Abstract
COVID-19 is an infectious respiratory disease caused by SARS-CoV-2, a new beta coronavirus that emerged in Wuhan, China. Being primarily a respiratory disease, it is highly transmissible through both direct and indirect contacts. It displays a range of symptoms in different individuals and thus has been grouped into mild, moderate, and severe diseases. The virus utilizes spike proteins present on its surface to recognize ACE-2 receptors present on the host cells to enter the cell cytoplasm and replicate. The viral invasion of cells induces damage response, pyroptosis, infiltration of immune cells, expression of pro-inflammatory cytokines (cytokine storm), and activation of the adaptive immune system. Depending on viral load and host factors like age and underlying medical conditions, the immune responses mounted against SARS-CoV-2 may cause acute respiratory distress syndrome (ARDS), multiple organ failure, and death. In this review, we specify and justify both viral and host therapeutic targets that can be modulated to relieve the symptoms and treat the disease. Furthermore, we discuss vaccine development in the time of pandemic and the most promising vaccine candidates by far, according to WHO database. Finally, we discuss the conventional re-purposed drugs and potential alternative treatments as adjuvants.
Keywords: COVID-19, SARS-CoV-2, Therapeutic targets, Re-purposed drugs, Vaccines
1. Introduction
Coronavirus disease 2019 or COVID-19 pandemic has infected 58,900,547 individuals and claimed 1,393,305 lives in 216 countries or territorial regions in approximately a year (WHO, 2020a). The pathogen responsible for COVID-19 was initially isolated from individuals with severe pneumonia of then-unknown cause in Wuhan city, Hubei Province, China, the epicenter of the outbreak in December 2019 (Lu et al., 2020a). The pathogen was identified as the seventh coronavirus that could infect humans and was named as SARS-CoV-2 due to its similarities with the sister virus, SARS-CoV (outbreak caused in 2003), (Gorbalenya et al., 2020 and Wu et al., 2020a). Since the initial cases in Wuhan were presented from and around the local seafood market or wet market, which is also a hub for live animal trading, an animal to human transmission of the virus was suggested (Singhal, 2020). Initially, the origin of SARS-CoV-2 has been controversial. While natural evolution from precursor viruses was a possibility, some scientists believed that it was genetically engineered in a laboratory that was working on bat CoV- RaTG13. However, the latter has been rejected since the nucleotide difference between the two viruses is distributed throughout the genome randomly instead of more targeted and logical insertions as would be expected in synthetic constructs, making RatG13 unlikely a direct source for SARS-Cov-2 (Liu et al., 2020). On a genomic level, SARS-CoV-2 is more similar to RatG13 (96.2%) and pangolin-CoV (91.02%) than SARS-CoV (70%), indicating both bats, and pangolins could be the reservoir hosts Zhang et al., 2020a. The SARS-CoV-2 could have been originated from a precursor that underwent natural selection in one of the animal hosts like bats or pangolins under high population density. Alternatively, it might have jumped to humans from the animal hosts and evolved through human to human transmissions. Finally, a less likely accidental laboratory release of a progenitor virus of SARS-CoV-2 or SARS-CoV-2 itself (Andersen et al., 2020) cannot be ruled out. Another debate that is uprising among the scientific community is the number of strains present for SARS-CoV-2. Genome analysis of the viral isolates from different countries report mutations in both coding and non-coding regions of the virus genome. Based on SNPs genotyping of 103 sequences, two subtypes of SARS-CoV-2 have been proposed-the S and the L subtype, where the L subtype has been evolved from S subtype (Tang et al., 2020a). A mutation in the spike protein D614G has been deemed to be of vital concern as the researchers claim it furnished high transmissibility to SARS-CoV-2 (Bhattacharyya et al., 2020). Nevertheless, the more important question is if these and many other mutations reported, give rise to different clinical manifestations as claimed on the basis of which we validate the presence of diverse strains of SARS-CoV-2.
SARS-CoV-2 is a 65–125 nm spherical enveloped virus composed of spike (S), membrane (M), envelope (E), and nucleocapsid (N) structural proteins. The N protein binds to the positive single-stranded RNA genome of the virus and forms nucleocapsid. The heavily phosphorylated nature of N protein not only enhances the affinity for viral RNA but also plays an essential role in virus replication. The M protein interacts with the nucleocapsid to give the virus its spherical shape and form an internal core. The E protein associates with the envelope and assists in the maturation and release of the virus. Finally, the spike (S) protein, a transmembrane glycoprotein of ~150 kDa protrudes from the surface of the virus, and aid viral entry into the host (Ashour et al., 2020) via Angiotensin-Converting Enzyme 2 (ACE 2) receptors. Spike is assembled as a homotrimeric surface protein and cleaved by the host cell protease into two subunits, viz., S1 and S2. The S1 head contains the receptor-binding domain (RBD), which provides cellular tropism while the S2 stalk mediates membrane fusion (Letko et al., 2020) of the transmitting host cells. Because alveolar type II cells in the lung abundantly express ACE2 receptors, SARS-CoV-2 infection primarily manifests pulmonary disease (Hamming et al., 2004). The modulation of the ACE2 expression on the epithelial cells due to underlying conditions like COPD and cigarette smoking may affect the susceptibility of an individual to SARS-CoV-2 infection (Lippi and Henry, 2020) (Brake et al., 2020)., Another imperative factor that influences viral entry is the presence of host proteolytic enzymes like TMPRSS2 and furin. Furin makes the first proteolytic cleavage at the S1/S2 interface followed by a second cleavage mediated by transmembrane protease type II (TMPRSS2) to activate S protein for effective membrane fusion (Hoffmann et al., 2020a). Once the virus-cell membrane fuse to the host's Hofmann and Pöhlmann, 2004), the RNA strand is injected into the cytoplasm of the host cell, where it hijacks the host's cellular machinery to make copies of the viral genome and translate viral proteins. Viral RNA dependent RNA polymerase (RdRp) is among the pioneer proteins to be translated for replicating viral genome and transcribing subgenomic mRNAs, which in turn code for structural and non-structural proteins. These proteins are then processed in the ER-Golgi apparatus and finally appropriately assembled with the viral RNA-nucleocapsid complex to bud out of the infected cell by exocytosis and invade the neighboring tissue (Shang et al., 2020a).
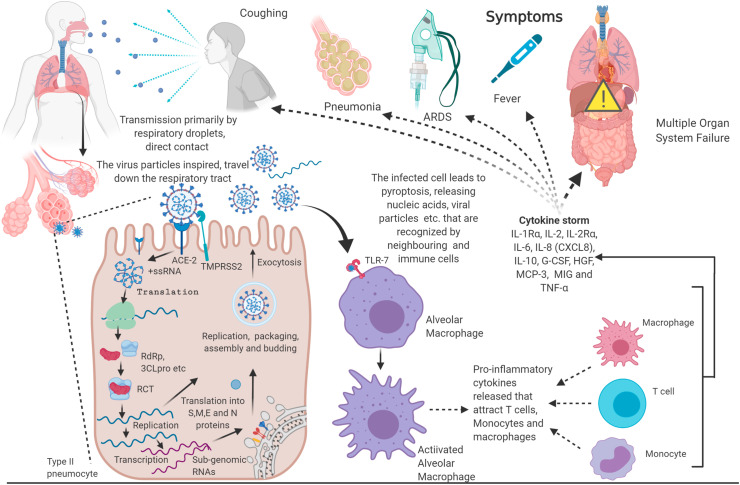
Virus particles (ssRNA, dsRNA, DNA, proteins) in the infected cells are recognized as pathogen-associated molecular patterns (PAMPs) by soluble pattern recognition receptors (PRRs) like NLRP3 inflammasome (da Costa et al., 2019). NLRP3 inflammasome is a multi-protein complex that once assembled and activated cleaves pro-caspase-1 into caspase 1, which in turn cleaves pro-IL-β, pro-IL-8 and pyroptotic factor gasdermin D (GSDMD) into their active form (Tang et al., 2020b). GDSMD makes the cell membrane porous inducing pyroptosis, an inflammatory programmed cell death, releasing PAMPS, DAMPS (Damage associated molecular patterns), IL-18, IL-β. These molecules are further recognized by toll-like receptors (TLRs), particularly TLR 3, 7, 8, and 9, present on the immune cells like - DCs, NKs, neutrophils, macrophages, T cells and B cells leading to their activation. Following this, these immune cells produce type 1 Interferon (IFN-1) and other pro-inflammatory cytokines (Prompetchara et al., 2020). The type of cytokines and chemokines released indicate the type of immune cells that are activated by the virus and the underlying disease mechanism. A recent meta-analysis study revealed that the levels of most cytokines- IL-1β, IL-1Rα, IL-2, IL-2Rα, IL-4, IL-5, IL-6, IL-7, IL-8 (CXCL8), IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, TNF-α, IFN-γ, IP-10 (CXCL10), GCSF, MIP-1α (CCL3), M-CSF, CTACK, GM-CSF, MCP-1 (CCL2), FGF basic, RANTES (CCL5), and Eotaxinin were elevated in COVID-19 patients when compared to healthy individuals. Among these, IL-1Rα, IL-2, IL-2Rα, IL-6, IL-8 (CXCL8), IL-10, G-CSF, HGF, MCP-3, and MIG were further elevated in severe infections whereas TNF-α was higher in patients in ICU care (Ramezani et al., 2020). Certain clinical reports have noted the increased concentration of TFH (follicular helper T Cells) cells, activated CD4+ and CD8+ T cells and antibody-secreting cells in COVID-19 patients (Thevarajan et al., 2020), (Xu et al., 2020a). In patients with severe infection, macrophages recruited neutrophils, and monocytic cells induce local inflammation while in moderate infections, macrophages induce T-cell proliferation was evident (Liao et al., 2020), (Merad and Martin, 2020). The extent of activation of these immune cells and the production of pro-inflammatory cytokines measures the severity of the disease pathology; a cytokine storm marks disease escalation. These cytokines are responsible for symptoms associated with COVID-19- fever, coughing, sneezing, chills, fatigue, pneumonia, haemoptysis, lymphopenia (systemic), ground-glass opacities in lungs and, in severe cases acute respiratory distress syndrome (ARDS) and multiple organ failure which are the cause of most mortalities (Rothan and Byrareddy, 2020) (Coperchini et al., 2020)., The respiratory droplets produced by infected individuals while coughing, sneezing and wheezing, direct, and indirect contact with the infected individuals are the primary source of transmission among individuals (World Health Organization, 2020) (Fig. 1 ).
Fig. 1.
Exclusive Features of COVID-19: SARS-CoV-2, the causative agent of COVID-19, is transmitted via direct, like coughing or indirect contact. SARS-CoV-2 invades the lung alveolar cells via ACE-2 receptors, wherein it replicates and assembles more virus particles, which then invade neighboring cells. Host immunity surveillance recognize the viral particles and mount an immune response that may be overdriven to cause ARDS, multi-system organ failure and death (Created with BioRender.com).
Nonetheless, the fecal-oral route of transmission is becoming more evident after a study wherein patients’ rectum swabs were positive for the virus even when their nasopharyngeal swabs were negative, making viral shedding from the digestive tract a potential route of transmission (Hindson 2020). Airborne transmission is uncommon as all the studies that have detected an RNA virus from air samples do not point to a viable transmissible virus. However, it is common in healthcare facilities during procedures that generate aerosols droplets of less than 5 μm in diameter (Wilson et al., 2020). The use of Personal Protective Equipment and surveillance of local infection and transmission is necessary for these healthcare settings. While social distancing, respiratory etiquette, maintaining self-hygiene, and environmental disinfection remain the WHO recommended preventive measures, an effective therapeutic measure is scarce. Herein, we review potential therapeutic targets and strategies, both conventional and alternative, along with vaccine candidates in clinical trials against COVID-19.
2. Viral and host therapeutic targets
The outbreak of the novel COVID-19 infection has put immense pressure on the research and clinical communities across the world for the development of therapeutics to combat the ever-growing pandemic. However, successful completion of drug development may require several years with no guarantee. Alternatively, the already established drugs can be repurposed to treat the COVID-19 infection. Whether it is the development of new drugs/vaccines or repurposing of commercial medications, precise information of the potential target(s) is required. These targets can be of two different types; they can be either of SARS-CoV-2 origin or can belong to the human immune system (Sanders et al., 2020). Sequencing of the viral genome and various computational methods has led (Wu et al., 2020b) to the identification of 18 viral proteins as a potential target for therapeutic intervention. These proteins can be distributed under the functions they perform 1) RNA synthesis and replication: 3CLpro(Mpro), PLpro, RdRp, helicase, Nsp3b, Nsp3e, Nsp7_Nsp8 complex, Nsp9-Nsp10, Nsp14-Nsp16, Nsp15, C-terminal RNA binding domain (CRBD) and N-terminal RNA binding domain (NRBD); 2) structural proteins: spike, TMPRSSS2, E-channel; 3) virulence factors: Nsp1, Nsp3c, ORF7a. In another study, a different viral protein called 2′-O-ribose methyltransferases, which also aids in replication by methylating the viral genome, was identified as a potential target for drug repurposing, (Kadioglu et al., 2020) (Sharma et al., 2020). The genetic material of SARS-CoV-2 has also been proposed as a potential target in a novel and efficient approach that employs CRISPR/Cas13 d system (Nguyen et al., 2020). The system uses three guide RNAs that target the ORF1ab and S sequences, separated by spacer sequences and Cas13 d, an RNA-cleaving enzyme, concisely delivered in an all-in-one adeno-associated virus (AAV) delivery.
Alternatively, the host factors that are involved in the pathological pathways could serve as potential drug targets. Affinity purification and mass-spectroscopy identified 67 human proteins that can be therapeutically targeted (O'Meara et al., 2020). Some of these proteins, for example, acyl-transferase complex, HDAC2, TRMT1, ANK-binding kinase 1 (TBK1), TANK-binding kinase 1-binding protein 1 (TBKBP1/SINTBAD), E3 ubiquitin ligases, IkB kinase, NF-KB signaling molecules, Tom70, NUP98-RAE1, etc. Can be either inhibited or stimulated to fight the SARS-CoV-2 infection. Because SARS-CoV-2 interacts with the ACE-2 receptor, it has become a significant therapeutic target to fight COVID-19 infection Zhang et al., 2020b. The components of the immune system have also been proposed as a therapeutic target; for instance, modulation of TLR5 has been shown to strike-off SARS-CoV-2 infection (Golonka et al., 2020), (Wang et al., 2020a).
Moreover, components of inflammatory pathways involved in the pathophysiology of COVID-19 are significant therapeutic targets (Channappanavar et al., 2016). For instance, high mobility group box 1 (HMGB1) protein induces inflammation and has been projected as a therapeutic target (Street 2020). A systematic analysis is necessary to evaluate the feasibility of these therapeutic targets by screening for the potential treatments available.
3. Lead candidates for vaccine
Conventionally, the development of a vaccine is a linear multi-step process that takes years to identify a vaccine candidate and many more years to accomplish multiple efficacy and safety checks, validation of the process, and large-scale manufacturing. However, during a pandemic, the linear steps are overlapped, and the system works in a parallel manner, making the potentialvaccine candidate less viable and the manufacturers at possible financial risk (Lurie et al., 2020). The current situation calls for strong international ties and coordinated efforts for the development of vaccines, policies, funding, manufacturing, and distribution. Given the urgent need for a vaccine to fight an infectious disease like COVID-19, already established platforms are being utilized for the development of vaccination by early 2021 (Le et al., 2020). According to WHO, currently, there are 180 vaccine candidates out of which 36 candidates are in the clinical trial (Table 1 ), while 145 are in pre-clinical investigations (WHO 2020c).
