Abstract
Polymer-based controlled-release formulations are gaining significant advantage over chemical fertilizers in recent years as they contribute to the preservation of soil fertility by reducing soil pollution in farm lands. In this work, urea (a nitrogen source fertilizer) has been entrapped within chitosan-alginate and gelatin-alginate composite beads at three different concentrations. The physical properties of the polymer composite beads namely the diameter, porosity, yield percentage, Carr's index and Hausner's ratio were determined. These fertilizer-loaded beads were also characterized by Scanning Electron Microscopy (SEM) and Fourier Transform-Infra Red (FT-IR) spectroscopy. Urea enhanced swelling of chitosan-alginate beads through the creation of pores whereas in the case of gelatin-alginate formulations, urea decreased the swelling. The swelling of the polymer composite beads was found to be maximum at pH of 5.6 when compared to that of pH conditions, 7 and 8.5. The chitosan-alginate composite beads were found to possess better fertilizer entrapping efficiency than the gelatin-alginate composite beads. The in vitro urea release studies demonstrated that the urea-entrapped gelatin-alginate beads exhibited slower urea release than that of the chitosan-alginate beads. These controlled release urea formulations were found to follow quasi-fickian diffusion mechanism.
Keywords: Urea, Alginate, Chitosan, Gelatin, Physical properties, Swelling, Controlled release, Quasi-fickian diffusion, Soil biology, Soil chemistry, Soil fertility, Soil health, Plant growth, Bioaccumulation, Agroecosystem
Urea, Alginate, Chitosan, Gelatin, Physical properties, Swelling, Controlled release, Quasi-fickian diffusion; Soil biology; Soil chemistry; Soil fertility; Soil health; Plant growth; Bioaccumulation; Agroecosystem.
1. Introduction
An agrochemical (a fertilizer/a pesticide/a herbicide) is defined to be ideal for its usage in agricultural lands when it is capable of supplying nutrients required for crops or efficient enough to kill pests/insects that infect crops and also possesses a sensible cost/benefit ratio by minimizing soil pollution (Gandeza et al., 1991). The efficiency of these agrochemicals can be improved by means of utilizing the Controlled Release (CR) property of polymers. Controlled release agrochemicals are granulated agrochemicals that release into the soil gradually from polymer-based formulations in a controlled and sustained fashion through the porous channels created in the polymers or polymer composites network. Polymers-based controlled-release formulations are gaining significant advantage over chemical fertilizers in recent years as they contribute to the preservation of soil fertility by reducing soil pollution. Table 1 presents the summary of some reports available in the literature on such CR agrochemical formulations.
Table 1.
Literature review on biodegradable polymer(s)-based CR agrochemicals.
| Polymer/polymer composite(s) | Type of the agrochemical | Name of the agrochemical | Characteristics of formulation | Reference(s) |
|---|---|---|---|---|
| Polymer(s): polyvinyl alcohol/alginate-montmorillonite (PVA/Alg-MMT); cross linker: glutaraldehyde | Pesticide | Neemazal | The swelling ratio of the nano composite decreased with introduction of MMT and increase in the PVA content. The release of neemazal was inhibited with the addition of sodium MMT. | Rashidzadeh et al. (2014) |
| Polymer(s): acrylic acid and acrylamide; crosslinker: glutaraldehyde, initiator: lipase | Nitrogen fertilizer | Urea | The interpenetrating acrylic acid-acrylamide hydrogel enhanced the water uptake capacity up to 6.2 % and 7.2 % in sandy loam and clay soil. The hydrogels followed non-fickian diffusion kinetics. | Saruchi et al. (2016) |
| Polymer(s): sulfur, polymer and sulphur in combination with polymer | Nitrogen fertilizer | Urea | Polymer-Coated Urea (PCU) exhibited the desired controlled release property than that of the Sulfur-Coated Urea (SCU) and Polymer-Coated Sulfur-Coated Urea (PCSCU). | Ransom et al. (2020) |
| Polymer(s): poly(hydroxybutyrate), poly(hydroxybutyrate-valerate) | Herbicide | Ametryn | The formulations were found to follow anomalous transport mechanism. | Grillo et al. (2011) |
| Polymer(s): DURAMON, controlled release DURAMON urea with 3% lignosulfonate-coated with humic acid, controlled release DURAMON urea with 5% lignosulfonate-coated with seaweed extracts | Nitrogen fertilizer | Urea | Humic acid polymeric fertilizer helped in maintaining efficient crop (wheat) management and also educed the impact created by nitrogen loss which is caused due to the chemical fertilizers. | Gil-Ortiz et al. (2020) |
| Polymer(s): alginate; adsorbents: bentonite, anthracite and activated carbon | Herbicides | Metribuzin and chloridazon | The sphere formulations made up of alginate along with adsorbents exhibited slow release rates than that of the formulations made up of only alginate. Activated carbon was found to be the most effective adsorbent than the other adsorbents in promoting slower release rate of herbicide. | Flores-Cespedes et al. (2007) |
| Polymer(s): sodium alginate; crosslinker: glutaraldehyde | Botanical insecticide | Azadirachtin (from neem oil) | The release rate of particles was faster in with particles having higher concentration of neem oil in its composition. Long-time exposure of particles to reticulation agent diminished the release rate of insecticide. | Kulkarni et al. (2000) |
| Polymer(s): tapioca starch modified by PVA and citric acid | Nitrogen fertilizer | Urea | The formulations were prepared by Fluidized bed. Longevity of nitrogen release from the formulation was achieved. | Azeem et al. (2020) |
| Polymer(s): chitosan and poly(acrylic acid-co-acrylamide) | Nitrogen phosphorous potassium (NPK) fertilizer, retention of water | nitrogen, phosphorous and potassium | The granular formulations showed slower fertilizer release rate and also enhanced the water retention capacity of the soil. | Wu and Liu (2008) |
| Polymer(s): ethyl cellulose, cellulose acetate butyrate, poly (methyl methacrylate) | Pesticide | Ethyl benzoate | The microspheres were prepared by solvent evaporation technique. Cellulose matrices were found to be efficient in delivering sustained release of ethyl benzoate than poly (methyl methacrylate) matrix. | El Bahri and Taverdet (2007) |
| Polymers: ethyl cellulose for internal coating and poly(acrylic acid co-acrylamide) for external coating | Nitrogen fertilizer | Urea | The dual-coated particles exhibited water absorption equivalent to 70 times their own weight and also exhibited slow fertilizer release trend. | Ni et al. (2009) |
| Polymers: polyvinyl alcohol, chitosan, the blend of polyvinyl alcohol and chitosan; cross linker: glutaraldeyde | Fertilizer | phosphorous | All the phosphorous entrapped CRF hydrogels exhibited quasi-fickian diffusion kinetics. | Jamnongkan and Kaewipirom (2010) |
| Polymers: starch and polyvinyl alcohol; cross linker: formaldehyde | Soluble fertilizers | Ammonium | Reduction in starch concentration affected the compatibility of films whereas the formaldehyde concentration influenced the absorption capacity of films. The concentration of PVA influenced the permeability of films to water and ammonium. | Han et al. (2009) |
| Polymers: gelatin; cross linker: glutaraldehyde | Nitrogen fertilizer | Urea | The gelatin microspheres prepared by emulsification method were found to follow fickian diffusion kinetics. The gelatin-urea ratio significantly affected the swelling nature of gelatin microspheres. | Tang et al. (2018) |
| Polymer(s): ethylcellulose | Herbicide | Alachlor | The microspheres enabled slow and gradual release of herbicide. Further, the use of ethylcellulose protected the herbicide from photolytic degradation. | Sopena et al. (2007) |
| Polymer(s): ethylcellulose | Herbicide | Norflurazon | The microspheres facilitated slow and gradual release of norflurazon. The use of ethylcellulose reduced the dosage of norflurazon for soil applications. | Sopena et al. (2011) |
