Abstract
Questing is a host-seeking behavior in which ticks ascend plants, extend their front legs, and wait poised for a chance to attach to a passing host. Hard ticks are ectoparasites of terrestrial vertebrates and because some species vector disease, they are among the most medically important of arthropod pests. All ixodid ticks require blood to survive and reproduce with the number of blood-hosts needed to complete their life cycle varying among species. The vast majority are three-host ticks requiring a different host for each developmental stage: larva, nymph, and adult. A few, including some of the most economically important species, are one-host ticks, that quest only in the larval stage. Questing is a rate-limiting behavior critical to tick survival and disease transmission. For the off-host larval stage, survival is highly dependent on ecological and physiological factors. Yet, off-host larval ecophysiology is often overlooked for the more obvious adult and nymphal tick-host interactions. This review summarizes the literature on ixodid larval questing with emphasis on how specific biotic and abiotic factors affect off-host survival.
Keywords: off-host ticks, tick larvae, Ixodidae, questing, climate change
Ticks are second only to mosquitoes as vectors of human disease worldwide, while within the United States, ticks account for 77% of vector-borne diseases as of 2016 (Rosenberg et al. 2018). As sanguinivorous, parasites of vertebrates, ticks vector microbes threaten humans, wildlife, pets, and livestock (Gage et al. 2008, Dantas-Torres et al. 2012). Tick feeding on a host can also cause allergic reactions, loss of blood (tick burden), reduced milk production, damage to hides, and in some cases paralysis (Doube 1975, Willadsen et al. 1978, Jonsson et al. 1998, McCulloch and Tungaraza 2015). For these reasons, ticks are among the most economically and medically important ectoparasites of domestic animals (Jongejan and Uilenberg 2004, Durrani and Shakoori 2009). Ticks consist of three families: Ixodidae, Argasidae, and the monotypic Nuttalliellae. The family Ixodidae (hard ticks), which comprises most tick species, are particularly pestiferous with the highest transmission rate of disease and disease causing agents among the three tick families (Hargis and Myers 2017). These ticks are intermittent ectoparasites that spend as much as 90% of their life cycle off-host (Needham and Teel 1991, Randolph 2004). Oviposition, hatching from eggs, metamorphosis, and molting between life stages typically takes place off-host. For these nonparasitic, transitional stages, survival is highly dependent on biotic and abiotic factors such as climate and predation (Mwangi, Newson, et al. 1991b, Randolph 2004, Daniel et al. 2015). Thus, the ability to find and attach to a host quickly is essential for survival.
To find a host, larval ticks engage in a behavior known as questing, arguably the most important phase in the life cycle in that the larvae suffer the greatest mortality. Depending on the species and habitat, certain factors have greater influence than others, determining not only survival but the level of questing activity. There are two strategies used by ticks in general, the ambush strategy and the hunter strategy. First, the ‘ambush’ strategy wherein ticks climb to the top of vegetation (or rocks), extend their front legs, and wait for a passing host. Second, the ‘hunter’ strategy wherein the ticks actively pursue a potential blood-host (Parola and Raoult 2001). Larval ixodids employ the ‘ambush’ strategy. Questing is metabolically costly, as larvae expend energy and run the risk of dehydration while receiving no nourishment (Lees 1946, Randolph 2004, Nicholson et al. 2019). It is at this stage that environmental factors directly affect questing behavior, at times forcing larvae to retreat to refugia when conditions are less favorable, thus limiting host interaction frequency. Under optimal conditions, larvae can survive off-host for 8–9 mo before starving to death (Hooker et al. 1912, Hitchcock 1955).
Because of their small size and clustered distribution, off-host ixodid larvae, sometimes called ‘seed ticks’, are not easily sampled in the field (Schulze et al. 1997, Ramos et al. 2014). Consequently, this phase remains the most nebulous phase of the tick’s life cycle. Knowledge of longevity, persistence, and the ecophysiological limitations of this nonparasitic transitional stage can help improve management practices. Therefore, this review summarizes the published knowledge on ixodid larval questing and the specific environmental factors that determine its success (Table 1).
Table 1.
Factors that influence off-host larval tick questing behavior
| Abiotic/biotic factors | Optimal for larval questing | Description |
|---|---|---|
| Humidity | ≥80–85% | Larvae restrict questing activity to optimal relative humidity levels. Dry conditions will cause larvae to dehydrate. |
| Temperature | 3–38°C | Ticks adapted to cold temperatures, such as I. ricinus, have been shown to commence in questing at temperatures as low as 3°C. While questing activity has been shown to be curtailed at ≥ 38°C. |
| Precipitation | Precipitation in late spring/early summer | Precipitation contributes to higher levels of larval questing by providing optimal humidity, milder temperatures, and lower saturation deficit. |
| Clustering | Larger clusters | Larger clusters reduce moisture loss and maximizes host attachment. |
| Photoperiod | Presence of light during appropriate photoperiod | Ticks commence in questing depending on the specific photoperiod of the species of tick. |
| Seasonality | Species dependent relationship between adaptive physiology and ecology | Fluctuation in climate, for a given region, dictate pronounce deviations in developmental maturation which create seasonal periods of questing. |
| Habitat | Sheltered site with optimal microclimate | Optimal habitats produce prime microclimate conditions for questing architecture. |
| Predation and natural enemies | Low predation | Predatory mites, ants, spiders, and carabid beetles. |
Ixodid Tick Life Cycle
The majority of ixodid ticks are associated with free-roaming hosts (Kolonin 2007). Ixodid ticks are slow-feeding parasites that attach to the host for days or weeks while feeding, and they are categorized by the number of hosts exploited during their life cycle. The main difference between one- and three-host ticks is the detachment and questing period between the stages. One-host ticks attach and complete their life cycle on a single host, while most species are three-host ticks that detach and drop off the host to molt in the environment between stages (Sutherst et al. 1978, Needham and Teel 1991, Sonenshine and Roe 1991, de Castro and Newson 1993). Very few species are two-host ticks. In the genus, Rhipicephalus with mostly three-host ticks, there are two-host tick species such as the red tick Rhipicephalus (Digenius) evertsi (Neumann). The red tick infests a single-host rabbit or hare as larvae, detaching to molt but remaining on the host during molt and reattachment as a nymph, later dropping off the host as an engorged nymph, eventually questing for an ungulate as the second host (Londt and Van Der Bijl 1977). Other species are functionally two-host ticks, responding to stress by not dropping from the host after larval engorgement. Climatic conditions and host type are the main factors influencing how many hosts ticks will use for development. For example, camel ticks (Hyalomma spp.) in the Saharan desert have the ability to develop on three or two hosts to complete the life cycle (Apanaskevich and Oliver 2014). Because the two-host ticks are derivates of the three-host life cycle, they detach from the host to molt, whereas one-host ticks remain attached to the host during the molt.
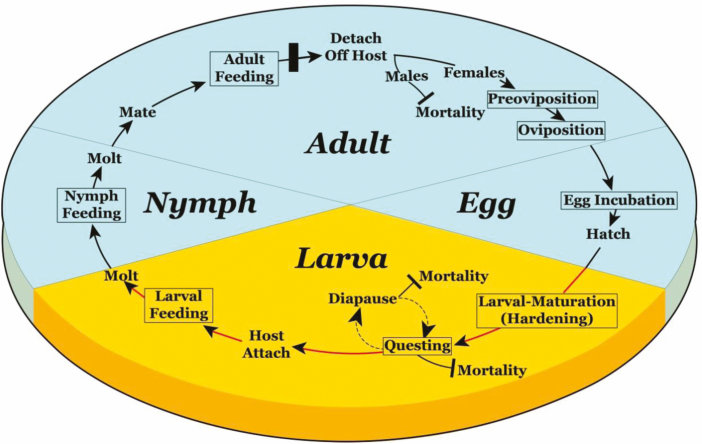
The life cycle for a one-host tick comprises eight stages: female preoviposition period, female oviposition period, egg incubation period, larval-maturation (hardening) stage, host-seeking (questing) larva, feeding larva, feeding nymph stage, and the feeding adult stage (Fig. 1; Loomis 1961, Eremeeva and Dasch 2015). In a three-host tick, the postembryonic life stages consist of larvae, nymphs, and adults in which all stages quest. Larvae typically require 1–7 d of cuticular sclerotization (hardening) before they become receptive to hosts (Gladney et al. 1970, Davey 1986). In some instances, it is the tick larvae that transmit the infectious agents such as the transfer of Babesia pathogens by Boophilus species (Howell et al. 2007, Labruna et al. 2009). Under optimal scenarios, one-host species can complete their life cycle and reproduce in 4–8 wk (Mount et al. 1991), allowing multiple generations within a year. Three-host ticks frequently require more than a year to complete a generation (Troughton and Levin 2007).
Fig. 1.
Ixodid one-host tick life cycle. The eight stages shown in boxes. Questing and host attachment occurs during larval development. If successful, the tick will remain on the host, continue to mature to an adult, mate and detach for oviposition. Under ideal conditions, one-host species can compete their life cycle and reproduce in 4–8 wk.
Perhaps, the best indicator of the precariousness of larval tick existence is the reproductive rate of adult females. Fully engorged females with heavier weights have more blood to convert to an egg mass compared with lighter females (Drummond et al. 1971, Campbell and Glines 1979). Once egg laying begins, a typical oviposition period for ixodid ticks is from 1–3 wk (Sweatman 1967, Drummond and Whetstone 1970). Ticks produce variable amounts of eggs for example, the female rabbit tick, Haemaphyalis leporispalustris (Packard), can produce up to 3,327 eggs in one egg mass (Campbell and Glines 1979), and the female horse tick, Anocentor nitens (Neumann), can produce 3,984 eggs in one egg mass (Despins 1992), while Amblyomma maculatum (Koch) females can produce as many as 9,963 eggs in an egg mass (Drummond and Whetstone 1970). If females need to produce 10,000 eggs to maintain the population then, applying the logistic equation (Cody 1966, Pianka 1972), the odds of larva finding/attaching to a suitable host and completing its development is around 5,000 to 1. Host range, habitat, and behavior are factors that influence clutch size. In contrast to the free-living ticks mentioned above, nidicolous tick, ectoparasites of nesting animals, have much smaller egg masses (Arthur 1951, Toutoungi et al. 1995). For example, the tree-hole tick, Ixodes arboricola (Schulze and Schlotke), has an egg mass of only 300–800 eggs (Heylen et al. 2014).
Larval Sensory Organs
Ticks orient to environmental stimuli using an array of sensory receptors that facilitate questing (Mitchell et al. 2017). In larvae, the main recognized sensory organs are the eyes, Haller’s organ, palpal organ, and integumentary sensilla. Collectively, these organs detect microclimatic changes eliciting a biological response that helps tick larvae navigate their surroundings, minimize metabolic loss, and ultimately attach to a host (Leonovich 2013).
Ixodid larvae have unipolar photoreceptor neurons that connect to the optic lobes of the brain via the optic nerve (Phillis and Cromroy 1977). The lens of the tick eye is unique in having light guiding pore canals that function as motion detectors picking up movement and contrasting silhouettes (Phillis and Cromroy 1977, Kaltenrieder 1990). Larvae are responsive to light as early as 4-d postemergence (Krijgsman 1937). The presence of light is a principal factor in influencing the ascent of larvae on available foliage, but the length of exposure can alter questing behaviors (Wilkinson 1953, Binnington 1972, Nuñez et al. 1982, Oliver 1989). For example, southern cattle fever tick, Rhipicephalus (Boophilus) microplus (Canestrini), larvae primarily quest from 10:00–14:00 h and do not descend to soil levels during this time frame (Short et al. 1989). However, during hours of strong UV or infrared exposure, some species of larvae will hide beneath the shaded portion of grass blades (Wilkinson 1953; Fig. 3). Amblyomma variegatum (Fabricius) and Rhipicephalus (Rhipicephalus) appendiculatus (Neumann) larvae are most active in the morning (09:00–10:00 h) and afternoon (after 15:00 h), retreating to the shade during the mid-day (12:00–15:00 h) (Pegram and Banda 1990). Temperature also influences this behavior and determining the dominant stimuli is sometimes difficult. Conversely, Rhipicephalus (Boophilus) australis (Fuller) larvae were observed to quest both day and night (Wilkinson 1961), which likely has much to do with host activity. Ixodes ricinus (L.) and Ixodes hexagonus (Leach) are known to be active at night because their preferred hosts (rodents, hedgehogs, and foxes) are nocturnal (Lees and Milne 1951, Matuschka et al. 1990, Perret et al. 2003). Photoperiodicity also plays a dominant role determining the onset of larval behavioral diapause (Oliver 1989, Cabrera and Labruna 2009).
Fig. 3.
Representative images of common questing and avoidance behavior in larvae of (A) Rhipicephalus (Boophilus) annulatus larvae taking refuge from the sun under a grass blade; (B) Rhipicephalus (Boophilus) annulatus larvae emerging in response to the approach of a host from a dry stem to form questing cluster; (C) Rhipicephalus (Boophilus) microplus larvae forming a questing cluster; and (D) larvae of Rhipicephalus (Boophilus) annulatus forming linked chain around a grass blade. Photos taken by Dr. Donald B. Thomas using a Cannon EOS Rebel T5i camera with EFS 18 × 15 mm Macro Lens.