Table 1.
Lead vaccine candidates in clinical trials.
| Types of Vaccine | ||||
|---|---|---|---|---|
| (NonReplicating Viral Vector) | Nucleic Acid | Inactivated | Protein Subunit | Others |
| ChAdOx1-S (Chimpanzee Adenovirus Vectored Vaccine Oxford 1 encoding S protein) University of Oxford/AstraZeneca Phase III |
mRNA-1273 LNP encapsulated mRNA Moderna/NIAID Phase III |
Wuhan Institute of Biological Products/Sinopharm Phase III |
NVX-CoV2373 Nanoparticle-based SARS CoV-2 spike protein vaccine with adjuvant Novavax Phase IIb |
Plant-based- viral particle (VPL) Medicago and Université Laval, Quebec, Canada Phase I |
| Adenovirus Type 5 Vector CanSino Biological Inc./Beijing Institute of Biotechnology Phase III |
BNT162 LNP-mRNAs BioNTech/Fosun Pharma/Pfizer Phase III |
Beijing Institute of Biological Products/Sinopharm Phase III |
S-Trimer subunit vaccine candidate (SCB-2019) Dynavax Technologies Corporation, and Clover Biopharmaceuticals, a China Phase I |
Replicating Measles based Vector Institute Pasteur/Themis/Univ. of Pittsburg CVR/Merck Sharp & Dohme Phase I |
| Gam-COVID-Vac Adeno Vector Gamaleya Research Institute of Epidemiology and Microbiology, Russia Phase III |
DNA plasmid vaccine with electroporation Inovio Pharmaceuticals Phase I/II |
Inactivated + Alum Sinovac Phase III |
COVAX-19 recombinant SARS-CoV-2 spike protein + polysaccharide-based adjuvant platform, Advax Vaxine, Australia and Medytox, South Korea Phase I |
Intranasal flu-based-RBD Replicating vector Beijing Wantai Biological Pharmacy/Xiamen University Phase I |
| Ad26COVS1 Janssen Pharmaceutical Companies Phase III |
LNP-nCoVsaRNA saRNA Imperial College London Phase I |
Inactivated Institute of Medical Biology and Chinese Academy of Medical Sciences Phase I/II |
Adjuvanted recombinant protein (RBD-Dimer) Anhui Zhifei Longcom Biopharmaceutical/Institute of Microbiology, Chinese Academy of Sciences Phase II |
RBD-HBsAg VLPs (Virus like Particle) SpyBiotech and Serum Institute of India Phase I/II |
| Research Institute for Biological Safety Problems, Rep of Kazakhstan Phase I/II |
CVnCoV mRNA CureVac Phase II |
COVAXIN Bharat Biotech and Indian Council of Medical Research Phase I/II |
KBP-201 RBD-Based Kentucky BioProcessing, Inc. Phase I/II |
|
| Adenoviral Vector based vaccine ReiThera (Rome)/LEUKOCARE (Munich)/Univercells (Brussels) Phase I |
DNA Plasmid Vaccine Zydus Cadila Healthcare Limited, India Phase I/II |
Inactivated SARS-CoV-2 Beijing Minhai Biotechnology Co., Ltd., China. Phase I |
Adjuvanted recombinant protein-based vaccine Sanofi Pasteur/GSK Phase I/II |
|
| VXA-CoV2-1 Ad5 adjuvanted Oral Vaccine platform Vaxart Phase I |
GX-19 DNA Vaccine Genexine Phase I/IIa |
Spike protein with MF59 adjuvant stabilized with molecular clamp University of Queensland/CSL/Seqirus Phase I |
||
| MVA-SARS-2-S Ludwig-Maximilians - University of Munich German Center for Infection Research Philipps University Marburg Medical Center Phase I |
DNA Plasmid + Adjuvant Takara Bio Inc., Japan with Osaka University and AnGes, Japan Phase I/II |
S–2P protein + CpG 1018 Medigen Vaccine Biologics Corp + NIAID + Dynavax Phase I |
||
| Ad5-nCoV CanSino Biological Inc/Institute of Biotechnology, Academy of Military Medical Sciences, PLA of China Phase I |
Arcovax mRNA People's Liberation Army (PLA) Academy of Military Sciences, Suzhou Abogen Biosciences and Walvax Biotechnology Co., Ltd Phase I |
Receptor Binding Domain + Adjuvant Instituto Finlay de Vacunas, Cuba Phase I |
||
| ARCT-021 mRNA-based Arcturus TherapeuticsInc. Phase I/II |
Peptide FBRI SRC VB VECTOR, Rospotrebnadzor, Koltsovo Phase I |
|||
| Receptor Binding Domain West China Hospital, Sichuan University Phase I |
||||
| SARS-CoV-2 HLA-DR peptides University Hospital Tuebingen Phase I |
||||
| UB-612 S1-RBD-protein COVAXX Phase I |
||||
Moderna, a Boston based company and researchers at the University of Oxford, published the results of their eagerly anticipated clinical trials, (Jackson et al., 2020) (Phillips et al., 2020). The Moderna mRNA-1273 is an mRNA-based vaccine that codes for a complete prefusion stabilized spike (S) protein of SARS-CoV-2 encapsulated in a novel lipid nanoparticle (LNP). Moderna trial included 45 healthy adults, 18–55 years of age. Individuals received two vaccinations, 28 days apart with dose ranging from 25 to 200 mg. The mRNA-1273 vaccine-elicited anti-SARS-CoV-2 immune response in all participants without any safety concern. Importantly, the 100 μg dose was found to induce a high neutralizing response. Unlike mRNA-1273, the oxford candidate vaccine (AZD1222) has been adapted from chimpanzee's adenovirus-vectored vaccine (ChAdOx1 nCoV-19) expressing the SARS-CoV-2 spike protein (Van Doremalen et al., 2020). Healthy adults aged 18–55 years randomly assigned (1:1) to receive ChAdOx1 nCoV-19 at a dose of 5 × 101⁰ viral particles or control meningococcal conjugate vaccine (MenACWY) as a single intramuscular injection. Neutralizing antibody responses against SARS-CoV-2 were detected in 35 (100%) participants when measured in PRNT50. Importantly, Neutralizing antibody responses correlated strongly with antibody levels measured by ELISA (R2 = 0.67 by Marburg VN; P < 0.001). Both candidate vaccines are now in the process of Phase II/III clinical trial.
Interestingly, a multi-center randomized, double-blind, placebo-controlled, phase 2 trial of the Ad5-vectored COVID-19 vaccine was conducted in Wuhan, China (Zhu et al., 2020). Healthy 508 adults aged 18 years or older randomly received the vaccine (1 × 1011 viral particles n = 253; 5 × 101⁰ viral particles n = 129) or placebo (n = 126). Both doses of the vaccine-induced significant neutralizing antibody responses against SARS-CoV-2. Severe adverse reactions were reported by 24 (9%) participants in the 1 × 1011 viral particles dose group and one (1%) participant in the 5 × 101⁰ viral particles dose group. The authors claimed that the Ad5-vectored COVID-19 vaccine at 5 × 101⁰ viral particles is safe and induced significant immune responses in the majority of recipients after a single immunization. Recently, Gam-COVID-Vac (NCT04436471, NCT04437875) (clinicaltrials.gov, 2020a) (clinicaltrials.gov, 2020b), an adeno-based vaccine candidate carrying the SARS-CoV-2 gene for spike protein manufactured by Gamaleya Research Institute of Epidemiology and Microbiology, Russia was declared safe and effective in eliciting an immune response by Russian officials. The corresponding two-stage, non-randomized clinical trials have recruited 38 participants in total; in the first stage, 18 participants will receive the drug with two different adenovirus components-rAd26 and rAd5. The vaccine is expected to enter phase III in August. In a global consortium involving BioNTech, Fosun Pharma, and Pfizer, four vaccine candidates were developed with different RNA formats with Lipid nanoparticle formulation: BNT162a1, BNT162b1, BNT162b2, and BNT162b3. Two of these candidates use modified mRNA (modRNA), while the third and fourth candidates are based on uridine RNA (uRNA) and self-amplifying RNA (saRNA). Three of these candidates- BNT162b1, BNT162b2, and BNT162b3 have now advanced to a randomized combined phase I/II/III clinical trial (NCT04368728) (clinicaltrials.gov, 2020c). The trial aims at evaluating the safety, tolerability, efficacy, and dosing of the three vaccine candidates with placebo control in 32000 volunteers distributed among three age groups (18–55, 65–85 and 18–85 years). Recently it was reported that the vaccine candidate- BNT162b2 was more than 90% effective in an initial phase three study (pfizer.com, 2020). A DNA vaccine candidate, INO-4800 that encodes for S protein, was developed and evaluated in pre-clinical trials by INOVIO Pharmaceuticals, USA, Wistar Institute, Philadelphia, USA, Public Health England, UK, Advaccine, China and University of Texas, USA (Smith et al., 2020). INO-4800 shows vigorous T-cell response and antibody neutralization to block binding of Spike protein to ACE2 receptor in mice and guinea pigs. The phase I trial concluded that the candidate was safe and well-tolerated among 40 participants with no adverse effects. Imperial College of London has successfully developed a saRNA based candidate vaccine encapsulated in a lipid-based nanoparticle; the vaccines candidate has moved from the initial phase to next where larger cohorts will be dosed (ISRCTN17072692) (clinicaltrials.gov, 2020d). CVnCoV, another mRNA-based vaccine candidate manufactured by CureVac has entered clinical trials44 (Le et al., 2020).
Sinopharm, China, has collaborated with Wuhan Institute of Biological Products and Beijing Institute of Biological, separately to develop and evaluate inactivated vaccine candidate that is currently being evaluated in phase III of clinical trials (ChiCTR2000034780) in Abu Dhabi, UAE in 5000 individuals aged 18–60 years (chictr.org.cn, 2020a). Sinovac Biotech Ltd has manufactured purified inactivated SARS-CoV-2 virus plus alum vaccine candidate (PicoVacc), which is currently being tested in Phase I/II clinical trials in healthy adults aged 18–59 years (NCT04352608) and in healthy adults aged ≥ 60 years (NCT04383574), (clinicaltrials.gov, 2020e) (clinicaltrials.gov, 2020f). In pre-clinical studies, Picovac was found to have broader neutralization against ten different strains of SARS-CoV-2 without inducing enhancement of infection (Gao et al., 2020). The Institute of Medical Biology at the Chinese Academy of Medical Sciences, China, has produced another inactivated candidate vaccine that has recently entered Phase II for clinical trials (clinicaltrials.gov, 2020g).
In another approach, Novavax, USA, has successfully manufactured a protein subunit vaccine candidate, NVX-CoV2373- nanoparticle-based prefusion, stable recombinant SARS-CoV-2 spike protein with Novavax’ proprietary Matrix-M as an adjuvant. The candidate is currently in Phase I of clinical trials being tested for safety and immunogenicity with or without the adjuvant in healthy subjects (clinicaltrials.gov, 2020h). In a collaboration tie, Clover Biopharmaceuticals, China, and Dynavax Technologies, California, USA, have begun Phase I clinical trials for their vaccine candidate, which is a spike-trimer protein of SARS-CoV2 along with a toll-like receptor 9 (TLR9) agonist adjuvant (dynavax.com, 2020). New viral candidates are being developed in different parts of the world, and an up-to-date brief summary of such candidates that have advanced to clinical trials can be found in Table 1.
4. Re-purposed small molecule drugs for COVID-19
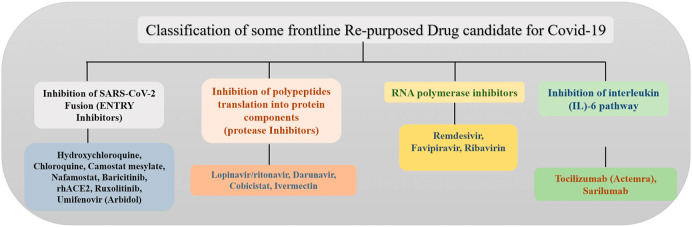
Undoubtedly, the development and manufacturing of a vaccine are crucial in preventing the COVID-19 pandemic. However, even under extreme pressure of a pandemic and with rigorous efforts being made, the development of a safe and effective vaccine is time-consuming. The repurposing of existing drugs against SARS, MERS, HIV/AIDS, and malaria has proven to be an effective alternate strategy for the treatment of COVID-19 (Li and De Clercq, 2020). Drug repurposing strategy can potentially shorten the time and as well as reduce the cost compared to develop novel drugs (N. Kumar et al., 2020). In this section, we have classified and discussed some frontline re-purposed drugs based on their mode of action against SARS-COV-2 (see Fig. 2, Fig. 11(Table 2 ).
Fig. 2.
Classification of some frontline repurposed drug candidates based on their mechanism of action against SARS-CoV-2.
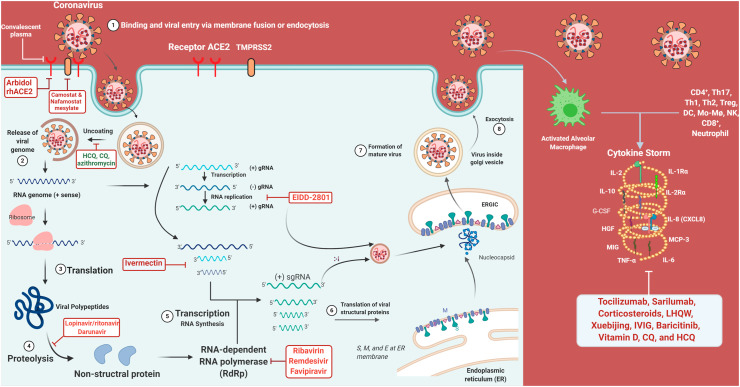
Fig. 11.
Repurposed drugs and their targets: Representation of inhibitory effects of the re-purposed drug on crucial steps of viral replication and disease manifestation (Created with BioRender.com).
Table 2.
