Key Points
Question
Do hearing impairment (HI) incidence and rates of change in pure-tone average (PTA) differ by generation of birth?
Findings
In this cohort study of data from 3651 adults in the Epidemiology of Hearing Loss Study and Beaver Dam Offspring Study, younger generations experienced decreased incidence of HI and slower rates of increase in PTA when controlling for known risk factors. Younger generations are experiencing better hearing during aging than previous generations.
Meaning
This study suggests that unknown, potentially modifiable risk factors may explain these generational differences in HI incidence and PTA change, and the future burden of age-related HI may be overestimated.
Abstract
Importance
Age-adjusted prevalence of hearing impairment (HI) decreased across generations in the 20th century, suggesting that HI is partially preventable. It is not known whether HI incidence differs by generation.
Objectives
To examine whether HI incidence and change in pure-tone average (PTA) differ by generation and identify factors underlying these differences.
Design, Setting, and Participants
This cohort study used data from the Epidemiology of Hearing Loss Study (EHLS) and Beaver Dam Offspring Study (BOSS), a pair of studies of adults in Beaver Dam, Wisconsin. Baseline examinations occurred from 1993 to 1995 in the EHLS and 2005 to 2008 in BOSS, with two 5-year follow-up examinations in each cohort. This longitudinal cohort study assessed 3651 participants without HI at baseline who had follow-up data.
Main Outcomes and Measures
The primary outcome was incident HI measured by pure-tone audiometry, defined as PTA greater than 25-dB hearing level (dB HL) in either ear. Associations of 5-year incidence were estimated by relative risks (RRs) and 10-year cumulative incidence with generation, as categorized by commonly used sociodemographic descriptors of year of birth, by hazard ratios (HRs). The 10-year change in PTA was investigated using a generation × time interaction term in generalized estimating equation models.
Results
Among the 3651 participants (mean [SD] age at baseline 53.1 [10.6] years; 2255 [61.8%] female; and 3567 [97.7%] non-Hispanic White), the 5-year HI incidence was 14.1% (95% CI, 13.0%-15.3%) and the 10-year cumulative incidence was 26.0% (95% CI, 24.6%-27.6%). The incidence increased with age. The risk of 5-year incident HI decreased by generation (RR, 0.80; 95% CI, 0.66-0.97) when adjusting for multiple covariates. The decreased risk was similar in the 10-year period (HR, 0.86; 95% CI, 0.73-1.01). The PTA change rate (per 5 years of follow-up) decreased by generation, with the Greatest Generation (born 1901-1924) experiencing the highest rate (7.03 dB HL). The rates were all significantly lower for the other generations (Silent Generation [born 1925-1945], 3.30 dB HL; Baby Boom Generation [born 1946-1964], 3.36 dB HL; and Generation X [born 1965-1984], 2.33 dB HL).
Conclusions and Relevance
This study suggests that the risk of HI and rate of PTA change is lower for the Silent Generation and Baby Boom Generation compared with the Greatest Generation. Part of this lower risk is likely associated with changes in modifiable factors. A potential continued benefit may exist for Generation X. Combined with the reduced risk of HI for the Silent Generation and Baby Boom Generation, this finding implies that the future HI burden may be lower than current estimates suggest.
This cohort study examines whether hearing impairment incidence and change in pure-tone average in adults differ by generation and identifies factors underlying these differences.
Introduction
Population-based studies1,2,3,4 of cardiovascular disease (CVD), dementia, and ocular conditions such as age-related macular degeneration have found lower risks of adverse outcomes in recent generations. These findings imply systematic generational differences in risk factors or exposures that have resulted in better age-related health outcomes over time. Understanding what factors (ie, cardiovascular, metabolic, or behavioral risk factors) explain these differences may help to better understand the causes of age-related disorder and identify primary prevention strategies. This understanding is especially important for conditions that affect a large proportion of the general population, such as hearing impairment (HI).
The Global Burden of Disease project reported HI as the fourth most common chronic disease worldwide, and there were steady increases in years lived with disability attributable to HI from 1997 to 2017.5,6 As the US population ages, researchers have estimated that the number of affected adults with HI will increase from 44.1 million in 2020 to 73.5 million in 2060.7 However, this estimate does not account for reported decreases in age-specific HI prevalence.8,9,10 It is important to determine whether HI incidence and pure-tone average (PTA) trajectories have changed across generations to correctly estimate future need for hearing health care. Understanding factors underlying generational changes could help identify prevention strategies to improve hearing health. This study investigates generational differences in HI incidence and PTA change in a US cohort of adults and examines whether known risk factors for HI, such as cardiovascular factors, metabolic factors, and work-related noise, are associated with any observed differences in HI or PTA by generation of birth.
