Abstract
The main objective of this work was to evaluate the effect that several plant extracts (currently sold as functional ingredients) have on gut microbiota community structure and functionality. Plant extracts were submitted to an in vitro digestion and fecal fermentation. Overall, plant extracts showed a marked inhibitory activity when compared to basal conditions. However, they also favored the growth of some bacteria such as Coprococcus and Butyricimonas, two butyrate producers. Especially interesting was tea extract which inhibited the growth of the genus Escherichia/Shigella, known to involve species related with gastrointestinal disorders. Additionally, tea extract increased the growth of Faecalibacterium, a known butyrate producer. Regarding short chain fatty acids production, while plant extracts reduced acetate production, butyrate was increased for most samples, especially tea extract. Propionate production was less affected in comparison with basal conditions. Fermentation by gut microbiota also modified the antioxidant capacity (assessed via DPPH, FRAP and Folin-Ciocalteu methods).
Keywords: Food science, Microbiology, Plant extract, Phytochemicals, Gut microbiota, Short chain fatty acids, Antioxidant capacity, Tea
Food Science; Microbiology; Plant extract; Phytochemicals; Gut microbiota; Short chain fatty acids; Antioxidant capacity; Tea
1. Introduction
Herbal extracts have been used since ancient times, specially represented by the traditional Chinese medicine, to promote general health but also to fight specific diseases or conditions (Huang et al., 2019). Later on, in the present days, herbal extracts have, again, gain the favor of the general population in their search of ‘natural’ remedies to current conditions (Shen et al., 2013). Since herbs used in traditional medicine have provided many active compounds for modern medicine (Huang et al., 2019; Pastoriza et al., 2017a; Pérez-Burillo et al., 2018a), a large number of research projects have focused on the potential use of these herbs to fight modern diseases or just to improve the general health state. Some studies have investigated the antioxidant capacity of plant extracts such as Securigera Securidaca (Alizadeh-Fanalou et al., 2020), Rosmarinus officinalis (Cheung and Tai, 2007), Ginkgo biloba (Singh et al., 2019) among others (Fragopoulou et al., 2018); other studies have focused on their effects against diabetes (Alizadeh-Fanalou et al., 2020; Huang et al., 2019), anti-proliferative effects (Cheung and Tai, 2007), platalet functionality (Gavriil et al., 2019), irritable bowel syndrome (Ko et al., 2011), gain of bone mineral density and height in children (Lee et al., 2005), hypertension (Micucci et al., 2016), metabolic syndrome (Villiger et al., 2015) or as neuroprotectors (Singh et al., 2019). However, to the best of our knowledge, very little research has been done to investigate their effects or influence on gut microbiota composition and/or functionality. We only found a research in relation to IBD in which an herbal formula (composed of 13 herbs) used in traditional Asian medicine in combination with a probiotic product (with 7 different probiotic bacteria) were tested (Ko et al., 2011).
There is currently an intense focus on gut microbiota due to its role on human health (Flint et al., 2015). Accordingly, the gut microbiota was pointed out as a major player in intestinal inflammatory disorders (Malinen et al., 2010), obesity (Ley et al., 2005), autism spectrum disorders (Strati et al., 2017) and immune system disorders (Salazar et al., 2009), among others. In addition, gut microbial functionality is just as important, if not more, than its composition since the resulting metabolites will have a great influence on the host health. Short chain fatty acids harbor special interest having demonstrated positive effects on different fronts: inhibition of pathogenic bacteria (Ríos-Covián et al., 2016), gut barrier integrity (Ríos-Covián et al., 2016) or a protective role in diet-induced obesity (Lin et al., 2012) and colorectal cancer by reducing inflammation and stimulating apoptosis (Donohoe et al., 2014).
Therefore, the main goal of this paper was to evaluate the influence of several herbal extracts on gut microbial community structure and functionality, after subjecting such extracts to an in vitro gastrointestinal digestion followed by an in vitro fermentation with human feces. As complementary analysis, antioxidant capacity released by the fermentation of such herbs was also measured.
2. Materials and methods
2.1. Reagents
2.1.1. Antioxidant experiments
2,4,6-Tri(2-pyridyl)-s-triazine (TPTZ), iron (III) chloride hexahydrate, 2,2-Diphenyl-1-picrylhydrazyl (DPPH), Trolox ((±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid), Folin-Ciocalteu reagent, calcium carbonate, gallic acid, and methanol. All reagents were obtained from Sigma-Aldrich (Germany).
2.1.2. In vitro digestion and fermentation
Potassium di-hydrogen phosphate, potassium chloride, magnesium chloride hexahydrate, sodium chloride, calcium chloride dihydrate, sodium mono-hydrogen carbonate, ammonium carbonate, hydrochloric acid, salivary alpha-amylase, pepsin from porcine, and bile extract from porcine (bile acids). For the in vitro fermentation, sodium di-hydrogen phosphate, sodium sulfide, tryptone, cysteine, and resazurin. All these reagents were obtained from Sigma-Aldrich (Germany).
2.1.3. SCFA identification
Standards of acetic acid, propionic acid, and butyric acid were also obtained from Sigma-Aldrich (Germany).
2.2. Samples
Herbal extracts were provided by Natac (Madrid, Spain). Samples comprise several plant extracts currently used as functional ingredients. Plant extracts were obtained from the corresponding part of the plant, which were freeze-dried, ground to powder and extracted with a mix of water:ethanol (50:50 v/v). Table 1 describes the plant and which part of it was used for each extract. Several extracts were added with specific phytochemicals (as they are commercially sold) at different concentrations (w/w) specified in Table 1.
Table 1.
Sample composition information.
| Plant | Part of the plant | Phytochemical added (w/w) |
|---|---|---|
| Olive | Leaves | 50% Oleanolic acid |
| Olive and apple (pomolive) | Leaves and fruit | |
| Olive | Leaves | 20% Oleuropein; 10% Oleanolic acid; 3% Maslinic Acid; 3% Hydroxytyrosol |
| Bearberry | Leaves | |
| Fennel | Fruit | |
| Cranberry | Fruit | |
| Oregano | Leaves | |
| Ginger | Root | |
| Apple | Fruit | |
| Cinnamon | Bark | |
| Olive and Vine (antioxidant complex) | Leaves and Fruit | |
| Rosemary | Leaves | 15% Diterpens |
| Pomegranate | Fruit | 40% Punicalagins |
| Salvia | Leaves | 15% Pentaciclic triterpenoids |
| Rosemary | Leaves | 6% Rosmarinic acid |
| Lemon verbena | Leaves | |
| Salvia | Leaves | 15% Ursolic acid |
| Grape | Fruit, peel | |
| Eucalyptus | Leaves | 10% polyphenols |
| Eucalyptus | Leaves | 10% ursolic acid |
| Grape | Seeds | 95% Anthocyanidins |
| Olive (Allolive) | fruit | |
| Chamomile | Flowers | |
| Pomegranate | Fruit | 40% Ellagic acid |
| Grape | Fruit, peel | 5% Resveratrol |
| Artichoke | leaves | |
| Coffee | Green fruits | |
| Olive | leaves | 40% oleuropein |
| Tea | leaves | |
| Olive | leaves | pentaciclic triterpenoids |
2.3. In vitro gastrointestinal digestion and in vitro fermentation
In vitro gastrointestinal digestion was performed as in Pérez-Burillo et al. (2018b). Briefly, 5mL of oral phase with alpha amylase (150 U/mL) were mixed with 5 g of plant extract and kept at 37 °C for 2 min. Afterwards, 10 mL of gastric phase with pepsin (4000 U/mL) were added to the mix, the pH adjusted to 3 and kept at 37 °C for 2 h. Finally, 20 mL of intestinal phase with pancreatin (26.74 mg/mL) and bile acids (20 mM) were added to the mix (final volume 40 mL), the pH adjusted to 7 and kept at 37 °C for 2 h. Enzyme activity was stopped by immersion in ice. The tubes were centrifuged at 4500 rpm for 10 min and the supernatant (absorbable fraction at the small intestine) separated from the solid residue (not absorbed in the small intestine, reaching the large intestine). The latter fraction was used for the gut microbiota in vitro fermentation.
