH, Cheng CL, Lin LJ, et al. Epidemiology and predictors of short-term mortality in symptomatic venous thromboembolism. Circ J. 2011;75:1998–2004. doi: 10.1253/circj.cj-10-0992. [DOI] [PubMed] [Google Scholar]
- 12.Lee CH, Lin LJ, Cheng CL, et al. Incidence and cumulative recurrence rates of venous thromboembolism in the Taiwanese population. J Thromb Haemost. 2010;8:1515–1523. doi: 10.1111/j.1538-7836.2010.03873.x. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura M, Miyata T, Ozeki Y, et al. Current venous thromboembolism management and outcomes in Japan. Circ J. 2014;78:708–717. doi: 10.1253/circj.cj-13-0886. [DOI] [PubMed] [Google Scholar]
- 14.Kucher N, Rossi E, De Rosa M, et al. Massive pulmonary embolism. Circulation. 2006;113:577–582. doi: 10.1161/CIRCULATIONAHA.105.592592. [DOI] [PubMed] [Google Scholar]
- 15.Klok FA, van der Hulle T, den Exter PL, et al. The post-PE syndrome: a new concept for chronic complications of pulmonary embolism. Blood Rev. 2014;28:221–226. doi: 10.1016/j.blre.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Pollack CV, Schreiber D, Goldhaber SZ, et al. Clinical characteristics, management, and outcomes of patients diagnosed with acute pulmonary embolism in the emergency department: initial report of EMPEROR (Multicenter Emergency Medicine Pulmonary Embolism in the Real World Registry). J Am Coll Cardiol. 2011;57:700–706. doi: 10.1016/j.jacc.2010.05.071. [DOI] [PubMed] [Google Scholar]
- 17.Keller K, Beule J, Balzer JO, et al. Typical symptoms for prediction of outcome and risk stratification in acute pulmonary embolism. Int Angiol. 2016;35:184–191. [PubMed] [Google Scholar]
- 18.Prandoni P, Lensing AW, Prins MH, et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016;375:1524–1531. doi: 10.1056/NEJMoa1602172. [DOI] [PubMed] [Google Scholar]
- 19.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism:clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999;353:1386–1389. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 20.Wells PS, Ginsberg JS, Anderson DR, et al. Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med. 1998;129:997–1005. doi: 10.7326/0003-4819-129-12-199812150-00002. [DOI] [PubMed] [Google Scholar]
- 21.Stein PD, Saltzman HA, Weg JG. Clinical characteristics of patients with acute pulmonary embolism. Am J Cardiol. 1991;68:1723–1724. doi: 10.1016/0002-9149(91)90339-m. [DOI] [PubMed] [Google Scholar]
- 22.Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12:464–474. doi: 10.1038/nrcardio.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med. 2007;120:871–879. doi: 10.1016/j.amjmed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shopp JD, Stewart LK, Emmett TW, et al. Findings from 12-lead electrocardiography that predict circulatory shock from pulmonary embolism: systematic review and meta-analysis. Acad Emerg Med. 2015;22:1127–1137. doi: 10.1111/acem.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller K, Beule J, Coldewey M, et al. Heart rate in pulmonary embolism. Intern Emerg Med. 2015;10:663–669. doi: 10.1007/s11739-015-1198-4. [DOI] [PubMed] [Google Scholar]
- 26.Keller K, Beule J, Balzer JO, et al. Right bundle branch block and SIQIII-type patterns for risk stratification in acute pulmonary embolism. J Electrocardiol. 2016;49:512–518. doi: 10.1016/j.jelectrocard.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Qaddoura A, Digby GC, Kabali C, et al. The value of electrocardiography in prognosticating clinical deterioration and mortality in acute pulmonary embolism: a systematic review and meta-analysis. Clin Cardiol. 2017;40:814–824. doi: 10.1002/clc.22742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodger M, Makropoulos D, Turek M, et al. Diagnostic value of the electrocardiogram in suspected pulmonary embolism. Am J Cardiol. 2000;86:807–809, A10. doi: 10.1016/s0002-9149(00)01090-0. [DOI] [PubMed] [Google Scholar]
- 29.McFarlane MJ, Imperiale TF. Use of the alveolar-arterial oxygen gradient in the diagnosis of pulmonary embolism. Am J Med. 1994;96:57–62. doi: 10.1016/0002-9343(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 30.Rodger MA, Bredeson CN, Jones G, et al. The bedside investigation of pulmonary embolism diagnosis study: a double-blind randomized controlled trial comparing combinations of 3 bedside tests vs ventilation-perfusion scan for the initial investigation of suspected pulmonary embolism. Arch Intern Med. 2006;166:181–187. doi: 10.1001/archinte.166.2.181. [DOI] [PubMed] [Google Scholar]
