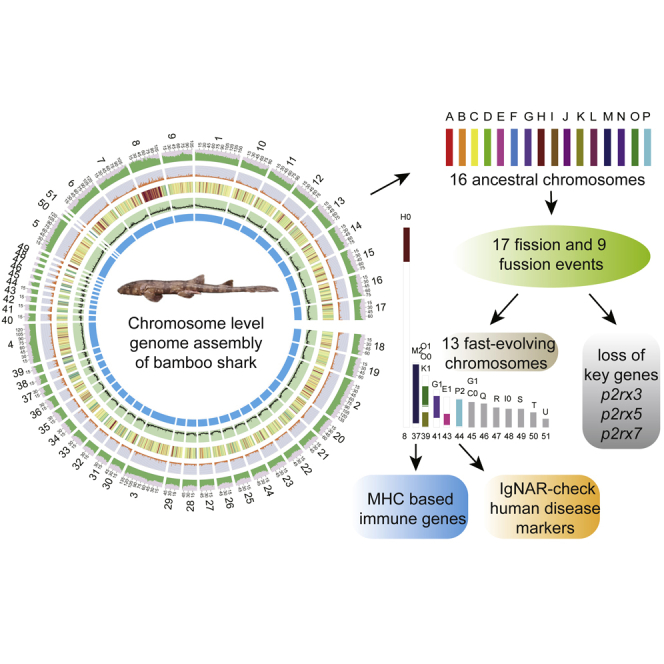
Summary
Chondrichthyan (cartilaginous fish) occupies a key phylogenetic position and is important for investigating evolutionary processes of vertebrates. However, limited whole genomes impede our in-depth knowledge of important issues such as chromosome evolution and immunity. Here, we report the chromosome-level genome of white-spotted bamboo shark. Combing it with other shark genomes, we reconstructed 16 ancestral chromosomes of bamboo shark and illustrate a dynamic chromosome rearrangement process. We found that genes on 13 fast-evolving chromosomes can be enriched in immune-related pathways. And two chromosomes contain important genes that can be used to develop single-chain antibodies, which were shown to have high affinity to human disease markers by using enzyme-linked immunosorbent assay. We also found three bone formation-related genes were lost due to chromosome rearrangements. Our study highlights the importance of chromosome rearrangements, providing resources for understanding of cartilaginous fish diversification and potential application of single-chain antibodies.
Subject Areas: Biological Sciences, Genetics, Genomics, Phylogenetics, Evolutionary Biology
Graphical Abstract
Highlights
-
•
Inferred ancestral chromosome karyotypes of cartilaginous fish
-
•
Chromosome rearrangements resulted in fast-evolving chromosomes and immune genes
-
•
Chromosome rearrangements led to deletion of bone formation-related genes
-
•
Proved that single-domain antibodies in shark have great potential application
Biological Sciences; Genetics; Genomics; Phylogenetics; Evolutionary Biology
Introduction
The white-spotted bamboo shark, Chiloscyllium plagiosum, (hereinafter referred to as bamboo shark) belongs to the class of Chondrichthyes, which is one of the oldest extant jawed vertebrate groups (McKenna, 1988). Cartilaginous fishes including Elasmobranchii and Holocephali shared a common ancestor with other vertebrates about 460–520 Ma, and then evolved independently to distinct lineages (Inoue et al., 2010). The phylogenetic evolution of cartilaginous fishes has been disputed for a long time (Cao et al., 1998; Janvier, 1996; Rasmussen and Arnason, 1999; Zardoya et al., 1998), especially the evolutionary relationships with bony fishes. Furthermore, most cartilaginous fishes have various chromosome karyotypes (2n = 66–104) (Rocco et al., 2003; Schwartz and Maddock, 1986), revealing interesting chromosome evolution processes. As known, immunoglobulins or lymphocyte receptors-based adaptive immunity is restricted to vertebrates (Litman et al., 2010). And as one of the extant early branching jawed vertebrates, cartilaginous fishes developed special immunity (for example, sharks comprise heavy-chain immunoglobulins, which are different from canonical antibodies consisting of both heavy and light chains, Könning et al., 2017), which makes them “immunologist's delight.”
The bamboo shark is a nocturnal reef-dwelling species and widely distributed in the Indo-West Pacific from India to Indonesia, southern China, and Japan (Kyne and Burgess, 2006). Its biological features, including docile nature, small body size (24–37 inches in length), convenient reproductivity, and longevity make it ideal for research. Its special immunoglobulins are superior in biological and medical applications and have been proposed for developing antibody drugs (Wesolowski et al., 2009; Zielonka et al., 2014). Despite its biological and application importance, previous researchers mostly focused on addressing its biology of hematology, reproduction, muscle activity, liver regeneration, and anatomy (Alexander et al., 2016; Maia and Wilga, 2013; Straube et al., 2016). Limited whole-genome sequencing of cartilaginous fishes including elephant shark (2014) (Venkatesh et al., 2014), whale shark (2017) (Read et al., 2017), brownbanded bamboo shark (2018) (Hara et al., 2018), cloudy catshark (2018) (Hara et al., 2018), and white shark (2019) (Marra et al., 2019) prevents our further understanding of genetic mechanisms for these species. Furthermore, these five genomes were all assembled to scaffold level, which also limits our investigation of chromosome rearrangements.
To better understand the evolution and special immunity of cartilaginous fishes, we sequenced and assembled a chromosome-level genome of a female bamboo shark, identifying dynamic chromosome rearrangement events and related evolutionary consequences. We carefully analyzed evolution of immune-related genes, which will provide new resources to understand their biology and applications in immunology. We also found that chromosome rearrangements delete three important bone formation-related genes, which may interpret chondrified endoskeleton of cartilaginous fishes.