In vitro fermentation was performed as in Pérez-Burillo et al. (2018b). Fecal material was obtained from volunteers not suffering from any illness or disorder and without any antibiotic prescription. Additionally, feces were taken from people with a body mass index (BMI) within the normal range (18,5–24,9). Fecal material was collected the morning of the experiment and recover in sterile containers which were kept at 4 °C until use. The fecal inoculum was composed of fecal material and phosphate buffer (0.1M, pH 7) at 32% (w/v). Fermentation medium was composed of peptone (15 g/L), cysteine (0.312 mg/L) and sodium sulfide (0.312 mg/L), adjusted to pH 7. Five hundred mg of digestion solid residue were placed into a screw cap 50 mL tube and mixed with 7.5 mL of fermentation medium and 2 mL of fecal inoculum. Nitrogen was bubbled for 2 min to obtain an anaerobic atmosphere. Tubes were then kept at 37 °C for 20 h under oscillation. Microbial activity was halted by immersion in ice and tubes were centrifuged at 4500 rpm for 10 min. Aliquots were taken and stored at -80 °C until analysis.
2.4. Antioxidant assays
2.4.1. TEACFRAP assay
The ability of each sample to reduce Fe3+ ions was interrogated following the procedure described by Benzie and Strain (1996). 280 μL of FRAP reagent were mixed in a 96-well microplate (Biogen Científica, Spain) with 20 μL sample (fermentation supernatant). The FRAP reagent was composed of 2.5 mL of 10 mM TPTZ solution, 2.5 mL of 20 mM FeCl3.6H20 and 25 mL of acetate buffer 0.3 M and pH 3.6. Absorbance (595 nm) readings were monitored for 30 min with a FLUOStar Omega microplate reader (BMG Labtech, Germany) at 37 °C. Calibration was based on different Trolox concentrations (0.01–1.00 mg/mL). Results were expressed as mmol Trolox equivalents per kg of sample.
2.4.2. TEACDPPH assay
DPPH was performed as in Yen & Chen (1995). 20 μL of sample (fermentation supernatant) were mixed with 280 μLDPPH reagent (7.4 mg/100 mL of methanol) in a transparent 96-well polystyrene microplate (Biogen Científica, Spain) plate. Absorbance (517nm) readings (517 nm) were monitored for 60 min using a FLUOStar Omega microplate reader (BMG Labtech, Germany) at 37 °C. Calibration was performed with Trolox (0.01–1.00 mg/mL). Results were expressed as mmol Trolox equivalents per kg of sample.
2.4.3. Folin-Ciocalteu assay
Although this method is widely used to estimate the amount of total phenolics in foods, it also expresses antioxidant capacity since it follows a similar chemistry than FRAP method. We adapted the procedure described by Singleton and Rossi (1965) to a microplate reader. 30 μL of sample were mixed in a microplate with 15 μL of Folin-Ciocalteu reagent and 255 μL of sodium carbonate 2.35%. All tests were run in triplicate. Absorbance readings were monitored at 725 nm for 60 min at 37 °C in a FLUOStar Omega microplate reader (BMG Labtech, Germany). Calibration was performed with Gallic acid (0.01–1.00 mg/mL) and results were expressed as mg of gallic acid per kg of sample.
2.5. DNA extraction and sequencing
Fecal microbial ecology was assessed by analyzing the 16S rRNA gene. MagNA Pure 24 platform from Roche was used to extract DNA from samples. To prepare the 16S rRNA gene Metagenomic Sequencing Library Preparation (Cod. 15044223 Rev. A), we used DNA at concentration of 5 ng/μl in 10 mM Tris (pH 8.5). The Illumina protocol was followed. The PCR primers used to target the 16S rRNA gene V3 and V4 regions were: Forward 5′TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCA-G3′ and Reverse 5′GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTA-CHVGGGTATCTAATCC3’. Primers were compatible with the Illumina Nextera XT Index kit (FC-131-1096). Amplicons were multiplexed and a Bioanalyzer DNA 1000 was used to check amplicon size (~550 bp). Finally, an Illumina MiSeq sequencer was used to sequence the libraries following the manufacturer's instructions in a 2 × 300 cycles paired-end run (MiSeq Reagent kit v3 MS-102-3001).
2.6. Bioinformatic analysis
Prinseq-lite program was used to assess the quality of the reads with the following parameters: a minimal length (min_length) of 50 nt and a quality score threshold of 30 from the 3′-end (trim_qual_right), using a mean quality score (trim_qual_type) calculated with a sliding window of 10 nucleotides (trim_qual_window). Read 1 and read 2 from Illumina sequencing where joined using fastq-join from the ea-tools suite. Taxonomic affiliations were assigned using the RDP_classifier (Cole et al., 2009) from the Ribosomal Database Project (RDP).
2.7. SCFA analysis
SCFA identification and quantification was performed as in Panzella et al. (2017). The equipment used was an Accela 600 HPLC (Thermo Scientific) with a quaternary pump, an autosampler and a UV-VIS PDA detector set at 210 nm. We used 0.1 M phosphate buffer (pH 2.8)/acetonitrile 99:1 v/v with a flow rate of 1.25 mL/min as mobile phase. An Aquasil C18 reverse phase (Thermo Scientific) (150 × 4.6 mm, 5 μm) was used with a run time of 30 min. The samples did not require any pretreatment before injecting except for centrifugation at 13000 rpm and further filtration through 0.22 μm nylon filter. One mL of fermentation supernatant was centrifuged and filtered prior injection of 20 μL. The SCFA standards (acetate, propionate and butyrate) were prepared in the mobile phase at concentrations ranging from 5 to 10000 ppm. Results were expressed as mM.
2.8. Statistical analysis
Principal Coordinates Analysis (PCoA) with unifrac distance was carried out with R software. Statistical significance of the data and differences among samples were tested by one-way ANOVA at p < 0.05 significance level. Evaluation of the relationship between bacteria relative abundance and SCFA concentration was carried out by computing the Pearson correlation coefficient at p < 0.05. These statistical analyses were performed using the Statgraphics Plus software (Statpoint Technologies, Inc., The plains, USA), version 5.1, 2001.
3. Results and discussion
3.1. Gut microbiota community structure and SCFA/functionality is affected by the different herbal extracts
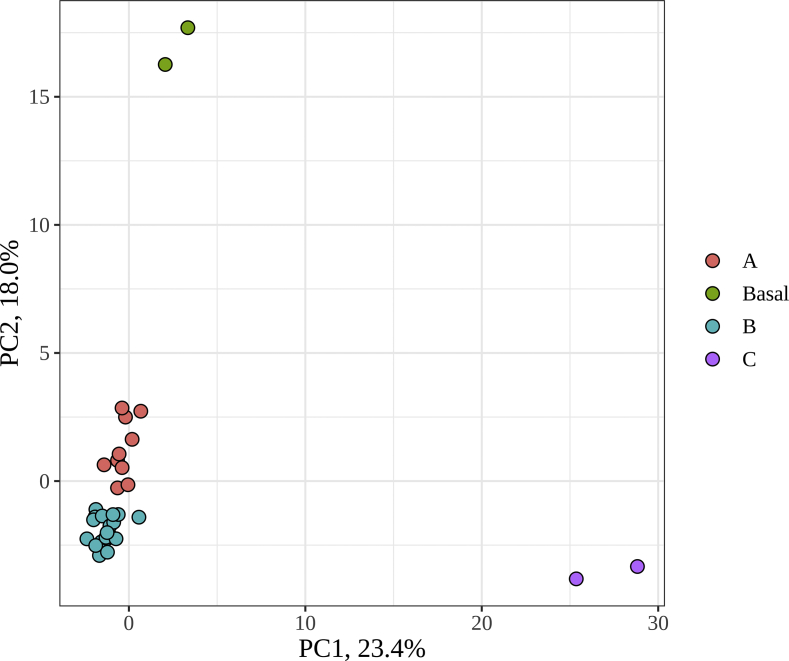
The different herbal extracts gave rise to distinct microbial communities, with no apparent similarities depending on the herbal origin or source. However, we observed through PCoA with phylogenetic dissimilarity distance Unifrac that gut microbial communities could be significantly grouped in four different groups: A: olive, pomolive, allolive, cranberry, apple, pomegranate, and chamomile; B: bearberry, fennel, oregano, ginger, cinnamon, antioxidant complex, rosemary, salvia, lemon verbena, grape, eucalyptus, artichoke, and green coffee; C: tea; and Basal conditions (Figure 1). As can be observed, all three groups modified greatly basal microbial communities.