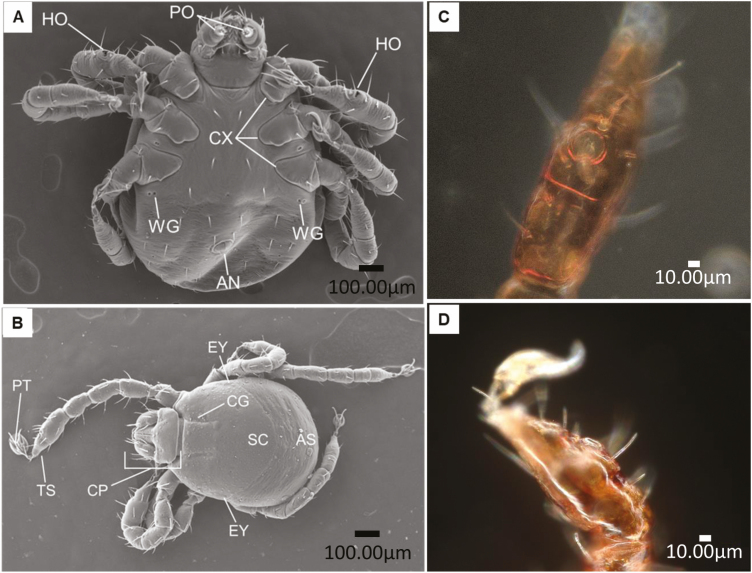
The Haller’s organ is a triple function sensillum (chemo-mechanoreceptor) used to detect heat, humidity, and odors (Nuttall et al. 1908, Lees 1948, Wilkinson 1953, Chu-Wang and Axtell 1973, Waladde 1976, Rechav et al. 1977). The organ is located on the dorsal side of the tarsus of each foreleg with a number of hairs positioned in the anterior pit and posterior capsule (Fig. 2A–D; Wilkinson 1953). These hairs act as the chemo-mechanoreceptors to environmental cues (Lees 1948), and at least in adults can detect infrared radiation (Mitchell et al. 2017). If the Haller’s organ is removed, the tick is not responsive to potential hosts (Morales 2019). The Haller’s organ detects host associated odors such as CO2 and ammonia (Sonenshine and Roe 1991). The palpal organ houses chemoreceptors assumed to be associated with feeding stimuli during and after attaching to a host (Lees 1948, Wilkinson 1953).
Fig. 2.
Representative Scanning Electron Microscope images of larvae of Rhipicephalus microplus identifying several different sensory organs taken by Brenda Leal and Alejandra Fuentes using a Hitachi TM4000 Scanning Electron Microscope. (A) ventral view; (B) dorsal view: papal organs( PO), Haller’s organ (HO), wax glands (WG), coxa (CX), anus (AN), scutum (SC), cervical groove (CG), alloscutum (AS), capitulum (CP), eyes (EY), pretarsus (PT), and tarsus (TS); and (C and D) Close up of the Haller’s organ taken by Dr. Donald B. Thomas using a Keyence VHX-7000 digital microscope.
Numerous sensilla comprise the integumentary sensory system of ixodid ticks. These vary in number and receptor type based on stage of life cycle and species. Along with the common sensory setae (sensilla trichoidea), Schulze (1942) first described four additional sensory organs in adult ticks: sensilla auriformia (ear-shaped), sensilla sagittiformia (arrow-shaped), sensilla hastiformia (spear-shaped), and sensilla laterniformia (lantern-shaped). Larvae have three of these, lacking the sensilla laterniformia, in the genera Rhipicephalus and Hyalomma (Dinnik and Zumpt 1949). The sensilla auriformia are proprioreceptive in nature detecting deformational changes in the cuticle (Schulze 1942). It is believed that the other three organs act as krobylophores (vibro-chemoreceptive organs) that react to chemical and seismic stimulation (Schulze 1942, Dinnik and Zumpt 1949, Wilkinson 1953). In addition, the sensilla sagittiformia in adult ticks secrete squalene, which protects some ticks from high-temperature stress, while in others, it deters ant predators (Yoder et al. 2009).
Humidity
Humidity is one of the most influential factors affecting questing activity and tick survival (Daniel and Dubabek 1994). Larval ticks tend to lose moisture rapidly through evaporation due to a high surface area to volume ratio (Randolph and Storey 1999). Larvae typically restrict questing to optimal relative humidity (RH) conditions close to saturation, below which larvae begin to dehydrate (Lees 1946, Yoder et al. 2006). When ambient conditions are desiccating the duration of larval questing is limited to shorter time periods, and if too dry, larvae may not quest at all (Randolph and Storey 1999, Ogden and Lindsay 2016). Larvae respond to low relative humidity levels using avoidance behaviors like moving to the ground surface, burrowing into the soil, hiding under rocks or into hollow stems, questing at night, or clustering when questing (Fig. 3A–D; Lees 1946, Camin and Drenner 1978, Hadley 1994, Boivin et al. 2006, Knap et al. 2009).
At higher humidity levels, larvae exhibit greater questing activity and have higher survival rates (Knülle and Devine 1972, Tukahirwa 1976, Davey et al. 1991). In general, survivorship is threatened by an RH under 65%, although it varies with species and temperature. For example, R. australis larvae quest at ≥66% RH, surviving at an RH as high as 95% RH (Wilkinson 1953, Roberts 1971). Larvae of the Bont tick, Amblyomma hebraeum (Koch), experienced mortality from loss of water at RH ˂70% RH (Londt and Whitehead 1972). Similarly, the threshold for survival in multiple species of Rhipicephalus larvae was reported at 66% RH (Tukahirwa 1976, Davey et al. 1991). The crucial factors in off-host survival under desiccating conditions are larva’s ability to balance moisture loss with moisture gain from the air (Garris and Popham 1990, Yoder et al. 2006). Larvae drink water from dew and absorb water from vapor when the air is at or near saturation (Lees 1946, Knülle 1966, Yoder and Spielman 1992, Benoit et al. 2007). Water vapor absorbed through the cuticle is the primary source of hydration for questing larvae (Lees 1946, Yoder et al. 2006). The moisture level is optimal at ≥80–85% RH, also known as the critical equilibrium humidity (CEH). At this level or above, larvae can absorb water vapor from the air (Lees 1946, Knülle 1966, Roberts 1971, Knülle and Devine 1972, Needham and Teel 1991, Yoder et al. 2006). Temperature can exacerbate the effects of low RH and these two factors are not independent when it comes to larval survivorship. High saturation deficits (SD) can prevent larvae from rehydrating (Boivin et al. 2006). Larvae that occupy semiarid environments are more resistant to desiccation and, if engorged, have the ability to molt over a range of SDs (Short and Norval 1981, Mooring et al. 1994). Rhipicephalus sanguineus (Latreille) larvae are tolerant to high temperatures and dry air, being able to molt over a range from 18 to 38°C with SDs up to 35 mm Hg. In contrast, Ixodes holocyclus (Neumann) larvae can molt at 18–28°C but experienced high mortality rates at ≥4 mm Hg SD with 100% mortality at 6–8 mm Hg SD (Heath 1981). At 10 mm Hg SD Haemaphysalis longicornis (Neumann) larvae had a mortality rate of 100%. Amblyomma triguttatum (Koch) larvae had shortened longevity when exposed to saturation deficits of 16–17 mm Hg at temperatures of 40°C (Norval 1977, Guglielmone 1992).
Some studies suggest that moisture stress can influence phenotype at subsequent stages of the life cycle. Yoder et al., (2006) reported that R. sanguineus larvae from mothers exposed to high humidities are much less tolerant of low humidities than larvae from mothers exposed to humidity stress (RH < 85%). This was traced to the ability to absorb moisture from saturated air. Larvae from females exposed to humidity stress were capable of absorbing moisture at 75–85% RH and have a mean longevity of 76 d. Larvae from females exposed to high humidity were only able to absorb moisture from air at 93–97% RH, thus shifting the CEH higher. The latter larvae had a mean longevity of only 13 d when kept below the CEH. The authors attributed this result to an alteration in the ability of larvae to absorb moisture from saturated air. Similarly, experiments with R. australis found that larval ticks from humidity stressed (higher SD) eggs had lower survivorship compared with controls. Larvae hatched from eggs that were incubated at 1 mm Hg suffered negligible mortality, whereas the percent survival of larvae decreased with increasing saturation deficit (5 and 20 mm Hg) (Sutherst and Bourne 2006).
Temperature
Off-host larval ticks can survive in a range of temperatures and handle moderate fluctuations through behavioral and phenotypic plasticity. One such adaptation is through physiological tolerance by production of heat shock proteins, protecting cellular functions during temperature stress (Busby et al. 2012). Larval ticks also employ avoidance strategies by modulating questing behavior, such as by adjusting questing height or moving to shade (Wilkinson 1953, Wilkinson and Wilson 1959, Davey 1986, McPherson et al. 2000). Exposure to temperature ranges of 40–45°C causes all terrestrial arthropods, including ticks, to lose their capacity to retain moisture (Londt and Whitehead 1972). At those temperatures, the lipid layer of the waxy cuticle depolarizes, rapidly increasing water loss, resulting in larval dehydration and the likelihood of death (Edney 1977). Like other off-host scenarios, in nonideal temperatures, larval survival is balanced between questing strategies that conserve/obtain water and those that promote attaching to a host.
Temperature tolerance varies among species and is influenced by evolutionary adaptation to the tick’s environment. For tick larvae, in general, longevity, questing activity, and survival are curtailed when ambient temperatures exceed 38°C (Sweatman 1967, Tukahirwa 1976). The larvae of R. appendiculatus are among the most heat tolerant surviving in ambient temperatures of ≥40°C even with low relative humidity (Tukahirwa 1976). At the other end of the spectrum Haemaphysalis flava (Neumann), an Asian tick found in cool mountain bamboo forests were absent in grasslands when temperatures exceeded 30°C (Kakuda et al. 1990). Gothe (1967) found that Margaropus winthemi (Karsch) larvae can withstand 24 h exposure to −15°C, whereas, Rhipicephalus decoloratus (Koch) can only withstand 24 h exposure to −10°C. Larvae of R. microplus die if exposed to −10°C for 24 h but can withstand freezing transition temperatures (0°C) for up to 72 h. The larvae of Ixodes scapularis (Say) are more resistant to low temperatures than nymphs, with 50% of the mortality (LT50) occurring at 12.3°C compared with the nymph which has LT50 at 18.5°C (2-h exposure) (Vandyk et al. 1996). In a study by Heath (1981), I. holocyclus and Ha. longicornis larvae were unable to survive temperatures of >28°C. Winter tick, Dermacentor albipictus (Packard), larvae have been shown to tolerate short-term cold shock down to −25°C (Holmes et al. 2018). Because of its tolerance of low temperatures, this species occurs as far north as 60° latitude, the equivalent of the 1,500 degree-days above 6°C isocline. Overall, the greatest tolerance to cold is found in the Antarctic tick, Ixodes uriae (White), a parasite of penguins and other polar seabirds. In laboratory studies, larvae survived −20°C and the egg stage survived −30°C when temperatures were lowered by 1°C/min (Lee and Baust 1987). Larvae were even observed to survive 208 d when submerged in water of freezing temperatures (0–7°C; Murray and Vestjens 1967).
Temporal variations in regional weather provide a more robust evaluation of impact on larval questing and survival. Optimal temperature range and duration at which the larvae are exposed to this range results in larger numbers of larvae (ELghali and Hassan 2010). The optimal range for survival in Hyalomma dromedarii (Koch) larvae was shown to be 21–34°C (Hagras and Khalil 1988). Under laboratory conditions, larvae survived up to 63 d at 35°C but only survived 5.5 d when the temperature was increased to 38°C (ELghali and Hassan 2010). For R. australis off-host stages (eggs and larvae), the optimal range for development is 28°C with thresholds of 12–40°C (Sutherst and Maywald 1985). Furthermore, temperature lows were shown to have a greater impact on stimulating questing activity in this species than temperature highs (Sutherst et al. 1978). The commencement of questing activity for I. ricinus was shown to begin at 3°C (Walker 2001), whereas in D. albipictus larvae, active questing was shown to begin only at temperatures higher than 10°C (Drew and Samuel 1985).
Temperature is a key factor in Climex models predicting the potential range of invasive tick species (Estrada-Peña 2001, Sutherst and Bourne 2009). For tropical species especially, tick distributions are constrained mainly by low temperatures (Estrada-Peña et al. 2004). In arid desert areas, high temperatures are the limiting factor. As an example, Aponomma hydrosauri (Denny) larvae have adapted to thrive in cooler conditions on the margins of the Australian outback (Chilton and Bull 1993). Survival of the off-host larvae decreased as temperature increased and was greater at cool temperatures than for A. limbatum, a warmer climate species that shares the same host (139 vs 21 d at 4°C and 80% RH). These two species of reptile ticks are displaced by different habitat tolerances. At 30°C, A. limbatum survived 65 versus 46 d for A. hydrosauri.