| Polymer(s): Fe(III)-carboxylates | Fertilizer | Phosphate | The photosensitive hydrogels were efficient enough to reclaim phosphates from waste solutions and able to serve as a controlled-release fertilizer. | Karunarathna et al. (2019) |
| Polymer(s): chitosan; inducers: peat, humic acid and humin | Nitrogen fertilizer | Urea | The release of urea from the formulations was found to be dependent on the pH conditions. The usage of peat and humic acid substances contribute to reduction of environmental pollution. | Araujo et al. (2017) |
| Polymer(s): starch-g-poly(vinyl acetate) (St-g-PVAc); initiator: K2S2O8 | Nitrogen fertilizer | Urea | In situ graft copolymerization method was adopted to prepare urea entrapped St-g-PVAc biodegradable films. The swelling ability of the films decreased in the introduction of hydrophobic PVAc. | Niu and Li (2012) |
| Polymer(s): chitosan and salicyladehyde | Nitrogen fertilizer | Urea | Urea-entrapped chitosan-salicyladehyde hydrogel formulations prepared by in situ hydrogelation technique served as an effective multifunctional soil conditioner. | Iftime et al. (2019) |
| Polymer(s): chitosan | Nitrogen fertilizer | Urea | Spherical nano-chitosan urea particles prepared by ionotropic gelation exhibited different sizes with respect to the concentration of urea used in the formulations. The pot study conducted with these nano fertilizers using potato plant indicated that the formulations enhanced the yield of potato than that of the commercial fertilizer. | Kalia et al. (2019) |
| Polymer(s): Poly(butylene succinate) and a butylene ester of dilinoleic acid | Nitrogen phosphorous potassium (NPK) fertilizer | Nitrogen, phosphorous and potassium | A conceptual model was formulated based on the concentration of mineral components inside the granules in order to describe the mechanism of fertilizer release. | Lubkowski et al. (2015) |
| Polymer(s): polyvinyl alcohol-modified starch biopolymer | Nitrogen fertilizer | Urea | The diffusion coefficient decreased with increase in the coating thickness. The coating thickness and integrity of the polymer composite films yielded formulations with improved controlled release characteristics. | Azeem et al. (2016) |
Urea is a leading nitrogen fertilizer in terms of economic value and utilization rate in agricultural farms. However, it possesses a drawback of its susceptibility to get volatilized when dispersed in the soil. About one-third of fertilizers used in agricultural lands are being lost from the soil due to volatilization of ammonia (Hargrove, 1988; Kissel and Cabrera, 1988). Researchers have reported that moisture regime, temperature and the physico-chemical properties of the soil are some of the factors which influence ammonia volatilization (Hargrove, 1988; Kissel and Cabrera, 1988). Due to repetitive usage of such fertilizers in agricultural farm lands, the texture and fertility of the soil ceases continuously day by day. These chemical fertilizers degrade soil fertility by elevating the alkaline condition of the soil. Ramirez et al. (2007) had stated that the Controlled Release Fertilizers (CRFs) made up of biodegradable carriers can minimize the application rate, prevent seeding damage, reduce leaching or volatilization and protects the ecosystem (Ramirez et al., 2007).
While looking in depth on the criteria to be satisfied for selecting polymers for CR formulations, one must choose polymers that can serve as a good soil-conditioner and able to biodegrade when added to the soil without leading to soil pollution. Chitosan is a biodegradable cationic polysaccharide polymer made up of repetitive units of ß-(1–4)-linked D-glucosamine (deacetylated unit) and N-acetyl-D-glucosamine (acetylated unit). Chitosan is derived by deacetylation of chitin (a polymer is present in crustacean and shrimp shells). Chitosan have a widespread range of applications. They have been used in different types of environmental (Yong et al., 2015; Kanmani et al., 2017) and biomedical applications (Singh and Ray, 2007; Zhao et al., 2018). Chitosan has been considered as an effective biodegradable polymer for its usage as a functional material in wide range of applications as they possess excellent properties such as biocompatibility, biodegradability, adsorption and non-toxicity (Ravi Kumar, 2000). The physical and mechanical properties of chitosan can be enhanced for practical applications by blending with other polymers (Shu and Zhu, 2000).
Gelatin is a biodegradable translucent, colourless, brittle and flavorless polymeric substance obtained from collagen of animal bones. It is made up of nineteen amino acids and hence it can be hydrolysed by several proteolytic enzymes to yield its constituent amino acids. Due to this degradation property, gelatine has been used by many researchers in different applications that require purposeful biodegradability (Chandra and Rustgi, 1998). Gelatin has good wound healing property (Jang et al., 2018) and it finds many of its applications in the field of medicine. Narrowed research has been undergone with this proteinaceous polymer in the sector of agriculture.
Sodium alginate is an organic linear copolymer made up of (1–4)-linked ß-D-mannuronate and α-L-guluronate residues. Alginate has been used in wide variety of applications. The ability of sodium alginate to solubilize in water, to form gels, to form films and fibres of calcium alginate are some of the key properties (http://www.fao.org/3/y4765e/y4765e08.htm) which enable sodium alginate to be used in several applications such as textile printing (Wang et al., 2014), food (Qin et al., 2018), biomedical (Lee and Mooney, 2012), enzyme (Zhao et al., 2015), agricultural (El-Rehim, 2006) and environmental (Wang et al., 2019) applications. Alginate gels has the tendency to serve as excellent matrices for Slow Release (SR) of agrochemicals in the field of agriculture as they are biodegradable and incorporation of agrochemicals using an aqueous system at ambient temperatures is easier (Nnamonu et al., 2012).
In this work, the mechanical stability of the chitosan and gelatin had enhanced by using sodium alginate as strengthening agent and entrapped urea within the biopolymer complexes in order to facilitate controlled release of urea. These controlled release formulations were characterized by Scanning Electron Microscopic (SEM) and Fourier Transform-Infra Red (FT-IR) spectroscopic analysis.
2. Experimental
2.1. Materials
Chitosan (medium molecular weight ~1,90,000–3,10,000 daltons, <200 mPa.s in viscosity, deacetylation degree ≥75%), gelatin (50,000–1,00,000 daltons in molecular weight and 5.10–5.80 mPa.s in viscosity), sodium alginate (216.12 g/mol in molecular weight), urea, calcium chloride (CaCl2), acetic acid glacial, potassium dihydrogen phosphate, dipotassium hydrogen phosphate, disodium hydrogen phosphate, sodium acetate, orthophosphoric acid, concentrated sulphuric acid, DiAcetylMonoxime (DAM), thiosemicarbazide, sodium chloride and ferric chloride was procured from Hi Media Laboratories Pvt. Ltd. Deionized water was used throughout the experiments.
2.2. Methodology
2.2.1. Preparation of beads
2.2.1.1. Chitosan-alginate beads with and without urea entrapment
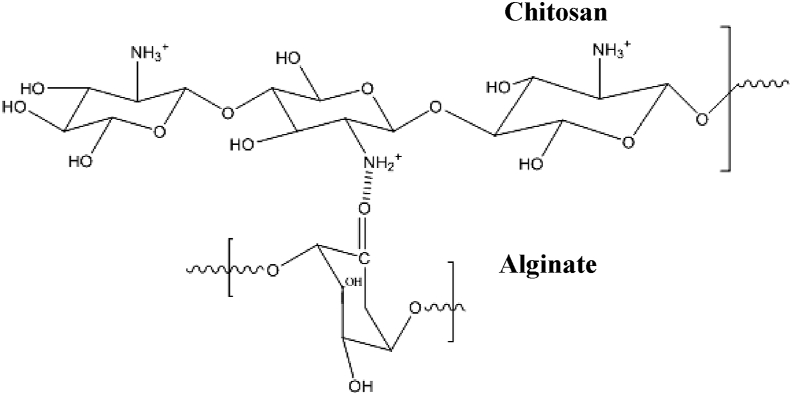
A 25 ml of emulsion containing 4% sodium alginate and 50 mg of urea was added as drops into 50 ml suspension containing 0.5 g of chitosan, 2% acetic acid and 0.2 M CaCl2. The glass beads obtained were allowed to stand still for 2 h. The beads were washed with distilled water and allowed to air-dry (Nnamonu et al., 2012). The same methodology was carried out to prepare beads with different urea concentrations (50mg, 100 mg and 150 mg). The beads containing 50 mg, 100 mg and 150 mg of urea were designated as CAu(1), CAu(2) and CAu(3). Followed by the preparation of urea-entrapped beads, chitosan-alginate beads devoid of urea (CA) was also prepared. A schematic representation of the anticipated intermolecular interaction between chitosan and alginate in chitosan-alginate composite beads is shown in Figure 1.
