Dear Editor,
Coronavirus disease-2019 (COVID-19) has been associated with life-threatening thromboembolic complications due to increased inflammation, marked hypercoagulability and endothelial activation.1, 2, 3 Preventing deep vein thrombosis (DVT) and pulmonary embolism is important, since ∼10% of COVID-19-related deaths are caused by pulmonary embolism complicating DVT.4 Recently, enhanced prophylactic or therapeutic anticoagulation dose regimens have been recommended by experts and adopted in some centres.5 , 6 However, direct comparative studies of the different anticoagulation regimens are lacking.
In our intensive care unit (ICU), we established a DVT prevalence of 46% in mechanically ventilated COVID-19 patients on standard prophylactic anticoagulation7 and subsequently, we increased anticoagulation to reduce thromboembolic complications. We designed this before-after observational exploratory study to evaluate the risk/benefit ratio of increased (IA) versus standard prophylactic anticoagulation (SPA) in mechanically ventilated COVID-19 patients. The study was part of the COVID-ICU and French COVID-19 cohort registries and received approval from the ethics committee of our institution (N°, IDRCB, 2020-A00256-33; CPP, 11-20.20.02.04.68737).
We included all consecutive patients admitted for COVID-19-related pneumonia requiring tracheal intubation. We excluded patients on long-term therapeutic anticoagulation before ICU admission. To diagnose DVT, an initial ultrasound was routinely performed during the first week after intubation, and in DVT-free patients, a second ultrasound was performed ∼1week later by certified sonographers (SV/PB) according to guidelines.8 The study was composed of two periods, defined according to the type of anticoagulation received from intubation to the first ultrasound examination. In both groups, if DVT was diagnosed, therapeutic anticoagulation was initiated.
Patients admitted from 2020/03/11 to 2020/04/01 (SPA group) received prophylactic anticoagulation with subcutaneous enoxaparin 40 mg once daily or unfractionated heparin 15000IU/day if creatinine clearance <15 mL/min. Patients admitted from 2020/04/02 to 2020/10/12 (IA group) received either prophylactic double-dose enoxaparin 40 mg twice daily or therapeutic anticoagulation with either enoxaparin 1 mg/kg twice daily or unfractionated heparin to reach plasma anti-Xa activity of 0.3–0.6IU/mL. Supportive care included optimized mechanical ventilation, vasopressors, sedation and muscular paralysis according to guidelines. Dexamethasone, antiviral and other immunomodulatory drugs were administered according to the physicians in charge.
The efficacy endpoint was the prevalence of femoral/popliteal DVT, known to be strongly associated with pulmonary embolism.9 The efficacy endpoint was also compared between patients treated with double-dose prophylactic enoxaparin (0.4 mg twice daily) and patients treated with standard enoxaparin prophylaxis (0.4 mg once daily). The safety endpoint was the number of patients with at least one major bleeding (MB) defined according to guidelines,10 i.e. bleedings causing death, decreasing hemoglobin by ≥2 g/dL, requiring transfusion of ≥2 blood units or occurring in a critical organ.
Quantitative variables are expressed as medians [25th–75th percentiles] and categorical variables as percentages. Parameters were compared between SPA and IA patients using Mann-Whitney and Fisher's exact tests as appropriate. An exploratory generalized multilinear regression model was built to adjust for parameters significantly different between groups. P-values ≤0.05 were considered significant. Based on the 26% prevalence of femoral/popliteal DVT in the SPA group and a presumed reduction to <5% in the IA group, 42 patients/group were required for 95% confidence interval and 80% statistical power.
Ninety-three patients were included, 50 in the SPA and 43 in the IA group. Baseline characteristics did not differ significantly between the groups (Table 1 ). The initial ultrasound was performed 2 days [1–4] post-intubation. Time from ICU admission to the first ultrasound was 4days [2–6] in the SPA versus 5days [3–8] in the IA group, P = 0.03. In 37 of the femoral/popliteal DVT-free patients, a second ultrasound was performed 8days [7–10] post-intubation. Anticoagulant treatment is presented in Table 1. At the time of the initial ultrasound C-reactive protein, fibrinogen and D-dimer were remarkably elevated at 223 mg/L [132–307], 7.6 g/L [6.2–8.6] and 3180 ng/mL [1495–6808], respectively. Twenty-nine patients (31%) required renal replacement therapy (RRT) while 12 (13%) were treated with extracorporeal membrane oxygenation (ECMO), 3/50 (6%) in the SPA and 9/43 (21%) in the IA group (P = 0.06).
Table 1.