Selected therapeutic drugs and their combinations for COVID-19 in clinical trials.
| Drug/Combination | Administration route/Posological data | Proposed Mechanism of Action against SARS CoV-2 |
Trial Phase | Common Repercussions | References |
|---|---|---|---|---|---|
| Acalabrutinib with supportive care | Oral route/NA | Bruton's tyrosine kinase inhibitor | I | headache, diarrhea | clinicaltrials.gov (2020o) |
| Arbidol (Umifenovir) | Oral route/2 tables per time, 3 times a day for 14–20 days | Antiviral | IV | Drug sensitization in children | clinicaltrials.gov (2020p) |
| Umifenovir, Interferon-β 1a, Lopinavir/Ritonavir, Single Dose of Hydroxychloroquine, Standards of Care |
Anti-viral | IV | NA | clinicaltrials.gov (2020q) | |
| Atorvastatin | Oral route/40 mg | Competitive inhibitor of HMG-CoA reductase | II | diarrhea, heartburn, muscle pains, | Clinicaltrials.gov (2020r) |
| Atzanavir | NA | Protease inhibitor | III | Gastrointestinal upset, nausea | clinicaltrials.gov (2020s) |
| Nitazoxanide and atazanavir/ritonavir | Oral/tablets | Protease inhibitor | II | Gastrointestinal upset, nausea | clinicaltrials.gov (2020t) |
| Avipatadil | 67 μg (nebulized) | inhibit IL6, and TNF-α production | I | Alteration in blood pressure, ECG or heart rate | clinicaltrials.gov (2020u) |
| Azithromycin | Oral route | Prevents translation of mRNA binding with the bacterial ribosome | IV | diarrhea, nausea, abdominal pain, and vomiting | clinicaltrials.gov (2020v) |
| Atovaquone/Azithromycin | Oral route | Antiprotozoal Agents Antiparasitic Agents |
II | clinicaltrials.gov (2020w) | |
| Ivermectin, Azithromycin, Cholecalciferol |
Oral route | Antihelmintic | NA | clinicaltrials.gov (2020x) | |
| Baricitinib | I.V/2 mg | Janus Kinase inhibitor | III | fever, sweating, muscle aches, | clinicaltrials.gov (2020y) |
| BDB-001 | Injection/NA | Toll-like receptor (TLR) against, Immunostimular | II | Nystagmus, dizziness | clinicaltrials.gov (2020z) |
| BLD-2660 | Oral route/NA | Antiviral, Calpains inhibitor | II | diarrhea, nausea, vomiting | (clinicaltrials.gov, 2020a’) |
| Canakinumab | I.V infusion/750 mg (max dose) | Anti-human-IL-1β monoclonal antibody, Prevent IL-6 release | III | Fever, sweating, stomach pain, diarrhea, | (clinicaltrials.gov, 2020b’) |
| CD24Fc | I.V./Single dose at Day 1, CD24Fc, 480 mg, diluted to 100 ml | Immunomodulator, suppress the expression of inflammatory cytokines | III | Safe | (clinicaltrials.gov, 2020c’) |
| Chlorhexidine, povidone-iodine, saline water. | Oral and nasal rinse/5 cc + 20 cc of nasal rinses + oral gargles, for 7 days- 4 times each day (or all three) | Antiseptic, binds with the anionic cell wall of bacteria this, at high concentration causes membrane disruption and cell death | II | Deafness, ARDS Kidney problem, high blood sodium, metabolic acidosis | clinicaltrials.gov, 2020d’ |
| Chloroquine | Oral Route/500 mg TID for 7 days | Antimalarial, lower endosomal PH, prevent glycosylation of ACE2 enzyme | III | mental problems, abdominal cramps, headache, diarrhea, etc. |
clinicaltrials.gov, 2020e’ |
| Chloroquine, telemedicine | Oral route/NA | IV | clinicaltrials.gov, 2020f’ | ||
| CHLORPROMAZINE (CPZ), Standard of Care (SOC) Chlorpromazine |
Oral route/50 mg | Antipsychotics | III III |
Drowsiness, dizziness, dry mouth |
clinicaltrials.gov, 2020g’ clinicaltrials.gov, 2020h’ |
| Clevudine | Oral route/120 mg once a day for 14 days | Antiviral, causes early viral DNA chain termination | II | May cause mitochondrial toxicity, | clinicaltrials.gov, 2020i’ |
| Corticosteroids | Oral route/40 mg q12 h for 5 days | Immunosuppressiveanti-inflammatory, anti-proliferative | NA | Hypertension, psychosis, hyperkalemia, | (clinicaltrials.gov, 2020j’) |
| Deferoxamine | I.V/NA | Iron chelator | II | Fever, vomiting, hearing loss | (clinicaltrials.gov, 2020k’) |
| Dexamethasone | I.V/1 mg/kg/day ivgtt for 7 days | Anti-inflammatory | III | Cataracts, bone loss, bruising, thrush | clinicaltrials.gov, 2020l’ |
| Ebastine | NA | Antihistamine, 2nd generation H1 receptor agoinst | II | Headache, dry mouth and drowsiness | chictr.org.cn (2020b) |
| EIDD-2801 | Oral route/200 mg | Antiviral | II | No severe side effects | clinicaltrials.gov, 2020m’ |
| Favipiravir | Oral route/NA | Inhibit viral RdRp | IV | Harm babies in pregnant women | clinicaltrials.gov, 2020n’ |
| Hydroxychloroquine (HCQ) | Oral route/400 mg | Lower endosomal PH | II | Nausea, vomiting, heart damage. | clinicaltrials.gov, 2020o’ |
| HCQ, Oseltamivir, Azithromycin | Oral route/200 mg; 75 mg; 500 mg | III | clinicaltrials.gov, 2020p’ | ||
| HCQ and Nitazoxanide | Oral route/200 mg; 500 mg | III | clinicaltrials.gov, 2020q’ | ||
| HCQ vs. Azithromycin | Oral route/400 mg; 500 mg | I | clinicaltrials.gov, 2020r’ | ||
| HCQ and favipiravir | NA | II | clinicaltrials.gov, 2020s’ | ||
| Ifenprodil | Oral route/20 mg TID | Anti-inflammatory | III | Can cause lung injury | clinicaltrials.gov, 2020t’ |
| IFX-1 with Best supportive care | I.V/NA | Targets pro-inflammatory | III | acute severe infusion reactions | clinicaltrials.gov, 2020u’ |
| Isotretinoin | Oral route/0.5 mg/kg/day | Down regulator of ACE2 receptor, PLpro inhibitor, increase CD4 count | III | Dry skin, itching, psychiatric disorders | clinicaltrials.gov, 2020v’ |
| Ivermectin | Oral route/a Single dose (3 mg) of Ivermectin | Ivermectin activates glutamate-gated chloride channels; | III | Stomach pain, headache, itching, vomiting, fever | clinicaltrials. gov, 2020w’ |
| Ivermectin, Nitazoxanide | Oral route/200mcg/kg of Ivermectin | Ivermectin activates glutamate-gated chloride channels; nitazoxanide interfere enzyme-dependent electron transfer | III | Stomach pain, headache, itching, vomiting, fever | clinicaltrials.gov, 2020x’ |
| Lenalidomide | Oral route/25 mg | Antiangiogenic agent, interact with the ubiquitin E3 ligase cereblon and target this enzyme to degrade the Ikaros transcription factors IKZF1, IKZF3 | IV | Vomiting, itching, joint pain, thrombosis, pulmonary embolus, hepatotoxicity, bone marrow toxicity etc. | clinicaltrials.gov, 2020y’ |
| Lopinavir/ritonavir | Oral route/200 mg lopinavir, 50 mg ritonavir Oral route/200 mg and 400 mg respectively |
Bind viral proteases | II | Diarrhea, nausea, vomiting, stomach pain | clinicaltrials.gov, 2020z’ |
| Lopinavir/ritonavir, Emtricitabine/tenofovir, raltegravir (RAL) | Oral/Lopinavir/Ritonavir 400/100 mg | III | clinicaltrials.gov, 2020a’’ | ||
| Lopinavir/rit., Ribavirin and Interferon beta-1b | 1 & 2 oral route, 3 S.C injection/400mg/100 mg, 400 mg′ 0.25 mg respectively | II | clinicaltrials.gov, 2020b’’ | ||
| Lopinavir/ritonavir, and HCQ | I.V infusion,/200 mg; oral route/400 mg lopinavir and 100 mg ritonavir; S.C injection, and 44 μg; 400 mg oral route respectively | III | clinicaltrials.gov, 2020c’’ | ||
| Losartan | Oral route/50 mg daily | Angiotensin receptor blocker | II | Diarrhea, insomnia, muscle pain, fetal death | clinicaltrials.gov, 2020d’’ |
| NA-831 | Oral route/30 mg | NA | II | Safer | clinicaltrials.gov, 2020e’’ |
| NA-831, Atazanavir, Dexamethasone |
Oral route/30 mg, 200 mg, 4 mg respectively | NA | III | Safer | |
| Olokizumab | Oral route/64 mg | Block IL-6 | III | side effect due to antimicrobial activity of IL-6 | clinicaltrials.gov, 2020f’’ |
| Piclidenoson | Oral route/ | A3 adenosine receptor against, deregulate Wnt/β-catenin pathway | II | No severe side effects | clinicaltrials.gov, 2020g’’ |
| Prazosin | Oral route | prevent cytokine storm, Alpha-blocker | II | Headache, nausea, blurredness | clinicaltrials.gov, 2020h’’ |
| Remdesivir | I.V infusion/NA | It mimics adenosine and interferes with RdRp | III | Hypertriglyceridemia, | clinicaltrials.gov, 2020i’’ |
| Remicade | NA | TNF-α inhibitor | II | Sinus infections | clinicaltrials.gov, 2020j’’ |
| Ruxolitinib | Oral route/5 mg | Kinase inhibitor | II | Pancytopenia, thrombocytopenia, anemia, neutropenia | clinicaltrials.gov, 2020k’’ |
| Sildenafil citrate | Oral route/0.1 g per day for 14 days | Phosphodiesterase inhibitor | III | Headache, flushing, upset stomach | clinicaltrials.gov, 2020l’’ |
| TAK 981 | Intravenous/60 min infusion (60 mg) | a selective inhibitor of SUMOlysation enzymatic cascade, | II | Potential liver toxicity | clinicaltrials.gov, 2020m’’ |
| Telmisartan | Oral route/40 mg | Angiotensin receptor blocker | II | Tachycardia, bardycardia, hypotension | clinicaltrials.gov, 2020n’’ |
| Oral route/80 mg twice daily | IV | clinicaltrials.gov, 2020o’’ | |||
| Tocilizumab | I.V injection/8 mg/kg (max 800mg/dose) | Immunosuppressive | II | Urinary tract infection, acute pyelonephritis | clinicaltrials.gov, 2020p’’ |
| Tocilizumab, Dexamethasone | Oral route/Tocilizumab: 8 mg/kg, Dexamethasone: 10 mg | Imunosuppressive | II | Urinary tract infection, acute pyelonephritis | clinicaltrials.gov, 2020q’’ |
| Tranexamic acid | Oral route/1300 mg p.o. 3 times/day for 5 days | Antifibrinolytics | II | Rare | clinicaltrials.gov, 2020r’’ |
| Vazegepant (BHV-3500) | Intranasal/10 mg intranasal for 14 days | Anti-inflammatory antagonist | III | Safer | clinicaltrials.gov, 2020s’’ |
| Vitamin-C (ascorbic acid) | Infusion/12 g twice a day for 7 days | Anti-inflammatory, | II | Nausea, abdominal cramps | clinicaltrials.gov, 2020t’’ |
| Vitamin-D (cholecalciferol) | Oral route/400,000 IU | Immunomodulatory; induces secretion of antimicrobial peptides | III | Increase in urination and thirst | clinicaltrials.gov, 2020u’’ |
| XAV-19 | Infusion/0.5 mg per kg | Heterologous glyco-humanized polyclonal antibody (GH-pAb) raised in swine binds spike protein of SARS-CoV-2 | II | Blood transfusion-related risks | clinicaltrials.gov, 2020v’’ |
4.1. Inhibition of SARS-CoV-2 fusion (ENTRY inhibitors)
The endocytic entry of coronavirus involves complexation of transmembrane spike (S) glycoprotein of the virus with angiotensin-converting enzyme 2 (ACE2) and TMPRSS2 (De Savi et al., 2020). Hence, targeting these interactions and blocking the endolysosomal pathway can lead to a potential treatment for SARS-CoV-2. Here, we describe the re-purposed drugs that can potentially target the fusion of SARS-CoV-2 into the host cells.
4.1.1. Hydroxychloroquine & chloroquine
For decades, Hydroxychloroquine (HCQ) and Chloroquine (CQ) (Fig. 3) have been well-known drugs to treat malaria. HCQ is also able to treat rheumatoid arthritis and systemic lupus erythematosus (Savarino et al., 2003). However, HCQ and CQ have some side effects as well, for instance, headache, dizziness, diarrhea, stomach cramps, and vomiting.
Fig. 3.
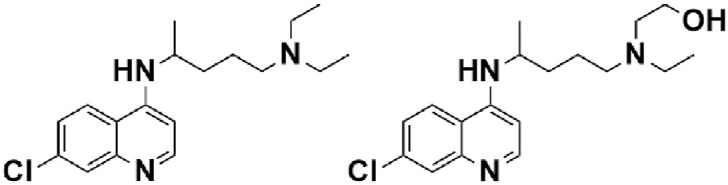
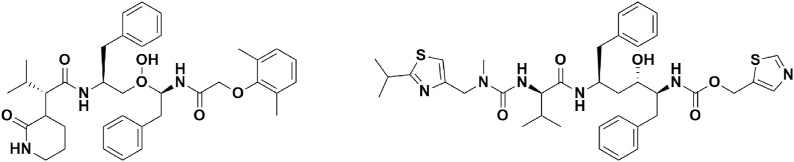
Chemical structure of Chloroquine and Hydroxychloroquine.
In malaria, CQ and HCQ affect heme polymerase process by the accumulation of cytotoxic heme that kills the parasite. Although the exact mechanism is not known, it is believed that these drugs enhance endosomal pH, thereby affecting the fusion of SARS-CoV-2 into host cells (Vincent et al., 2005). In addition, HCQ and CQ can also diminish the cytokine storm by interfering with the IL-6 pathway. There are over 77 clinical trials that have been registered on HCQ by various companies and institutions. The combination therapy of these two drugs with azithromycin and zinc have also been proposed but led to some severe side effects such as fatal arrhythmia (Gautret et al., 2020). On June 5, 2020, RECOVERY trial recommended that there are no significant improvements in hospitalized COVID-19 patients after treating with HCQ (recoverytrial.net, 2020a). A randomized study was conducted on 1542 patients, and compared with 3132 patients with standard care, it was observed that there was no substantial difference in the 28-day mortality endpoint (25.7% HCQ vs. 23.5% standard care; p = 0.10). As on June 15, 2020, FDA revoked the emergency use of hydroxychloroquine and chloroquine to treat patients with COVID-19, considering the emerging cases of side effects associated with these two drugs (fda.gov, 2020a). However, in a clinical trial involving 2541 patients, treated with HCQ or azithromycin or a combination of two showed encouraging results and It was concluded that when controlling for COVID-19 risk factors, treatment with the only HCQ and along with azithromycin was associated with a reduction in COVID-19 associated mortality (Arshad et al., 2020).
4.1.2. Umifenovir (arbidol)
Arbidol (Fig. 4) is a promising antiviral agent currently being used in Russia and China for the treatment of COVID-19 based on its previous studies. It is an indole-based antiviral drug with no significant side effects. It is established in numerous in-vitro and in-vivo studies (Blaising et al., 2014) that arbidol has a broad-spectrum activity against the various infectious disease, which makes arbidol a potential drug candidate against SARS-CoV-2. Arbidol targets the interactions of spike glycoprotein (S) of coronavirus and ACE2 and inhibits the membrane fusion of the viral envelope (Kadam and Wilson, 2017). Currently, five clinical trials have been registered, which involve arbidol. In March 2020, a study conducted in China observed that 69 COVID-19 patients who were given arbidol showed lower mortality rates and higher discharge rates in a median duration of 9 days (Wang et al., 2020b). The efficacy of arbidol is also being evaluated in combination with Bromhexine Hydrochloride tablets and recombinant human interferon-α 2 b (clinicaltrials.gov, 2020i). In June 2020, DCGI, Government of India gave its approval to CSIR-Central Drug Research Institute (CDRI) Lucknow, to commence randomized, placebo, and double-blind phase III clinical trial to authenticate efficacy and safety of arbidol (csir.res.in., 2020) (CSIR, 2020). Arbidol hydrochloride is currently in randomized phase IV clinical trials in the USA, wherein 380 COVID-19 patients will be given a dose of 200 mg arbidol orally every 8 h to evaluate efficacy and safety of this drug (NCT04260594) (clinicaltrial.gov, 2020j). The requirement of a large number of doses to achieve peak plasma concentration and therapeutic efficacy is one of the major drawbacks of the drug.
