Graphical abstract
Keywords: Glioma, Glioblastoma multiforme, miRNA-21, microRNA, oncomiR
Highlights
-
•
MIRNA-21 is consistently upregulated in gliomas.
-
•
It suppresses apoptosis and promotes tumor invasion.
-
•
It is involved in resistance against chemo- and radiotherapy.
-
•
Research should focus on inhibiting MiRNA-21 in human brains.
Abstract
Gliomas are the most common primary brain tumors in adults. They are generally very resistant to treatment and are therefore associated with negative outcomes. MicroRNAs (miRNAs) are small, non-coding RNA molecules that affect many cellular processes by regulating gene expression and, post-transcriptionally, the translation of mRNAs. MiRNA-21 has been consistently shown to be upregulated in glioma and research has shown that it is involved in a wide variety of biological pathways, promoting tumor cell survival and invasiveness. Furthermore, it has been implicated in resistance to treatment, both against chemotherapy and radiotherapy. In this review, we gathered the existent data on miRNA-21 and gliomas, in terms of its expression levels, association with grade and prognosis, the pathways it involves and its targets in glioma, and finally how it leads to treatment resistance. Furthermore, we discuss how this knowledge could be applied in clinical practice in the years to come. To our knowledge, this is the first review to assess in extent and depth the role of miRNA-21 in gliomas.
1. Introduction
Gliomas are the most common and lethal primary brain tumors in adults. They are characterized by rapid growth rates, high invasion capacity, and resistance to treatment [1].Cancerous cells, as well as a variety of stromal cells make up the main tumor mass [2]; notably, the malignant cells reprogram the surrounding healthy cells, reforming the extracellular matrix and promoting angiogenesis in order to survive and proliferate [1,2].
Glioma grading is based on WHO criteria, with Grades I and II referred to as “low grade”, Grade III referred to as “high grade” or “anaplastic”, and Grade IV, the most aggressive, referred to as “glioblastoma multiforme” (GBM) (Grades III and IV often termed together as just “high grade”) [3].Treatment options include surgical resection, chemotherapy and radiotherapy. However, due to the location of most gliomas and their high invasiveness, total surgical removal is difficult to achieve while recurrence rates remain high [4]. Only recently has immunotherapy gained some ground in the treatment of gliomas, with clinical trials of advanced stages currently being conducted [5], and the combination of several solitary immune therapies carrying much promise as a treatment alternative in the future [6]. For the aforementioned reasons however, gliomas have a notoriously bad prognosis, with only 5% of the patients surviving beyond a year after initial diagnosis [7]; patients with GBM, under the standard radiation and temozolomide chemotherapy regimen, survive per average just a few months over a year, and without treatment, they live per average for less than five months [8].
MicroRNAs (miRNAs) are small, non-coding RNA molecules that are approximately 18–25 nucleotides long. They influence many important cell processes by regulating gene expression and post-transcriptionally, the translation of mRNA into proteins [9].MiRNAs are transcribed from genes localized in introns of protein-coding or non-coding genes, or in exons, partly overlapping with coding areas, or in intergenic regions; in humans, most miRNAs are independently transcribed [10,11]. The primary transcript is processed by the RNAase III DROSHA complex, forming the precursor miRNA (pre-miRNA), which is then further processed by the RNAase III Dicer in the cytoplasm, leading to the creation of two miRNA strands. The one is chosen to mature and becomes the final, functional miRNA molecule, while the other is degraded. The mature miRNA molecule binds to its complementary mRNA sequence, hindering its translation and, therefore, regulating the levels of the protein product; a single miRNA can bind to many different mRNAs, while a single mRNA can be regulated by many different miRNAs as well [9].
MiRNAs are known to impact many processes, including cancer cell functions, with their levels being reported deregulated in a wide variety of diseases. Some miRNAs have been shown to promote tumor growth much like oncogenes, and are widely referred to as “oncomiRs” [12]; they have also been gaining considerable ground as circulating tumor biomarkers [13]. One of these onco-miRNAs most extensively studied is miRNA-21. In fact, it was the only one found increased in all types of solid cancers [14], and the first one to be found deregulated in human glioblastoma [15]. Recent studies continue to confirm its oncogenic potential and upregulation in a plethora of common and lethal malignancies besides glioblastoma [16], such as colorectal cancer [17], prostate cancer [18], and lung cancer [19]. In fact, in several reports, miR-21 has been proposed as a possible diagnostic biomarker, with better potential than other deregulated miRNAs [18].
In recent years, it has been consistently reported as increased in glioma [1]and many studies have tried to elucidate its decisive role. There is mounting evidence that targeting miRNA-21 may indeed pave the way for novel therapeutic applications concerning glioma management, and all these subjects will be covered in the sections to follow. In this review, therefore, we summarize the currently available data on this miRNA both in in vitro and human glioma tissue samples, its association with prognosis and tumor grade, and its involvement in glioma growth and invasion, focusing on the pathways and studies conducted on glioma cells. More importantly, we also address the ways via which this molecule mediates resistance to treatment, and how its inhibition can assist treatment. To our knowledge, this is the first review to assess in extent and depth the role of miRNA-21 in gliomas, with an emphasis on the implicated mechanisms and its therapeutic potential.
2. MiRNA-21 upregulation and diagnostic potential
Several studies have assessed the expression levels of miRNA-21, both in human samples and in glioma cell lines, as summarized in Table 1 and Table 2 respectively.
Table 1.
Studies on miRNA-21 levels in human samples.
| Author, Year | WHO Grade | Human Sample | MiRNA-21 levels |
|---|---|---|---|
| Chan et al., 2005 | IV | BT* | Significantly increased (5- to 100-fold; p < 0.05) |
| Ciafrè et al., 2005 | IV | BT | Increased (C/P** ratio 1.81- to 9.3-fold in 4 out of 9 samples) |
| Gabriely et al., 2008 | II-IV | BT | Significantly increased in grade IV (10- to 45-fold) |
| Silber et al., 2008 | III-IV | BT | Increased (5- to 30-fold) |
| Conti et al., 2009 | II-IV | BT | Increased (8.76 ± 1.25-fold in grade II, 9.39 ± 1.46-fold in grade III, and 9.18 ± 2.54-fold in grade IV) |
| Sasayama et al., 2009 | IV | BT | Increased (C/P** ratio 1.94- to 5.16-fold) |
| Guan et al., 2010 | III-IV | BT | Significantly increased in grade IV (∼3-fold log2 ratio; p < 0.001) |
| Rao et al., 2010 | III-IV | BT | Significantly increased (∼37-fold in III, p = 0.0049 and ∼58-fold in IV, p < 0.0001) |
| Shi et al., 2010 | II-IV | BT | Significantly increased in grades III & IV (∼15-fold in III and ∼50-fold in IV; p < 0.001) |
| Zhi et al., 2010 | I-IV | BT | Significantly increased (mean fold tumor/normal adjacent tissue 2.3308; p = 0.003845) |
| Gaur et al., 2011 | IV | BT | Increased (∼8- to ∼35-fold) |
| Lages et al., 2011 | GBM*3 & ODG*4 | BT | Significantly increased (87.8-fold in GBM and 9-fold in ODG as indicated by real time-PCR, 4.8-fold in GBM and 1.3-fold in ODG as reported by hybridisation; p < 0.05) |
| Lakomy et al., 2011 | IV | BT | Significantly increased (3.45-fold; p = 0.02550) |
| Han et al., 2012 | I-IV | BT | Remain low in grades I-II Significantly increased in grades III-IV (∼3-fold in III-IV; p < 0.05) |
| Wu et al., 2013 | I-IV | BT | Significantly increased in grades III –IV (∼20-fold in III and ∼40-fold in IV;p < 0.001) |
| Barbano et al., 2014 | II-IV | BT | Significantly increased in grades III-IV (∼6-fold;p = 0.008) |
| Piwecka et al., 2015 | III-IV | BT | Significantly increased in grade IV (2.847-fold;p = 0.003267) |
| Shang et al., 2015 | IV | BT | Significantly increased (∼1.6-fold compared to paracancerous tissues;p < 0.05) |
| Qu et al., 2016 | II-IV | BT& CSF | Significantly increased (>1.5-fold in BT. ∼3-fold in CSF;p = 0.004) |
| Sippl et al., 2019 | IV | BT | Significantly increased (1.51 ± 1.35 within tumor tissues, whereas 0.31 ± 0.51 within controls;p < 0.001) |
| Sathyan et al., 2015 | IV | BT | Variation in expression patterns |
| Baraniskin et al., 2012 | II-IV | CSF | Significantly increased (∼20-fold;p < 0.05) |
| Teplyuk et al., 2012 | IV | CSF | Significantly increased (∼13-fold;p < 0.001 when normalized to miR-24, whereas ∼2-fold when normalized to miR120b) |
| Wang et al., 2012 | II-IV | Plasma | Significantly increased (∼2-fold in II and ∼3-fold in III-IV;p < 0.008) |
| Ilhan-Mutlu et al., 2012 | IV | Plasma | Significantly increased (∼4-fold;p = 0.02) |
| Mao et al., 2014 | IV | Serum | Significantly increased (∼2-fold;p < 0.0001) |
| Ivo D’Urso et al., 2015 | II-IV | Serum | Significantly increased (Ct in glioma patients = 31.30626 versus controls = 32.4941;p < 0.01) |
| Zhi et al., 2015 | II-IV | Serum | Increased not significantly |
| Siegal et al., 2016 | III-IV | Serum | Significantly increased (∼5-fold;p < 0.02) |
| ParvizHamidi et al., 2019 | IV | Serum | Significantly increased |
| Akers et al., 2013 | IV | CSF EVs & Serum EVs | Significantly increased in CSF EVs (0.14–1.04 copies/EV in patients and 5.26 × 10−4 to 1.48 × 10−1 copies/EV in non-oncologic patients ;p < 0.001) Not significantly different in serum EVs |
| Shi et al., 2015 | I-IV | CSF exosomes & Serum exosomes | Significantly increased in CSF exosomes (∼9-fold in III-IV, p < 0.01; ∼3-fold in I-II,p < 0.05) Not significantly different in serum exosomes |
| Santangelo et al., 2018 | I-IV | Serum exosomes | Significantly increased in grades III-IV (∼7-fold) Similar to healthy controls in grades I-II |
*Brain Tissue. **Central tumor area (C) and Peripheral glial area (P). *3Glioblastoma multiforme. *4Oligodendroglioma (Not otherwise specified).
Table 2.
Studies on miRNA-21 levels in glioma cell lines.
| Author, Year | Cell Lines | MiRNA-21 Levels |
|---|---|---|
| Chan et al., 2005 | A172, U87,U373, LN229, LN428, LN308 | Increased (5- to 30- fold) |
| Ciafrè et al., 2005 | DBTRG-05MG, U118,U87, A172, LN18, M059 J, M059 K, LN229, T98 G, U138MG | Significantly increased (L/B* ratio = 1.61;p = 0.00818) |
| Rao et al., 2010 | U138, U251, U343, U373, U87, LN18 and LN229 | Increased (log2 ratio in normal samples ranging from -2 to 2; log2 ratio in cell lines ranging from 5.5–9) |
| Shi et al., 2010 | U87MG | Significantly increased (∼60-fold;p < 0.01) |
| Zhou et al., 2010 | U251, TJ866, TJ905, TJ899, A172, H4 | Significantly increased (7-fold;p < 0.05) |
| Gaur et al., 2011 | SNB19, U251, U87, SF767 | Increased (∼15- to ∼55-fold) |
| Yang et al., 2014 | U87, MT330, SJ-G2 | Increased (∼19-fold in U87, ∼27-fold in MT330 and ∼24-fold in SJ-G2 compared to normal fibroblasts) |
*Averaged cell lines sample values (L) and control sample values (B).
The first study on miRNA-21 levels in patients with GBM was conducted by Chan et al. (2005), who evaluated miRNA expression in patients’ tissues and reported miRNA-21 upregulation. Numerous subsequent studies have also reported elevated expression of miRNA-21 in patients’ tumor tissues affected by gliomas of various grades [7,15,[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37]].
Baraniskin et al. were the first to quantify miRNA in cerebrospinal fluid (CSF) samples of patients with glioma, and reported significantly increased miRNA-21 levels compared to healthy controls [38]. In agreement with these results, Teplyuk et al. performed miRNA profiling in the CSF of GBM patients and found a significant increase in miRNA-21 levels [39].
Due to the difficulty of sampling CSF, Wang et al. examined the miRNA levels in the plasma of low grade (LGG) and high grade glioma (HGG) patients, and they reported significantly higher miRNA-21 levels in grade II-IV glioma patients, compared to controls [40]. Consistent with these results, Ilhan-Mutlu et al. reported elevated miRNA-21 levels in plasma samples of GBM patients [41].
In their attempt to find a reliable biomarker for primary central nervous system (CNS) lymphoma (PCNSL), Mao et al. measured miRNA-21 levels in patients with PCNSL and various neurologic disorders, including GBM, as well as in healthy controls. They found that miRNA-21 levels in the serum of GBM patients were significantly higher compared to healthy individuals [42]. A similar study with 30 glioma, 36 PCNSL, 30 brain metastases and 30 other neurological conditions blood samples reported increased miR-21 levels in the glioma samples [43], something also shown in another study of the same year involving glioma serum samples though it failed to reach the statistical significance threshold [44]. A significant elevation in serum miRNA-21 levels was also reported by Siegal et al. and Parviz Hamidi et al., who examined miRNA levels in HGG and GBM patients, respectively [45,46].
Akers et al. investigated miRNA levels in CSF and serum extracellular vesicles (EVs, cell-derived lipid bilayer membranous structures) of GBM patients, and reported a significant increase in miRNA-21 levels in CSF EVs, but failed to detect a statistically significant difference in serum EVs [47]. Similarly, Shi et al. examined miRNA-21 expression in exosomes (small vesicles between a range of 40∼100 nm in size) derived from CSF, as well as serum of glioma patients, and found markedly elevated miRNA-21 levels only in CSF exosomes [48]. That being said, conflicting results were reported by Santangelo et al., who observed significantly increased levels of miRNA-21 in serum exosomes of HGG patients, but reported similar levels in LGG patients and healthy controls [49]. Conclusively, these reports raise the expectation that miRNA-21, being a miRNA produced by the tumor cells, is more accurately represented by CSF measurements, since this fluid is more closely associated with the organ harboring the pathology, i.e. the brain.
Recent bioinformatics studies have also involved miR-21. Sathyan et al. explored miRNA-21 levels on glioma database and their own samples, and concluded that miRNA-21 is not unanimously elevated but has several expression patterns [50]. On the contrary, Candido et al. aimed to find miRNAs deregulated in both the setting of GBM and Alzheimer’s disease. Running their analyses on numerous GBM databases, they reported miR-21 as among most strongly upregulated [16]. Wang et al. used the National Center for Biotechnology Information Gene Expression Omnibus (GEO) database and brain tissue samples to study miR-455-3p, and concomitantly reported miR-21-3p as upregulated [51].
Accumulating evidence has also reported elevated miRNA-21 levels in numerous glioma cell lines including A172 [15,21,52], U87 [15,21,23,30,33,53], U373 [15,30], U251 [23,30,52], U138 and LN18 [21,30], LN229 [15,21,30], U118, M059 J, M059 K and DBTRG-05MG, T98 G [21], U343 [30], LN428 and LN308 [15], TJ866, TJ905, TJ899 GB lines, and H4 [52], SNB19 and SF767 [23], SJ-G2 and MT330 [53]. The aforementioned studies are only indicative of the numerous studies available on glioma lines. Most of the studies that examined miRNA-21 targets and influenced pathways also examined miRNA levels and found them consistently increased. This shows that in vitro studies on glioma cell lines produce the same results with human studies, and reach the unanimous conclusion that miR-21 is elevated in the setting of glioma.
MiRNAs have been gaining considerable ground in terms of diagnosis, and a recent meta-analysis of deregulated miRNAs in the serum/plasma of glioma patients reported a pooled sensitivity, specificity and area under the curve (AUC) of 0.87, 0.86 and 0.93 respectively [54]. Taking into consideration how unanimous the results of studies on miRNA-21 levels in gliomas have been, it is only reasonable to see it being discussed as a potential biomarker as well [55]. For example, miR-21 alongside miR-16 could differentiate glioma patients from patients of other brain-affecting diseases with a sensitivity of 90 % and a specificity of 100 % [43], and many of the aforementioned studies referred to its potential as a diagnostic biomarker. As such, it will not be a surprise if miR-21, alone or combined with other miRNAs, is included in the diagnostic test array for gliomas, although solid statistical analysis on its potential is still needed.
3. Correlation of miRNA-21 levels with glioma grade and prognosis
3.1. Glioma grade
Several studies have evaluated the association between miRNA-21 levels and glioma grade, and are summarized in Table 3.
Table 3.
