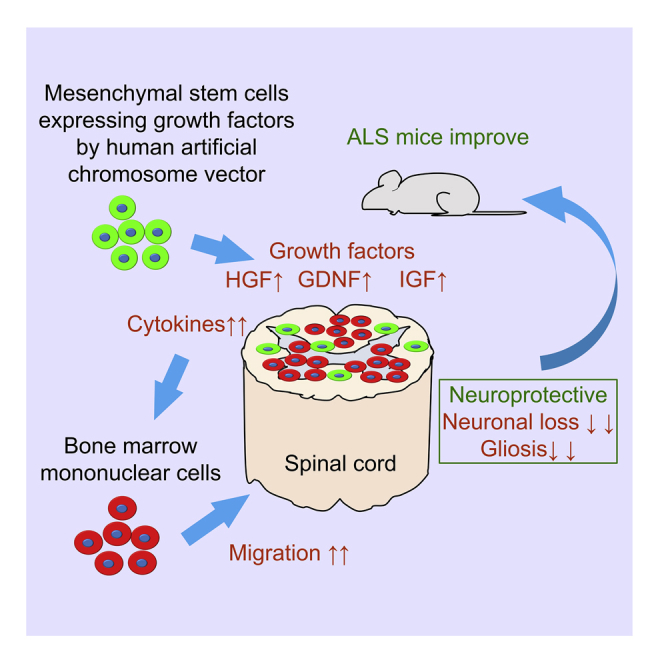
Summary
Several treatments have been attempted in amyotrophic lateral sclerosis (ALS) animal models and patients. Recently, transplantation of bone marrow-derived mononuclear cells (MNCs) was investigated as a regenerative therapy for ALS, but satisfactory treatments remain to be established. To develop an effective treatment, we focused on mesenchymal stem cells (MSCs) expressing hepatocyte growth factor, glial cell line-derived neurotrophic factor, and insulin-like growth factor using human artificial chromosome vector (HAC-MSCs). Here, we demonstrated the transplantation of MNCs with HAC-MSCs in ALS mice. As per our results, the progression of motor dysfunction was significantly delayed, and their survival was prolonged dramatically. Additional analysis revealed preservation of motor neurons, suppression of gliosis, engraftment of numerous MNCs, and elevated chemotaxis-related cytokines in the spinal cord of treated mice. Therefore, growth factor-expressing MSCs enhance the therapeutic effects of bone marrow-derived MNCs for ALS and have a high potential as a novel cell therapy for patients with ALS.
Subject Areas: Molecular Neuroscience, Cellular Neuroscience, Stem Cells Research
Graphical Abstract
Highlights
-
•
MNCs with growth factor-expressing MSCs is an effective cell therapy for ALS mice
-
•
The MSCs enhance therapeutic effects by migration of MNCs into ALS mice spinal cord
-
•
This cell therapy suppresses neuronal loss and gliosis in ALS mice spinal cord
-
•
This cell therapy induces several cytokines expression in ALS mice spinal cord
Molecular Neuroscience; Cellular Neuroscience; Stem Cells Research
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease that results in muscle weakness caused by the loss of functioning of upper and lower motor neurons (Mitchell and Borasio, 2007; Robberecht and Philips, 2013). Most patients with ALS are sporadic, but approximately 10% of the patients with ALS have the disease traits in their genetic background (Pasinelli and Brown, 2006). Mutations in the superoxide dismutase (SOD) 1 gene is one of the most frequent causes of familial ALS in Asians, although the GGGGCC (G4C2) repeat expansion in C9orf72 is the most commonly found mutation in Caucasians (McCauley and Baloh, 2019; Rosen et al., 1993; Zou et al., 2017). Transgenic mice models that show an overly high expression of the mutant SOD1 protein result in induced neuronal cell death in the central nervous system and have been used as the representative animal models of ALS in a variety of experiments (Gurney et al., 1994). The hSOD1 G93A transgenic (SOD1-tg) mouse is the most commonly used ALS mouse model (Gurney et al., 1994; Tu et al., 1996). Many researchers have used this mouse model and reported pathological findings in the spinal cord and brain of mice (Magrané et al., 2012; Tu et al., 1996). There is no established treatment for ALS, and many studies continue to conduct research to develop a cure (Abati et al., 2019a; Baloh et al., 2018).
Stem cell therapy or regenerative therapy is an attractive strategy to provide great hope for the treatment of an incurable disease like ALS (Abati et al., 2019b; Chen et al., 2016; Goutman et al., 2018). In regenerative therapy, transplantation not only replaces damaged neurons but may also reverse their functioning by protecting weakened neurons and modulating the surrounding niche via the secretion of neurotrophic factors (Kim et al., 2014; Krakora et al., 2013; Tang, 2017). As a regenerative therapy for ALS in mice models, we previously have performed transplantation therapy of stem cell factor-modified bone marrow-derived mononuclear cells (MNCs) and have reported their therapeutic effects in ALS mice (Terashima et al., 2014). Our results showed that bone marrow-derived cells migrated into the spinal cord of ALS mice and their neuroprotective effects were also observed (Terashima et al., 2014). However, the effects were partial and we postulated that additional modulation of the spinal cord niche would be required for the successful engraftment of the transplanted cells.
As an alternative strategy for the treatment of ALS in mice models, we previously generated trophic factor-expressing mesenchymal stem cells (MSCs)using human artificial chromosome vectors (Watanabe et al., 2015). Artificial chromosome vectors have been developed and applied to novel therapeutic strategies for cell-based therapies (Kouprina et al., 2014; Ikeno et al., 2020; Sinenko et al., 2020; Suzuki et al., 2020). One of them, the 21st human chromosome vector (21HAC) has been engineered previously based on truncation of human chromosome 21 by Oshimura lab for gene therapeutic applications (Katoh et al., 2004). As a gene therapy vector, this 21HAC featured sustained gene expression, ability to insert large genomic loci, and efficient insertion of multiple genes, and lack of insertional mutagenesis. Based on the 21HAC vector (hereafter termed as HAC), we developed a gene therapeutic vector expressing three growth factors: hepatocyte growth factor (HGF), glial cell line-derived neurotrophic factor (GDNF), and insulin-like growth factor (IGF) (Watanabe et al., 2015). The cell lines established using HAC technology have been named HAC-MSCs and have been used for studies on ALS treatment in experimental mice models (Watanabe et al., 2015; Nakanishi et al., 2019). We focused on HAC-MSCs expressing the three growth factors, which have been reported to have effects on ALS rodents (Dodge et al., 2008; Kaspar et al., 2003; Sun et al., 2002; Wang et al., 2002), because HAC-MSCs are expected to improve the environment and the niche surrounding neurons in the spinal cord of ALS. This might provide successful engraftment of bone marrow-derived MNCs in ALS mouse model.
Therefore, in the present study, we have demonstrated a new transplantation therapy of bone marrow-derived MNCs with HAC-MSCs expressing growth factors for the ALS mouse model with an aim to increase the therapeutic effects of the transplanted cells. We have also analyzed the engraftment of transplanted cells in ALS mice. Additionally, we successfully demonstrated the efficacy of the combination transplantation therapy.
Results
Preparation of MNCs and HAC-MSCs and Experimental Design for the Treatment of SOD1-tg
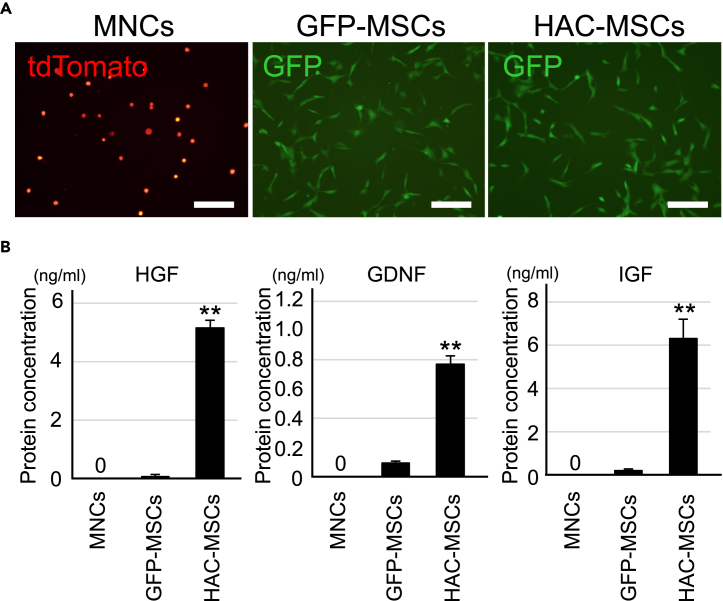
For bone marrow transplantation therapy in SOD1-tg mice, MSCs expressing HGF, GDNF, and IGF by HAC (HAC-MSCs, Figures S1 and 1), MSCs containing the control HAC without growth factor expression but with green fluorescent protein (GFP) (GFP-MSCs, Figures S1 and 1), and MNCs were prepared (Figure 1). GFP-MSCs and HAC-MSCs were confirmed to express GFP under a microscope (Figure 1A). In contrast, MNCs, which were isolated from bone marrow of tdTomato red fluorescent protein (hereafter tdTomato)-expressing transgenic mice, were distinguished from the HAC-MSCs population by differences in expressing-fluorescent protein (Figure 1A). HAC-MSCs expressed significantly higher levels of the three growth factors as compared with GFP-MNCs as shown in the HGF, GDNF, and IGF enzyme-linked immunosorbent assay (ELISA) analysis, whereas MNCs did not show any expression (Figure 1B). These cells were then used for cell therapy in SOD1-tg mice. Eight-week-old female SOD1-tg mice were transplanted as follows: only MNCs (MNCs group), MNCs with control MSCs (MNCs + GFP-MSCs group), or MNCs with HAC-MSCs (MNCs + HAC-MSCs group) (Figure S2). The transplanted mice were monitored for their body weight, neurological behavior, and survival rate to evaluate the therapeutic effects of the transplanted cells and also analyzed for their histological and biological features (Figure S2).