- 31.Wicki J, Perneger TV, Junod AF, et al. Assessing clinical probability of pulmonary embolism in the emergency ward: a simple score. Arch Intern Med. 2001;161:92–97. doi: 10.1001/archinte.161.1.92. [DOI] [PubMed] [Google Scholar]
- 32.Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165–171. doi: 10.7326/0003-4819-144-3-200602070-00004. [DOI] [PubMed] [Google Scholar]
- 33.van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289–297. doi: 10.1016/S0140-6736(17)30885-1. [DOI] [PubMed] [Google Scholar]
- 34.Lucassen W, Geersing GJ, Erkens PM, et al. Clinical decision rules for excluding pulmonary embolism: a meta-analysis. Ann Intern Med. 2011;155:448–460. doi: 10.7326/0003-4819-155-7-201110040-00007. [DOI] [PubMed] [Google Scholar]
- 35.Gibson NS, Sohne M, Kruip MJ, et al. Further validation and simplification of the Wells clinical decision rule in pulmonary embolism. Thromb Haemost. 2008;99:229–234. doi: 10.1160/TH07-05-0321. [DOI] [PubMed] [Google Scholar]
- 36.Douma RA, Gibson NS, Gerdes VE, et al. Validity and clinical utility of the simplified Wells rule for assessing clinical probability for the exclusion of pulmonary embolism. Thromb Haemost. 2009;101:197–200. [PubMed] [Google Scholar]
- 37.Douma RA, Mos IC, Erkens PM, et al. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism: a prospective cohort study. Ann Intern Med. 2011;154:709–718. doi: 10.7326/0003-4819-154-11-201106070-00002. [DOI] [PubMed] [Google Scholar]
- 38.Ceriani E, Combescure C, Le Gal G, et al. Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:957–970. doi: 10.1111/j.1538-7836.2010.03801.x. [DOI] [PubMed] [Google Scholar]
- 39.Ishimaru N, Ohnishi H, Yoshimura S, et al. The sensitivities and prognostic values of the Wells and revised Geneva scores in diagnosis of pulmonary embolism in the Japanese population. Respir Investig. 2018;56:399–404. doi: 10.1016/j.resinv.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Luo Q, Xie J, Han Q, et al. Prevalence of venous thromboembolic events and diagnostic performance of the Wells score and revised Geneva scores for pulmonary embolism in patients with interstitial lung disease: a prospective study. Heart Lung Circ. 2014;23:778–785. doi: 10.1016/j.hlc.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 41.Di Nisio M, Squizzato A, Rutjes AW, et al. Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: a systematic review. J Thromb Haemost. 2007;5:296–304. doi: 10.1111/j.1538-7836.2007.02328.x. [DOI] [PubMed] [Google Scholar]
- 42.Stein PD, Hull RD, Patel KC, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med. 2004;140:589–602. doi: 10.7326/0003-4819-140-8-200404200-00005. [DOI] [PubMed] [Google Scholar]
- 43.Carrier M, Righini M, Djurabi RK, et al. VIDAS D-dimer in combination with clinical pre-test probability to rule out pulmonary embolism. A systematic review of management outcome studies. Thromb Haemost. 2009;101:886–892. [PubMed] [Google Scholar]
- 44.Kearon C, Ginsberg JS, Douketis J, et al. An evaluation of D-dimer in the diagnosis of pulmonary embolism: a randomized trial. Ann Intern Med. 2006;144:812–821. doi: 10.7326/0003-4819-144-11-200606060-00007. [DOI] [PubMed] [Google Scholar]
- 45.van Belle A, Büller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA. 2006;295:172–179. doi: 10.1001/jama.295.2.172. [DOI] [PubMed] [Google Scholar]
- 46.Geersing GJ, Erkens PM, Lucassen WA, et al. Safe exclusion of pulmonary embolism using the Wells rule and qualitative D-dimer testing in primary care: prospective cohort study. BMJ. 2012;345:e6564. doi: 10.1136/bmj.e6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geersing GJ, Janssen KJ, Oudega R, et al. Excluding venous thromboembolism using point of care D-dimer tests in outpatients: a diagnostic meta-analysis. BMJ. 2009;339:b2990. doi: 10.1136/bmj.b2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354:2317–2327. doi: 10.1056/NEJMoa052367. [DOI] [PubMed] [Google Scholar]
- 49.Perrier A, Roy PM, Sanchez O, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med. 2005;352:1760–1768. doi: 10.1056/NEJMoa042905. [DOI] [PubMed] [Google Scholar]
- 50.Righini M, Le Gal G, Aujesky D, et al. Diagnosis of pulmonary embolism by multidetector CT alone or combined with venous ultrasonography of the leg: a randomised non-inferiority trial. Lancet. 2008;371:1343–1352. doi: 10.1016/S0140-6736(08)60594-2. [DOI] [PubMed] [Google Scholar]