Methods
Study Population
This cohort study includes combined data from the Epidemiology of Hearing Loss Study (EHLS) and the Beaver Dam Offspring Study (BOSS). The EHLS (baseline: 1993-1995) is a longitudinal, population-based study of aging, sensory function, and cognition based in Beaver Dam, Wisconsin (adults 48-92 years of age at baseline). BOSS (baseline: 2005-2008) is a longitudinal investigation of aging in the adult offspring (21-84 years of age at baseline) of EHLS participants. Follow-up examinations were performed every 5 years, with high retention in both studies (84%). Ten years of follow-up data are used (EHLS: 1993-1995,1998-2000, and 2003-2005 examinations; BOSS: 2005-2008, 2010-2013, and 2015-2017 examinations). At their respective baseline examinations, 1931 EHLS and 2395 BOSS participants were free of HI. Additional EHLS and BOSS details are provided in previous reports.8,11,12,13 The University of Wisconsin Health Sciences Institutional Review Board approved these studies. Written informed consent was obtained from participants before each examination. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Measurements
All measurements were conducted identically in the EHLS and BOSS, except where noted. Audiometric testing was performed following American Speech-Language-Hearing Association guidelines in compliance with the American National Standards Institute standards.14,15,16 Testing occurred in sound-treated booths using TDH-50P earphones or ER3-A inserts when probable ear canal collapse was present. Inserts were used for participants who were tested at satellite clinics or in homes (any examination: n = 171, baseline: n = 15, 5-year: n = 74, 10-year: n = 112). These examination types were rare, and American National Standards Institute standards were confirmed by ambient noise–level measurement. Pure-tone air-conduction thresholds were measured at 0.5, 1, 2, 3, 4, 6, and 8 kHz. Masking was used when a 40-dB hearing level (dB HL) or higher or a 60 dB HL or higher air-conduction difference occurred between the ears or between the test-ear air conduction and the non–test ear bone conduction when using TDH-50P or insert earphones, respectively, or when the air-conduction threshold was worse at a level of 15 dB HL or greater than the bone-conduction threshold of either ear. The PTA was calculated using 0.5-, 1-, 2-, and 4-kHz thresholds, with HI defined as a PTA greater than 25 dB HL in either ear.
Generation was classified according to birth year as delineated by commonly used sociodemographic descriptors. The Greatest Generation included the birth years 1901 to 1924 (n = 291), the Silent Generation included the birth years 1925 to 1945 (n = 1499), the Baby Boom Generation included the birth years 1946 to 1964 (n = 1372), and Generation X included the birth years 1965 to 1984 (n = 489). Data on birth year were available for all participants.
Height, weight, and waist circumference were measured. Waist circumference was measured at the level of the umbilicus and recorded to the nearest 0.5 cm. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Blood pressure was measured with random-zero sphygmomanometers in accordance with the Hypertension Detection and Follow-up Program protocol in the EHLS and with an automated blood pressure machine (Dinamap; GE Healthcare) in BOSS.17 Hypertension was considered present if systolic blood pressure was 140 mm Hg or higher, diastolic blood pressure was 90 mm Hg or higher, or if the participant reported physician diagnosis of high blood pressure with current antihypertensive medication use. High-resolution B-mode carotid artery ultrasonography (AU4; Biosound Esaote) was performed to determine mean carotid intima-media thickness (IMT) and carotid artery plaque presence.18,19 Carotid IMT and plaque were first measured at baseline in BOSS and at 5-year follow-up in the EHLS.
Glycosylated hemoglobin was measured at the 5-year EHLS phase, and glycated hemoglobin (HbA1c) was measured at baseline in BOSS in nonfasting whole blood samples. Diabetes was defined as a glycosylated hemoglobin level greater than 8% (HbA1c≥6.5%), a diabetes diagnosis, or suspected diabetes with current treatment. Baseline nonfasting serum cholesterol levels were measured. Non–high-density lipoprotein cholesterol (HDL-C) was calculated as the difference between total cholesterol and HDL-C. Serum inflammatory markers, high-sensitivity C-reactive protein, and interleukin 6 were measured at the University of Minnesota Advanced Research and Diagnostic Laboratory from samples collected at the 5-year EHLS and baseline BOSS phases.4,20 Levels of high-sensitivity C-reactive protein were grouped according to clinical cut points as less than 1.0 mg/L, 1.0-3.0 mg/L, and greater than 3.0 mg/L. Interleukin 6 was grouped by tertiles.
Self-reported demographic and behavioral information were obtained by interview, including age, sex, race/ethnicity, educational level (≥16 vs <16 years), smoking status, exercise (at least once per week, long enough to sweat), currently having or ever having had work-related noise exposure, and CVD history (participant-reported physician-diagnosed angina, myocardial infarction, or stroke).
Statistical Analysis
Five-year incidence models were constructed using a modified Poisson regression approach with a robust error variance.21 Ten-year cumulative incidence by generation and age groups were calculated using Kaplan-Meier (product-limit) survival analysis.22 Age- and sex-adjusted 10-year cumulative incidence with corresponding 95% CIs were obtained using the Breslow estimator for the cumulative baseline function.23 To estimate strength of association between generation and 10-year cumulative HI incidence, a discrete-time hazard model with a binomial distribution and a complementary log-log transformation link was fit. This model corresponds to the Cox proportional hazards regression continuous-time hazard model.24,25 In both 5-year incidence and 10-year cumulative incidence models, generation was treated as an ordered factor, resulting in estimated relative risks (RRs) associated with one generation compared with the previous generation. Similar models were built using indicator variables for generation. The PTA change was analyzed using linear generalized estimating equations with generation × time interaction terms to estimate the rate of change by generation. Resulting estimates for this interaction represent the difference in PTA change for each generation compared with the rate in the Greatest Generation (1901-1924).
We calculated RRs for 5-year incidence, hazard ratios (HRs) for 10-year cumulative incidence, and β values for 10-year PTA change with corresponding 95% CIs. Models were initially age and sex adjusted. Subsequent models additionally adjusted for factors associated with increased HI risk, BMI, waist circumference, hypertension, carotid IMT, carotid plaque, CVD history, diabetes, cholesterol, inflammatory markers, educational level, smoking status, exercise, and work-related noise to investigate their associations with relationships between generation and hearing. A manual stepwise approach was used to develop final, reduced models. Sex-stratified analyses were conducted to test for possible differential generational associations by sex. A sensitivity analysis was conducted by removing participants seen outside the main study clinic to ensure that site differences did not affect results. Estimated 10-year cumulative HI incidence plots by age and generation were generated from the final reduced multivariable model. Plots of 10-year PTA change by generation were produced using the final reduced multivariable model. All analyses were completed using SAS statistical software, version 9.4 (SAS Institute Inc). P values were 2 sided, and P ≤ .05 was considered statistically significant.