Figure 1.
Principal Coordinates Analysis (PCoA) with phylogenetic dissimilarity distance Unifrac. A circles: olive, pomolive, allolive, cranberry, apple, pomegranade, and chamomile; B circles: bearberry, fennel, oregano, ginger, cinnamon, antioxidant complex, rosmary, salvia, lemon verbena, grape, eucalyptus, artichoke, and green coffee; C circles: tea. Also depicted Basal conditions.
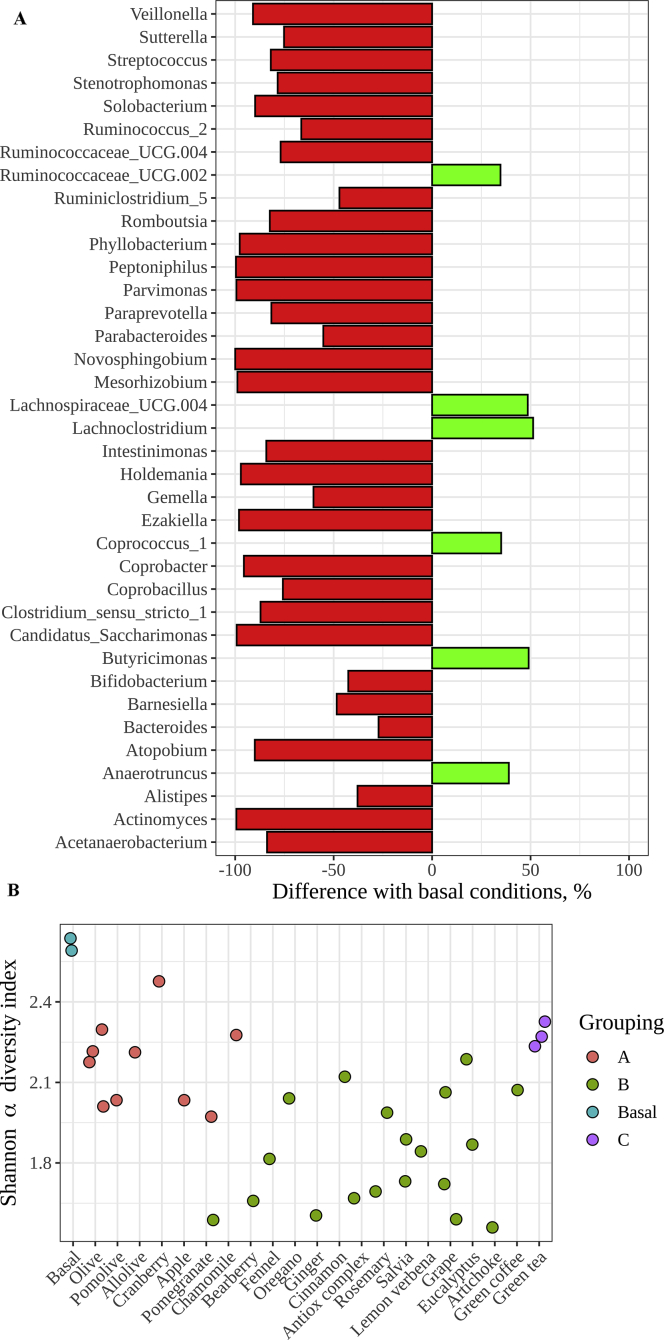
After comparing relative abundances of the different genera in each group against the basal conditions, we observed that all samples had a marked inhibitory effect in many genera. Specifically, 47 genera were found in lower abundance in group A, 48 in group B and 44 in group C, whereas 31 were found in lower abundance in all three groups (Figure 2A). This inhibitory effect was also supported by a lower alpha diversity (less diverse microbial community) in comparison with basal conditions (Figure 2B). Such inhibitory effect could be due to some phytochemicals, such as some polyphenols, present in those herbs acting as bactericides. For instance, even though some polyphenols such as ellagitannins, tannins orchlorogenic acids can be metabolized by gut microbes (Rowland et al., 2018), some others have been described as possible inhibitors for some genera (Poirier et al., 2016). Accordingly, it was reported that ferulate and quercetin could affect negatively the growth of some gut bacterial groups in charge of degrading in rats, leading to less SCFA produced and more oligosaccharides excreted (Zhang et al., 2018). However, there were also some genera whose growth was stimulated by those herbs; in group A, 34 different genera were found in higher abundances than in basal conditions, 33 in group B, and 36 in group C. Moreover, 6 different genera were found to be stimulated in all three groups (Figure 2A).
Figure 2.
A: Bacteria stimulated or inhibited by all plant groups (expressed as percentage with respect to basal conditions). Red bars represent the percentage of reduction in abundance with respect to basal conditions and green bars represent the percentage of increase. B: Alpha diversity, as Shannon index, of each gut microbial community after in vitro fermentation of each plant extract.
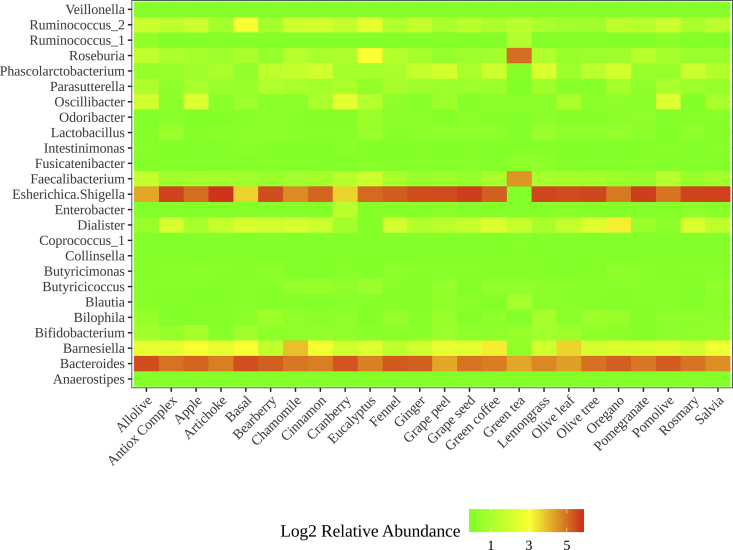
After consulting bibliography, we observed that some of these genera (with similar growth or inhibition compared to basal conditions in the three groups) are involved in host health. Among them, we found SCFA producers or genera involved in inflammatory processes, obesity, etc (Table 2). For instance, as depicted in Figure 2A, while two butyrate producers (Coprococcus and Butyricimonas) were stimulated by all plant extracts, other potentially beneficial genera were negatively affected in all three groups: Bifidobacterium (which is a known probiotic with reduced abundance in colorectal cancer and type I diabetes (Murri et al., 2013)) or Barnesiella (which according to Bilinski et al. (2017) could prevent infections by antibiotic-resistant bacteria), among others. The relative abundances of the different bacteria are represented in Figure 3.