- 51.Stein PD, Goodman LR, Hull RD, et al. Diagnosis and management of isolated subsegmental pulmonary embolism: review and assessment of the options. Clin Appl Thromb Hemost. 2012;18:20–26. doi: 10.1177/1076029611422363. [DOI] [PubMed] [Google Scholar]
- 52.Hutchinson BD, Navin P, Marom EM, et al. Overdiagnosis of pulmonary embolism by pulmonary CT angiography. AJR Am J Roentgenol. 2015;205:271–277. doi: 10.2214/AJR.14.13938. [DOI] [PubMed] [Google Scholar]
- 53.Stein PD, Athanasoulis C, Alavi A, et al. Complications and validity of pulmonary angiography in acute pulmonary embolism. Circulation. 1992;85:462–468. doi: 10.1161/01.cir.85.2.462. [DOI] [PubMed] [Google Scholar]
- 54.Diffin DC, Leyendecker JR, Johnson SP, et al. Effect of anatomic distribution of pulmonary emboli on interobserver agreement in the interpretation of pulmonary angiography. AJR Am J Roentgenol. 1998;171:1085–1089. doi: 10.2214/ajr.171.4.9763002. [DOI] [PubMed] [Google Scholar]
- 55.Huang WC, Hsu CH, Sung SH, et al. 2018 TSOC guideline focused update on diagnosis and treatment of pulmonary arterial hypertension. J Formos Med Assoc. 2019;118:1584–1609. doi: 10.1016/j.jfma.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 56.PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA. 1990;263:2753–2759. doi: 10.1001/jama.1990.03440200057023. [DOI] [PubMed] [Google Scholar]
- 57.Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA. 2007;298:2743–2753. doi: 10.1001/jama.298.23.2743. [DOI] [PubMed] [Google Scholar]
- 58.Stein PD, Chenevert TL, Fowler SE, et al. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III). Ann Intern Med. 2010;152:434–443, W142-3. doi: 10.1059/0003-4819-152-7-201004060-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Revel MP, Sanchez O, Couchon S, et al. Diagnostic accuracy of magnetic resonance imaging for an acute pulmonary embolism: results of the ‘IRM-EP’ study. J Thromb Haemost. 2012;10:743–750. doi: 10.1111/j.1538-7836.2012.04652.x. [DOI] [PubMed] [Google Scholar]
- 60.Revel MP, Sanchez O, Lefort C, et al. Diagnostic accuracy of unenhanced, contrast-enhanced perfusion and angiographic MRI sequences for pulmonary embolism diagnosis: results of independent sequence readings. Eur Radiol. 2013;23:2374–2382. doi: 10.1007/s00330-013-2852-8. [DOI] [PubMed] [Google Scholar]
- 61.Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 62.Miniati M, Monti S, Pratali L, et al. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med. 2001;110:528–535. doi: 10.1016/s0002-9343(01)00693-3. [DOI] [PubMed] [Google Scholar]
- 63.Bova C, Greco F, Misuraca G, et al. Diagnostic utility of echocardiography in patients with suspected pulmonary embolism. Am J Emerg Med. 2003;21:180–183. doi: 10.1016/s0735-6757(02)42257-7. [DOI] [PubMed] [Google Scholar]
- 64.Dresden S, Mitchell P, Rahimi L, et al. Right ventricular dilatation on bedside echocardiography performed by emergency physicians aids in the diagnosis of pulmonary embolism. Ann Emerg Med. 2014;63:16–24. doi: 10.1016/j.annemergmed.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 65.Fields JM, Davis J, Girson L, et al. Transthoracic echocardiography for diagnosing pulmonary embolism: a systematic review and meta-analysis. J Am Soc Echocardiogr. 2017;30:714–723. doi: 10.1016/j.echo.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 66.Hull RD, Hirsh J, Carter CJ, et al. Pulmonary angiography, ventilation lung scanning, and venography for clinically suspected pulmonary embolism with abnormal perfusion lung scan. Ann Intern Med. 1983;98:891–899. doi: 10.7326/0003-4819-98-6-891. [DOI] [PubMed] [Google Scholar]
- 67.British Thoracic Society Standards of Care Committee Pulmonary Embolism Guideline Development Group. British Thoracic Society guidelines for the management of suspected acute pulmonary embolism. Thorax. 2003;58:470–483. doi: 10.1136/thorax.58.6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turkstra F, Kuijer PM, van Beek EJ, et al. Diagnostic utility of ultrasonography of leg veins in patients suspected of having pulmonary embolism. Ann Intern Med. 1997;126:775–781. doi: 10.7326/0003-4819-126-10-199705150-00005. [DOI] [PubMed] [Google Scholar]
- 69.Barritt DW, Jordan SC. Anticoagulant drugs in the treatment of pulmonary embolism. A controlled trial. Lancet. 1960;1:1309–1312. doi: 10.1016/s0140-6736(60)92299-6. [DOI] [PubMed] [Google Scholar]