Temperature plays a significant role in ticks’ life cycle, thus determining the number of larvae and generation times (Branagan 1973; Davey et al. 1980, 1982; Ouhelli et al. 1982; Chilton and Bull 1994). Depending on the temperature tolerance and optimal temperatures favored by the species of tick, temperature can either lengthen or shorten certain life cycle stages or the entire life cycle itself. Sutherst and Bourne (2006) observed that R. australis larvae derived from eggs held at 35°C had half the life expectancy compared with those from eggs developed at 30°C. Similarly, R. microplus had the longest survival when larvae hatched from eggs incubated at 20°C (Davey et al. 1991). In this species, temperature affected duration of embryonic incubation and larval hardening (Sutherst et al. 1978, Ogden et al. 2004). For example, Davey (1986) reported that for Rhipicephalus (Boophilus) annulatus (Say), the incubation period is 52 d at 20°C, but only 16 d at 35°C. Likewise, for Amblyomma limbatum (Neumann) incubation lasts 51 d at 23°C; but 19 d at 34°C (Chilton and Bull 1994). In contrast, I. scapularis larvae did not hatch from eggs held at 32°C (Ogden et al. 2004).
For ixodid ticks, exposure to extreme temperatures can be the determining factor for the activation and termination of diapause (delayed development; Chilton and Bull 1994, Randolph et al. 2002, Ogden et al. 2004). Diapause aids in the survival of larvae during periods of nonconductive temperatures by lowering their metabolic rate and reducing energy sapping activities, such as questing (Belozerov 1982, Gray et al. 2016). When conditions turn favorable, larvae can behaviorally exit diapause (Gray et al. 2016). Behavioral diapause is manifested in Amblyomma cajennense (Fabricius) larvae remaining quiescent on the ground under canopied vegetation during temperature stress. These larvae remained at soil level under the vegetation for 7–10 wk in diapause induced by high temperatures (≈25°C) and long day length (>12 h) (Cabrera and Labruna 2009). At high temperatures, H. dromedarii larvae can shift from a three-host tick to two-host life cycle to avoid desiccation of the host (Delpy and Gouchey 1937, Hoogstraal 1956). Once ixodid larvae hatch from the eggs, their primary instinct is to move to avoid microclimate stress (Davey 1986). Wilkinson (1953) noted that R. australis larvae on grass blades would move from more to less exposed situations over the course of the day. In an experiment by Wilkinson and Wilson (1959), it was observed that larvae move to the shaded side of stems by midday. Adjusting questing height is another way for ticks to avoid temperature stress. McPherson et al. (2000) found that height of questing clusters of the winter tick was negatively correlated with ambient temperature.
Climate change and temperature range have epidemiological implications. In the northern hemisphere, Ogden et al. (2006) predicts that due to climate change, the degree-day >0°C line will move north by 300–500 km over the next 20–50 yr. If so, this will allow ticks and their tick-borne diseases, such as I. scapularis and Lyme disease, to extend much further north than at present. Within the temperature range of 12–30°C, I. scapularis larvae were able to develop in a time span of 2–3 wk (Ogden et al. 2004). Similarly, Jones and Kitron (2000) found a positive correlation between accumulated degree-days and larval populations of that species.
Precipitation
The influence of precipitation on larval questing and survivorship is predominantly indirect and dependent on the distribution and amount of rainfall. An increase in precipitation can provide optimal humidity, milder temperatures, lower saturation deficits, and an increase in vegetation, all promoting larval survivorship (Kaiser et al. 1988, Garcia et al. 2011, Medlin et al. 2015). Periods of heavy precipitation in late spring/early summer contributes to higher levels of eclosion, larval questing, and survival while reducing diapause in Ixodes spp. (Knülle and Rudolph 1982, Eisen et al. 2016). In sandy grassland habitats, R. appendiculatus and A. hebraeum larvae were observed to be more abundant during the wet season (Mooring et al. 1994). Increased larval questing in A. cajennense coincided with low temperatures at the end of the rainy season (Labruna et al. 2002). Off-host populations of R. microplus larvae survive longer during periods of increased rainfall but during low precipitation and high temperatures longevity is negatively affected (Garris et al. 1990). In most of these cases, it is likely that rain altered the amount of water vapor thereby affecting questing larval hydration (Lees 1946, Yoder et al. 2006). However, larvae may not survive when conditions are excessively wet with decreased reproductive success from drowning and/or breakup of egg masses by heavy rain (Davey et al. 1991, Mooring et al. 1994, Leal et al. 2018).
Precipitation is a significant indirect factor related to the occurrence of certain tick-borne diseases, as it correlates with tick abundance and influences phenology of host-seeking behavior, which, in turn, increases the occurrence of tick-borne diseases such as Borreliosis (Daniels et al. 1996, Gage et al. 2008). There is a higher incidence of Lyme disease following seasons of heavy rainfall than in dry, hot seasons (McCabe and Bunnell 2004, Burtis et al. 2016).
In the mid-western United States, greater seasonal rainfall leads to higher oak masting. In turn, more acorns lead to higher numbers of deer and rodent which are ideal tick hosts. The white footed mouse population escalated after acorn production and this directly resulted in large numbers of I. scapularis larvae (Lindsay et al. 1999, Jones and Kitron 2000). These studies documented that higher rainfall led to a larger host density thence to successful larval survivorship and more host infestations, in turn raising the probability of transmitting diseases such as Lyme disease, Rocky Mountain spotted fever, and Human Granulocyctic Anaplasmosis (Jones and Kitron 2000, Gage et al. 2008, Andersen and Davis 2017).
Clustering
Larval clustering accomplishes two functions that benefit larval survival (Fig. 3A-D). Firstly, reduces moisture loss; secondly, it is an important strategy to maximize host attachment. As described in previous sections, larvae are prone to desiccation when exposed to high saturation deficits. To reduce this water loss, questing larvae will form tight clusters to conserve moisture. Lees (1947) labeled this clustering behavior as ‘stereokinesis’ in his studies of I. ricinus larvae (Lees 1947). Clusters of R. microplus have been observed to contain 70–261 larvae (Wilkinson 1953, Utech et al. 1983). Clustering has been shown to significantly increase survivorship of the individuals in the aggregate compared with single larva (Garris and Popham 1990, Tsunoda 2008). Larvae of Ha. longicornis and H. dromedarii had higher clustering activity under drier conditions as compared with wet conditions and even small aggregations of Ha. longicornis had significantly increased longevity over single larvae from 90 to 150 d (Hafez et al. 1970, Tsunoda 2008). In another study, Yoder and Knapp (1999) observed that larvae of the American dog tick, Dermacentor variabilis (Say), could retain water more effectively when in groups of 10–20 individuals compared with a single isolated larva. When clustered together, I. uriae larvae had the rate of water loss drop by 30% compared with the rate of water loss by isolated individuals. Larvae of I. uriae demonstrated a 10–15% reduction in moisture loss in clusters of ≈5 compared with single isolated larvae, while in clusters of ≈10 water loss was suppressed by 65% (Benoit et al. 2007).
Another advantage that accrues from larval clustering is that aggregates of larvae in grasping each other are conveyed on to the passing host at the same time. Numerous studies provide evidence that clustering increases the number of larvae that successfully attach to a host, thus increasing survivorship (Dunn 1918, (Sutherst et al. 1978, Sutherst and Maywald 1985, Oliver 1989), with enhanced mating and reproductive success (Lees 1947, Drew and Samuel 1985, Ogden et al. 2000).
Seasonality
Annual fluctuations in weather within a given region drive rates of developmental maturation creating seasonal periods of questing or quiescence. Seasonality results from multiple over-lapping but largely predictable, variables within the environment to which ticks adapt their phenology.
In the Köppen classification system (Köppen 1936), regions are categorized into one of four different climates: polar, arid, temperate, and tropical. The polar and arid regions have little seasonality and few ticks. The winter tick, D. albipictus, one of the most cold adopted, does not occur north of the arctic circle and polar bears are not known to have ticks (Hueffer et al. 2011). The south polar ticks are ectoparasites of pelagic birds including penguins. The feeding period for such ticks is restricted to the brief time when the birds are on the nesting grounds. The life cycle of I. uriae can last 2–7 yr depending on host availability; the typical life cycle is 3–4 yr. The ticks are adapted to survive off-host until the following or succeeding year if a nest is not re-occupied. The attachment/feeding phase lasts only about 6–12 d. Once engorged, larvae still require 73–105 d before they molt into nymphs. Following molting, the I. uriae nymphs are quiescent until the following feeding season (Murray and Vestjens 1967, Eveleigh and Threlfall 1975, Frenot et al. 2001). The general pattern in development is for the eggs to be laid in summer in the first year: larvae hatch in autumn or the following spring: larvae feed and molt in the second year: nymphs feed and molt the third year: and adults feed and reproduce in the fourth year.
An example of species adapted to the arid zone are the camel ticks. In the harshest of Saharan desert habitats, H. dromedarii can survive by curtailing the life cycle. Under stress, it can molt on the camel host becoming essentially a two-host or even a one-host tick (Fard et al. 2012). This species is especially pliant regarding host range (Van Straten and Jongejan 1993). They spend the dry hot summer on the host camel as adults (May to September) (Gharbi et al. 2013) with the peak of nymphal infestation on the camel in January (Van Straten and Jongejan 1993). There is virtually no information on questing by the larvae, but by default it must be in the early winter months, which is also the only time that rain falls. Another desert adapted tick is A. hydrosauri, which specializes on the sleepy lizard in the outback of Australia. The sleepy lizard is so-called because it is dormant most of the year, restricting its activity to the mild spring months (August–September). Because development is accelerated by the high temperatures, the tick has two generations a year. The larvae quest in the spring (mainly in leaf litter under bushes where the lizards rest) and in the fall, mainly in the burrows where the lizards hide (Bull and Sharrad 1980).
Seasonality in temperate regions is characterized by cold winters and warm summers, whereas tropical regions are characterized by the dry and rainy seasons. In general, larvae of temperate zone ticks pass the winter feeding on the host or in diapause off of the host (Wilson and Spielman 1985, Randolph 1997). In northern Canada, larvae of D. albipictus (the winter tick) have been shown to quest in early September through late autumn (Kutz et al. 2009). In the winter, this one-host tick spends all three (larval, nymph, and adult) developmental stages on the host. In the northern United States, the off-host larvae enter diapause in the spring and remain quiescent until autumn when questing activity peaks. Diapause is terminated by shortening photoperiods, induced by the hormone ecdysone (Wright 1969, Drew and Samuel 1985). Questing is highly seasonal for D. variabilis larvae in the northern part of its range. In Canada, larval population numbers peak during the spring (Garvie et al. 1978). The questing larvae of the Lone Star tick, Amblyomma americanum (L.), in the central United States are present in late summer (Semtner and Hair 1973, Kollars et al. 2000). High temperatures and low relative humidity extend the larval hardening period for this species from 10 to 29 d (Semtner and Hair 1973).
For ticks in tropical zones, seasonality is driven largely by avoidance of the dry season. In São Paulo, Brazil, A. cajennense larvae enter behavioral diapause in springe, marked by long days (photoperiods > 12 h) lasting until the end of summer (October–March). Diapause terminates during autumn (April–May), synchronizing the life cycle with the rainy season (Cabrera and Labruna 2009). In equatorial Africa, there are three seasons: the rainy season, a cool dry season, and a hot dry season. In Zimbabwe, larvae of the bont tick, A. hebraeum are only active during the rainy season (Mooring et al. 1994). In contrast, blue tick, R. decoloratus larvae have been shown to quest during both dry and wet seasons but are more abundant in dry years (Walker et al. 2003). The neotropical tick, Amblyomma inornatum (Banks), has a 1-yr life cycle. In the northern part of its range in south Texas, it has a very distinct seasonality. The questing larvae are found only in the fall, from August to November (Medlin et al. 2015). In this same region, populations of R. microplus, quest and increase activity in late spring and early summer, referred to as the ‘spring rise’ (Davey et al. 1994, Leal et al. 2018). Similarly, Zamora et al. (2020) showed an increase in larval questing activity by the cattle fever tick, R. annulatus, in spring in south Texas.
The vast majority of Ixodes species have a 1- to 2-yr life cycle (Semtner and Hair 1973, Daniels et al. 1996, Randolph et al. 2002). With a 2-yr life cycle, the peak season for the larval questing activity of I. scapularis in northeastern North America and I. ricinus in Europe is in mid-summer of the first year. The 2-yr life cycle is temperature driven, and thus, their life cycle can last up to 6 yr in northern latitudes (Schulz et al. 2013, Jore et al. 2014, Burtis et al. 2016, Eisen et al. 2016). In Scotland, United Kingdom, I. ricinus larvae are most active during spring and early summer, times of high temperature and low humidity (Walker 2001). At the other extreme, toward the southern end of the range during warmer years, Ixodes ticks can complete one generation and begin a second generation later in the same year. In that case, larvae hatching in the fall enter diapause and overwinter as unfed larvae (Daniels et al. 1996, Randolph et al. 2002). In contrast, Ixodes pacificus (Cooley) larvae emerge from the eggs in mid to late summer, enter diapause through winter, then commence questing in spring (Padgett and Lane 2001).