Fig. 4.
Chemical structure of Umifenovir (Arbidol).
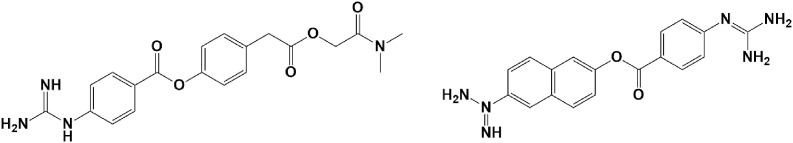
4.1.3. Camostat & nafamostat
Camostat (Fig. 5) is a serine protease inhibitor manufactured by Ono Pharmaceutical Co. Ltd in 1977. The current form of the drug is also valuable in the treatment of cancer (Okuno et al., 2002) and some viral infections (Hsieh and Hsu., 2007). Nafamostat mesylate(Fig. 5), another serine protease inhibitor, is commonly used as an anticoagulant (Al‐Horani and Desai, 2014), and also as an antiviral and anticancer drug (Chen et al., 2019).
Fig. 5.
Chemical structure of Camostat and Nafamostat mesylate.
Both Camostat and Nafamostat mesylate are reported to block the entry of the virus through inhibiting the host serine protease TMPRSS2 Hoffmann et al., 2020b. Camostat confirmed to partly block SARS-CoV by inhibiting the TMPRSS2 in HeLa cell lines (Kawase et al., 2012). Recently, a study reported that camostat could block SARS-CoV-2 in a mouse model at a concentration that is tolerated in humans (clinicaltrials.gov, 2020k). These studies make this drug a suitable candidate to treat patients infected with SARS-CoV-2. Currently, there are six clinical trials registered to substantiate the efficacy of Camostat for COVID-19 in Germany, Netherland, Denmark, and Japan (clinicaltrials.gov, 2020l).
4.1.4. Baricitinib
Baricitinib (Fig. 6) , a Janus kinase (JAK) inhibitor, is currently sold for the treatment of rheumatoid arthritis (RA) (fda.gov, 2020b). It selectively and reversibly binds JAK receptors and is successful in inhibiting JAK1/2 subtype. It effectively disrupts the cytokine-mediated signal transduction through JAKs and discourages the development of inflammatory response (Genovese et al., 2016).
Fig. 6.
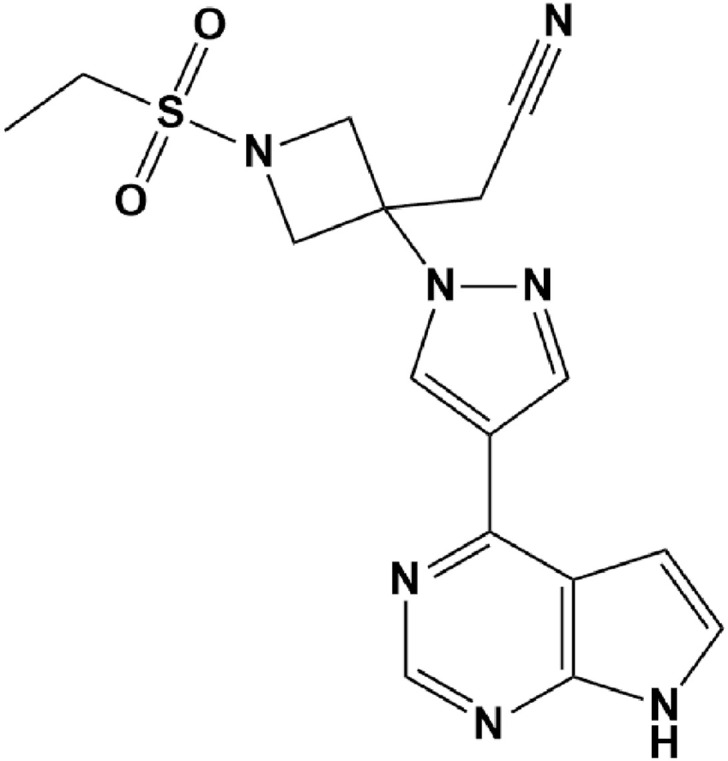
Chemical structure of Baricitinib.
Baricitinib also prevents the fusion of the virus into the host cells by inhibiting the AAK1 receptor, a member of the kinase family, and one of the regulators of endocytosis (Lu et al., 2020b). Thus, Baricitinib may be a potential option to treat Covid-19. It has been reported that 2–4 mg of Baricitinib is suitable for inhibiting the AAK1 receptor and may be useful in the treatment of COVID patients (Favalli et al., 2020a). Pharmacokinetic studies suggest that there is 79% oral bioavailability in humans, and approximately 1hr is sufficient to reach peak plasma concentration. Some of the side effects associated with this drug are malignancy and thrombosis (Kuriya et al., 2017). There are sixteen clinical trials registered by various organizations to determine the efficacy of this drug in different combinations such as hydroxychloroquine, ritonavir (clinicaltrials.gov, 2020m), and remdesivir (nih.gov, 2020) against COVID-19.
4.2. Inhibition of Polypeptide translation into protein components (protease inhibitors)
Upon the fusion of viral and host cell membranes, SARS-CoV-2 injects the positive-sense single-stranded RNA (+SSRNA) into the cytoplasm. The RNA binds to the ribosomes for translation of polyproteins, which in turn give rise to structural and non-structural proteins. Among these, 3-chymotrypsin-like protease (3CLpro) and papain-like protease (PLpro) play an essential role in the virus’ life cycle since they cleave the polyproteins (PP1A and PP1AB) into functional viral proteins, making them a suitable therapeutic target (Chen et al., 2020). Computer-aided drug design has been employed to annotate the potential inhibitors for proteases (bD. Kumar et al., 2020).
4.2.1. Lopinavir/ritonavir (Kaletra, LPV/r)
Lopinavir/ritonavir (LPV/r) (Fig. 7) , a fixed-dose combination of two protease inhibitors-lopinavir and ritonavir is sold under the trade name Kaletra for the treatment and prevention of HIV/AIDS.
Fig. 7.
Chemical structure of Lopinavir & ritonavir.
During the 2003 SARS outbreak, LPV/r was shown to be effective against inhibiting SARS-CoV replication by inhibiting protease function (Chu et al., 2004). Moreover, triple combination therapy with LPV/r, ribavirin, and IFN-α has produced impressive results against MERS (Kim et al., 2016). Despite the fact that HIV and SARS-COV-2 proteases belong to a different family (Hsu et al., 2004), a combinational therapy of Chinese medicine, LPV, interferon, RTV, and corticosteroids (3–5 days) has shown excellent results in the treatment of 50 patients in China (Jian-ya, 2020). An older adult was successfully cured with LPV and RTV (LPV 200 mg/RTV50 mg) in South Korea (Lu et al., 2020). Cao group has reported promising results of LPV/r monotherapy in 199 patients in Wuhan (Cao et al., 2020). However, it has been concluded in a new study that the LPV combination should be retained until the completion of the WHO SOLIDARITY trial (Dalerba et al., 2020). On June 25, 2020, the RECOVERY trial concluded that there are no significant improvements in hospitalized COVID-19 patients after treating with LPV/r (recoverytrial.net, 2020b) (see ).
4.3. RNA polymerase inhibitors
RNA dependent RNA polymerase (RdRp) is a multisubunit protein that plays a critical role in the replication and transcription of the viral genome; therefore, RdRp emerges as a primary target to disrupt viral infection. Essentially there are two types of polymerase inhibitors based on their mode of action-nucleos(t)ide inhibitors (NIs) and non-nucleoside inhibitors (NNIs). NIS when get phosphorylated bind the polymerase at its active site and inhibit chain elongation. Despite their excellent efficacy, they implicate toxic side effects-hepatotoxicity, nephrotoxicity, reproductive toxicity and thus their use is discouraged in pregnant women and babies. Nevertheless, certain studies published on the use of remdesivir in pregnant COVID-19 patients did not cause an adverse site effect (Igbinosa et al., 2020). On the other hand, NNIs bind the polymerase at its allosteric site, modifying the conformation and thus the polymerase activity is halted with low toxicity and side effects. Nucleotide analogs have been used as successful antiviral agents as they can effectively block RdRp activity and inhibit viral replication. In this section, we have discussed the potential use of RdRp inhibitors, such as remdesivir and favipiravir.
4.3.1. Remdesivir
Remdesivir (RDV) (Fig. 8) , previously known as GS-5734, is one of the frontline drug candidates for SARS-CoV-2. Remdesivir is an analog of 1′-cyano-substituted adenosine triphosphate nucleoside and is effective against a broad spectrum for viral infections. Moreover, it is a phosphoramidate prodrug, which gets metabolized into the body as GS-441524, a ribonucleotide analog. Gilead Sciences developed remdesivir as a potential drug for Ebola and Marburg virus disease in 2005 (Scavone et al., 2020). Soon after, in-vivo studies of remdesivir demonstrated efficacy against SARS-CoV and MERS-CoV (Sheahan et al., 2017). Some of the side effects associated with this drug are low blood pressure, inflammation in the liver, and sweating (ema.europa.eu, 2020)ema.europa.eu, 2020.
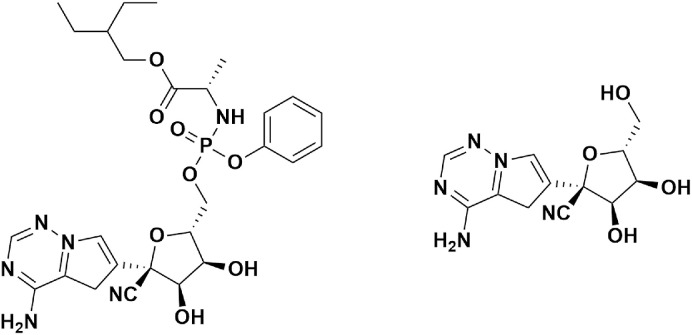
Fig. 8.
Chemical structure of Remdesivir (GS-5734) & Active form GS-441524.
In April 2020, remdesivir was considered a potential candidate drug and included in clinical trials by SOLIDARITY and European Discovery trial to establish efficacy against COVID-19 (Karki, 2020), (inserm.fr., 2020). Wang et al. reported promising in-vitro studies of remdesivir on human cell lines against SARS-CoV-2 infection40 (Wang et al., 2020a). In January 2020, the first US COVID-19 infected patient (35-year-old man) recovered after intravenous administration of remdesivir without any adverse side effects (Holshue et al., 2020). According to the recent clinical trials, the current dose for the infected patient is 200 mg on day one, followed by 100 mg daily intravenously administration of remdesivir for 5–10 days (clinicaltrials.gov, 2020n). Currently, 19 clinical trials are underway to evaluate the efficacy of remdesivir. As of June 1, 2020, Gilead Sciences announced Phase III trial results of remdesivir in patients with moderate to severe COVID-19. It is reported that moderate cases of COVID-19 patients show 65% of clinical improvement on a 5-day dose of remdesivir than patients who had standard care of 11 days (P = 0.077) (gilead.com, 2020). Also, in another published report, 64% and 54% of severe COVID-19 patients who were treated with five and a 10-day course of remdesivir, respectively, have shown clinical improvement by day 14 compared to those who received standard care (Goldman et al., 2020). Though the results are not statistically significant, remdesivir has emerged as a leading drug for the SARS-CoV-2. The US, India, Singapore, and Japan approved remdesivir for emergency treatment of hospitalized patients with severe Covid-19. However, On October 15, 2020, WHO concluded in their solidarity trials that Remdesivir have little or no effect on hospitalized COVID Patients (Pan et al., 2020).
The administration of the Remdesivir in the patients has been a major problem since the drug gets largely metabolized by the body in the oral delivery and does not affect the viral infection site effectively. Recently, to enhance the absorption rate and efficacy of the Remdesivir, a research group in University of Texas has developed inhaled form of Remdesivir (dry powder) using thin film freezing (TFF) (Sahakijpijarn et al., 2020). It was observed in the in-vivo studies that only 10 mg/kg dose of dry powder form of Remdesivir achieved necessary EC50 value to inhibit the viral infection. On July 8, 2020, Gilead Sciences has also initiated randomized, placebo-controlled Phase 1a clinical trials of inhaled solution of Remdesivir to evaluate safety, pharmacokinetics and tolerability of the drug in the non-hospitalized patients (Gilead.com, 2020).
4.3.2. Favipiravir
Favipiravir (FPV) (Fig. 9) , sold under the brand name Aviga® is an antiviral agent developed for the treatment of influenza ABC viruses in Japan (Du, and Chen, 2020). Favipiravir is an analog of pyrazine carboxamide. Once administered, its rapid phosphorylation generates pharmacologically active form favipiravir-RTP (ribofuranosyl-5′-triphosphate). The FPV-RTP serves as a substrate for RdRp, which results in the termination of the nascent RNA chain and inhibition of virus replication (Furuta et al., 2005). It has shown efficacy against a broad spectrum of viruses such as Ebola, arenavirus, H1N1, filovirus, and bunyaviruses, (Delang et al., 2018) (Sissoko et al., 2016).
Fig. 9.
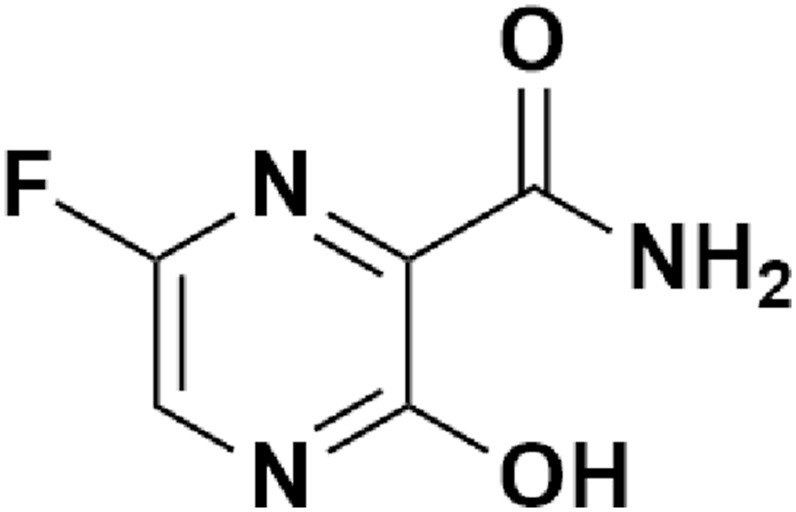
Chemical structure of Favipiravir.