Figure 1.
A schematic representation of the `intermolecular interaction between chitosan and alginate in chitosan-alginate composite beads.
2.2.1.2. Gelatin-alginate beads with and without urea entrapment
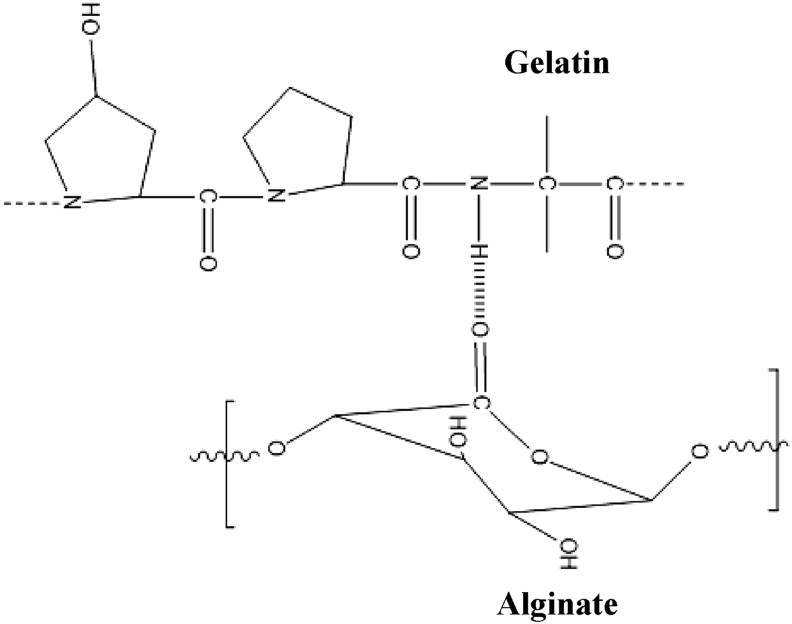
A 25 ml of emulsion was prepared by dissolving 0.5 g of gelatin and 4% sodium alginate in distilled water. The emulsion was constantly stirred using magnetic stirrer for 1 h in order to obtain a homogenous suspension (solution ‘A’). The glass beads were produced by adding solution ‘A’ as drops in 0.2 M CaCl2 solution. The so formed round beads were allowed to stand still for 2 h. The glass beads were then washed with distilled water and allowed to air-dry. These urea-free glass beads were named as GA beads (Roy et al., 2009). In order to prepare the urea-entrapped gelatin-alginate composite beads, 50 mg of urea was added to solution ‘A’ and stirred well. The resultant suspension was then suspended in the form of drops in 0.2 M CaCl2 solution, allowed to stand still for 2 h and then washed with distilled water and air-dried to obtain urea-entrapped gelatin-alginate GAu(1) beads (Roy et al., 2009). The same methodology was carried out to produce gelatin-alginate composite beads loaded with 100 mg and 150 mg of urea. These beads were designated as GAu(2) and GAu(3). Figure 2 presents a schematic representation of the anticipated intermolecular interaction between the polymers, gelatin and alginate in the gelatin-alginate composite beads.
Figure 2.
A schematic representation of the `intermolecular interaction between gelatin and alginate in gelatin - alginate composite beads.
2.2.2. Physical properties of the beads
The parameters such as diameter, porosity, yield percentage, Carr's index, Hausner's ratio, equilibrium water content (EWC) and swelling properties of the urea-entrapped chitosan-alginate (CAu) and gelatine-alginate (GAu) beads were determined in order to understand the physical properties of the beads.
2.2.2.1. Diameter of beads
About 20 beads were taken in random and its diameter was measured using a screw gauge. The least count (LC) of the screw gauge used was 0.01 mm (Nnamonu et al., 2012).
2.2.2.2. Porosity
The porosity of the chitosan-alginate beads and gelatine-alginate beads without and with urea were determined by volume/density method reported by Nnamonu et al. (2012). A definite mass of beads were taken in a 10 mL measuring cylinder and tapped well. Total volume (Vt) and the volume occupied by the beads (Vb) were noted. The pore volume (Vp) was calculated using the following formula (Nnamonu et al., 2012):
| Pore volume (Vp) = Total volume (Vt) – Volume of beads (Vb) | (1) |
| (2) |
2.2.2.3. Yield
The yield percentage of beads was determined by the following formula:
| (3) |
Where, W1 = Sum of the weight of fertilizer, polymer and cross-linking agents
W2 = Weight of the beads generated
2.2.2.4. Carr's index and Hausner's ratio
The Carr's index value and Hausner's ratio of beads was calculated using the following equation (Asha et al., 2011):
| (4) |
| (5) |
2.2.2.5. Equilibrium water content (EWC)
The imbibition of water by the beads was determined by calculating the EWC of beads. About 30 mg of beads were taken in a known volume of deionized water and allowed to remain still for a period of 24 h. After 24 h, these water-imbibed beads were taken and its EWC was calculated using the following formula (Thakur et al., 2012):
| (6) |
where, W24h is the weight of the beads at equilibrium (at time, t = 24 h) and W0 is the initial weight of beads (at time, t = 0).
2.2.2.6. Swelling properties
Known weight of beads were taken and allowed for swelling in acetate buffer of pH 5.6, phosphate buffer of pH 7 and 8.5 at room temperature for a period of 3 h. The swollen beads were carefully taken using tissue paper at a periodic time interval of 30 min and their weight was measured. The Swelling Ratio of the beads was then calculated using the following formula:
| (7) |
2.2.3. Urea entrapment by beads
The concentration of urea entrapped by CAu(1), CAu(2), CAu(3), GAu(1), GAu(2) and GAu(3) beads was determined by DiAcetyl Monoxime (DAM) method using HITACHI U-2900 Spectrophotometer. 50 mg of urea-entrapped chitosan-alginate and gelatine-alginate beads were immersed in 5 ml of distilled water and allowed to swell for 48 h. After 48 h of swelling, 0.5 ml of the solution was taken, made into aliquots and then treated with DAM reagent. The Optical Density (OD) of the DAM treated sample aliquot was measured spectrophotometrically at 540 nm. The concentration of urea entrapped by the beads was determined using the calibration curve constructed for urea in prior by DAM method.
2.2.4. Characterization of beads
The morphological characteristics of the CA, GA, CAu(1) and GAu(1) beads were recorded using ZEISS EVO Series SEM model EVO 50 available at Karunya University, Coimbatore, Tamil Nadu, India. The variation in vibrational characteristics of CA, GA, CAu(1) and GAu(1) beads was determined using Perkin Elmer SPECTRA 100 Fourier Transform-Infra Red Spectrometer using KBr pellet at Department of Nano Technology, K.S. Rangasamy College of Technology, Tiruchengode, Tamil Nadu, India. The transparent pellet was prepared by mixing 100 mg of KBr with 1 mg of powdered bead and pressed under a pressure of 10,000 to 15,000 pounds per square inch with special disc.
2.2.5. Estimation of urea entrapped in beads
The concentration of entrapped urea was determined by DiAcetyl Monoxime (DAM) method using HITACHI U-2900 Spectrophotometer. 50 mg of CAu(1), CAu(2), CAu(3), GAu(1), GAu(2) and GAu(3) beads were immersed in 5 mL of distilled water and allowed to swell for 48 h. After 48 h of swelling, the solution was filtered to remove polymeric particles. 0.5 mL of the solution was taken and its absorbance was measured spectrophotometrically using DAM reagent at 540 nm.
2.2.6. Kinetics of fertilizer release
The diffusion kinetics of the beads [CA, CAu(1), CAu(2), CAu(3), GA, GAu(1), GAu(2) and GAu(3)] in swelling media (pH 5.6) were studied using a semi-empirical model called as Power law or Korsmeyer-Peppas model (Korsmeyer et al., 1986):
| (8) |
where Mt is the amount of fertilizer released at time ‘t’, M∞ is the amount of fertilizer released at equilibrium and Mt/M∞ is the fractional release of fertilizer at time ‘t’. The value of power law exponent ‘n’ will imply the mechanism of fertilizer release. The power law exponent was determined from the slope of the plot logarithm of (Mt/M∞) versus log t.