Habitat Associations
Ticks are adapted to the environment of their hosts, which limits the types of habitat that a given tick species can successfully exploit (Walker et al. 2003). The time and place of detachment from the host by the replete female determines the habitat where eggs are laid and consequently where the larvae quest, typically ascending the plant nearest to the emergence site. Notably, some plants act as repellents and are thus avoided by larvae (Malonza et al. 1992) or have sticky trichomes, which trap the tick larvae (Fernandez-Ruvalcaba et al. 1999). Anti-tick grasses (Thompson et al. 1978, Wilson et al. 1989) and certain pasture legumes, produce a sticky secretion that can trap ascending larvae before they reach the tip of the stem (Sutherst et al. 1988).
Sutherst et al. (1986) found that lateral movement (movement away from the emergence site) by R. australis larvae was negligible. In another study, R. microplus larvae were shown to quest within 2 m of the oviposition site (Wilkinson 1953, 1957). Rechav (1979) measured lateral movement by R. appendiculatus and A. hebraeum larvae to be very short, around 80 cm. For R. evertsi, it was slightly greater, around 120 cm. Similarly, Daniels and Fish (1990) measured only 4% of I. scapularis moving >3 m from the oviposition site. However, Daniels and Fish (1990) also reported finding unfed larvae on nonhost mammals, raccoons and possums, suggesting that ‘transport hosts’ may disperse larvae over greater distances. Lewis (1970) found that winds can move ticks up to 30 m, but such dispersal would break up clusters leading to lower survival.
In theory, engorged ticks will drop most of the time in habitats most frequented by their host. For the off-host, engorged female, finding a favorable oviposition site translates to increased larval numbers (Sutherst et al. 1988). Typically, engorged females crawl to a sheltered site, but body weight limits the female from moving a significant distance (Hitchcock 1955, Balashov 1973). Basically, the habitat they fall into is where they oviposit. If conditions are optimal for oviposition, females still face two major threats: desiccation and predation (Bull et al. 1988). Branagan (1978) reported that desiccation of eggs was the ultimate limiting factor in determining the distribution of R. appendiculatus with oviposition failures occurring during maximum vapor saturation deficit. Similarly, Teel (1984) found that as saturation deficit increased, egg hatch would decrease in R. microplus and R. annulatus. Unlike adults or immatures, the eggs cannot absorb moisture even when humidity exceeds 95% (Yoder et al. 2015). As hatching success can be influenced by temperature and RH, these microhabitat conditions determine larval numbers (Sweatman 1967, Sonenshine and Tigner 1969, Oliver 1989, Despins 1992, Chilton and Bull 1994, Ogden et al. 2004, Sutherst and Bourne 2006). In many species, including I. scapularis and R. sanguineus, temperatures below 20°C lengthen the duration of oviposition (Sweatman 1967, Ogden et al. 2004). Conversely, temperatures above 30°C shortens the duration of the oviposition period and/or reduce total egg production in H. leporispalustris (Campbell and Glines 1979). In the Australian outback, A. limbatum and A. hydrosauri eggs failed to hatch below 21°C, and for both species, hatching time decreased with increasing temperatures (Chilton and Bull 1994). Hitchcock (1955) reported that for eggs of R. australis, hatching success was best when the temperature ranged between 21 and 37°C and at humidity levels at ≥70%. For the eggs of Amblyomma lepidum (Donitz), optimal temperature and humidity levels for development ranged from 27 to 35°C and above 90% RH (Binni et al. 2010).
Because of the movements of free-roaming hosts and limited movements of off-host females, the resulting questing ticks are subjected to a range of habitats which differ in microclimate. In Oklahoma, the larvae of A. americanum were shown to have lower survivorship in meadows compared to oak-hickory bottom lands (Semtner et al. 1971, Robertson et al. 1975). Numbers of A. hebraeum larvae were shown to be higher in dense acacia grasslands with well-drained soils compared with tree-shaded habitats with leaf-litter groundcover (Norval 1977). In Japan, Ha. flava larvae were shown to quest in late summer in woodlands compared with larvae being absent in grasslands in the same region at the same tie. This was attributed to high soil temperatures measured in the grasslands (Kakuda et al. 1990). In a study in Puerto Rico, R. microplus was shown to have maximum larval survivorship in wooded and grassy-wooded environments (Garris and Popham 1990, Garris et al. 1990). Similarly, larvae of Rhipicephalus (Boophilus) spp. survive longer in canopied habitats compared with exposed habitats (Teel et al. 1997, Corson et al. 2001, Leal et al. 2018, Zamora et al. 2020). In open pastures, Wilkinson (1953) observed that R. australis larvae would conceal themselves beneath grass blades, leaf axils, and seed heads to protect themselves from the midday sun (Fig. 3). Sonenshine and Stout (1968) reported the preference of D. variabilis larvae for grassy, herbaceous vegetation. Similarly, Civitello et al. (2008) found this same species near the edge of the forest where environmental conditions were more favorable compared with open fields. In Scotland, I. ricinus larvae were found to be most abundant in coniferous woodlands (Walker et al. 2001). For I. scaularis, shrubs, wooded pastures, and deciduous forests were all favorable habitats as long as they were moist (Carroll and Schmidtmann 1996, Lubelczyk et al. 2004). Larvae of the paralysis tick, Ixodes pilosus (Koch) were observed to mainly quest in habitats of short vegetation covered by a canopy of trees (Londt and Whitehead 1972). Of course, circumstances can be quite dynamic as climatic factors can make a favorable habitat in one season become suboptimal the next. For example, in sandy grassland habitats R. appendiculatus larvae were shown to be more abundant during years of high rainfall compared with low rainfall (Mooring et al. 1994). It is not uncommon for multiple species of ticks to share a habitat. At one site in Japan, Tsunoda (2008) found two co-occuring species questing on sedges, Ha. longicornis and Haemaphysalis megaspinosa (Sito). Normally, they are segregated by season, but when temporally overlapping Ha. longicornis shifted their aggregations to lower stem tips to avoid the competitor. Nevertheless, mixed clusters of both species were also found.
There is evidence that larvae seek the optimal height of vegetation corresponding to the height of their host. In the case of A. hebraeum, which use birds as larval hosts, larvae will quest at ˃40 cm height vegetation (Londt and Whitehead 1972). Larvae of D. albipictus were reported clustering 50–190 cm above ground, about the height of elk, moose, and deer (McPherson et al. 2000). Conversely, Ha. leporispalustris larvae only climb to heights of 7–19 cm, approximately, the height of rabbits (Camin and Drenner 1978). The larvae of Amblyomma incisum (Neumann) quest at ≈40–50 cm height within the vegetation, the larval host is unknown (Szabó et al. 2006, 2009). Microhabitats used by ticks can be host associated. Haemaphyalis silacea (Robinson) larvae are found in leaf litter in the dense vegetation zone on the margins of rivers; the larvae are generalists on many species of mammals and birds (Norval 1977). Engorged females of Haemphysalis spinigera (Neumann) the monkey tick, oviposit on the leaf litter on the florest floor, hence it is likely the larvae attach to monkeys foraging on the forest floor (Sadanandane et al. 2018). By questing for hosts in their burrows, nidicolous ticks experience less abiotic stress. Larave of I. hexagonus are found in the burrows of their preferred hosts, hedgehogs (Pfäffle et al. 2011). In contrast, pine martens that do not live in burrows were not suitable hosts for I. hexagonus larvae according to Christian (2012). In the case of A. limbatum, the majority of larvae were found in burrows or under bushes, used as shelters by their preferred hosts, the Australian sleepy lizard (Tiliqua rugosa) (Kerr and Bull 2006). Ixodes vespertilionis (Koch) larvae were observed questing on the walls of caverns that supported large bat populations only during March to June and were absent the rest of the year (Hornok et al. 2014). For Ixodes simplex (Neumann), questing activity was consistent year-round when parasitizing cave-dwelling bats (Lourenço and Palmeirim 2008). In tree-holes or nest boxes of roosting or brooding birds, warmer temperatures provide optimal developmental ranges and attachment sites for Ixodes arboricola (Schulze and Schlottke) larvae (Heylen et al. 2014). For camel ticks, H. dromedarii, the majority of larvae are found in crevices around the watering holes and attach to hosts that are resting at desert caravansaries (Higgins 1984, Sonenshine and Roe 1991). Hyalomma anatolicum (Koch) second to H. dromedarii larvae in parasitizing camels, most are commonly found in rodent burrows (Higgins 1984). In the Antarctic Peninsula, I. uriae larvae remain at close proximity to penguin rookeries by residing under nesting rocks and debris, only leaving their shelter to feed (Benoit et al. 2007).
Predators and Natural Enemies of Tick Larvae
During the off-host stage, larvae are vulnerable to predation by natural enemies that share the same habitat (Holm and Wallace 1989), but there is precious little empirical evidence of the impact. Although there is potential for biological control, it has not been proven feasible (Knipling 1992). And thus far, all known parasitoids target adults or nymphs, not larvae or eggs (Mwangi et al. 1991a). The intensive grazing of a pasture by livestock results in some accidental ingestion of larval Boophilus cattle ticks (Sutherst et al. 1978), and along with trampling, over-stocking can significantly reduce the larval population density. In parts of the southern United States, populations of Lone Star ticks, A. americanum, have been dramatically reduced by the imported fire ants, Solenopsis invicta (Burns and Melancon 1977). In a study done in Australia, predatory mites, Anysitidae (Holm and Wallace 1989) and Trombiculidae (Meyer and Hill 2014), showed the ability to prey on the I. holocyclus and R. microplus larvae. Ants, spiders, and carabid beetles are generalist predators that feed on larvae and eggs advantageously (Samish and Alekseev 2001). Such natural enemies tend to be habitat specific rather than prey specific. For example, it is hypothesized that fiddler crabs may limit larval tick populations in coastal areas (Showler et al. 2019), although predation was not actually observed. Pathogens may also be important under high humidity, conditions otherwise conducive to survival, as acaropathogenic fungi may cause significant mortality to larvae and eggs of ticks (Tuininga et al. 2009).
Summary Statement
Questing behavior and phenology adapt larval ticks to specific hosts and habitats.
Although ticks have their greatest impact on hosts, ticks spend a much greater proportion of their life cycle of off-host.
In population dynamic terms, the larvae are crucial as this stage experiences the greatest proportion of mortality.
A common denominator in larval tick ecology is a vulnerability to desiccation.
Epidemiologically, the larval stage is critical because in cases of transovarial transmission of the etiological agent, it is the larvae that vector disease.
Future Issues
The gaps in our knowledge of tick biology is greatest for the off-host stages. Very little is known about predation on the off-host stages. Longevity and persistence of off-host larvae is known for a very few species. Techniques to sample or census ticks off-host are not efficient, especially for the larval stage.
Acknowledgments
We would like to give special thanks to Alexis Racelis and Christopher Vitek of the University of Texas Rio Grande Valley, Michael Moses, Jason Tidwell, Summer De Luna, Joni Ortiz, Ruby Martinez, Bethany Olivarez, James Hellums, Cesario Agádo, Homero Vazquez, Ariel Hinojosa, and Charluz Arocho Rosario of the United States Department of Agriculture for their unwavering support and technical assistance. Also, thanks to Alejandra Fuentes for access and assistance with the USDA-APHIS scanning electron microscope at the Moore Air Base facility. This research was conducted in part to complete the requirements of the Master of Science Degree in the Department of Biology at the University of Texas Rio Grande Valley. Mention of trade names or commercial products in this article is for information purposes only and does not constitute endorsement by the USDA. The USDA is an equal opportunity provider and employer. In conducting the research described in this report, the investigators adhered to the ‘Guide for the Care and Use of Laboratory Animals’, as promulgated by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council. The facilities are fully accredited by the American Association of Laboratory Animal Care. This project was funded in part by the United States Department of Agriculture (USDA)-Agricultural Research Service, National Program 104, and Project #3094-32000-039-00D, STEP 2 USDA Research Success program (USDA grant 2015-38422-24061) and the National Institute of General Medical Sciences (1R25GM100866).