Studies on miRNA-21 levels and glioma WHO grade (I, III, III and IV).
| Author, Year | Samples, Grades | MiRNA-21 Levels Results |
|---|---|---|
| Gabriely et al., 2008 | BT*, grades II-IV | Significantly higher in IV (up to ∼40 times higher in IV than II-III, p < 0.05) |
| Conti et al., 2009 | BT, grades II-IV | No difference between grades (compared to NBT**, 8.76 ± 1.25-fold in I-II, 9.39 ± 1.46-fold in III, and 9.18 ± 2.54-fold in IV) |
| Lages et al., 2011 | BT, OGD*** vs. IV | Could differentiate between OGD and IV*4 (9.8 times higher in IV than OGD, p < 0.05) |
| Han et al., 2012 | BT, grades I-IV | Progressive increase, highest in IV (compared to NBT, 2−2.5 higher in I-II, 2.5−4.5 higher in III-IV, p < 0.05) |
| Hermansen et al., 2012 | BT, grades I-IV | Progressive increase, highest in IV [significant correlation with grade, p = 0.027, rs = 0.161, 95 % confidence interval (CI), 0.015–0.301 |
| Yang et al., 2014 | BT, grades II-III vs. IV | Significantly higher in IV (∼2 times higher in IV than I-II, p < 0.0001) |
| Barbano et al., 2014 | BT, grade II vs. III-IV | Significantly higher in III-IV (up to ∼50 times higher compared to NBT, p = 0.008, and compared to II, p = 0.005) |
| Shi et al., 2015 | BT and CSF, grades I-IV | Progressive increase, CSF could differentiate between I-II and III-IV (CSF: up to 1-fold higher in I-II compared to NBT, p < 0.05, up to 2-fold higher in III-IV compared to NBT, p < 0.01) |
| Piwecka et al., 2015*5 | BT, grade III vs. IV | No statistically significant difference between III and IV (statistical details shown only for significantly different miRNAs) |
| Li et al., 2016*5 | BT, grades I-IV | Significant correlation between grade and levels, highest in IV (HR = 2.936, 95 % CI 0.155–5.718, P = 0.039) |
| Yang et al., 2017 | BT, grade II vs. III | Significantly higher in III than II (p < 0.0001) |
*Brain Tissue. **Normal brain tissue. ***Oligodendroglioma *4 Within a panel of miRNAs. *5Meta-analysis.
Gabriely et al. studied miR-21 levels in fresh frozen grade II, III and IV gliomas and normal brain tissue samples via quantitative reverse-transcriptase PCR (qRT-PCR). They found that the levels were low in grade II and most of grade III tumors, while they were significantly higher in GBM samples [7]. Similarly, Han et al. studied 93 human glioma brain tissue samples of all grades, assessing miR-21 levels via in situ hybridization and qRT-PCR. Both methods showed relatively low miR-21 expression in grades I and II, which progressively increased in grades III and IV, reaching their peak in GBM samples [25]. Hermansen et al. also reported similar results, by applying in situ hybridization in 193 glioma tissue samples of various grades, and found increasedmiR-21 levels as the grade got higher, peaking in GBM as well [56]. Yang et al. also reported a significant difference in miR-21 levels, measured with qRT-PCR, between LGG and GBM samples [53], and Shi et al. further reported a correlation between miR-21 levels and tumor grade in 198 glioma samples; both tissue and exosomal CSF levels could effectively differentiate between higher and lower grade gliomas [48]. Similarly, Santangelo et al. found increased serum exosomal miR-21 levels in HGG blood samples when compared to LGG [49].Yang et al. studied miR-21 levels in grade II and III samples, and found that they were significantly higher in grade III than in grade II [57]. In a similar vein, Barbano et al. found a significant difference of miR-21 levels between grade II and grade III + IV glioma samples [20]. Additionally, Lages et al. included miR-21 in an array of 7 miRNAs that could differentiate oligodendrogliomas from the aggressive grade IV glioblastomas [26]. Finally, Li et al. conducted a meta-analysis of the studies available up to April of 2016, and reached the conclusion that miR-21 levels correlated with the WHO grading system of gliomas [58].
On the contrary, Conti et al. used qRT-PCT to assess miRNA-21 levels in grade II to IV astrocytoma samples from 28 patients, and they found them higher in all tumor samples when compared to normal tissue, but with no differences between the various tumor grades [22], a finding replicated by a later study as well [40]. Similarly, Piwecka et al., despite reporting miR-21 as consistently upregulated in glioma tissue samples, could not find a significant difference between grade III and grade IV samples. This finding was replicated in their meta-analysis [28]. Taken together, however, the majority of studies seem to agree that miR-21 levels correlate with tumor grade, with GBM, the most aggressive form, consistently presenting the highest values.
In order to explain why miRNA-21 levels were the highest in GBM samples compared to the rest of the grades, Gabriely et al. proposed that this miRNA is most likely involved in angiogenesis and reorganization of the extracellular matrix, and in tumor proliferation capacity, i.e. the main features that are evaluated in appointing a grade IV to a glioma, and, to this end, they studied this notion further [7]; their findings are discussed in the sections to follow. Hermansen et al., in their in situ hybridization study, localized the expression of miRNA-21 in tumor cells and blood vessels, as no expression was found in adjacent non-malignant parenchyma. In lower grades, the miR-21+ cells were few, both in tumors and blood vessels, while in grade III samples, most cells and their capillary networks were miR-21 + . In GBM, tumor-characteristic formations, such as giant multinucleated cells and glomeruloid vessels, and necrotic areas were also frequently found to be miRNA-21+ [56]. These findings support the notion that the role of miR-21 in glioma pathogenesis is very intricate, and that it probably involves several different pathways pertaining to cancerous proliferation.
3.2. Prognosis
Several studies have reported a link between miRNA-21 levels and prognosis, summarized in Table 4. Lakomy et al. studied surgically-resected brain tissue samples from 38 GBM patients and found miR-21 significantly upregulated in the subset of patients that presented fast disease progression after the operation (<6 months). They reported that the combination of miR-21 and miR-181c could predict the patients whose tumor would have progressed within 6 months with 92 % sensitivity and 81 % specificity [27]. Ilhan-Mutlu et al. reported a decrease in miR-21 plasma levels following tumor resection in all but one patients; this patient presented with neurological symptoms shortly after the blood sample was taken, and MRI scans showed tumor progression after 2 months [41]. These findings indicate how increased levels of this molecule closely reflect tumor progression. Yang et al. also tested tumor samples from patients that survived for either one or two years following the operation. They found higher levels in patients with shorter survival and associated miR-21 with poorer prognosis [53]. Moreover, Zhi et al. studied the levels of miRNA-21 via qRT-PCR in 84 astrocytoma and 40 normal adjacent tissue samples, and they validated their results with another 40 astrocytoma brain tissue samples. They reported that higher miRNA-21 levels inversely associated with patient survival, independent of other clinical and pathological traits [37]. Hermansen et al. further found that after adjusting for clinical parameters such as grade and age, only the levels of miR-21 in tumor cells correlated with poor prognosis and survival in their study [56]. Among a cluster of miRNAs, Wang et al. analyzed 108 glioma and 95 normal brain tissue samples, and found that only 3, miR-21-3p included, reached the significance levels for prognosis association. They divided patients into high-risk and low-risk depending on their miRNA levels, and the high-risk group had significantly worse 1500-day survival rates [51]. Barbano et al. also found a significant inverse correlation between miR-21 levels and overall survival in tissue samples from 32 glioma patients [20]. Finally, in the meta-analysis by Li et al., miR-21 levels were inversely associated with overall survival in glioma and GBM patients. Yang et al. further confirmed that miR-21 levels are associated with worse survival in grade II and III cases as well [57,58].
Table 4.
Studies on miRNA-21 levels and glioma prognosis.
| Author, Year | Samples | Results |
|---|---|---|
| Zhi et al., 2010 | BT* | Inverse association with survival (p = 0.061, mean survival of patients with high miR-21 levels: 52.9 months, with low miR-21 levels: 70.8 months) |
| Lakomy et al., 2011 | BT, grade IV | Significantly higher in patients with faster disease progression (p = 0.0143), could differentiate these patients** (miR-181c and miR-21 as predictors of time to progression within 6 months of diagnosis: 92% sensitivity, 81% specificity, p < 0.0001) |
| Ilhan-Mutlu et al., 2012 | Plasma | High in one patient with recurrence |
| Hermansen et al., 2012 | BT | Inverse association with survival [p = 0.049, hazard ratio (HR) = 1.545, 95 % confidence interval (CI), 1.002–2.381] |
| Teplyuk et al., 2012 | CSF | Higher in active cancer/brain metastases (up tp ∼60 times higher in recurrent glioma), could differentiate glioma from metastases*** |
| Yang et al., 2014 | BT | Higher in survival of 1 year vs. 2 years (∼30 % higher in 1-year survival) |
| Barbano et al., 2014 | BT | Inverse association with survival (Univariable Cox regression: HR of death = 1.26; 95 %CI 1.06–1.48, p = 0.007, multivariable Cox regression: HR = 1.19; 95 %CI 1.01–1.41, p = 0.04) |
| Shi et al., 2015 | CSF | Inverse association with survival (p < 0.05, for grades II-IV), significantly higher in diffuse dissemination cases (p < 0.001) |
| Sathyan et al., 2015 | BT, grade IV | No significant association with survival, except when combined with levels of Sox2; low levels of miRNA-21 and high levels of Sox2 predict longer survival (p = 0.0088) |
| Li et al., 2016*4 | BT | Inverse association with survival (Asians: HR = 2.200, 95 %Cl 1.357–3.042, p < 0.001, non-Asians: HR = 1.293, 95 % Cl 1.113–1.473, p < 0.001) |
| Yang et al., 2017 | BT, grade II-III | Inverse association with survival (p < 0.05 for miR-21-3p and -5p association with short survival, in sample groups divided both by tumor mass and miR-21 expression) |
| Sippl et al., 2019 | BT, grade IV | No association with survival |
| Wang et al., 2019 | BT | Inverse association with survival (p < 0.00001) |
*Brain Tissue. **Alongside miR-181c. ***In a panel of other miRNAs. *4Meta-analysis.
On the contrary, in one recent study on GBM samples, the levels of miRNA-21 were not associated with overall survival [35], something also encountered in the Sathyan et al. study that assessed databases and various glioma-associated cellular and biopsy samples, and reported that miRNA-21 levels were not significantly associated with survival; however, when low miRNA-21 was combined with high levels of Sox2, those patients did present longer survival [50].
Concerning CSF values, the study by Shi et al. linked higher CSF exosomal miRNA-21 levels to poorer survival and higher rates of diffuse intracranial and spinal dissemination. However, these findings did not correspond to those obtained from serum samples, where no association was found [48]. Additionally, Teplyuk et al. included miR-21 in an array of 7 miRNA molecules whose CSF levels could very accurately differentiate GBM patients from patients with brain metastases of other cancers. Of note, they additionally reported that miR-21 levels were significantly lower in patients in remission, as opposed to patients with active GBM or brain metastases. This finding further enhances the notion that miR-21 reflects tumor activity and progression [39].
Collectively, most of the aforementioned studies seem to agree that higher miR-21 levels reflect a worse outcome for glioma patients, since they have been associated with more advanced tumor grades, and therefore this miRNA could be a very useful prognostic biomarker. The assessment of miR-21 levels in resected tumors could provide further insight on the aggressiveness of the tumor, and guide how invasive the following treatment should be. Although more research on the accuracy of its measurement in CSF and other biological samples is urgently needed, a pre-operative prognostic assessment could be performed in the future, in order to aid clinicians in therapeutic decisions, early enough and even before surgery for glioma is planned.
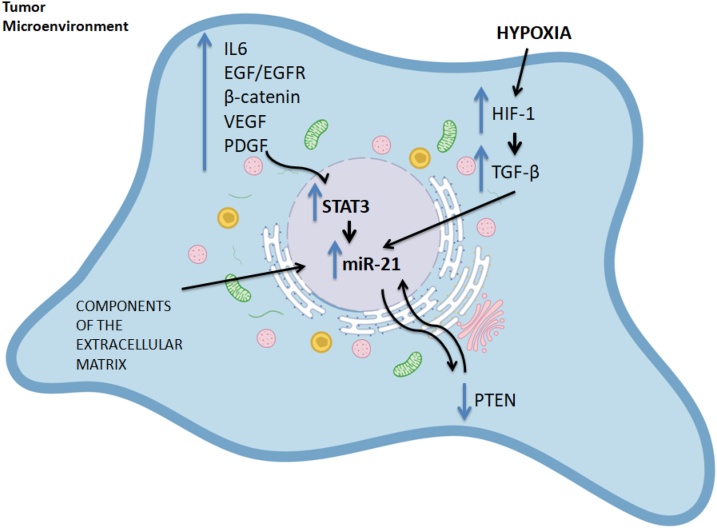
4. MiRNA-21 upregulation factors
MiRNA-21 increase in glioma is now established. Nevertheless, the mechanisms behind it remain poorly understood, as no single causative factor can be identified. Few studies have hypothesized how this miRNA is found consistently upregulated. The possible factors implicated in miRNA-21 upregulation in glioma are schematically represented in Fig. 1.
Fig. 1.
Factors leading to the Upregulation of miRNA-21 in Glioma.
MiRNA-21 is independently transcribed from its gene in chromosome 17q23, which contains two highly conserved STAT3 binding sites [59]. STAT3, a part of the Signal Transducer and Activator of Transcription (STAT) family of transcription factors, has been shown to play a very important role in gliomatumorigenesis, by promoting tumor growth, angiogenesis, and invasion [60], and is considered a crucial inductor of mesenchymal transformation in gliomas [61].Additionally, the persistent activation of STAT3, described in glioma, is thought to exert a tolerogenic action on the host immune system, sustaining cancer growth [62]. MiR-21 can also be activated by a wide variety of other growth factor receptors and cytokines, such as EGFR, IL-6R, JAK and other kinases [63]. In greater detail, it is known that the IL-6/STAT3 signaling axis holds great importance in glioma [64] and that EGFR is overexpressed in GBM [65].This could explain the upregulation of miR-21 via the subsequent induction of STAT3. An interesting study by Ren et al. showed that STAT3 and miRNA-21 closely interact and that there is a regulatory loop between STAT3 and miR-21. The researchers reported lower levels of STAT3 after inhibiting miRNA-21 and treating cells with taxol. Such a finding suggests that miRNA-21 provides some regulatory feedback to STAT3. They also noted that EGFR levels decreased as well, upon taxol treatment [66].
The Wnt/β-catenin pathway is known to coordinate cell differentiation and proliferation, targeting the transcription of many genes such as STAT3, cyclin D and c-Myc [25,67]. Additionally, the prolonged activation of the β-catenin pathway has been reported in glioma [68]. Han et al. further provided proof of STAT3’s effect on miRNA-21 levels, by showing that the β-catenin pathway regulates miR-21 in a STAT3-dependent way as well. The β-catenin elevation was accompanied by an increase in miR-21 levels, while its knockdown led to a decrease in miR-21 levels. When STAT3 was inhibited, however,β-catenin could not induce miR-21 [25], suggesting that STAT3 is imperative for miR-21 expression. Furthermore, Zhang et al. showed that EGFR regulated miR-21 via the β-catenin pathway, and they described a feedback loop between them [69], which will be further described in the section to follow.
Hypoxia, a common trait in cancerous microenvironments, is frequently encountered in gliomas [70]. Studies have also shown that necrotic foci around gliomas are severely hypoxic [71], in turn activating hypoxia-inducible factors 1 and 2 (Hif-1/2). This promotes angiogenesis [72]. Hif-1 has been shown to be extensively activated in glioma tumorigenesis, resulting in the activation of other proliferative factors, such as VEGF/VEGFR and TGF-α/β [70].The role of hypoxia in glioma is crucial, as higher incidence of brain malignancy has been noted in the longitudinal follow-up of ischemic stroke patients, while interestingly, histological staining for Hif-1 was only found positive in glioma patients that had suffered a stroke in the past [73].Hypoxia can also induce miR-21 [74] and Hif-1 has also been shown to regulate miRNA-21 in a feedback loop [75]. Therefore, this common glioma trait, hypoxia, may be another culprit behind the upregulation of miRNA-21. Moreover, TGF-β is also implicated in miR-21 regulation, as Davis et al. reported that miRNA-21 levels increased upon TGF-βstimulation [76], and TGF-β aberrant signaling is a trait of glioma [77]. VEGF is also elevated in patients with glioma, such as in the study assessing on urinary metabolites by Smith et al. [78]. Thus both these factors may also contribute to the increase of miRNA-21 in glioma.
The study of Kwak et al. showed that a glycosaminoglycan of the extracellular matrix, hyaluronan, also induces miR-21 in glioma cells but not in normal astrocytes, which further facilitates glioma invasion via various pathways that will be discussed in the section to follow. The authors also showed that miR-21 expression was upregulated by growth factors of the cancer micro-environment, such as PDGF, EGF and bFGF [79], which are known to be abundant and important in gliomas [[80], [81], [82]].Moreover, PTEN, a known tumor suppressor that is frequently suppressed in gliomas, had a negative impact on miR-21 levels [79]. PTEN interacts with miR-21 in a perplexed way (see below).
Taken together, a plethora of factors pertaining to gliomas, such as hypoxia and increased levels of cytokines and growth factors/growth factor receptors, appear to affect the levels of miRNA-21, which in turn facilitates tumor growth and invasion through various mechanisms.