3. Results and discussions
3.1. Physical characteristics of beads
Table 2 presents the various physical properties of the CA, CAu(1), CAu(2), CAu(3), GA, GAu(1), GAu(2) and GAu(3) beads synthesized in this work. From Table 2, it is evident that type of the biopolymer and presence of urea had significant impact on the physical characteristics of beads.
Table 2.
Physical characteristics of urea entrapped chitosan-alginate and gelatin-alginate bead formulations.
| Evaluation Parameters | Chitosan-Alginate Beads |
Gelatin-Alginate Beads |
||||||
|---|---|---|---|---|---|---|---|---|
| CA | CAu(1) | CAu(2) | CAu(3) | GA | GAu(1) | GAu(2) | GAu(3) | |
| Diameter (mm) | 0.9205 ± 0.03 | 0.8375 ± 0.04 | 0.892 ± 0.03 | 0.8366 ± 0.02 | 1.4 ± 0.02 | 1.403 ± 0.04 | 1.285 ± 0.03 | 1.004 ± 0.03 |
| Yield (%) | 47.82 | 51.06 | 41.66 | 40.41 | 55.31 | 52.08 | 58.36 | 54.34 |
| Porosity (%) | 75 | 75 | 77 | 78 | 72 | 72 | 73 | 74 |
| Bulk density (g/cm3) | 0.77 ± 0.01 | 0.77 ± 0.01 | 0.83 ± 0.01 | 0.87 ± 0.01 | 0.69 ± 0.01 | 0.69 ± 0.01 | 0.71 ± 0.01 | 0.74 ± 0.01 |
| Tapped density (g/cm3) | 0.8 ± 0.01 | 0.8 ± 0.01 | 0.87 ± 0.01 | 0.91 ± 0.01 | 0.71 ± 0.01 | 0.71 ± 0.01 | 0.74 ± 0.01 | 0.77 ±0.01 |
| Carr's Index | 3.8 | 3.8 | 4.1 | 4.35 | 3.45 | 3.45 | 3.57 | 3.70 |
| Hausner's Ratio | 1.04 | 1.04 | 1.04 | 1.05 | 1.04 | 1.04 | 1.04 | 1.04 |
| EWC (%) | 94.44 | 94.91 | 95.58 | 95.83 | 97.72 | 96.84 | 96.62 | 96.25 |
It has been observed that the beads made up of gelatine-alginate mixture exhibited larger diameter than the beads made up of chitosan-alginate. Klokk and Melvik had illustrated in their study that diameter of alginate gel beads produced by means of electrostatic potential bead generator was found to increase with increase in concentration as well as the viscosity of alginate solution (Klokk and Melvik, 2002). In our study, we found that GAu(1) beads was found to have larger diameter followed by GAu(3), GAu(2), GA, CA, CAu(2), CAu(1) and CAu(3) beads. Thus, we anticipate that the larger diameter of gelatine-alginate beads is possibly attributed to the gelling effect of gelatine and alginate.
With respect to the yield percentage of the beads, GAu(2) beads exhibited a maximum yield percentage of 58.36 % whereas the CAu(3) beads exhibited a minimum yield percentage of 40.41 %.
It is interesting to note that among the CA and GA beads which are devoid of urea, the CA beads exhibited higher porosity than the GA beads indicating the fact that chitosan contributes in the enhancement of porosity of beads. It can be also observed that apart from the biopolymer used in conjugation with gelatin, the presence of urea also has its influence on the porous nature of beads. CAu(3) beads containing higher concentration of urea possessed a maximum porosity percentage of 78% whereas GA beads without urea showed the lowest porosity percentage of 72%. Thus, a direct correlation was observed between the porous nature of the beads and the concentration of urea used in the formulations.
Moreover, CAu(3) beads exhibited maximum bulk and tapped densities of 0.87 ± 0.01 g/cm3 and 0.91 ± 0.01 g/cm3 while GA and GAu(1) beads exhibited a minimum bulk and tapped densities of 0.69 ± 0.01 g/cm3 and 0.71 ± 0.01 g/cm3 respectively. The Carr's index and Hausner's ratio calculated for the CA and GA beads with and without urea are also shown in Table 2.
EWC of beads was found to be influenced by the hydrophilic nature of the biopolymer and the presence of urea (see Table 2). Urea-free GA beads were found to exhibit a maximum EWC of 97.72% whereas incorporation of urea into the gelatine-alginate composite decreased the EWC percentage. Thus, we anticipate that the hydrophilic nature of gelatine has led to increased water uptake by GA beads. However, addition of urea could have lead to degradation of gelatine (as gelatine is a proteinaceous polymer), thereby leading to reduced ability of gelatine-alginate beads to retain water. On the other hand, CA beads with urea had led to increased water uptake of the beads than that of the urea-free CA beads by facilitating the absorption of water molecules. This could be attributed to the hydrophobicity of chitosan and impregnation of urea in the chitosan-alginate complex.
3.1.1. Swelling behaviour of the beads
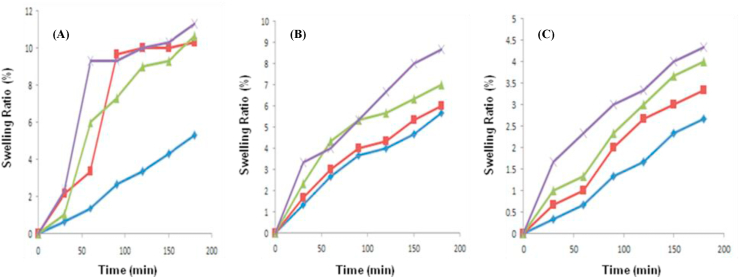
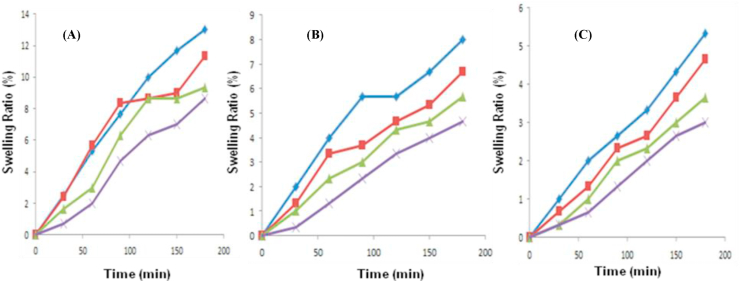
The swelling characteristic analysis of CA, CAu(1), CAu(2), CAu(3), GA, GAu(1), GAu(2) and GAu(3) beads in sodium acetate buffer (pH 5.6) and phosphate buffer (pH 7 & 8.5) at room temperature revealed that swelling of chitosan and gelatin beads was influenced by the pH of the swelling media as well as concentration of urea entrapped within beads. Figures 1(A-C) and 2(A-C) presents the variation in swelling behaviour of chitosan-alginate [CA, CAu(1), (CAu(2) and CAu(3)] beads and gelatine-alginate composite [GA, GAu(1), (GAu(2) and GAu(3)] beads loaded without and with urea at pH 5.6, 7 and 8.5.
(a) Effect of pH: The swelling of CA and GA beads was found to increase at acidic pH conditions (as shown in Figures 3 and 4). In case of CA beads, the swelling nature of chitosan increased with decrease in the ionic strength of the swelling medium (Figure 3A-3C) (Yao et al., 1994; Muzzarelli et al., 1999; Pourjavadi et al., 2009). In case of GA beads, at alkaline environment conditions, the dissociation of –COOH functional groups results in weakening of physical forces between the two polymers, gelatin and alginate. This ultimately results in degradation of beads leading to reduced swelling at alkaline pH conditions (as shown in Figure 4B and 4C) (Roy et al., 2009).
Figure 3.
Swelling behaviour chitosan-alginate beads at pH conditions of (A) 5.6, (B) 7 and (C) 8.5. (CA[ ], CAu(1) [
], CAu(1) [ ], CAu(2) [
], CAu(2) [ ]and CAu(3) [
]and CAu(3) [ ]).
]).
Figure 4.
Swelling behaviour gelatin-alginate beads at pH conditions of (A) 5.6, (B) 7 and (C) 8.5. (GA [ ], GAu(1) [
], GAu(1) [ ], GAu(2) [
], GAu(2) [ ]and GAu(3) [
]and GAu(3) [ ]).