References Cited
- Andersen, L. K., and Davis M. D.. 2017. Climate change and the epidemiology of selected tick-borne and mosquito-borne diseases: update from the international society of dermatology climate change task force. Int. J. Dermatol. 56: 252–259. [DOI] [PubMed] [Google Scholar]
- Apanaskevich, D. A., and Oliver J. H.. 2014. Life cycles and natural history of ticks, pp. 59–73. InSonenshine D. E. and Roe R. M. (eds.), Biology of Ticks. Oxford University Press, New York. [Google Scholar]
- Arthur, D. R. 1951. The bionomics of Ixodes hexagonus Leach in Britain. Parasitology. 41: 82–90. [DOI] [PubMed] [Google Scholar]
- Balashov, Y. S. 1973. Bloodsucking ticks (Ixodoidea) – vectors of disease in man and animals, pp. 682–682. In H. Hoogstraal and R. J. Tatchell (eds.), Miscellaneous publications of the Entomological Society of America. The American Society of Tropical Medicine and Hygiene, College Park, MD. [Google Scholar]
- Belozerov, V. N. 1982. Diapause and biological rhythms in ticks, pp. 469–500. In Obenchain F. D. and Galun R. (eds.), Physiology of ticks. Pergamon, Oxford, England. [Google Scholar]
- Benoit, J. B., Yoder J. A., Lopez-Martinez G., Elnitsky M. A., R. E.Lee, Jr, and Denlinger D. L.. 2007. Habitat requirements of the seabird tick, Ixodes uriae (Acari: Ixodidae), from the Antarctic Peninsula in relation to water balance characteristics of eggs, nonfed and engorged stages. J. Comp. Physiol. B. 177: 205–215. [DOI] [PubMed] [Google Scholar]
- Binni, E. A., Yagi A. I., and Mohammed A. S.. 2010. The influence of temperature and humidity on oviposition and hatchability of Amblyomma lepidum (Dönitz, 1808) (Acarina: Ixodidae) under laboratory conditions. Vet. Parasitol. 170: 344–347. [DOI] [PubMed] [Google Scholar]
- Binnington, K. C. 1972. The distribution and morphology of probable photoreceptors in eight species of ticks (Ixodoidea). Z. Parasitenkd. 40: 321–332. [DOI] [PubMed] [Google Scholar]
- Boivin, G., Kollier-Ott U. M., Bale J. S., and Bigler F.. 2006. Assessing the establishment potential of inundative biological control agents, pp. 98–113. InBigler F., Babendreier D., and Kuhlmann U. (eds.), Environmental impact of invertebrates for biological control of arthropods: methods and risk assessment. CABI Publishing, Cambridge, MA. [Google Scholar]
- Branagan, D. 1973. The developmental periods of the Ixodid tick Rhipicephalus appendiculatus Neum. under laboratory conditions. Bull. Entomol. Res. 63: 155–168. [Google Scholar]
- Branagan, D. 1978. Observations on the development and survival of the ixodid tick Rhipicephalus appendiculatus Neumann, 1901 under quasi-natural conditions in Kenya. Trop. Anim. Health Prod. 5: 153–165. [DOI] [PubMed] [Google Scholar]
- Bull, C. M., and Sharrad R. D.. 1980. Seasonal activity of the reptile tick, Aponomma hydrosauri (Denny) (Acari: Ixodidae) in experimental enclosures. Aust. J. Entomol. 19: 47–52. [Google Scholar]
- Bull, C. M., Chilton N. B. I., and Sharrad R. D.. 1988. Risk of predation for two reptile tick species. Exp. Appl. Acarol. 5: 93–94. [Google Scholar]
- Burns, E. C., and Melancon D. G.. 1977. Effect of imported fire ant (Hymenoptera: Formicidae) invasion on lone star tick (Acarina: Ixodidae) populations. J. Med. Entomol. 14: 247–249. [DOI] [PubMed] [Google Scholar]
- Burtis, J. C., Sullivan P., Levi T., Oggenfuss K., Fahey T. J., and Ostfeld R. S.. 2016. The impact of temperature and precipitation on blacklegged tick activity and Lyme disease incidence in endemic and emerging regions. Parasit. Vectors. 9: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby, A. T., Ayllón N., Kocan K. M., Blouin E. F., de la Fuente G., Galindo R. C., Villar M., and de la Fuente J.. 2012. Expression of heat shock proteins and subolesin affects stress responses, Anaplasma phagocytophilum infection and questing behaviour in the tick, Ixodes scapularis. Med. Vet. Entomol. 26: 92–102. [DOI] [PubMed] [Google Scholar]
- Cabrera, R. R., and Labruna M. B.. 2009. Influence of photoperiod and temperature on the larval behavioral diapause of Amblyomma cajennense (Acari: Ixodidae). J. Med. Entomol. 46: 1303–1309. [DOI] [PubMed] [Google Scholar]
- Camin, J. H., and Drenner R. W.. 1978. Climbing behavior and host-finding larval rabbit ticks (Haemaphysalis leporispalustris). J. Parasitol. 64: 905–909. [PubMed] [Google Scholar]
- Campbell, A., and Glines M. V.. 1979. Development, survival, and oviposition of the rabbit tick, Haemaphysalis leporispalustris (Packard) (Acari: Ixodidae), at constant temperatures. J. Parasitol. 65: 777–781. [PubMed] [Google Scholar]
- Carroll, J. F., and Schmidtmann E. T.. 1996. Dispersal of blacklegged tick (Acari:Ixodidae) nymphs and adults at the woods-pasture interface. J. Med. Entomol. 33: 554–558. [DOI] [PubMed] [Google Scholar]
- de Castro, J. J., and Newson R. M.. 1993. Host resistance in cattle tick control. Parasitol. Today. 9: 13–17. [DOI] [PubMed] [Google Scholar]
- Chilton, N. B., and Bull C. M.. 1993. A comparison of the off-host survival times of larvae and nymphs of two species of reptile ticks. Inter. J. Parasitol. 23: 693–696. [Google Scholar]
- Chilton, N. B., and Bull C. M.. 1994. Influence of environmental factors on oviposition and egg development in Amblyomma limbatum and Aponomma hydrosauri (Acari: Ixodidae). Int. J. Parasitol. 24: 83–90. [DOI] [PubMed] [Google Scholar]
- Christian, A. 2012. Tick infestation (Ixodes) on the Eurasian otter (Lutra lutra) - a long-term study. Soil Org. 84: 481–487. [Google Scholar]
- Chu-Wang, I., and Axtell R. C.. 1973. Structure of ventral and lateral tarsal sensilla hard tick, Amblyomma americanum. Ann. Entomol. Soc. Am. 67: 453–457. [Google Scholar]
- Civitello, D. J., Flory S. L., and Clay K.. 2008. Exotic grass invasion reduces survival of Amblyomma americanum and Dermacentor variabilis ticks (Acari: Ixodidae). J. Med. Entomol. 45: 867–872. [DOI] [PubMed] [Google Scholar]
- Cody, M. L. 1966. A general theory of clutch size. Evolution. 20: 174–184. [DOI] [PubMed] [Google Scholar]
- Corson, M. S., Teel P. D., and Grant W. E.. 2001. Influence of acaricide resistance on cattle-fever tick (Boophilus spp.) infestations in semi-arid thornshrublands: a simulation approach. Exp. Appl. Acarol. 25: 171–184. [DOI] [PubMed] [Google Scholar]
- Daniel, M., and Dubabek F.. 1994. Micrometerological and microhabitat factors affecting maintenance and dissemination of tick-borne diseases in the environment, pp. 91–138. InSonenshine D. E. and Mather T. N. (eds.), Ecological Dynamics of Tick-Borne Zoonoses. Oxford University Press, New York. [Google Scholar]
- Daniel, M., Malý M., Danielová V., Bohumír K., and Nuttall P.. 2015. Abiotic predictors and annual seasonal dynamics of Ixodes ricinus, the major disease vector of Central Europe. Parasit. Vectors. 8: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels, T. J., and Fish D.. 1990. Spatial distribution and dispersal of unfed larval Ixodes dammini (Acari: Ixodidae) in Southern New York. Environ. Entomol. 19: 1029–1033. [Google Scholar]
- Daniels, T. J., Falco R. C., Curran K. L., and Fish D.. 1996. Timing of Ixodes scapularis (Acari: Ixodidae) oviposition and larval activity in southern New York. J. Med. Entomol. 33: 140–147. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres, F., Chomel B. B., and Otranto D.. 2012. Ticks and tick-borne diseases: a One Health perspective. Trends Parasitol. 28: 437–446. [DOI] [PubMed] [Google Scholar]
- Davey, R. B. 1986. Daily dynamics of egg development and fecundity and effect of age of larvae on attachment rate to cattle in Boophilus annulatus. Southwest. Entomol. 11: 17–22. [Google Scholar]
- Davey, R. B., Garza J., Thompson G. D., and Drummond O.. 1980. Ovipositional biology of the cattle tick, Boophilus annulatus (Acari: Ixodidae) in the laboratory. J. Med. Entomol. 17: 287–289. [Google Scholar]
- Davey, R. B., J.Garza, Jr, and Thompson G. D.. 1982. Seasonal observations on the development and ovipositional capability of Boophilus annulatus and B. microplus (Acari: Ixodidae) reared on bovines. J. Med. Entomol. 19: 24–28. [DOI] [PubMed] [Google Scholar]
- Davey, R. B., Cooksey L. M., and Despins J. L.. 1991. Survival of larvae of Boophilus annulatus, Boophilus microplus, and Boophilus hybrids (Acari: Ixodidae) in different temperature and humidity regimes in the laboratory. Vet. Parasitol. 40: 305–313. [DOI] [PubMed] [Google Scholar]
- Davey, R. B., Pound J. M., and Cooksey L. M.. 1994. Comparative reproduction and nonparasitic development of Boophilus microplus and hybridized Boophilus ticks (Acari: Ixodidae) under natural field conditions in subtropical south Texas. Exp. Appl. Acarol. 18: 185–200. [DOI] [PubMed] [Google Scholar]
- Delpy, L., and Gouchey S. H.. 1937. Biologie de Hyalomma dromedarii (Koch 1844). Ann. Parasitol. Humanie Comp. 15: 487–499. [Google Scholar]
- Despins, J. L. 1992. Effects of temperature and humidity on ovipositional biology and egg development of the tropical horse tick, Dermacentor (Anocentor) nitens. J. Med. Entomol. 29: 332–337. [DOI] [PubMed] [Google Scholar]
- Dinnik, J., and Zumpt F.. 1949. The integumentary sense organs of the larvae of Rhipicephalinae (Acarina). Psyche (Stuttg). 56: 1–18. [Google Scholar]
- Doube, B. M. 1975. Cattle and the paralysis tick Ixodes holocyclus. Aust. Vet. J. 51: 511–515. [DOI] [PubMed] [Google Scholar]
- Drew, L., and Samuel W. M.. 1985. Factors affecting transmission of larval winter ticks, Dermacentor albipictus (Packard), to moose, Alces alces L., in Alberta, Canada. J. Wildl. Dis. 21: 274–282. [DOI] [PubMed] [Google Scholar]
- Drummond, R. O., and Whetstone T. M.. 1970. Oviposition of the gulf coast tick. J. Econ. Entomol. 63: 1547–1551. [Google Scholar]
- Drummond, R. O., Whetstone T. M., Ernst S. E., and Gladney W. J.. 1971. Oviposition of the american dog tick (Acarina: Ixodidae). Ann. Entomol. Soc. Am. 64: 1305–1309. [Google Scholar]
- Dunn, L. H. 1918. Studies on the iguana tick, Amblyomma dissimile, in Panama. J. Parasitol. 5: 1–10. [Google Scholar]
- Durrani, A. Z., and Shakoori A. R.. 2009. Study on ecological growth conditions of cattle Hyalomma ticks in Punjab, Pakistan. Iran. J. Parasitol. 4: 19–25. [Google Scholar]
- Edney, E. B. 1977. Water balance in land arthropods. Springer, Berlin, Germany. [Google Scholar]
- Eisen, R. J., Eisen L., Ogden N. H., and Beard C. B.. 2016. Linkages of weather and climate with Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae), Enzootic transmission of Borrelia burgdorferi, and Lyme Disease in North America. J. Med. Entomol. 53: 250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elghali, A., and Hassan S. M.. 2010. Drop-off rhythms and survival periods of Hyalomma dromedarii (Acari: Ixodidae) fed on camels (Camelus dromedarius) in the Sudan. Vet. Parasitol. 170: 302–306. [DOI] [PubMed] [Google Scholar]
- Eremeeva, M. E., and Dasch G. A.. 2015. Challenges posed by tick-borne Rickettsiae: eco-epidemiology and public challenges. Front. Public Heal. 3: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Peña, A. 2001. Forecasting habitat suitability for ticks and prevention of tick-borne diseases. Vet. Parasitol. 98: 111–132. [DOI] [PubMed] [Google Scholar]
- Estrada-Peña, A., Guglielmone A. A., and Mangold A. J.. 2004. The distribution and ecological preferences of the tick Amblyomma cajennense (Acari: Ixodidae), an ectoparasite of humans and other mammals in the Americas. Ann. Trop. Med. Parasitol. 98: 283–292. [DOI] [PubMed] [Google Scholar]