5. MiRNA-21-induced glioma proliferation
MiRNA-21 seems to modulate a wide variety of cellular processes and studies have uncovered many of its targets, via the regulation of which this miRNA facilitates glioma growth. These studies have been performed on glioma cellular lines (in vitro)and some have replicated their results in vivo, by applying a xenograft model, mainly in mice. Some researchers have also used patient resection tissue samples to perform additional metrics. The cellular lines used and details of the studies are described in Table 5.
Table 5.
Studies on miRNA-21 in correlation with glioma proliferation.
| Author, Year | Cellular Line | MiRNA-21 Target/Result |
|---|---|---|
| Chan et al., 2005 | A172, U87, LN229, LN428, U373, LN308 | Increased apoptotic rates by miR-21 inhibition |
| Gabriely et al., 2008 | A172, U87,LN229 | RECK, TIMP3/Raise in MMP-2/-9 |
| Chen et al., 2008 | T98 G, A172, U87, U251 | PDCD4/Suppressed apoptosis |
| Papagiannakopoulos et al., 2008 | U251, U87, HeLa | Numerous targets/Suppression of mitochondrial apoptosis, TGF-βand p53 pathways |
| Li et al., 2009 | U373MG | LLRFIP1/NF-κBenhancement |
| Zhou et al., 2010 | U251, TJ866,TJ905, TJ899, A172 | TIMP3, PTEN/Suppressed apoptosis |
| Zhou et al., 2010B | U251, LN229 | PTEN/EGFR enhancement, suppressed apoptosis |
| Kwak et al., 2011 | U373MG U87MG, LN428 | Spry2/Activation of Ras/MAPK |
| Gaur et al., 2011 | SNB19, U251, U87,SF767 | PDCD4/Suppressed apoptosis |
| Li et al., 2011 | U251 | Cdc25/Suppressed apoptosis |
| Han et al., 2012 | U251, LN229, SNB19 | RECK/Decreased invasiveness |
| Quintavalle et al., 2012 | TB10, LN229, T98 G, LN18 | Tap63/P53 suppression |
| Zhang et al., 2014 | LN229, U87, U251 | VHL, PPARa/ EGFR enhancement |
| Yang et al., 2014 | U87, MT330, SJ-G2 | IGFBP3/Enhanced proliferation |
| Shi et al., 2015* | U251 | PTEN, RECK, PDCD4/Validation of earlier studies, EGFR enhancement |
| Sathyan et al., 2015 | GSC** lines | Sox2/suppresses expression |
| Luo et al., 2017 | U87, A172, T98,U343 | Sox2 (incr.)/β-catenin enhancement |
| Abels et al., 2019 | GL261 | Btg2, PTEN/Microglia reprogramming and proliferation |
| Seo et al., 2019 | U87 | Upregulation of PTEN following miR-21 inhibition |
*Used CSF exosomes.*Glioblastoma stem-like cells.
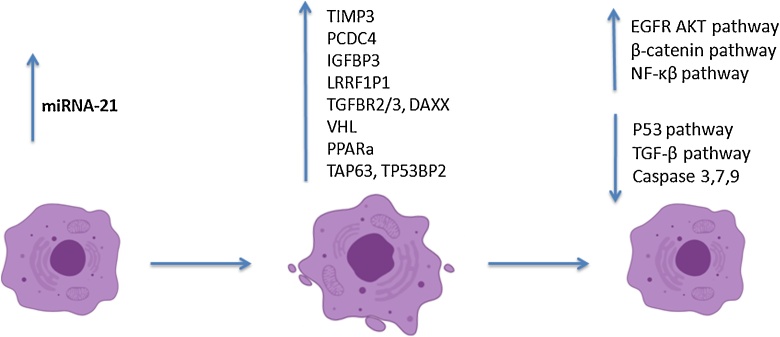
As it is well known, apoptosis can be induced via three main pathways: a) the extrinsic pathway, which makes use of death receptors (such as TNFR and FAS) and caspase-8, b) the intrinsic/mitochondrial pathway, which activates caspase-9 upon cellular stress, and c) the granzyme B pathway, which requires the delivery of the granzyme B protease to sensitive cells, which have been chosen to undergo apoptosis. All three pathways intersect at the apoptotic phase that requires the activation of caspase-3 and/or -7 [83,84]. This knowledge will help us interpret some of the findings reported in various studies, and an overview of how apoptosis can be inhibited via miR-21 can be seen in Fig. 2.
Fig. 2.
Schematic Representation of MiRNA-21-induced Apoptosis Inhibition Mechanisms.
Gabriely et al. observed that miRNA-21 regulates several angiogenic and apoptotic genes, involved in processes that are crucial for glioma [7]. Additionally, among the genes downregulated by miR-21 were the RECK and TIMP3(tissue inhibitor of metalloproteinase-3) genes, which are both known suppressors of cancerous processes and important inhibitors of matrix metalloproteinases (MMPs).The researchers found lower mRNA expression of those two genes in glioma samples with higher tumor grades, which had higher miR-21 levels. Upon knocking-down miR-21, they found an elevation in RECK and TIMP3,confirming that they are targeted by miR-21, and a subsequent reduction in MMP-2 activity and cellular motility; the results were replicated in an in vivo model too [7].MMPs break down components of the extracellular matrix, participating in glioma cell motility and tissue invasion [85,86], and triggering angiogenesis [87];they have further been found overexpressed in glioma [78]. MMP-2 and MMP-9 in particular have been associated with tumor invasiveness [88]. Smith et al. studied urinary levels of MMPs and found that both MMP-2 and MMP-9 were elevated in samples of glioma patients compared to controls; the levels dropped following the surgical resection of the tumor [78]. Finally, Kwak et al. evaluated the association between miR-21 and MMP-9 and showed a decrease in hyaluronan-induced MMP-9 levels and invasiveness when miR-21 was suppressed, as well as a respective increase when miR-21 was overexpressed [79].
The disruption of the extracellular matrix plays a key part in oncogenesis [89], and MMPs, as important peptidases with the potential of remodeling the extracellular matrix in favor of tumorigenic processes, are a common “weapon” in the arsenal of cancers. For this reason, they are physiologically under strict regulation [90], and when carcinogenic processes shift their balance, tumor growth is favored. This dysregulation is met in several malignancies, and miR-21 has also emerged in the relevant literature [91,92]. For instance, MMPs are induced in the setting of melanoma, and the overexpression of MMP-9 in this malignancy has also been the target of therapeutic efforts, with MMP-9 inhibitors being tried in clinical trials [93]. However, the therapeutic results of MMP inhibitors in pancreatic cancer [94], ovarian cancer [95], and non-small-cell lung cancer [96] have been rather disappointing. Regardless, MMPs are not obsolete in terms of cancer research, as they considerably facilitate tumor growth and invasion, and MMP inhibitors may be eventually considered an additive to many preexistent treatments.
Moving on, glioma cells communicate and influence their surrounding environment via EVs. These carry a specific RNA cargo, which gets transferred from the donor to the recipient cells. Because of their lipid membranes, they are relatively protected from degradation and reach adjacent and non-adjacent cells [97]. Via EVs, glioma cells affect nearby non-malignant CNS cells, such as microglia and astrocytes, promoting the release of various cytokines and agents that can stimulate glioma proliferation [98]. Abels et al., in an in vivo model, described the transfer of functional miR-21 from glioma cells to nearby miR-21-null microglia via spontaneous EV release, leading to miR-21-target downregulation. In particular, the inhibition of Btg2 by miR-21 led to increased microglia proliferation [1]. Btg2 belongs to a family of genes involved in cellular proliferation; it negatively controls proliferation by suppressing cyclin D1 [99]. Consequently, by suppressing this inhibitor, miR-21 promotes the reprogramming and the multiplication of microglia, which in turn create a microenvironment friendly for glioma cells.
Shi et al. (2015) validated the results of Gabriely et al. by suppressing miR-21 expression in cell cultures, and found an increase in RECK [48]. Similar results were reported by Han et al., where miR-21 knockdown led to a rise in RECK levels and a subsequent decrease in cellular invasiveness; this translated in decreased tumor growth in a xenograft murine model [25]. The main focus of this study was the β-catenin pathway. As already mentioned above, the researchers showed that β-catenin regulated miR-21 levels via STAT3, and that the entire pathway of β-catenin/STAT3/miR-21, via RECK’s involvement as a downstream target of miR-21, mediates glioma invasion [25]; these findings were replicated in other studies as well, such as the one by Pu et al. where knockdown of Wnt and β-catenin in glioma cells reduced motility and invasion, and induced apoptosis [100]. Luo et al. showed that miRNA-21 also regulated β-catenin, via overexpression of the Sox2 protein. In greater detail, a rise in miR-21 led to increased Sox2 and cellular invasion and migration; however, Sox2 inhibition induced miR-21-related cellular migration and invasion suppression. Additionally, inhibition of Sox2 or miR-21 significantly decreased β-catenin levels; this decrease in β-catenin led to diminished invasiveness. When miR-21 and Sox2 were suppressed, a β-catenin agonist compound could restore the invasion potential of glioma cells, while a β-catenin signaling inhibitor significantly hindered the miR-21/Sox2-induced invasion potential, showing that the miR-21/Sox2/β-catenin axis promotes glioma proliferation [101]. Sox2 is an important transcription factor, whose overexpression has been reported in glioma [102], and its role in glioma growth has been established; Sox2 suppression in glioblastoma cells led to a loss of tumorigenicity [103]. Moreover, Sox2 has been shown to be hypoxia-induced [104], and since miR-21 is also hypoxia-induced, it is possible that miR-21, following a hypoxia triggering signal, leads to this increase in Sox2, which subsequently activates the β-catenin pathway and facilitates tumor invasion.
Chan et al. first described the considerable upregulation of miR-21 in all glioma cell lines under study, and also reported that knockdown of miR-21 led to caspase activation and increased apoptosis. In greater detail, the activity of caspase-3 and -7, the key mediators of apoptosis, was found significantly increased in cells transfected with an anti-miR-21 anti-sense oligonucleotide (ASO). This was accompanied by a significant rise in apoptotic nuclei and fragmented DNA [15]. Similar findings were reported in the study by Zhou et al., where ASO downregulation of miR-21 led to increased caspase-3 and caspase-9 activities, which in turn led to mitochondrial apoptosis induction in glioma cells; this was in vivo reflected in a marked difference in tumor size between anti-miR-21 ASO transfected and non-transfected murine xenograft subjects. The authors also described an increase in TIMP3, confirming that it is regulated by miR-21 [105].TIMP3,described in the study of Gabriely et al. as a target of miRNA-21 [7], promotes apoptosis in glioma cells via caspase activation and TNF-a-converting enzyme inhibition, which leads to a stabilization of TNFa receptors on cell surfaces [106,107]. This suggests that miR-21 promotes glioma survival by inhibiting apoptosis via all of the apoptotic pathways, namely the mitochondrial, intrinsic pathway (caspase-9) and extrinsic (TIMP3-TNFR interaction).
It also worth raising the issue of PTEN. PTEN is a tumor suppressor gene whose suppression, which could occur due to a deletion or mutation, is a frequent observation in glioma [108]. PTEN regulates the EGFR/Akt signaling pathway; a consequent upregulation of this pathway, via PTEN suppression, has been described in the setting of glioma [109], although the role of miR-21 and PTEN’s interaction in glioma is still unclear. Abels et al. described a decrease in PTEN in microglia transfected with glioma-derived miR-21-carrying EVs [1]. Belter et al. reported an increase in PTEN when miR-21 was depleted with specifically designed enzymes [110], while Seo et al. inhibited miR-21 in the transplanted tumors of murine subjects and found an upregulation of PTEN, overall confirming that PTEN is in fact targeted by miR-21 [111].Similarly, Shi et al. showed an increase in PTEN levels following miR-21 inhibition in a glioma cellular culture [48], a finding first reported by Zhou et al.. These authors described a rise in PTEN when they inhibited miR-21 with an ASO, and a decrease in EGFR (alongside Bcl-2, Ki67, Cyclin-D1 and AKT-2). However, they found an increase in apoptosis and a tumor growth halt in their in vivo study on cellular lines with both wild-type and mutant PTEN, showing that the effect of miR-21on tumor survival is most likely PTEN-independent [52]. The researchers had initially hypothesized that since a large proportion of gliomas are PTEN-deficient and PTEN can even predict the prognosis of patients, the glioma line carrying a wild-type PTEN, instead of a deficient or deleted PTEN, would respond better to ASO treatment. Nevertheless, this hypothesis was rejected because the apoptotic rates were similar in PTEN wild-type and deficient lines [52]. These results suggest that miR-21 acts in ways surpassing the tumor suppressor ability of PTEN. Furthermore, the aforementioned study by Chan et al. involved a cellular line that does not express PTEN, and still showed growth restriction when miR-21 was inhibited [15]. Ren et al. conducted an interesting study on chemotherapy response, which will be discussed in the relevant section. However, it is worth mentioning here that miR-21 inhibition led to enhanced apoptosis and responsiveness to taxol in cellular lines either PTEN-wild type or PTEN-deficient. These findings showed that miR-21 interacts with the EGFR pathway in a PTEN-independent way [66] and suggest that the interplay between PTEN and miR-21 has not been fully clarified yet.
Zhang et al. proposed a different mechanism via which miRNA-21 may regulate EGFR/AKT signaling, by examining two different miR-21 targets, VHL (von Hippel-Lindau) and PPARa (peroxisome-proliferator-activated receptor a) [69].They initially showed the importance of EGF by suppressing miR-21 with an ASO and subsequently administrating recombinant human EGF in glioma cells; the administration could partly reverse the inhibition of apoptosis and invasion that followed the miR-21 inhibition. The miR-21 suppression also led to increased levels of VHL and PPARa, validating them as targets [69]. VHL reduces β-catenin [112], and β-catenin regulates the EGFR/AKT pathway, which prinicipally contributes to glioma progression [113]. MiRNA-21 inhibition or VHL enhancement produced similar results, namely β-catenin reduction and EGFR/AKT signaling suppression. Therefore, the researchers concluded that miR-21 regulates EGFR/AKT with the targeting of VHL. Furthermore, the EGFR gene is targeted by the AP-1 complex [114], while PPARa regulates AP-1, and is targeted by miR-21 [115]. These facts suggest that miR-21 also regulates the EGFR axis via PPARa/AP-1 modification as well. Conclusively, it was shown that miR-21 suppresses VHL and PPARa, thus cancelling their inhibitory effects on β-catenin and AP-1 activation, and leading to an enhancement of the EGFR pathway, and simultaneously inducing a further rise in miR-21 levels, in a positive feedback circuit [69].
Moving on, PTEN, discussed above, and miR-21 might be implicated in glioma invasion via a different pathway previously mentioned, namely MMPs. Park et al. used hyaluronan to enhance invasiveness in PTEN wild-type and deficient glioma cellular lines. In deficient lines, hyaluronan led to an increase in MMP-9 and invasion, while in PTEN-wild type, hyaluronan protected the cells against MMP-9 overexpression [116]. The same research group later showed that wild type PTEN could suppress hyaluronan-induced miR-21 upregulation, while PTEN knockdown gave way to miR-21 potentiation, showing that there is a negative feedback loop between PTEN and miR-21 [79]. Their results suggest that miR-21 is crucial in hyaluronan-enhanced glioma invasion, via MMP-9, in PTEN-deficient cells [79], showing that PTEN interacts with miR-21 in several ways.
Another tumor suppressor gene that is targeted by miR-21 is PDCD4. PDCD4 is a pro-apoptotic molecule, with a known role in several malignancies [117,118]. Chen et al. found an inverse correlation of miR-21 levels with PDCD4 in four glioma cellular lines. Overexpression of miR-21 further suppressed PDCD4-induced apoptosis, while its inhibition restored PDCD4 levels [119]. These results were replicated in the study of Gaur et al., where miR-21 knockdown led to PDCD4 increase, which in turn led to increased apoptosis in glioma cell cultures. An in vivo inhibition of miR-21 or an overexpression of PDCD4 in mice led to tumors of smaller size (up to 90 % smaller when compared to tumor-controls), or led to no tumorigenicity potential. When both miR-21 and PDCD4 were inhibited, the tumors regained their growth potential, highlighting the role of PDCD4 inhibition, as a downstream target of miR-21, in glioma proliferation [23]. Shi et al. published similar results, validating PCDC4 as a miR-21 target in gliomas, and showed higher apoptotic rates following miR-21 inhibition [48]. Finally, Abels et al., in their study on the EV-mediated miRNA transfer from glioma to microglia cells, showed that PDCD4 levels significantly dropped in microglia following the transfer of miR-21, but the downregulation was incomplete, an observation they attributed to the relatively limited amount of miR-21 transferred within the EV [1].
Yang et al. showed that miR-21 also targets IGFBP3 (insulin-like growth factor -binding protein-3), which serves as a tumor suppressor in glioma cells [53]. More specifically, miR-21 inhibition or IGFBP3 overexpression led to decreased proliferation in vitro and smaller tumors in vivo, while RNA analysis in samples from GBM patients showed an inverse association between IGFBP3 and miR-21 levels. The researchers also reported that increased levels of IGFBP3 in GBM samples were significantly associated with better prognosis, expressed as a survival beyond 2 years [53], an observation that has emerged in other studies on glioma prognosis as well [120].The researchers further highlighted the importance of IGFBP3 inhibition in glioma progress by simultaneously knocking down miR-21 and IGFBP3, and found that the cells’ tumorigenic potential was restored [53]. IGFBP3 is a member of the IGFBP family that binds to IGFs, which have been shown to promote tumor growth [121].Overexpression of IGF-1 and its receptor IGFR1 has been reported in glioblastoma, where it mediates therapy resistance and leads to worse patient survival rates [122,123]. IGFBP3 is the key binding protein of IGF-1, so its tumor-suppressive ability derives from regulating IGF-1 bioavailability and blocking its growth-promoting action [124].