Table 2.
Beneficial or detrimental effects of bacteria on human health.
| Bacteria | Health effect | References | |
|---|---|---|---|
| Akkermansia spp. | + | It helps to control diet induced obesity and associated metabolic disorders | Everard et al. (2013) |
| Bacteroides | + | Polysaccharide degrader | Patnode et al. (2019) |
| Bilophila | +/- | More abundant in dysbiosis, diets with high intake of sugars and fats; associated with obesity and inflammation. Reduced abundance in autism spectrum disorders subjects. | Sen et al. (2017) |
| Enterobacter | - | member of the ESKAPE pathogens | Pendleton et al. (2013) |
| Escherichia/Shigella | - | Responsible for gastrointestinal disorders such as constipation, diarrheic symptoms. | Verbeke et al. (2014) |
| Odoribacter | + | Butyrate producer; lower abundance was associated with higher blood pressure in pregnant women and regulation of blood sugar. . | Gomez-Arango et al. (2016); Salomäki-Myftari et al. (2016) |
| Faecalibacterium spp. | + | Produces butyrate, it helps to regulate the immune system, possible positive effect on Chron's disease | Flint et al. (2015) |
| Veillonella spp. | + | Produces propionate | Flint et al. (2015) |
| Bifidobacterium spp. | + | Lower abundance in colorectal cancer and in type I diabetes | Murri et al. (2013) |
| Collinsella spp. | + | Lower abundance in irritable bowel syndrome patients with more severe symptoms | Malinen et al. (2010) |
| Barnesiella spp. | + | Possible prevention/treatment of infections by antibiotic resistant bacterias | Bilinski et al. (2017) |
| Blautia spp. | + | Related to decreased inflammation in cirrhosis and hepatic encephalopathy, reduced in colorectal cancer and type I diabetes | Murri et al. (2013) |
| Fusicatenibacter spp. | + | Reduced in ulcerative colitis patients and possible anti-inflammatory function | Takeshita et al. (2016) |
| Roseburia spp. | + | Linked to weight loss and reduced glucose intolerance, lower abundance in ulcerative colitis patients, differential abundance in diabetes type II and healthy people | Ryan et al. (2014) |
| Anaerostipes spp. | + | Produces acetic, lactic and butyric acid | Flint et al. (2015); Ríos-Covián et al. (2016) |
| Coprococcus spp. | + | Produces acetic and butyric acid, and lower amounts of propionic or formic acid | Flint et al. (2015); Ríos-Covián et al. (2016) |
| Butyricimonas spp. | + | Produces butyric acid | Flint et al. (2015); Ríos-Covián et al. (2016) |
| Intestinimonas spp. | + | Produces butyric acid | Flint et al. (2015); Ríos-Covián et al. (2016) |
| Butyricicoccus spp. | + | Produces butyric acid, reduced in ulcerative colitis patients and patients with inflammatory disease in general | Flint et al. (2015); Ríos-Covián et al. (2016) |
| Ruminococcus spp. | + | Key role in degradation of resistant starch | Ze et al. (2012) |
| Oscillibacter spp. | + | Increased in depression and in high-fat diet | Jung et al. (2016) |
| Parasutterella spp. | - | Related with Crohn's disease and with dysbiosis in hypertriglyceridemia associated to necrotizing pancreatitis | Chiodini et al. (2015) |
Figure 3.
Heatmap of most abundant genera across plant extracts. Note the log2 scale.
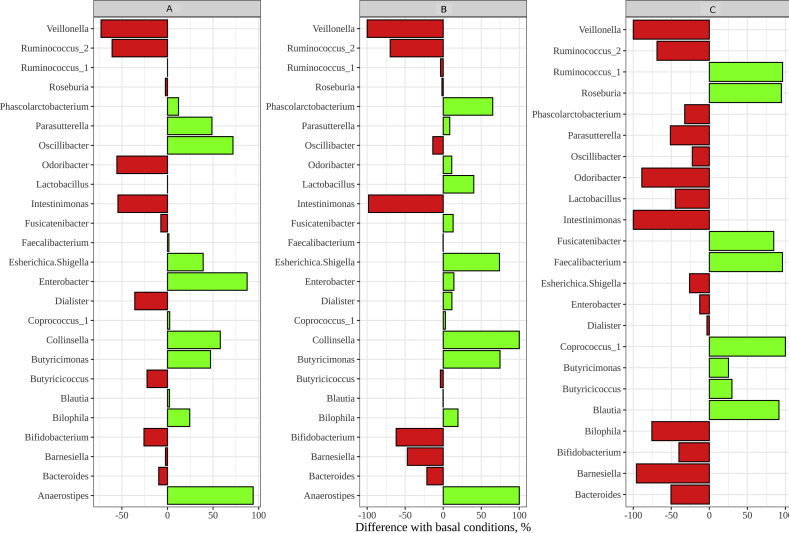
Secondly, by comparing how each group affected basal gut microbial communities, we could extract some interesting information (Figure 4). Groups A and B stimulated the growth of Escherichia/Shigella and Enterobacter, being the first one responsible for gastrointestinal disorders such as diarrheic symptoms (Verbeke et al., 2014), and the later a member of the ESKAPE pathogens (Pendleton et al., 2013). However, group C inhibited the growth of such genera, which could, therefore, lead to consider tea extract as a potential agent to fight an eventual overgrowth of such enteric bacteria. Parasutterella, which according to Chiodini et al. (2015) would have a detrimental health effect due to its relation with Crohn's disease and dysbiosis, is stimulated in groups A and B, whereas it is inhibited by group C. Oscillibacter, on the other hand, which is associated with depression and it is increased in high fat diet (Jung et al., 2016), is stimulated in group A whereas it is inhibited in groups B and C. Fusicatenibacter, which has been linked to anti-inflammatory properties (Takeshita et al., 2016), followed the same trend as Oscillibacter. Finally, Collinsella, which has been found reduced in irritable bowel syndrome patients (Malinen et al., 2010), was stimulated only by groups A and B.
Figure 4.
Stimulation or inhibition activity exerted by each plant group on the different genera (expressed as percentage with respect to basal conditions). Panel A: plant group A; panel B: plant group B; panel C: plant group C. Red bars represent inhibition and green bars stimulation.
As stated above, we also found different behaviors in SCFA producing genera. This is important since a stimulation of SCFA production could lead to health benefits for the host. For instance, butyrate and propionate have been related to protection against obesity (Lin et al., 2012), or to anti-inflammatory effects (Ríos-Covián et al., 2016). Accordingly, we found that two main acetate producers, Bifidobacterium and Bacteroides (Ríos-Covián et al., 2016; Flint et al., 2015), were inhibited by the three groups of extracts, whereas Collinsella (another acetate producer) was only stimulated by groups A and B (Figure 4). These results could lead to consider these two groups of extracts as better option to favor acetate production. In the case of propionate producers (Flint et al., 2015; Ríos-Covián et al., 2016) Veillonella and Ruminococcus_2 were found in lower abundances than in basal conditions in all three groups. On the other hand, Ruminococcus_1 and Blautia were highly stimulated by group C and very little by group A. Phascolarctobacterium, was, however, stimulated by groups A and B and inhibited by group C whereas Dialister was only stimulated by group B. Finally, regarding butyrate, Faecalibacterium and Roseburia, (which are two of the main butyrate producers for human beings), were only stimulated by group C. On the other hand, Coprococcus_1 was stimulated by all three groups, but whereas in group C its abundance increased greatly in comparison with basal conditions, in groups A and B such increase was almost insignificant. Anaerostipes was only stimulated by groups A and B.