Main characteristics, biological parameters, anticoagulant treatment and outcome in 93 mechanically ventilated COVID-19 patients.
| Parameters | All patients (N = 93) |
Standard prophylaxis (N = 50) |
Increased anticoagulation (N = 43) |
P |
|---|---|---|---|---|
| Patient characteristics | ||||
| Male gender, N (%) | 64 (69) | 36 (72) | 28 (65) | 0.51 |
| Age (years) | 63 [56–71] | 62 [54–69] | 65 [58–73] | 0.14 |
| Body mass index (kg/m2) | 29 [25–32] | 28 [25–31] | 30 [25–34] | 0.18 |
| Past hypertension, N (%) | 49 (53) | 23 (46) | 26 (60) | 0.21 |
| Diabetes, N (%) | 36 (39) | 22 (44) | 14 (33) | 0.29 |
| Ischemic heart disease, N (%) | 11 (12) | 9 (18) | 2 (5) | 0.58 |
| SOFA score on admission | 6 [3–8] | 6 [4–9] | 5 [3–8] | 0.14 |
| Main biological parameters | ||||
| PaO2/FiO2 (mmHg) | 151 [113–240] | 179 [117–258)] | 141 [110–188] | 0.15 |
| PT (ratio of normal) | 1.18 [1.12–1.27] | 1.17 [1.11–1.25] | 1.19 [1.14–1.31] | 0.17 |
| APTT (ratio of normal) | 1.23 [1.12–1.50] | 1.21 [1.10–1.43] | 1.29 [1.20–1.65] | 0.04 |
| Plasma fibrinogen (g/L) | 7.6 [6.2–8.6] | 8.1 [6.7–8.8] | 7.2 [6.1–8.1] | 0.07 |
| Plasma D-dimer (ng/mL) | 3180 [1495–5808] | 3500 [2000–7760] | 2710 [1465–4135] | 0.10 |
| White blood cells (G/L) | 10.4 [7.8–14.0] | 10.5 [8.0–13.8] | 9.8 [7.1–14.3] | 0.57 |
| Lymphocytes (G/L) | 0.72 [0.42–1.20] | 0.74 [0.45–1.14] | 1.0 [0.43–1.24] | 0.74 |
| Platelets (G/L) | 274 [197–367] | 271 [200–365] | 274 [194–372] | 0.99 |
| CRP (mg/L) | 223 [132–307] | 246 [180–304] | 180 [117–307] | 0.18 |
| Serum creatinine (µmol/L) | 98 [67–154] | 94 [70–150] | 99 [63–173] | 0.87 |
| Serum ALT (IU/L) | 33 [23–50] | 32 [21–48] | 34 [25–54] | 0.46 |
| Anti-COVID-19 and supportive treatments | ||||
| Lopinavir/ritonavir combination, N (%) | 12 (13) | 12 (24) | 0 (0) | 0.003 |
| Azithromycin, N (%) | 42 (45) | 16 (32) | 26 (61) | 0.01 |
| Hydroxychloroquine, N (%) | 25 (27) | 14 (28) | 11 (26) | 0.82 |
| Dexamethasone, N (%) | 41 (44) | 13 (26) | 28 (65) | 0.0002 |
| Vasopressor treatment, N (%) | 45 (49) | 27 (54) | 18 (42) | 0.21 |
| Renal replacement therapy, N (%) | 29 (31) | 14 (28) | 15 (35) | 0.51 |
| ECMO, N (%) | 12 (13) | 3 (6) | 9 (21) | 0.06 |
| Anticoagulation regimen | ||||
| Standard prophylaxis before initial ultrasound, N (%) | 50 (54) | 50 (100) | 0 (0) | <0.0001 |
| Standard prophylactic enoxaparin, N (%) | 42 (45) | 42 (84) | 0 (0) | <0.0001 |
| Standard prophylactic unfractionated heparin, N (%) | 8 (9) | 8 (16) | 0 (0) | 0.05 |
| Double-dose prophylactic enoxaparin, N (%) | 25 (27) | 0 (0) | 25 (58) | <0.0001 |
| Therapeutic anticoagulation before initial ultrasound, N (%) | 18 (19) | 0 (0) | 18 (54) | <0.0001 |
| Therapeutic enoxaparin before initial ultrasound, N (%) | 6 (6) | 0 (0) | 6 (14) | 0.01 |
| Therapeutic unfractionated heparin before ultrasound, N (%) | 12 (13) | 0 (0) | 12 (28) | <0.0001 |
| Endpoints | ||||
| Femoral/popliteal DVT, N (%) | 15 (16) | 13 (26) | 2 (5) | 0.01 |
| Femoral/popliteal DVT, on enoxaparin prophylaxis, N (%) | 13 (14) | 11 (22) | 1 (2) | 0.02 |
| DVT below the popliteal level, N (%) | 23 (25) | 12 (24) | 11 (26) | 1.0 |
| Major bleeding, N (%) | 18 (19) | 7 (14) | 11 (26) | 0.19 |
| Therapeutic anticoagulation at major bleeding, N (%) | 16 (17) | 9 (18) | 8 (19) | 1.0 |
| Death, N (%) | 38(44) | 18 (34) | 20 (56) | 0.08 |
DVT, deep vein thrombosis; SOFA score, Sepsis-related Organ Failure Assessment score; PaO2/FiO2, oxygen arterial partial pressure/fraction of inspired oxygen ratio; PT, prothrombin time; APTT, activated partial thromboplastin time; CRP, C-reactive protein; ALT, alanine-aminotransferase; ECMO, extracorporeal membrane oxygenation.