]).
(b) Effect of urea: Urea is a carbamide and hence it increases the porous nature of hydrogels (Tyliszczak et al., 2009). So, CA beads with higher concentration of urea (CAu(3) exhibited a maximum swelling rate when compared with the rest of the CA beads due to the pores created in the beads by urea molecules (as shown in Figure 3A-3C). In case of GA beads, increase in urea concentration reduced the swelling efficiency of beads (as shown in Figure 4A-4C). This could be possibly due to the fact that, urea (a protein denaturant) denatures gelatin in the gelatin-alginate formulation and causes disintegration of beads, thereby leading to reduced swelling. Thus, urea-free GA beads showed improved swelling than urea-entrapped beads. Decreased swelling may also result from the “salting-out” effect. Gelatin being a proteinaceous polymer consists of greater amount of hydrophobic amino acid residues. Swelling of beads in the greater ionic strength medium exposes hydrophobic patches of protein. As a result, the solubility of gelatin also enhances. Above the saturation point, excess ionic strength in the swelling medium has the tendency to decrease solubility of gelatin. So, urea at increased concentration decreased the swelling nature of GA beads (GAu(3) at all the three pH conditions. Urea-free gelatin-alginate (GA) beads showed good swelling ratio when compared with the rest at all the three pH conditions (as shown in Figure 4A-4C).
3.2. Characterization of beads
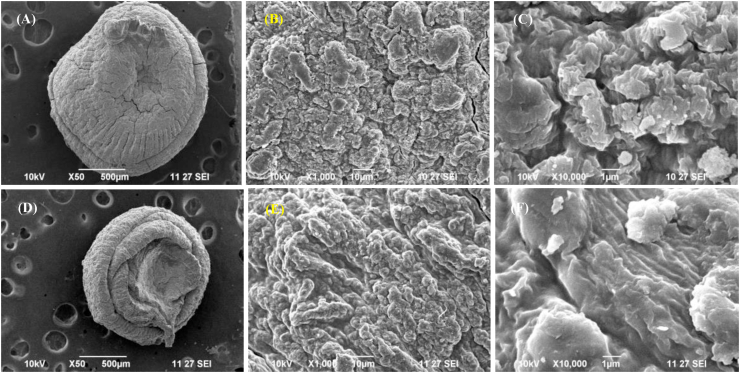
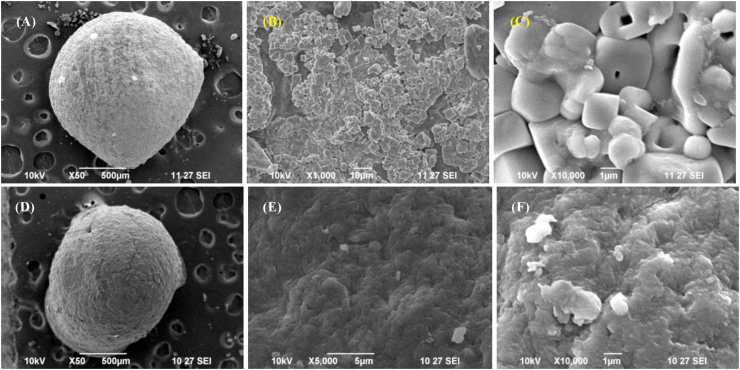
Figures 5 and 6 presents the SEM micrographs of CA (Figure 5A-5C), CAu(1) (Figure 5D-5F), GA (Figure 6A-6C) and GAu(1) (Figure 6D-6F) beads recorded at 50 X, 1000 X and 10000 X. It is evident from Figure 3 that the chitosan-alginate beads had rough and irregular morphology (Nnamonu et al., 2012). Gelatin-alginate beads had a regular and well-packed cubical arrangement in the same magnification. This arrangement was disordered as a result of incorporation of urea. Addition of urea within the polymer matrix had a significant impact on the morphology of chitosan-alginate and gelatin-alginate composites.
Figure 5.
SEM micrographs of a CA bead at (A) 50 X, (B) 1000 X, (C) 10,000 X magnification, CAu(1) bead at (D) 50 X, (E) 1000 X and (F) 10,000 X magnification.
Figure 6.
SEM micrographs of a GA bead at (A) 50 X, (B) 1000 X, (C) 10,000 X magnification, GAu(1) bead at (D) 50 X, (E) 1000 X and (F) 10,000 X magnification.
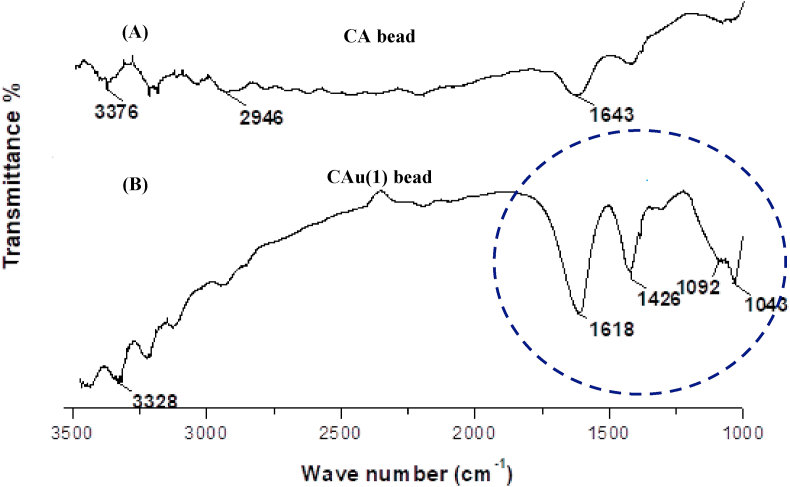
The urea-free chitosan-alginate (CA), gelatin-alginate beads (GA) and urea entrapped chitosan-alginate [CAu(1)], gelatin-alginate [GAu(1)] beads were characterized by FT-IR analysis. Figures 7 and 8 presents the overlay of FT-IR spectra of CA and CAu(1) beads. In the FT-IR spectrum 7A, the existence of alginate is defined by the stretching of carboxylate (C=O) ions at 1643 cm−1. The presence of chitosan is evident by the stretching of N–H group between 3400 cm−1 and 3300 cm−1 and C–H stretching between 3000 cm−1 and 2850 cm−1 respectively. In the spectra 7B, the presence of urea was confirmed by the stretching of primary amine (NH2) at 3328 cm−1. A band of strong intensity at 1618 cm−1 was attributed to the characteristic of C=O group. A band of medium intensity was observed at 1426 cm−1 which may be due to the vibrational stretching of the C–N bond of urea (Tang et al., 2018). The presence of C–O–C link is represented by the stretching of bands at 1092 cm−1 and 1043 cm−1 (Nnamonu et al., 2012). Thus, the variation in the vibrational stretching of CAu(1) beads (as shown in Figure 7B) in comparison with that of CA beads (Figure 7A) confirms the impregnation of urea in chitosan-alginate matrix.
Figure 7.
FT-IR spectra of chitosan-alginate bead: (A) without urea [CA] and (B) with urea [CAu(1)].
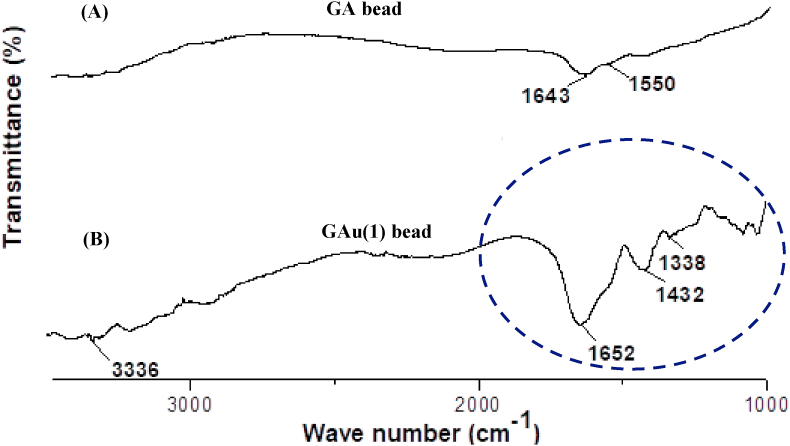
Figure 8.