Results
Genome Assembly and Annotation
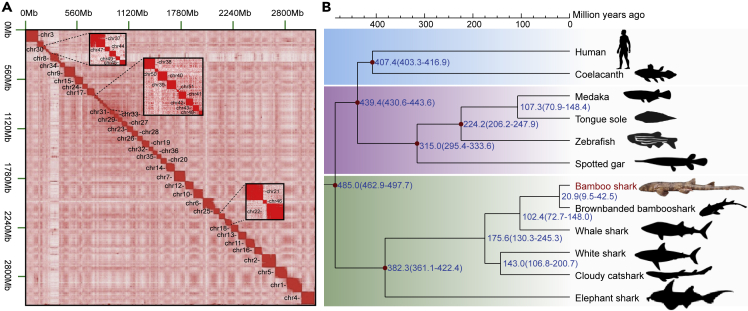
We assembled a 3.85-Gb genome assembly with 51 chromosomes supported by chromatin interaction relationships with Hi-C sequencing data (Figure 1A and Tables S1 and S2) and karyotype analysis (Ma et al., 2008), and we annotated 19,595 protein coding genes (Table S3) and 63.53% of repeat content (Table S4) in this genome assembly. Comparison of repeat content among cartilaginous fishes and bony fishes shows that cartilaginous fish genomes contain higher proportion of repeated sequences (Table S5). The GC content and repeat and gene density distributed in 51 chromosomes are shown in Figures S1 and S2. And ~95.8% of the Benchmarking Universal Single-Copy Orthologs (BUSCOs) (Simao et al., 2015) were identified to be complete in this genome (Table S6). Syntenic relationships revealed unambiguous alignments of 41 bamboo shark chromosomes to 29 chromosomes of chicken (the tetrapod species with most stable karyotypes, Ellegren, 2010, Figure S3), whereas the alignments between bamboo shark and zebrafish chromosomes (Figure S4) are disordered. Extensive inter-chromosomal rearrangements have been described previously in zebrafish genome (Kasahara et al., 2007). Therefore, these results suggest the bamboo shark genome also kept relatively conserved chromosome karyotypes without many inter-chromosomal rearrangements.
Figure 1.
Genome Assembly and Phylogeny of Bamboo Shark
(A) Heatmap of chromatin interaction relationships at 125 kb resolution of 51 chromosomes.
(B) Phylogenetic tree and divergence time estimation of cartilaginous fish and representative bony fishes. Red dots mean reference times from TimeTree database (http://www.timetree.org/).
The assembled genome size of bamboo shark is ~3.85 Gb, larger than elephant shark (~974 Mb) (Venkatesh et al., 2014), whale shark (~2.93 Gb) (Read et al., 2017), and most bony fishes (340 Mb–2.97 Gb). Whole-genome duplication (WGD) is one of reasons that result in larger genome sizes (Grover and Wendel, 2010). Thus we investigate whether there is a third WGD event in bamboo shark apart from the common two rounds of WGD of vertebrates (Grover and Wendel, 2010). First, we found only one peak on the 4-fold synonymous third-codon transversion rates (4DTv) distribution of bamboo shark genome, which represented the recent common WGD event of all vertebrates (Figure S5). We also checked the 4DTv distribution of elephant shark and zebrafish, finding that elephant shark also has only one peak, which is similar to bamboo shark, but zebrafish has another peak representing the third WGD of teleost fish (Glasauer and Neuhauss, 2014), indicating the reliability of our results. Second, we checked HOX (homeobox) genes, which are highly conserved in vertebrates and always clustered together (Santini et al., 2003) and thus have become reliable markers of WGD events (Kuraku, 2011). We only identified three HOX clusters in bamboo shark, compared with seven clusters in zebrafish, which experienced a third WGD. Similar HOX clusters were also found in cattle, whale shark and elephant shark genomes (Figure S6). These two results suggest that bamboo shark genome did not experience a third WGD event. Therefore, the larger genome size of bamboo shark should result from the burst of repeated sequences.
Molecular Phylogeny
In consideration of the important evolutionary position of cartilaginous fishes, we inferred phylogenetic relationship of bamboo shark with other five cartilaginous fishes, five representative bony fishes (four ray-finned fishes and one lobefin fish), and humans. Based on coding sequences of 823 single-copy orthologous genes identified using TreeFam (Li et al., 2006), we constructed phylogenetic trees using both maximum-likelihood and Bayesian methods and generated identical results (Figure 1B). The tree topology for cartilaginous and bony fishes is consistent with previous researches (Hara et al., 2018; Marra et al., 2019). And we estimated divergence times of cartilaginous and bony fishes, Elasmobranchii and Holocephali; white spotted bamboo shark; and brownbanded bamboo shark to be about 485.0, 382.3, and 20.9 Ma, respectively.
Reconstruct Ancestral Chromosome
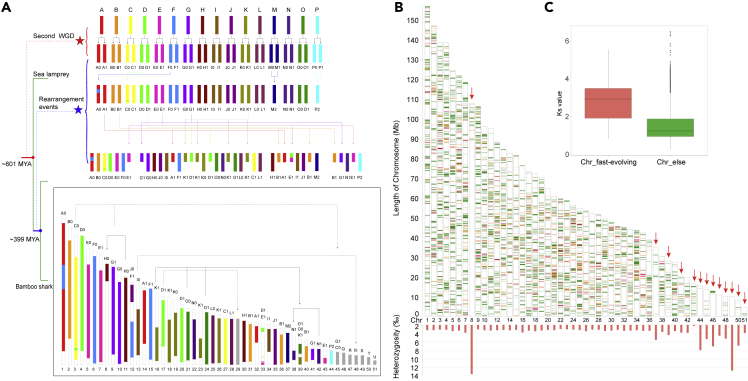
This chromosome-level genome makes it possible to study chromosome evolution of cartilaginous fishes. Thus we reconstructed ancestral chromosome karyotypes of cartilaginous fishes by identifying paralogous and orthologous genes between the bamboo shark and elephant shark genomes (Venkatesh et al., 2014) following a previously described method (Salse et al., 2009) (Table S7). Finally, we constructed 16 putative ancestral chromosomes and illustrated an evolutionary scenario during which eight fission and five fusion events occurred (Figure 2A, colored arrows), possibly for all cartilaginous fishes. As for the bamboo shark, nine fission and four fusion events (black and dotted arrows) occurred, resulting in six candidate daughter chromosomes (Chr8, Chr29, Chr38 and Chr39, Chr45, Chr48) (Figures 2A and S7 and Table S7). All these rearrangements ultimately gave rise to 51 chromosomes of the bamboo shark genome.
Figure 2.