Results
Of 4326 participants free of HI at baseline, 3651 (84.4%) (mean [SD] age at baseline 53.1 [10.6] years; 2255 [61.8%] female; and 3567 [97.7%] non-Hispanic White) had the necessary data for this investigation (mean follow-up, 8.4 years), 249 (5.8%) participated at follow-up but had no audiometric examination, 174 (4.0%) died before follow-up, and 252 (5.8%) did not participate. Included and excluded participants were similar (eTable 1 in the Supplement).
Five-Year Incidence
The 5-year HI incidence was 14.1% (exact binomial 95% CI, 13.0%-15.3%). Age group–specific incidence was highest in the Greatest Generation (1901-1924) (Table 1). For example, among participants 60 to 69 years of age, the 5-year incidence was 36.8% (95% CI, 21.8%-54.0%) in the Greatest Generation (1901-1924), 22.1% (95% CI, 18.8%-25.7%) in the Silent Generation (1925-1945), and 12.5% (95% CI, 2.7%-32.4%) in the Baby Boom Generation (1946-1964).
Table 1. The 5-Year Incidence and 10-Year Cumulative Incidence of Hearing Impairment by Generation and Age Groupa.
| Age range, y | Greatest Generation (1901-1924) | Silent Generation (1925-1945) | Baby Boom Generation (1946-1964) | Generation X (1965-1984) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At risk, No. | Incidence per 100 population (95% CI) | At risk, No. | Incidence per 100 population (95% CI) | At risk, No. | Incidence per 100 population (95% CI) | At risk, No. | Incidence per 100 population (95% CI) | |||||
| 5 Years | 10 Years | 5 Years | 10 Years | 5 Years | 10 Years | 5 Years | 10 Years | |||||
| 22-39 | NA | NA | NA | NA | NA | NA | NA | NA | NA | 374 | 2.0 (0.8-4.0) | 4.8 (2.9-7.8) |
| 40-49 | NA | NA | NA | 72 | 7.1 (2.4-15.9) | 13.1 (7.1-23.8) | 713 | 6.1 (4.5-8.2) | 12.1 (9.8-15.0) | 115 | 7.5 (3.3-14.2) | 11.9 (6.9-20.2) |
| 50-59 | NA | NA | NA | 811 | 12.1 (9.9-14.6) | 22.8 (19.9-25.9) | 635 | 10.2 (8.0-12.9) | 22.0 (18.8-25.7) | NA | NA | NA |
| 60-69 | 38 | 36.8 (21.8-54.0) | 57.9 (41.7-75.0) | 597 | 22.1 (18.8-25.7) | 44.5 (40.3-48.8) | 24 | 12.5 (2.7-32.4) | 28.9 (14.0-53.9) | NA | NA | NA |
| 70-79 | 230 | 48.2 (41.5-55.0) | 73.5 (67.0-79.6) | 19 | 21.1 (6.1-45.6) | 52.6 (29.9-79.3) | NA | NA | NA | NA | NA | NA |
| 80-89 | 23 | 100 (83.9-100.0) | 100 (83.9-100.0) | NA | NA | NA | NA | NA | NA | NA | NA | NA |
Abbreviation: NA, not applicable.
eFigure in the Supplement provides greater detail on baseline age by generation.
Age- and sex-adjusted 5-year HI incidence risk decreased by generation (RR, 0.81; 95% CI, 0.67-0.98). Younger generations had lower 5-year RRs (Silent Generation: RR, 0.72; 95% CI, 0.55-0.93; Baby Boom Generation: RR, 0.60; 95% CI, 0.41-0.89; Generation X: RR, 0.59; 95% CI, 0.28-1.25) compared with the Greatest Generation. In the final reduced multivariable model further adjusted for educational level, BMI, and current smoking, the generational association was similar, with an approximately 20% reduction (RR, 0.80; 95% CI, 0.66-0.97) for each successive generation. In multivariable models that used categorical indicators for generation (Table 2), the Silent Generation and Baby Boom Generation had significantly lower 5-year RRs and effect sizes remained similar.
Table 2. Multivariable Adjusted Risk of 5- and 10-Year Incident Hearing Impairment by Generationa.