- Eveleigh, E. S., and Threlfall W.. 1975. The biology of Ixodes (Ceratixodes) uriae White, 1852 in Newfoundland. Acarologia. 16: 621–635. [PubMed] [Google Scholar]
- Fard, S. R., Fathi S., Asl E. N., Nazhad H. A., and Kazeroni S. S.. 2012. Hard ticks on one-humped camel (Camelus dromedarius) and their seasonal population dynamics in southeast, Iran. Trop. Anim. Health Prod. 44: 197–200. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruvalcaba, M., Cruz-Vazquez C., Solano-Vergara J., and Garcia-Vazquez Z.. 1999. Anti-tick effects of Stylosanthes humilis and Stylosanthes hamata on plots experimentally infested with Boophilus microplus larvae in Morelos, Mexico. Exp. Appl. Acarol. 23: 171–175. [DOI] [PubMed] [Google Scholar]
- Frenot, Y., de Oliveira E., Gauthier-Clerc M., Deunff J., Bellido A., and Vernon P.. 2001. Life cycle of the tick Ixodes uriae in penguin colonies: relationships with host breeding activity. Int. J. Parasitol. 31: 1040–1047. [DOI] [PubMed] [Google Scholar]
- Gage, K. L., Burkot T. R., Eisen R. J., and Hayes E. B.. 2008. Climate and vectorborne diseases. Am. J. Prev. Med. 35: 436–450. [DOI] [PubMed] [Google Scholar]
- Garcia, M. V., Monteiro A. C., Szabó M. P., Mochi D. A., Simi L. D., Carvalho W. M., Tsuruta S. A., and Barbosa J. C.. 2011. Effect of Metarhizium anisopliae fungus on off-host Rhipicephalus (Boophilus) microplus from tick-infested pasture under cattle grazing in Brazil. Vet. Parasitol. 181: 267–273. [DOI] [PubMed] [Google Scholar]
- Garris, G. I., and Popham T. W.. 1990. Vertical distribution and longevity of Boophilus microplus (Acari: Ixodidae) larvae in a moist tropical grass environment in Puerto Rico. Environ. Entomol. 19: 1403–1409. [Google Scholar]
- Garris, G. I., Popham T. W., and Zimmerman R. H.. 1990. Boophilus microplus (Acari: Ixodidae): oviposition, egg viability, and larval longevity in grass and wooded environments of Puerto Rico. Environ. Entomol. 19: 66–75. [Google Scholar]
- Garvie, M. B., McKiel J. A., Sonenshine D. E., and Campbell A.. 1978. Seasonal dynamics of American dog tick, Dermacentor variabilis (Say), populations in southwestern Nova Scotia. Can. J. Zool. 56: 28–39. [DOI] [PubMed] [Google Scholar]
- Gharbi, M., Moussi N., Jedidi M., Mhadhbi M., Sassi L., and Darghouth M. A.. 2013. Population dynamics of ticks infesting the one-humped camel (Camelus dromedarius) in central Tunisia. Ticks Tick. Borne. Dis. 4: 488–491. [DOI] [PubMed] [Google Scholar]
- Gladney, W. J., Drummond R. O., Whetstone T. M., and Ernst S. E.. 1970. Effect of age on the attachment rate of the parasitic stages of the lone star tick, Amblyomma americanum (Linnaeus) (Acarina: Ixodidae), in the laboratory. J. Med. Entomol. 7: 92–95. [DOI] [PubMed] [Google Scholar]
- Gothe, R. 1967. Investigations into the cold resistance of the eggs and larvae of Boophilus decoloratus (Koch, 1844), Boophilus microplus (Canestrini, 1888) and Margaropus winthemi Karsch, 1879. Onderstepoort J. Vet. Res. 34: 109–127. [PubMed] [Google Scholar]
- Gray, J. S., Kahl O., Lane R. S., Levin M. L., and Tsao J. I.. 2016. Diapause in ticks of the medically important Ixodes ricinus species complex. Ticks Tick. Borne. Dis. 7: 992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmone, A. A. 1992. The effect of temperature and humidity on development and longevity of Amblyomma triguttatum (Acarina: Ixode). Bull. Entomol. Res. 82: 203–208. [Google Scholar]
- Hadley, N. 1994. Water relations of terrestrial arthropods, 1st ed.Academic Press Cambridge, MA. [Google Scholar]
- Hafez, M., El-Ziady S., and Hefnawy T.. 1970. Biochemical and physiological studies of certain ticks (Ixodoidea). Uptake of water vapor by different developmental stages of Hyalomma (H.) dromedarii Koch (Ixodidae) and Ornithodoros (O.) savignyi (Audouin) (Argasidae). J. Parasitol. 56: 354–361. [PubMed] [Google Scholar]
- Hagras, A. E., and Khalil G. M.. 1988. Effect of temperature on Hyalomma (Hyalomma) dromedarii Koch (Acari: Ixodidae). J. Med. Entomol. 25: 354–359. [DOI] [PubMed] [Google Scholar]
- Hargis, A. M., and Myers S.. 2017. Pathologic basis of veterinary disease, pp. 1009–1146. InZachary J. F. (ed.), Pathologic basis of veterinary disease. Elsevier, St. Louis, MO. [Google Scholar]
- Heath, A. C. G. 1981. The temperature and humidity preferences of Haemaphysalis longicornis, Ixodes holocyclus and Rhipicephalus sanguineus (Ixodidae): studies on engorged larvae. Int. J. P. 11: 169–175. [DOI] [PubMed] [Google Scholar]
- Heylen, D. J. A., Van Oosten A. R., Devriendt N., and Elst J.. 2014. Seasonal feeding activity of the tree-hole tick, Ixodes arboricola. Parasitology. 141: 1044–1051. [DOI] [PubMed] [Google Scholar]
- Higgins, A. J. 1984. The camel in health and disease. Introduction. Br. Vet. J. 140: 482–484. [PubMed] [Google Scholar]
- Hitchcock, L. F. 1955. Studies on the non-parasitic stages of the cattle tick, Boophilus microplus (Canestrini) (Acarina: Ixodidae). Aust. J. Zool. 3: 295–311. [Google Scholar]
- Holm, E., and Wallace M. M.. 1989. Distribution of some anystid mites (Acari: Anystidae) in Australia and Indonesia and their role as possible predators of the cattle tick, Boophilus microplus (Acari: Ixodidae). Exp. Appl. Acarol. 6: 77–83. [DOI] [PubMed] [Google Scholar]
- Holmes, C. J., Dobrotka C. J., Farrow D. W., Rosendale A. J., Benoit J. B., Pekins P. J., and Yoder J. A.. 2018. Low and high thermal tolerance characteristics for unfed larvae of the winter tick Dermacentor albipictus (Acari: Ixodidae) with special reference to moose. Ticks Tick. Borne. Dis. 9: 25–30. [DOI] [PubMed] [Google Scholar]
- Hoogstraal, H. 1956. African ixodidae. Vol. I. Ticks of the Sudan (with special reference to Equatoria Province and with preliminary reviews of the genera Boophilus, Margaropus, and Hyalomma). United States Navy Department. Bureau of Medicine and Surgery, Washington D.C. [Google Scholar]
- Hooker, W. A., Bishopp F. C., and Wood H. P.. 1912. The life history of bionomics of some north American ticks, 106th ed.U.S Department of Agriculture - Bureau of Entomology, Washington, DC. [Google Scholar]
- Hornok, S., Kontschán J., Kováts D., Kovács R., Angyal D., Görföl T., Polacsek Z., Kalmár Z., and Mihalca A. D.. 2014. Bat ticks revisited: Ixodes ariadnae sp. nov. and allopatric genotypes of I. vespertilionis in caves of Hungary. Parasit. Vectors. 7: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell, J. M., Ueti M. W., Palmer G. H., Scoles G. A., and Knowles D. P.. 2007. Transovarial transmission efficiency of Babesia bovis tick stages acquired by Rhipicephalus (Boophilus) microplus during acute infection. J. Clin. Microbiol. 45: 426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueffer, K., Hara T. M. O., and Follmann E. H.. 2011. Adaptation of mammalian host-pathogen interactions in a changing arctic environment. Acta Vet. Scand. 53: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, C. J., and Kitron U. D.. 2000. Populations of Ixodes scapularis (Acari: Ixodidae) are modulated by drought at a Lyme disease focus in Illinois. J. Med. Entomol. 37: 408–415. [DOI] [PubMed] [Google Scholar]
- Jongejan, F., and Uilenberg G.. 2004. The global importance of ticks. Parasitology. 129: S4–S14. [DOI] [PubMed] [Google Scholar]
- Jonsson, N. N., Mayer D. G., Matschoss A. L., Green P. E., and Ansell J.. 1998. Production effects of cattle tick (Boophilus microplus) infestation of high yielding dairy cows. Vet. Parasitol. 78: 65–77. [DOI] [PubMed] [Google Scholar]
- Jore, S., Vanwambeke S. O., Viljugrein H., Isaksen K., Kristoffersen A. B., Woldehiwet Z., Johansen B., Brun E., Brun-Hansen H., Westermann S., et al. 2014. Climate and environmental change drives Ixodes ricinus geographical expansion at the northern range margin. Parasit. Vectors. 7: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser, M. N., Sutherst R. W., Bourne A. S., Gorissen L., and Floyd R. B.. 1988. Population dynamics of ticks on Ankole cattle in five ecological zones in Burundi and strategies for their control. Prev. Vet. Med. 6: 199–222. [Google Scholar]
- Kakuda, H., Shiraishi S., and Uchida T. A.. 1990. Seasonal fluctuations of populations and effects of temperatures on development and growth in the tick, Haemaphysalis flava. J. Fac. Agric. Kyushu Univ. 35: 17–26. [Google Scholar]
- Kaltenrieder, M. 1990. Scototaxis and target perception in the camel tick Hyalomma dromedarii. Exp. Appl. Acarol. 9: 267–278. [DOI] [PubMed] [Google Scholar]
- Kerr, G. D., and Bull C. M.. 2006. Interactions between climate, host refuge use, and tick population dynamics. Parasitol. Res. 99: 214–222. [DOI] [PubMed] [Google Scholar]
- Knap, N., Durmisi E., Saksida A., Korva M., Petrovec M., and Avsic-Zupanc T.. 2009. Influence of climatic factors on dynamics of questing Ixodes ricinus ticks in Slovenia. Vet. Parasitol. 164: 275–281. [DOI] [PubMed] [Google Scholar]
- Knipling, E. F. 1992. Principles of insect parasitism analyzed From new perspectives practical implications for regulating insect populations by biological means, 1st ed.United States Department of Agriculture, Washington, DC. [Google Scholar]
- Knülle, W. 1966. Equilibrium humidities and survival of some tick larvae. J. Med. Entomol. 2: 335–338. [DOI] [PubMed] [Google Scholar]
- Knülle, W., and Devine T. L.. 1972. Evidence for active and passive components of sorption of atmospheric water vapour by larvae of the tick Dermacentor variabilis. J. Insect Physiol. 18: 1653–1664. [DOI] [PubMed] [Google Scholar]
- Knülle, W., and Rudolph D.. 1982. Humidity relationships and water balance of ticks. pp. 43–70. In Obenchain F. D. and Galun R. (eds.), Phsiology of ticks. Pergamon Press, Oxford, England. [Google Scholar]
- Kollars, T. M., Jr, J. H.Oliver, Jr, Durden L. A., and Kollars P. G.. 2000. Host association and seasonal activity of Amblyomma americanum (Acari: Ixodidae) in Missouri. J. Parasitol. 86: 1156–1159. [DOI] [PubMed] [Google Scholar]
- Kolonin, G. V. 2007. Mammals as hosts of Ixodid ticks (Acarina, Ixodidae). Entomol. Rev. 87: 401–412. [Google Scholar]
- Köppen, W. 1936. Das geographische system der klimate, pp. 1–44. InHeandb. Der Klimatologie. Verlag von Gebrüder Borntl’aeger, Borntraeger, Berlin, Germany. [Google Scholar]
- Krijgsman, B. J. 1937. Stimulus physiological studies on blood-sucking arthropods related to your nutritional choice. Arch. Néerlandaises Zool. 2: 401–413. [Google Scholar]
- Kutz, S. J., Jenkins E. J., Veitch A. M., Ducrocq J., Polley L., Elkin B., and Lair S.. 2009. The Arctic as a model for anticipating, preventing, and mitigating climate change impacts on host-parasite interactions. Vet. Parasitol. 163: 217–228. [DOI] [PubMed] [Google Scholar]
- Labruna, M. B., Kasai N., Ferreira F., Faccini J. L., and Gennari S. M.. 2002. Seasonal dynamics of ticks (Acari: Ixodidae) on horses in the state of São Paulo, Brazil. Vet. Parasitol. 105: 65–77. [DOI] [PubMed] [Google Scholar]
- Labruna, M. B., Naranjo V., Mangold A. J., Thompson C., Estrada-peña A., Guglielmone A. A., Jongejan F., and De Fuente J.. 2009. Allopatric speciation in ticks: genetic and reproductive divergence between geographic strains of Rhipicephalus (Boophilus) microplus. BMC Evol. Biol. 46: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal, B., Thomas D. B., and Dearth R. K.. 2018. Population dynamics of off-host Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) larvae in response to habitat and seasonality in south Texas. Vet. Sci. 5: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, R. E., and Baust J. G.. 1987. Cold-hardiness in the Antarctic Tick, Ixodes uriae. Univ. Chicago Press Journal1. 60: 499–506. [Google Scholar]