Figure 1.
Characterization of MNCs, GFP-MSCs, and HAC-MSCs
(A) Images of primary MNCs from tdTomato transgenic mice and cultured GFP-MSCs and HAC-MSCs. Scale bar, 20 μm.
(B) ELISA analysis of HGF, GDNF, and IGF in the media of MNCs (n = 8), GFP-MSCs (n = 8), and HAC-MSCs (n = 8) after 3 days of culture. ∗∗p < 0.01 between HAC-MSCs and the others. Error bars represent the mean + SD. GDNF, glial cell line-derived neurotrophic factor; GFP, green fluorescent protein; HAC, human artificial chromosome vectors; HGF, hepatic growth factor; IGF, insulin-like growth factor; MNCs, mononuclear cells; MSCs, mesenchymal stem cells.
Effects of Combined Bone Marrow Transplantation Therapy in SOD1-tg Mice
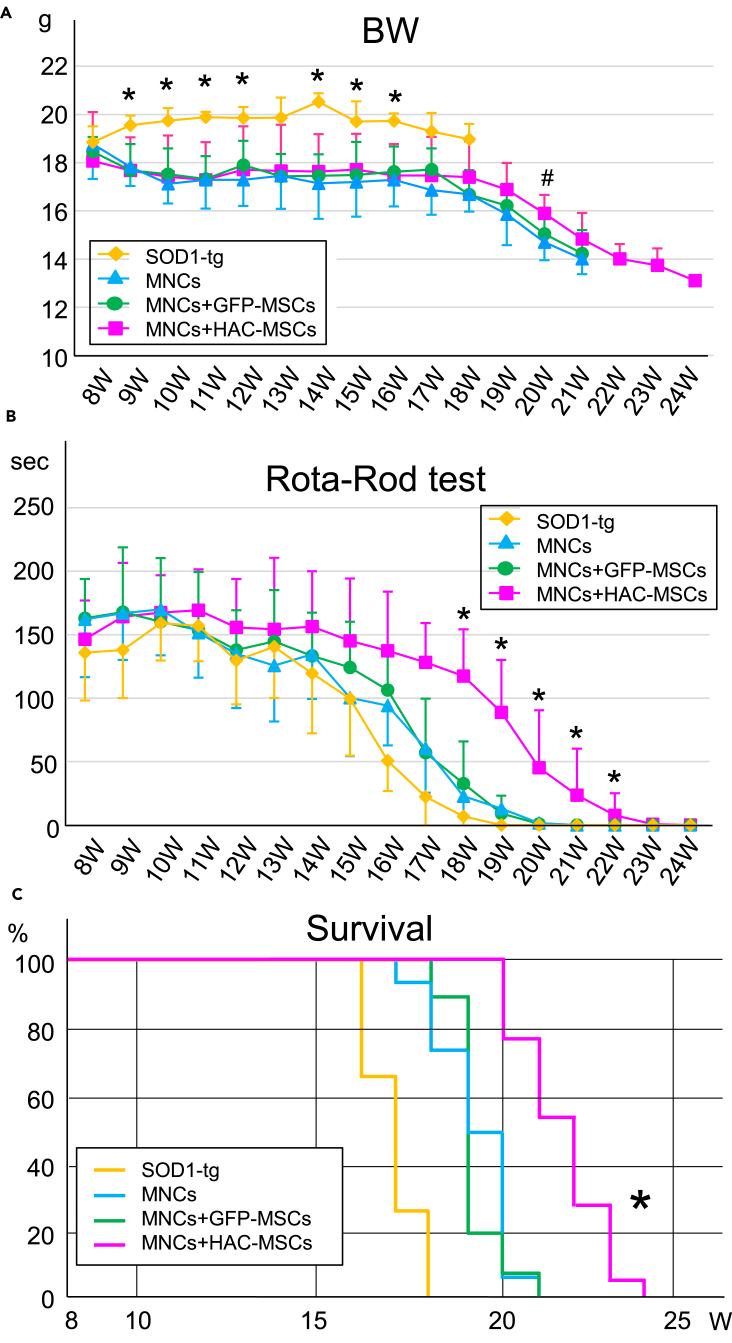
Eight-week-old female SOD1-tg mice underwent cell therapy after initial examination for motor behavior using the Rota-Rod test and full body irradiation. Bodyweight, motor function, and survival rates were investigated every week until the Rota-Rod test was zero seconds, and the results were compared with those of the non-transplanted SOD1-tg mice (SOD1-tg group) (Figure 2). Body weight of the SOD1-tg group was heavier than that of the three treatment groups at some time points, but there was no significant difference among the three cell therapy groups throughout the monitoring period except at 20 W (Figure 2A). Only at 20 W, body weight in the MNCs + HAC-MSCs group was significantly heavier than that in the other two cell therapy groups (Figure 2A). Next, three bone marrow transplantation groups showed a trend toward improved motor behavior compared with the no-treatment SOD1-tg mice (Figure 2B). In particular, the therapeutic effects were considerably enhanced in the MNCs + HAC-MSCs group as compared with all other groups at 18–22 W (Figure 2B). For the survival rate, the MNCs + HAC-MSCs group showed the most effective results among all the groups, similar to that of the motor function test (Figure 2C).
Figure 2.
Bone Marrow Transplantation Effects on Bodyweight, Motor Function, and Survival in SOD1-tg
(A) Bodyweight (BW) was measured once a week in the SOD1-tg mice without treatment (n = 15) or with MNCs (n = 15), MNCs + GFP-MSCs (n = 16), or MNCs + HAC-MSCs transplantation (n = 18). ∗p < 0.05 between the SOD1-tg group and the others. #p < 0.05 between the MNCs + HAC-MSCs group and the other two treatment groups. The error bars represent the mean ± SD.
(B) Rota-rod test was performed in same four groups. ∗p < 0.05 between MNCs + HAC-MSCs group and others at each time point. The error bars represent the mean ± SD.
(C) Survival curves were analyzed in same four groups. ∗p < 0.05 between the MNCs + HAC-MSCs group and the others using the log rank test.