Chromosome Evolution of Bamboo Shark
(A) Construction of the ancestral chromosome model of elephant shark and bamboo shark. The lines on the left represent the phylogenetic tree. The dotted lines on the left represent the second WGD event for vertebrates and rearrangement events of ancestral chromosomes of sharks. Letters from A to P represent constructed ancestral chromosomes. Letters Q, R, S, T, and U represent small chromosomes that may be the result of more recent fissions from the larger chromosomes. The colored arrows represent rearrangements of chromosomes. The black arrows represent rearrangements specific to the bamboo shark. The dotted arrows represent potential rearrangements that are supported by gene pairs. The red star represents the second whole-genome duplication occurred before the divergence of sea lamprey and gnathostome (Smith et al., 2013) (earlier than ~601 Ma). The blue star represents rearrangement events of shark ancestral chromosomes. The times are cited from the TimeTree database.
(B) (Top): Distribution of conserved genes of elephant shark, bamboo shark, whale shark, brownbanded bamboo shark, cloudy catshark, white shark and medaka. Magenta, conserved regions shared by four species (R1). Orange, common regions in six shark genomes excluding R1. Green, regions shared between the bamboo shark and medaka excluding R1. The red arrows point out 13 fast-evolving chromosomes. (Bottom) Heterozygosity of 51 chromosomes in bamboo shark genome.
(C) Comparison of Ks values of single-copy orthologous genes between 13 fast-evolving chromosomes and other chromosomes.
Fast-Evolving Chromosomes and Immune Genes
Plenty of chromosome rearrangements play a role in fast-evolving gene families and in fostering large-scale changes in gene order (Eichler and Sankoff, 2003). To identify potential causes and consequences of dynamic chromosome rearrangements in cartilaginous fishes, we further analyzed distribution of conserved protein-coding genes of cartilaginous fishes along bamboo shark chromosomes. We identified 2,323 orthologous genes (~12.90% of total genes) shared among bamboo shark, elephant shark, whale shark, brown-banded bamboo shark, cloudy catshark, and white shark (Figure S8). After exclusion of genes shared among these six cartilaginous fishes and representative bony fishes (medaka, Kasahara et al., 2007, Figure S9, and spotted gar Braasch et al. 2015, Figure S10), we finally identified 1,359 genes conserved only in cartilaginous fishes (Figure 2B). Interestingly, we found those genes to be unevenly distributed along bamboo shark chromosomes with conserved genes on chromosomes 8, 37, 39, 41, 43, 44, 45, 46, 47, 48, 49, 50, and 51—notably fewer than (average: 2.6 genes) those of other chromosomes (average: 34.9 genes, (Mann-Whitney U test, p value < 0.001) (Figures 2B and Table S8). We then evaluated the evolutionary rate by calculating KS (synonymous substitutions per synonymous site) values of orthologous genes on these 13 chromosomes (mean KS value: 2.79), which was significantly higher than that of other chromosomes (mean KS value: 1.54, Mann-Whitney U test, p value < 0.001, Figure 2C). In addition, we found heterozygous SNPs in the genome of this individual we sequenced to be notably more frequent on these 13 chromosomes (except Chr43) than other chromosomes (Mann-Whitney U test, p value < 0.001, Figure 2B). All these findings suggest that these 13 chromosomes are fast-evolving. Enrichment analysis (according to the Kyoto Encyclopedia of Genes and Genomes [KEGG]-assigned gene functions and pathways) showed that genes on these 13 fast-evolving chromosomes are significantly enriched in immune-related pathways with 171 immune-related genes (p value < 0.01, Tables S9 and S10). These include allograft rejection, antigen processing and presentation, as well as intestinal immune network for IgA production.
Analysis of MHC-Related Genes
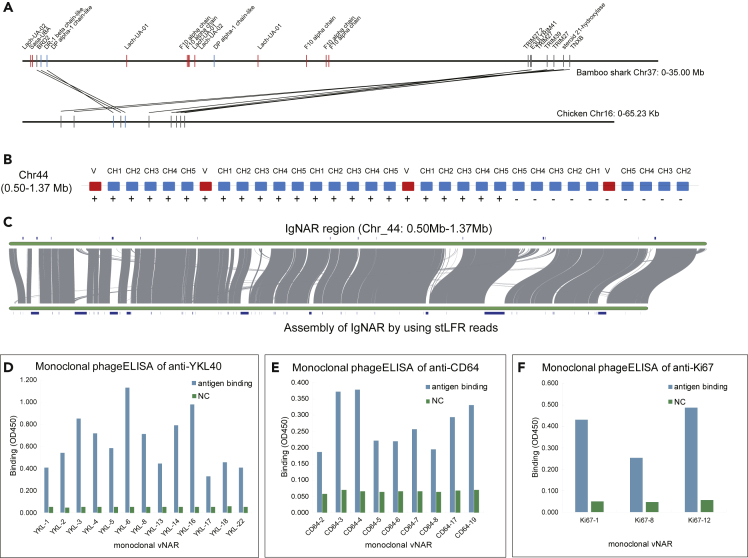
Among these 13 fast-evolving chromosomes, we found that Chr37 and Chr44 likely underwent a special self-fusion event after a possible chromosome or large segment duplication event (Figure 2A). We also found that major histocompatibility complex (MHC) genes (11 class I and 3 class II genes) are notably enriched on Chr37 (11 genes, Figure 3A), except for those on unanchored scaffolds. MHC genes were not found in the amphioxus genome, whereas one fragment of a possible MHC class II gene was found in sea lamprey (Gene ID: PMZ_0007681-RA; KEGG function: K06752 MHC, class II) (Smith et al., 2018). Upon further investigation of MHC gene numbers in other species, we found both MHC class I and class II genes in cartilaginous fishes and bony fishes except for the elephant shark genome, which lacked MHC class II genes according to our analysis (Tables S11, S12, and S13 and Figure S11). These results suggest that the innate immune system accompanied with adaptive system based on variable lymphocyte receptors (Pancer et al., 2004; Saha et al., 2010) played a major role in defending against infections in amphioxus and sea lamprey, whereas cartilaginous and bony fishes evolved with acquiring a complete MHC-based adaptive immune system. The differences in these immune systems may have arisen from the fast-evolving chromosomes. Moreover, we suggest that MHC class II genes were likely acquired before MHC class I genes based on our identification of an MHC class II-like fragment in the starlet sea anemone genome (NCBI Accession: XP_001628845.1, identified by aligning PMZ_0007681-RA using BLAST, Altschul et al., 1990) and sea lamprey genome (Smith et al., 2018), potentially resolving a long debate about MHC evolution (Flajnik et al., 1991; Kaufman, 1988, 2011, 2018; Kaufman et al., 1984; Rock et al., 2016; Zhang et al., 2014). In addition, we found that tripartite motif-containing protein 69 (TRIM69) gene family was expanded significantly in cartilaginous fishes (average 18 copies) compared with ray-finned fishes (less than 3 copies) (Figure S12). Also, in bamboo shark genome, 13 copies of TRIM69 were also located on Chr37. TRIM69 can function as an antiviral defense (Rihn et al., 2019; Wang et al., 2018), playing important roles in innate immune system and class I MHC-mediated antigen processing and presentation.