- 70.Carrier M, Le Gal G, Wells PS, et al. Systematic review: case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med. 2010;152:578–589. doi: 10.7326/0003-4819-152-9-201005040-00008. [DOI] [PubMed] [Google Scholar]
- 71.Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135:98–107. doi: 10.7326/0003-4819-135-2-200107170-00010. [DOI] [PubMed] [Google Scholar]
- 72.Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. 2014;311:1117–1124. doi: 10.1001/jama.2014.2135. [DOI] [PubMed] [Google Scholar]
- 73.Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–3069, 69a-69k. doi: 10.1093/eurheartj/ehu283. [DOI] [PubMed] [Google Scholar]
- 74.van Es N, Kraaijpoel N, Klok FA, et al. The original and simplified Wells rules and age-adjusted D-dimer testing to rule out pulmonary embolism: an individual patient data meta-analysis. J Thromb Haemost. 2017;15:678–684. doi: 10.1111/jth.13630. [DOI] [PubMed] [Google Scholar]
- 75.Freund Y, Cachanado M, Aubry A, et al. Effect of the pulmonary embolism rule-out criteria on subsequent thromboembolic events among low-risk emergency department patients: the PROPER randomized clinical trial. JAMA. 2018;319:559–566. doi: 10.1001/jama.2017.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van der Pol LM, Tromeur C, Bistervels IM, et al. Pregnancy-adapted YEARS algorithm for diagnosis of suspected pulmonary embolism. N Engl J Med. 2019;380:1139–1149. doi: 10.1056/NEJMoa1813865. [DOI] [PubMed] [Google Scholar]
- 77.Hirsh J, Bates SM. Prognosis in acute pulmonary embolism. Lancet. 1999;353:1375–1376. doi: 10.1016/S0140-6736(99)90053-3. [DOI] [PubMed] [Google Scholar]
- 78.Wicki J, Perrier A, Perneger TV, et al. Predicting adverse outcome in patients with acute pulmonary embolism: a risk score. Thromb Haemost. 2000;84:548–552. [PubMed] [Google Scholar]
- 79.Aujesky D, Obrosky DS, Stone RA, et al. A prediction rule to identify low-risk patients with pulmonary embolism. Arch Intern Med. 2006;166:169–175. doi: 10.1001/archinte.166.2.169. [DOI] [PubMed] [Google Scholar]
- 80.Jimenez D, Yusen RD, Otero R, et al. Prognostic models for selecting patients with acute pulmonary embolism for initial outpatient therapy. Chest. 2007;132:24–30. doi: 10.1378/chest.06-2921. [DOI] [PubMed] [Google Scholar]
- 81.Jimenez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010;170:1383–1389. doi: 10.1001/archinternmed.2010.199. [DOI] [PubMed] [Google Scholar]
- 82.Barra S, Paiva L, Providencia R, et al. LR-PED rule: low risk pulmonary embolism decision rule - a new decision score for low risk pulmonary embolism. Thromb Res. 2012;130:327–333. doi: 10.1016/j.thromres.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 83.Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. 1997;30:1165–1171. doi: 10.1016/s0735-1097(97)00319-7. [DOI] [PubMed] [Google Scholar]
- 84.Otero R, Trujillo-Santos J, Cayuela A, et al. Haemodynamically unstable pulmonary embolism in the RIETE Registry: systolic blood pressure or shock index? Eur Respir J. 2007;30:1111–1116. doi: 10.1183/09031936.00071007. [DOI] [PubMed] [Google Scholar]
- 85.Kohn CG, Mearns ES, Parker MW, et al. Prognostic accuracy of clinical prediction rules for early post-pulmonary embolism all-cause mortality: a bivariate meta-analysis. Chest. 2015;147:1043–1062. doi: 10.1378/chest.14-1888. [DOI] [PubMed] [Google Scholar]
- 86.Becattini C, Agnelli G. Risk stratification and management of acute pulmonary embolism. Hematology Am Soc Hematol Educ Program. 2016;2016:404–412. doi: 10.1182/asheducation-2016.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Venetz C, Jimenez D, Mean M, et al. A comparison of the original and simplified pulmonary embolism severity index. Thromb Haemost. 2011;106:423–428. doi: 10.1160/TH11-04-0263. [DOI] [PubMed] [Google Scholar]
- 88.Laporte S, Mismetti P, Decousus H, et al. Clinical predictors for fatal pulmonary embolism in 15,520 patients with venous thromboembolism: findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) Registry. Circulation. 2008;117:1711–1716. doi: 10.1161/CIRCULATIONAHA.107.726232. [DOI] [PubMed] [Google Scholar]
- 89.Aujesky D, Roy PM, Verschuren F, et al. Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet. 2011;378:41–48. doi: 10.1016/S0140-6736(11)60824-6. [DOI] [PubMed] [Google Scholar]