FT-IR spectra of gelatin-alginate bead: (A) without urea [GA] and (B) with urea [GAu(1)].
A FT-IR spectrum of urea-free GA beads is shown in Figure 8A. The presence of alginate was evident by the stretching of carboxylate (C=O) ions at 1643 cm−1 and bending of amine (N–H) group at 1550 cm−1 confirmed the presence of gelatin. Figure 8B illustrates the FT-IR spectrum of GAu(1) beads. The primary amine (NH2) stretching at 3328 cm−1 reveals the evidence of urea and stretching at 1652 cm−1 is the characteristic of C=O group. The stretching of band at 1432 cm−1 may be attributed to the C–N bond of urea (Tang et al., 2018), thereby confirming the vibrational changes induced by urea in the gelatin-alginate complex in the beads.
3.3. Estimation of urea and kinetics of fertilizer release
3.3.1. Urea entrapped in beads
The concentration of urea entrapped in the beads CAu(1), CAu(2), CAu(3), GAu(1), GAu(2) and GAu(3) was determined spectrophotometrically using DiAcetyl Monoxime (DAM) method at 540 nm. Presence of urea in the beads resulted in the formation of purple-color complex in presence of DAM reagent in the hot acidic medium. CAu(3) beads was found to entrap urea at a maximum concentration of 38 μg/mg of beads, followed by GAu(3), CAu(2), GAu(2), CAu(1) and GAu(1). Table 3 describes the concentration of urea entrapped by the beads and diffusion kinetics of the beads.
Table 3.
Evaluation of fertilizer release kinetics from CRF beads.
| S. No. | CRF beads | Concentration of urea entrapped by beads (μg/mg) | n | K | Kinetics of fertilizer release | R2 | Equation |
|---|---|---|---|---|---|---|---|
| 01. | CAu(1) | 11.9 | 0.3222 | 1.2912 | quasi-fickian diffusion | 0.9115 | y = 0.0407 x + 3.2459 |
| 02. | CAu(2) | 22 | 0.3247 | 1.3656 | quasi-fickian diffusion | 0.8978 | y = 0.0369 x + 3.1755 |
| 03. | CAu(3) | 38 | 0.3131 | 1.3710 | quasi-fickian diffusion | 0.9016 | y = 0.0398 x + 3.2047 |
| 04. | GAu(1) | 12.4 | 0.3178 | 1.3600 | quasi-fickian diffusion | 0.9041 | y = 0.039 x + 3.2629 |
| 05. | GAu(2) | 20.2 | 0.3659 | 1.3466 | quasi-fickian diffusion | 0.8612 | y = 0.0289 x + 2.9637 |
| 06. | GAu(3) | 36.6 | 0.4029 | 1.3400 | quasi-fickian diffusion | 0.8164 | y = 0.0227 x + 2.7198 |
3.3.2. Determination of urea release kinetics
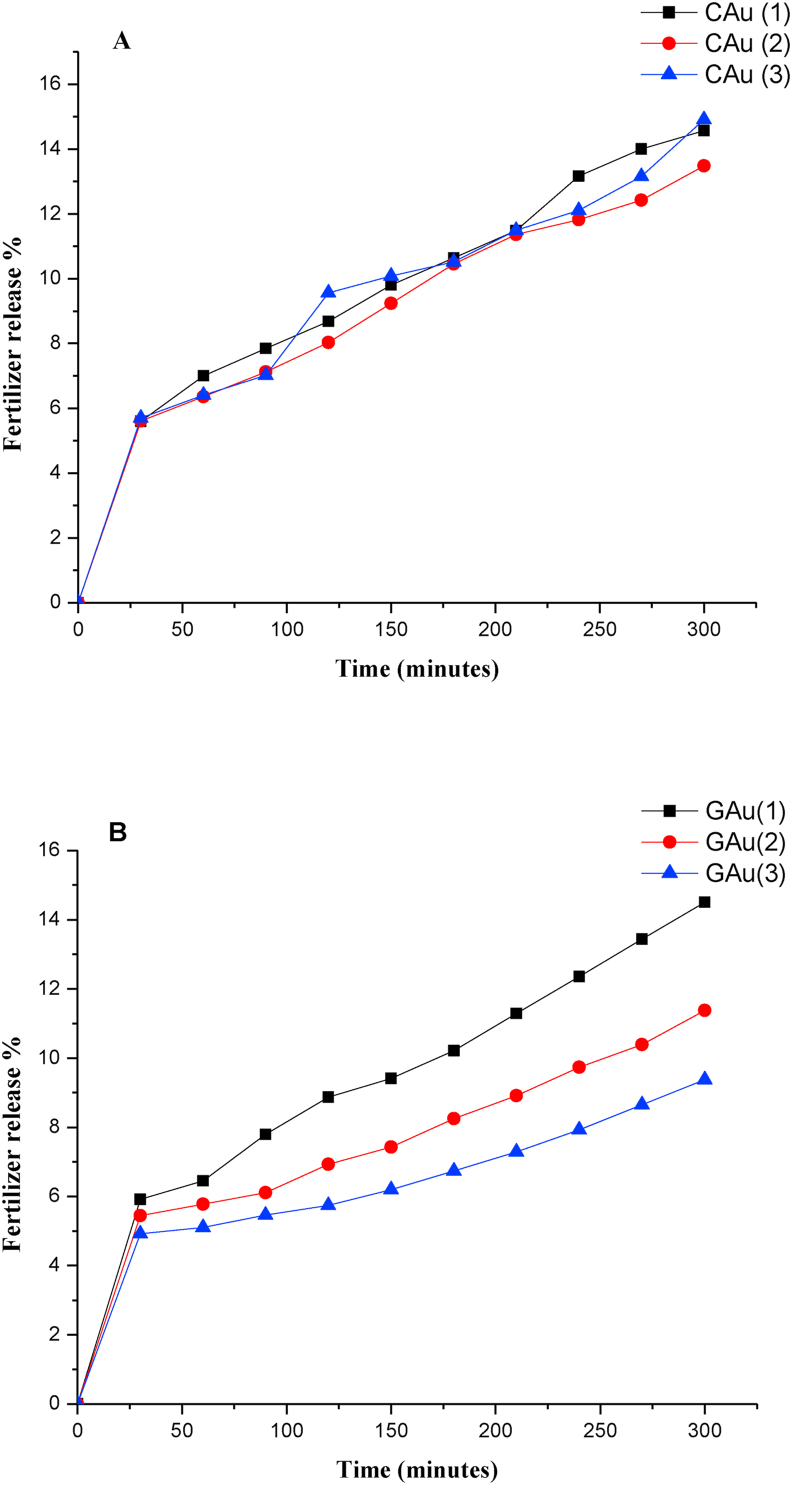
Following the estimation of urea concentration entrapped by the chitosan-alginate and gelatin-alginate beads, efforts were made to understand the release kinetics adopted by the beads in sodium acetate buffer (pH 5.6). Figures 9A and 9B represents the urea release profile of urea-entrapped polymer composite beads (CAu and GAu). It can be observed from Figure 9 that 10% of urea released at 175 min by CAu beads and 10, 8 & 6% of urea released at 175 min by GAu(1), GAu(2) & GAu(3) beads respectively. Thus urea-entrapped gelatin-alginate (GA) beads exhibited slower urea release than that of the chitosan-alginate (CA) beads at the end of 300 min.
Figure 9.
Fertilizer release profile of (A) chitosan-alginate beads and (B) gelatin-alginate beads.