Figure 3.
Specific Immune-Related Genes of Bamboo Shark
(A) Distribution of MHC genes on chromosome 37. The red and blue rectangles represent MHC class I and class II genes, respectively. The gray rectangles represent non-MHC genes. Here, we only show syntenic genes and shark MHC genes compared with chicken.
(B) Distribution of identified IgNAR loci on chromosome 44.
(C) Syntenic relationship of assemblies of IgNAR region by using shotgun WGS and stLFR data. The gray lines represent consistent region. The blue rectangles represent gaps.
(D, E, and F) Binding efficiency of cloned vNARs to human disease markers (D) YKL40, (E) CD64, and (F) Ki67 by using monoclonal phage ELISA. NC represents negative control. The vertical axis represents the signal of binding measured using optical density (OD450).
Identification and Amplification of IgNAR
In contrast to MHC genes found on Chr37, we identified that the immunoglobulin new antigen receptor (IgNAR) (Feige et al., 2014) gene loci (four complete IgNAR structure, V-CH1-CH2-CH3-CH4-CH5, and two incomplete IgNAR) was located on Chr44 (Figure 3B). To obtain and verify the complete sequence assembly of IgNAR region, we sequenced ~124-fold new single-tube long fragment reads (stLFR) (Wang et al., 2019) to re-assemble it. The good syntenic relationship (Figure 3C) and the distribution of paired-end reads (Figure S13) reveal high-quality assembly of IgNAR region. Because of the application potential of single-domain antibodies (sdAbs) in biotechnical and therapeutic use, we tried to check the diagnostic potential of IgNAR in bamboo shark. We first designed primers (Table S14) based on IgNAR sequences of bamboo shark and specifically amplified the variable domain of New Antigen Receptors (vNARs) from peripheral blood leukocytes and spleen tissue of five bamboo shark individuals. Amplified vNARs were inserted into phagemid vector pMECS and then into E. coli TG1 competent cells to produce the vNAR-phage display library. Sanger sequencing of randomly selected ~100 clones shows low repetition and high diversity of vNARs, especially the complementarity-determining region 3 (CDR3) (Figure S14). We then chose several human disease biomarkers: YKL40 (Kastrup, 2012; Rathcke and Vestergaard, 2009) for cardiovascular disease, CD64 for infectious disease (Hoffmann, 2009), and Ki67 for lung cancer (Li et al., 2015) as targets of four rounds screened monoclonal vNARs. Monoclonal phage enzyme-linked immunosorbent assay (ELISA) shows high affinity to targets of those positive clones. In detail, we obtained 13 unique YKL40-binding clones with signal at least ~6-fold (highest: ~20-fold) than negative control (NC) (Figures 3D), 9 unique CD64-binding clones with signal at least ~3-fold than NC (Figure 3E), and 3 unique Ki67-binding clones with signal at least ~5-fold than NC (Figure 3F). Although further study should be carried out to verify the affinity of those positive clones and their diagnostic function by using real samples, we believe that this work is of significance for using genome data to develop sdAbs.
P2X Gene Family
Chromosome rearrangements would remove genes that may first become pseudogenes because selective pressure acting on them was relaxed when new phenotypic traits arose or they may have very little effect on its adaptations. Syntenic comparison among chicken, zebrafish, medaka, and bamboo shark showed at least four possible genome rearrangement events occurred in bamboo shark that may result in the deletion of gene, p2rx5 (Purinergic Receptor P2X, Ligand-Gated Ion Channel, 5) after this gene became redundant or non-functioning due to selective pressures acting on it (Figure 4A). The loss of this gene was also supported by checking RNA sequence data of 14 tissues including blood, eye, gill, heart, liver, muscle, spleen, stomach, dorsal fin, tail fin, pancreas, leptospira, two capsulogenous gland, and two kidney samples. And this gene has been previously reported to be involved in bone development and homeostasis (Nicolaidou et al., 2012; Sitcheran et al., 2003; Solle et al., 2001; Sun et al., 2013; Syberg et al., 2012; Takahashi et al., 1988; Thaler et al., 2014). Furthermore, analysis of the whole gene family of purinergic receptor P2X in sea lamprey, six sharks, and representative bony fishes showed that p2rx3, p2rx5, and p2rx7 genes were lost in six cartilaginous fishes, whereas at least six paralogs (p2rx1, p2rx2, p2rx3, p2rx4, p2rx5, p2rx7) with multiple copies were found in bony fishes (Figure 4B and Tables S15 and S16). P2X receptors contain ligand-gated ion channels and activate receptor triggers signaling pathways associated with Ca2+ influx (Burnstock, 2012; Jing et al., 2014; Rodrigues-Ribeiro et al., 2015). Moreover, p2rx3, p2rx5, and p2rx7 receptors have been shown to participate in differentiation and proliferation of osteoblast (Nakamura et al., 2000; Nicolaidou et al., 2012; Rodrigues-Ribeiro et al., 2015), bone formation, and resorption (Grol et al., 2009; Kim et al., 2018; Syberg et al., 2012). However, p2rx1 receptor negatively regulates bone mineralization (Lenertz et al., 2015). P2rx2 receptor, which mainly functions in sensory neurons, neuromuscular junction formation, and hearing (Yan et al., 2013), has nothing to do with bone formation. P2rx4 also functions in response to ATP binding, and there are few researches that show its role in bone formation to date. Thus, it is reasonable to infer that loss of those genes, together with loss of spp1 gene identified previously (Venkatesh et al., 2014), may further explain the establishment of chondrification of the endoskeleton in cartilaginous fishes.