- 90.Grifoni S, Olivotto I, Cecchini P, et al. Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation. 2000;101:2817–2822. doi: 10.1161/01.cir.101.24.2817. [DOI] [PubMed] [Google Scholar]
- 91.Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J. 2008;29:1569–1577. doi: 10.1093/eurheartj/ehn208. [DOI] [PubMed] [Google Scholar]
- 92.Becattini C, Agnelli G, Vedovati MC, et al. Multidetector computed tomography for acute pulmonary embolism: diagnosis and risk stratification in a single test. Eur Heart J. 2011;32:1657–1663. doi: 10.1093/eurheartj/ehr108. [DOI] [PubMed] [Google Scholar]
- 93.Meinel FG, Nance JW Jr, Schoepf UJ, et al. Predictive value of computed tomography in acute pulmonary embolism: systematic review and meta-analysis. Am J Med. 2015;128:747–759. doi: 10.1016/j.amjmed.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 94.Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation. 2007;116:427–433. doi: 10.1161/CIRCULATIONAHA.106.680421. [DOI] [PubMed] [Google Scholar]
- 95.Klok FA, Mos IC, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med. 2008;178:425–430. doi: 10.1164/rccm.200803-459OC. [DOI] [PubMed] [Google Scholar]
- 96.Hellenkamp K, Schwung J, Rossmann H, et al. Risk stratification of normotensive pulmonary embolism: prognostic impact of copeptin. Eur Respir J. 2015;46:1701–1710. doi: 10.1183/13993003.00857-2015. [DOI] [PubMed] [Google Scholar]
- 97.Kostrubiec M, Labyk A, Pedowska-Wloszek J, et al. Neutrophil gelatinase-associated lipocalin, cystatin C and eGFR indicate acute kidney injury and predict prognosis of patients with acute pulmonary embolism. Heart. 2012;98:1221–1228. doi: 10.1136/heartjnl-2012-301884. [DOI] [PubMed] [Google Scholar]
- 98.Barco S, Mahmoudpour SH, Planquette B, et al. Prognostic value of right ventricular dysfunction or elevated cardiac biomarkers in patients with low-risk pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2019;40:902–910. doi: 10.1093/eurheartj/ehy873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lankeit M, Jimenez D, Kostrubiec M, et al. Predictive value of the high-sensitivity troponin T assay and the simplified pulmonary embolism severity index in hemodynamically stable patients with acute pulmonary embolism: a prospective validation study. Circulation. 2011;124:2716–2724. doi: 10.1161/CIRCULATIONAHA.111.051177. [DOI] [PubMed] [Google Scholar]
- 100.Sanchez O, Trinquart L, Planquette B, et al. Echocardiography and pulmonary embolism severity index have independent prognostic roles in pulmonary embolism. Eur Respir J. 2013;42:681–688. doi: 10.1183/09031936.00097512. [DOI] [PubMed] [Google Scholar]
- 101.Jimenez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2014;189:718–726. doi: 10.1164/rccm.201311-2040OC. [DOI] [PubMed] [Google Scholar]
- 102.Vanni S, Nazerian P, Pepe G, et al. Comparison of two prognostic models for acute pulmonary embolism: clinical vs. right ventricular dysfunction-guided approach. J Thromb Haemost. 2011;9:1916–1923. doi: 10.1111/j.1538-7836.2011.04459.x. [DOI] [PubMed] [Google Scholar]
- 103.Barco S, Lankeit M, Binder H, et al. Home treatment of patients with low-risk pulmonary embolism with the oral factor Xa inhibitor rivaroxaban. Rationale and design of the HoT-PE Trial. Thromb Haemost. 2016;116:191–197. doi: 10.1160/TH16-01-0004. [DOI] [PubMed] [Google Scholar]
- 104.Barco S, Schmidtmann I, Ageno W, et al. Early discharge and home treatment of patients with low-risk pulmonary embolism with the oral factor Xa inhibitor rivaroxaban: an international multicentre single-arm clinical trial. Eur Heart J. 2020;41:509–518. doi: 10.1093/eurheartj/ehz367. [DOI] [PubMed] [Google Scholar]
- 105.Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002;121:877–905. doi: 10.1378/chest.121.3.877. [DOI] [PubMed] [Google Scholar]
- 106.Miller GA, Sutton GC, Kerr IH, et al. Comparison of streptokinase and heparin in treatment of isolated acute massive pulmonary embolism. Br Med J. 1971;2:681–684. doi: 10.1136/bmj.2.5763.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tibbutt DA, Davies JA, Anderson JA, et al. Comparison by controlled clinical trial of streptokinase and heparin in treatment of life-threatening pulmonay embolism. Br Med J. 1974;1:343–347. doi: 10.1136/bmj.1.5904.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goldhaber SZ, Haire WD, Feldstein ML, et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet. 1993;341:507–511. doi: 10.1016/0140-6736(93)90274-k. [DOI] [PubMed] [Google Scholar]