Histological Analysis of the Spinal Cord and Muscle in SOD1-tg Mice after Cell Therapy
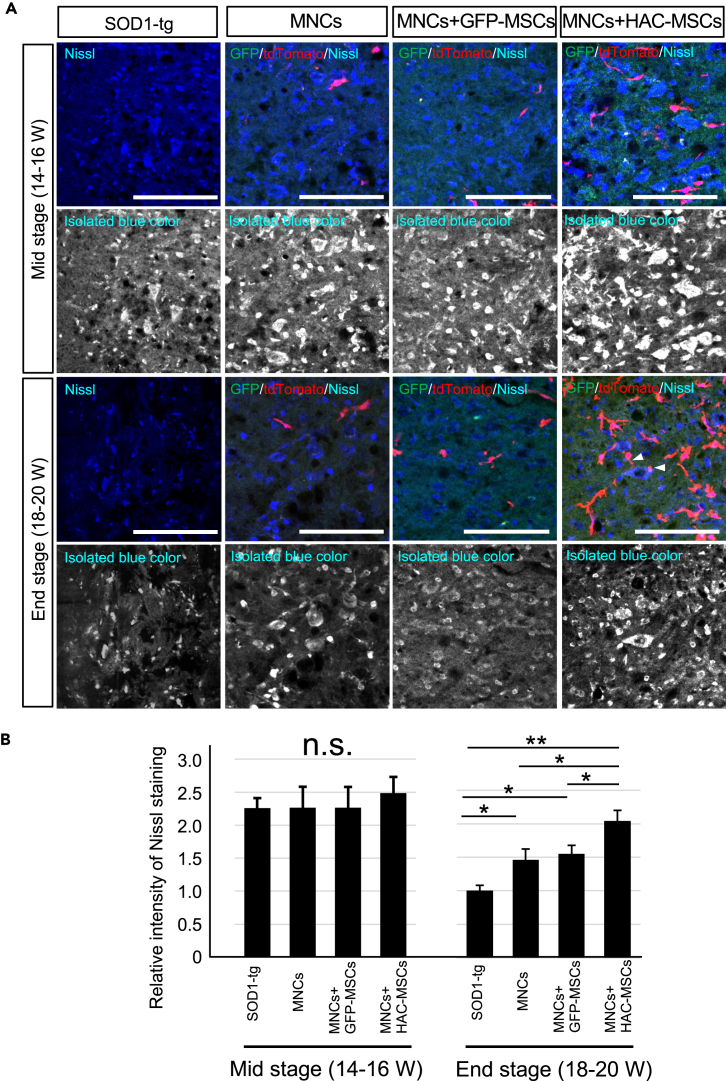
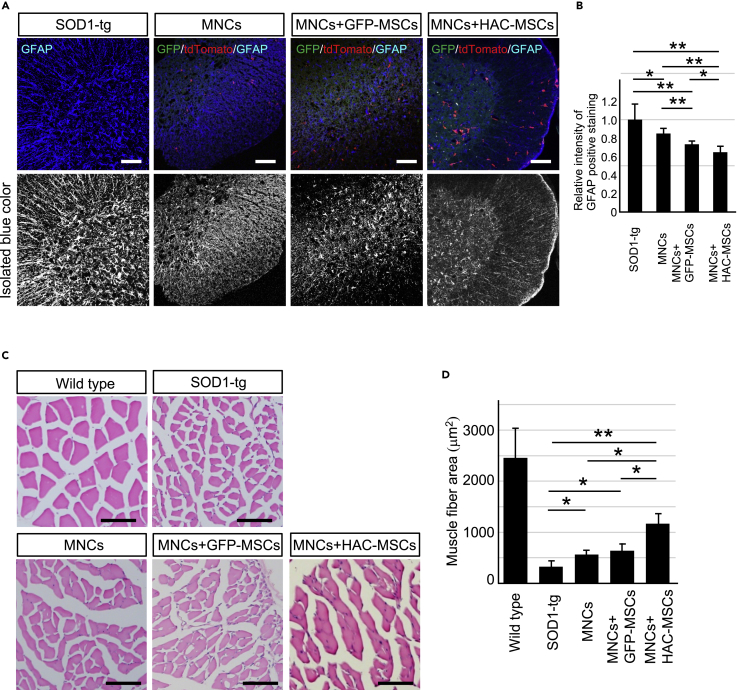
To evaluate the effects of cell therapy, Nissl staining was performed to observe motor neuron survival in the spinal cord at middle (14–16 W) and end stages (18–20 W) of disease after cell therapy (Figure 3). At the middle stage of the disease, Nissl-positive staining was the same level among the three treatment groups and SOD1-tg disease control group (Figure 3A upper 2 rows and 3B left side). These results suggested that the motor neurons were still preserved in the SOD1-tg group compared with the other three cell therapy groups. However, Nissl staining was decreased at the end stage of disease in the SOD1-tg group (Figure 3A lower 2 rows and 3B right side). In contrast, MNCs, MNCs + GFP-MSCs, and MNCs + HAC-MSCs groups showed significantly higher intensity of Nissl staining when compared with the SOD1-tg group. In addition, the MNCs + HAC-MSCs group showed the highest staining intensity among all the four groups (Figures 3A and 3B). As another finding, a few MNCs were observed to be attached to neurons in the MNCs + HAC-MSCs group (Figure 3A arrowheads). These results suggest that neurons were somehow influenced by MNCs and that neuronal loss from middle to end stage of the disease was suppressed in the MNCs + HAC-MSCs group. Next, gliosis in the spinal cord of SOD1-tg mice was evaluated after cell therapy using GFAP immunostaining (Figure 4A). GFAP staining in the three cell therapy groups was significantly weaker than that in the SOD1-tg group at the end stage of the disease (Figure 4B). The MNCs + HAC-MSCs group showed the weakest staining in the four groups. These results were similar to those of Nissl staining, but GFAP staining in MNCs + GFP-MSCs was weaker than that in MNCs (Figures 4A and 4B). These results indicate that the MNCs + HAC-MSCs therapy group was most effective for the suppression of gliosis and that the MSCs likely contributed to the neuroprotective effect compared with MNCs. Muscle degeneration was also evaluated by measuring the muscle fiber area in the anterior tibial muscle of SOD1-tg mice after cell therapy (Figures 4C and 4D). The muscle fiber area was preserved in the three cell therapy groups and was most prominent in the MNCs + HAC-MSCs group (Figure 4D). However, the effects of the three cell therapy groups for the suppression of gliosis and muscle degeneration were shown only at the end stage and not at the middle stage, similar to the effect observed on motor neurons (data not shown).
Figure 3.
Histological Analysis of Motor Neurons in the Spinal Cord in SOD1-tg After Cell Transplantation Therapy
(A) Nissl stain (blue) with GFP (GFP-MSCs or HAC-MSCs; green) and tdTomato (MNCs; red) signals in anterior horns of the spinal cords in the SOD1-tg, MNCs, MNCs + GFP-MSCs, and MNCs + HAC-MSCs groups at the middle stage (Mid: 14–16 W) and the end stage (End: 18–20 W) of the disease. Upper row in each stage shows the color images and lower row in each stage shows black and white images of blue color (Nissl staining) isolated from the corresponding upper row. The arrowheads show the MNCs attached to the Nissl positive neurons. Scale bar, 100 μm.
(B) Relative intensity of Nissl staining as seen in the SOD1-tg, MNCs, MNCs + GFP-MSCs, and MNCs + HAC-MSCs groups at the middle stage (Mid: 14–16 W) (n = 5 in each group) and the end stage (End: 18–20 W) (n = 5 at each group) of the disease. The intensity of Nissl staining was measured in the black and white image in (A) using the ImageJ software and the ratio was calculated against the intensity of that in SOD1-tg at end stage. ∗p < 0.05. ∗∗p < 0.01. n.s.: not significant. The error bars represent the mean + SD.
Figure 4.
Histological Analysis of Gliosis in Spinal Cord and Muscle Degeneration in SOD1-tg After Cell Transplantation Therapy
(A) GFAP immunohistochemistry (blue) with GFP (GFP-MSCs or HAC-MSCs; green) and tdTomato (MNCs; red) signals in anterior horns of the spinal cords in SOD1-tg, MNCs, MNCs + GFP-MSCs, and MNCs + HAC-MSCs groups at 18–20 weeks old. Upper row in each stage shows the color images and lower row in each stage shows black and white images of blue color (GFAP staining) isolated from the corresponding upper row. Scale bar, 100 μm.
(B) The bar graph shows relative intensity of GFAP positive staining in same four groups at the 18–20 weeks old (n = 5 at each group). The intensity of GFAP staining was measured in the black and white image as shown in lower picture of left side by ImageJ software and the ratio was calculated against the intensity of that in SOD1-tg. ∗p < 0.05. ∗∗p < 0.01. Scale bar, 100 μm. Error bars represent the mean + SD.
(C) Hematoxylin-eosin stain of anterior tibial muscle in SOD1-tg, MNCs, MNCs + GFP-MSCs and MNCs + HAC-MSCs groups and wild-type mice at 18–20 weeks old. Scale bar, 100 μm.
(D) Bar graph indicates the area of each muscle fiber in five groups. The average areas of muscle fibers were compared among the four disease groups (n = 5 at each group). ∗p < 0.05. ∗∗p < 0.01. Error bars represent the mean + SD.
Histological Analysis of Transplanted Cells in the Spinal Cord and Bone Marrow Tissues of SOD1-tg Mice
Next, to analyze the mechanism by which the MNCs + HAC-MSCs group showed an increase in the therapeutic effects observed above, histological experiments were performed on spinal cord and bone marrow tissues of 16-week-old SOD1-tg mice in three therapeutic groups. Sections of the spinal cord and bone marrow were prepared and observed for GFP (green) and tdTomato (red) fluorescent signals under a fluorescence microscope (Figure S3). In the spinal cord sections, GFP signals were observed occasionally in MNCs + GFP-MSCs and MNCs + HAC-MSCs groups (Figure S3A). However, an increased number of red signals were observed in the spinal cord sections of MNCs + HAC-MSCs group when compared with the other two groups (Figure S3A). These results showed that many MNCs have migrated into the spinal cord of the SOD1-tg mice transplanted with MNCs + HAC-MSCs. In bone marrow tissues, many GFP-positive cells were observed in MNCs + GFP-MSCs and in MNCs + HAC-MSCs groups, whereas the tdTomato-positive MNCs were diffusely present in all groups (Figure S3B). These results suggest that each population of transplanted cells colonized the bone marrow of the recipient SOD1-tg mice.