Figure 4.
The Loss of p2rx3, p2rx5, and p2rx7 Genes in Cartilaginous Fishes
(A) The syntenic relationship of the p2rx5 region among bamboo shark, chicken, medaka, and zebrafish. The red rectangles represent P2X genes. The lines represent syntenic genes. The numbers besides the pink dots represent gene number in the gap regions. From the figure, we can see the gene loss of p2rx5 in bamboo shark genome after at least four chromosome rearrangements.
(B) Phylogenetic tree of the P2X gene family. Dan: zebrafish, GAD: Atlantic cod, Gas: three-spined sticklebacks, Ory: medada, Lat: coelacanth, Sal: Atlantic salmon, Fug: torafugu, Lar: large yellow croaker, Rhi: whale shark, Cal: elephant shark, Bam: bamboo shark, Chp: brownbanded bamboo shark, Cac: white shark, Scy: cloudy catshark, PMZ: sea lamprey. The shark's P2X genes are marked red. Although many p2rx5 genes exist in PMZ, all these genes were less than 246 aa in length (10 of 14 less than 200 aa). This tree was constructed with protein sequences of these genes by using software of MUSCLE (v3.8.31) (Edgar, 2004) and FastTree (v2.1.10) (Price et al., 2010).
Discussion
Because of the therapeutic potential of single-domain antibodies, sharks have drawn scientists' interest for many years. With ideal biological features of bamboo shark, we selected this species and were able to obtain high-quality samples for further research. Combining paired-end, mate-paired, stLFR, and Hi-C sequencing strategies, we successfully assembled a chromosome-level reference genome of bamboo shark. In the present study, we mainly focused chromosome evolution and t fast-evolving chromosomes and immune gene families of cartilaginous fishes. Also, we investigated that burst of repeat that caused larger genome size of bamboo shark and inferred phylogenetic topologies between sequenced cartilaginous fishes and bony fishes, which is important for exploring evolutionary process of vertebrates.
Using this genome, we inferred ancestral chromosomes of cartilaginous fishes and found dynamical rearrangements. Based on chromosome evolutionary processes and comparative genomic analysis, we were able to identify fast-evolving chromosomes and immune-related genes. Moreover, chromosome fusions and fissions would also cause DNA damages, deletion of genes, and formation of new genes that may be functionally important and closely associated to species-specific features. Based on this, we found gene loss events associated with phenotypic diversity, for example, chondrification of the endoskeleton. Thus, our study highlights the importance of chromosome rearrangements in the diversification of cartilaginous fishes. With effective methods described in this study, more chromosome-level genomes can be obtained in the future to further elucidate the early evolution of jawed vertebrates as well as extant jawed vertebrate lineages.
Shark-specific immunocompetence always attracts researchers. Investigating immune genes could help to understand evolutionary processes of immune system of cartilaginous fishes compared with jawless species. MHC-I-like and MHC-II-like genes found in cartilaginous fishes revealed the possible time of acquirement of MHC-based adaptive immunity. Bamboo shark chromosomes (Chr37 and Chr44) enriched with immune-related gene may play vital roles in its powerful immunity, and more chromosome-level genomes of cartilaginous fishes should be accomplished to further confirm this conclusion. Shark single-domain antibodies have shown prospects in therapeutic use and our ELISA experiments also proved their potential use in human diseases. Thus, assembly of IgNAR sequences will accelerate development of antibodies for future medicine. In summary, our results provide valuable resources and will be significant for future research about vertebrate evolution and pharmaceutical development.
Limitations of the Study
In this study, we assembled bamboo shark genome, analyzed chromosome rearrangements, and performed ELISA experiments. However, this genome is the only chromosome-level cartilaginous fish genome and more high-quality genomes should be assembled to further verify our chromosome evolution analysis. Besides, more functional experiments should be performed to further validate candidate functional genes.
Resource Availability
Lead Contact
Further information and requests for materials should be directed to and will be fulfilled by the lead contact, Xin Liu (liuxin@genomics.cn).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
The accession numbers for the genome sequencing data, RNA sequencing data, and genome assembly reported in this paper NCBI: PRJNA478295.This Whole Genome Shotgun project has been deposited to National Center for Biotechnology Information (NCBI) under the accession: QPFF00000000 referring project: PRJNA478295. Raw RNA sequencing reads have also been uploaded to the SRA database under accession: SRP154403. The assembled genome can also be obtained from CNSA (CNGB Nucleotide Sequence Archive) by assembly ID: CNA0000025.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We would like thank Dr. Lynn Fink for revising these manuscripts. This work was supported by National Key R&D Program of China (2018YFD0900301), Shenzhen-Hongkong Collaboration Fund JCYJ20170412152916724 (20170331), State Key Laboratory of Agricultural Genomics (No. 2011DQ782025), and Strategic Priority Research Program of the Chinese Academy of Sciences (XDA19060403).
Author Contributions
X.L., N.Y., G.F., and X.X. designed and managed this project. M.W., C.L., H.X., L.W., H.R., Y.X., Q.X., and S.P. were responsible for collecting samples, library construction, sequencing, and co-drafting the manuscript. Y.Z., H.G., and J.G. worked on genome assembly, annotation, chromosome evolution, gene family analysis, transcriptome, and co-drafting the manuscript. J.W., M.L., X.G., Q.L., and Y.S. performed data processing, whole-genome duplication, Hox gene clusters, and repeat analysis. H.L., B.O., Y.G., B.R., X.D., and Y.Y. performed ELSA experiment. S.L., J.W., Y.J., J.S., S.L., L.M., J.S.M.S., M.J.S., M.K., N.H.H., H.Y., J.W., and S.M.-Y.L. helped to revise the manuscript. All authors took part in the interpretation of data.
Declaration of Interests
The authors declare no competing interests.
Published: November 20, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101754.
Contributor Information
Guangyi Fan, Email: fanguangyi@genomics.cn.
Naibo Yang, Email: yangnaibo@genomics.cn.
Xin Liu, Email: liuxin@genomics.cn.