| Variable | 5-Year incidence, RR (95% CI) | 10-Year cumulative incidence, HR (95% CI) | ||
|---|---|---|---|---|
| Full (n = 3301) | Reduced (n = 3432) | Full (n = 3377) | Reduced (n = 3552) | |
| Generation | ||||
| Greatest Generation (1901-1924) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Silent Generation (1925-1945) | 0.70 (0.54-0.93) | 0.71 (0.54-0.93) | 0.73 (0.57-0.95) | 0.74 (0.58-0.95) |
| Baby Boom Generation (1946-1964) | 0.61 (0.41-0.92) | 0.60 (0.40-0.89) | 0.68 (0.47-0.98) | 0.68 (0.48-0.96) |
| Generation X (1965-1984) | 0.60 (0.27-1.32) | 0.56 (0.26-1.20) | 0.76 (0.40-1.45) | 0.75 (0.40-1.40) |
| Age (per 5 y) | 1.38 (1.28-1.49) | 1.37 (1.28-1.48) | 1.51 (1.41-1.61) | 1.50 (1.41-1.60) |
| Sex (male) | 2.06 (1.75-2.43) | 2.06 (1.76-2.42) | 2.08 (1.79-2.41) | 2.11 (1.83-2.44) |
| Educational level (≥16 y vs all others) | 0.73 (0.59-0.91) | 0.70 (0.57-0.87) | 0.71 (0.59-0.85) | 0.68 (0.56-0.81) |
| BMI (per 1 unit) | 1.02 (1.01-1.04) | 1.02 (1.01-1.04) | 1.02 (1.01-1.03) | 1.02 (1.01-1.04) |
| Current smoker | 1.11 (0.86-1.43) | 1.20 (0.95-1.51) | 1.20 (0.97-1.50) | 1.29 (1.05-1.57) |
| Exercise (once or more per week) | 0.92 (0.78-1.09) | NA | 0.91 (0.79-1.06) | NA |
| Hypertension | 1.05 (0.89-1.25) | NA | 1.11 (0.95-1.30) | NA |
| Glycated hemoglobin (per 1%) | 1.06 (0.97-1.15) | NA | 1.02 (0.92-1.12) | NA |
| Non–HDL-C (per 20 mg/dL) | 0.98 (0.95-1.02) | NA | 0.98 (0.95-1.01) | NA |
| Baseline work–related noise exposure | 1.11 (0.84-1.48) | NA | 1.18 (0.94-1.49) | NA |
Abbreviations: BMI, body mass index (weight in kilograms divided by height in meters squared); HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; NA, not applicable; RR, relative risk.
Generation modeled as an indicator variable, with the Greatest Generation as reference.
Ten-Year Cumulative Incidence
The 10-year cumulative HI incidence was 26.0% (Kaplan-Meier 95% CI, 24.6%-27.6%). The 10-year cumulative HI incidence was highest for the Greatest Generation and increased by age group and decreased by generation (Table 1).
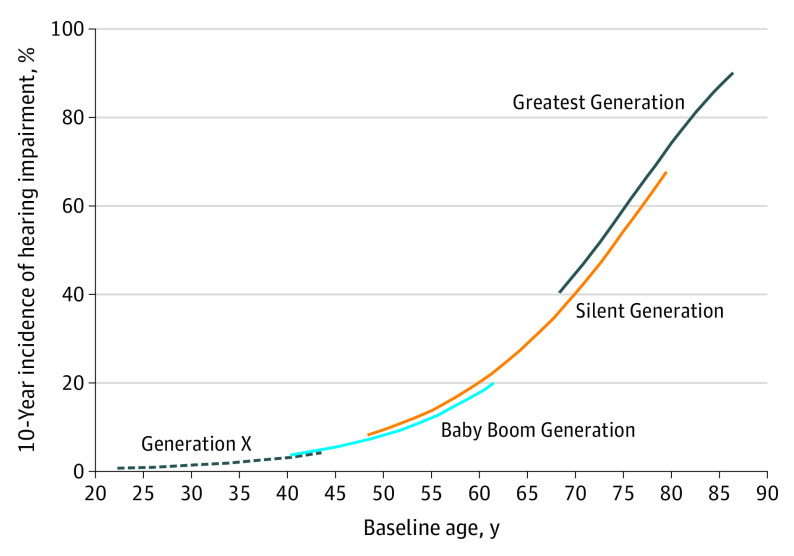
Age- and sex-adjusted 10-year cumulative incidence decreased from the earliest generation to the most recent (age- and sex-adjusted incidence, 61.5%; 95% CI, 54.8%-67.2% for the Greatest Generation; 26.1%; 95% CI, 23.7%-28.4% for the Silent Generation; 14.5%; 95% CI, 12.4%-16.5% for the Baby Boom Generation; and 4.3%; 95% CI, 2.0%-6.5% for Generation X). The lower RRs seen by generation during the first 5-year follow-up were similar for the 10-year period (HR, 0.86; 95% CI, 0.73-1.01). Estimates for the second 5-year period (from 5 to 10 years) were also lower (HR, 0.78; 95% CI, 0.47-1.31). When data were analyzed using indicator variables, the Silent Generation (HR, 0.73; 95% CI, 0.57-0.93) and Baby Boom Generation (HR, 0.67; 95% CI, 0.48-0.93) had significantly lower 10-year HI risk than the Greatest Generation, whereas the decreased risk for Generation X was not, although the effect size was similar (HR, 0.70; 95% CI, 0.38-1.28).
In multivariable modeling, a protective association was observed for the Silent Generation and the Baby Boom Generation compared with the Greatest Generation when adjusting for age, sex, educational level, BMI, smoking status, exercise, hypertension, HbA1c level, non–HDL-C levels, and work-related noise (Table 2). Compared with age- and sex-adjusted models, the HRs were only slightly attenuated, with the final reduced model having nearly identical results (Silent Generation: HR, 0.74; 95% CI, 0.58-0.95; Baby Boom Generation: HR, 0.68; 95% CI, 0.48-0.96; and Generation X: HR, 0.75; 95% CI, 0.40-1.40). Ten-year cumulative incidence by age and generation estimated from the reduced multivariable model are shown in Figure 1. Sex-stratified analyses showed similar estimates for generational effect for men and women (see eTable 2 in the Supplement). Sensitivity analysis removing those seen outside the main clinic had nearly identical results.
Figure 1. Estimated 10-Year Cumulative Hearing Impairment Incidence by Age and Generation.