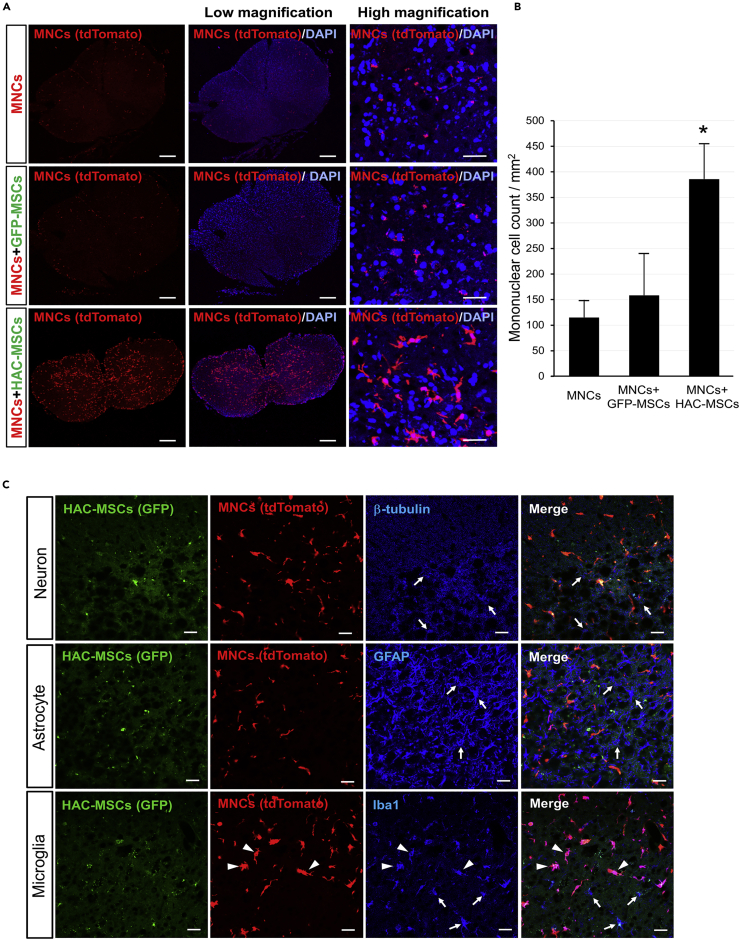
In addition, we compared the population of tdTomato-positive MNCs among the three kinds of bone marrow transplantations because tdTomato-positive cells in the MNCs + HAC-MSCs group migrated in large numbers to the spinal cord. Spinal cord sections were obtained from MNCs, MNCs + GFP-MSCs, and MNCs + HAC-MSCs groups and seen for the presence of red fluorescence (Figure 5). In all three groups, tdTomato-positive MNCs were diffusely observed in 14- to 16-week-old SOD1-tg mice after transplantation (Figure 5A). However, the number of MNCs (red fluorescent-positive cells) increased markedly in only the MNCs + HAC-MSCs group (Figures 5A and 5B). The number of tdTomato positive cells were approximately three times higher in the MNCs + HAC-MSCs group than in the MNCs and MNCs + GFP-MSCs groups (Figure 5B).
Figure 5.
Accumulation and Characteristic Analysis of MNCs in the Spinal Cord of the SOD1-tg Mice After Cell Transplantation Therapy
(A) tdTomato (MNCs; red and left side) signals with a nuclear stain (DAPI, blue, middle, and right side) in the sections of whole spinal cords of 14 to 16-week-old SOD1-tg mice from the MNCs, MNCs + GFP-MSCs, and MNCs + HAC-MSCs transplantation groups. Scale bars, 100 μm.
(B) The number of MNCs in spinal cord sections of 14- to 16-week-old SOD1-tg mice after transplantation of MNCs (n = 5), MNCs + GFP-MSCs (n = 5), and MNCs + HAC-MSCs (n = 5) groups. ∗p < 0.05 between the MNCs + HAC-MSCs group and the others. The error bars represent the mean + SD.
(C) Counterstain of spinal cord sections of 14- to 16-week-old SOD1-tg mice from the MNCs + HAC-MSCs group was performed for β-tubulin in neuron (left side lane), for GFAP in astrocyte (middle lane), and for Iba1 in microglia (right side lane). HAC-MSCs shows GFP protein (green) and MNCs shows tdTomato protein (red). The staining of β-tubulin, GFAP, and Iba1 are shown in blue color. Arrows indicate endogenous β-tubulin-positive neurons, GFAP-positive astrocytes, and Iba1-positive microglia (blue), and arrowheads indicate Iba1-positive cells that originated from transplanted MNCs. Scale bars, 20 μm.
Furthermore, we also performed counter-staining of the migrated MNCs to clarify their characteristics. Sections were prepared from spinal cords of 16-week-old SOD1-tg mice after transplantation of MNCs + HAC-MSCs. Histological analysis of the sections was performed using β-tubulin immunostaining as a neuronal marker, glial fibrillary acidic protein (GFAP) as an astrocyte marker, and ionized calcium-binding adapter molecule 1 (Iba1) as a microglia marker (Figure 5C). Red fluorescent signals for tdTomato protein were diffused in the spinal cord, and over half of the signals co-localized with Iba1 staining pattern but not with β-tubulin and GFAP staining (Figure 5C). These results suggest that a large number of MNCs that have migrated to the spinal cord showed microglia-like features but did not seem to differentiate into neurons.
Expression of Growth Factors and Reporter Genes in the Transplanted SOD1-tg Mice
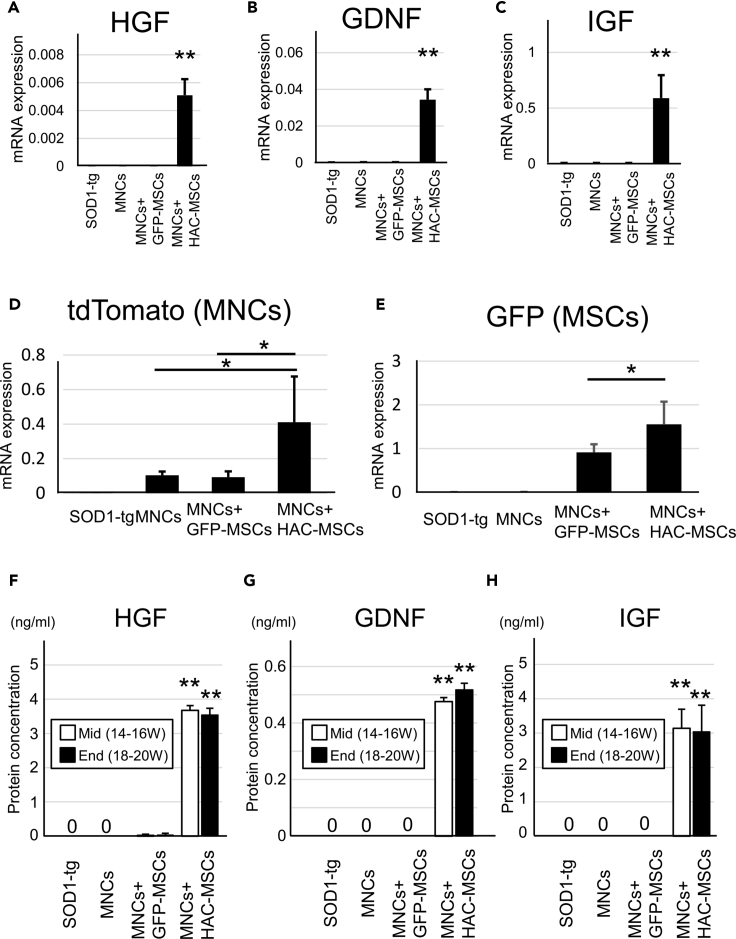
Spinal cord tissues were obtained from 16- to 18-week-old SOD1-tg mice after transplantation of the three cell therapy groups. Quantitative PCR of HGF, GDNF, and IGF was performed in spinal cord tissues of no treatment, MNCs, MNCs + GFP-MSCs, and MNCs + HAC-MSCs groups (Figures 6A–6C). Significant mRNA expression of human HGF, GDNF, and IGF genes was recognized only in the MNCs + HAC-MSCs group (Figures 6A–6C). These results suggest that the transgenes from HAC-MSCs were specifically expressed at the pathological lesion sites of the spinal cords. Additionally, the tdTomato and GFP genes in the spinal cord of SOD1-tg mice were quantitated by PCR after each transplantation (Figures 6D and 6E). tdTomato gene expression was much higher in the MNCs + HAC-MSCs group than in the MNCs and MNCs + GFP-MSCs groups (Figure 6D). These results were consistent with the histological analysis of tdTomato-positive MNCs (Figures 5A and 5B). GFP gene expression, which originated from MSCs, was recognized in only the MNCs + GFP-MSCs and MNCs + HAC-MSCs groups (Figure 6E). The expression level of GFP was significantly higher in the MNCs + HAC-MSCs group than in the MNCs + GFP-MSCs group (Figure 6E). These results suggest that the transplanted MSCs have to a higher degree migrated and colonized the spinal cords in the MNCs + HAC-MSCs group than in the MNCs + GFP-MSCs group. Next, ELISA of HGF, GDNF, and IGF was performed to evaluate the protein expression level in spinal cord tissues from 14 to 16 W (middle stage) and 18–20 W (end stage) SOD1-tg mice after transplantation therapy (Figures 6F–6H). Expression levels of HGF, GDNF, and IGF were observed at a significantly high level in the spinal cord of MNCs + HAC-MSCs group at both the middle and end stages, whereas the SOD1-tg group and the other two groups hardly expressed any of the three proteins in both stages. However, the expression levels of the three proteins were not different between the two stages in the MNCs + HAC-MSCs group (Figures 6F–6H).
Figure 6.
Analysis of mRNA and Protein Levels in the Spinal Cord of SOD1-tg After Transplantation of HAC-MSCs and MNCs
(A–C) mRNA expression level of growth factors including HGF (A), GDNF (B), and IGF (C) in the spinal cord of 16- to 18-week-old SOD1-tg mice were analyzed and compared among the control SOD1-tg (n = 5), MNCs (n = 5), MNCs + GFP-MSCs (n = 5), and MNCs + HAC-MSCs (n = 5) groups. The y axis shows mRNA expression standardized with β-actin mRNA expression. ∗∗p < 0.01 between the MNCs + HAC-MSCs group and the others. Error bars represent the mean + SD.