Recently, In-vitro studies of favipiravir in Vero E6 cells established EC50 of 61.88 μMol and showed efficacy against SARS-CoV-2 (Wang et al., 2020a). Also, in another recent clinical trial in China reported promising results in chest imaging in the favipiravir arm (91.43%/62%) (Cai et al., 2020). However, several clinical trials are underway to evaluate the efficacy of favipiravir against SARS-CoV-2. Currently, Japan and the USA have commenced phase 3 and phase 2 clinical trials, respectively (reuters.com, 2020). Russian Ministry of Health has also recommended the use of favipiravir (rdif.ru, 2020) after observing the efficacy of favipiravir >80% in COVID-19 patients. As of June 19, 2020, the Government of India has given regulatory approval to the Glenmark Pharmaceutical company for oral antiviral drug Favipiravir (under the brand name FabiFlu® to treat mild to moderate COVID-19 patients in India after promising results of phase 3 clinical trials (glenmarkpharma.com, 2020a). Glenmark recommended 1800 mg dose twice daily on day one, followed by 800 mg twice daily up to day 14 (glenmarkpharma.com, 2020b). Glenmark has also commenced clinical trials of favipiravir and umifenovir as a combination therapy in India (clinicaltrialsarena.com, 2020).
4.4. Inhibition of interleukin (IL)-6 pathway
The most striking clinical feature of COVID-19 is the presence of elevated pro-inflammatory cytokines in the sera of COVID-19 patients. Severe cases display even higher levels of cytokines giving rise to “cytokine storm,” which further increases disease severity leading to ARDS, multiple organ failure, and death. Therefore, targeting cytokine production could be a potential therapeutic option for severely infected patients with COVID-19 (Coperchini et al., 2020). The elevated level of IL-6 has been linked to the severity of the disease and as a prognostic marker for the negative outcome. Tocilizumab and sarilumab are some of the repurposed drugs, which can inhibit the IL-6+ receptor and downstream signaling cascade.
4.4.1. Tocilizumab (Actemra)
Tocilizumab (Fig. 10) is an immunosuppressant drug that is sold under the brand name Actemra® and was developed by Hoffmann-La Roche and Chugai (Venkiteshwaran, 2009). It is a recombinant humanized monoclonal antibody of IgG1 subclass, which targets IL-6R, both membrane-bound and soluble forms. Binding of tocilizumab to IL-6R prevents its dimerization and interaction with GP130 transmembrane receptor leading to inhibition of downstream JAK-STAT or MAPK signaling pathways and attenuation of inflammatory responses. Tocilizumab is approved in the EU to treat rheumatoid arthritis (Oldfield et al., 2009), and is administered intravenously (dose-8 mg/kg) in combination with methotrexate. Tocilizumab follows both linear and nonlinear pharmacokinetics with a half-life of 11hr for 4kmg/kg dose. In China, out of 21 COVID-19 patients who were treated with tocilizumab (400 mg per day), 20 recovered within two weeks without any side effects associated with tocilizumab (Xu et al., 2020b). A recent study has reported promising results of intravenous tocilizumab in severely infected patients (Guaraldi et al., 2020). Treatment with tocilizumab reduced excessive use of ventilation and reduced the death count with an adjusted hazard ratio of 0·61, 95% CI 0·40–0·92; P = 0·020. In order to evaluate the efficacy and safety of tocilizumab and remdesivir, Genentech has initiated a Phase III, randomized, double-blind, multicenter study (REMDACTA) (gene.com, 2020).
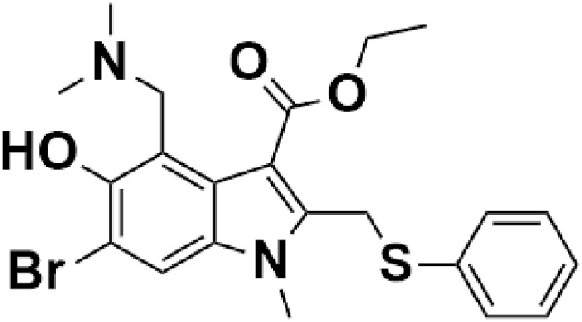
Fig. 10.
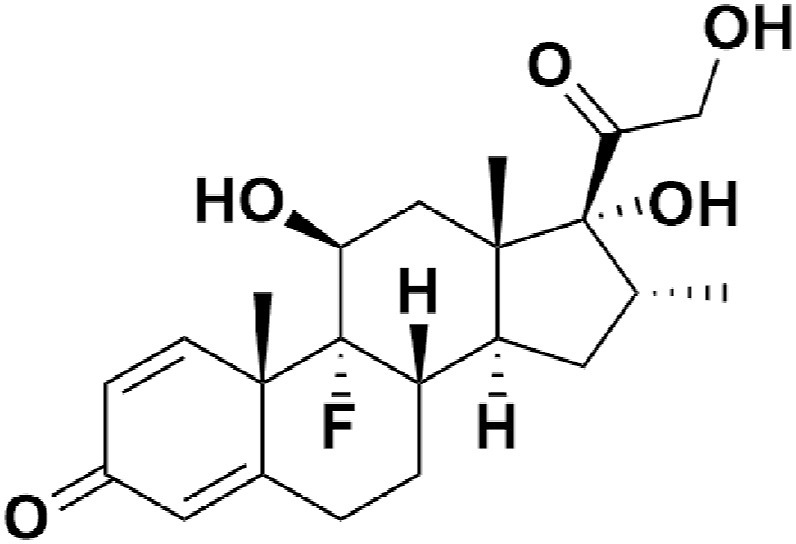
Chemical structure of dexamethasone.
5. Corticosteroids
Corticosteroids are broad-spectrum drugs, known to attenuate inflammation. They are used for the treatment of autoimmune and inflammatory diseases, including inflammatory bowel disease, asthma, rheumatoid arthritis, and allergy (Schäcke et al., 2002). Their use is controversial due to the side effects associated with their use, such as Cushing's syndrome, diabetes, hypertension, and skin atrophy, which questions the safety and effectiveness of the drug (Becker 2013). Some of the previous clinical trials using corticosteroids for the treatment of SARS, MERS, and H1N1 were limited due to the suppression of host immune responses (Arabi et al., 2018) (Han et al., 2011) (Stockman et al., 2006). Nevertheless, reduced mortality rates and lesser hospital stays of 401 patients infected with SARS (Chen et al., 2006) were reported. A recent study involving 10 COVID-19 patients, administered with a dose of 190 mg/day of corticosteroid and a dose of 20 g/day of immunoglobulin, showed improved results without any adverse side effects (Zhou et al., 2020). However, in a separate study of 416 COVID-19 patients, it was observed that improved results by administering corticosteroid and gamma globulin are associated with low lymphocyte counts in patients (Shang et al., 2020b).
Corticosteroids are currently administered in the systematic and inhaled form. Administration of inhaled corticosteroids (ICS) is still in the shadow of uncertainty, since a study of OpenSAFELY group suggested that ICS in 966,461 patients with asthma and chronic disease did not show good results (Schultze et al., 2020). Nonetheless, ICS has been proven to be effective in reducing 50% ARDS in high risk patients (Ortiz-Diaz et al., 2011). In-vitro studies in infected epithelial cells also support role of ICS in inhibiting replication step of coronavirus (Yamaya et al., 2020). Inhaled Ciclesonide has also been reported effective in blocking SARS-CoV-2 replication in vitro studies with EC90 0.55 μM (Matsuyama et al., 2020). Inhaled Ciclesonide has also successfully treated three mild cases of covid patients in Hong Kong in low dose of drug, however efficacy of this drug cannot be established in such small sample (Iwabuchi et al., 2020).
5.1. Dexamethasone
Dexamethasone, cheaply available corticosteroids, was approved for the treatment of inflammatory diseases (Fischer and Ganellin, 2010). On June 16, 2020, researchers from Oxford University announced a major breakthrough in a clinical trial of severely ill COVID-19 patients. It was observed that the administration of dexamethasone significantly reduced the deaths of patients (ox.ac.uk, 2020) ox.ac.uk, 2020.
This trial commenced in March 2020 under the randomized RECOVERY program in which 2104 patients were given 6 mg per day dose of dexamethasone (oral/intravenous) for ten days compared to 4321 patients who received standard care treatment. It was observed that dexamethasone significantly reduced deaths by one-third (p = 0.0003) and one-fifth (p = 0.0021) in patients receiving ventilators support and supplemental oxygen, respectively in the 28-days mortality endpoint. However, no benefits were observed in mild to moderate patients who did not require any respiratory care.
On September 2, 2020, based on the findings WHO, it was recommended that 6 mg of dexamethasone orally or intravenously daily for 7–10 days in patients with severe COVID-19 (WHO, 2020d).
6. Convalescent Plasma therapy
Convalescent plasma therapy (CPT) is a passive immunization initially used against Diptheria in 1890 (Behring, 1890). Convalescent Plasma is obtained from recovered patients, who developed humoral immunity. Convalescent Plasma consists of neutralizing antibodies (Nabs) as a critical component that reduces viremia. Additional components include non-neutralizing antibodies (Non-Nabs), antithrombotic factors, immunoglobulin, and anti-inflammatory cytokine, which may be immunomodulatory in effect (Pandey et al., 2020). Convalescent plasma therapy has been effective in treating SARS-1 and MERS patients and has shown immediate but temporary immunity in COVID-19 patients (Duan et al., 2020). Hence, in the absence of any effective therapeutic strategy against SARS-CoV-2, CPT may turn out to be an important tool for treating COVID-19 patients. On March 24, 2020, FDA approved Convalescent plasma therapy and allowed emergency use of investigational new drug (IND) pathway to access CP (fda.gov, 2020c).
To test the effectiveness of CP, a pilot study involving ten critically ill COVID-19 patients, median aged 52.5 years, was conducted. It was established that each patient developed high NAbs titre with significant viral shedding and improved clinical symptoms (fever, trouble breathing, cough, etc.) within three days of 200 ml CP infusion. Alongside elevated oxyhaemoglobin, improvement in lung lesions and lymphocyte numbers, and decreased inflammation was observed (Bloch et al., 2020). In a second study, five critically ill COVID-19 patients, aged 18–60 were subjected to CP transfusion with a nCoV-2019 specific antibody (IgG) and neutralizing antibody. Within a few days of CP transfusion, a decline in the SARS CoV-2 RNA load and improved clinical conditions were observed (Shen et al., 2020). An open-label clinical study demonstrated that 80% of CP recipients developed appreciably high antibody titer three days post-infusion of 400 ml CP dose, irrespective of donor antibody titer. Moreover, repeat administration of CP dose in one patient was well tolerated, and CPT did not result in any adverse effects (Madariaga et al., 2020).
In the first randomized controlled clinical trial in 103 patients, 52 were administered with CP along with standard care, while 51 received standard care only. The results indicate that the use of CP failed to accomplish clinical improvements in critically ill patients. However, clinical symptoms improved in severely ill patients within 28 days of symptom onset. The study suggests that viral elimination and inflammation reduction might occur before clinical benefits. Therefore, the right phase and right time for CP injection is a must for its replete therapeutic utilization (Zeng et al., 2020). CPT may have a synergistic effect in treating COVID-19 in combination with antiviral drugs such as remdesivir (Casadevall and Scharff, 1995).
Although initial CPT studies have shown promising results, further clinical trials and multi-center studies are required for ensuring the reliability of CPT against novel coronavirus. Donor criteria, optimum dose, and serological assay (to determine SARS CoV-2 in serum) are still needed to be precisely ascertained.
7. Alternative medicines: Traditional Chinese Medicine (TCM) & ayurveda
The lack of an effective therapeutic strategy against COVID-19 has prompted scientists to investigate the use of alternative medicines in the fight against coronavirus infection. China initiated this approach, wherein it dexterously utilized its concept of Traditional Chinese Medicine (TCM). An in-silico study yielded 13 molecular compounds found in 230 medicinal herbs used in TCM, to target SARS-CoV-2 associated factors such as the spike protein, 3CLpro, and PLpro. By screening for herbs that contain more than one of these 13 compounds, and have been used as a part of TCM to treat respiratory viral infections, 26 medicinal herbs were identified against COVID-19 infection (Zhang et al., 2020c). According to the seventh trial version of “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia” published by the National Health Commission of the People's Republic of China on March 3, 2020, TCM remedies namely Huoxiang Zhengqi capsules, Jinhua Qinggan granules, Lianhua Qingwen capsules, Shufeng Jiedu capsules, and Qingfei Paidu are recommended for various levels of clinical manifestations. Among these, Qingfei Paidu decoction (QFPD) has gained much attention after curing 99.28% of the infected individuals in 66 major Chinese cities (satcm.gov.cn, 2020). An in silico that Ma Xing Shi Gan component of QFPD inhibits the inflammatory response by interfering with the Toll-like receptor signaling pathway, and eventually ameliorating the symptoms of cytokine storm that leads to ARDS (Yang et al., 2020).
Ayurveda, an alternative medicine rooted in India, has also been suggested to be an effective therapeutic intervention against COVID-19 (Pandey et al., 2013). Ayurveda broadly comprises of three types of therapeutic interventions, which act as its three pillars: Rasayana, local and systemic prophylaxis (Tillu et al., 2020). Local and systematic prophylaxis include warm water gargling, nasal oil application, etc., and meditation, diet, and other good lifestyle measures, respectively, to block physically viral infection and improve immunity. In silico methods have shown that active compounds in Ashwagandha and Amritaballi block the viral entry by destabilizing the ACE-2-S interactions. In addition, there are basic remedies and regimens that in previous studies have shown to induce both T cell-mediated and B cell-mediated immune response in other infections (Gayatri et al., 2020). In a case study recently reported, a COVID-19 patient was cured within a short duration of time depending entirely on Ayurvedic intervention and diet regulation, demonstrating, Ayurvedic as a potential mainstream treatment option (Girija and Sivan, 2020). However, such studies are one of its kind; absence of mechanistic insights of these herbs, robust clinical trials, and Ayurveda induced toxicity (hepatotoxicity) makes the use of Ayurvedic intervention alone debatable. To regulate the use of Ayurveda during COVID-19 pandemic, Ministry of Ayush, Government of India has issued guidelines and protocols for Ayurveda practitioners in the fight against COVID-19 (ayush.gov.in, 2020a) and self-care guidelines the general public ayush. gov.in, 2020b().
8. Global response to COVID-19 epidemic
The current SARS-CoV-2 epidemic crushed healthcare infrastructures globally. With no effective pharmaceutical treatments available at present, most nations have adopted preventive measures, including social distancing measures, contact tracing, quarantine, and nation-wide lockdowns to control the spread of coronavirus. After the initial outbreak in China, the epicenter of the coronavirus has now shifted to the USA and Brazil. Countries like China and South Korea took aggressive preventive measures to contain the rapid spread of the virus.
Report by Eurasia Group indicates that countries like New Zealand, Taiwan, South Korea, and Australia handled the current pandemic better than the rest of the world in terms of healthcare management, financial policy response, and political response (time.com, 2020). In the initial days of COVID-19, Taiwan and Singapore were expected to become next epicenter since they share borders and undertake huge export-import trade business with China, respectively. However, these two countries learned from the previous SARS attack and took proactive measures such as; aggressive contact-tracing, widespread testing, closing borders, and banning the export of healthcare facilities. It is reported that strict travel restrictions imposed by the countries have reduced at least 50% transmission of the coronavirus (Chinazzi et al., 2020). In June, New Zealand lifted all the COVID restrictions and declared itself a COVID-19-free country. Some of the countries like Greece, UAE, Argentina, Iceland, Germany, and Canada also handled the current pandemic reasonably well. Mitigation strategies by governments and sudden lockdowns triggered a massive economic crisis in most of the countries. It is expected that the global economy may fall between 6% and 7.6% in 2020. To combat these future outcomes, governments around the globe took essential measures (thompsonphine.com, 2020) such as (a) Health & Safety Measures, (b) Labor & Employment Measures, (c) Economic Measures, (d) Export/Import Measures. They announced big budget packages, tax deferments, loans, and financial Stimulus to support their citizens among financial crises triggered by strict preventive measures. The new President elect of the USA, Joe Biden has formulated a task force of healthcare experts and physicians to take scientific studies and facts into consideration for shaping guidelines and policies to curb the surge in COVID-19 cases.