- 109.Piazza G, Hohlfelder B, Jaff MR, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv. 2015;8:1382–1392. doi: 10.1016/j.jcin.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 110.Jerjes-Sanchez C, Ramirez-Rivera A, de Lourdes Garcia M, et al. Streptokinase and heparin versus heparin alone in massive pulmonary embolism: a randomized controlled trial. J Thromb Thrombolysis. 1995;2:227–229. doi: 10.1007/BF01062714. [DOI] [PubMed] [Google Scholar]
- 111.Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347:1143–1150. doi: 10.1056/NEJMoa021274. [DOI] [PubMed] [Google Scholar]
- 112.Sharifi M, Bay C, Skrocki L, et al. Moderate pulmonary embolism treated with thrombolysis (from the "MOPETT" trial). Am J Cardiol. 2013;111:273–277. doi: 10.1016/j.amjcard.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 113.Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402–1411. doi: 10.1056/NEJMoa1302097. [DOI] [PubMed] [Google Scholar]
- 114.Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost. 2014;12:459–468. doi: 10.1111/jth.12521. [DOI] [PubMed] [Google Scholar]
- 115.Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129:479–486. doi: 10.1161/CIRCULATIONAHA.113.005544. [DOI] [PubMed] [Google Scholar]
- 116.Tapson VF, Sterling K, Jones N, et al. A randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate-risk pulmonary embolism: the OPTALYSE PE trial. JACC Cardiovasc Interv. 2018;11:1401–1410. doi: 10.1016/j.jcin.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 117.Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA. 2014;311:2414–2421. doi: 10.1001/jama.2014.5990. [DOI] [PubMed] [Google Scholar]
- 118.Meneveau N, Seronde MF, Blonde MC, et al. Management of unsuccessful thrombolysis in acute massive pulmonary embolism. Chest. 2006;129:1043–1050. doi: 10.1378/chest.129.4.1043. [DOI] [PubMed] [Google Scholar]
- 119.Keeling WB, Sundt T, Leacche M, et al. Outcomes after surgical pulmonary embolectomy for acute pulmonary embolus: a multi-institutional study. Ann Thorac Surg. 2016;102:1498–1502. doi: 10.1016/j.athoracsur.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 120.Lee T, Itagaki S, Chiang YP, et al. Survival and recurrence after acute pulmonary embolism treated with pulmonary embolectomy or thrombolysis in New York State, 1999 to 2013. J Thorac Cardiovasc Surg. 2018;155:1084–1090. doi: 10.1016/j.jtcvs.2017.07.074. [DOI] [PubMed] [Google Scholar]
- 121.Lee CH, Fang CC, Tsai LM, et al. Changing treatment patterns in patients with venous thromboembolism in Taiwan. Circ J. 2020;84:283–293. doi: 10.1253/circj.CJ-19-0741. [DOI] [PubMed] [Google Scholar]
- 122.Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–2352. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 123.EINSTEIN Investigators. Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 124.EINSTEIN-PE Investigators. Büller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–1297. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 125.Agnelli G, Büller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808. doi: 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- 126.Hokusai-VTE Investigators. Büller HR, Decousus H, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406–1415. doi: 10.1056/NEJMoa1306638. [DOI] [PubMed] [Google Scholar]
- 127.Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129:764–772. doi: 10.1161/CIRCULATIONAHA.113.004450. [DOI] [PubMed] [Google Scholar]
- 128.van Es N, Coppens M, Schulman S, et al. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124:1968–1975. doi: 10.1182/blood-2014-04-571232. [DOI] [PubMed] [Google Scholar]
- 129.van Es J, Douma RA, Kamphuisen PW, et al. Clot resolution after 3 weeks of anticoagulant treatment for pulmonary embolism: comparison of computed tomography and perfusion scintigraphy. J Thromb Haemost. 2013;11:679–685. doi: 10.1111/jth.12150. [DOI] [PubMed] [Google Scholar]
- 130.Paraskos JA, Adelstein SJ, Smith RE, et al. Late prognosis of acute pulmonary embolism. N Engl J Med. 1973;289:55–58. doi: 10.1056/NEJM197307122890201. [DOI] [PubMed] [Google Scholar]