Generation modeled as an ordered factor. Adjusted for age, sex, educational level, body mass index, and smoking. Generation categorized by year of birth: Greatest Generation (1901-1924), Silent Generation (1925-1945), Baby Boom Generation (1946-1964), and Generation X (1965-1984).
PTA Change
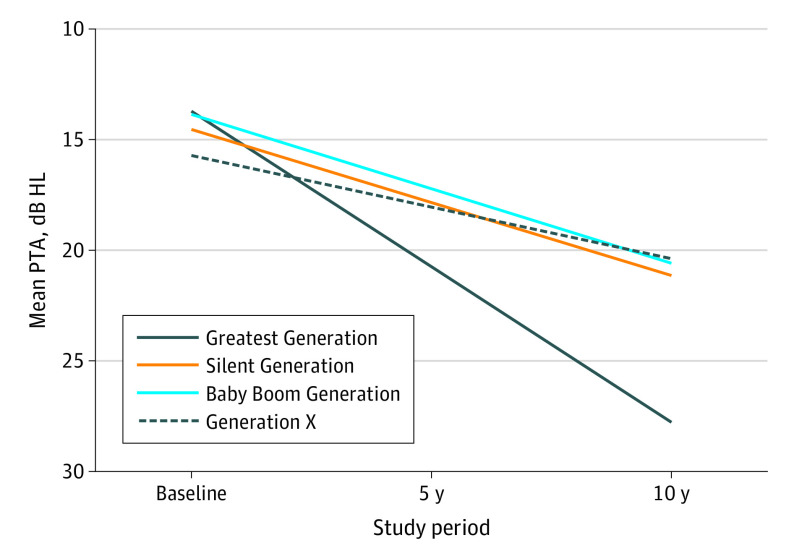
During the 10-year follow-up period, age- and sex-adjusted mean PTA increased from 18.2 to 31.2 dB HL for the Greatest Generation, from 13.9 to 20.4 dB HL for the Silent Generation, from 10.6 to 17.3 dB HL for the Baby Boom Generation, and from 7.5 to 12.2 dB HL for Generation X. The Greatest Generation experienced a rate of 7.12–dB HL PTA increase per 5 years of follow-up, whereas the Silent Generation (3.36 dB HL), Baby Boom Generation (3.35 dB HL), and Generation X (2.32 dB HL) had significantly lower rates of increase (Table 3).
Table 3. Worse Ear PTA Change (per 5 Years) by Generationa.
| Variable | Age and sex adjusted | Multivariable adjusted | ||
|---|---|---|---|---|
| PTA, dB HL | P value | PTA, dB HL | P value | |
| Generation | ||||
| Greatest Generation (1901-1924) | 7.12 | 1 [Reference] | 7.03 | 1 [Reference] |
| Silent Generation (1925-1945) | 3.36 | <.001 | 3.30 | <.001 |
| Baby Boom Generation (1946-1964) | 3.35 | <.001 | 3.36 | <.001 |
| Generation X (1965-1984) | 2.32 | <.001 | 2.33 | <.001 |
| Covariates | ||||
| Age (per 5 y) | 1.87 | <.001 | 1.81 | <.001 |
| Male sex | 3.91 | <.001 | 3.21 | <.001 |
| College education | −1.27 | <.001 | ||
| Waist circumference (per 10 cm) | NA | NA | 0.41 | <.001 |
| Current smoker | NA | NA | 1.08 | . <.001 |
| Exercise | NA | NA | −0.63 | .007 |
| Hypertension | NA | NA | 0.20 | .41 |
| Work-related noise exposure | NA | NA | 0.39 | .25 |
| Glycated hemoglobin | NA | NA | 0.08 | .60 |
Abbreviations: HL, hearing level; NA, not applicable; PTA, pure-tone average.
Generation modeled as an indicator variable, with the Greatest Generation as reference.
In addition, the PTA increase rate for Generation X was lower (−0.82 dB HL per 5 years of follow-up; 95% CI, −1.05 to −0.58) than for the Silent Generation, whereas no difference was found between the Baby Boom Generation (0.09 dB HL per 5 years of follow-up; 95% CI, −0.29 to 0.10) and Silent Generation. In models additionally adjusted for college educational level, waist circumference, smoking status, exercise, hypertension, work-related noise exposure, and HbA1c level, results were virtually unchanged, with the Silent Generation, Baby Boom Generation, and Generation X experiencing a slower rate of PTA increase (3.73 dB HL less change per 5 years of follow-up in the Silent Generation, 3.67 dB HL less change per 5 years of follow-up in the Baby Boom Generation, and 4.70 dB HL less change per 5 years in Generation X) (Table 3). The observed 10-year change in PTA by generation from multivariable models is shown in Figure 2. The rate of hearing decline for the Greatest Generation was significantly greater than for all other generations. In addition, Generation X’s rate of change was significantly smaller than the Silent Generation’s rate, whereas no difference was found between the Baby Boom Generation and the Silent Generation. With the Silent Generation (3.36 dB HL change per 5 years) as reference, the 5-year rate of change in Generation X was 1.04 dB HL slower (2.32) (P < .001). The rate of change in the Baby Boom Generation (3.35 dB HL per 5 years) was nearly identical to that in the Silent Generation (P = .99).
Figure 2. Smoothed Multivariable-Adjusted Worse Ear Mean PTA by Generation at Baseline and 5- and 10-Year Follow-up.
Generation modeled as an indicator variable, with the Greatest Generation as reference. Adjusted for age, sex, college education, waist circumference, current smoking, exercise, hypertension, work exposure to noise, and glycated hemoglobin. Generation categorized by year of birth: Greatest Generation (1901-1924), Silent Generation (1925-1945), Baby Boom Generation (1946-1964), and Generation X (1965-1984). HL indicates hearing level; PTA, pure-tone average.