- Lees, A. D. 1946. The water balance in Ixodes ricinus L. and certain other species of ticks. Parasitology. 37: 1–20. [DOI] [PubMed] [Google Scholar]
- Lees, A. D. 1947. Transpiration and the structure of the epicuticle in ticks. J. Exp. Biol. 23: 379–410. [DOI] [PubMed] [Google Scholar]
- Lees, A. D. 1948. The sensory physiology of the sheep tick, Ixodes ricinus L. J. Exp. Biol. 25: 145–207. [Google Scholar]
- Lees, A. D., and Milne A.. 1951. The seasonal and diurnal activities of individual sheep ticks (Ixodes ricinus L). Parasitology. 41: 189–208. [DOI] [PubMed] [Google Scholar]
- Leonovich, S. A. 2013. The main evolutionary trends in sensory organs and questing behavior of Parasitiform ticks and mites. Entomol. Rev. 93: 1190–1191. [PubMed] [Google Scholar]
- Lewis, I. J. 1970. Observations on the dispersal of larvae of the cattle tick Boophilus microplus (Can.). Bull. Entomol. Res. 59: 595–604. [DOI] [PubMed] [Google Scholar]
- Lindsay, L. R., Mathison S. W., Barker I. K., McEwen S. A., and Surgeoner G. A.. 1999. Abundance of Ixodes scapularis (Acari: Ixodidae) larvae and nymphs in relation to host density and habitat on Long Point, Ontario. J. Med. Entomol. 36: 243–254. [DOI] [PubMed] [Google Scholar]
- Londt, G. H., and Van Der Bijl E. B.. 1977. The life cycle of the two-host tick Rhipicephalus evertsi evertsi Neumann, 1897, under laboratory conditions (Acarina: Ixodidae). Onderstepoort J. Vet. Res. 44: 21–28. [PubMed] [Google Scholar]
- Londt, J. G. H., and Whitehead G. B.. 1972. Parasitology ecological studies of larval ticks in south Africa (Acarina: Ixodidae). Parasitology. 65: 469–490. [DOI] [PubMed] [Google Scholar]
- Loomis, E. C. 1961. Life histories of ticks under laboratory conditions (Acarina: Ixodidae and Argasidae). J. Parasitol. 47: 91–99. [PubMed] [Google Scholar]
- Lourenço, S., and Palmeirim J. M.. 2008. Which factors regulate the reproduction of ectoparasites of temperate-zone cave-dwelling bats? Parasitol. Res. 104: 127–134. [DOI] [PubMed] [Google Scholar]
- Lubelczyk, C. B., Elias S. P., Rand P. W., Holman M. S., Lacombe E. H., and Smith R. P.. 2004. Habitat associations of Ixodes scapularis (Acari: Ixodidae) in Maine. Environ. Entomol. 33: 900–906. [Google Scholar]
- Malonza, M. M., Dipeolu O. O., Amoo A. O., and Hassan S. M.. 1992. Laboratory and field observations on anti-tick properties of the plant Gynandropsis gynandra (L.) Brig. Vet. Parasitol. 42: 123–136. [DOI] [PubMed] [Google Scholar]
- Matuschka, F. R., Richter D., Fischer P., and Spielman A.. 1990. Nocturnal detachment of the tick Ixodes hexagonus from nocturnally active hosts. Med. Vet. Entomol. 4: 415–420. [DOI] [PubMed] [Google Scholar]
- McCabe, G. J., and Bunnell J. E.. 2004. Precipitation and the occurrence of Lyme Disease in the northeastern United States. Vector-borne Zoonotic Dis. 4: 129–134. [DOI] [PubMed] [Google Scholar]
- McCulloch, B., and Tungaraza R.. 2015. Skin disease in cattle hides and goat skins in North-Western Tanzania. East African Agric. For. J. 32: 240–245. [Google Scholar]
- McPherson, M., Shostak A. W., and Samuel W. M.. 2000. Climbing simulated vegetation to heights of ungulate hosts by larvae of Dermacentor albipictus (Acari: Ixodidae). J. Med. Entomol. 37: 114–120. [DOI] [PubMed] [Google Scholar]
- Medlin, J. S., Cohen J. I., and Beck D. L.. 2015. Vector potential and population dynamics for Amblyomma inornatum. Ticks Tick. Borne. Dis. 6: 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, J. M., and Hill C. A.. 2014. Tick genetics, genomics, and transformation, pp. 61–87. InSonenshine D. E. and Roe R. M. (eds.), Biology of Ticks. Oxford University Press, New York. [Google Scholar]
- Mitchell, R. D., 3rd, Zhu J., Carr A. L., Dhammi A., Cave G., Sonenshine D. E., and Roe R. M.. 2017. Infrared light detection by the Haller’s organ of adult american dog ticks, Dermacentor variabilis (Ixodida: Ixodidae). Ticks Tick. Borne. Dis. 8: 764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooring, M. S., Mazhowu W., and Scott C. A.. 1994. The effect of rainfall on tick challenge at Kyle Recreational Park, Zimbabwe. Exp. Appl. Acarol. 18: 507–520. [DOI] [PubMed] [Google Scholar]
- Morales, J. 2019. Removing black-legged tick (Ixodes scapularis) Haller’s organs affects their ability to detect a carbon dioxide gradient. Sr. Proj. Spring 2019. 10: 1–38. [Google Scholar]
- Mount, G. A., Haile D. G., Davey R. B., and Cooksey L. M.. 1991. Computer simulation of Boophilus cattle tick (Acari: Ixodidae) population dynamics. J. Med. Entomol. 28: 223–240. [DOI] [PubMed] [Google Scholar]
- Murray, M. D., and Vestjens W. J. M.. 1967. Studies on the ectoparasites of seals and penguins. III. The distribution of the tick Ixodes uriae White and the flea Parapsyllus magellanicus heardi de Meillon on Macquarie Island. Aust. J. Zool. 15: 715–725. [Google Scholar]
- Mwangi, E. N., Dipeolu O. O., Newson R. M., Godwin P., Hassan S. M., and Hassan K.. 1991a. Predators, parasitoids and pathogens of ticks: a review. Biocontrol Sci. Technol. 1: 147–159. [Google Scholar]
- Mwangi, E. N., Newson R. M., and Kaaya G. P.. 1991b. Predation of free-living engorged female Rhipicephalus appendiculatus. Exp. Appl. Acarol. 12: 153–162. [DOI] [PubMed] [Google Scholar]
- Needham, G. R., and Teel P. D.. 1991. Off-host physiological ecology of ixodid ticks. Annu. Rev. Entomol. 36: 659–681. [DOI] [PubMed] [Google Scholar]
- Nicholson, W. L., Sonenshine D. E., Noden B. H., and Brown R. N.. 2019. Ticks (Ixodida), 3rd ed, Medical and Veterinary Entomology. Elsevier Inc., London, United Kingdom. [Google Scholar]
- Norval, R. A. I. 1977. Studies on the ecology of the tick Amblyomma hebraeum Koch in the eastern cape province of south Africa. II. Survival and development. J. Parasitol. 63: 740–747. [PubMed] [Google Scholar]
- Nuñez, J. L., Muñoz Cobeñas M. E., and Moltedo H. L.. 1982. Boophilus microplus: la garrapta común del ganado vacuno. Editorial Hemisferio SUR, 1982, Buenos Aires, Argentina. [Google Scholar]
- Nuttall, G. H. F., Cooper W. F., and Robinson L. E.. 1908. On the structure of ‘Haller’s organ’ in the Ixodoidea. Parasitology. 1: 238–242. [Google Scholar]
- Ogden, N. H., and Lindsay L. R.. 2016. Effects of climate and climate change on vectors and vector-borne diseases: ticks are different. Trends Parasitol. 32: 646–656. [DOI] [PubMed] [Google Scholar]
- Ogden, N. H., Cripps P., Davison C. C., Owen G., Parry J. M., Timms B. J., and Forbes A. B.. 2000. The ixodid tick species attaching to domestic dogs and cats in Great Britain and Ireland. Med. Vet. Entomol. 14: 332–338. [DOI] [PubMed] [Google Scholar]
- Ogden, N. H., Lindsay L. R., Beauchamp G., Charron D., Maarouf A., O’Callaghan C. J., Waltner-Toews D., and Barker I. K.. 2004. Investigation of relationships between temperature and developmental rates of tick Ixodes scapularis (Acari: Ixodidae) in the laboratory and field. J. Med. Entomol. 41: 622–633. [DOI] [PubMed] [Google Scholar]
- Ogden, N. H., Trudel L., Artsob H., Barker I. K., Beauchamp G., Charron D. F., Drebot M. A., Galloway T. D., O’Handley R., Thompson R. A., et al. 2006. Ixodes scapularis ticks collected by passive surveillance in Canada: analysis of geographic distribution and infection with Lyme borreliosis agent Borrelia burgdorferi. J. Med. Entomol. 43: 600–609. [DOI] [PubMed] [Google Scholar]
- Oliver, J. H. 1989. Biology and systematics of ticks (Acari: Ixodida). Annu. Rev. Ecol. Syst. 20: 397–430. [Google Scholar]
- Ouhelli, H., Pandey V. S., and Choukri M.. 1982. The effects of temperature, humidity, photoperiod and weight of the engorged female on oviposition of Boophilus annulatus (Say, 1821). Vet. Parasitol. 11: 231–239. [DOI] [PubMed] [Google Scholar]
- Padgett, K. A., and Lane R. S.. 2001. Life cycle of Ixodes pacificus (Acari: Ixodidae): timing of developmental processes under field and laboratory conditions. J. Med. Entomol. 38: 684–693. [DOI] [PubMed] [Google Scholar]
- Parola, P., and Raoult D.. 2001. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin. Infect. Dis. 32: 897–928. [DOI] [PubMed] [Google Scholar]
- Pegram, R. G., and Banda D. S.. 1990. Ecology and phenology of cattle ticks in Zambia: development and survival of free-living stages. Exp. Appl. Acarol. 8: 291–301. [DOI] [PubMed] [Google Scholar]
- Perret, J. L., Guerin P. M., Diehl P. A., Vlimant M., and Gern L.. 2003. Darkness induces mobility, and saturation deficit limits questing duration, in the tick Ixodes ricinus. J. Exp. Biol. 206: 1809–1815. [DOI] [PubMed] [Google Scholar]
- Pfäffle, M., Petney T., Skuballa J., and Taraschewski H.. 2011. Comparative population dynamics of a generalist (Ixodes ricinus) and specialist tick (I. hexagonus) species from European hedgehogs. Exp. Appl. Acarol. 54: 151–164. [DOI] [PubMed] [Google Scholar]
- Phillis, W. A., 3rd, and Cromroy H. L.. 1977. The microanatomy of the eye of Amblyomma americanum (Acari: Ixodidae) and resultant implications of its structure. J. Med. Entomol. 13: 685–698. [DOI] [PubMed] [Google Scholar]
- Pianka, E. R. 1972. r and K selection or b and d selection? Am. Soc. Nat. 106: 581–588. [Google Scholar]
- Ramos, V. D. O. N., Osava C. F., Piovezan U., and Szabó M. P. J.. 2014. Complementary data on four methods for sampling free-living ticks in the Brazilian Pantanal. Rev. Bras. Parasitol. Vet. 23: 516–521. [DOI] [PubMed] [Google Scholar]
- Randolph, S. E. 1997. Abiotic and biotic determinants of the seasonal dynamics of the tick Rhipicephalus appendiculatus in South Africa. Med. Vet. Entomol. 11: 25–37. [DOI] [PubMed] [Google Scholar]
- Randolph, S. E. 2004. Tick ecology: processes and patterns behind the epidemiological risk posed by ixodid ticks as vectors. Parasitology. 129 Suppl: S37–S65. [DOI] [PubMed] [Google Scholar]
- Randolph, S. E., and Storey K.. 1999. Impact of microclimate on immature tick-rodent host interactions (Acari: Ixodidae): implications for parasite transmission. J. Med. Entomol. 36: 741–748. [DOI] [PubMed] [Google Scholar]
- Randolph, S. E., Green R. M., Hoodless A. N., and Peacey M. F.. 2002. An empirical quantitative framework for the seasonal population dynamics of the tick Ixodes ricinus. Int. J. Parasitol. 32: 979–989. [DOI] [PubMed] [Google Scholar]
- Rechav, Y. 1979. Migration and dispersal patterns of three African ticks. J. Med. Entomol. 16: 150–163. [Google Scholar]
- Rechav, Y., Terry S., Knight M. M., and Cross R. H.. 1977. Chemoreceptor organs used in detection of pheromone(s) of the tick Amblyomma hebraeum (Acarina: Ixodidae). J. Med. Entomol. 14: 395–400. [DOI] [PubMed] [Google Scholar]
- Roberts, J. A. 1971. Behavior of larvae of the cattle tick, Boophilus microplus (Canestrini), on cattle of differing degrees of resistance. J. Parasitol. 57: 651–656. [PubMed] [Google Scholar]
- Robertson, A. S., Patrick C. D., Semtner P. J., and Hair J. A.. 1975. The ecology and behavior of the lone star tick (Acarina: Ixodidae). VII. Pre- and post-molt behavior of engorged nymphs and larvae. J. Med. Entomol. 12: 530–534. [DOI] [PubMed] [Google Scholar]