- 131.Hall RJ, Sutton GC, Kerr IH. Long-term prognosis of treated acute massive pulmonary embolism. Br Heart J. 1977;39:1128–1134. doi: 10.1136/hrt.39.10.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brekelmans MPA, Büller HR, Mercuri MF, et al. Direct oral anticoagulants for pulmonary embolism: importance of anatomical extent. TH Open. 2018;2:e1–e7. doi: 10.1055/s-0037-1615251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brekelmans MP, Ageno W, Beenen LF, et al. Recurrent venous thromboembolism in patients with pulmonary embolism and right ventricular dysfunction: a post-hoc analysis of the Hokusai-VTE study. Lancet Haematol. 2016;3:e437–e445. doi: 10.1016/S2352-3026(16)30080-1. [DOI] [PubMed] [Google Scholar]
- 134.Simes J, Becattini C, Agnelli G, et al. Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation. 2014;130:1062–1071. doi: 10.1161/CIRCULATIONAHA.114.008828. [DOI] [PubMed] [Google Scholar]
- 135.Weitz JI, Lensing AWA, Prins MH, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376:1211–1222. doi: 10.1056/NEJMoa1700518. [DOI] [PubMed] [Google Scholar]
- 136.Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368:709–718. doi: 10.1056/NEJMoa1113697. [DOI] [PubMed] [Google Scholar]
- 137.Raskob G, Ageno W, Cohen AT, et al. Extended duration of anticoagulation with edoxaban in patients with venous thromboembolism: a post-hoc analysis of the Hokusai-VTE study. Lancet Haematol. 2016;3:e228–e236. doi: 10.1016/S2352-3026(16)00023-5. [DOI] [PubMed] [Google Scholar]
- 138.Wang KL, van Es N, Cameron C, et al. Extended treatment of venous thromboembolism: a systematic review and network meta-analysis. Heart. 2019;105:545–552. doi: 10.1136/heartjnl-2018-313617. [DOI] [PubMed] [Google Scholar]
- 139.Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: burden, mechanisms, and management. Thromb Haemost. 2017;117:219–230. doi: 10.1160/TH16-08-0615. [DOI] [PubMed] [Google Scholar]
- 140.Timp JF, Braekkan SK, Versteeg HH, et al. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122:1712–1723. doi: 10.1182/blood-2013-04-460121. [DOI] [PubMed] [Google Scholar]
- 141.Sorensen HT, Mellemkjaer L, Olsen JH, et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 142.Hisada Y, Geddings JE, Ay C, et al. Venous thrombosis and cancer: from mouse models to clinical trials. J Thromb Haemost. 2015;13:1372–1382. doi: 10.1111/jth.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 144.Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25:5490–5505. doi: 10.1200/JCO.2007.14.1283. [DOI] [PubMed] [Google Scholar]
- 145.Lyman GH, Bohlke K, Khorana AA, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology Clinical Practice guideline update 2014. J Clin Oncol. 2015;33:654–656. doi: 10.1200/JCO.2014.59.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 147.Lee AYY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314:677–686. doi: 10.1001/jama.2015.9243. [DOI] [PubMed] [Google Scholar]
- 148.Carrier M, Prandoni P. Controversies in the management of cancer-associated thrombosis. Expert Rev Hematol. 2017;10:15–22. doi: 10.1080/17474086.2017.1257935. [DOI] [PubMed] [Google Scholar]
- 149.Imberti D, Agnelli G, Ageno W, et al. Clinical characteristics and management of cancer-associated acute venous thromboembolism: findings from the MASTER registry. Haematologica. 2008;93:273–278. doi: 10.3324/haematol.11458. [DOI] [PubMed] [Google Scholar]
- 150.Spirk D, Ugi J, Korte W, et al. Long-term anticoagulation treatment for acute venous thromboembolism in patients with and without cancer. The SWIss Venous ThromboEmbolism Registry (SWIVTER) II. Thromb Haemost. 2011;105:962–967. doi: 10.1160/TH11-01-0002. [DOI] [PubMed] [Google Scholar]
- 151.van der Hulle T, den Exter PL, Kooiman J, et al. Meta-analysis of the efficacy and safety of new oral anticoagulants in patients with cancer-associated acute venous thromboembolism. J Thromb Haemost. 2014;12:1116–1120. doi: 10.1111/jth.12605. [DOI] [PubMed] [Google Scholar]
- 152.Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378:615–624. doi: 10.1056/NEJMoa1711948. [DOI] [PubMed] [Google Scholar]
- 153.Mulder FI, van Es N, Kraaijpoel N, et al. Edoxaban for treatment of venous thromboembolism in patient groups with different types of cancer: results from the Hokusai VTE Cancer study. Thromb Res. 2020;185:13–19. doi: 10.1016/j.thromres.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 154.Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol. 2018;36:2017–2023. doi: 10.1200/JCO.2018.78.8034. [DOI] [PubMed] [Google Scholar]
- 155.McBane RD, 2nd, Wysokinski WE, Le-Rademacher JG, et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: the ADAM VTE trial. J Thromb Haemost. 2020;18:411–421. doi: 10.1111/jth.14662. [DOI] [PubMed] [Google Scholar]