Discussion
This cohort study aimed to investigate generational associations with HI incidence and changes in PTA and to examine whether known risk factors for HI may explain any observed differences. The multivariable-adjusted HI incidence risk decreased by 25% or more for those born during the Silent Generation and Baby Boom Generation compared with the Greatest Generation. The rate of PTA increase was more than doubled for the Greatest Generation compared with the subsequent 2 generations (7.1 dB vs 3.4 dB per 5 years of follow-up). These results are consistent with previous reports8,9,10 of prevalence in this cohort and from the National Health and Nutrition Examination Survey, which found a 25% improvement in better-ear HI for adults 65 to 74 years of age when comparing the 1999-2006 data with the National Health Examination Survey 1959-1962 data, and adults 20 to 69 years of age in the 2011-2012 survey were 30% less likely to have HI in the worse ear than those included in the 1999-2004 survey. Although the National Health and Nutrition Examination reports involve birth cohort effects on prevalence and our results report generational associations with incidence, the trends of decreasing HI over time are similar and likely associated with one another. Although a Swedish study26 found no difference in hearing among 75-year-old people born in 3 different decades, their birth years were 1901, 1915, and 1930, which corresponds mostly to the Greatest Generation (1901-1924) in the present study. More recent generations have experienced better health outcomes in CVD, dementia, and vision.1,2,3,4 The observed hearing health changes appear to coincide with gains in other health outcomes, implying a common protective factor. Adjusting for known risk factors for HI incidence, including HbA1c levels, cardiovascular risk factors, and work-related noise, did not eliminate generational differences. Although these factors have strong associations with HI incidence, including them in models did not alter the observed generational differences in the current study. This finding suggests that the differences in HbA1c levels, CVD, and work-related noise by generation do not likely explain the generational differences, and as-yet-unidentified factors are also important to age-related hearing changes.
The gains in healthy aging could be attributable to lower childhood disease rates or infections generally, with continued improvements in vaccination, public sanitation, water quality, and disease surveillance. If differences in infection prevalence by generation exist, HI-related infection reductions could explain a portion of the observed generational association. A previous study27 found that childhood diphtheria was associated with increased hearing loss odds in adulthood. A study28 in the United Kingdom found that those with childhood tonsillitis, otorrhea, bronchitis, or severe respiratory infections were more likely to have HI as adults. US diphtheria rates decreased greatly between the 1920s and 1940s and even more greatly after universal childhood vaccination began during the late 1940s.29 Reductions in other childhood infections also occurred with increasing antibiotic use and vaccinations.30 Infectious agents have been implicated as possible risk factors for other outcomes with a generational decrease, such as CVD, because infections may contribute to atherosclerotic processes.31,32,33 Improvements in infectious disease prevention and treatment may partially explain the decrease in CVD and HI by generation.
Nutritional changes and neurotoxin or other environmental exposures are also potential explanations for decreased HI risk and slower PTA increase in generations subsequent to the Greatest Generation. A previous study34 observed the importance of nutrition at wide-ranging life stages to hearing outcomes. One study35 found that iron deficiency at birth was associated with abnormal auditory neural maturation, and another study36 found that adult β-carotene, β-cryptoxanthin, and folate intake were associated with lower hearing loss risk. A study in rural Nepal37 found an association between childhood undernutrition and adult hearing loss. The undernutrition in that study could be compared with what the Greatest Generation experienced as young adults during the Great Depression and may have had similar associations. Adults of more recent generations in the US have not experienced a similar food scarcity period.
Previous studies38,39 found associations between HI and bone and blood lead levels. In a recent report from BOSS,40 participants with higher levels of blood cadmium (a neurotoxin with common exposures, including smoking and food sources) had an increased HI risk, although no association was observed between lead and HI. Because these neurotoxin measures are not available in the EHLS, it is unknown whether generational differences in exposure to cadmium and lead influenced the findings in the present study. Because regulations for control of neurotoxic chemicals and other environmental factors have changed over time (eg, the banning of leaded gasoline), lifetime exposure differences may exist by generation and could explain part of the difference in risk by generation.
Just as some chemical exposures could be work related, exposure to noise at work must also be considered. The inclusion of current work-related noise exposure in models did not affect estimates for generational associations in the current study, perhaps because the older generations had larger proportions of retired individuals, who would no longer be exposed to work-related noise. However, inclusion of ever having work-related noise exposure similarly did not affect generational estimates. This finding indicates that, although noise exposure may be important to hearing health, a change in work environments by generation to occupations with less noise exposure, such as a shift away from factory or production employment, does not explain the decreasing HI incidence.
This study reports the largest gains in hearing health occurred between the Greatest Generation and the Silent Generation (Figure 1). As Generation X continues to age, this population may also demonstrate a significantly lower incidence than previous generations. The 5-year rate of PTA increase in Generation X was significantly lower than that of other generations (2.3 dB), which may suggest long-term reduced risk. Additional follow-up is necessary to confirm this trend, which would imply that additional gains in healthy aging are still occurring and future HI burden estimates should be adjusted accordingly.