As the temperature of northern hemisphere would drop in the month of November, the 2nd wave of coronavirus is expected to affect countries like Belgium, Canada, China, France, Germany, India, Japan, Mexico, Russia, Spain, Sweden, Switzerland, and United Kingdom. However, in the opinion of the Chinese Center for Disease Control and Prevention (CDC), the 2nd wave of the coronavirus would be less worrying because of the prior strict preventive measures taken by the countries around the globe. This may be true for the COVID-19 cases in China which, successfully managed 2nd wave of the coronavirus by mass testing and contact tracing. Unfortunately, the second wave of the coronavirus has already struck European countries dangerously. France, Italy and Spain are recording more than 15,000 cases on the daily basis in the month of October. Situation may get worse if effective measures are not taken sooner. To this end, Spain and France are imposing partial and planned lockdowns instead of complete shutdown to contain the virus, which could be an effective approach to combat the virus.
9. Conclusion
The current coronavirus pandemic is unprecedented and requires extraordinary efforts from the scientific community across the globe. Incidentally, SARS, and MERS outbreaks have provided important insights to understand SARS-CoV-2 pathogenesis and execute effective therapeutic strategies to combat COVID-19. However, rigorous research and human clinical trials of repurposed drugs and future vaccines are required to authenticate the efficacy and safety of proposed treatment options. Repurposed drugs such as Remdesivir, Favipiravir, and Dexamethasone, reportedly decreased fatality rates and improved recovery rates in several human clinical trials. Nonetheless, the development and distribution of a safe and effective vaccine is a crucial preventive strategy to limit the COVID-19 pandemic. In the meantime, preventive measures imposed by governments and public corporations are proving to be successful in containing the spread of SARS-CoV-2.
Authors contribution
Conception, literature research, and writing were performed by H.C, A.A & Y.A. Additional data, proofreading, and critical review for the manuscript were delivered by H.C., A.A. R.G, G.D & RC.
CRediT authorship contribution statement
Heerak Chugh: contributed equally as First author, Conceptualization, literature research, Writing - original draft, Additional data, proofreading, and critical review. Amardeep Awasthi: contributed equally as First author, Conceptualization, literature research, Writing - original draft, Additional data, proofreading, and critical review. Yashi Agarwal: Conceptualization, literature research, Writing - original draft. Rajesh K. Gaur: Additional data, proofreading, and critical review. Gagan Dhawan: Additional data, proofreading, and critical review. Ramesh Chandra: Additional data, proofreading, and critical review.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgment
RC would like to thank the University of Delhi for providing infrastructural and lab facilities. H.C is thankful to Science and Engineering Research Board(SERB), Deparmtent od Science and Technology (DST), Government of lndia and Confederation of Indian Industries (CII) for the award of Prime Minister Fellowship Scheme for Doctoral Research (SERB/PM Fellow/CII-FICCI/Meeting/2018); and University Grant Commission for Junior Research Fellowship (528/(CSIR-UGC NET DEC. 2017)). A. A gratefully acknowledges the award of Senior Research Fellowship (IF170256) under DST-INSPIRE program .
References
- clinicaltrials. gov Ivermectin in treatment of COVID-19. 2020. https://clinicaltrials.gov/ct2/show/NCT04445311 Available at:
- CSIR-CDRI’s COVID-19 drug candidate Umifenovir secures DCGI approval for phase III clinical trial (2020) Available at: https://www.csir.res.in/slider/csir-cdri%E2%80%99s-covid-19-drug-candidate-umifenovir-secures-dcgi-approval-phase-iii-clinical-trial (accessed 26 June, 2020).
- (ox.ac.uk, 2020) Dexamethasone reduces death in hospitalised patients with severe respiratory complications of COVID-19. Available at: http://www.ox.ac.uk/news/2020-06-16-dexamethasone-reduces-death-hospitalised-patients-severe-respiratory-complications (accessed on 1st July 2020).
- Government Measures_Taken_in_Response_to_COVID-19.pdf. Available at: https://www.thompsonhine.com/uploads/1345/doc/Country-by-Country_Guide. (accessed on 28 June, 2020).
- Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J. Med. Virol. 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH clinical trial testing antiviral remdesivir plus anti-inflammatory drug baricitinib for COVID-19 begins. 2020. Available at: https://www.nih.gov/news-events/news-releases/nih-clinical-trial-testing-antiviral-remdesivir-plus-anti-inflammatory-drug-baricitinib-covid-19-begins, (accessed May 16,
- RDIF and ChemRar announce the Russian Ministry ofHealth has included Avifavir in the list of nationally recommended drugs for the treatment of COVID-19. https://rdif.ru/Eng_fullNews/5224/ (accessed on 4th June 2020).
- Rigel launches Phase II trial to access fostamatinib for Covid-19 patients https://www.clinicaltrialsarena.com/news/glenmark-favipiravir-combo-trial. (accessed on 4th July 2020).
- Shang J., Du R., Lu Q., Wu J., Xu S., Ke Z., Cai Z., Gu Y., Huang Q., Zhan Y., Yang J. 2020. The treatment and outcomes of patients with COVID-19 in Hubei, China: a multi-centered, retrospective, observational study. [DOI] [Google Scholar]
- Tang D., Comish P., Kang R. The hallmarks of COVID-19 disease. PLoS Pathog. 2020;16(5) doi: 10.1371/journal.ppat.1008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (ema.europa.eu, 2020) Summary on compassionate use. Available at: https://www.ema.europa.eu/en/documents/other/summary-compassionate-use remdesivirgi lead_en.pdf. (accessed on 29 April 2020).
- The Best Global Responses to COVID-19 Pandemic. Available at: https://time.com/5851633/best-global-responses-covid-19/ (accessed on 28 June, 2020).
- Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical infectious diseases; 2020. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2020c World Health Organization, 2020. DRAFT landscape of COVID-19 candidate vaccines. World.
- Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020 doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Wu Q., Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 2020 doi: 10.1016/j.cub.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Horani R.A., Desai U.R. Recent advances on plasmin inhibitors for the treatment of fibrinolysis‐related disorders. Med. Res. Rev. 2014;34(6):1168–1216. doi: 10.1002/med.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A., Jose J., Pinto R., Al-Omari A., Kharaba A., Almotairi A. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am. J. Respir. Crit. Care Med. 2018 doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- Arshad S., Kilgore P., Chaudhry Z.S., Jacobsen G., Wang D.D., Huitsing K., Brar I., Alangaden G.J., Ramesh M.S., McKinnon J.E., O'Neill W. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour H.M., Elkhatib W.F., Rahman M., Elshabrawy H.A. Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks. Pathogens. 2020;9(3):186. doi: 10.3390/pathogens9030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ayush. gov.in . 2020. Ministry of Ayush 2020. Guidelines for Ayurveda Practitioners for COVID 19.https://www.ayush.gov.in/docs/ayurved-guidlines.pdf Available at: [Google Scholar]
- ayush. gov.in Ayurveda's immunity boosting measures for self-care during COVID 19 crisis. 2020. https://www.ayush.gov.in/docs/123.pdf Ministry of Ayush. 2020a, Available at:
- Becker D.E. Basic and clinical pharmacology of glucocorticosteroids. Anesth. Prog. 2013;60(1):25–32. doi: 10.2344/0003-3006-60.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behring E.V. Ueber das zustandekommen der diphtherie-immunität und der tetanus-immunität bei thieren. mBio. 1890 doi: 10.1128/mBio.00117-17. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya C., Das C., Ghosh A., Singh A.K., Mukherjee S., Majumder P.P., Basu A., Biswas N.K. Global spread of SARS-CoV-2 subtype with spike protein mutation D614G is shaped by human genomic variations that regulate expression of TMPRSS2 and MX1 genes. bioRxiv. 2020 doi: 10.1101/2020.05.04.075911. [DOI] [Google Scholar]
- Blaising J., Polyak S.J., Pécheur E.I. Arbidol as a broad-spectrum antiviral: an update. Antivir. Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch E.M., Shoham S., Casadevall A., Sachais B.S., Shaz B., Winters J.L., van Buskirk C., Grossman B.J., Joyner M., Henderson J.P., Pekosz A. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J. Clin. Invest. 2020;130(6):2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake S.J., Barnsley K., Lu W., McAlinden K.D., Eapen M.S., Sohal S.S. Smoking upregulates angiotensin-converting enzyme-2 receptor: a potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19) J. Clin. Med. 2020 doi: 10.3390/jcm9030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., Liao X., Gu Y., Cai Q., Yang Y., Shen C. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A., Scharff M.D. Return to the past: the case for antibody-based therapies in infectious diseases. Clin. Infect. Dis. 1995;21(1):150–161. doi: 10.1093/clinids/21.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19(2):181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.C., Tang X.P., Tan S.Y., Liang B.L., Wan Z.Y., Fang J.Q., Zhong N. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129:1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Xu Z., Zeng S., Wang X., Liu W., Qian L., Wei J., Yang X., Shen Q., Gong Z., Yan Y. The molecular aspect of antitumor effects of protease inhibitor nafamostat mesylate and its role in potential clinical applications. Front Oncol. 2019;9:852. doi: 10.3389/fonc.2019.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- chictr. org.cn A Phase III clinical trial for inactivated novel coronavirus pneumonia (COVID-19) vaccine (Vero cells) 2020. Available at: http://www.chictr.org.cn/showprojen.aspx?proj=56651, accessed on 15th June 2020.
- chictr. org.cn Multi-center clinical study on the treatment of patients with novel coronavirus pneumonia (COVID-19) by ebastine. 2020. http://www.chictr.org.cn/showprojen.aspx?proj=49790 Available at:
- Chinazzi M., Davis J.T., Ajelli M., Gioannini C., Litvinova M., Merler S., y Piontti A.P., Mu K., Rossi L., Sun K., Viboud C. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. doi: 10.1126/science.aba9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S., Kao R.Y., Poon L.L., Wong C.L., Guan Y., Peiris J.S., Yuen K.Y. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- clinicaltrials. gov . 2020. An Open Study of the Safety.https://clinicaltrials.gov/ct2/show/NCT04436471 Tolerability and Immunogenicity of the Drug "Gam-COVID-Vac" Vaccine Against COVID-19- Available at. [Google Scholar]
- clinicaltrials. gov ReSafety and antiviral activity of BLD-2660 in COVID-19 hospitalized subjects. 2020. https://clinicaltrials.gov/ct2/show/NCT04334460 Available at:
- clinicaltrials. gov Study comparing lopinavir/ritonavir (LPV/r) + emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) with a nucleoside sparing regimen consisting of lopinavir/ritonavir + raltegravir (RAL) (PROGRESS) 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT00711009.
- clinicaltrials. gov . 2020. An Open Study of the Safety, Tolerability and Immunogenicity of "Gam-COVID-Vac Lyo" Vaccine against COVID-19- [Google Scholar]
- clinicaltrials. gov Study of efficacy and safety of canakinumab treatment for CRS in participants with COVID-19-induced pneumonia (CAN-COVID) 2020. https://clinicaltrials.gov/ct2/show/NCT04362813 Available at:
- clinicaltrials. gov Lopinavir/ritonavir, ribavirin and IFN-beta combination for nCoV treatment. 2020. https://www.clinicaltrials.gov/ct2/show/NCT04276688 Available at:
- clinicaltrials. gov Study to describe the safety, tolerability, immunogenicity, and efficacy of RNA vaccine candidates against COVID-19 in healthy adults. 2020. https://clinicaltrials.gov/ct2/show/NCT04368728?term=vaccine&cond=covid-19&draw=3 Available at:
- clinicaltrials. gov CD24Fc as a non-antiviral immunomodulator in COVID-19 treatment (SAC-COVID) 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04317040.
- clinicaltrials. gov Comparison of lopinavir/ritonavir or hydroxychloroquine in patients with mild coronavirus disease (COVID-19) 2020. https://clinicaltrials.gov/ct2/show/NCT04307693 Available at:
- clinicaltrials. gov Clinical trial to assess the safety of a coronavirus vaccine in healthy men and women. 2020. Available at: [DOI]
- clinicaltrials. gov Gargling and nasal rinses to reduce oro- and nasopharyngeal viral load in patients with COVID-19. 2020. https://clinicaltrials.gov/ct2/show/NCT04344236 Available at:
- clinicaltrials. gov Losartan for patients with COVID-19 requiring hospitalization. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04312009.
- clinicaltrials. gov . 2020. Safety and Immunogenicity Study of Inactivated Vaccine for Prophylaxis of SARS CoV-2 Infection (COVID-19)https://clinicaltrials.gov/ct2/show/NCT04352608?term=Sinovac&cntry=CN&draw=2 Avaialble at. [Google Scholar]
- clinicaltrials. gov Efficacy of chloroquine or hydroxychloroquine in COVID-19 treatment. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04353336.
- clinicaltrials. gov NA-831, atazanavir and dexamethasone combination therapy for the treatment of COVID-19 infection (NATADEX) 2020. https://clinicaltrials.gov/ct2/show/NCT04452565 Available at:
- clinicaltrials. gov Safety and immunogenicity study of inactivated vaccine for prevention of SARS-CoV-2 infection (COVID-19) 2020. https://clinicaltrials.gov/ct2/show/NCT04383574?term=covid19&cond=vaccine&cntry=CN&draw=2 Available at:
- clinicaltrials. gov Chloroquine as antiviral treatment in coronavirus infection 2020. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04331600.
- clinicaltrials. gov Study of the efficacy and safety of a single administration of olokizumab and RPH-104 with standard therapy in patients with severe severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) 2020. https://clinicaltrials.gov/ct2/show/NCT04380519 Available at:
- clinicaltrials. gov Safety and immunogenicity study of an inactivated SARS-CoV-2 vaccine for preventing against COVID-19. 2020. https://clinicaltrials.gov/ct2/show/NCT04412538?term=vaccine&cond=covid-19&draw=2 Available at:
- clinicaltrials. gov Repurposing of chlorpromazine in covid-19 treatment (reCoVery) 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04366739.
- clinicaltrials. gov Piclidenoson for treatment of COVID-19. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04333472.
- clinicaltrials. gov Evaluation of the safety and immunogenicity of a SARS-CoV-2 rS (COVID-19) nanoparticle vaccine with/without matrix-M adjuvant) 2020. https://clinicaltrials.gov/ct2/show/NCT04368988?term=vaccine&recrs=a&cond =covid19&draw=2 Available at: (accessed on 15th June 2020)
- clinicaltrials. gov Administration of chlorpromazine as a treatment for COVID-19. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04354805.
- clinicaltrials. gov Prazosin to prevent COVID-19 (PREVENT-COVID trial) (PREVENT) 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04365257.