Several other possible miRNA-21 targets have been previously identified. Li et al. showed that miR-21 targets the LRRFIP1 [leucine rich repeat (in FLII) interacting protein 1 gene], which encodes TRIP[tumor necrosis factor receptor (TNFR)-associated factor (TRAF) interacting protein], an inhibitor of the NF-κB pathway [125]. NF-κBsignaling mediates cellular activation and protection against apoptosis [126]. Therefore, miR-21suppresses one of its inhibitors and gives way to its overactivation, promoting glioma proliferation. In addition, Li et al. studied the resistance to radiotherapy, which will be discussed later, and found an inverse correlation between miR-21 levels and Cdc25A (cell division cycle 25 A). Cdc25A is an isoform of the Cdc25 protein family [127],which is thought to be crucial for p53-independent cell-cycle arrest, a regulator of a cell-cycle checkpoint following DNA damage induced by factors such as radiation or oxidative stress [128]. Therefore, miR-21 may suppress apoptosis via this molecule as well. Finally, Sathyan et al. identified Sox2, a protein necessary for “stemness” in neural cells [129], as another miR-21 target [50]. The researchers studied the miRNA-21-Sox2 axis, finding that it is implicated in neuronal development and neuronal stem cells. They also reported distinct glioma phenotypes based on the miR-21/Sox2 ratios, and claimed that a classification into high miR-21/low Sox2 and low miR-21/high Sox2 better reflects patient prognosis, with the former correlating to worse survival [50].
The aforementioned Cdc25A may act independently from the p53 pathway, but miRNA-21 regulates factors involved in this pathway as well. Papagiannakopoulos et al. reported the miR-21 targeting of p53, TGF-β and mitochondrial apoptosis components, such as p53 homologue, p63, JMY, TOPORS, TP53BP2, DAXX, HNRPK, TAp63, TGFBR2/3, CASP3 and APAF1. In this study, miR-21 knockdown led to the reactivation of these biological pathways, a consequent increase of the repressed genes and proteins, and a rise in apoptosis and cell-cycle arrest [130]. Additionally, Quintavalle et al. also showed that miR-21 targeted TAp63 of the p53 pathway, an important transcription factor that regulates several apoptosis-related genes [131]. The p53 pathway is known to be suppressed in the setting of glioma [113], a molecular process that could be explained via the action of miR-21, which, per the aforementioned studies, downregulates many p53 activating cofactors and homologues that aid in the expression of proapoptotic genes [132]. Similarly, TGF-β (transforming growth factor-β) is the cornerstone cytokine of growth suppression that can also lead to apoptosis [133]. Glioblastomas are particularly resistant to the activity of TGF-β, possibly via the action of miR-21, which downregulates some of its key factors, namely the TGF-β receptors, TGFBR2 and TGFBR3, and the apoptotic inducer DAXX, which is central to the pathway [130,134]. Finally, the miR-21-induced suppression of components such as caspase-3 and APAF1 (cytosolic apoptotic peptidase activating factor 1) leads to diminished cytochrome c release and mitochondrial apoptosis, which is a mechanism very frequently deregulated in malignancies [130,135]. TAp63, a target of miR-21, also regulates the APAF1 expression [136], besides the p53 pathway genes. Therefore, miR-21 leads to the deregulation of these pathways in a variety of ways and therefore significantly promotes glioma proliferation.
Another pathway that seems to be involved in miR-21-assisted glioma progression is the Ras/MAPK signaling, which is often abnormally activated in gliomas [137]. Kwak et al. showed that miR-21 targets Spry2, a negative feedback regulator of Ras, and consequently amplifies Ras/MAPK signaling [79]. In this study, Spry2 levels were inversely correlated with miR-21 levels, and significantly decreased in glioma samples of grades II to IV, but not in non-invasive grade I samples or normal tissues. Spry2 depletion cancelled its inhibitory action on Ras/MAPK signaling, which became more pronounced and led to increased cell invasion. Cell invasion could be hindered by miR-21inhibition, and overexpression of Spry2 could protect against growth-factor-induced cellular invasion [79]. The RAS genes are considered oncogenes, and RAS also regulates several other biological pathways involved in processes such as cell proliferation and tumorigenesis [137]. Therefore, miR-21 may promote glioma growth via Spry2 inhibition and the subsequent overactivation of Ras/MAPK.
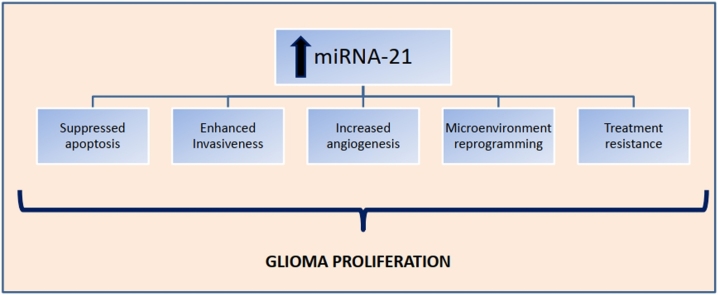
Conclusively, miR-21 is a key player in glioma processes. It suppresses apoptosis, promotes infiltration and invasion in surrounding tissues, by targeting several genes, proteins and biological pathways. A schematic overview of the mechanisms involved can be found in Fig. 2.
6. MiRNA-21-mediated treatment resistance
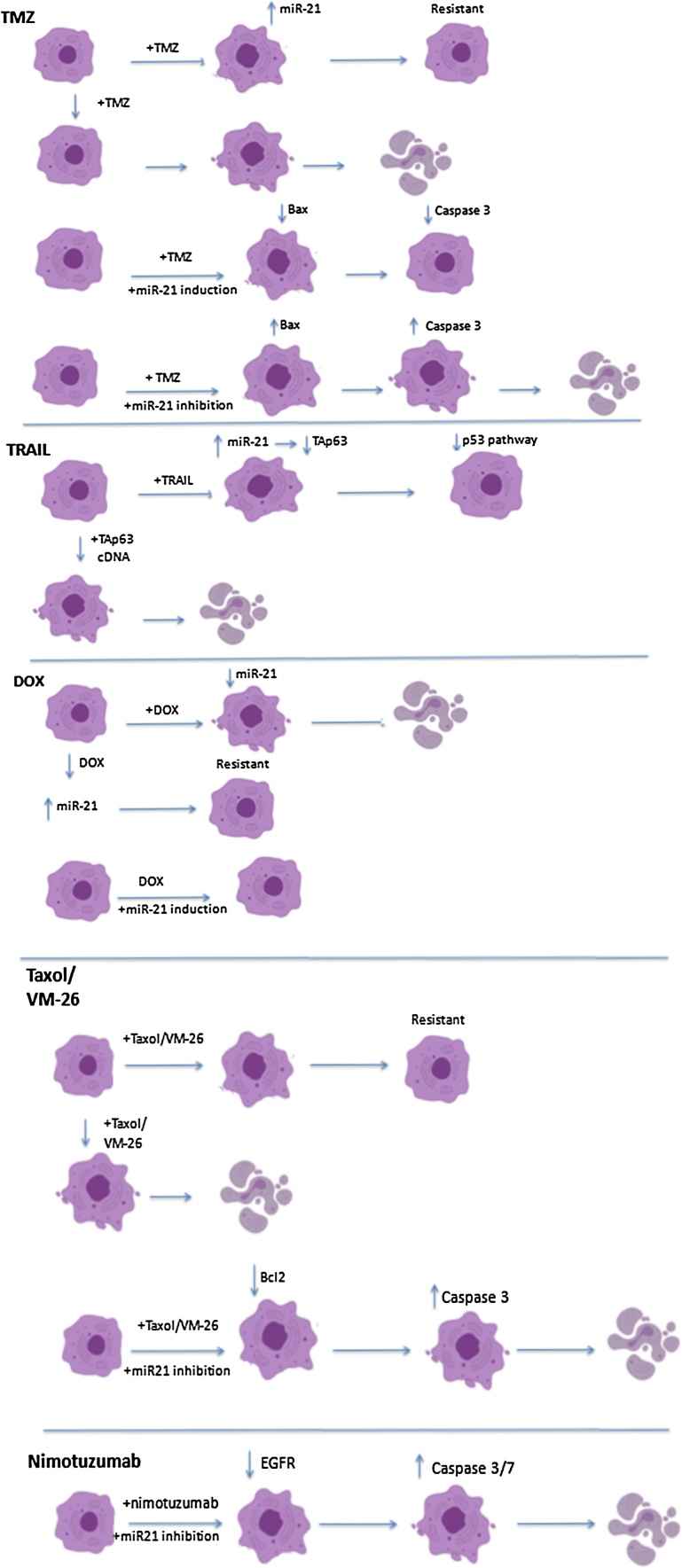
MiRNA-21 not only helps gliomas grow and expand, but also helps them survive against treatments; it has been shown that miRNA-21 is crucially involved in mechanisms underlying therapy resistance. In this section we describe the studies that highlight miR-21’s implication and the possible underlying mechanisms, and a schematic overview of Fig. 3.
Fig. 3.
MiRNA-21-induced Treatment Resistance.
6.1. Chemotherapy resistance
The standard treatment for GBM includes maximal safe surgical resection, concomitant TMZ administration and external beam radiation [138]; as such, several studies have focused on resistance to temozolomide (TMZ). These in vitro studies on treatment resistance used glioma cellular lines and are summarized in Table 6.
Table 6.
Studies on the effects of cell lines’ exposure to a variety of agents.
| Author, Year | Cell. Line | Agent | Results |
|---|---|---|---|
| Shi et al., 2010 | U87MG | TMZ* | MiR-21 induction led to resistance, lower Bax/Bcl2 ratio and caspase-3 activity |
| Zhang et al., 2012 | U251MG | TMZ | MiR-21 inhibition led to higher Bax and caspase-3 activity, and lower Bcl2 |
| Wong et al., 2012 | D54MG | TMZ | Chronic exposure developed resistant cells with higher miR-21 levels/ MiR-21 inhibition and TMZ in resistant cells resulted in higher apoptotic rates |
| Rodrigues et al., 2019 | U343MG | TMZ/ IR** |
Increase in miR-21 levels in neurosphere cells upon exposure to TMZ and IR |
| Li et al., 2009 | U373MG | VM-26*** | Dose-dependent reduced survival with miR-21 inhibition |
| Quintavalle et al., 2012 | T98 G, LN18 | TRAIL | Significant increase in miR-21 in resistant cells |
| Giunti et al., 2015 | A172, T98GU87MG | DOX*4 | MiR-21 inhibition led to increased apoptotic rates upon DOX treatment in resistant cells |
| Papagiannakopoulos et al. 2008 | U251, U87 | DOX | Cells with overexpressing miR-21 were resistant towards DOX |
| Ren et al., 2010 | U251, LN229 | Taxol | MiR-21 inhibition led to increased apoptotic rates upon taxol treatment independent of PTEN status |
| Zhang et al., 2014 | U87 | Nimotuzumab | MiR-21 inhibition led to enhanced nimotuzumab effectiveness, in vitro and in vivo |
| Li et al., 2011 | U251 | IR | IR led to increased miR-21 expression, miR-21 inhibition combined with IR led to increased apoptosis |
| Chaudhry et al., 2010 | M059 J, M059K | IR | Increased miR-21 levels upon IR exposure in M059K |
| Gwak et al., 2012 | U87, U373, LN428, LN18 | IR | IR led to increased miR-21 expression, miR-21 inhibition sensitized PTEN-deficient cells to IR |
| Seo et al., 2019 | U87 | NPs*5 with anti-miR-21 agents | 30−40% reduced cell viability and increased sensitivity to TMZ |
*Temozolomide. **Ionizing Radiation. ***Teniposide. *4Doxorubicin. *5Nanoparticles.
Shi et al. exposed glioma cells to TMZ and found that an induction of miR-21 before treatment administration significantly decreased the apoptotic rates induced by the agent (from 53 % in TMZ only, to 39 % in TMZ and pre-induction of miR-21). The researchers found that miR-21 led to a decrease in the pro-apoptotic protein Bax and an increase in the anti-apoptotic Bcl2, shifting the Bax/Bcl2 ratio and decreasing the activity of caspase-3 (more than 30 %), thus enhancing cancer survival [33].Similar results were reported by Zhang et al., in glioma stem cells of a different line. Following TMZ treatment or miR-21 inhibition, the stem cells showed no signs of apoptosis; however, when the two were combined, apoptosis was significantly enhanced. Additionally, it was reported that pretreatment with a miR-21 inhibitor prior to TMZ administration, led to decreased Bcl2 and increased Bax and caspase-3, compared to TMZ administration alone [139]. Bax and Bcl2 are known to regulate apoptosis in glioma [140] and the bcl-2 family plays a major role in treatment resistance [141]; a lower Bax/Bcl2 ratio has long been described in glioma patients as well [142]. Caspase-3 is a downstream molecule of the Bax/Bcl2 apoptotic pathway [143]. Therefore, its decreased activation was to be expected upon the suppression of apoptosis via miR-21 overexpression.
Wong et al. chronically exposed a GBM cellular line to TMZ, in order to develop a resistant subclone; these resistant cells presented significantly higher levels of miR-21 [144], showing that this molecule is possibly overexpressed when the cells are exposed to treatment, since it harbors anti-apoptotic abilities. The researchers also reported that miR-21 inhibition alone led to higher apoptotic levels; however, inhibition and subsequent TMZ treatment resulted in an apoptotic rate of 53 %, compared to 10.8 % in cells without miR-21 inhibition. The authors proposed that miR-21 levels could eventually be used as a marker of treatment resistance [144]. Rodrigues et al. studied miR-21 levels in neurosphere (cells that possess remarkable regenerative and differentiating ability) and adjacent cells of a glioma cellular line following TMZ administration and ionizing radiation (IR) exposure. They reported that immediately after TMZ administration, miR-21 levels were significantly higher in the adjacent cells,compared to the neurosphere cells. On the contrary, when they compared the levels2 days after TMZ and IR administration, neurospherecells had significantly higher miR-21 levels [145]. The decrease in miR-21, after the treatment, in the surrounding cells could be associated with treatment effectiveness and the process of apoptosis commences, while the increase noted in the neurosphere cells could represent a compensation mechanism activated by the treatment, in order to prevent apoptosis. Finally, Seo et al. presented the efficacy of nanoparticles in delivering anti-miR-21 agents in gliomas of murine subjects. In their study, this injection led to a decrease in cell viability, while it increased tumor sensitivity against TMZ [111].
Another interesting subject in terms of glioma treatment and resistance, is TRAIL. TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) represents an attempt to reactivate the TNF pathway and induce apoptosis in malignant cells, without affecting normal parenchyma [146]. Methods to enhance its effectiveness are being studied in glioma as well [147], and will be discussed in the section to follow; however, some cancers have shown resistance to its action. Quintavalle et al. studied TRAIL-resistant and TRAIL-sensitive glioma cells and found markedly raised miR-21 levels in the resistant cells [131]. As previously mentioned, the researchers identified TAp63 as a miR-21 target and insinuated that there is a causative link between the downregulation of TAp63 and the p53 pathway, and resistance to TRAIL. When they transfected cells with miR-21 and TAp63 cDNA, the cells were rendered sensitive to TRAIL again, and this finding strengthened the above hypothesis [131].
Li et al. studied resistance to VM-26 (teniposide), a topoisomerase II inhibitor, and they found that pre-treatment with a miR-21-ASO led to increased treatment effectiveness; therefore, miR-21 contributes to chemoresistance against this agent [125]. Teniposide has yielded mediocre results in glioma patients, since only 50 % of the patients seem to profit from it as a first-line agent,per some earlier reports [148]. It seems to act synergistically with other chemotherapeutic agents [125], though more recent reports do not seem to endorse its co-administration due to toxicity [149].Additionally, researchers pinpointed LRRFIP1, an inhibitor of the NF-κBpathway, as a miR-21 target [125]. The overactivation of this biological pathway has been described in many malignancy settings and has been further implicated in chemoresistance [150]. Therefore miR-21 may mediate resistance to treatment in gliomas via suppressing LRRFIP1 and potentiating this biological pathway.
Giunti et al. studied the effect of doxorubicin (DOX) in three glioma lines, pinpointing one of them as resistant. They reported a decrease in miR-21 levels following DOX treatment in the sensitive cells. In order to validate the involvement of miR-21, the researchers transfected the resistant cells with a miR-21 inhibitor and found increased apoptotic rates after DOX treatment. More specifically, concomitant miR-21 inhibition led to a 23 % rise in apoptosis when compared to DOX treatment alone, while very few apoptotic cells were found in cells treated with a miR-21 inhibitor or DOX alone [151]. Papagiannakopoulos et al. also exposed cells either with or without miR-21 overexpression, to different concentrations of DOX and reported that cells expressing miR-21 did not respond to treatment, even at higher doses [130]. MiR-21 has been involved in resistance to DOX in other forms of cancer [152] as well, and these studies in glioma cells show that miR-21 may mediate resistance to DOX in brain cancer and merit more research as DOX presents an attractive treatment option in gliomas, since it seems to potentiate the effect of TMZ [153].