Till date, a limited number of reports are available in the literature where researchers have studied agrochemical release kinetics (Grillo et al., 2011; Jamnongkan and Kaewipirom, 2010; Saruchi et al., 2016; Tang et al., 2018). Jamnongkan and Kaewipirom (2010) studied the mechanism of phosphorous release from the CRF hydrogels prepared from polyvinyl alcohol (PVA), chitosan (CS) and the blend of these two polymers using Korsmeyer-Peppas equation (Jamnongkan and Kaewipirom, 2010). If n = 0.5, the release mechanism is fickian's diffusion. If the value of n lies between 0.5 and 1, then the release is non-fickian diffusion release. In n = 1, then the release kinetics is non-fickian case II kinetics. If n < 0.5, the fertilizer release kinetics follows quasi-fickian diffusion. In case of all the phosphorous entrapped in CRF hydrogels, the researchers found the n values than 0.5, further confirming the nutrient release kinetics from CRF hydrogels to be a quasi-Fickian diffusion (Jamnongkan and Kaewipirom, 2010). In this study, it was observed that the urea-entrapped chitosan-alginate and gelatin-alginate beads followed quasi-fickian diffusion kinetics in accordance with the n values (Table 3) calculated using Korsmeyer-Peppas equation [equation (8)]. Tang and co-workers (2018) had reported that the gelatin microspheres prepared by emulsification method using glutaraldehyde as cross-linker exhibited fickian diffusion kinetics (Tang et al., 2018). The authors have reported the gelatin-urea ratio significantly affected the swelling nature of gelatin microspheres (Tang et al., 2018). Thus, this work illustrates that the type of polymer-based formulation, preparation methodology, the cross-linker used, the concentration of polymers/copolymers and the concentration of urea entrapped in the formulations influence the fertilizer release from the polymer composites.
4. Conclusions
Chitosan-alginate and gelatine-alginate composite beads entrapping three different concentrations of urea were prepared and characterized by SEM and FT-IR analysis. The physical properties of the beads were evaluated. The porous nature of chitosan and gelatin was modified due to alginate crosslinking and urea entrapment. The diameter of chitosan-alginate beads obtained was smaller than the gelatin-alginate beads. The swelling studies indicated that chitosan-alginate and gelatin–alginate beads exhibit enhanced swelling at acidic pH conditions. In case of chitosan-alginate beads, urea enhanced the swelling of beads whereas in gelatin-alginate beads, urea decreased the swelling. Chitosan-alginate beads were found to possess good urea entrapping efficiency than gelatine-alginate beads. Gelatin-alginate beads exhibited slower fertilizer release than that of the chitosan-alginate beads. The chitosan-alginate and gelatin-alginate controlled release of urea formulations followed quasi-fickian diffusion kinetics. Our studies demonstrate that these biodegradable polymers-based urea formulations can serve as effective soil-conditioners, facilitate slow and gradual release of urea into the soil, and thereby assist in minimizing soil pollution.
Declarations
Author contribution statement
Indumathi Sathisaran: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Murugesan Balasubramanian: Conceived and designed the experiments; Wrote the paper.
Funding statement
This work was supported by Department of Biotechnology, K. S. Rangasamy College of Technology, Tiruchengode, Tamil Nadu.
Data availability statement
Data included in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors gratefully acknowledge Department of Biotechnology, K. S. Rangasamy College of Technology, Tiruchengode, Tamil Nadu for providing research lab facilities to carry out this work. The authors are very grateful to the Department of Nanoscience Science and Technology, K. S. Rangasamy College of Technology, Tiruchengode, Tamil Nadu and Karunya University, Coimbatore, Tamil Nadu for providing access to Fourier Transform Infra Red spectroscopy and Scanning Electron Microscopy respectively.
References
- Araujo B.R., Romao L.P.C., Doumer M.E., Mangrich A.S. Evaluation of the interactions between chitosan and humics in media for the controlled release of nitrogen fertilizer. J. Environ. Manag. 2017;190:122–131. doi: 10.1016/j.jenvman.2016.12.059. [DOI] [PubMed] [Google Scholar]
- Asha K., Dash V., Maiti B.C. Formulation and evaluation of calcium alginate beads from plant extract. Indian J. Nov. Drug Deliv. 2011;3(3):197–205. [Google Scholar]
- Azeem B., KuShaari K., Man Z. 2016, effect of coating thickness on release characteristics of controlled release urea produced in fluidized bed using waterborne starch biopolymer as coating material. Proc. Eng. 2016;148:282–289. [Google Scholar]
- Azeem B., KuShaari K., Naqvi M., Keong L.K., Almesfer M.K., Al-Qodah Z., Naqvi S.R., Elboughdiri N. Production and characterization of controlled release urea using biopolymer and geopolymer as coating materials. Polymers. 2020;12(2):400. doi: 10.3390/polym12020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra R., Rustgi R. Biodegradable polymers. Prog. Polym. Sci. 1998;23:1273–1335. [Google Scholar]
- El Bahri Z., Taverdet J.-L. Elaboration and characterisation of microparticles loaded by pesticide model. Powder Technol. 2007;172:30–40. [Google Scholar]
- El-Rehim H.A.A. 2006, Characterization and possible agricultural application of polyacrylamide/sodium alginate crosslinked hydrogels prepared by ionizing radiation. J. Appl. Polym. Sci. 2006;101(6):3572–3580. [Google Scholar]
- Flores Cespedes F., Villafranca Sanchez M., Perez Garcia S., Fernandez Perez M. Modifying sorbents in controlled release formulations to prevent herbicides pollution. Chemosphere. 2007;69:785–794. doi: 10.1016/j.chemosphere.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Gandeza A.T., Shoji S., Yamada I. Simulation of crop response to Polyolefin-coated urea: I. Field dissolution. Soil Sci. Soc. Am. J. 1991;55:1462–1467. [Google Scholar]
- Gil-Ortiz R., Naranjo M.A., Ruiz-Navarro A., Caballero-Molada M., Atares S., Garcia C., Vicente O. New eco-friendly polymeric-coated urea fertilizers enhanced crop yield in wheat. Agronomy. 2020;10:438. [Google Scholar]
- Grillo R., Pereira A.E.S., Melo N.F.S., Porto R.M., Feitosa L.O., Tonello P.S., Filho N.L.D., Rosa A.H., Lima R., Fraceto L.F. Controlled release system for ametryn using polymer microspheres: preparation, characterization and release kinetics in water. J. Hazard Mater. 2011;186:1645–1661. doi: 10.1016/j.jhazmat.2010.12.044. [DOI] [PubMed] [Google Scholar]
- Han X., Chen S., Hu X. Controlled-release fertilizer encapsulated by starch/polyvinyl alcohol coating. Desalination. 2009;240:21–26. [Google Scholar]
- Hargrove W.L. Soil, environmental, and management factors influencing ammonia volatilization under field conditions. In: Bock B.R., Kissel D.E., editors. Ammonia Volatilization from Urea Fertilizers. National Fertilizer Development Centre, Muscle Shoals; AL: 1988. pp. 17–36. [Google Scholar]
- Iftime M.M., Ailiesei G.L., Ungureanu E., Marin L. Designing chitosan based eco-friendly multifunctional soil conditioner systems with urea controlled release and water retention. Carbohydr. Polym. 2019;223:115040. doi: 10.1016/j.carbpol.2019.115040. [DOI] [PubMed] [Google Scholar]
- Jamnongkan T., Kaewipirom S. Controlled-release fertilizer based on chitosan hydrogel: phosphorous release kinetics. Sci. J. UBU. 2010;1(1):43–50. [Google Scholar]
- Jang H.J., Kim Y.M., Yoo B.Y., Seo Y.K. Wound-healing effects of human dermal components with gelatin dressing. J. Biomater. Appl. 2018;32(6):716–724. doi: 10.1177/0885328217741758. https://www.fao.org/3/y4765e/y4765e08.html. [DOI] [PubMed] [Google Scholar]
- Kalia A., Luthra K., Sharma S.P., Dheri G.S., Taggar M.S., Gomes C. International Symposium on Horticulture: Priorities and Emerging Trends. 2019. Chitosan-urea nano-formulation: synthesis, characterization and impact on tuber yield of potato. ISHS Acta Horticulturae 1255. [Google Scholar]