(D and E) mRNA expression level of tdTomato (D) and GFP gene (E) was observed in control SOD1-tg (n = 5), MNCs (n = 5), MNCs + GFP-MSCs (n = 5), and MNCs + HAC-MSCs (n = 5) groups. ∗p < 0.05 between MNCs + HAC-MSCs group and others. Error bars represent the mean + SD.
(F–H) ELISA analysis of growth factors including HGF (F), GDNF (G), and IGF (H) in spinal cord at SOD1-tg, MNCs, MNCs + GFP-MSCs, and MNCs + HAC-MSCs groups at middle stage (Mid: 14–16 W) (n = 5 at each group) and end stage (End: 18–20 W) (n = 5 at each group) of disease. ∗∗p < 0.01 between MNCs + HAC-MSCs group and others. Error bars represent the mean + SD.
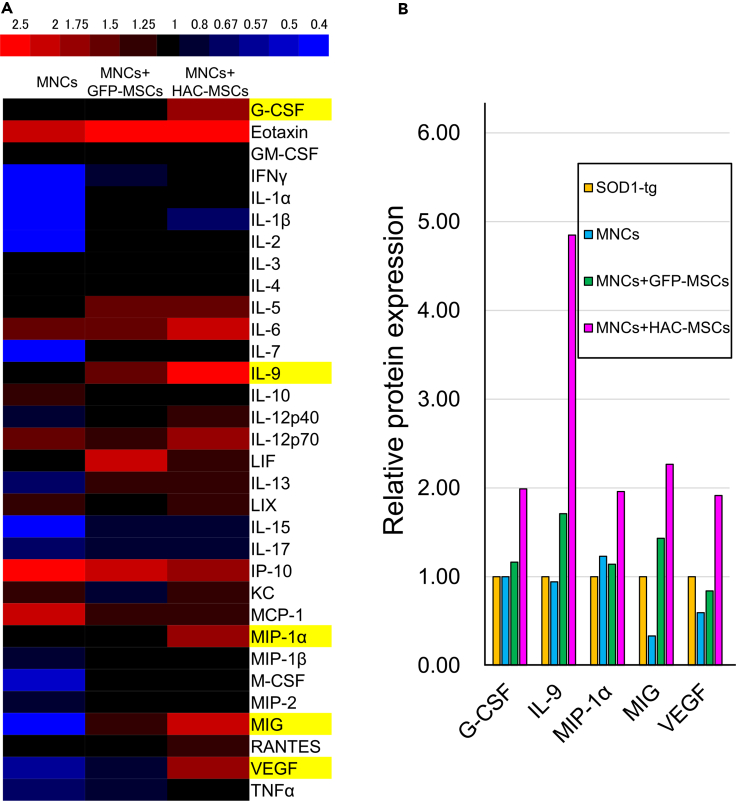
Expression of Cytokines in the Spinal Cords of SOD1-tg Mice
Spinal cord tissues were collected from 16- to 18-week-old SOD1-tg mice after MNCs, MNCs + GFP-MSCs, and MNCs + HAC-MSCs transplantation procedure was carried out. The concentrations of 32 cytokines were measured in the spinal cord tissues of the three groups (Figure 7). Cytokine expression was represented on a red-blue color scale against the expression level of no-treatment SOD1-tg mice (Figure 7A). The expression of only five cytokines, granulocyte-colony-stimulating factor (G-CSF), interleukin (IL)-9, macrophage inflammatory protein (MIP)-1α, monokine induced by interferon-γ (MIG), and vascular endothelial growth factor (VEGF), increased gradually from the MNCs group to the MNCs + GFP-MSCs groups and from MNCs + GFP-MSCs to MNCs + HAC-MSCs groups (Figure 7A, yellow highlight). The above-mentioned five cytokines were elevated by the addition of MSCs and further elevated by the expression of three growth factors by HAC-MSCs (Figure 7B). The concentration of IL-9 in the spinal cord of the MNCs + HAC-MSCs group was four times higher than that in the no-treatment SOD1-tg mice, which showed the highest rate among the five cytokines (Figure 7B). The concentrations of the other four cytokines were approximately two times higher than those in the no-treatment SOD1-tg mice (Figure 7B). Conversely, IL-1β decreased in the MNCs + HAC-MSCs group when compared with no-treatment SOD1-tg mice. However, the level of reduction was smaller than in the MNCs group (Figure 7A). These results did not seem to reflect the additional effects of HAC-MSCs into the MNCs group.
Figure 7.
Quantitative Analysis of Cytokines in the Spinal Cord of SOD1-tg After Transplantation of HAC-MSCs and MNCs
(A) The protein concentration of 32 cytokines was measured in the spinal cord combined from over three 16- to 18-week-old SOD1-tg mice in the control SOD1-tg, MNCs, MNCs + GFP-MSCs, and MNCs + HAC-MSCs groups. The ratio of the protein concentration of the cytokines in each group was compared against that of the control SOD1-tg to each MNCs, MNCs + GFP-MSCs, and MNCs + HAC-MSCs groups. Red color indicates the upregulation of cytokines expression and blue color indicates the downregulation against the SOD1-tg group. Yellow highlight indicates the cytokines with gradual increase in expression levels from MNCs to MNCs + HAC-MSCs. Red bars indicate the elevated cytokine levels, and blue bars indicate the decreased cytokines in the MNCs group when compared with the SOD1-tg group.
(B) After picking up five yellow-highlighted cytokines, their protein expression ratio was calculated against control SOD1-tg in MNCs, MNCs + GFP-MSCs, and MNCs + HAC-MSCs groups.
Discussion
Primary bone marrow MNCs are a heterogeneous population that includes hematopoietic lineage cells such as lymphocytes, monocytes, hematopoietic stem cells, and progenitor cells. MSCs are multipotent cells that can differentiate into osteoblasts, chondrocytes, myocytes, and adipocytes and are known to provide a supportive microenvironmental niche for hematopoietic stem cells. In this study, we used immortalized MSCs, which were originally derived from the bone marrow. We used a combination of MNCs and MSCs because immortalized MSCs would support the survival of primary MNCs and the secreted neurotrophic factors would further strengthen the trophic effect of MSCs.
This study showed that a combined bone marrow transplantation (BMT) of MNCs and growth factor-expressing MSCs (HAC-MSCs) enhanced the effect of BMT therapy of MNCs on ALS disease progression and survival in mice models. After MNCs + HAC-MSCs therapy, the number of bone marrow-derived MNCs that homed to the spinal cord in SOD1-tg mice increased markedly, as detected by the expression of the tdTomato protein. This phenomenon was remarkably the most different in MNCs + HAC-MSCs therapy compared with that in MNCs and MNCs + GFP-MSCs transplantation groups, which could be related to the expression of cytokines in the spinal cord and their therapeutic effects in SOD1-tg mice.
Previous reports have shown that BMT preserved motor function and prolonged the survival time of SOD1-tg mice (Ohta et al., 2011; Silani et al., 2004; Venturin et al., 2016). BMT has been experimentally used for the treatment of patients with ALS in conjunction with total body irradiation (Appel et al., 2008). Similarly, we have also reported the therapeutic effects of stem cell factor-modified bone marrow transplantation in SOD1-tg mice (Terashima et al., 2014). In this study, the transplanted cells did not differentiate into neurons. Instead, the neuroprotective effects of the transplant were observed because the expression of inflammatory cytokines was suppressed, which improved the pathological conditions in the spinal cord of SOD1-tg mice via microglia-like cells of donor origin (Ohta et al., 2011; Terashima et al., 2014; Venturin et al., 2016). In the current study, migration of a large number of transplanted MNCs was observed in the spinal cord, and some of them showed expression of microglial markers and were also likely attached to neurons. This migration and expression could be the reason for the therapeutic effects that are observed as is consistent with a previous report (Terashima et al., 2014).