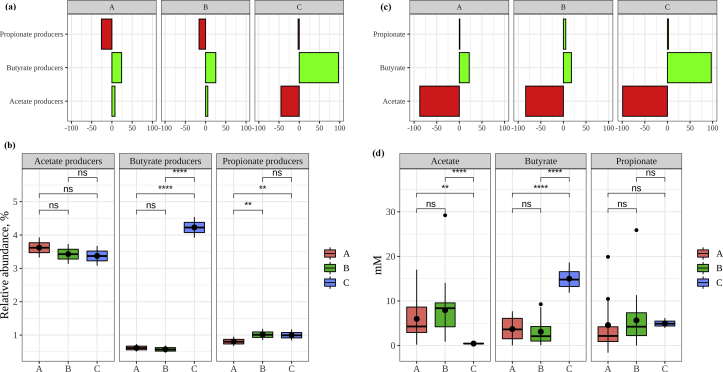
Focusing on the relative abundance of the different genera (Figure 4), the most distinguishing information was the inhibition of Escherichia/Shigella and the high relative abundance of Faecalibacterium and Roseburia achieved by green tea (group C).
However, all this information did not translate into a clear pattern since producers of the same SCFA often behaved differently across groups. Therefore, with the aim to simplify information and to try and find a clearer pattern, we also compare the sum of the different SCFA producers (Figure 5(a)). Overall, acetate producers were stimulated by groups A and B, whereas group C inhibited their growth. However, the general stimulation in comparison with basal conditions was small. Moreover, there were not significant differences (p > 0.05) in the average relative abundance of acetate producers between groups (Figure 5(b)). Secondly, propionate producers were usually inhibited by all groups whereas butyrate producers were stimulated by all groups, though more prominently by group C. However, the relative abundance of propionate producers in group A were significantly lower than that of the other groups. Finally, the mean relative abundance of butyrate producers was significantly higher in group C than in groups A and B.
Figure 5.
(a): stimulation or inhibition activity exert by each plant group on SCFA producing genera expressed as percentage with respect to basal conditions. Panel titles A-C refer to plant groups A-C. (b): relative abundance (%) of SCFA producing genera. Labels: ns: not significant, ∗∗: p < 0.01, ∗∗∗∗: p < 0.001. (c): increase or decrease of SCFA production with respect to basal conditions. Panel titles A-C refer to plant groups A-C. (d): concentration of SCFA (mM) produced by each group. Labels: ns: not significant, ∗∗: p < 0.01, ∗∗∗∗: p < 0.001.
These results, regarding SCFA production, could lead to several hypothesis; a) compared to basal conditions, acetate production could increase with the intake of groups A and B plant extracts, whereas it would decrease with tea extract. Propionate production, on the other hand, should decrease with all the groups and butyrate production should increase, being tea extract the one with higher potential butyrate production (Figure 5(b)); b) acetate production should be similar in all three groups since there is no significant differences between the average relative abundance of this SCFA producers, whereas propionate production should be lower in group A and butyrate production should be much higher in group C (Figure 5(b)).
In order to answer these questions, we also quantified SCFA production (Figures 5(c)–(d)). According to our results, compared to basal conditions, all three groups decreased acetate production whereas propionate and butyrate levels increased with the intake of all three groups, specially butyrate production. These results did not match what we found with SCFA producing bacteria, which could be related to some other genera involved in SCFA metabolism not considered in bibliography.
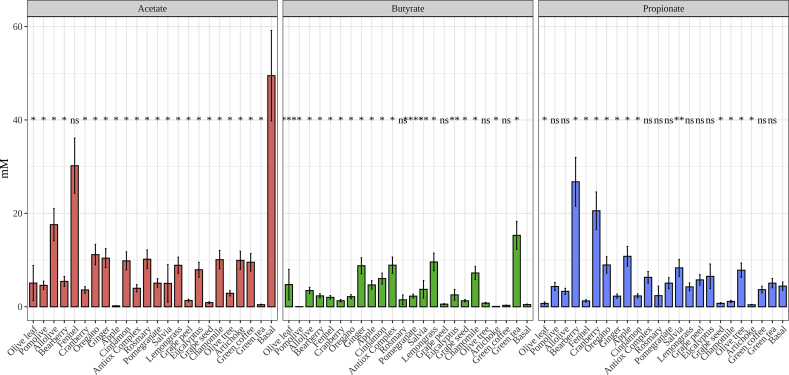
Regarding actual SCFA concentrations, as stated before, acetate production did not behave as expected as it was found in much lower concentrations in group C than in the other two groups (Figure 5(d)). This information led us to hypothesize again that should be other bacteria involved in acetate production which were not considered (or reported) in bibliography. In relation to propionate production, there were not significant differences between all three groups, though the average production of group A tended to be lower than that of the other groups. Butyrate production, however, resulted as expected, since group C achieved a much higher production than the other two groups. Focusing on individual plant extracts, the highest acetate production was achieved by fennel, whereas regarding propionate it was bearberry, and green tea in the case of butyrate (Figure 6). These results confirmed that green tea could be considered as a potential source for increasing butyrate production in the colon, which could also be accompanied by an inhibition of potentially harmful bacteria such as Escherichia/Shigella and Enterobacter.
Figure 6.
Short chain fatty acids production of each plant extract. Statistical comparisons were made using Basal conditions as reference group. Significance labels: ns: not significant; ∗: p < 0.05; ∗∗: p < 0.01.
Finally, we also performed spearman correlations between the relative abundance of SCFA producers and SCFA concentration. This could help to stablish a direct relation (positive or negative) between certain genera and a specific SCFA. Accordingly, we found statistically significant (p < 0.05) correlations between acetate concentration and Bacteroides or Collinsella, propionate- Dialister and butyrate- Faecalibacterium.
3.2. Effect of specific phytochemicals on short chain fatty acids production
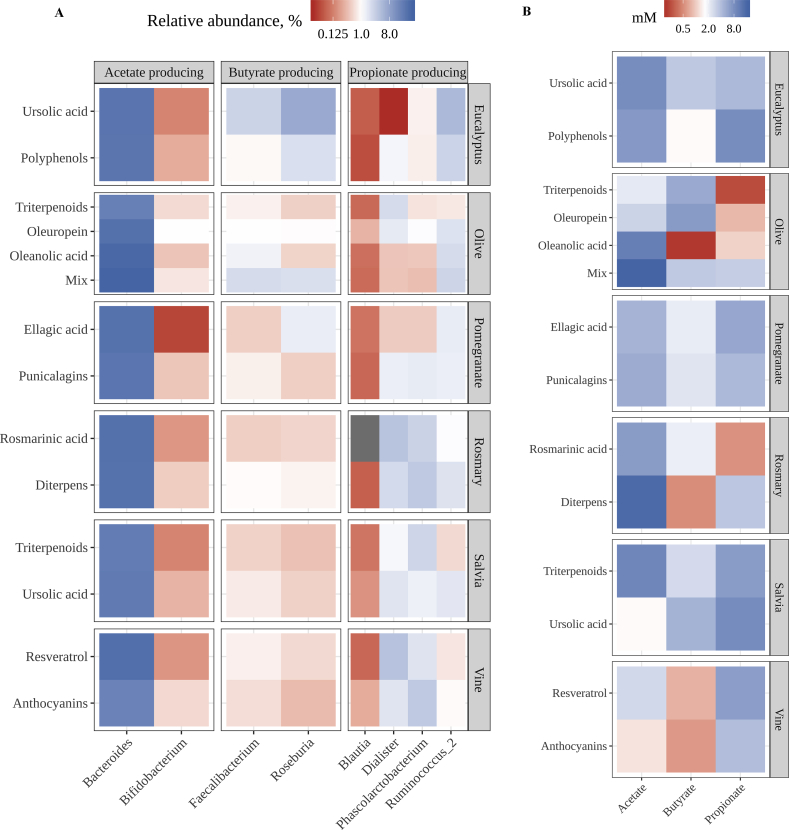
We investigated whether the addition of specific bioactive compounds to the plant extracts had any influence on SCFA producing bacteria and hence, on SCFA production (Figures 7A-B). We observed that Bifidobacterium was specially inhibited by ellagic acid in pomegranate extracts. However, it did not translate into a significantly lower amount of acetate production. Lowest acetate values were observed in vine and salvia with added ursolic acid extracts. As stated above, this could be due to other genera involved in acetate production, which were not considered in bibliography as acetate producers or no changes in their relative abundance were found.