- Kanmani P., Aravind J., Kamaraj M., Sureshbabu P., Karthikeyan S. Environmental applications of chitosan and cellulosic biopolymers: a comprehensive outlook. Bioresour. Technol. 2017;242:295–303. doi: 10.1016/j.biortech.2017.03.119. [DOI] [PubMed] [Google Scholar]
- Karunarathna M.H.J.S., Hatten Z.R., Bailey K.M., Lewis E.T., Morris A.L., Kolk A.R., Laib J.C., Tembo N., Williams R.A., III, Phillips B.T., Ash B.L., Midden W.R., Ostrowski A.D. Reclaiming phosphate from waste solutions with Fe (III) – polysaccharide hydrogel beads for photo-controlled-release fertilizer. J. Agric. Food Chem. 2019;67(44):12155–12163. doi: 10.1021/acs.jafc.9b02860. [DOI] [PubMed] [Google Scholar]
- Kissel D.E., Cabrera M.L. Factors affecting urea hydrolysis. In: Bock B.R., Kissel D.E., editors. Ammonia Volatilization from Urea Fertilizers. National Fertilizer Development Centre, Muscle Shoals; AL: 1988. pp. 53–66. [Google Scholar]
- Klokk T.I., Melvik J.E. Controlling the size of alginate gel beads by use of a high electrostatic potential. J. Microencapsul. 2002;19(4):415–424. doi: 10.1080/02652040210144234. [DOI] [PubMed] [Google Scholar]
- Korsmeyer R.W., Meerwall E.V., Peppas N.A. Solute and penetrant diffusion in swellable polymers. II. Verification of theoretical models. J. Polym. Sci. B Polym. Phys. 1986;24:409–434. [Google Scholar]
- Kulkarni A.R., Soppimath K.S., Aminabhavi T.M. Glutaraldehyde crosslinked sodium alginate beads containing liquid pesticide for soil application. J. Control Release Off. J. Control Release Soc. 2000;63:97–105. doi: 10.1016/s0168-3659(99)00176-5. [DOI] [PubMed] [Google Scholar]
- Lee K.Y., Mooney D.J. Alginate: properties and biomedical applications. Prog. Polym. Sci. 2012;37(1):106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubkowski K., Smorowska A., Grzmil B., Kozlowska A. Controlled-release fertilizer prepared using a biodegradable aliphatic copolyster of poly(butylene succinate) and dimerized fatty acid. J. Agric. Food Chem. 2015;63(10):2597–2605. doi: 10.1021/acs.jafc.5b00518. [DOI] [PubMed] [Google Scholar]
- Muzzarelli R.A., Mattioli-Belmonte M., Pugnaloni A., Biagini G. Biochemistry, histology and clinical uses of chitins and chitosans in wound healing. In: Jolles P., Muzzarelli R.A.A., editors. Chitin and Chitinases. Birkhauser; Basel: 1999. [DOI] [PubMed] [Google Scholar]
- Ni B., Liu M., Lu S. Multifunctional slow-release urea fertilizer from ethylcellulose and superabsorbent coated formulations. Chem. Eng. J. 2009;155:892–898. [Google Scholar]
- Niu Y., Li H. Controlled release of urea encapsulated by starch-g-poly(vinyl acetate) Ind. Eng. Chem. Res. 2012;51(38):12173–12177. [Google Scholar]
- Nnamonu L.A., Ato R.S., Onyido I. Alginate reinforced chitosan and starch beads in slow release formulation of imazaquin herbicide-preparation and characterization. Mater. Sci. Appl. 2012;3:566–574. [Google Scholar]
- Pourjavadi A., Soleyman R., Bardajee G.R., Ghavami S. BA-crosslinked Na-Alg/CMC as a smart full-polysaccharide superabsorbent hydrogels. Bull. Kor. Chem. Soc. 2009;30:2680–2686. [Google Scholar]
- Qin Y., Jiang J., Zhao L., Zhang J., Wang F. Chapter 13- applications of alginate as a functional food ingredient. Biopolym. Food Des. 2018:409–429. [Google Scholar]
- Ramirez F., Varela G., Delgado E., Lopez D.F., Zuniga V., Gonzalez V., Faix O., Meier D. Reactions, characterization and uptake of ammoxidized kraft lignin. Bioresour. Technol. 2007;98:1494. doi: 10.1016/j.biortech.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Ransom C.J., Jolley V.D., Blair T.A., Sutton L.E., Hopkins B.G. Nitrogen release rates from slow- and controlled-release fertilizers influenced by placement and temperature. PloS One. 2020 doi: 10.1371/journal.pone.0234544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashidzadeh A., Olad A., Salari D., Hejazi M.J. On the encapsulation of natural pesticide using polyvinyl alcohol/alginate-montmorillonite nanocomposite for controlled release application. Polym. Eng. Sci. 2014;54(12):2707–2714. [Google Scholar]
- Ravi Kumar M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000;46:1–27. [Google Scholar]
- Roy A., Bajpai J., Bajpai A.K. Development of calcium alginate-gelatin based microspheres for release of endosulfan as a model pesticide. Indian J. Chem. Technol. 2009;16:388–395. [Google Scholar]
- Saruchi Kaith B.S., Kumar V., Jindal R. Biodegradation study of enzymatically catalyzed interpenetrating polymer network: evaluation of agrochemical release and impact on soil fertility. Biotechnol. Rep. 2016;9:74–81. doi: 10.1016/j.btre.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu X.Z., Zhu K.J. A novel approach to prepare tripolyphosphate/chitosan complex beads for controlled release drug delivery. Int. J. Pharm. 2000;201:51–58. doi: 10.1016/s0378-5173(00)00403-8. [DOI] [PubMed] [Google Scholar]
- Singh D.K., Ray A.R. Biomedical applications of chitin, chitosan, and their derivatives. J. Macromol. Sci., Part C. 2007;40(1):69–83. [Google Scholar]
- Sopena F., Maqueda C., Morillo E. Norflurazon mobility, dissipation, activity, and persistence in a sandy soil as influenced by formulation. J. Agric. Food Chem. 2007;55:3561–3567. doi: 10.1021/jf070064u. [DOI] [PubMed] [Google Scholar]
- Sopena F., Villaverde J., Maqueda C., Morillo E. Photostabilization of the herbicide norflurazon microencapsulated with ethylcellulose in the soil-water system. J. Hazard Mater. 2011;195:298–305. doi: 10.1016/j.jhazmat.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Tang J., Hong J., Liu Y., Wang B., Hua Q., Liu L., Ying D. Urea controlled-release fertilizer based on gelatin microspheres. J. Polym. Environ. 2018;26:1930–1939. [Google Scholar]
- Thakur G., Mitra A., Basak A., Sheet D. Characterization and scanning electron microscopic investigation of crosslinked freeze dried gelatine matrices for study of drug diffusivity and release kinetics. Micron. 2012;43(2-3):311–320. doi: 10.1016/j.micron.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Tyliszczak B., Polaczek J., Pielichowski K. PAA-based hybrid organic-inorganic fertilizers with controlled release. Pol. J. Environ. Stud. 2009;18:475–479. [Google Scholar]
- Wang L., Liu B., Yang Q., Lu D. Rheological studies of mixed printing pastes from sodium alginate and modified xanthan and their application in the reactive printing of cotton. Color. Technol. 2014;130(4):273–279. [Google Scholar]
- Wang B., Wan Y., Zheng Y., Lee X., Liu T., Yu Z., Huang J., Ok Y.S., Chen J., Gao B. Alginate-based composites for environmental applications: a critical review. Crit. Rev. Environ. Sci. Technol. 2019;49(4):318–356. doi: 10.1080/10643389.2018.1547621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Liu M. Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water retention. Carbohydr. Polym. 2008;72(2):240–247. [Google Scholar]
- Yao K., Peng T., Xu M., Yuan C., Goosen M.F.A., Zhang Q., Ren L. pH-dependent hydrolysis and drug release of chitosan/polyether interpenetrating polymer network hydrogel. Polym. Int. 1994;34(2):213–219. [Google Scholar]
- Yong S.K., Shrivastava M., Srivastava P., Kunhikrishnan A., Bolan N. Environmental applications of chitosan and its derivatives. Rev. Environ. Contam. Toxicol. 2015;233:1–43. doi: 10.1007/978-3-319-10479-9_1. [DOI] [PubMed] [Google Scholar]
- Zhao F., Li H., Wang X., Wu L., Hou T., Guan J., Jiang Y., Xu H., Mu X. CRGO/alginate microbeads: an enzyme immobilization system and its potential application for a continuous enzymatic reaction. J. Math. Chem. B. 2015;3:9315–9322. doi: 10.1039/c5tb01508a. [DOI] [PubMed] [Google Scholar]
- Zhao D., Yu S., Sun B., Gao S., Guo S., Zhao K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers. 2018;10(4):462. doi: 10.3390/polym10040462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article.