In the current study, transplantation of MNCs + GFP-MSCs did not show enhanced effects on motor function and survival compared with the MNCs group; however, it demonstrated the therapeutic effects discussed above when compared with the no-treatment group. MSCs have been widely researched for their usage in regenerative therapies for neuronal diseases such as stroke and neurodegenerative diseases (Tanna and Sachan, 2014). MSCs transplantation has been shown to be effective in the mice model of ALS, and the mechanisms underlying the therapeutic effects of the transplantation appear to be highly dependent on two major outcomes: motor neuron survival and an increase in regeneration of the diseased neuronal tissues (Kim et al., 2010; Zhang et al., 2009). MSCs have already been used in clinical studies for ALS treatment (Mazzini et al., 2010, 2012). In these studies, the MSCs were directly administered into ALS mice and patients through intrathecal or local injection (Kim et al., 2010; Mazzini et al., 2010, 2012; Zhang et al., 2009). We have also previously shown effective results with the intrathecal administration route for injection of the cells (Watanabe et al., 2015). However, this study used BMT strategy through intravenous injection, and this was different from the methods used in previous studies. Owing to the differences between these approaches, MNCs + GFP-MSCs may result in no enhanced effects on motor function and survival compared with MNCs. Moreover, a combination of human MSCs and human chromosome vectors was used to transplant mice in this study since the development of human chromosome vectors is considered to be widely used in future clinical settings. Therefore, histocompatibility-related issues could occur with regard to xenogeneic human MCSs and allogeneic mouse MNCs, even though immunosuppressants were used in this study. In mouse MSCs, HAC is less stable, possibly because of differences in centromeres between humans and mice. Therefore, we started to use human MSCs for HAC in the series of our experiments; however, it was recently reported that a novel version of mouse artificial chromosome (MAC), a mouse counterpart of HAC, is stably maintained in mouse cells (Shimohara et al., 2017). If a combination of mouse MSCs and MAC is used, it may have yielded better results. Despite the probability of histocompatibility-related issues occurring in our experimental settings, the MNCs + HAC-MSCs transplant group showed a remarkable therapeutic effect. As an evidence in support of the therapeutic effect, it is worth noting that human HGF, GDNF, and IGF are shown to have biological activities in mice (Seishima et al., 2019; Tamura et al., 1989; Zhang et al., 2018). Considering HAC-MSCs have overcome negative factors such as histocompatibility-related problems against mouse and have shown therapeutic effect in this study, we expect that the usage of HAC-MSCs could bring more therapeutic effects to patients with ALS with allogenic administration.
The effects of either MNCs or MSCs transplantation monotherapy are limited, and improvements are expected. In this study, we have shown the advantage of using a combined cell transplantation as the therapeutic strategy as compared with monotherapy with just MNCs or MSCs. Reasons for this advantage can be attributed to the expression of the growth factors including HGF, GDNF, and IGF, and it is this expression that majorly confers the overall therapeutic effects observed in this study. Supplementation of growth factors has been classically attempted for a long time (Azzouz et al., 2004; Dodge et al., 2008; Kaspar et al., 2003; Wang et al., 2002). HGF, GDNF, and IGF are the most widely used growth factors in ALS therapeutic research, as some studies have shown their usage to be effective in mice models for ALS (Dodge et al., 2008; Kaspar et al., 2003; Sun et al., 2002; Wang et al., 2002). Clinical trials have also been conducted for the use of HGF, GDNF, and IGF in the ALS therapy in several countries (Sakowski et al., 2009; Sufit et al., 2017). HGF is known to act on epithelial, endothelial, and hematopoietic cells and is related to organ development and regeneration (Nakamura et al., 2011). In an ALS mouse model, HGF suppressed neuronal apoptosis and degeneration (Sun et al., 2002). GDNF and IGF have also shown to suppress apoptosis of motor neurons in ALS mice (Dodge et al., 2008; Kaspar et al., 2003; Wang et al., 2002). Additionally, GDNF and IGF are shown to induce brain development and neurite extension similar to that induced by the TGF family of growth factors (Connor and Dragunow, 1998). These factors exert their trophic effects through common signaling pathways such as the AKT and ERK pathway by binding to their respective activating receptors: RET/GFRα for GDNF, C-MET for HGF, and IGF-1R for IGF-1. We used these three growth factors simultaneously because the expression of each of these receptors, i.e., c-RET, C-MET, and IGF-1R, is decreased in the spinal cord motor neurons in ALS (Kato et al., 2003; Ryu et al., 2011; Steyn et al., 2012). Since there is a decrease in all the three receptor expression levels, we hypothesized that using just one or two factors would be a weak strategy to obtain a considerable therapeutic effect. Hence, the higher therapeutic effects of using all the three growth factors simultaneously as a treatment strategy in the SOD1-tg mice was considered as an acceptable result by us, as we observed high levels of their expression in the spinal cord of these mice.
Furthermore, five kinds of cytokines, namely, G-CSF, IL-9, MIP-1α, MIG, and VEGF, showed an increased level of expression in the spinal cord of SOD1-tg after MNCs + HAC-MSCs transplantation. These results could explain the increased number of bone marrow-derived MNCs that homed specifically to the spinal cord in the SOD1-tg mice. G-CSF, IL-9, MIP-1α, and MIG are known to promote differentiation, multiplication, and chemotaxis of hematopoietic cells (Keller et al., 1994; Liao et al., 1995; Miller and Krangel, 1992; Wang et al., 1999; Welte et al., 1985). Therefore, these cytokines might also be related to the migration of MNCs in the HAC-MSCs transplant group. G-CSF has also been reported to directly suppress disease progression and improve survival rates in ALS mice models (Pitzer et al., 2008). VEGF is known to promote angiogenesis and is regulated by HGF expression (Xin et al., 2001). Thus, the elevation of VEGF in this study would have been caused due to the overexpression of HGF. VEGF expression has been shown to be induced by hypoxia and oxidative stress (Shweiki et al., 1992). However, VEGF was not elevated under oxidative conditions in an ALS mouse model (Moreau et al., 2006). ALS disease conditions progress faster by the deletion of the hypoxia-response element in the VEGF promoter (Oosthuyse et al., 2001). Therefore, a decrease in VEGF expression is intrinsically related to ALS progression, and supplementation with VEGF has therapeutic potential for ALS. Thus, VEGF induced by HGF may play a pivotal role in the amelioration of the disease in this study.
The theory of non-cell autonomous neuronal cell death is well known in the pathogenesis of neurodegenerative diseases (Ilieva et al., 2009; Tang, 2017). This theory states that immune cells, endothelial cells, and astrocytes surrounding the neurons are also subjected to pathological changes in the course of the disease (Ilieva et al., 2009; Tang, 2017). In our study, the expression of HGF, GDNF, and IGF was increased after the transplantation of HAC-MSCs, but HAC-MSCs were not observed in large numbers in the spinal cord after treatment. Instead, MNCs migrated very well into the spinal cord only in the MNCs + HAC-MSCs transplant group. Therefore, our results clearly indicate that MSCs provided no direct neuroprotective effect but instead indirectly affected the spinal cord promoting chemotaxis of MNCs. This migration could have promoted a change in the spinal cord niche leading to a minimized inflammatory and oxidative condition due to the elevated expression levels of cytokines and growth factors.
Considering all the results together observed in the current study, we suggest a timeline wherein the migration of MNCs and growth factor expression precede histological and behavioral effects of the combination therapy of MNCs and HAC-MSCs transplantation. Therefore, the two events of MNCs migration and growth factor expression could be the reasons to explain the remarkable therapeutic effect observed during this combination therapy. We believe that improvement in the microenvironmental niche of the spinal cord was induced majorly by the HAC-MSCs-derived cytokines promoting the migration of MNCs to the spinal cord region. The expression of the three growth factors that are also from HAC-MSCs confer a direct neuroprotective effect on the neuronal cells and the spinal cord tissue. Thus, we postulate that the simultaneous action of the MNCs migration coupled with the expression of growth factors, all which occur driven by the presence of HAC-MSCs, brought about the distinct and precise therapeutic effect in the ALS mouse models.
Despite recent advances in stem cell therapies, ALS remains non-curable and clinical remission or reversal remains to be achieved. Although bone marrow or MSCs transplantation has already been attempted in ALS mice and patients, their efficacy has been limited (Appel et al., 2008; Kim et al., 2010; Mazzini et al., 2010, 2012; Ohta et al., 2011; Silani et al., 2004; Venturin et al., 2016; Zhang et al., 2009). Therefore, to improve the therapeutic effects, we demonstrated a new therapeutic approach that combines MNCs and MSCs with the overexpression of growth factors such as HGF, GDNF, and IGF, whose efficacy has been reported for ALS (Dodge et al., 2008; Kaspar et al., 2003; Sun et al., 2002; Wang et al., 2002). The results of our combined transplantation strategy have conferred a clear therapeutic advantage, compared with the transplantation of either MNCs or MSCs, which can be attributed to the migration of a large number of MNCs to the spinal cord and also the elevation of several cytokine expression levels. We conclude that growth factor-expressing mesenchymal stem cells improve the therapeutic effects of bone marrow-derived mononuclear cells for ALS. Additionally, we suggest that this therapy may be a promising new strategy for the treatment of ALS patients.
Limitations of the Study
We developed a novel cell therapy for amyotrophic lateral sclerosis (ALS) using combined transplantation of bone marrow-derived mononuclear cells (MNCs) and growth factor-expressing mesenchymal stem cells (HAC-MSCs). The transplanted MNCs with the aid of MSCs expressing HGF, GDNF, and IGF significantly improved their therapeutic efficacy for ALS. Although this novel cell therapy has a high therapeutic potential for the treatment of patients with ALS, it is necessary to further investigate the detailed mechanisms of the effects brought about by this combined transplantation strategy, the safety of artificial chromosome vectors, immortalized MSCs, and the risk of bone marrow transplantations in clinical applications. In particular, the possibility of tumorigenesis with artificial chromosome vectors and immortalized MSCs should be studied in detail, and adaptation to this therapy should be considered carefully owing to the side effects of whole-body irradiation used during bone marrow transplantations.
Resource Availability
Lead Contact
Additional information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Tomoya Terashima (tom@belle.shiga-med.ac.jp).
Materials Availability
Novel materials generated in this study are available from the Lead Contact on reasonable request.