Strengths and Limitations
This study has strengths and limitations. Its strengths include repeated standardized measurements of hearing and potential confounders in a well-established, long-term population-based cohort. A large sample size, with a high retention rate and minimal missing data, and wide age range provide the necessary power to detect generational differences in risk across most of the cohort, although the small number of cases in the youngest generation may be a limitation. Familial relationships between the EHLS and BOSS cohorts reduce the possibility that observed generational differences are heritable in nature, implying they are attributable to modifiable risk factors, although epigenetic factors could play a role in the observed differences. Rapidly decreasing incidence is also considered strong evidence that a disorder is at least partially preventable because genetic changes are slow. A limitation is that certain potential confounders, such as carotid IMT, were not available at baseline in the EHLS. As a result, 5-year follow-up data were used in the analysis. Ideally, baseline IMT measures would be used from both studies; however, IMT progression has been reported to be slow.41 Another limitation is that the EHLS and BOSS are racially/ethnically homogeneous, with nearly all participants of non-Hispanic White background. As a result, these findings may not be generalizable to other racial/ethnic groups. Generational HI risk differences should be investigated in diverse populations to assess whether trends exist among other backgrounds.
Conclusions
In this study, HI risk and PTA increase were lower for the Silent Generation and the Baby Boom Generation compared with the Greatest Generation. Part of this lower risk is likely attributable to changes in modifiable risk factors, although these factors could not be identified in the present study. A potential continued benefit, identified by a lower PTA change rate, may exist for Generation X. Combined with the reduced HI risk for the Silent Generation and Baby Boom Generation, future HI burden may be lower than current estimates suggest.
eTable 1. Age, Sex, and Generation by Inclusion Status
eFigure. Baseline Age by Generation, Defined By Birth Year
eTable 2. Sex-stratified Sensitivity Analyses of Incident Hearing Impairment and PTA Change (per 5 Years) by Generation of Birth
References
- 1.Ford ES, Roger VL, Dunlay SM, Go AS, Rosamond WD. Challenges of ascertaining national trends in the incidence of coronary heart disease in the United States. J Am Heart Assoc. 2014;3(6):e001097. doi: 10.1161/JAHA.114.001097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aparicio HJ, Himali JJ, Satizabal CL, et al. Temporal trends in ischemic stroke incidence in younger adults in the Framingham Study. Stroke. 2019;50(6):1558-1560. doi: 10.1161/STROKEAHA.119.025171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374(6):523-532. doi: 10.1056/NEJMoa1504327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruickshanks KJ, Nondahl DM, Johnson LJ, et al. Generational differences in the 5-year incidence of age-related macular degeneration. JAMA Ophthalmol. 2017;135(12):1417-1423. doi: 10.1001/jamaophthalmol.2017.5001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saunders JE, Rankin Z, Noonan KY. Otolaryngology and the global burden of disease. Otolaryngol Clin North Am. 2018;51(3):515-534. doi: 10.1016/j.otc.2018.01.016 [DOI] [PubMed] [Google Scholar]
- 6.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789-1858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goman AM, Reed NS, Lin FR. Addressing estimated hearing loss in adults in 2060. JAMA Otolaryngol Head Neck Surg. 2017;143(7):733-734. doi: 10.1001/jamaoto.2016.4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhan W, Cruickshanks KJ, Klein BE, et al. Generational differences in the prevalence of hearing impairment in older adults. Am J Epidemiol. 2010;171(2):260-266. doi: 10.1093/aje/kwp370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman HJ, Dobie RA, Ko CW, Themann CL, Murphy WJ. Hearing threshold levels at age 70 years (65-74 years) in the unscreened older adult population of the United States, 1959-1962 and 1999-2006. Ear Hear. 2012;33(3):437-440. doi: 10.1097/AUD.0b013e3182362790 [DOI] [PubMed] [Google Scholar]
- 10.Hoffman HJ, Dobie RA, Losonczy KG, Themann CL, Flamme GA. Declining prevalence of hearing loss in US adults aged 20 to 69 Years. JAMA Otolaryngol Head Neck Surg. 2017;143(3):274-285. doi: 10.1001/jamaoto.2016.3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruickshanks KJ, Wiley TL, Tweed TS, et al. ; The Epidemiology of Hearing Loss Study . Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin. Am J Epidemiol. 1998;148(9):879-886. doi: 10.1093/oxfordjournals.aje.a009713 [DOI] [PubMed] [Google Scholar]
- 12.Cruickshanks KJ, Tweed TS, Wiley TL, et al. The 5-year incidence and progression of hearing loss: the Epidemiology of Hearing Loss Study. Arch Otolaryngol Head Neck Surg. 2003;129(10):1041-1046. doi: 10.1001/archotol.129.10.1041 [DOI] [PubMed] [Google Scholar]
- 13.Nash SD, Cruickshanks KJ, Klein R, et al. The prevalence of hearing impairment and associated risk factors: the Beaver Dam Offspring Study. Arch Otolaryngol Head Neck Surg. 2011;137(5):432-439. doi: 10.1001/archoto.2011.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Speech-Language-Hearing Association (ASHA) Guidelines for manual pure-tone threshold audiometry. ASHA. 1978;20(4):297-301. [PubMed] [Google Scholar]
- 15.American National Standards Institute Maximum Permissible Ambient Noise Levels for Audiometric Test Rooms (ANSI S3.1-1999). American National Standards Institute; 1999. [Google Scholar]
- 16.American National Standards Institute Specification for Audiometers (ANSI S3. 6.-). American National Standards Institute; 2010. [Google Scholar]