- 156.Agnelli G, Becattini C, Meyer G, et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382:1599–1607. doi: 10.1056/NEJMoa1915103. [DOI] [PubMed] [Google Scholar]
- 157.Giustozzi M, Agnelli G, Del Toro-Cervera J, et al. Direct oral anticoagulants for the treatment of acute venous thromboembolism associated with cancer: a systematic review and meta-analysis. Thromb Haemost. 2020;120:1128–1136. doi: 10.1055/s-0040-1712098. [DOI] [PubMed] [Google Scholar]
- 158.Moik F, Posch F, Zielinski C, et al. Direct oral anticoagulants compared to low-molecular-weight heparin for the treatment of cancer-associated thrombosis: updated systematic review and meta-analysis of randomized controlled trials. Res Pract Thromb Haemost. 2020;4:550–561. doi: 10.1002/rth2.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mulder FI, Bosch FTM, Young AM, et al. Direct oral anticoagulants for cancer-associated venous thromboembolism: a systematic review and meta-analysis. Blood. 2020;136:1433–1441. doi: 10.1182/blood.2020005819. [DOI] [PubMed] [Google Scholar]
- 160.Yusuff HO, Zochios V, Vuylsteke A. Extracorporeal membrane oxygenation in acute massive pulmonary embolism: a systematic review. Perfusion. 2015;30:611–616. doi: 10.1177/0267659115583377. [DOI] [PubMed] [Google Scholar]
- 161.Kjaergaard B, Kristensen JH, Sindby JE, et al. Extracorporeal membrane oxygenation in life-threatening massive pulmonary embolism. Perfusion. 2019;34:467–474. doi: 10.1177/0267659119830014. [DOI] [PubMed] [Google Scholar]
- 162.Lavonas EJ, Drennan IR, Gabrielli A, et al. Part 10: special circumstances of resuscitation: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S501–S518. doi: 10.1161/CIR.0000000000000264. [DOI] [PubMed] [Google Scholar]
- 163.Rosovsky R, Chang Y, Rosenfield K, et al. Changes in treatment and outcomes after creation of a pulmonary embolism response team (PERT), a 10-year analysis. J Thromb Thrombolysis. 2019;47:31–40. doi: 10.1007/s11239-018-1737-8. [DOI] [PubMed] [Google Scholar]
- 164.Wright C, Elbadawi A, Chen YL, et al. The impact of a pulmonary embolism response team on the efficiency of patient care in the emergency department. J Thromb Thrombolysis. 2019;48:331–335. doi: 10.1007/s11239-019-01875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kabrhel C, Rosovsky R, Channick R, et al. A multidisciplinary pulmonary embolism response team: initial 30-month experience with a novel approach to delivery of care to patients with submassive and massive pulmonary embolism. Chest. 2016;150:384–393. doi: 10.1016/j.chest.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 166.den Exter PL, van Es J, Kroft LJ, et al. Thromboembolic resolution assessed by CT pulmonary angiography after treatment for acute pulmonary embolism. Thromb Haemost. 2015;114:26–34. doi: 10.1160/TH14-10-0842. [DOI] [PubMed] [Google Scholar]
- 167.Kahn SR, Hirsch AM, Akaberi A, et al. Functional and exercise limitations after a first episode of pulmonary embolism: results of the ELOPE prospective cohort study. Chest. 2017;151:1058–1068. doi: 10.1016/j.chest.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 168.Albaghdadi MS, Dudzinski DM, Giordano N, et al. Cardiopulmonary exercise testing in patients following massive and submassive pulmonary embolism. J Am Heart Assoc. 2018;7:e006841. doi: 10.1161/JAHA.117.006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kim NH, Delcroix M, Jais X, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;53:1801915. doi: 10.1183/13993003.01915-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sista AK, Klok FA. Late outcomes of pulmonary embolism: the post-PE syndrome. Thromb Res. 2018;164:157–162. doi: 10.1016/j.thromres.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 171.Wells PS, Hirsh J, Anderson DR, et al. Accuracy of clinical assessment of deep-vein thrombosis. Lancet. 1995;345:1326–1330. doi: 10.1016/s0140-6736(95)92535-x. [DOI] [PubMed] [Google Scholar]
- 172.Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350:1795–1798. doi: 10.1016/S0140-6736(97)08140-3. [DOI] [PubMed] [Google Scholar]
- 173.Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227–1235. doi: 10.1056/NEJMoa023153. [DOI] [PubMed] [Google Scholar]
- 174.Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041–1046. doi: 10.1164/rccm.200506-862OC. [DOI] [PMC free article] [PubMed] [Google Scholar]