Figure 7.
A: heatmap representing the relative abundance of SCFA producers divided regarding plant extract and added phytochemical. B: heatmap of SCFA concentration produced by plant extracts with added phytochemicals.
Butyrate producers were specially stimulated by eucalyptus extract containing ursolic acid, whereas they were highly inhibited by olive extract containing oleuropein, and rosemary containing diterpens (Figures 7A-B). Regarding butyrate production, there was a fitter relationship between producers and butyrate levels compared to acetate. Eucalyptus containing ursolic acid produced high amounts of butyrate, though it was not the highest value. On the other hand, rosemary containing diterpens produced indeed one of the lowest amounts of butyrate, though olive extract enriched with oleanolic acid showed lower amounts. Vine, with either of the added compounds, gave also some of the lowest butyrate levels.
In the case of propionate producers, their abundance was generally low. Blautia was specially inhibited in eucalyptus containing ursolic acid, whereas Dialister and Phascolarctobacterium were not favored by olive extracts containing either oleanolic acid or the mix, and pomegranade containing ellagic acid (Figures 7A-B). However, the lowest amounts of propionate were found in olive extracts containing either the mix of bioactive compounds and in rosemary enriched in rosmarinic acid.
According to these results, we can conclude that in the case of SCFA production (though it obviously will depend on the presence and abundance of the producing bacteria) is not specially affected by the addition of specific phytochemicals, since the overall effect of the extract is higher than that of a single compound. Therefore, it was difficult to correlate SCFA production to actual abundances of producing bacteria. Different genera involved in these metabolites production (and not considered here) could also increase the difficulty of extracting correlations. In fact, the best relationship was found with butyrate, where producing genera has been better identified.
3.3. Antioxidant capacity
Dietary phytochemicals are known to possess chemopreventive properties (Cheung and Tai, 2007) being their antioxidant effects one of the most studied (Bonanni et al., 2007) and clearly linked to the phenolic content (Wojdyło et al., 2007) although they can be modified by thermal processing (Rufián-Henares et al., 2006; Pastoriza et al., 2017b). Therefore, we performed three antioxidant assays to assess the antioxidant capacity of the herbal extracts investigated after submitting them to an in vitro gastrointestinal digestion and fermentation. This procedure helped simulate physiological antioxidants extraction in a closer manner than regular procedures based on organic solvents (Granato et al., 2018).
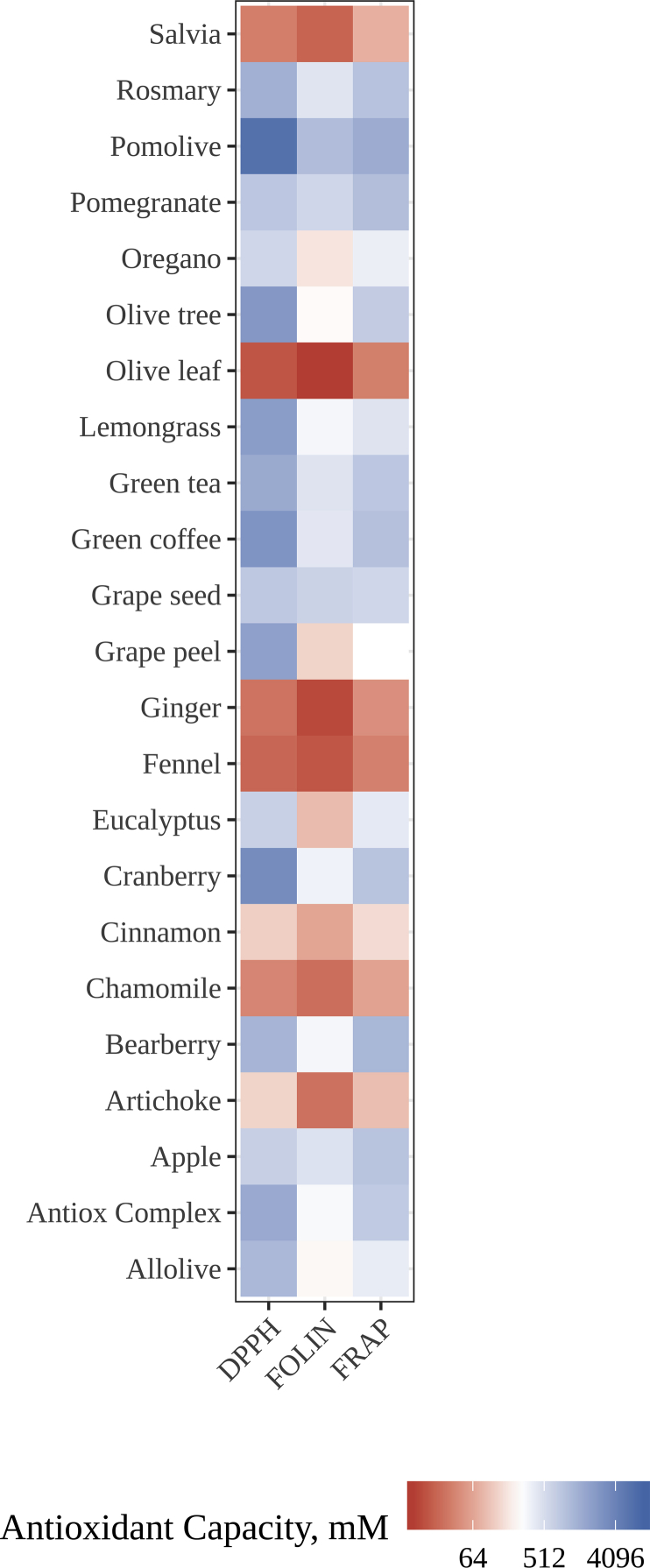
In this case, as PCA demonstrated (data not shown), plants could not be grouped in those three clusters. In fact, samples did not group together in any way. This could be due to their different nature and composition in phytochemicals, mainly phenolic compounds, responsible for their antioxidant capacity. All three antioxidant methodologies showed results in the same line (Figure 8). Salvia, olive leaf, ginger, fennel, and chamomile were the samples with lower antioxidant capacity. On the other hand, pomolive and cranberry showed the highest values whereas the rest of the samples showed very similar antioxidant capacity. In general, the antioxidant capacity of these extracts was much higher than those reported by our research group (Jiménez-Zamora et al., 2016) for regular plant infusions. On one hand this could be related with a higher concentration on plant extracts compared to infusions. On the other hand, is has been previously demonstrated that in vitro digestion and fermentation increase the antioxidant capacity of foods (Pérez-Burillo et al., 2018b).
Figure 8.
Heatmap of the antioxidant capacity (DPPH, FOLIN, FRAP) for each plant extract.
4. Conclusions
The tested plant extracts greatly modified gut microbial basal conditions after in vitro fermentation. Overall, plant extracts had a marked inhibitory effect, though there were also some genera whose growth was stimulated. Coprococcus and Butyricimonas (butyrate producing bacteria) were in general stimulated by all extracts, therefore potentially increasing the healthy butyrate production. A specially interesting result was the inhibition showed by tea extracts on Escherichia/Shigella genera, known species causing gastrointestinal disorders. Tea extract also showed an especially high abundance of Faecalibacterium and Roseburia, which led to a higher butyrate production. This could in turn favor the host health.