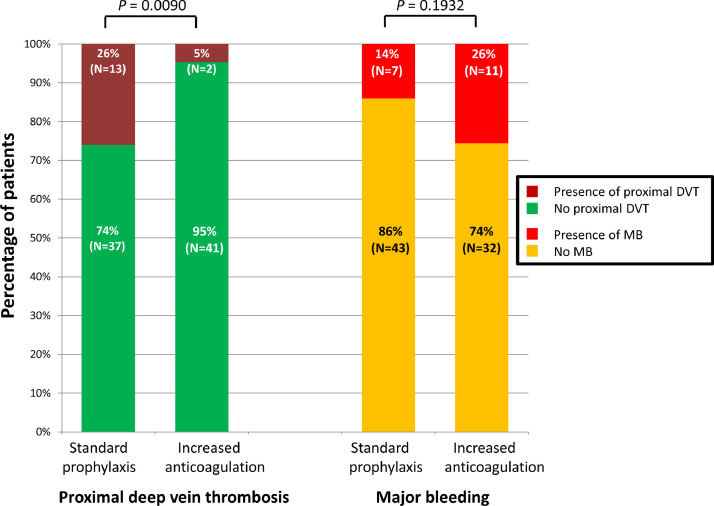
Prevalence of femoral/popliteal DVT was significantly reduced in the IA in comparison with the SPA group (two (5%) versus 13 (26%), P = 0.01; Fig. 1 ). The two DVT in the IA group and one DVT in the SPA group were associated with femoral central venous catheters. After adjustment for parameters significantly different between groups, anticoagulant treatment was the only factor associated with DVT (P = 0.02). Prevalence of femoral/popliteal DVT was decreased, i.e. 1/25 patients treated with enoxaparin 0.4 mg twice/day (2% of the IA group) versus 11/42 patients treated with enoxaparin 0.4 mg/day (22% of the SPA group), P = 0.02.
Fig. 1.
Before-after comparison of proximal deep vein thrombosis (DVT) and major bleeding (MB) prevalence in 93 mechanically ventilated COVID-19 patients.
MB occurred 10 days [8–13] post-intubation in 11 patients (26%) in the IA group versus seven patients (14%) in the SPA group (P = 0.19). MB occurred while on ECMO and/or RRT in 15/18 cases (83%). One patient died of intracranial hemorrhage. In patients treated with 0.4 mg/day enoxaparin in the SPA group, one MB occurred versus none in patients treated with enoxaparin 0.4 mg twice/day in the IA group. In the SPA group, 17/50 (34%) died as compared to 19/43 (44%) in the IA group (P = 0.08), while 7/43 (16%) are still hospitalized.
Our most important finding is that IA is associated with decreased femoral/popliteal DVT prevalence in comparison with SPA in mechanically ventilated COVID-19 patients. The second important finding is that enoxaparin 0.4 mg twice daily regimen5 seems effective for DVT prophylaxis compared with standard enoxaparin prophylaxis.
To our knowledge, this is the first study comparing SPA with IA strategies and showing a significant reduction in femoral/popliteal DVT using systematic ultrasound screening. Our data suggests that double-dose enoxaparin prophylaxis (40 mg twice daily) may have a favorable risk/benefit ratio, worth exploring in further studies. DVT below the popliteal level were not reduced in the IA group, suggesting that at this level, venous stasis and/or endothelial lesion may play a more important role than hypercoagulation.
Our study strength is that ultrasound was performed in all patients, avoiding biases related to the absence of systematic screening. Limitations include absence of randomization and small sample size precluding assessment of the effect on mortality.
In conclusion, using systematic ultrasound screening, we observed a decrease in femoral/popliteal DVT prevalence while increasing anticoagulation compared to standard prophylaxis in mechanically ventilated COVID-19 patients. The favorable risk/benefit ratio of prophylactic double-dose enoxaparin 40 mg twice-daily regimen is worth exploring in future studies.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgments
Consent for publication
All the authors agree to publish.