Ren et al. explored the effect of miR-21 on taxol sensitivity and the role of PTEN on this interaction. MiR-21 inhibition led to significant increase in apoptosis following taxol treatment in PTEN-wild type and PTEN-mutant cellular lines; in PTEN-mutant cells the interaction was additive, while in PTEN-wild type, it was synergistic, showing that miR-21 inhibition can aid treatment independent of the PTEN status. The researchers showed that miR-21 inhibition enhances taxol sensitivity (marked decrease in IC50 values), and respectively, taxol increases the efficacy of miR-21 inhibition (20 % cellular viability was noted upon the combination treatment, compared to 89 % upon miR-21 inhibition alone). They also studied Bcl2, MMP-2 and -9, and caspase-3 levels, finding the strongest reduction in the former three and the strongest increase in the latter, upon combination treatment [66].As mentioned, PTEN regulates EGFR/Akt signaling, although the action of miRNA-21 does not seem to closely depend on PTEN, but rather enhances the aberrant expression of this pathway in other ways. On this matter, Zhang et al. studied the anti-EGFR agent nimotuzumab, which has been proven superior to other similar agents and with fewer side-effects [154], and anti-miR-21 ASO, after finding that this miRNA targets several regulating factors of the pathway. They reported that concomitant administration of nimotuzumab and a miR-21 inhibitor led to significantly decreased cellular invasion, and increased apoptotic rates and caspase-3 and caspase-7 activity. The enhanced effectiveness of the combined treatment was also shown in a murine brain cancer xenograft model, as subjects with miR-21 inhibition prior to nimotuzumab administration responded considerably better to the agent [69].
Finally, an additional mechanism that may possibly lead to resistance could involve IGFBP3. Yang et al. showed that miR-21 targets this binding protein, giving way to higher IGF-1 bioavailability [53]. IGF-1/IGFR has been associated with chemoresistance [155], although studies exploring the effect of miR-21 knockdown on IGF-1 and response to treatment are still lacking.
6.2. Radiotherapy resistance
Chaudhry et al. exposed two glioma cell lines to IR and studied the expression of several miRNA molecules. In one of them, which normally expressed DNA-PK, an enzyme crucial to DNA repair following IR, miR-21 was found significantly upregulated in the first hours after IR [156]. In the study by Rodrigues et al. on neurospheres, miR-21 was overexpressed in cells upon TMZ and IR treatment [145]. Similarly, Li et al. showed that miRNA-21 was involved in radiotherapy resistance by exposing glioma cells to IR and then finding the levels of miR-21 elevated; a miR-21 inhibitor also led to increased IR-induced apoptosis, caspase-3 and caspase-7 activity (about 39 % increased upon combination, when compared to miR-21 inhibition alone), and cellular growth arrest. As previously mentioned, the researchers showed that miR-21 targets Cdc25A, an important regulator of the G2-M cellular transition, supporting the notion that miR-21 affects response to radiation via Cdc25A [127].
Gwak et al. also studied several glioma cellular lines and found that miR-21 levels correlated with resistance to radiotherapy, and that miRNA-21 levels increased upon treatment with radiation in a dose-dependent manner. Additionally, suppressing miR-21 with an inhibitor sensitized cells to IR, increasing parameters pertaining to autophagy, G2-M transition, and apoptosis. The researchers hypothesized that this inhibition deactivates the PI3K/AKT pathway, a pathway that facilitates DNA repair and hinders the effectiveness of radiation [157,158]. The study included both PTEN-wild type and PTEN-deficient lines. It showed that miR-21 inhibition in PTEN-deficient cells led to radiosensitization, whereas miR-21 overexpression in PTEN-wild type cells led to radioresistance, as inhibition of miR-21 in wild-type cells did not produce the same radiosensitizing effect. Additionally, the authors reported significantly higher miR-21 levels and a larger miRNA-21 increase upon radiation in PTEN-deficient lines [157]. These results suggest that the PTEN-miRNA-21 interplay is an important factor in radioresistance of gliomas, although other studies indicate that miRNA-21 may act in ways independent of PTEN as well.
7. Possible therapeutic interventions
Collectively, it is evident that miRNA-21 is involved in a wide array of mechanisms that eventually lead to increased proliferation, high invasiveness and treatment resistance, worsening the survival prospects of glioma patients. What remains to be explored are the translational implications of these findings, and ways to apply this knowledge in clinical practice.
Most in vitro and in vivo studies that are described above, in the quest of validating their findings, suppressed miRNA-21 and produced results that unanimously show the large impact that this inhibition has on cancerous cell survival. Upon miR-21 inhibition, apoptotic rates increased, and the invasion capacity of glioma cells markedly decreased, with several mechanisms being involved. All of these studies provide remarkable ideas for the development of future therapeutic strategies for glioma, either via miR-21 itself, or via its targets. Furthermore, a wide array of different studies showed how miR-21 inhibition re-sensitized resistant-to-treatment cells, or led to higher treatment effectiveness. This is of great importance, since gliomas are particularly resistant, and therefore associated with a worse prognosis despite access to several therapeutic options. However, inhibiting miRNA-21 in cellular cultures and in glioma patients are two completely different matters, and despite the encouraging results of the aforementioned studies, the scientific community needs to assess whether developing a treatment focused on miRNA-21 is a realistic and feasible goal.
In the in vitro studies, several techniques were applied for the inhibition of miRNA-21. Most of them used antisense-oligonucleotides, which are designed to specifically inhibit miR-21. These ASOs are often chemically enhanced with several molecular additions that facilitate cellular delivery and make them resistant to degradation, ensuring that they are delivered to the cultured cells in order to bind to their target, and are more effective in xenograft studies [159]. Belter et al. also developed special anti-miR-21 hammerhead ribozymes and DNA-zymes targeting miR-21 and its precursors, leading to the depletion of this molecule’s cellular pool and suggesting that this method could also be potentially applied in future treatment strategies [110]. The in vivo models mostly used a heterotopic xenograft method, creating subcutaneous tumors from the cellular lines involved in the in vitro parts of the studies. In these studies, the researchers could easily inject ASOs and then move on to measure tumor sizes or apply treatment, depending on the study objective. Krützfeld et al. intravenously injected ASOs in mice, which inhibited their targets in most tissues, but presented no effect in the brain due to the blood-brain barrier (BBB); when ASOs were injected in the cortex, satisfying knockdown was reported [160].However, a direct injection in the cortex is not a technique that can easily be performed in clinical practice, thus an alternative route needs to be found.
The application of an orthotopic xenograft model, where tumors are developed in the respective organs, represents a more realistic approach, as it resembles clinical practice. Corsten et al. described the use of neuronal precursor cells (NPCs) expressing a secretable form of TRAIL (S-TRAIL), in an effort to overcome the obstacle of the BBB and selectively migrate and target malignant cells in loco. In their study, they first transfected human glioma cell cultures with an anti-miR-21 and then intracranially injected the cells in mice, which showed a decrease in glioma burden when compared to control-ASO. After in vitro showing that miR-21 inhibition and S-TRAIL acted synergistically and led to higher apoptotic rates, they injected the transfected cells, either mixed with the aforementioned NPCs or not, and they found that their combination resulted in a significant decrease in glioma volumes, eventually leading to their eradication. Histological examination showed that the NPCs were located solely inside the glioma mass, and not in the surrounding normal parenchyma [161]. This also shows another advantage of NPCs, since their use could potentially involve fewer side-effects, if normal tissue is left unscathed by the therapeutic intervention. This research group, in previous publications, also showed that NPCs can migrate between hemispheres to reach their target and satellite tumors [162], and that the intraventricular administration is the optimal route for the treatment’s maximum efficacy [163]. The mechanism underlying this ability has been studied, and it seems to involve several mediators of chemotaxis [164]. TRAIL is considered a promising anti-cancer agent; however, challenges regarding its administration to the brain, given its short half-life, have been highlighted [165]. Studies in animals have proposed ways to overcome these limitations and include the intranasal application of stem cells expressing TRAIL [166] or nanoparticles [167]. However, the efficacy of these applications has yet to be evaluated in humans; clinical trials on a TRAIL-inducing compound are currently underway and seem to be promising as well [168]. Furthermore, a way to combine TRAIL and miR-21 inhibition in a realistic clinical setting is still much further ahead and more research towards this direction is required.
Concerning RNA nanoparticles (RNP), Lee et al. described the formation of a novel RNP, which could deliver anti-miR-21 sequences to gliomas and inhibit miR-21, leading to increased PTEN and PDCD4, and tumor growth suppression. The researchers used an orthotopic xenograft model and then systematically administered their RNP. They found that it reached its tumor target, not accumulating in normal tissues or other organs, and after five RNP injections, mice that received the anti-miR-21 regimen demonstrated decreased tumor growth when compared to controls. The mice that received this treatment also had higher survival rates [169]. In a similar vein, Seo et al. showed that NPs injected into orthotopically transplanted gliomas of murine subjects led to reduced cellular survival and a greater efficacy of TMZ treatment, while in detail describing their methodology in creating these nanoparticles [111]. These studies are of particular importance, because they show that targeted therapy against miRNA-21 might be feasible, and more studies in this direction, possibly combining chemotherapy or radiotherapy regimens with RNPs like this, will be more than welcomed in the future.
As Hanna et al. elegantly reviewed recently, miRNAs are steadily being more involved in clinical trials; mimics, to enhance their expression, or repressors, to diminish their function, can be systemically administered via intravenous injection, while they can also be injected directly inside the target tumors, further potentiating their effect [170]. However, they seem to be efficient for peripheral targets and the brain remains a territory rather untouched by those techniques. CSF administration would be an alternative option in general, but it is invasive and only small, lipophilic substances can penetrate through the BBB [170]. In glioma, a second barrier, i.e. the blood brain tumor barrier, is created via the newly formed vessels of the cancer. This acts to further inhibit the transfer or substances and drugs, adding to the BBB obstacle [171]. In this line of thought, Corsten et al. had also suggested that a lipophilic packaging of oligonucleotides could facilitate the BBB crossing and intratumoral delivery of the agents [161]; this approach has been translated into endeavors exploiting lipophilicity. Regarding miRNAs, exosomes, which are EVs secreted by cells, seem to be a promising therapeutic candidate, since they are created by cellular membranes and can cross the BBB; consequently, they have been used to deliver compounds through the BBB and into the brain. In an interesting study by Yang et al., EVs carrying a silencing RNA (siRNA) for VEGF passed the molecule through the BBB and into the cancerous cells of xenotransplanted tumors in zebrafish [172]. Several aspects of EVs, such as their pharmacokinetic abilities and the optimal choice of donor cells, need further evaluation if they are to be applied in clinical practice. Finally, other options for facilitating drug delivery via the BBB include hyperosmotic solutions, such as mannitol, which can be used to dilate tight junctions and facilitate drug permeation, and strategies involving viral vectors, and potentiating active transporters [171].
Another invasive but possibly promising option seems to be a convention-enhanced delivery via catheters used to directly administer the compounds to the tumor site. A study on the transfer of a miRNA into xenograft-derived GBMs in mice via this method, showed that it was in general well tolerated and effective, since the target of the miRNA was found downregulated [173]. If this method could eventually be applied to humans, the transfer of anti-miR-21 ASOs could greatly improve therapeutic options, since it has been shown that miR-21 inhibition leads to greater response rates. The aforementioned nanoparticles are also interesting, since they can be manufactured to access the tumor site, and in the case of gliomas, to cross the BBB in the way. Targeted liposomes of 100 nm are the carriers most commonly encountered in literature so far, and animal studies are also being conducted on this matter [174], such as the one by Brown et al., which provided methodological recommendations on the systemic delivery of miRNAs via nanoparticles and the assessment of whether they adequately reached their targets [175]. Nanoparticles carrying anti-miR-21 nucleotides could be the future of glioma treatment, should their safety and efficacy be shown in clinical trials.
In conclusion, there are several ideas for new therapeutic interventions. The research should now focus on finding ways to safely inhibit miRNA-21, or influence some of its targeted molecules, in human gliomas. It is not a trivial task, since the brain is a challenging organ to approach, but it is certainly very promising and showing considerable realistic potential for future interventions.