Declarations
Author contribution statement
S. Pérez-Burillo: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
D. Hinojosa-Nogueira: Performed the experiments; Analyzed and interpreted the data.
S. Pastoriza: Conceived and designed the experiments; Wrote the paper.
J. A. Rufián-Henares: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Spanish Ministry of Economy and Competitiveness (AGL2014-53895-R). J. A. Rufián-Henares was supported by the European Regional Development Fund (FEDER).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We acknowledge Natac for providing plant extracts for analyses.
References
- Alizadeh-Fanalou S., Babaei M., Hosseini A., Azadi N., Nazarizadeh A., Shojaii A., Borji M., Malekinejad H., Bahreini E. Effects of Securigera Securidaca seed extract in combination with glibenclamide on antioxidant capacity, fibroblast growth factor 21 and insulin resistance in hyperglycemic rats. J. Ethnopharmacol. 2020;248:112331. doi: 10.1016/j.jep.2019.112331. [DOI] [PubMed] [Google Scholar]
- Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bilinski J., Grzesiowski P., Sorensen N., Madry K., Muszynski J., Robak K., Wroblewska M., Dzieciatkowski T., Dulny G., Dwilewicz-Trojaczek J., Wiktor-Jedrzejczak W., Basak G.W. Fecal microbiota transplantation in patients with blood disorders inhibits gut colonization with antibiotic-resistant bacteria: results of a prospective, single-center study. Clin. Infect. Dis. 2017;65(3):364–370. doi: 10.1093/cid/cix252. [DOI] [PubMed] [Google Scholar]
- Bonanni A., Campanella L., Gatta T., Gregori E., Tomassetti M. Evaluation of the antioxidant and prooxidant properties of several commercial dry spices by different analytical methods. Food Chem. 2007;102(3):751–758. [Google Scholar]
- Cheung S., Tai J. Anti-proliferative and antioxidant properties of rosemary Rosmarinus officinalis. Oncol. Rep. 2007;17(6):1525–1531. [PubMed] [Google Scholar]
- Chiodini R.J., Dowd S.E., Chamberlin W.M., Galandiuk S., Davis B., Glassing A. Microbial population differentials between mucosal and submucosal intestinal tissues in advanced Crohn’s disease of the ileum. PloS One. 2015;10(7) doi: 10.1371/journal.pone.0134382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J.R., Wang Q., Cardenas E., Fish J., Chai B., Farris R.J., Kulam-Syed-Mohideen A.S., McGarrell D.M., Marsh T., Garrity G.M., Tiedje J.M. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37(Database issue):D141–145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe D.R., Holley D., Collins L.B., Montgomery S.A., Whitmore A.C., Hillhouse A., Curry K.P., Renner S.W., Greenwalt A., Ryan E.P., Godfrey V., Haise M.T., Threadgill D.S., Han A., Swenberg J.A., Threadgill D.W., Bultman S.J. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Canc. Discov. 2014;4(12):1387–1397. doi: 10.1158/2159-8290.CD-14-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard A., Belzer C., Geurts L., Ouwerkerk J.P., Druart C., Bindels L.B., Guiot Y., Derrien M., Muccioli G.G., Delzenne N.M., de Vos W.M., Cani P.D. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(22):9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint H.J., Duncan S.H., Scott K.P., Louis P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015;74(1):13–22. doi: 10.1017/S0029665114001463. [DOI] [PubMed] [Google Scholar]
- Fragopoulou E., Gavriil L., Argyrou C., Malagaris I., Choleva M., Antonopoulou S., Afxentiou G., Nikolaou E. Suppression of DNA/RNA and protein oxidation by dietary supplement which contains plant extracts and vitamins: a randomized, double-blind, placebo-controlled trial. Lipids Health Dis. 2018;17(1):187. doi: 10.1186/s12944-018-0836-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavriil L., Detopoulou M., Petsini F., Antonopoulou S., Fragopoulou E. Consumption of plant extract supplement reduces platelet activating factor-induced platelet aggregation and increases platelet activating factor catabolism: a randomised, double-blind and placebo-controlled trial. Br. J. Nutr. 2019;121(9):982–991. doi: 10.1017/S0007114519000308. [DOI] [PubMed] [Google Scholar]
- Granato D., Shahidi F., Wrolstad R., Kilmartin P., Melton L.D., Hidalgo F.J., Miyashita K., Camp J. van, Alasalvar C., Ismail A.B., Elmore S., Birch G.G., Charalampopoulos D., Astley S.B., Pegg R., Zhou P., Finglas P. Antioxidant activity, total phenolics and flavonoids contents: should we ban in vitro screening methods? Food Chem. 2018;264:471–475. doi: 10.1016/j.foodchem.2018.04.012. [DOI] [PubMed] [Google Scholar]
- Gomez-Arango L.F., Barrett H.L., McIntyre H.D., Callaway L.K., Morrison M., Nitert M.D. Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Novelty and significance. Hypertension. 2016;68(4):974–981. doi: 10.1161/HYPERTENSIONAHA.116.07910. [DOI] [PubMed] [Google Scholar]
- Huang Y.-H., Chen S.-T., Liu F.-H., Hsieh S.-H., Lin C.-H., Liou M.-J., Wang C.-C., Huang C.-H., Liu G.-H., Lin J.-R., Yang L.-Y., Hsu T.-Y., Lee M.-C., Huang C.-T., Wu Y.-H. The efficacy and safety of concentrated herbal extract granules, YH1, as an add-on medication in poorly controlled type 2 diabetes: a randomized, double-blind, placebo-controlled pilot trial. PloS One. 2019;14(8) doi: 10.1371/journal.pone.0221199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Zamora A., Delgado-Andrade C., Rufián-Henares J.A. Antioxidant capacity, total phenols and color profile during the storage of selected plants used for infusion. Food Chem. 2016;199:339–346. doi: 10.1016/j.foodchem.2015.12.019. [DOI] [PubMed] [Google Scholar]
- Jung M.-J., Lee J., Shin N.-R., Kim M.-S., Hyun D.-W., Yun J.-H., Kim P.S., Whon T.W., Bae J.-W. Chronic repression of mTOR complex 2 induces changes in the gut microbiota of diet-induced obese mice. Sci. Rep. 2016;6:srep30887. doi: 10.1038/srep30887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko S.-J., Ryu B., Kim J., Hong B.-G., Yeo I., Lee B.-J., Lee J.-M., Park J.-W. Effect of herbal extract granules combined with probiotic mixture on irritable bowel syndrome with diarrhea: study protocol for a randomized controlled trial. Trials. 2011;12(1):219. doi: 10.1186/1745-6215-12-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.S., Park K.-W., Park J.-S., Kim H.-J., Moon S.-R. Effects of nutritional supplement with herbal extract on bone mineral density and height in prepubescent children – a preliminary study. Phytother Res. 2005;19(9):810–811. doi: 10.1002/ptr.1718. [DOI] [PubMed] [Google Scholar]
- Ley R.E., Bäckhed F., Turnbaugh P., Lozupone C.A., Knight R.D., Gordon J.I. Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.V., Frassetto A., Kowalik E.J., Jr., Nawrocki A.R., Lu M.M., Kosinski J.R., Hubert J.A., Szeto D., Yao X., Forrest G., Marsh D.J. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PloS One. 2012;7(4) doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinen E., Krogius-Kurikka L., Lyra A., Nikkilä J., Jääskeläinen A., Rinttilä T., Vilpponen-Salmela T., von Wright A.J., Palva A. Association of symptoms with gastrointestinal microbiota in irritable bowel syndrome. World J. Gastroenterol. 2010;16(36):4532–4540. doi: 10.3748/wjg.v16.i36.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micucci M., Angeletti A., Cont M., Corazza I., Aldini R., Donadio E., Chiarini A., Budriesi R. Hibiscus sabdariffa L. Flowers and olea europea L. Leaves extract-based formulation for hypertension care: in vitro efficacy and toxicological profile. J. Med. Food. 2016;19(5):504–512. doi: 10.1089/jmf.2015.0072. [DOI] [PubMed] [Google Scholar]