Data and Code Availability
The data that support the findings of this study are available from the Lead Contact on reasonable request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We would like to thank the investigators at the Central Research Laboratory of the Shiga University of Medical Science for their technical support. This work was supported by MEXT KAKENHI grants JP18K07498.
Authors Contribution
Conceptualization, T.T. and Y.W.; Methodology, M.N., N.H., and N.O.; Investigation, T.T., S.K., and M.K.; Visualization, T.T.; Writing – Original Draft, T.T.; Funding Acquisition, T.T.; Resources, Y.W. and M.N.; Supervision, H.K.
Declaration of Interests
The authors declare no competing interests.
Published: November 20, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101764.
Supplemental Information
References
- Abati E., Bresolin N., Comi G., Corti S. Advances, challenges, and perspectives in translational stem cell therapy for amyotrophic lateral sclerosis. Mol. Neurobiol. 2019;56:6703–6715. doi: 10.1007/s12035-019-1554-x. [DOI] [PubMed] [Google Scholar]
- Abati E., Bresolin N., Comi G.P., Corti S. Preconditioning and cellular engineering to increase the survival of transplanted neural stem cells for motor neuron disease therapy. Mol. Neurobiol. 2019;56:3356–3367. doi: 10.1007/s12035-018-1305-4. [DOI] [PubMed] [Google Scholar]
- Appel S.H., Engelhardt J.I., Henkel J.S., Siklos L., Beers D.R., Yen A.A., Simpson E.P., Luo Y., Carrum G., Heslop H.E. Hematopoietic stem cell transplantation in patients with sporadic amyotrophic lateral sclerosis. Neurology. 2008;71:1326–1334. doi: 10.1212/01.wnl.0000327668.43541.22. [DOI] [PubMed] [Google Scholar]
- Azzouz M., Ralph G.S., Storkebaum E., Walmsley L.E., Mitrophanous K.A., Kingsman S.M., Carmeliet P., Mazarakis N.D. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- Baloh R.H., Glass J.D., Svendsen C.N. Stem cell transplantation for amyotrophic lateral sclerosis. Curr. Opin. Neurol. 2018;31:655–661. doi: 10.1097/WCO.0000000000000598. [DOI] [PubMed] [Google Scholar]
- Chen K.S., Sakowski S.A., Feldman E.L. Intraspinal stem cell transplantation for amyotrophic lateral sclerosis. Ann. Neurol. 2016;79:342–353. doi: 10.1002/ana.24584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor B., Dragunow M. The role of neuronal growth factors in neurodegenerative disorders of the human brain. Brain Res. Rev. 1998;27:1–39. doi: 10.1016/s0165-0173(98)00004-6. [DOI] [PubMed] [Google Scholar]
- Dodge J.C., Haidet A.M., Yang W., Passini M.A., Hester M., Clarke J., Roskelley E.M., Treleaven C.M., Rizo L., Martin H. Delivery of AAV-IGF-1 to the CNS extends survival in ALS mice through modification of aberrant glial cell activity. Mol. Ther. 2008;16:1056–1064. doi: 10.1038/mt.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutman S.A., Brown M.B., Glass J.D., Boulis N.M., Johe K., Hazel T., Cudkowicz M., Atassi N., Borqes L., Patil P.G. Long-term Phase 1/2 intraspinal stem cell transplantation outcomes in ALS. Ann. Clin. Transl. Neurol. 2018;5:730–740. doi: 10.1002/acn3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney M.E., Pu H., Chiu A.Y., Dal Canto M.C., Polchow C.Y., Alexander D.D., Caliendo J., Hentati Y.W., Kwon Y.W., Deng H.X. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Ikeno M., Hasegawa Y. Applications of bottom-up human artificial chromosomes in cell research and cell engineering. Exp. Cell Res. 2020;390:111793. doi: 10.1016/j.yexcr.2019.111793. [DOI] [PubMed] [Google Scholar]
- Ilieva H., Polymenidou M., Cleveland D.W. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J. Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar B.K., Lladó J., Sherkat N., Rothstein J.D., Gage F.H. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- Kato S., Funakoshi H., Nakamura T., Kato M., Nakano I., Hirano A., Ohama E. Expression of hepatocyte growth factor and c-Met in the anterior horn cells of the spinal cord in the patients with amyotrophic lateral sclerosis (ALS): immunohistochemical studies on sporadic ALS and familial ALS with superoxide dismutase 1 gene mutation. Acta Neuropathol. 2003;106:112–120. doi: 10.1007/s00401-003-0708-z. [DOI] [PubMed] [Google Scholar]
- Katoh M., Ayabe F., Norikane S., Okada T., Masumoto H., Horike S., Shirayoshi Y., Oshimura M. Construction of a novel human artificial chromosome vector for gene delivery. Biochem. Biophys. Res. Commun. 2004;321:280–290. doi: 10.1016/j.bbrc.2004.06.145. [DOI] [PubMed] [Google Scholar]
- Keller J.R., Bartelmez S.H., Sitnicka E., Ruscetti F.W., Ortiz M., Gooya J.M., Jacobsen S.E. Distinct and overlapping direct effects of macrophage inflammatory protein-1α and transforming growth factor β on hematopoietic progenitor/stem cell growth. Blood. 1994;84:2175–2181. [PubMed] [Google Scholar]
- Kim H., Kim H.Y., Choi M.R., Hwang S., Nam K.H., Kim H.C., Han J.S., Kim K.S., Yoon H.S., Kim S.H. Dose-dependent efficacy of ALS-human mesenchymal stem cells transplantation into cisterna magna in SOD1-G93A ALS mice. Neurosci. Lett. 2010;468:190–194. doi: 10.1016/j.neulet.2009.10.074. [DOI] [PubMed] [Google Scholar]
- Kim K.S., Lee H.J., An J., Kim Y.B., Ra J.C., Lim I., Kim S.U. Transplantation of human adipose tissue-derived stem cells delays clinical onset and prolongs life span in ALS mouse model. Cell Transpl. 2014;23:1585–1597. doi: 10.3727/096368913X673450. [DOI] [PubMed] [Google Scholar]
- Kouprina N., Tomilin A.N., Masumoto H., Earnshaw W.C., Larionov V. Human artificial chromosome-based gene delivery vectors for biomedicine and biotechnology. Expert Opin. Drug Deliv. 2014;11:517–535. doi: 10.1517/17425247.2014.882314. [DOI] [PubMed] [Google Scholar]
- Krakora D., Mulcrone P., Meyer M., Lewis C., Bernau K., Gowing G., Zimprich C., Aebischer P., Stevendsen C.N., Suzuki M. Synergistic effects of GDNF and VEGF on lifespan and disease progression in a familial ALS rat model. Mol. Ther. 2013;21:1602–1610. doi: 10.1038/mt.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao F., Rabin R.L., Yannelli J.R., Koniaris L.G., Vanguri P., Farber J.M. Human mig chemokine: biochemical and functional characterization. J. Exp. Med. 1995;182:1301–1314. doi: 10.1084/jem.182.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrané J., Sahawneh M.A., Przedborski S., Estévez Á.G., Manfredi G. Mitochondrial dynamics and bioenergetic dysfunction is associated with synaptic alterations in mutant sod1 motor neurons. J. Neurosci. 2012;32:229–242. doi: 10.1523/JNEUROSCI.1233-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzini L., Ferrero I., Luparello V., Rustichelli D., Gunetti M., Mareschi K., Testa L., Stecco A., Tarletti R., Miqlioretti M. Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: a Phase I clinical trial. Exp. Neurol. 2010;223:229–237. doi: 10.1016/j.expneurol.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Mazzini L., Mareschi K., Ferrero I., Miglioretti M., Stecco A., Servo S., Carriero A., Monaco F., Faqioli F. Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: a long-term safety study. Cytotherapy. 2012;14:56–60. doi: 10.3109/14653249.2011.613929. [DOI] [PubMed] [Google Scholar]
- McCauley M.E., Baloh R.H. Inflammation in ALS/FTD pathogenesis. Acta Neuropathol. 2019;137:715–730. doi: 10.1007/s00401-018-1933-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.D., Krangel M.S. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit. Rev. Immunol. 1992;12:17–46. [PubMed] [Google Scholar]
- Mitchell J., Borasio G. Amyotrophic lateral sclerosis. Lancet. 2007;369:2031–2041. doi: 10.1016/S0140-6736(07)60944-1. [DOI] [PubMed] [Google Scholar]
- Moreau C., Devos D., Brunaud-Danel V., Defebvre L., Perez T., Destée A., Tonnel A.B., Lassalle P., Just N. Paradoxical response of VEGF expression to hypoxia in CSF of patients with ALS. J. Neurol. Neurosurg. Psychiatry. 2006;77:255–257. doi: 10.1136/jnnp.2005.070904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi M., Watanabe Y., Honda N., Une M., Kazuki K., Kazuki Y., Terashima T., Katagi M., Nakashima K., Hanajima R. Dynamics of host and graft after cell sheet transplantation: basic study for the application of amyotrophic lateral sclerosis. Brain Res. 2019;1724:146444. doi: 10.1016/j.brainres.2019.146444. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Sakai K., Nakamura T., Matsumoto K. Hepatocyte growth factor twenty years on: much more than a growth factor. J. Gastroenterol. Hepatol. 2011;26:188–202. doi: 10.1111/j.1440-1746.2010.06549.x. [DOI] [PubMed] [Google Scholar]