8. Conclusions
MiRNA-21 seems to play a crucial role in several aspects of glioma pathogenesis. The results produced until today have been very consistent; MiRNA-21is upregulated in glioma cells, with its levels correlating to higher grade and worse prognosis for the patient. Various research groups have elucidated many of the underlying mechanisms of its action, showing that it promotes glioma survival and invasion, while its inhibition leads to increased apoptosis and reduced invasiveness. Additionally, its inhibition can make tumor cells more responsive to treatment, an issue of great importance since gliomas are one of the most resilient tumors and patients have very low chances of survival beyond a year, despite receiving treatment. The task to be resolved remains, however; the technology to inhibit miR-21 in humans still lacks, since crossing the BBB and accessing the brain has always been a great hurdle, with miRNAs not being an exception. Novel strategies have emerged, however, and we believe it is only a matter of time before a way to treat glioma with the involvement of miR-21 makes its way to everyday clinical practice. To this end, focused research is still required, especially regarding safe and effective ways to deliver inhibitory molecules against miR-21 into glioma cells.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Abels E.R., Maas S.L.N., Nieland L., Wei Z., Cheah P.S., Tai E., Kolsteeg C.-J., Dusoswa S.A., Ting D.T., Hickman S., El Khoury J., Krichevsky A.M., Broekman M.L.D., Breakefield X.O. Glioblastoma-Associated Microglia Reprogramming Is Mediated by Functional Transfer of Extracellular miR-21. Cell Rep. 2019;28 doi: 10.1016/j.celrep.2019.08.036. 3105-3119.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hambardzumyan D., Gutmann D.H., Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 2016;19:20–27. doi: 10.1038/nn.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis D.N., Ohgaki H., Wiestler O.D., Cavenee W.K., Burger P.C., Jouvet A., Scheithauer B.W., Kleihues P. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. (Berl.). 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore L.M., Zhang W. Targeting miR-21 in glioma: a small RNA with big potential, Expert Opin. Ther. Targets Neurol. Dis. 2010;14:1247–1257. doi: 10.1517/14728222.2010.527334. [DOI] [PubMed] [Google Scholar]
- 5.Christofi T., Baritaki S., Falzone L., Libra M., Zaravinos A. Current perspectives in Cancer immunotherapy. Cancers. 2019;11 doi: 10.3390/cancers11101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGranahan T., Therkelsen K.E., Ahmad S., Nagpal S. Current state of immunotherapy for treatment of glioblastoma. Curr. Treat. Options Oncol. 2019;20 doi: 10.1007/s11864-019-0619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabriely G., Wurdinger T., Kesari S., Esau C.C., Burchard J., Linsley P.S., Krichevsky A.M. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol. Cell. Biol. 2008;28:5369–5380. doi: 10.1128/MCB.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo J.W., Wang X., Yang Y., Mao Q. Role of micro-RNA (miRNA) in pathogenesis of glioblastoma. Eur. Rev. Med. Pharmacol. Sci. 2015;19:1630–1639. [PubMed] [Google Scholar]
- 9.Dardiotis E., Aloizou A.-M., Siokas V., Patrinos G.P., Deretzi G., Mitsias P., Aschner M., Tsatsakis A. The role of MicroRNAs in patients with amyotrophic lateral sclerosis. J. Mol. Neurosci. MN. 2018;66:617–628. doi: 10.1007/s12031-018-1204-1. [DOI] [PubMed] [Google Scholar]
- 10.Goodall E.F., Heath P.R., Bandmann O., Kirby J., Shaw P.J. Neuronal dark matter: the emerging role of microRNAs in neurodegeneration. Front. Cell. Neurosci. 2013;7:178. doi: 10.3389/fncel.2013.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinlan S., Kenny A., Medina M., Engel T., Jimenez-Mateos E.M. MicroRNAs in neurodegenerative diseases. Int. Rev. Cell Mol. Biol. 2017;334:309–343. doi: 10.1016/bs.ircmb.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Esquela-Kerscher A., Slack F.J. Oncomirs - microRNAs with a role in cancer. Nat. Rev. Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 13.Tuaeva N.O., Falzone L., Porozov Y.B., Nosyrev A.E., Trukhan V.M., Kovatsi L., Spandidos D.A., Drakoulis N., Kalogeraki A., Mamoulakis C., Tzanakakis G., Libra M., Tsatsakis A. Translational application of circulating DNA in oncology: review of the last decades achievements. Cells. 2019;8 doi: 10.3390/cells8101251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volinia S., Calin G.A., Liu C.-G., Ambs S., Cimmino A., Petrocca F., Visone R., Iorio M., Roldo C., Ferracin M., Prueitt R.L., Yanaihara N., Lanza G., Scarpa A., Vecchione A., Negrini M., Harris C.C., Croce C.M. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. U. S. A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan J.A., Krichevsky A.M., Kosik K.S. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 16.Candido S., Lupo G., Pennisi M., Basile M.S., Anfuso C.D., Petralia M.C., Gattuso G., Vivarelli S., Spandidos D.A., Libra M., Falzone L. The analysis of miRNA expression profiling datasets reveals inverse microRNA patterns in glioblastoma and Alzheimer’s disease. Oncol. Rep. 2019;42:911–922. doi: 10.3892/or.2019.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falzone L., Scola L., Zanghì A., Biondi A., Cataldo A.D., Libra M., Candido S. Integrated analysis of colorectal cancer microRNA datasets: identification of microRNAs associated with tumor development. Aging. 2018;10:1000. doi: 10.18632/aging.101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou H., Zhu X. MicroRNA-21 and microRNA-30c as diagnostic biomarkers for prostate cancer: a meta-analysis. Cancer Manag. Res. 2019;11:2039–2050. doi: 10.2147/CMAR.S189026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bica-Pop C., Cojocneanu-Petric R., Magdo L., Raduly L., Gulei D., Berindan-Neagoe I. Overview upon miR-21 in lung cancer: focus on NSCLC. Cell. Mol. Life Sci. CMLS. 2018;75 doi: 10.1007/s00018-018-2877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbano R., Palumbo O., Pasculli B., Galasso M., Volinia S., D’Angelo V., Icolaro N., Coco M., Dimitri L., Graziano P., Copetti M., Valori V.M., Maiello E., Carella M., Fazio V.M., Parrella P. PLoS One. 2014;9:e108950. doi: 10.1371/journal.pone.0108950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciafrè S.A., Galardi S., Mangiola A., Ferracin M., Liu C.-G., Sabatino G., Negrini M., Maira G., Croce C.M., Farace M.G. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem. Biophys. Res. Commun. 2005;334:1351–1358. doi: 10.1016/j.bbrc.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 22.Conti A., Aguennouz M., La Torre D., Tomasello C., Cardali S., Angileri F.F., Maio F., Cama A., Germanò A., Vita G., Tomasello F. miR-21 and 221 upregulation and miR-181b downregulation in human grade II-IV astrocytic tumors. J. Neurooncol. 2009;93:325–332. doi: 10.1007/s11060-009-9797-4. [DOI] [PubMed] [Google Scholar]
- 23.Gaur A.B., Holbeck S.L., Colburn N.H., Israel M.A. Downregulation of Pdcd4 by mir-21 facilitates glioblastoma proliferation in vivo. Neuro-Oncol. 2011;13:580–590. doi: 10.1093/neuonc/nor033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan Y., Mizoguchi M., Yoshimoto K., Hata N., Shono T., Suzuki S.O., Araki Y., Kuga D., Nakamizo A., Amano T., Ma X., Hayashi K., Sasaki T. MiRNA-196 is upregulated in glioblastoma but not in anaplastic astrocytoma and has prognostic significance. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010;16:4289–4297. doi: 10.1158/1078-0432.CCR-10-0207. [DOI] [PubMed] [Google Scholar]
- 25.Han L., Yue X., Zhou X., Lan F.-M., You G., Zhang W., Zhang K.-L., Zhang C.-Z., Cheng J.-Q., Yu S.-Z., Pu P.-Y., Jiang T., Kang C.-S. MicroRNA-21 expression is regulated by β-catenin/STAT3 pathway and promotes glioma cell invasion by direct targeting RECK. CNS Neurosci. Ther. 2012;18:573–583. doi: 10.1111/j.1755-5949.2012.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lages E., Guttin A., Atifi M.E., Ramus C., Ipas H., Dupré I., Rolland D., Salon C., Godfraind C., deFraipont F., Dhobb M., Pelletier L., Wion D., Gay E., Berger F., Issartel J.-P. MicroRNA and target protein patterns reveal physiopathological features of glioma subtypes. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020600. e20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lakomy R., Sana J., Hankeova S., Fadrus P., Kren L., Lzicarova E., Svoboda M., Dolezelova H., Smrcka M., Vyzula R., Michalek J., Hajduch M., Slaby O. MiR-195, miR-196b, miR-181c, miR-21 expression levels and O-6-methylguanine-DNA methyltransferase methylation status are associated with clinical outcome in glioblastoma patients. Cancer Sci. 2011;102:2186–2190. doi: 10.1111/j.1349-7006.2011.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piwecka M., Rolle K., Belter A., Barciszewska A.M., Żywicki M., Michalak M., Nowak S., Naskręt-Barciszewska M.Z., Barciszewski J. Comprehensive analysis of microRNA expression profile in malignant glioma tissues. Mol. Oncol. 2015;9:1324–1340. doi: 10.1016/j.molonc.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qu K., Lin T., Pang Q., Liu T., Wang Z., Tai M., Meng F., Zhang J., Wan Y., Mao P., Dong X., Liu C., Niu W., Dong S. Extracellular miRNA-21 as a novel biomarker in glioma: evidence from meta-analysis, clinical validation and experimental investigations. Oncotarget. 2016;7:33994–34010. doi: 10.18632/oncotarget.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao S.A.M., Santosh V., Somasundaram K. Genome-wide expression profiling identifies deregulated miRNAs in malignant astrocytoma. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 2010;23:1404–1417. doi: 10.1038/modpathol.2010.135. [DOI] [PubMed] [Google Scholar]
- 31.Sasayama T., Nishihara M., Kondoh T., Hosoda K., Kohmura E. MicroRNA-10b is overexpressed in malignant glioma and associated with tumor invasive factors, uPAR and RhoC. Int. J. Cancer. 2009;125:1407–1413. doi: 10.1002/ijc.24522. [DOI] [PubMed] [Google Scholar]
- 32.Shang C., Guo Y., Hong Y., Liu Y., Xue Y. MiR-21 up-regulation mediates glioblastoma cancer stem cells apoptosis and proliferation by targeting FASLG. Mol. Biol. Rep. 2015;42:721–727. doi: 10.1007/s11033-014-3820-3. [DOI] [PubMed] [Google Scholar]
- 33.Shi L., Chen J., Yang J., Pan T., Zhang S., Wang Z. MiR-21 protected human glioblastoma U87MG cells from chemotherapeutic drug temozolomide induced apoptosis by decreasing Bax/Bcl-2 ratio and caspase-3 activity. Brain Res. 2010;1352 doi: 10.1016/j.brainres.2010.07.009. 255–264. [DOI] [PubMed] [Google Scholar]
- 34.Silber J., Lim D.A., Petritsch C., Persson A.I., Maunakea A.K., Yu M., Vandenberg S.R., Ginzinger D.G., James C.D., Costello J.F., Bergers G., Weiss W.A., Alvarez-Buylla A., Hodgson J.G. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;6:14. doi: 10.1186/1741-7015-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sippl C., Teping F., Ketter R., Braun L., Tremmel L., Schulz-Schaeffer W., Oertel J., Urbschat S. The influence of distinct regulatory miRNAs of the p15/p16/RB1/E2F pathway on the clinical progression of glioblastoma multiforme. World Neurosurg. 2019;132 doi: 10.1016/j.wneu.2019.07.134. e900–e908. [DOI] [PubMed] [Google Scholar]
- 36.Wu L., Li G., Feng D., Qin H., Gong L., Zhang J., Zhang Z. MicroRNA-21 expression is associated with overall survival in patients with glioma. Diagn. Pathol. 2013;8:200. doi: 10.1186/1746-1596-8-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhi F., Chen X., Wang S., Xia X., Shi Y., Guan W., Shao N., Qu H., Yang C., Zhang Y., Wang Q., Wang R., Zen K., Zhang C.-Y., Zhang J., Yang Y. The use of hsa-miR-21, hsa-miR-181b and hsa-miR-106a as prognostic indicators of astrocytoma. Eur. J. Cancer. 2010;46:1640–1649. doi: 10.1016/j.ejca.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Baraniskin A., Kuhnhenn J., Schlegel U., Maghnouj A., Zöllner H., Schmiegel W., Hahn S., Schroers R. Identification of microRNAs in the cerebrospinal fluid as biomarker for the diagnosis of glioma. Neuro-Oncol. 2012;14:29–33. doi: 10.1093/neuonc/nor169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teplyuk N.M., Mollenhauer B., Gabriely G., Giese A., Kim E., Smolsky M., Kim R.Y., Saria M.G., Pastorino S., Kesari S., Krichevsky A.M. MicroRNAs in cerebrospinal fluid identify glioblastoma and metastatic brain cancers and reflect disease activity. Neuro-Oncol. 2012;14:689–700. doi: 10.1093/neuonc/nos074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Q., Li P., Li A., Jiang W., Wang H., Wang J., Xie K. Plasma specific miRNAs as predictive biomarkers for diagnosis and prognosis of glioma. J. Exp. Clin. Cancer Res. CR. 2012;31:97. doi: 10.1186/1756-9966-31-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ilhan-Mutlu A., Wagner L., Wöhrer A., Furtner J., Widhalm G., Marosi C., Preusser M. Plasma MicroRNA-21 concentration may be a useful biomarker in glioblastoma patients. Cancer Invest. 2012;30:615–621. doi: 10.3109/07357907.2012.708071. [DOI] [PubMed] [Google Scholar]
- 42.Mao X., Sun Y., Tang J. Serum miR-21 is a diagnostic and prognostic marker of primary central nervous system lymphoma. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2014;35:233–238. doi: 10.1007/s10072-013-1491-9. [DOI] [PubMed] [Google Scholar]
- 43.Ivo D’Urso P., Fernando D’Urso O., Damiano Gianfreda C., Mezzolla V., Storelli C., Marsigliante S. miR-15b and miR-21 as Circulating Biomarkers for Diagnosis of Glioma. Curr. Genomics. 2015;16:304–311. doi: 10.2174/1389202916666150707155610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhi F., Shao N., Wang R., Deng D., Xue L., Wang Q., Zhang Y., Shi Y., Xia X., Wang S., Lan Q., Yang Y. Identification of 9 serum microRNAs as potential noninvasive biomarkers of human astrocytoma. NeuroOncol. 2015;17:383–391. doi: 10.1093/neuonc/nou169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ParvizHamidi M., Haddad G., Ostadrahimi S., Ostadrahimi N., Sadeghi S., Fayaz S., Fard-Esfahani P. Circulating miR-26a and miR-21 as biomarkers for glioblastoma multiform. Biotechnol. Appl. Biochem. 2019;66:261–265. doi: 10.1002/bab.1707. [DOI] [PubMed] [Google Scholar]
- 46.Siegal T., Charbit H., Paldor I., Zelikovitch B., Canello T., Benis A., Wong M.L., Morokoff A.P., Kaye A.H., Lavon I. Dynamics of circulating hypoxia-mediated miRNAs and tumor response in patients with high-grade glioma treated with bevacizumab. J. Neurosurg. 2016;125:1008–1015. doi: 10.3171/2015.8.JNS15437. [DOI] [PubMed] [Google Scholar]
- 47.Akers J.C., Ramakrishnan V., Kim R., Skog J., Nakano I., Pingle S., Kalinina J., Hua W., Kesari S., Mao Y., Breakefield X.O., Hochberg F.H. E.G. Van Meir, B.S. Carter, C.C. Chen, MiR-21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): a platform for glioblastoma biomarker development. PLoS One. 2013;8 doi: 10.1371/journal.pone.0078115. e78115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi R., Wang P.-Y., Li X.-Y., Chen J.-X., Li Y., Zhang X.-Z., Zhang C.-G., Jiang T., Li W.-B., Ding W., Cheng S.-J. Exosomal levels of miRNA-21 from cerebrospinal fluids associated with poor prognosis and tumor recurrence of glioma patients. Oncotarget. 2015;6:26971–26981. doi: 10.18632/oncotarget.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santangelo A., Imbrucè P., Gardenghi B., Belli L., Agushi R., Tamanini A., Munari S., Bossi A.M., Scambi I., Benati D., Mariotti R., Di Gennaro G., Sbarbati A., Eccher A., Ricciardi G.K., Ciceri E.M., Sala F., Pinna G., Lippi G., Cabrini G., Dechecchi M.C. A microRNA signature from serum exosomes of patients with glioma as complementary diagnostic biomarker. J. Neurooncol. 2018;136:51–62. doi: 10.1007/s11060-017-2639-x. [DOI] [PubMed] [Google Scholar]
- 50.Sathyan P., Zinn P.O., Marisetty A.L., Liu B., Kamal M.M., Singh S.K., Bady P., Lu L., Wani K.M., Veo B.L., Gumin J., Kassem D.H., Robinson F., Weng C., Baladandayuthapani V., Suki D., Colman H., Bhat K.P., Sulman E.P., Aldape K., Colen R.R., Verhaak R.G.W., Lu Z., Fuller G.N., Huang S., Lang F.F., Sawaya R., Hegi M., Majumder S. Mir-21–Sox2 Axis delineates glioblastoma subtypes with prognostic impact. J. Neurosci. 2015;35:15097–15112. doi: 10.1523/JNEUROSCI.1265-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang W., Mu S., Zhao Q., Xue L., Wang S. Identification of differentially expressed microRNAs and the potential of microRNA-455-3p as a novel prognostic biomarker in glioma. Oncol. Lett. 2019;18:6150–6156. doi: 10.3892/ol.2019.10927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou X., Ren Y., Moore L., Mei M., You Y., Xu P., Wang B., Wang G., Jia Z., Pu P., Zhang W., Kang C. Downregulation of miR-21 inhibits EGFR pathway and suppresses the growth of human glioblastoma cells independent of PTEN status. Lab. Investig. J. Tech. Methods Pathol. 2010;90:144–155. doi: 10.1038/labinvest.2009.126. [DOI] [PubMed] [Google Scholar]