- Murri M., Leiva I., Gomez-Zumaquero J.M., Tinahones F.J., Cardona F., Soriguer F., Queipo-Ortuño M.I. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Med. 2013;11:46. doi: 10.1186/1741-7015-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzella L., Pérez-Burillo S., Pastoriza S., Martín M.Á., Cerruti P., Goya L., Ramos S., Rufián-Henares J.Á., Napolitano A., d’Ischia M. High antioxidant action and prebiotic activity of hydrolyzed spent coffee grounds (HSCG) in a simulated digestion–fermentation model: toward the development of a novel food supplement. J. Agric. Food Chem. 2017;65(31):6452–6459. doi: 10.1021/acs.jafc.7b02302. [DOI] [PubMed] [Google Scholar]
- Pastoriza S., Mesías M., Cabrera C., Rufián-Henares J.A. Healthy properties of green and white teas: an update. Food Funct. 2017;8:2650–2662. doi: 10.1039/c7fo00611j. [DOI] [PubMed] [Google Scholar]
- Pastoriza S., Álvarez J., Vérgvári A., Montilla-Gómez J., Cruz-López O., Delgado-Andrade C., Rufián-Henares J.A. Relationship between HMF intake and SMF formation in vivo: an animal and human study. Mol. Nutr. Food Res. 2017;61:1600773. doi: 10.1002/mnfr.201600773. [DOI] [PubMed] [Google Scholar]
- Patnode M.L., Beller Z.W., Han N.D., Cheng J., Peters S.L., Terrapon N., Henrissat B., Gall S.L., Saulnier L., Hayashi D.K., Meynier A., Vinoy S., Giannone R.J., Hettich R.L., Gordon J.I. Interspecies competition impacts targeted manipulation of human gut bacteria by fiber-derived glycans. Cell. 2019;179(1):59–73. doi: 10.1016/j.cell.2019.08.011. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendleton J.N., Gorman S.P., Gilmore B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti-infect. Ther. 2013;11(3):297–308. doi: 10.1586/eri.13.12. [DOI] [PubMed] [Google Scholar]
- Pérez-Burillo S., Ginénez R., Rufián-Henares J.A., Pastoriza S. Effect of brewing time and temperature on antioxidant capacity and phenols of white tea: relationship with sensory properties. Food Chem. 2018;248:111–118. doi: 10.1016/j.foodchem.2017.12.056. [DOI] [PubMed] [Google Scholar]
- Pérez-Burillo S., Rufián-Henares J.A., Pastoriza S. Towards an improved global antioxidant response method (GAR+): physiological-rese.....mbling in vitro digestion-fermentation method. Food Chem. 2018;239(Supplement C):1253–1262. doi: 10.1016/j.foodchem.2017.07.024. [DOI] [PubMed] [Google Scholar]
- Poirier S., Bize A., Bureau C., Bouchez T., Chapleur O. Community shifts within anaerobic digestion microbiota facing phenol inhibition: towards early warning microbial indicators? Water Res. 2016;100:296–305. doi: 10.1016/j.watres.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Ríos-Covián D., Ruas-Madiedo P., Margolles A., Gueimonde M., de los Reyes-Gavilán C.G., Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016;7 doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland I., Gibson G., Heinken A., Scott K., Swann J., Thiele I., Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 2018;57(1):1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufián-Henares J.A., Delgado-Andrade C., Morales F.J. Relationship between acrylamide and thermal-processing indexes in commercial breakfast cereals: a survey of Spanish breakfast cereals. Mol. Nutr. Food Res. 2006;50:756–762. doi: 10.1002/mnfr.200600039. [DOI] [PubMed] [Google Scholar]
- Ryan K.K., Tremaroli V., Clemmensen C., Kovatcheva-Datchary P., Myronovych A., Karns R., Wilson-Pérez H.E., Sandoval D.A., Kohli R., Bäckhed F., Seeley R.J. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar N., Ruas-Madiedo P., Kolida S., Collins M., Rastall R., Gibson G., de los Reyes-Gavilán C.G. Exopolysaccharides produced by Bifidobacterium longum IPLA E44 and Bifidobacterium animalis subsp. lactis IPLA R1 modify the composition and metabolic activity of human faecal microbiota in pH-controlled batch cultures. Int. J. Food Microbiol. 2009;135(3):260–267. doi: 10.1016/j.ijfoodmicro.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Salomäki-Myftari H., Vähätalo L.H., Ailanen L., Pietilä S., Laiho A., Hänninen A., Pursiheimo J.-P., Munukka E., Rintala A., Savontaus E., Pesonen U., Koulu M. Neuropeptide Y overexpressing female and male mice show divergent metabolic but not gut microbial responses to prenatal metformin exposure. PloS One. 2016;11(9) doi: 10.1371/journal.pone.0163805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen T., Cawthon C.R., Ihde B.T., Hajnal A., DiLorenzo P.M., de La Serre C.B., Czaja K. Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity. Physiol. Behav. 2017;173:305–317. doi: 10.1016/j.physbeh.2017.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B., Truong J., Helliwell R., Govindaraghavan S., Sucher N.J. An in vitro study of neuroprotective properties of traditional Chinese herbal medicines thought to promote healthy ageing and longevity. BMC Compl. Alternative Med. 2013;13(1):373. doi: 10.1186/1472-6882-13-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.K., Srivastav S., Castellani R.J., Plascencia-Villa G., Perry G. Neuroprotective and antioxidant effect of Ginkgo biloba extract against AD and other neurological disorders. Neurotherapeutics. 2019;16(3):666–674. doi: 10.1007/s13311-019-00767-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton V.L., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16(3):144–158. [Google Scholar]
- Strati F., Cavalieri D., Albanese D., De Felice C., Donati C., Hayek J., Jousson O., Leoncini S., Renzi D., Calabrò A., De Filippo C. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome. 2017;5(1) doi: 10.1186/s40168-017-0242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita K., Mizuno S., Mikami Y., Sujino T., Saigusa K., Matsuoka K., Naganuma M., Sato T., Takada T., Tsuji H., Kushiro A., Nomoto K., Kanai T. A single species of Clostridium subcluster XIVa decreased in ulcerative colitis patients. Inflamm. Bowel Dis. 2016;22(12):2802–2810. doi: 10.1097/MIB.0000000000000972. [DOI] [PubMed] [Google Scholar]
- Verbeke K.A., Boesmans L., Boets E. Modulating the microbiota in inflammatory bowel diseases: prebiotics, probiotics or faecal transplantation? Proc. Nutr. Soc. 2014;73(4):490–497. doi: 10.1017/S0029665114000639. [DOI] [PubMed] [Google Scholar]
- Villiger A., Sala F., Suter A., Butterweck V. In vitro inhibitory potential of Cynara scolymus, Silybum marianum, Taraxacum officinale, and Peumus boldus on key enzymes relevant to metabolic syndrome. Phytomedicine. 2015;22(1):138–144. doi: 10.1016/j.phymed.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Wojdyło A., Oszmiański J., Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105(3):940–949. [Google Scholar]
- Yen G.-C., Chen H.-Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995;43(1):27–32. [Google Scholar]
- Ze X., Duncan S.H., Louis P., Flint H.J. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012;6:1535–1543. doi: 10.1038/ismej.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Dong M., Xu Guangyong, Yuan Tian, Tang H., Wang Y. Metabolomics reveals that dietary ferulic acid and quercetin modulate metabolic homeostasis in rats. J. Agric. Food Chem. 2018;66(7):1723–1731. doi: 10.1021/acs.jafc.8b00054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.