- Ohta Y., Nagai M., Miyazaki K., Tanaka N., Kawai H., Mimoto T., Morimoto N., Kurata T., Ikeda Y., Matsuura T., Abe K. Neuroprotective and angiogenic effects of bone marrow transplantation combined with granulocyte colony-stimulating factor in a mouse model of amyotrophic lateral sclerosis. Cell Med. 2011;2:69–84. doi: 10.3727/215517910X582779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosthuyse B., Moons L., Storkebaum E., Beck H., Nuyens D., Brusselmans K., Van Dorpe J., Hellings P., Gorselink M., Heymans S. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat. Genet. 2001;28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- Pasinelli P., Brown R.H. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat. Rev. Neurosci. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- Pitzer C., Krüger C., Plaas C., Kirsch F., Dittgen T., Müller R., Laage R., Kastner S., Suess S., Spoelgen R. Granulocyte-colony stimulating factor improves outcome in a mouse model of amyotrophic lateral sclerosis. Brain. 2008;131:3335–3347. doi: 10.1093/brain/awn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberecht W., Philips T. The changing scene of amyotrophic lateral sclerosis. Nat. Rev. Neurosci. 2013;14:248–264. doi: 10.1038/nrn3430. [DOI] [PubMed] [Google Scholar]
- Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A., Donaldson D., Goto J., O'Reqan J.P., Deng H.X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Ryu H., Jeon G.S., Cashman N.R., Kowall N.W., Lee J. Differential expression of c-Ret in motor neurons versus non-neuronal cells is linked to the pathogenesis of ALS. Lab. Invest. 2011;91:342–352. doi: 10.1038/labinvest.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakowski S.A., Schuyler A.D., Feldman E.L. Insulin-like growth factor-I for the treatment of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2009;10:63–73. doi: 10.1080/17482960802160370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seishima R., Leung C., Yada S., Murad K.B.A., Tan L.T., Hajamohideen A., Tan S.H., Itoh H., Murakami K., Ishida Y. Neonatal Wnt-dependent Lgr5 positive stem cells are essential for uterine gland development. Nat. Commun. 2019;10:5378. doi: 10.1038/s41467-019-13363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimohara T., Kazuki K., Ogonuki N., Morinoto H., Matoba S., Hiramatsu K., Honma K., Suzuki T., Hara T., Ogura A. Transfer of a mouse artificial chromosome into spermatogonial stem cells generates transchromosomic mice. Stem Cell Rep. 2017;9:1180–1191. doi: 10.1016/j.stemcr.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shweiki D., Itin A., Soffer D., Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Silani V., Cova L., Corbo M., Ciammola A., Polli E. Stem-cell therapy for amyotrophic lateral sclerosis. Lancet. 2004;364:200–202. doi: 10.1016/S0140-6736(04)16634-8. [DOI] [PubMed] [Google Scholar]
- Sinenko S.A., Ponomartsev S.V., Tomilin A.N. Human artificial chromosomes for pluripotent stem cell-based tissue replacement therapy. Exp. Cell Res. 2020;389:111882. doi: 10.1016/j.yexcr.2020.111882. [DOI] [PubMed] [Google Scholar]
- Steyn F.J., Ngo S.T., Lee J.D., Leong J.W., Buckley A.J., Veldhuis J.D., McCombe P.A., Chen C., Bellingham M.C. Impairments to the GH-IGF-I axis in hSOD1 G93A mice give insight into possible mechanisms of GH dysregulation in patients with amyotrophic lateral sclerosis. Endocrinology. 2012;153:3735–3746. doi: 10.1210/en.2011-2171. [DOI] [PubMed] [Google Scholar]
- Sufit R.L., Ajroud-Driss S., Casey P., Kessler J.A. Open label study to assess the safety of VM202 in subjects with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemperol Degener. 2017;18:269–278. doi: 10.1080/21678421.2016.1259334. [DOI] [PubMed] [Google Scholar]
- Sun W., Funakoshi H., Nakamura T. Overexpression of HGF retards disease progression and prolongs life span in a transgenic mouse model of ALS. J. Neurosci. 2002;22:6537–6548. doi: 10.1523/JNEUROSCI.22-15-06537.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Kazuki Y., Hara T., Oshimura M. Current advances in microcell-mediated chromosome transfer technology and its applications. Exp. Cell Res. 2020;390:111915. doi: 10.1016/j.yexcr.2020.111915. [DOI] [PubMed] [Google Scholar]
- Tamura K., Kobayashi M., Ishii Y., Tamura T., Hashimoto K., Nakamura S., Niwa M., Zapf J. Primary structure of rat insulin-like growth factor-I and its biological activities. J. Biol. Chem. 1989;264:5616–5621. [PubMed] [Google Scholar]
- Tang B.L. The use of mesenchymal stem cells (MSCs) for amyotrophic lateral sclerosis (ALS) therapy - a perspective on cell biological mechanisms. Rev. Neurosci. 2017;28:725–738. doi: 10.1515/revneuro-2017-0018. [DOI] [PubMed] [Google Scholar]
- Tanna T., Sachan V. Mesenchymal stem cells: potential in treatment of neurodegenerative diseases. Curr. Stem Cell Res. Ther. 2014;9:513–521. doi: 10.2174/1574888x09666140923101110. [DOI] [PubMed] [Google Scholar]
- Terashima T., Kojima H., Urabe H., Yamakawa I., Ogawa N., Kawai H., Chan L., Maegawa H. Stem cell factor-activated bone marrow ameliorates amyotrophic lateral sclerosis by promoting protective microglial migration. J. Neurosci. Res. 2014;92:856–869. doi: 10.1002/jnr.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu P.H., Raju P., Robinson K.A., Gurney M.E., Trojanowski J.Q., Lee V.M.Y. Transgenic mice carrying a human mutant superoxide dismutase transgene develop neuronal cytoskeletal pathology resembling human amyotrophic lateral sclerosis lesions. Proc. Natl. Acad. Sci. U S A. 1996;93:3155–3160. doi: 10.1073/pnas.93.7.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturin G.T., Greggio S., Zanirati G., Marinowic D.R., de Oliveira I.M., Pêgas Henriques J.A., DaCosta J.C. Transplantation of bone marrow mononuclear cells prolongs survival, delays disease onset and progression and mitigates neuronal loss in pre-symptomatic, but not symptomatic ALS mice. Neurosci. Lett. 2016;633:182–188. doi: 10.1016/j.neulet.2016.09.030. [DOI] [PubMed] [Google Scholar]
- Wang L.J., Lu Y.Y., Muramatsu S., Ikeguchi K., Fujimoto K., Okada T., Mizukami H., Matsushita T., Hanazono Y., Kume A. Neuroprotective effects of glial cell line-derived neurotrophic factor mediated by an adeno-associated virus vector in a transgenic animal model of amyotrophic lateral sclerosis. J. Neurosci. 2002;22:6920–6928. doi: 10.1523/JNEUROSCI.22-16-06920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.Y., Gelfanov V., Sun H.B., Tsai S., Yang Y.C. Distinct actions of interleukin-9 and interleukin-4 on a hematopoietic stem cell line. Emlc1. Exp. Hematol. 1999;27:139–146. doi: 10.1016/s0301-472x(98)00002-2. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Kazuki Y., Kazuki K., Ebiki M., Nakanishi M., Nakamura K., Yoshida-Yamanaka M., Hosokawa H., Ohbayashi T., Oshimura M. Use of a human artificial chromosome for delivering trophic factors in a rodent model of amyotrophic lateral sclerosis. Mol. Ther. 2015;4:e253. doi: 10.1038/mtna.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte K., Platzer E., Gabrilove J.L., Lu L., Levi E., Polivka A., Mertelsmann R., Moore M.A. Purification to apparent homogeneity and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Hamatol. Bluttransfus. 1985;29:398–401. doi: 10.1007/978-3-642-70385-0_82. [DOI] [PubMed] [Google Scholar]
- Xin X., Yang S., Ingle G., Zlot C., Rangell L., Kowalski J., Schwall R., Ferrara N., Gerritsen M.E. Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am. J. Pathol. 2001;158:1111–1120. doi: 10.1016/S0002-9440(10)64058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Zhou C., Teng J.J., Zhao R.L., Song Y.Q. Multiple administrations of human marrow stromal cells through cerebrospinal fluid prolong survival in a transgenic mouse model of amyotrophic lateral sclerosis. Cytotherapy. 2009;11:299–306. doi: 10.1080/14653240902806986. [DOI] [PubMed] [Google Scholar]
- Zhang W., Wan H., Feng G., Qu J., Wang J., Jing Y., Ren R., Liu Z., Zhang L., Chen Z. SIRT6 deficiency results in developmental retardation in cynomolgus monkeys. Nature. 2018;560:661–665. doi: 10.1038/s41586-018-0437-z. [DOI] [PubMed] [Google Scholar]
- Zou Z.Y., Zhou Z.R., Che C.H., Liu C.Y., He R.L., Huang H.P. Genetic epidemiology of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatr. 2017;88:540–549. doi: 10.1136/jnnp-2016-315018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the Lead Contact on reasonable request.