- 53.Yang C.H., Yue J., Pfeffer S.R., Fan M., Paulus E., Hosni-Ahmed A., Sims M., Qayyum S., Davidoff A.M., Handorf C.R., Pfeffer L.M. MicroRNA-21 promotes glioblastoma tumorigenesis by down-regulating insulin-like growth factor-binding protein-3 (IGFBP3) J. Biol. Chem. 2014;289:25079–25087. doi: 10.1074/jbc.M114.593863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma C., Nguyen H.P.T., Luwor R.B., Stylli S.S., Gogos A., Paradiso L., Kaye A.H., Morokoff A.P. A comprehensive meta-analysis of circulation miRNAs in glioma as potential diagnostic biomarker. PLoS One. 2018;13 doi: 10.1371/journal.pone.0189452. e0189452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silantyev A.S., Falzone L., Libra M., Gurina O.I., Kardashova K.S., Nikolouzakis T.K., Nosyrev A.E., Sutton C.W., Mitsias P.D., Tsatsakis A. Current and Future Trends on Diagnosis and Prognosis of Glioblastoma: From Molecular Biology to Proteomics. Cells. 2019;8 doi: 10.3390/cells8080863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hermansen S.K., Dahlrot R.H., Nielsen B.S., Hansen S., Kristensen B.W. MiR-21 expression in the tumor cell compartment holds unfavorable prognostic value in gliomas. J. Neurooncol. 2013;111:71–81. doi: 10.1007/s11060-012-0992-3. [DOI] [PubMed] [Google Scholar]
- 57.Yang M., Bai H.X., Yang L. MicroRNA-21 expression in the prognosis of low-grade gliomas: data from the cancer genome atlas (TCGA) project. J. Neurooncol. 2017;134:241–242. doi: 10.1007/s11060-017-2500-2. [DOI] [PubMed] [Google Scholar]
- 58.Li C., Sun J., Xiang Q., Liang Y., Zhao N., Zhang Z., Liu Q., Cui Y. Prognostic role of microRNA-21 expression in gliomas: a meta-analysis. J. Neurooncol. 2016;130:11–17. doi: 10.1007/s11060-016-2233-7. [DOI] [PubMed] [Google Scholar]
- 59.Löffler D., Brocke-Heidrich K., Pfeifer G., Stocsits C., Hackermüller J., Kretzschmar A.K., Burger R., Gramatzki M., Blumert C., Bauer K., Cvijic H., Ullmann A.K., Stadler P.F., Horn F. Interleukin-6 dependent survival of multiple myeloma cells involves the Stat3-mediated induction of microRNA-21 through a highly conserved enhancer. Blood. 2007;110:1330–1333. doi: 10.1182/blood-2007-03-081133. [DOI] [PubMed] [Google Scholar]
- 60.Ganguly D., Fan M., Yang C.H., Zbytek B., Finkelstein D., Roussel M.F., Pfeffer L.M. The critical role that STAT3 plays in glioma-initiating cells: STAT3 addiction in glioma. Oncotarget. 2018;9:22095–22112. doi: 10.18632/oncotarget.25188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carro M.S., Lim W.K., Alvarez M.J., Bollo R.J., Zhao X., Snyder E.Y., Sulman E.P., Anne S.L., Doetsch F., Colman H., Lasorella A., Aldape K., Califano A., Iavarone A. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463:318–325. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piperi C., Papavassiliou K.A., Papavassiliou A.G. Pivotal role of STAT3 in shaping glioblastoma immune microenvironment. Cells. 2019;8 doi: 10.3390/cells8111398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim J.E., Patel M., Ruzevick J., Jackson C.M., Lim M. STAT3 activation in glioblastoma: biochemical and therapeutic implications. Cancers. 2014;6:376–395. doi: 10.3390/cancers6010376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.West A.J., Tsui V., Stylli S.S., Nguyen H.P.T., Morokoff A.P., Kaye A.H., Luwor R.B. The role of interleukin-6-STAT3 signalling in glioblastoma. Oncol. Lett. 2018;16:4095–4104. doi: 10.3892/ol.2018.9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hatanpaa K.J., Burma S., Zhao D., Habib A.A. Epidermal growth factor receptor in glioma: signal transduction, neuropathology, imaging, and radioresistance. Neoplasia N. Y. N. 2010;12:675–684. doi: 10.1593/neo.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ren Y., Zhou X., Mei M., Yuan X.-B., Han L., Wang G.-X., Jia Z.-F., Xu P., Pu P.-Y., Kang C.-S. MicroRNA-21 inhibitor sensitizes human glioblastoma cells U251 (PTEN-mutant) and LN229 (PTEN-wild type) to taxol. BMC Cancer. 2010;10:27. doi: 10.1186/1471-2407-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang K., Zhang J.-X., Han L., You Y.-P., Jiang T., Pu P.-Y., Kang C.-S. MicroRNA roles in beta-catenin pathway. Mol. Cancer. 2010;9:252. doi: 10.1186/1476-4598-9-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rossi M., Magnoni L., Miracco C., Mori E., Tosi P., Pirtoli L., Tini P., Oliveri G., Cosci E., Bakker A. β-catenin and Gli1 are prognostic markers in glioblastoma. Cancer Biol. Ther. 2011;11:753–761. doi: 10.4161/cbt.11.8.14894. [DOI] [PubMed] [Google Scholar]
- 69.Zhang K.-L., Han L., Chen L.-Y., Shi Z.-D., Yang M., Ren Y., Chen L.-C., Zhang J.-X., Pu P.-Y., Kang C.-S. Blockage of a miR-21/EGFR regulatory feedback loop augments anti-EGFR therapy in glioblastomas. Cancer Lett. 2014;342:139–149. doi: 10.1016/j.canlet.2013.08.043. [DOI] [PubMed] [Google Scholar]
- 70.Kaur B., Khwaja F.W., Severson E.A., Matheny S.L., Brat D.J. E.G. Van Meir, Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro-Oncol. 2005;7:134–153. doi: 10.1215/S1152851704001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brat D.J., Castellano-Sanchez A.A., Hunter S.B., Pecot M., Cohen C., Hammond E.H., Devi S.N., Kaur B., Van Meir E.G. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64:920–927. doi: 10.1158/0008-5472.can-03-2073. [DOI] [PubMed] [Google Scholar]
- 72.Zagzag D., Lukyanov Y., Lan L., Ali M.A., Esencay M., Mendez O., Yee H., Voura E.B., Newcomb E.W. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab. Investig. J. Tech. Methods Pathol. 2006;86:1221–1232. doi: 10.1038/labinvest.3700482. [DOI] [PubMed] [Google Scholar]
- 73.Chen C.-W., Cheng T.-J., Ho C.-H., Wang J.-J., Weng S.-F., Hou Y.-C., Cheng H.-C., Chio C.-C., Shan Y.-S., Chang W.-T. Increased risk of brain cancer incidence in stroke patients: a clinical case series, population-based and longitudinal follow-up study. Oncotarget. 2017;8:108989–108999. doi: 10.18632/oncotarget.22480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kulshreshtha R., Ferracin M., Wojcik S.E., Garzon R., Alder H., Agosto-Perez F.J., Davuluri R., Liu C.-G., Croce C.M., Negrini M., Calin G.A., Ivan M. A microRNA signature of hypoxia. Mol. Cell. Biol. 2007;27:1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y., Nie H., Zhang K., Ma D., Yang G., Zheng Z., Liu K., Yu B., Zhai C., Yang S. A feedback regulatory loop between HIF-1α and miR-21 in response to hypoxia in cardiomyocytes. FEBS Lett. 2014;588:3137–3146. doi: 10.1016/j.febslet.2014.05.067. [DOI] [PubMed] [Google Scholar]
- 76.Davis B.N., Hilyard A.C., Lagna G., Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han J., Alvarez-Breckenridge C.A., Wang Q.-E., Yu J. TGF-β signaling and its targeting for glioma treatment. Am. J. Cancer Res. 2015;5:945–955. [PMC free article] [PubMed] [Google Scholar]
- 78.Smith E.R., Zurakowski D., Saad A., Scott R.M., Moses M.A. Urinary biomarkers predict brain tumor presence and response to therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008;14:2378–2386. doi: 10.1158/1078-0432.CCR-07-1253. [DOI] [PubMed] [Google Scholar]
- 79.Kwak H.-J., Kim Y.-J., Chun K.-R., Woo Y.M., Park S.-J., Jeong J.-A., Jo S.H., Kim T.H., Min H.S., Chae J.S., Choi E.-J., Kim G., Shin S.-H., Gwak H.-S., Kim S.-K., Hong E.-K., Lee G.-K., Choi K.-H., Kim J.H., Yoo H., Park J.B., Lee S.-H. Downregulation of Spry2 by miR-21 triggers malignancy in human gliomas. Oncogene. 2011;30:2433–2442. doi: 10.1038/onc.2010.620. [DOI] [PubMed] [Google Scholar]
- 80.Chen X.-C., Wei X.-T., Guan J.-H., Shu H., Chen D. EGF stimulates glioblastoma metastasis by induction of matrix metalloproteinase-9 in an EGFR-dependent mechanism. Oncotarget. 2017;8:65969–65982. doi: 10.18632/oncotarget.19622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dhandapani K.M., Khan M.M., Wade F.M., Wakade C., Mahesh V.B., Brann D.W. Induction of transforming growth factor-beta1 by basic fibroblast growth factor in rat C6 glioma cells and astrocytes is mediated by MEK/ERK signaling and AP-1 activation. J. Neurosci. Res. 2007;85:1033–1045. doi: 10.1002/jnr.21182. [DOI] [PubMed] [Google Scholar]
- 82.Nazarenko I., Hede S.-M., He X., Hedrén A., Thompson J., Lindström M.S., Nistér M. PDGF and PDGF receptors in glioma. Ups. J. Med. Sci. 2012;117:99–112. doi: 10.3109/03009734.2012.665097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bodmer J.L., Holler N., Reynard S., Vinciguerra P., Schneider P., Juo P., Blenis J., Tschopp J. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat. Cell Biol. 2000;2:241–243. doi: 10.1038/35008667. [DOI] [PubMed] [Google Scholar]
- 84.Salvesen G.S., Dixit V.M. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 85.Nakada M., Okada Y., Yamashita J. The role of matrix metalloproteinases in glioma invasion. Front. Biosci. J. Virtual Libr. 2003;8 doi: 10.2741/1016. e261-269. [DOI] [PubMed] [Google Scholar]
- 86.Noha M., Yoshida D., Watanabe K., Teramoto A. Suppression of cell invasion on human malignant glioma cell lines by a novel matrix-metalloproteinase inhibitor SI-27: in vitro study. J. Neurooncol. 2000;48:217–223. doi: 10.1023/a:1006424424119. [DOI] [PubMed] [Google Scholar]
- 87.Bergers G., Brekken R., McMahon G., Vu T.H., Itoh T., Tamaki K., Tanzawa K., Thorpe P., Itohara S., Werb Z., Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wild-Bode C., Weller M., Wick W. Molecular determinants of glioma cell migration and invasion. J. Neurosurg. 2001;94:978–984. doi: 10.3171/jns.2001.94.6.0978. [DOI] [PubMed] [Google Scholar]
- 89.Walker C., Mojares E., Del Río Hernández A. Role of extracellular matrix in development and Cancer progression. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lu P., Takai K., Weaver V. Z. Z, Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu Q., Wang Z., Hu Y., Li J., Li X., Zhou L., Huang Y. miR-21 promotes migration and invasion by the miR-21-PDCD4-AP-1 feedback loop in human hepatocellular carcinoma. Oncol. Rep. 2012;27:1660–1668. doi: 10.3892/or.2012.1682. [DOI] [PubMed] [Google Scholar]
- 92.Li L., Li H. Role of microRNA-mediated MMP regulation in the treatment and diagnosis of malignant tumors. Cancer Biol. Ther. 2013;14:796–805. doi: 10.4161/cbt.25936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Napoli S., Scuderi C., Gattuso G., Di Bella V., Candido S., Basile M.S., Libra M., Falzone L. Functional roles of matrix metalloproteinases and their inhibitors in melanoma. Cells. 2020;9 doi: 10.3390/cells9051151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moore M., Hamm J., Dancey J., Eisenberg P., Dagenais A., Fields K., Hagan B., Greenberg B., Colwell B., Zee D., Tu J., Ottaway R., Humphrey L.Seymour. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer institute of Canada Clinical Trials Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003;21 doi: 10.1200/JCO.2003.02.098. [DOI] [PubMed] [Google Scholar]
- 95.Hirte H., Vergote I., Jeffrey J., Grimshaw R., Schwartz S.S.B., Tu D., Sadura A., Brundage M., Seymour L. A phase III randomized trial of BAY 12-9566 (tanomastat) as maintenance therapy in patients with advanced ovarian cancer responsive to primary surgery and paclitaxel/platinum containing chemotherapy: a National Cancer institute of Canada Clinical Trials Group Study. Gynecol. Oncol. 2006;102 doi: 10.1016/j.ygyno.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 96.Bissett D., O’Byrne K., j von Pawel, Gatzemeier U., Price A., Nicolson M., Mercier R., Mazabel E., Penning C., Zhang M., Collier M., Shepherd F. Phase III study of matrix metalloproteinase inhibitor prinomastat in non-small-cell lung cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005;23 doi: 10.1200/JCO.2005.03.170. [DOI] [PubMed] [Google Scholar]
- 97.van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 98.Broekman M.L., Maas S.L.N., Abels E.R., Mempel T.R., Krichevsky A.M., Breakefield X.O. Multidimensional communication in the microenvirons of glioblastoma. Nat. Rev. Neurol. 2018;14:482–495. doi: 10.1038/s41582-018-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Farioli-Vecchioli S., Tanori M., Micheli L., Mancuso M., Leonardi L., Saran A., Ciotti M.T., Ferretti E., Gulino A., Pazzaglia S., Tirone F. Inhibition of medulloblastoma tumorigenesis by the antiproliferative and pro-differentiative gene PC3. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007;21:2215–2225. doi: 10.1096/fj.06-7548com. [DOI] [PubMed] [Google Scholar]
- 100.Pu P., Zhang Z., Kang C., Jiang R., Jia Z., Wang G., Jiang H. Downregulation of Wnt2 and beta-catenin by siRNA suppresses malignant glioma cell growth. Cancer Gene Ther. 2009;16:351–361. doi: 10.1038/cgt.2008.78. [DOI] [PubMed] [Google Scholar]
- 101.Luo G., Luo W., Sun X., Lin J., Wang M., Zhang Y., Luo W., Zhang Y. MicroRNA-21 promotes migration and invasion of glioma cells via activation of Sox2 and β-catenin signaling. Mol. Med. Rep. 2017;15:187–193. doi: 10.3892/mmr.2016.5971. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 102.Annovazzi L., Mellai M., Caldera V., Valente G., Schiffer D. SOX2 expression and amplification in gliomas and glioma cell lines. Cancer Genomics Proteomics. 2011;8:139–147. [PubMed] [Google Scholar]
- 103.Gangemi R.M.R., Griffero F., Marubbi D., Perera M., Capra M.C., Malatesta P., Ravetti G.L., Zona G.L., Daga A., Corte G. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells Dayt. Ohio. 2009;27:40–48. doi: 10.1634/stemcells.2008-0493. [DOI] [PubMed] [Google Scholar]
- 104.Heddleston J.M., Li Z., Lathia J.D., Bao S., Hjelmeland A.B., Rich J.N. Hypoxia inducible factors in cancer stem cells. Br. J. Cancer. 2010;102:789–795. doi: 10.1038/sj.bjc.6605551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou X., Zhang J., Jia Q., Ren Y., Wang Y., Shi L., Liu N., Wang G., Pu P., You Y., Kang C. Reduction of miR-21 induces glioma cell apoptosis via activating caspase 9 and 3. Oncol. Rep. 2010;24:195–201. doi: 10.3892/or_00000846. [DOI] [PubMed] [Google Scholar]
- 106.Lam P., Sian Lim K., Mei Wang S., Hui K.M. A microarray study to characterize the molecular mechanism of TIMP-3-mediated tumor rejection. Mol. Ther. J. Am. Soc. Gene Ther. 2005;12:144–152. doi: 10.1016/j.ymthe.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 107.Mannello F., Gazzanelli G. Tissue inhibitors of metalloproteinases and programmed cell death: conundrums, controversies and potential implications. Apoptosis Int. J. Program. Cell Death. 2001;6:479–482. doi: 10.1023/a:1012493808790. [DOI] [PubMed] [Google Scholar]
- 108.Kato H., Kato S., Kumabe T., Sonoda Y., Yoshimoto T., Kato S., Han S.Y., Suzuki T., Shibata H., Kanamaru R., Ishioka C. Functional evaluation of p53 and PTEN gene mutations in gliomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2000;6:3937–3943. [PubMed] [Google Scholar]
- 109.Guillamo J.-S., de Boüard S., Valable S., Marteau L., Leuraud P., Marie Y., Poupon M.-F., Parienti J.-J., Raymond E., Peschanski M. Molecular mechanisms underlying effects of epidermal growth factor receptor inhibition on invasion, proliferation, and angiogenesis in experimental glioma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009;15:3697–3704. doi: 10.1158/1078-0432.CCR-08-2042. [DOI] [PubMed] [Google Scholar]
- 110.Belter A., Rolle K., Piwecka M., Fedoruk-Wyszomirska A., Naskręt-Barciszewska M.Z., Barciszewski J. Inhibition of miR-21 in glioma cells using catalytic nucleic acids. Sci. Rep. 2016;6:24516. doi: 10.1038/srep24516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Seo Y., Suh H., Bahal R., Josowitz A., Zhang J., Song E., Cui J., Noorbakhsh S., Jackson C., Bu T., Piotrowski-Daspit A., Bindra R., Saltzman W. Nanoparticle-mediated intratumoral inhibition of miR-21 for improved survival in glioblastoma. Biomaterials. 2019;201 doi: 10.1016/j.biomaterials.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Berndt J.D., Moon R.T., Major M.B. Beta-catenin gets jaded and von Hippel-Lindau is to blame. Trends Biochem. Sci. 2009;34:101–104. doi: 10.1016/j.tibs.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 113.Paul I., Bhattacharya S., Chatterjee A., Ghosh M.K. Current understanding on EGFR and wnt/β-Catenin signaling in glioma and their possible crosstalk. Genes Cancer. 2013;4:427–446. doi: 10.1177/1947601913503341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hsieh H.-L., Sun C.-C., Wu C.-B., Wu C.-Y., Tung W.-H., Wang H.-H., Yang C.-M. Sphingosine 1-phosphate induces EGFR expression via Akt/NF-kappaB and ERK/AP-1 pathways in rat vascular smooth muscle cells. J. Cell. Biochem. 2008;103:1732–1746. doi: 10.1002/jcb.21563. [DOI] [PubMed] [Google Scholar]