- Rosenberg, R., Lindsey N. P., Fischer M., Gregory C. J., Hinckley A. F., Mead P. S., Paz-Bailey G., Waterman S. H., Drexler N. A., Kersh G. J., et al. 2018. Vital signs: trends in reported vectorborne disease cases—United States and territories, 2004–2016. Morb. Mortal. Wkly. Rep. 67: 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadanandane, C., Gokhale M. D., Elango A., Yadav P., Mourya D. T., and Jambulingam P.. 2018. Prevalence and spatial distribution of Ixodid tick populations in the forest fringes of Western Ghats reported with human cases of Kyasanur forest disease and monkey deaths in South India. Exp. Appl. Acarol. 75: 135–142. [DOI] [PubMed] [Google Scholar]
- Samish, M., and Alekseev E.. 2001. Arthropods as predators of ticks (Ixodoidea). J. Med. Entomol. 38: 1–11. [DOI] [PubMed] [Google Scholar]
- Schulz, M., Mahling M., and Pfister K.. 2013. Abundance and seasonal activity of questing Ixodes ricinus ticks in their natural habitats in southern Germany in 2011. J. Vector Ecol. 39: 56–65. [DOI] [PubMed] [Google Scholar]
- Schulze, P. 1942. Die morphologische bedeutung des afters und seiner umgebung bei den zecken. Zeitschrift für Morphol. und Ökologie der Tiere. 38: 630–658. [Google Scholar]
- Schulze, T. L., Jordan R. A., and Hung R. W.. 1997. Biases associated with several sampling methods used to estimate abundance of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 34: 615–623. [DOI] [PubMed] [Google Scholar]
- Semtner, P. J., and Hair J. A.. 1973. The ecology and behavior of the lone star tick (Acarina: Ixodidae). IV. The daily and seasonal activity patterns of adults in different habitat types. J. Med. Entomol. 10: 337–344. [DOI] [PubMed] [Google Scholar]
- Semtner, P. J., Barker R. W., and Hair J. A.. 1971. The ecology and behavior of the lone star tick (Acarina: Ixodidae). II. Activity and survival in different ecological habitats. J. Med. Entomol. 8: 719–725. [DOI] [PubMed] [Google Scholar]
- Short, N. J., and Norval R. A.. 1981. The seasonal activity of Rhipicephalus appendiculatus Neumann 1901 (Acarina: Ixodidae) in the highveld of Zimbabwe Rhodesia. J. Parasitol. 67: 77–84. [PubMed] [Google Scholar]
- Short, N. J., Floyd R. B., Norval R. A. I., and Sutherst R. W.. 1989. Survival and behaviour of unfed stages of the ticks Rhipicephalus appendiculatus, Boophilus decoloratus, and B. microplus under field conditions in Zimbabwe. Exp. Appl. Acarol. 6: 215–236. [DOI] [PubMed] [Google Scholar]
- Showler, A. T., Osbrink W. L. A., Abrigo V., and Phillips P. L.. 2019. Relationships of salinity, relative humidity, mud flat fiddler crabs, ants, and sea ox-eye daisy with ixodid distribution and egg survival on the South Texas coastal plains. Environ. Entomol. 48: 733–746. [DOI] [PubMed] [Google Scholar]
- Sonenshine, D. E., and Roe M. R.. 1991. Biology of ticks, 2nd ed.Oxford University Press, New York. [Google Scholar]
- Sonenshine, D. E., and Stout I. J.. 1968. Use of old-field habitats by the American Dog Tick, Dermacentor variabilis. Ann. Entomol. Soc. Am. 61: 678–686. [DOI] [PubMed] [Google Scholar]
- Sonenshine, D. E., and Tigner J. A.. 1969. Oviposition and hatching in two species of ticks in relation to moisture deficit. J. Econ. Entomol. 62: 628–640. [DOI] [PubMed] [Google Scholar]
- van Straten, M., and Jongejan F.. 1993. Ticks (Acari: Ixodidae) infesting the Arabian camel (Camelus dromedarius) in the Sinai, Egypt with a note on the acaricidal efficacy of ivermectin. Exp. Appl. Acarol. 17: 605–616. [DOI] [PubMed] [Google Scholar]
- Sutherst, R. W., and Bourne A. S.. 2006. The effect of desiccation and low temperature on the viability of eggs and emerging larvae of the tick, Rhipicephalus (Boophilus) microplus (Canestrini) (Ixodidae). Int. J. Parasitol. 36: 193–200. [DOI] [PubMed] [Google Scholar]
- Sutherst, R. W., and Bourne A. S.. 2009. Modelling non-equilibrium distributions of invasive species: a tale of two modelling paradigms. Biol. Invasions. 11: 1231–1237. [Google Scholar]
- Sutherst, R. W., and Maywald G. F.. 1985. A computerised system for matching climates in ecology. Agric. Ecosyst. Environ. 13: 281–299. [Google Scholar]
- Sutherst, R. W., Wharton R. H., and Utech K. B. W.. 1978. Guide to studies on tick ecology. CSIRO, Melbourne, Australia. [Google Scholar]
- Sutherst, R. W., Floyd R. B., Bourne A. S., and Dallwitz M. J.. 1986. Cattle grazing behavior regulates tick populations. Experientia. 42: 194–196. [Google Scholar]
- Sutherst, R. W., Wilson L. J., Reid R., and Kerr J. D.. 1988. A survey of the ability of tropical legumes in the genus Stylosanthes to trap larvae of the cattle tick, Boophilus microplus (Ixodidae). Aust. J. Exp. Agric. 28: 473–479. [Google Scholar]
- Sweatman, G. K. 1967. Physical and biological factors affecting the longevity and oviposition of engorged Rhipicephalus sanguineus female ticks. J. Parasitol. 53: 432–445. [PubMed] [Google Scholar]
- Szabó, M. P., Labruna M. B., Castagnolli K. C., Garcia M. V., Pinter A., Veronez V. A., Magalhães G. M., Castro M. B., and Vogliotti A.. 2006. Ticks (Acari: Ixodidae) parasitizing humans in an Atlantic rainforest reserve of Southeastern Brazil with notes on host suitability. Exp. Appl. Acarol. 39: 339–346. [DOI] [PubMed] [Google Scholar]
- Szabó, M. P. J., Labruna M. B., Garcia M. V., Pinter G. A., Castagnolli K. C., Pacheco R. C., Castro M. B., Veronez V. A., Magalhaes G. M., Vogliotti A., et al. 2009. Ecological aspects of the free-living ticks (Acari: Ixodidae) on animal trails within Atlantic rainforest in south – eastern. Ann. Trop. Med. Parasitol. 103: 57–72. [DOI] [PubMed] [Google Scholar]
- Teel, P. D. 1984. Effect of saturation deficit on eggs of Boophilus annulatus and B. microplus (Acari: Ixodidae). Ann. Entomol. Soc. Am. 77: 65–68. [Google Scholar]
- Teel, P. D., Marin S., Grant W. E., and Stuth J. W.. 1997. Simulation of host-parasite-landscape interactions : influence of season and habitat on cattle fever tick (Boophilus sp.) population dynamics in rotational grazing systems. Ecol. Modell. 97: 87–97. [Google Scholar]
- Thompson, K. C., Roa E J., and Romero N T.. 1978. Anti-tick grasses as the basis for developing practical tropical tick control packages. Trop. Anim. Health Prod. 10: 179–182. [DOI] [PubMed] [Google Scholar]
- Toutoungi, L. N., Gern L., and Aeschlimann A.. 1995. Biology of Ixodes (Pholeoixodes) hexagonus under laboratory conditions. Part II. Effect of mating on feeding and fecundity of females. Exp. Appl. Acarol. 19: 233–245. [DOI] [PubMed] [Google Scholar]
- Troughton, D. R., and Levin M. L.. 2007. Life cycles of seven ixodid tick species (Acari: Ixodidae) under standardized laboratory conditions. J. Med. Entomol. 44: 732–740. [DOI] [PubMed] [Google Scholar]
- Tsunoda, T. 2008. Influence of aggregation and relative humidity on longevity of unfed bush tick, Haemaphysalis longicornis Neumann (Acari: Ixodidae). J. Parasitol. 94: 990–992. [DOI] [PubMed] [Google Scholar]
- Tuininga, A. R., Miller J. L., Morath S. U., Daniels T. J., Falco R. C., Marchese M., Sahabi S., Rosa D., and K. C.Stafford, 3rd. 2009. Isolation of entomopathogenic fungi from soils and Ixodes scapularis (Acari: Ixodidae) ticks: prevalence and methods. J. Med. Entomol. 46: 557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukahirwa, E. M. 1976. The effects of temperature and relative humidity on the development of Rhipicephalus appendiculatus Neumann (Acarina, Ixodidae). Bull. Entomol. Res. 66: 301–312. [Google Scholar]
- Utech, K. B. W., Sutherst R. W., Dallwitz M. T., Wharton R. H., Maywald G. F., and Sutherland I. D.. 1983. A model of the survival of larvae of the cattle tick, Boophilus microplus, on pasture. Aust. J. Agric. Res. 34: 63–72. [Google Scholar]
- Vandyk, J. K., Bartholomew D. M., Rowley W. A., and Platt K. B.. 1996. Survival of Ixodes scapularis (Acari: Ixodidae) exposed to cold. J. Med. Entomol. 33: 6–10. [DOI] [PubMed] [Google Scholar]
- Waladde, S. M. 1976. The sensory nervous system of the adult cattle tick Boophilus microplus (Canestrini) Ixodidae part I light microscopy. Aust. J. Entomol. 15: 379–387. [Google Scholar]
- Walker, A. R. 2001. Age structure of a population of Ixodes ricinus (Acari: Ixodidae) in relation to its seasonal questing. Bull. Entomol. Res. 91: 69–78. [PubMed] [Google Scholar]
- Walker, A. R., Alberdi M. P., Urquhart K. A., and Rose H.. 2001. Risk factors in habitats of the tick Ixodes ricinus influencing human exposure to Ehrlichia phagocytophila bacteria. Med. Vet. Entomol. 15: 40–49. [DOI] [PubMed] [Google Scholar]
- Walker, A. R., Bouattour A., Camicas J.-L., Estrada-Pena A., Horak I. G., Latif A. A., Pegram R. G., and Preston P. M.. 2003. Ticks of domestic animals in Africa: a guide to identification of species. Bioscience Reports, Edinburgh, United Kingdom. [Google Scholar]
- Wilkinson, P. R. 1953. Observations on the sensory physiology and behaviour of larvae of the cattle tick, Boophilus microplus (Can) (Ixodidae). Aust. J. Zool. 1: 345–356. [Google Scholar]
- Wilkinson, P. R. 1957. The spelling of pasture in cattle tick control. Aust. J. Agric. Res. 8: 414–423. [Google Scholar]
- Wilkinson, P. R. 1961. The use of sampling methods in studies of the distribution of larvae of Boophilus microplus on pastures. Aust. J. Zool. 9: 752–783. [Google Scholar]
- Wilkinson, P. R., and Wilson J. T.. 1959. Survival of cattle ticks in central Queensland pastures. Aust. J. Agric. Res. 10: 129–143. [Google Scholar]
- Willadsen, P., Williams P. G., Roberts J. A., and Kerr J. D.. 1978. Responses of cattle to allergens from Boophilus microplus. Int. J. Parasitol. 8: 89–95. [DOI] [PubMed] [Google Scholar]
- Wilson, M. L., and Spielman A.. 1985. Seasonal activity of immature Ixodes dammini (Acari: Ixodidae). J. Med. Entomol. 22: 408–414. [DOI] [PubMed] [Google Scholar]
- Wilson, L. J., Sutherst R. W., and Kerr J. D.. 1989. Trapping of larvae of the cattle tick Boophilus microplus by Stylosanthes scabra under grazing conditions. Aust. J. Agric. Res. 40: 1301–1308. [Google Scholar]
- Wright, J. E. 1969. Hormonal termination of larval diapause in Dermacentor albipictus. Science. 163: 390–391. [DOI] [PubMed] [Google Scholar]
- Yoder, J. A., and Knapp D. C.. 1999. Cluster-promoted water conservation by larvae of the American dog tick, Dermacentor variabilis (Acari: Ixodidae). Int. J. Acarol. 25: 55–57. [Google Scholar]
- Yoder, J. A., and Spielman A.. 1992. Differential capacity of larval deer ticks (Ixodes dammini) to imbibe water from subsaturated air. J. Insect Physiol. 38: 862–869. [Google Scholar]
- Yoder, J. A., Tank J. L., and Rellinger E. J.. 2006. Evidence of a maternal effect that protects against water stress in larvae of the American dog tick, Dermacentor variabilis (Acari: Ixodidae). J. Insect Physiol. 52: 1034–1042. [DOI] [PubMed] [Google Scholar]
- Yoder, J. A., Benoit J. B., Bundy M. R., Hedges Z., and Gribbins K. M.. 2009. Functional morphology of secretion by the large wax glands (sensilla sagittiformia) involved in tick defense. Pysche. 631030. [Google Scholar]
- Yoder, J. A., Pekins P. J., Jones H. F., Nelson B. W., Lorenz A. L., and Jajack A. J.. 2015. Water balance attributes for off-host survival in larvae of the winter tick (Dermacentor albipictus; Acari: Ixodidae) from wild moose. Int. J. Acarol. 42: 26–33. [Google Scholar]
- Zamora, E. J., Leal B., Thomas D. B., and Dearth R. K.. 2020. Survival of off-host unfed Rhipicephalus (Boophilus) annulatus larvae in study arenas in relation to climatic factors and habitats in South Texas, USA. Ticks Tick. Borne. Dis. 11: 101317. [DOI] [PMC free article] [PubMed] [Google Scholar]