Funding
The study, analysis, and manuscript preparation were completed as part of official duties at the university hospital. There was no additional funding.
Ethics approval and consent to participate
The study was part of the COVID-ICU and French COVID-19 cohort registries and approved by our institutional ethics committee (N°, IDRCB, 2020-A00256-33; CPP, 11-20-20.02.04.68737).
Availability of data and materials
Drs. Voicu, Mebazaa and Mégarbane contributed to the study concept and design. Drs. Voicu, Choustermann, Deye, Malissin, Le Gall, Barthélémy, Sutterlin, Naïm, Mrad, Pépin-Lehalleur, Le Dorze, de Roquetaillade, Ekhérian, Gayat, Sidéris, Mebazaa, and Mégarbane contributed to the patient management. Drs. Voicu and Bonin performed lower limbs systematic duplex ultrasound. All authors contributed to the acquisition, analysis, or interpretation of data. Drs. Voicu and Mégarbane contributed to drafting the manuscript. All authors contributed to the critical revision of the manuscript for important intellectual content. Dr. Mégarbane has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
The authors would like to thank Drs. Virginie Siguret and Alain Stépanian for performing the coagulation tests and Mrs. Alison Good (Scotland, UK) for her helpful review of the manuscript.
References
- 1.Brendish N.J., Poole S., Naidu V.V., Mansbridge C.T., Norton N., Borca F., et al. Clinical characteristics, symptoms and outcomes of 1054 adults presenting to hospital with suspected COVID-19: a comparison of patients with and without SARS-CoV-2 infection. J Infect. 2020 doi: 10.1016/j.jinf.2020.09.033. S0163-4453(20)30638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L., Feng X., Zhang D., Jiang C., Mei H., Wang J., et al. Deep vein thrombosis in hospitalized patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020;142:114–128. doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- 3.Voicu S., Delrue M., Chousterman B.G., Stépanian A., Bonnin P., Malissin I., et al. Imbalance between procoagulant factors and natural coagulation inhibitors contributes to hypercoagulability in the critically ill COVID-19 patient: clinical implications. Eur Rev Med Pharmacol Sci. 2020;24:9161–9168. doi: 10.26355/eurrev_202009_22866. [DOI] [PubMed] [Google Scholar]
- 4.Edler C., Schröder A.S., Aepfelbacher M., Fitzek A., Heinemann A., Heinrich F., et al. Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020;134:1275–1284. doi: 10.1007/s00414-020-02317-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Susen S., Tacquard C.A., Godon A., Mansour A., Garrigue D., Nguyen P., et al. Prevention of thrombotic risk in hospitalized patients with COVID-19 and hemostasis monitoring. Crit Care. 2020;24:364. doi: 10.1186/s13054-020-03000-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chowdhury J.F., Moores L.K., Connors J.M. Anticoagulation in hospitalized patients with COVID-19. N Engl J Med. 2020;383:1675–1678. doi: 10.1056/NEJMclde2028217. [DOI] [PubMed] [Google Scholar]
- 7.Voicu S., Bonnin P., Stépanian A., Chousterman B.G., Le Gall A., Malissin I., et al. High prevalence of deep vein thrombosis in mechanically ventilated COVID-19 patients. J Am Coll Cardiol. 2020;76:480–482. doi: 10.1016/j.jacc.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Needleman L., Cronan J.J., Lilly M.P., Merli G.J., Adhikari S., Hertzberg B.S., et al. Ultrasound for lower extremity deep venous thrombosis: multidisciplinary recommendations from the society of radiologists in ultrasound consensus conference. Circulation. 2018;137:1505–1515. doi: 10.1161/CIRCULATIONAHA.117.030687. [DOI] [PubMed] [Google Scholar]
- 9.Konstantinides S.V., Meyer G., Becattini C., Bueno H., Geersing G.J., Harjola V.P., et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur Heart J. 2020;41:543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 10.Schulman S., Kearon C. Subcommittee on control of anticoagulation of the scientific and standardization committee of the international society on thrombosis and haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Drs. Voicu, Mebazaa and Mégarbane contributed to the study concept and design. Drs. Voicu, Choustermann, Deye, Malissin, Le Gall, Barthélémy, Sutterlin, Naïm, Mrad, Pépin-Lehalleur, Le Dorze, de Roquetaillade, Ekhérian, Gayat, Sidéris, Mebazaa, and Mégarbane contributed to the patient management. Drs. Voicu and Bonin performed lower limbs systematic duplex ultrasound. All authors contributed to the acquisition, analysis, or interpretation of data. Drs. Voicu and Mégarbane contributed to drafting the manuscript. All authors contributed to the critical revision of the manuscript for important intellectual content. Dr. Mégarbane has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.