- 17.Borhani NO, Kass EH, Langford HG, et al. The hypertension detection and follow-up program: hypertension detection and follow-up program cooperative group. Prev Med. 1976;5(2):207-215. doi: 10.1016/0091-7435(76)90039-6 [DOI] [PubMed] [Google Scholar]
- 18.Zhong W, Cruickshanks KJ, Schubert CR, et al. Carotid atherosclerosis and 10-year changes in cognitive function. Atherosclerosis. 2012;224(2):506-510. doi: 10.1016/j.atherosclerosis.2012.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schubert CR, Cruickshanks KJ, Fischer ME, et al. Carotid intima media thickness, atherosclerosis, and 5-year decline in odor identification: the Beaver Dam Offspring Study. J Gerontol A Biol Sci Med Sci. 2015;70(7):879-884. doi: 10.1093/gerona/glu158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nash SD, Cruickshanks KJ, Zhan W, et al. Long-term assessment of systemic inflammation and the cumulative incidence of age-related hearing impairment in the Epidemiology of Hearing Loss Study. J Gerontol A Biol Sci Med Sci. 2014;69(2):207-214. doi: 10.1093/gerona/glt075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou G A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457-81. doi: 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 23.Breslow NE Discussion of the paper by D. R. Cox. J R Stat Soc Series B. 1972;34:216–217. [Google Scholar]
- 24.Singer JD, Willet JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford University Press; 2003. doi: 10.1093/acprof:oso/9780195152968.001.0001 [DOI] [Google Scholar]
- 25.Alisson PD Survival Analysis Using the SAS System: A Practical Guide. SAS Institute; 1995. [Google Scholar]
- 26.Rosenhall U, Möller C, Hederstierna C. Hearing of 75-year old persons over three decades: has hearing changed? Int J Audiol. 2013;52(11):731-739. doi: 10.3109/14992027.2013.808381 [DOI] [PubMed] [Google Scholar]
- 27.Schubert CR, Cruickshanks KJ, Wiley TL, Klein R, Klein BEK, Tweed TS. Diphtheria and hearing loss. Public Health Rep. 2001;116(4):362-368. doi: 10.1016/S0033-3549(04)50058-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearson F, Mann KD, Rees A, Davis A, Pearce MS. The effect of childhood infection on hearing function at age 61 to 63 years in the Newcastle Thousand Families Study. Ear Hear. 2015;36(2):185-190. doi: 10.1097/AUD.0000000000000094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67(2):1-44. doi: 10.15585/mmwr.rr6702a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamborsky J, Kroger A, Wolfe S, eds. Epidemiology and Prevention of Vaccine-Preventable Diseases. 13th ed Centers for Disease Control and Prevention Public Health Foundation; 2015. [Google Scholar]
- 31.Nieto FJ Infections and atherosclerosis: new clues from an old hypothesis? Am J Epidemiol. 1998;148(10):937-948. doi: 10.1093/oxfordjournals.aje.a009570 [DOI] [PubMed] [Google Scholar]
- 32.Campbell LA, Rosenfeld ME. Infection and atherosclerosis development. Arch Med Res. 2015;46(5):339-350. doi: 10.1016/j.arcmed.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pothineni NVK, Subramany S, Kuriakose K, et al. Infections, atherosclerosis, and coronary heart disease. Eur Heart J. 2017;38(43):3195-3201. doi: 10.1093/eurheartj/ehx362 [DOI] [PubMed] [Google Scholar]
- 34.Puga AM, Pajares MA, Varela-Moreiras G, Partearroyo T. Interplay between nutrition and hearing loss: state of art. Nutrients. 2018;11(1):35. doi: 10.3390/nu11010035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choudhury V, Amin SB, Agarwal A, Srivastava LM, Soni A, Saluja S. Latent iron deficiency at birth influences auditory neural maturation in late preterm and term infants. Am J Clin Nutr. 2015;102(5):1030-1034. doi: 10.3945/ajcn.115.113084 [DOI] [PubMed] [Google Scholar]
- 36.Curhan SG, Stankovic KM, Eavey RD, Wang M, Stampfer MJ, Curhan GC. Carotenoids, vitamin A, vitamin C, vitamin E, and folate and risk of self-reported hearing loss in women. Am J Clin Nutr. 2015;102(5):1167-1175. doi: 10.3945/ajcn.115.109314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emmett SD, Schmitz J, Karna SL, et al. Early childhood undernutrition increases risk of hearing loss in young adulthood in rural Nepal. Am J Clin Nutr. 2018;107(2):268-277. doi: 10.1093/ajcn/nqx022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park SK, Elmarsafawy S, Mukherjee B, et al. Cumulative lead exposure and age-related hearing loss: the VA Normative Aging Study. Hear Res. 2010;269(1-2):48-55. doi: 10.1016/j.heares.2010.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi YH, Hu H, Mukherjee B, Miller J, Park SK. Environmental cadmium and lead exposures and hearing loss in U.S. adults: the National Health and Nutrition Examination Survey, 1999 to 2004. Environ Health Perspect. 2012;120(11):1544-1550.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22851306&dopt=Abstract doi: 10.1289/ehp.1104863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dalton DS, Schubert CR, Pinto A, et al. Cadmium, obesity, and education, and the 10-year incidence of hearing impairment: the Beaver Dam Offspring Study. Laryngoscope. 2020;130(6):1396-1401.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31424564&dopt=Abstract doi: 10.1002/lary.28244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chambless LE, Folsom AR, Davis V, et al. Risk factors for progression of common carotid atherosclerosis: the Atherosclerosis Risk in Communities Study, 1987-1998. Am J Epidemiol. 2002;155(1):38-47. doi: 10.1093/aje/155.1.38 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Age, Sex, and Generation by Inclusion Status
eFigure. Baseline Age by Generation, Defined By Birth Year
eTable 2. Sex-stratified Sensitivity Analyses of Incident Hearing Impairment and PTA Change (per 5 Years) by Generation of Birth