- 115.Zhou J., Wang K.-C., Wu W., Subramaniam S., Shyy J.Y.-J., Chiu J.-J., Li J.Y.-S., Chien S. MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc. Natl. Acad. Sci. U. S. A. 2011;108:10355–10360. doi: 10.1073/pnas.1107052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Park M.-J., Kim M.-S., Park I.-C., Kang H.-S., Yoo H., Park S.H., Rhee C.H., Hong S.-I., Lee S.-H. PTEN suppresses hyaluronic acid-induced matrix metalloproteinase-9 expression in U87MG glioblastoma cells through focal adhesion kinase dephosphorylation. Cancer Res. 2002;62:6318–6322. [PubMed] [Google Scholar]
- 117.Lankat-Buttgereit B., Göke R. Programmed cell death protein 4 (pdcd4): a novel target for antineoplastic therapy? Biol. Cell. 2003;95:515–519. doi: 10.1016/j.biolcel.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 118.Zhu S., Wu H., Wu F., Nie D., Sheng S., Mo Y.-Y. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 119.Chen Y., Liu W., Chao T., Zhang Y., Yan X., Gong Y., Qiang B., Yuan J., Sun M., Peng X. MicroRNA-21 down-regulates the expression of tumor suppressor PDCD4 in human glioblastoma cell T98G. Cancer Lett. 2008;272:197–205. doi: 10.1016/j.canlet.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 120.Elstner A., Stockhammer F., Nguyen-Dobinsky T.-N., Nguyen Q.L., Pilgermann I., Gill A., Guhr A., Zhang T., von Eckardstein K., Picht T., Veelken J., Martuza R.L., von Deimling A., Kurtz A. Identification of diagnostic serum protein profiles of glioblastoma patients. J. Neurooncol. 2011;102:71–80. doi: 10.1007/s11060-010-0284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vincent A.M., Feldman E.L. Control of cell survival by IGF signaling pathways. Growth Horm. IGF Res. Off. J. Growth Horm. Res. Soc. Int. IGF Res. Soc. 2002;12:193–197. doi: 10.1016/s1096-6374(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 122.Trojan J., Blossey B.K., Johnson T.R., Rudin S.D., Tykocinski M., Ilan J., Ilan J. Loss of tumorigenicity of rat glioblastoma directed by episome-based antisense cDNA transcription of insulin-like growth factor I. Proc. Natl. Acad. Sci. U. S. A. 1992;89:4874–4878. doi: 10.1073/pnas.89.11.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu D., Watanabe H., Shibuya H., Miura M. Redundancy of radioresistant signaling pathways originating from insulin-like growth factor I receptor. J. Biol. Chem. 2003;278:6702–6709. doi: 10.1074/jbc.M209809200. [DOI] [PubMed] [Google Scholar]
- 124.Trojan J., Cloix J.-F., Ardourel M.-Y., Chatel M., Anthony D.D. Insulin-like growth factor type I biology and targeting in malignant gliomas. Neuroscience. 2007;145:795–811. doi: 10.1016/j.neuroscience.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 125.Li Y., Li W., Yang Y., Lu Y., He C., Hu G., Liu H., Chen J., He J., Yu H. MicroRNA-21 targets LRRFIP1 and contributes to VM-26 resistance in glioblastoma multiforme. Brain Res. 2009;1286:13–18. doi: 10.1016/j.brainres.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 126.Lee S.Y., Lee S.Y., Choi Y. TRAF-interacting protein (TRIP): a novel component of the tumor necrosis factor receptor (TNFR)- and CD30-TRAF signaling complexes that inhibits TRAF2-mediated NF-kappaB activation. J. Exp. Med. 1997;185:1275–1285. doi: 10.1084/jem.185.7.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li Y., Zhao S., Zhen Y., Li Q., Teng L., Asai A., Kawamoto K. A miR-21 inhibitor enhances apoptosis and reduces G(2)-M accumulation induced by ionizing radiation in human glioblastoma U251 cells. Brain Tumor Pathol. 2011;28:209–214. doi: 10.1007/s10014-011-0037-1. [DOI] [PubMed] [Google Scholar]
- 128.Ray D., Kiyokawa H. CDC25A phosphatase: a rate-limiting oncogene that determines genomic stability. Cancer Res. 2008;68:1251–1253. doi: 10.1158/0008-5472.CAN-07-5983. [DOI] [PubMed] [Google Scholar]
- 129.Suh H., Consiglio A., Ray J., Sawai T., D’Amour K., Fh F. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1 doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Papagiannakopoulos T., Shapiro A., Kosik K.S. MicroRNA-21 targets a network of key tumor-suppressive pathways in glioblastoma cells. Cancer Res. 2008;68:8164–8172. doi: 10.1158/0008-5472.CAN-08-1305. [DOI] [PubMed] [Google Scholar]
- 131.Quintavalle C., Donnarumma E., Iaboni M., Roscigno G., Garofalo M., Romano G., Fiore D., De Marinis P., Croce C.M., Condorelli G. Effect of miR-21 and miR-30b/c on TRAIL-induced apoptosis in glioma cells. Oncogene. 2013;32:4001–4008. doi: 10.1038/onc.2012.410. [DOI] [PubMed] [Google Scholar]
- 132.Coutts A.S., La Thangue N.B. The p53 response: emerging levels of co-factor complexity. Biochem. Biophys. Res. Commun. 2005;331:778–785. doi: 10.1016/j.bbrc.2005.03.150. [DOI] [PubMed] [Google Scholar]
- 133.Siegel P.M., Massagué J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat. Rev. Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 134.Perlman R., Schiemann W.P., Brooks M.W., Lodish H.F., Weinberg R.A. TGF-beta-induced apoptosis is mediated by the adapter protein Daxx that facilitates JNK activation. Nat. Cell Biol. 2001;3:708–714. doi: 10.1038/35087019. [DOI] [PubMed] [Google Scholar]
- 135.Lopez J., Tait S.W.G. Mitochondrial apoptosis: killing cancer using the enemy within. Br. J. Cancer. 2015;112:957–962. doi: 10.1038/bjc.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gressner O., Schilling T., Lorenz K., Schulze Schleithoff E., Koch A., Schulze-Bergkamen H., Lena A.M., Candi E., Terrinoni A., Catani M.V., Oren M., Melino G., Krammer P.H., Stremmel W., Müller M. TAp63alpha induces apoptosis by activating signaling via death receptors and mitochondria. EMBO J. 2005;24:2458–2471. doi: 10.1038/sj.emboj.7600708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mao H., LeBrun D.G., Yang J., Zhu V.F., Li M. Deregulated signaling pathways in glioblastoma multiforme: molecular mechanisms and therapeutic targets. Cancer Invest. 2012;30:48–56. doi: 10.3109/07357907.2011.630050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bush N.A.O., Chang S.M., Berger M.S. Current and future strategies for treatment of glioma. Neurosurg. Rev. 2017;40:1–14. doi: 10.1007/s10143-016-0709-8. [DOI] [PubMed] [Google Scholar]
- 139.Zhang S., Wan Y., Pan T., Gu X., Qian C., Sun G., Sun L., Xiang Y., Wang Z., Shi L. MicroRNA-21 inhibitor sensitizes human glioblastoma U251 stem cells to chemotherapeutic drug temozolomide. J. Mol. Neurosci. MN. 2012;47:346–356. doi: 10.1007/s12031-012-9759-8. [DOI] [PubMed] [Google Scholar]
- 140.Manero F., Gautier F., Gallenne T., Cauquil N., Grée D., Cartron P.-F., Geneste O., Grée R., Vallette F.M., Juin P. The small organic compound HA14-1 prevents Bcl-2 interaction with Bax to sensitize malignant glioma cells to induction of cell death. Cancer Res. 2006;66:2757–2764. doi: 10.1158/0008-5472.CAN-05-2097. [DOI] [PubMed] [Google Scholar]
- 141.Kouri F., Jensen S., Stegh A. The role of Bcl-2 family proteins in therapy responses of malignant astrocytic gliomas: Bcl2L12 and beyond. ScientificWorldJournal. 2012;2012 doi: 10.1100/2012/838916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shinoura N., Yoshida Y., Nishimura M., Muramatsu Y., Asai A., Kirino T., Hamada H. Expression level of Bcl-2 determines Anti- or proapoptotic function. Cancer Res. 1999;59:4119–4128. [PubMed] [Google Scholar]
- 143.Salakou S., Kardamakis D., Tsamandas A.C., Zolota V., Apostolakis E., Tzelepi V., Papathanasopoulos P., Bonikos D.S., Papapetropoulos T., Petsas T., Dougenis D. Increased Bax/Bcl-2 ratio up-regulates caspase-3 and increases apoptosis in the thymus of patients with myasthenia gravis. In Vivo. 2007;21:123–132. [PubMed] [Google Scholar]
- 144.Wong S.T.S., Zhang X.-Q., Zhuang J.T.-F., Chan H.-L., Li C.-H., Leung G.K.K. MicroRNA-21 inhibition enhances in vitro chemosensitivity of temozolomide-resistant glioblastoma cells. Anticancer Res. 2012;32:2835–2841. [PubMed] [Google Scholar]
- 145.Rodrigues A.R., Neto F.S.L., Lourenço L.G., Trevisan F.A., Cirino M.L.A., Nery B., Peria F.M., Pereira-da-Silva G., Tazima M.F.G.S., Tirapelli L.F., Tiezzi D.G., Junior C.G.C., Tirapelli D.P.C. Expression of oncogenic microRNA-21 in neurospheres and attached cells of a glioblastoma cell line increased after treatment with temozolomide and ionizing radiation. Genet. Mol. Res. 2019;18 doi: 10.4238/gmr18095. [DOI] [Google Scholar]
- 146.Gonzalvez F., Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene. 2010;29:4752–4765. doi: 10.1038/onc.2010.221. [DOI] [PubMed] [Google Scholar]
- 147.Deng X., Zhao W., Song L., Ying W., Guo X. Pro-apoptotic effect of TRAIL-transfected endothelial progenitor cells on glioma cells. Oncol. Lett. 2018;15:5004–5012. doi: 10.3892/ol.2018.7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sklansky B.D., Mann-Kaplan R.S., Reynolds A.F., Rosenblum M.L., Walker M.D. Proceedings: 4’-Demethyl-epipodophyllotoxin-beta-D-thenylidene-glucoside (PTG) in the treatment of malignant intracranial neoplasms. Cancer. 1974;33:460–467. doi: 10.1002/1097-0142(197402)33:2<460::aid-cncr2820330222>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 149.Mack F., Schäfer N., Kebir S., Stuplich M., Schaub C., Niessen M., Scheffler B., Herrlinger U., Glas M. Carmustine (BCNU) plus teniposide (VM26) in recurrent malignant glioma. Oncology. 2014;86:369–372. doi: 10.1159/000360295. [DOI] [PubMed] [Google Scholar]
- 150.Li F., Sethi G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim. Biophys. Acta. 2010;1805:167–180. doi: 10.1016/j.bbcan.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 151.Giunti L., da Ros M., Vinci S., Gelmini S., Iorio A.L., Buccoliero A.M., Cardellicchio S., Castiglione F., Genitori L., de Martino M., Giglio S., Genuardi M., Sardi I. Anti-miR21 oligonucleotide enhances chemosensitivity of T98G cell line to doxorubicin by inducing apoptosis. Am. J. Cancer Res. 2014;5:231–242. [PMC free article] [PubMed] [Google Scholar]
- 152.Tao J., Lu Q., Wu D., Li P., Xu B., Qing W., Wang M., Zhang Z., Zhang W. microRNA-21 modulates cell proliferation and sensitivity to doxorubicin in bladder cancer cells. Oncol. Rep. 2011;25:1721–1729. doi: 10.3892/or.2011.1245. [DOI] [PubMed] [Google Scholar]
- 153.Villodre E.S., Kipper F.C., Silva A.O., Lenz G., Lopez P.Lda C. Low dose of doxorubicin potentiates the effect of temozolomide in glioblastoma cells. Mol. Neurobiol. 2018;55:4185–4194. doi: 10.1007/s12035-017-0611-6. [DOI] [PubMed] [Google Scholar]
- 154.Boland W.K., Bebb G. Nimotuzumab: a novel anti-EGFR monoclonal antibody that retains anti-EGFR activity while minimizing skin toxicity. Expert Opin. Biol. Ther. 2009;9:1199–1206. doi: 10.1517/14712590903110709. [DOI] [PubMed] [Google Scholar]
- 155.Zheng Y., Bao J., Zhao Q., Zhou T., Sun X. A spatio-temporal model of macrophage-mediated drug resistance in glioma immunotherapy. Mol. Cancer Ther. 2018;17:814–824. doi: 10.1158/1535-7163.MCT-17-0634. [DOI] [PubMed] [Google Scholar]
- 156.Chaudhry M.A., Sachdeva H., Omaruddin R.A. Radiation-induced micro-RNA modulation in glioblastoma cells differing in DNA-repair pathways. DNA Cell Biol. 2010;29:553–561. doi: 10.1089/dna.2009.0978. [DOI] [PubMed] [Google Scholar]
- 157.Gwak H.-S., Kim T.H., Jo G.H., Kim Y.-J., Kwak H.-J., Kim J.H., Yin J., Yoo H., Lee S.H., Park J.B. Silencing of microRNA-21 confers radio-sensitivity through inhibition of the PI3K/AKT pathway and enhancing autophagy in malignant glioma cell lines. PLoS One. 2012;7:e47449. doi: 10.1371/journal.pone.0047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Toker A., Yoeli-Lerner M. Akt signaling and cancer: surviving but not moving on. Cancer Res. 2006;66:3963–3966. doi: 10.1158/0008-5472.CAN-06-0743. [DOI] [PubMed] [Google Scholar]
- 159.Li C., Zamore P.D. Preparation of antisense oligonucleotides to inhibit miRNA function. Cold Spring Harb. Protoc. 2018;2018 doi: 10.1101/pdb.prot097527. [DOI] [PubMed] [Google Scholar]
- 160.Krützfeldt J., Kuwajima S., Braich R., Rajeev K.G., Pena J., Tuschl T., Manoharan M., Stoffel M. Specificity, duplex degradation and subcellular localization of antagomirs. Nucleic Acids Res. 2007;35:2885–2892. doi: 10.1093/nar/gkm024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Corsten M.F., Miranda R., Kasmieh R., Krichevsky A.M., Weissleder R., Shah K. MicroRNA-21 knockdown disrupts glioma growth in vivo and displays synergistic cytotoxicity with neural precursor cell delivered S-TRAIL in human gliomas. Cancer Res. 2007;67:8994–9000. doi: 10.1158/0008-5472.CAN-07-1045. [DOI] [PubMed] [Google Scholar]
- 162.Shah K., Bureau E., Kim D.-E., Yang K., Tang Y., Weissleder R., Breakefield X.O. Glioma therapy and real-time imaging of neural precursor cell migration and tumor regression. Ann. Neurol. 2005;57:34–41. doi: 10.1002/ana.20306. [DOI] [PubMed] [Google Scholar]
- 163.Tang Y., Shah K., Messerli S.M., Snyder E., Breakefield X., Weissleder R. In vivo tracking of neural progenitor cell migration to glioblastomas. Hum. Gene Ther. 2003;14:1247–1254. doi: 10.1089/104303403767740786. [DOI] [PubMed] [Google Scholar]
- 164.Qin E.Y., Cooper D.D., Abbott K.L., Lennon J., Nagaraja S., Mackay A., Jones C., Vogel H., Jackson P.K., Monje M. Neural precursor-derived pleiotrophin mediates subventricular zone invasion by glioma. Cell. 2017;170 doi: 10.1016/j.cell.2017.07.016. 845-859.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Alexiou G.A., Tsamis K.I., Kyritsis A.P. Targeting Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL): A Promising Therapeutic Strategy in Gliomas. Semin. Pediatr. Neurol. 2015;22:35–39. doi: 10.1016/j.spen.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 166.Balyasnikova I.V., Prasol M.S., Ferguson S.D., Han Y., Ahmed A.U., Gutova M., Tobias A.L., Mustafi D., Rincón E., Zhang L., Aboody K.S., Lesniak M.S. Intranasal delivery of mesenchymal stem cells significantly extends survival of irradiated mice with experimental brain tumors. Mol. Ther. J. Am. Soc. Gene Ther. 2014;22:140–148. doi: 10.1038/mt.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Perlstein B., Finniss S.A., Miller C., Okhrimenko H., Kazimirsky G., Cazacu S., Lee H.K., Lemke N., Brodie S., Umansky F., Rempel S.A., Rosenblum M., Mikklesen T., Margel S., Brodie C. TRAIL conjugated to nanoparticles exhibits increased anti-tumor activities in glioma cells and glioma stem cells in vitro and in vivo. Neuro-Oncol. 2013;15:29–40. doi: 10.1093/neuonc/nos248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ralff M.D., Lulla A.R., Wagner J., El-Deiry W.S. ONC201: a new treatment option being tested clinically for recurrent glioblastoma. Transl. Cancer Res. 2017;6:S1239–S1243. doi: 10.21037/tcr.2017.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lee T.J., Yoo J.Y., Shu D., Li H., Zhang J., Yu J.-G., Jaime-Ramirez A.C., Acunzo M., Romano G., Cui R., Sun H.-L., Luo Z., Old M., Kaur B., Guo P., Croce C.M. RNA nanoparticle-based targeted therapy for glioblastoma through inhibition of oncogenic miR-21. Mol. Ther. J. Am. Soc. Gene Ther. 2017;25:1544–1555. doi: 10.1016/j.ymthe.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hanna J., Hossain G.S., Kocerha J. The potential for microRNA therapeutics and clinical research. Front. Genet. 2019;10:478. doi: 10.3389/fgene.2019.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dong X. Current strategies for brain drug delivery. Theranostics. 2018;8:1481–1493. doi: 10.7150/thno.21254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yang T., Fogarty B., LaForge B., Aziz S., Pham T., Lai L., Bai S. Delivery of small interfering RNA to inhibit vascular endothelial growth factor in zebrafish using natural brain endothelia cell-secreted exosome nanovesicles for the treatment of brain Cancer. AAPS J. 2017;19:475–486. doi: 10.1208/s12248-016-0015-y. [DOI] [PubMed] [Google Scholar]
- 173.Halle B., Marcusson E.G., Aaberg-Jessen C., Jensen S.S., Meyer M., Schulz M.K., Andersen C., Kristensen B.W. Convection-enhanced delivery of an anti-miR is well-tolerated, preserves anti-miR stability and causes efficient target de-repression: a proof of concept. J. Neurooncol. 2016;126:47–55. doi: 10.1007/s11060-015-1947-2. [DOI] [PubMed] [Google Scholar]
- 174.Banelli B., Forlani A., Allemanni G., Morabito A., Pistillo M.P., Romani M. MicroRNA in glioblastoma: an overview. Int. J. Genomics. 2017;2017 doi: 10.1155/2017/7639084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Brown R.A.M., Richardson K.L., Kalinowski F.C., Epis M.R., Horsham J.L., Kabir T.D., De Pinho M.H., Beveridge D.J., Stuart L.M., Wintle L.C., Leedman P.J. Evaluation of MicroRNA delivery in vivo, methods mol. Biol. Clifton NJ. 2018;1699:155–178. doi: 10.1007/978-1-4939-7435-1_12. [DOI] [PubMed] [Google Scholar]