Supplemental Information
References
- Alexander A.B., Parkinson L.A., Grant K.R., Carlson E., Campbell T.W. The hemic response of white-spotted bamboo sharks (Chiloscyllium plagiosum) with inflammatory disease. Z. Biol. 2016;35:251–259. doi: 10.1002/zoo.21280. [DOI] [PubMed] [Google Scholar]
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Braasch I., Gehrke A.R., Smith J.J., Kawasaki K., Manousaki T., Pasquier J., Amores A., Desvignes T., Batzel P., Catchen J. The spotted gar genome illuminates vertebrate evolution and facilitates human-teleost comparisons. Nat. Genet. 2015;47:427. doi: 10.1038/ng.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling: its unpopular beginning, its acceptance and its exciting future. Bioessays. 2012;34:218–225. doi: 10.1002/bies.201100130. [DOI] [PubMed] [Google Scholar]
- Cao Y., Janke A., Waddell P.J., Westerman M., Takenaka O., Murata S., Okada N., Pääbo S., Hasegawa M. Conflict among individual mitochondrial proteins in resolving the phylogeny of eutherian orders. J. Mol. Evol. 1998;47:307–322. doi: 10.1007/pl00006389. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler E.E., Sankoff D. Structural dynamics of eukaryotic chromosome evolution. science. 2003;301:793–797. doi: 10.1126/science.1086132. [DOI] [PubMed] [Google Scholar]
- Ellegren H. Evolutionary stasis: the stable chromosomes of birds. Trends Ecol. Evol. 2010;25:283–291. doi: 10.1016/j.tree.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Feige M.J., Gräwert M.A., Marcinowski M., Hennig J., Behnke J., Ausländer D., Herold E.M., Peschek J., Castro C.D., Flajnik M. The structural analysis of shark IgNAR antibodies reveals evolutionary principles of immunoglobulins. Proc. Natl. Acad. Sci. U S A. 2014;111:8155–8160. doi: 10.1073/pnas.1321502111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajnik M.F., Canel C., Kramer J., Kasahara M. Which came first, MHC class I or class II? Immunogenetics. 1991;33:295–300. doi: 10.1007/BF00216688. [DOI] [PubMed] [Google Scholar]
- Glasauer S.M., Neuhauss S.C. Whole-genome duplication in teleost fishes and its evolutionary consequences. Mol. Genet. genomics. 2014;289:1045–1060. doi: 10.1007/s00438-014-0889-2. [DOI] [PubMed] [Google Scholar]
- Grol M.W., Panupinthu N., Korcok J., Sims S.M., Dixon S.J. Expression, signaling, and function of P2X7 receptors in bone. Purinergic Signal. 2009;5:205. doi: 10.1007/s11302-009-9139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover C.E., Wendel J.F. Recent insights into mechanisms of genome size change in plants. J. Bot. 2010;2010:1–8. [Google Scholar]
- Hara Y., Yamaguchi K., Onimaru K., Kadota M., Koyanagi M., Keeley S.D., Tatsumi K., Tanaka K., Motone F., Kageyama Y. Shark genomes provide insights into elasmobranch evolution and the origin of vertebrates. Nat. Ecol. Evol. 2018;2:1761. doi: 10.1038/s41559-018-0673-5. [DOI] [PubMed] [Google Scholar]
- Hoffmann J.J. Neutrophil CD64: a diagnostic marker for infection and sepsis. Clin. Chem. Lab. Med. 2009;47:903–916. doi: 10.1515/CCLM.2009.224. [DOI] [PubMed] [Google Scholar]
- Inoue J., Donoghue P.C., Yang Z. The impact of the representation of fossil calibrations on Bayesian estimation of species divergence times. Syst. Biol. 2010;59:74–89. doi: 10.1093/sysbio/syp078. [DOI] [PubMed] [Google Scholar]
- Janvier P.. Early Vertebrates. Oxford University Press; 1996. [Google Scholar]
- Jing D., Baik A.D., Lu X.L., Zhou B., Lai X., Wang L., Luo E., Guo X.E. In situ intracellular calcium oscillations in osteocytes in intact mouse long bones under dynamic mechanical loading. FASEB J. 2014;28:1582–1592. doi: 10.1096/fj.13-237578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara M., Naruse K., Sasaki S., Nakatani Y., Qu W., Ahsan B., Yamada T., Nagayasu Y., Doi K., Kasai Y. The medaka draft genome and insights into vertebrate genome evolution. Nature. 2007;447:714. doi: 10.1038/nature05846. [DOI] [PubMed] [Google Scholar]
- Kastrup J. Can YKL-40 be a new inflammatory biomarker in cardiovascular disease? Immunobiology. 2012;217:483–491. doi: 10.1016/j.imbio.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Vertebrates and the evolution of the major histocompatibility complex (MHC) class I and class II molecules. Verh. Dtsch. Zool. Ges. 1988;81:131–144. [Google Scholar]
- Kaufman J. The evolutionary origins of the adaptive immune system of jawed vertebrates. In: Kaufmann S.H.E., Rouse B.T., Sacks D.L., editors. The Immune Response to Infection. American Society of Microbiology; 2011. pp. 41–55. [Google Scholar]
- Kaufman J. Unfinished business: evolution of the MHC and the adaptive immune system of jawed vertebrates. Annu. Rev. Immunol. 2018;36:383–409. doi: 10.1146/annurev-immunol-051116-052450. [DOI] [PubMed] [Google Scholar]
- Kaufman J.F., Auffray C., Korman A.J., Shackelford D.A., Strominger J. The class II molecules of the human and murine major histocompatibility complex. Cell. 1984;36:1–13. doi: 10.1016/0092-8674(84)90068-0. [DOI] [PubMed] [Google Scholar]
- Kim H., Kajikawa T., Walsh M.C., Takegahara N., Jeong Y.H., Hajishengallis G., Choi Y. The purinergic receptor P2X5 contributes to bone loss in experimental periodontitis. BMB Rep. 2018;51:468. doi: 10.5483/BMBRep.2018.51.9.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraku S. Hox gene clusters of early vertebrates: do they serve as reliable markers for genome evolution? Genomics, proteomics bioinformatics. 2011;9:97–103. doi: 10.1016/S1672-0229(11)60012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyne P.M., Burgess G.H. Vol. 2006. 2006. (Chiloscyllium plagiosum. . The IUCN Red List of Threatened Species). eT60222A12325334. [Google Scholar]