- clinicaltrials. gov Evaluating the efficacy and safety of bromhexine hydrochloride tablets combined with standard treatment/standard treatment in patients with suspected and mild novel coronavirus pneumonia (COVID-19) 2020. https://clinicaltrials.gov/ct2/show/NCT04273763 Available at: April 9, 2020.
- clinicaltrials. gov The phase 2 study to evaluate the safety and efficacy of clevudine in patients with moderate COVID-19. 2020. https://clinicaltrials.gov/ct2/show/NCT04347915 Available at:, accessed on.
- clinicaltrials. gov Study to evaluate the safety and antiviral activity of remdesivir (GS-5734™) in participants with moderate coronavirus disease (COVID-19) compared to (clinicaltrials.gov. 2020. Standard of Care Treatment. Available at: https://clinicaltrials.gov/ct2/show/NCT04292730.
- clinicaltrials. gov Clinical study of arbidol hydrochloride tablets in the treatment of pneumonia caused by novel coronavirus adjuvant) 2020. https://clinicaltrials.gov/ct2/show/NCT04260594?term=NCT04260594&draw=2&rank=1 Available at:
- clinicaltrials. gov Efficacy and safety of corticosteroids in COVID-19. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04273321.
- clinicaltrials. gov Phase 2 trial of infliximab in coronavirus disease 2019 (COVID-19) 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04425538.
- clinicaltrials. gov . 2020. The Impact of Camostat Mesilate on COVID-19 Infection (CamoCO-19)https://clinicaltrials.gov/ct2/show/NCT04321096 NCT04321096. Available at: [Google Scholar]
- clinicaltrials. gov Application of desferal to treat COVID-19. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04333550.
- clinicaltrials. gov Ruxolitinib for treatment of covid-19 induced lung injury ARDS (RuXoCoil) 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04359290.
- clinicaltrials. gov Study to describe the safety, tolerability, immunogenicity, and efficacy of RNA vaccine candidates against COVID-19 in healthy adults. 2020. https://clinicaltrials.gov/ct2/results?cond=&term=camostat&cntry=&state=&city=&dist= Available at:
- clinicaltrials. gov Dexamethasone for COVID-19 related ARDS: a multicenter. 2020. https://clinicaltrials.gov/ct2/show/NCT04395105 Randomized Clinical Available at:
- clinicaltrials. gov Pilot study of sildenafil in COVID-19. 2020. https://clinicaltrials.gov/ct2/show/NCT04304313 Available at:
- clinicaltrials. gov . 2020. Clinical Trial to Evaluate Efficacy of 3 Types of Treatment in Patients with Pneumonia by COVID-19.https://clinicaltrials.gov/ct2/show/NCT04346147 Available at: [Google Scholar]
- clinicaltrials. gov A safety, tolerability and efficacy of EIDD-2801 to eliminate infectious virus detection in persons with COVID-19. 2020. https://clinicaltrials.gov/ct2/show/NCT04405570 Available at:
- clinicaltrials. gov A study to evaluate the safety, tolerability and pharmacokinetics (PK) of TAK-981 in adult participants with advanced or metastatic solid tumors or relapsed/refractory hematologic malignancies and in a subset with coronavirus disease 2019 (COVID-19) 2020. https://clinicaltrials.gov/ct2/show/NCT04338126 Available at:
- clinicaltrials. gov . 2020. Severe 2019-nCoV Remdesivir RCT - Full Text View - ClinicalTrials.Gov.https://clinicaltrials.gov/ct2/show/NCT04257656 (web archive link, 09 April 2020), (n.d.). Available at: [Google Scholar]
- clinicaltrials. gov Favipiravir in hospitalized COVID-19 patients (FIC)h. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04359615.
- clinicaltrials. gov Telmisartan in respiratory failure due to COVID-19 (STAR-COVID) 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04510662.
- clinicaltrials. gov Acalabrutinib study with best supportive care in participants hospitalized with COVID-19. 2020. https://clinicaltrials.gov/ct2/show/NCT04497948 Available at:
- clinicaltrials. gov Hydroxychloroquine in the prevention of COVID-19 infection in healthcare workers. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04333225. accessed on.
- clinicaltrials. gov Telmisartan for treatment of COVID-19 patients. 2020. https://www.clinicaltrials.gov/ct2/show/NCT04355936 Available at:
- clinicaltrials. gov Clinical study of arbidol hydrochloride tablets in the treatment of pneumonia caused by novel coronavirus. 2020. https://clinicaltrials.gov/ct2/show/NCT04260594 Available at:
- clinicaltrials. gov Hydroxychloroquine, oseltamivir and azithromycin for the treatment of COVID-19 infection: an RCT (PROTECT) 2020. https://clinicaltrials.gov/ct2/show/NCT04338698 Available at:
- clinicaltrials. gov Tocilizumab in COVID-19 pneumonia (TOCIVID-19) 2020. https://clinicaltrials.gov/ct2/show/NCT04317092 Available at:
- clinicaltrials. gov Umifenovir in hospitalized COVID-19 patients (UAIIC) 2020. https://clinicaltrials.gov/ct2/show/NCT04350684 Available at:
- clinicaltrials. gov Hydroxychloroquine and nitazoxanide combination therapy for COVID-19) 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04361318.
- clinicaltrials. gov Comparison of tocilizumab plus dexamethasone vs. Dexamethasone for patients with covid-19 (TOCIDEX) 2020. https://clinicaltrials.gov/ct2/show/NCT04476979 Available at:
- clinicaltrials. gov Atorvastatin as adjunctive therapy in COVID-19 (STATCO19) 2020. https://clinicaltrials.gov/ct2/show/NCT04380402 Available at:
- clinicaltrials. gov Efficacy and safety of hydroxychloroquine and azithromycin for the treatment of ambulatory patients with mild COVID-19. 2020. https://clinicaltrials.gov/ct2/show/NCT04348474 Available at:
- clinicaltrials. gov Tranexamic acid (TXA) and corona virus 2019 (COVID19) in inpatients (TCInpatient) 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04338074.
- clinicaltrials. gov NA-831, atazanavir and dexamethasone combination therapy for the treatment of COVID-19 infection (NATADEX) 2020. https://clinicaltrials.gov/ct2/show/NCT04452565 Available at:
- clinicaltrials. gov Favipiravir vs hydroxychloroquine in COVID -19. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04387760.
- clinicaltrials. gov Safety and efficacy trial of zavegepant* intranasal for hospitalized patients with COVID-19 requiring supplemental oxygen. 2020. https://clinicaltrials.gov/ct2/show/NCT04346615 Available at:
- clinicaltrials. gov The nitazoxanide plus atazanavir for COVID-19 study (NACOVID) 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04459286.
- clinicaltrials. gov Safety and efficacy of NP-120 (ifenprodil) for the treatment of hospitalized patient with confirmed COVID-19 disease. 2020. https://clinicaltrials.gov/ct2/show/NCT04382924 Available at:
- clinicaltrials. gov Vitamin C infusion for the treatment of severe 2019-nCoV infected pneumonia. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04264533.
- clinicaltrials. gov Inhaled aviptadil for the prevention of COVID-19 related ARDS. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04536350.
- clinicaltrials. gov Randomized, controlled study of IFX-1 in patients with severe COVID-19 pneumonia (PANAMO) 2020. https://clinicaltrials.gov/ct2/show/NCT04333420 Available at:
- clinicaltrials. gov COVID-19 and vitamin D supplementation: a multicenter randomized controlled trial of high dose versus standard dose vitamin D3 in high-risk COVID-19 patients (CoVitTrial) 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04344041.
- clinicaltrials. gov Azithromycin for COVID-19 treatment in outpatients nationwide (ACTION) 2020. https://clinicaltrials.gov/ct2/show/NCT04332107 Available at:
- clinicaltrials. gov Isotretinoin in treatment of COVID-19 (randomized) 2020. https://clinicaltrials.gov/ct2/show/NCT04361422 Available at:
- clinicaltrials. gov Study to evaluate the safety and efficacy of XAV-19 in patients with COVID-19 induced moderate pneumonia (POLYCOR) 2020. https://clinicaltrials.gov/ct2/show/NCT04453384 Available at:
- clinicaltrials. gov Atovaquone and azithromycin combination for confirmed COVID-19 infection. 2020. https://clinicaltrials.gov/ct2/show/NCT04339426 Available at:
- clinicaltrials. gov Ivermectin-azithromycin-cholecalciferol (IvAzCol) combination therapy for COVID-19 (IvAzCol) 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04399746.
- clinicaltrials. gov Ivermectin and nitazoxanide combination therapy for COVID-19. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04360356.
- clinicaltrials. gov Study of baricitinib (LY3009104) in participants with COVID-19 (COV-barrier) 2020. https://clinicaltrials.gov/ct2/show/NCT04421027 Available at:
- clinicaltrials. gov Low-dose lenalidomide for non-severe COVID-19 treatment trial (GETAFE) 2020. https://clinicaltrials.gov/ct2/show/NCT04361643 Available at:
- clinicaltrials. gov Efficacy and safety study of BDB-001 in severe COVID-19 with ALI/ARDS. 2020. https://clinicaltrials.gov/ct2/show/NCT04449588 Available at:
- clinicaltrials. gov Lopinavir/ritonavir for the treatment of COVID-19 positive patients with cancer and immune suppression in the last year. 2020. https://clinicaltrials.gov/ct2/show/NCT04455958 Available at:
- Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020 doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa L.S., Outlioua A., Anginot A., Akarid K., Arnoult D. RNA viruses promote activation of the NLRP3 inflammasome through cytopathogenic effect-induced potassium efflux. Cell Death Dis. 2019;10(5):1–15. doi: 10.1371/journal.pone.0229570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalerba P., Levin B., Thompson J.L. A trial of lopinavir–ritonavir in covid-19. J Heart Lung Transplant. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2008043. [DOI] [PubMed] [Google Scholar]
- De Savi C., Hughes D.L., Kvaerno L. Quest for a COVID-19 cure by repurposing small molecule drugs: mechanism of action, clinical development, synthesis at scale, and outlook for supply. Org. Process Res. Dev. 2020 doi: 10.1021/acs.oprd.0c00233. [DOI] [PubMed] [Google Scholar]
- Delang L., Abdelnabi R., Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antivir. Res. 2018;153:85–94. doi: 10.1016/j.antiviral.2018.03.003. [DOI] [PubMed] [Google Scholar]
- Du Y.X., Chen X.P. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019‐nCoV infection. Clin. Pharmacol. Ther. 2020 doi: 10.1002/cpt.1844. [DOI] [PubMed] [Google Scholar]
- Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Y., Peng C. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U. S. A. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynavax. Clover Biopharmaceuticals Announce Research Collaboration to Evaluate Coronavirus . 2020. COVID-19) Vaccine Candidate with CpG 1018 Adjuvant.http://investors.dynavax.com/news-releases/news-release-details/dynavax-and-clover-biopharmaceuticals-announce-research Available at: accessed on 09 July, 2020. [Google Scholar]
- Favalli E.G., Ingegnoli F., De Lucia O., Cincinelli G., Cimaz R., Caporali R. COVID-19 infection and rheumatoid arthritis: faraway, so close. Autoimmun. Rev. 2020:102523. doi: 10.1016/j.autrev.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- fda. gov . 2020. Letter Revoking EUA for Chloroquine Phosphate and Hydroxychloroquine Sulfate, 6/15/2020.https://www.fda.gov/media/138945/download June 2020. Available at: (accessed on 15th June 2020) [Google Scholar]
- fda. gov . 2020. Drug Trials Snapshots: Olumiant. U.S. Food and Drug Administration (FDA). May 2018. Available at: https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-olumiant. [Google Scholar]
- fda. gov . 2020. US Food & Drug Administration Development & Approval Process (CBER) Investigational COVID-19 Convalescent Plasma - Emergency INDs.https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-processcber/recommendation-investigational-covid-19-convalescent-plasma Available at: [Google Scholar]
- Fischer J., Ganellin C.R., editors. Analogue-based Drug Discovery. Wiley-VCH; Hoboken, NJ: 2010. 978-3-527-31257-3. [Google Scholar]
- Furuta Y., Takahashi K., Kuno-Maekawa M., Sangawa H., Uehara S., Kozaki K., Nomura N., Egawa H., Shiraki K. Mechanism of action of T-705 against influenza virus. Antimicrob. Agents Chemother. 2005;49:981–986. doi: 10.1128/AAC.49.3.981-986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., Li Y., Zhu L., Wang N., Lv Z., Gao H. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020 doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P., Lagier J.C., Parola P., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honoré S. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayatri R., Lavanya S., Hussain M., Veslin J. The new pandemic covid-19: treatment options and developments. Asian J. Bio. Sci. 2020;113 doi: 10.9734/ajob/2020/v9i330086. [DOI] [Google Scholar]
- Genentech Initiates Phase III Clinical trial of Actemra plus remdesivir in hospitalized patients with severe COVID-19 pneumonia. 2020. https://www.gene.com/media/press-releases/14855/2020-05-27/genentech-initiates-phase-iii-clinical-t Available at:, (accessed on 29th May 2020)
- Genovese M.C., Kremer J., Zamani O., Ludivico C., Krogulec M., Xie L., Beattie S.D., Koch A.E., Cardillo T.E., Rooney T.P., Macias W.L. Baricitinib in patients with refractory rheumatoid arthritis. N. Engl. J. Med. 2016;374:1243–1252. doi: 10.1056/NEJMoa1507247. [DOI] [PubMed] [Google Scholar]
- Girija P.L.T., Sivan N. Ayurvedic Treatment of COVID-19/SARS-CoV-2: a case report. J. Ayurveda Integr. Med. 2020 doi: 10.1016/j.jaim.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- glenmarkpharma.com Unlocking the treatment for mild to moderate covid-19 in India. 2020. https://www.glenmarkpharma.com/sites/default/files/Glenmark-FabiFlu-Press-Brief.pdf Available at:
- glenmarkpharma.com 2020. https://www.glenmarkpharma.com/sites/default/files/Indian-regulator-approves-Favipiravir-for-the-treatment-of-mild-to-moder.pdf
- Goldman J.D., Lye D.C., Hui D.S., Marks K.M., Bruno R., Montejano R., Spinner C.D., Galli M., Ahn M.Y., Nahass R.G., Chen Y.S. Remdesivir for 5 or 10 days in patients with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golonka R.M., Saha P., Yeoh B.S., Chattopadhyay S., Gewirtz A.T., Joe B., Vijay-Kumar M. Harnessing innate immunity to eliminate SARS-CoV-2 and ameliorate COVID-19 disease. Physiol. Genom. 2020 doi: 10.1152/physiolgenomics.00033.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R., Groot R.J.D., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D. Severe acute respiratory syndrome-related coronavirus: the species and its viruses–a statement of the Coronavirus Study Group. Nat. Microbiol. 2020 doi: 10.1038/s41564-020-0695-z. [DOI] [Google Scholar]
- Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M., Franceschini E., Cuomo G., Orlando G., Borghi V., Santoro A. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K., Ma H., An X., Su Y., Chen J., Lian Z., Zhao J., Zhu B.P., Fontaine R.E., Feng Z., Zeng G. Early use of glucocorticoids was a risk factor for critical disease and death from pH1N1 infection. Clin. Infect. Dis. 2011;53:326–333. doi: 10.1093/cid/cir398. [DOI] [PubMed] [Google Scholar]
- Hindson J. COVID-19: faecal–oral transmission. Nat. Rev. Gastroenterol. Hepatol. 2020;17(5):259. doi: 10.1038/s41575-020-0295-7. 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Pöhlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol. Cell. 2020 doi: 10.1016/j.molcel.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann H., Pöhlmann S. Cellular entry of the SARS coronavirus. Trends Microbiol. 2004;12(10):466–472. doi: 10.1016/j.tim.2004.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H.P., Hsu J.T.A. Strategies of development of antiviral agents directed against influenza virus replication. Curr. Pharmaceut. Des. 2007;13(34):3531–3542. doi: 10.2174/138161207782794248. [DOI] [PubMed] [Google Scholar]
- Hsu J.T.A., Kuo C.J., Hsieh H.P., Wang Y.C., Huang K.K., Lin C.P.C., Huang P.F., Chen X., Liang P.H. Evaluation of metal‐conjugated compounds as inhibitors of 3CL protease of SARS‐CoV. FEBS Lett. 2004;574(1–3):116–120. doi: 10.1016/j.febslet.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igbinosa I., Miller S., Bianco K., Nelson J., Kappagoda S., Blackburn B.G., Grant P., Subramanian A., Lyell D.J., El-Sayed Y.Y., Aziz N. Use of remdesivir for pregnant patients with severe novel coronavirus disease 2019. Am. J. Obstet. Gynecol. 2020 doi: 10.1016/j.ajog.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi K., Yoshie K., Kurakami Y., Takahashi K., Kato Y., Morishima T. Therapeutic potential of ciclesonide inahalation for COVID-19 pneumonia: report of three cases. J. Infect. Chemother. 2020 doi: 10.1016/j.jiac.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J. An mRNA vaccine against SARS-CoV-2 preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian-ya G. Clinical characteristics of 51 patients discharged from hospital with COVID-19 in Chongqing, China. medRxivb. 2020 [Google Scholar]
- Kadam R.U., Wilson I.A. Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc. Natl. Acad. Sci. U. S. A. 2017;114(2):206–214. doi: 10.1073/pnas.1617020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadioglu O., Saeed M., Johannes Greten H., Efferth T. Identification of novel compounds against three targets of SARS CoV-2 coronavirus by combined virtual screening and supervised machine learning. Bull. World Health Organ. Comput. Biol. Med. 2020 doi: 10.1016/j.compbiomed.2020.103848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki N. “Solidarity trial”: a feeling of trust towards COVID-19 treatments. J. Lumbini Med. Coll. 2020;8(1):2. doi: 10.22502/jlmc.v8i1.335. [DOI] [Google Scholar]
- Kawase M., Shirato K., van der Hoek L., Taguchi F., Matsuyama S. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J. Virol. 2012;86(12):6537–6545. doi: 10.1128/JVI.00094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U.J., Won E.J., Kee S.J., Jung S.I., Jang H.C. Combination therapy with lopinavir/ritonavir, ribavirin and interferon-α for middle east respiratory syndrome. Antivir. Ther. 2016;21:455–459. doi: 10.3851/IMP3002. [DOI] [PubMed] [Google Scholar]
- Kumar N., Awasthi A., Kumari A., Sood D., Jain P., Singh T., Sharma N., Grover A., Chandra R. Antitussive noscapine and antiviral drug conjugates as arsenal against COVID-19: a comprehensive chemoinformatics analysis. J. Biomol. Struct. Dyn. 2020:1–16. doi: 10.1080/07391102.2020.1808072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D., Kumari K., Jayaraj A., Kumar V., Kumar R.V., Dass S.K., Chandra R., Singh P. Understanding the binding affinity of noscapines with protease of SARS-CoV-2 for COVID-19 using MD simulations at different temperatures. J. Biomol. Struct. Dyn. 2020:1–14. doi: 10.1080/07391102.2020.1752310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriya B., Cohen M.D., Keystone E. Baricitinib in rheumatoid arthritis: evidence-to-date and clinical potential. Ther. Adv. Musculoskelet. Dis. 2017;9(2):37–44. doi: 10.1177/1759720X16687481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INSERM . 22 March 2020. Launch of a European Clinical Trial against COVID-19.https://presse.inserm.fr/en/launch-of-a-european-clinical-trial-against-covid-19/38737/ Available at: (accessed on 1 May 2020) [Google Scholar]
- Le T.T., Andreadakis Z., Kumar A., Roman R.G., Tollefsen S., Saville M., Mayhew S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020;19(5):305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5(4):562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19(3):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F., Liu L. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020:1–3. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- Lippi G., Henry B.M. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19) Respir. Med. 2020;167:105941. doi: 10.1016/j.rmed.2020.105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.L., Saif L.J., Weiss S.R., Su L. No credible evidence supporting claims of the laboratory engineering of SARS-CoV-2. Emerg. Microb. Infect. 2020;9(1):505–507. doi: 10.1080/22221751.2020.1733440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 vaccines at pandemic speed. N. Engl. J. Med. 2020;382(21):1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- Madariaga M.L.L., Guthmiller J., Schrantz S., Jansen M., Christenson C., Kumar M., Prochaska M., Wool G., Durkin A., Oh W.H., Trockman L. Clinical predictors of donor antibody titer and correlation with recipient antibody response in a COVID-19 convalescent plasma clinical trial. medRxiv. 2020 doi: 10.1111/joim.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Kawase M., Nao N., Shirato K., Ujike M., Kamitani W., Shimojima M., Fukushi S. The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting viral replication-transcription complex in culture cells. J. Virol. 2020 doi: 10.1128/JVI.01648-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020:1–8. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Administration of Traditional Chinese Medicine of China Qingfei Paidu Decoction is an effective prescription for the treatment of mild common pneumonia. Available at: http://www.satcm.gov.cn/xinxifabu/meitibaodao/2020-04-23/14821.html, Accessed on.
- Nguyen T.M., Zhang Y., Pandolfi P.P. Virus against virus: a potential treatment for 2019-nCov (SARS-CoV-2) and other RNA viruses. Cell Res. 2020 doi: 10.1038/s41422-020-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Meara, M.J., Guo, J.Z., Swaney, D.L., Tummino, T.A. and Hüttenhain, R., A SARS-CoV-2-human protein-protein interaction map reveals drug targets and potential drug-repurposing. Nature. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed]
- Okuno M., Kojima S., Akita K., Matsushima-Nishiwaki R., Adachi S., Sano T., Takano Y., Takai K., Obora A., Yasuda I., Shiratori Y. Retinoids in liver fibrosis and cancer. Front. Biosci. 2002;7:d204–d218. doi: 10.2741/a775. [DOI] [PubMed] [Google Scholar]
- Oldfield V., Dhillon S., Plosker G.L. Tocilizumab. Drugs. 2009;69(5):609–632. doi: 10.2165/00003495-200969050-00007. [DOI] [PubMed] [Google Scholar]
- Ortiz-Diaz E., Li G., Kor D. Preadmission use of inhaled corticosteroids is associated with a reduced risk of direct acute lung injury/acute respiratory distress syndrome. Chest. 2011;140:912A. doi: 10.1378/chest.1110134. [DOI] [Google Scholar]
- Pandey M.M., Rastogi S., Rawat A.K.S. Indian traditional ayurvedic system of medicine and nutritional supplementation. Evid Based Complement Alternat. Med. 2013 doi: 10.1155/2013/376327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A., Nikam A.N., Shreya A.B., Mutalik S.P., Gopalan D., Kulkarni S., Padya B.S., Fernandes G., Mutalik S., Prassl R. Life Sci.; 2020. Potential Therapeutic Targets for Combating SARS-CoV-2: Drug Repurposing, Clinical Trials and Recent Advancements; p. 117883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFIZER AND BIONTECH ANNOUNCE VACCINE CANDIDATE AGAINST COVID-19 ACHIEVED SUCCESS First interim analysis from phase 3 study. Available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-vaccine-candidate-against.
- Phillips R.O., Robert J., Abass K.M., Thompson W., Sarfo F.S., Wilson T., Sarpong G., Gateau T., Chauty A., Omollo R., Otieno M.O. Rifampicin and clarithromycin (extended release) versus rifampicin and streptomycin for limited Buruli ulcer lesions: a randomised, open-label, non-inferiority phase 3 trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)30047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- Ramezani M., Nemati H., Najafi F., Sayad B., Sadeghi M. Chemokines in COVID-19 Patients: A Systematic Review and Meta-Analysis. 2020. Evaluation of blood levels of cytokines/chemokines in COVID-19 patients: a systematic review and meta-analysis. 4/20/2020. [DOI] [Google Scholar]
- recoverytrial. net . 2020. No Clinical Benefit from Use of Hydroxychloroquine in Hospitalised Patients with COVID-19. 5 June 2020.https://www.recoverytrial.net/news/statement-from-the-chief-investigators-of-the-randomised-evaluation-of-covid-19-therapy-recovery-trial-on-hydroxychloroquine-5-june-2020-no-clinical-benefit-from-use-of-hydroxychloroquine-in-hospitalised-patients-with-covid-19 Available at: (accessed on 5th June 2020) [Google Scholar]
- recoverytrial. net No clinical benefit from use of lopinavir-ritonavir in hospitalized COVID-19 patients studied in Recovery. 2020. https://www.recoverytrial.net/news/no-clinical-benefit-from-use-of-lopinavir-ritonavir-in-hospitalised-covid-19-patients-studied-in-recovery Available at:, (accessed on 29th June 2020)
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahakijpijarn S., Moon C., Koleng J.J., Christensen D.J., Williams R.O. Development of remdesivir as a dry powder for inhalation by thin film freezing. bioRxiv. 2020 doi: 10.1101/2020.07.26.222109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. Jama. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases. Lancet Infect. Dis. 2003;3(11):722–727. doi: 10.1016/s1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavone C., Brusco S., Bertini M., Sportiello L., Rafaniello C., Zoccoli A., Berrino L., Racagni G., Rossi F., Capuano A. Current pharmacological treatments for COVID‐19: what's next? Br. J. Pharmacol. 2020 doi: 10.1111/bph.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäcke H., Döcke W.D., Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 2002;96(1):23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- Schultze A., Walker A.J., McKenna B. 2020. Inhaled Corticosteroid Use and Risk COVID-19 Related Death Among 966,461 Patients with COPD or Asthma: an OpenSAFELY Analysis. medRxiv.https://www.medrxiv.org/content/10.1101/2020.06.19.20135491v1 (preprint [Google Scholar]
- Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K., Morla S., Goyal A., Kumar S. Computational guided drug repurposing for targeting 2'-O-ribose methyltransferase of SARS-CoV-2. ChemRxiv. 2020 doi: 10.26434/chemrxiv.12111138.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9(396) doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L., Wei J. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. Jama. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J. Pediatr. 2020:1–6. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sissoko D., Laouenan C., Folkesson E., M'Lebing A.B., Beavogui A.H., Baize S., Camara A.M., Maes P., Shepherd S., Danel C., Carazo S. Correction: experimental treatment with favipiravir for Ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med. 2016;13(4) doi: 10.1371/journal.pmed.1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T.R., Patel A., Ramos S., Elwood D., Zhu X., Yan J., Gary E.N., Walker S.N., Schultheis K., Purwar M., Xu Z. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020;11(1):1–13. doi: 10.1038/s41467-020-16505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street M.E. HMGB1: a possible crucial therapeutic target for COVID-19? Horm Res. Paediatr. 2020:1–3. doi: 10.1159/000508291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Wu C., Li X., Song Y., Yao X., Wu X., Duan Y., Zhang H., Wang Y., Qian Z., Cui J. On the origin and continuing evolution of SARS-CoV-2. Natl. Sci. Rev. 2020 doi: 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevarajan I., Nguyen T.H., Koutsakos M., Druce J., Caly L., van de Sandt C.E., Jia X., Nicholson S., Catton M., Cowie B., Tong S.Y. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. 2020;26(4):453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillu G., Chaturvedi S., Chopra A., Patwardhan B. Public health approach of Ayurveda and Yoga for COVID-19 prophylaxis. Altern. Complement. Med. 2020;26(5):360–364. doi: 10.1089/acm.2020.0177. [DOI] [PubMed] [Google Scholar]
- Van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J.N., Port J.R., Avanzato V., Bushmaker T., Flaxman A., Ulaszewska M., Feldmann F. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. bioRxiv. 2020 doi: 10.1101/2020.05.13.093195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkiteshwaran A. September. Tocilizumab. mAbs. 2009;5(1):432–438. doi: 10.4161/mabs.1.5.9497. MAbs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2(1):1–10. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Coronavirus Disease (COVID-19)https://www.who.int/emergencies/diseases diseases /novel-coronavirus-2019/situation-reports [Google Scholar]
- World Health Organization Corticosteroids for COVID-19. 2020. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1.
- Wilson N.M., Norton A., Young F.P., Collins D.W. Airborne transmission of severe acute respiratory syndrome coronavirus‐2 to healthcare workers: a narrative review. Anaesthesia. 2020 doi: 10.1111/anae.15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2020. Modes of Transmission of Virus Causing COVID-19: Implications for IPC Precaution Recommendations: Scientific Brief. 27 March 2020 (No. WHO/2019-nCoV/Sci_Brief/Transmission_modes/2020.1. [Google Scholar]
- Wu Y., Ho W., Huang Y., Jin D.Y., Li S., Liu S.L., Liu X., Qiu J., Sang Y., Wang Q., Yuen K.Y. SARS-CoV-2 is an appropriate name for the new coronavirus. Lancet. 2020;395(10228):949–950. doi: 10.1016/s0140-6736(20)30557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respiratory Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaya M., Nishimura H., Deng X. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir Investig. 2020;58:155–168. doi: 10.1016/j.resinv.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Liu H., Bai C., Wang Y., Zhang X., Guo R., Wu S., Wang J., Leung E., Chang H., Li P. Chemical composition and pharmacological mechanism of Qingfei Paidu decoction and Ma xing Shi Gan decoction against coronavirus disease 2019 (COVID-19): in silico and experimental study. Pharmacol. Res. 2020 doi: 10.1016/j.phrs.2020.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q.L., Yu Z.J., Gou J.J., Li G.M., Ma S.H., Zhang G.F., Xu J.H., Lin W.B., Cui G.L., Zhang M.M., Li C. Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. J. Infectious Dis. 2020;222(1):38–43. doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.H., Wu K.L., Zhang X., Deng S.Q., Peng B. In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J Integr Med. 2020;18(2):152–158. doi: 10.1016/j.joim.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Xie S., Zhang J., Zheng F., Jiang D., Li K., Zuo Q., Yan Y., Liu J., Xie Y., Peng H., Zhang L. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi: 10.20944/preprints202003.0065.v1. [DOI] [Google Scholar]
- Zhu F.C., Guan X.H., Li Y.H., Huang J.Y., Jiang T., Hou L.H., Li J.X., Yang B.F., Wang L., Wang W.J., Wu S.P. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (gilead.com, 2020) Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19. doi: 10.1021/acscentsci.0c00489 . Available at: https://www.gilead.com/news-and-press/press-room/press-releases/2020/6/gilead-announces-results-from-phase-3-trial-of-remdesivir-in-patients-with-moderate-covid-19. (accessed on 29th June 2020). [DOI] [PMC free article] [PubMed]