- Könning D., Zielonka S., Grzeschik J., Empting M., Valldorf B., Krah S., Schröter C., Sellmann C., Hock B., Kolmar H. Camelid and shark single domain antibodies: structural features and therapeutic potential. Curr. Opin. Struct. Biol. 2017;45:10–16. doi: 10.1016/j.sbi.2016.10.019. [DOI] [PubMed] [Google Scholar]
- Lenertz L.Y., Baughman C.J., Waldschmidt N.V., Thaler R., van Wijnen A.J. Control of bone development by P2X and P2Y receptors expressed in mesenchymal and hematopoietic cells. Gene. 2015;570:1–7. doi: 10.1016/j.gene.2015.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Coghlan A., Ruan J., Coin L.J., Heriche J.K., Osmotherly L., Li R., Liu T., Zhang Z., Bolund L. TreeFam: a curated database of phylogenetic trees of animal gene families. Nucleic Acids Res. 2006;34:D572–D580. doi: 10.1093/nar/gkj118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.T., Jiang G., Chen Q., Zheng J.N. Ki67 is a promising molecular target in the diagnosis of cancer. Mol. Med. Rep. 2015;11:1566–1572. doi: 10.3892/mmr.2014.2914. [DOI] [PubMed] [Google Scholar]
- Litman G.W., Rast J.P., Fugmann S.D. The origins of vertebrate adaptive immunity. Nat. Rev. Immunol. 2010;10:543–553. doi: 10.1038/nri2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Wang S.-f., Wang J., Su Y.-q. Analysis of the karyotype of Chiloscyllium plagiosum [J] J. Xiamen Univ. (Nat. Sci.) 2008;6 [Google Scholar]
- Maia A., Wilga C.D. Anatomy and muscle activity of the dorsal fins in bamboo sharks and spiny dogfish during turning maneuvers. J. Morphol. 2013;274:1288–1298. doi: 10.1002/jmor.20179. [DOI] [PubMed] [Google Scholar]
- Marra N.J., Stanhope M.J., Jue N.K., Wang M., Sun Q., Pavinski Bitar P., Richards V.P., Komissarov A., Rayko M., Kliver S. White shark genome reveals ancient elasmobranch adaptations associated with wound healing and the maintenance of genome stability. Proc. Natl. Acad. Sci. U S A. 2019;116:4446–4455. doi: 10.1073/pnas.1819778116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna M.C. The vertebrates updated: vertebrate paleontology and evolution. Science. 1988;239:512–513. doi: 10.1126/science.239.4839.512. [DOI] [PubMed] [Google Scholar]
- Nakamura E.I., Uezono Y., Narusawa K.I., Shibuya I., Oishi Y., Tanaka M., Yanagihara N., Nakamura T., Izumi F. ATP activates DNA synthesis by acting on P2X receptors in human osteoblast-like MG-63 cells. Am. J. Physiol. Cell Physiol. 2000;279:C510–C519. doi: 10.1152/ajpcell.2000.279.2.C510. [DOI] [PubMed] [Google Scholar]
- Nicolaidou V., Wong M.M., Redpath A.N., Ersek A., Baban D.F., Williams L.M., Cope A.P., Horwood N.J. Monocytes induce STAT3 activation in human mesenchymal stem cells to promote osteoblast formation. PLoS One. 2012;7:e39871. doi: 10.1371/journal.pone.0039871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancer Z., Amemiya C.T., Ehrhardt G.R., Ceitlin J., Gartland G.L., Cooper M.D.J.N. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. 2004;430:174. doi: 10.1038/nature02740. [DOI] [PubMed] [Google Scholar]
- Price M.N., Dehal P.S., Arkin A.P. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen A.-S., Arnason U. Molecular studies suggest that cartilaginous fishes have a terminal position in the piscine tree. Proc. Natl. Acad. Sci. U S A. 1999;96:2177–2182. doi: 10.1073/pnas.96.5.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathcke C.N., Vestergaard H. YKL-40-an emerging biomarker in cardiovascular disease and diabetes. Cardiovasc. diabetol. 2009;8:61. doi: 10.1186/1475-2840-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read T.D., Petit R.A., Joseph S.J., Alam M.T., Weil M.R., Ahmad M., Bhimani R., Vuong J.S., Haase C.P., Webb D.H. Draft sequencing and assembly of the genome of the world’s largest fish, the whale shark: rhincodon typus Smith 1828. BMC genomics. 2017;18:532. doi: 10.1186/s12864-017-3926-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihn S.J., Aziz M.A., Stewart D.G., Hughes J., Turnbull M.L., Varela M., Sugrue E., Herd C.S., Stanifer M., Sinkins S.P. TRIM69 inhibits vesicular stomatitis Indiana virus. J. Virol. 2019;93 doi: 10.1128/JVI.00951-19. e00951–00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco L., Costagliola D., Liguori I., Stingo V. Cytogenetic and molecular studies in the nurse shark, Ginglymostoma cirratum (Galeomorphii, Heterodontiformes) Annales de Genetique. 2003;46:98. [Google Scholar]
- Rock K.L., Reits E., Neefjes J. Present yourself! by MHC class I and MHC class II molecules. Trends Immunol. 2016;37:724–737. doi: 10.1016/j.it.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Ribeiro R., Alvarenga E.C., Calio M.L., Paredes-Gamero E.J., Ferreira A.T. Dual role of P2 receptors during osteoblast differentiation. Cell Biochem. Biophys. 2015;71:1225–1233. doi: 10.1007/s12013-014-0332-7. [DOI] [PubMed] [Google Scholar]
- Saha N.R., Smith J., Amemiya C.T. Seminars in Immunology. Elsevier; 2010. Evolution of adaptive immune recognition in jawless vertebrates; pp. 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salse J., Abrouk M., Murat F., Quraishi U.M., Feuillet C. Improved criteria and comparative genomics tool provide new insights into grass paleogenomics. Brief. Bioinformatics. 2009;10:619–630. doi: 10.1093/bib/bbp037. [DOI] [PubMed] [Google Scholar]
- Santini S., Boore J.L., Meyer A. Evolutionary conservation of regulatory elements in vertebrate Hox gene clusters. Genome Res. 2003;13:1111–1122. doi: 10.1101/gr.700503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz F.J., Maddock M.B. Indo-Pacific fish biology Ichthyological Society of Japan; 1986. Comparisons of karyotypes and cellular DNA contents within and between major lines of elasmobranchs; pp. 148–157. [Google Scholar]
- Simao F.A., Waterhouse R.M., Ioannidis P., Kriventseva E.V., Zdobnov E.M. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31:3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- Sitcheran R., Cogswell P.C., Baldwin A.S. NF-κB mediates inhibition of mesenchymal cell differentiation through a posttranscriptional gene silencing mechanism. Genes Dev. 2003;17:2368–2373. doi: 10.1101/gad.1114503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.J., Kuraku S., Holt C., Sauka-Spengler T., Jiang N., Campbell M.S., Yandell M.D., Manousaki T., Meyer A., Bloom O.E. Sequencing of the sea lamprey (Petromyzon marinus) genome provides insights into vertebrate evolution. Nat. Genet. 2013;45:415. doi: 10.1038/ng.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.J., Timoshevskaya N., Ye C., Holt C., Keinath M.C., Parker H.J., Cook M.E., Hess J.E., Narum S.R., Lamanna F. The sea lamprey germline genome provides insights into programmed genome rearrangement and vertebrate evolution. Nat. Genet. 2018;50:270. doi: 10.1038/s41588-017-0036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solle M., Labasi J., Perregaux D.G., Stam E., Petrushova N., Koller B.H., Griffiths R.J., Gabel C.A. Altered cytokine production in mice lacking P2X7Receptors. J. Biol. Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- Straube N., Lampert K.P., Geiger M.F., Weiss J.D., Kirchhauser J.X. First record of second-generation facultative parthenogenesis in a vertebrate species, the whitespotted bambooshark Chiloscyllium plagiosum. J. fish Biol. 2016;88:668–675. doi: 10.1111/jfb.12862. [DOI] [PubMed] [Google Scholar]
- Sun D., Junger W.G., Yuan C., Zhang W., Bao Y., Qin D., Wang C., Tan L., Qi B., Zhu D. Shockwaves induce osteogenic differentiation of human mesenchymal stem cells through ATP release and activation of P2X7 receptors. Stem Cells. 2013;31:1170–1180. doi: 10.1002/stem.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syberg S., Petersen S., Beck Jensen J.-E., Gartland A., Teilmann J., Chessell I., Steinberg T.H., Schwarz P., Jørgensen N.R. Genetic background strongly influences the bone phenotype of P2X7 receptor knockout mice. J. Osteoporos. 2012;2012:391097. doi: 10.1155/2012/391097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Akatsu T., Udagawa N., Sasaki T., Yamaguchi A., Moseley J.M., Martin T.J., Suda T. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 1988;123:2600–2602. doi: 10.1210/endo-123-5-2600. [DOI] [PubMed] [Google Scholar]
- Thaler R., Sturmlechner I., Spitzer S., Riester S.M., Rumpler M., Zwerina J., Klaushofer K., Van Wijnen A.J., Varga F. Acute-phase protein serum amyloid A3 is a novel paracrine coupling factor that controls bone homeostasis. FASEB J. 2014;29:1344–1359. doi: 10.1096/fj.14-265512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh B., Lee A.P., Ravi V., Maurya A.K., Lian M.M., Swann J.B., Ohta Y., Flajnik M.F., Sutoh Y., Kasahara M. Elephant shark genome provides unique insights into gnathostome evolution. Nature. 2014;505:174. doi: 10.1038/nature12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Zou C., Wang X., Huang C., Feng T., Pan W., Wu Q., Wang P., Dai J. Interferon-stimulated TRIM69 interrupts dengue virus replication by ubiquitinating viral nonstructural protein 3. PLoS Pathog. 2018;14:e1007287. doi: 10.1371/journal.ppat.1007287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang O., Chin R., Cheng X., Wu M.K.Y., Mao Q., Tang J., Sun Y., Anderson E., Lam H.K., Chen D. Efficient and unique cobarcoding of second-generation sequencing reads from long DNA molecules enabling cost-effective and accurate sequencing, haplotyping, and de novo assembly. Genome Res. 2019;29:798–808. doi: 10.1101/gr.245126.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowski J., Alzogaray V., Reyelt J., Unger M., Juarez K., Urrutia M., Cauerhff A., Danquah W., Rissiek B., Scheuplein F. Single domain antibodies: promising experimental and therapeutic tools in infection and immunity. Med. Microbiol. Immunol. 2009;198:157–174. doi: 10.1007/s00430-009-0116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D., Zhu Y., Walsh T., Xie D., Yuan H., Sirmaci A., Fujikawa T., Wong A.C.Y., Loh T.L., Du L. Mutation of the ATP-gated P2X2 receptor leads to progressive hearing loss and increased susceptibility to noise. Proc. Natl. Acad. Sci. U S A. 2013;110:2228–2233. doi: 10.1073/pnas.1222285110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zardoya R., Cao Y., Hasegawa M., Meyer A. Searching for the closest living relative (s) of tetrapods through evolutionary analyses of mitochondrial and nuclear data. Mol. Biol. Evol. 1998;15:506–517. doi: 10.1093/oxfordjournals.molbev.a025950. [DOI] [PubMed] [Google Scholar]
- Zhang P., Julia I., Leu J., Murphy M.E., George D.L., Marmorstein R. Crystal structure of the stress-inducible human heat shock protein 70 substrate-binding domain in complex with peptide substrate. PLoS One. 2014;9:e103518. doi: 10.1371/journal.pone.0103518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielonka S., Weber N., Becker S., Doerner A., Christmann A., Christmann C., Uth C., Fritz J., Schäfer E., Steinmann B. Shark attack: high affinity binding proteins derived from shark vNAR domains by stepwise in vitro affinity maturation. J. Biotechnol. 2014;191:236–245. doi: 10.1016/j.jbiotec.2014.04.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession numbers for the genome sequencing data, RNA sequencing data, and genome assembly reported in this paper NCBI: PRJNA478295.This Whole Genome Shotgun project has been deposited to National Center for Biotechnology Information (NCBI) under the accession: QPFF00000000 referring project: PRJNA478295. Raw RNA sequencing reads have also been uploaded to the SRA database under accession: SRP154403. The assembled genome can also be obtained from CNSA (CNGB Nucleotide Sequence Archive) by assembly ID: CNA0000025.