Abstract
This cohort study assesses the concordance of 3 models to stratify risk for progression to multiple myeloma in an independent cohort of patients with smoldering multiple myeloma.
Smoldering multiple myeloma (SMM) is a plasma cell disorder with the potential to evolve to multiple myeloma.1 Several risk stratification models—commonly, the Mayo Clinic Risk Stratification Model 2008, the Programa para el Tratamiento de Hemopatias Malignas (PETHEMA) model, and the newer Mayo Clinic Risk Stratification Model 2018—have been developed to prognosticate the risk of progression.2,3 These models use clinical variables of disease burden rather than biological tumor characteristics to predict low risk (LR), intermediate risk (IR), and high risk (HR) for progression to multiple myeloma (MM). Multiple clinical trials are investigating the role of treatment in HR SMM. Interpreting the results of clinical trials investigating the role of treatment in HR SMM is problematic owing to variation in the risk stratification criteria used.4 Our study attempts to assess the concordance of these 3 models in a cohort of patients with SMM from 2 clinical trials.
Methods
In this retrospective analysis of data from 2 clinical trials, NCT01572480 and NCT01109407, which were approved by the National Cancer Institute Institutional Review Board and had written patient informed consent attained, patient records sequentially assigned a diagnosis of SMM (using International Myeloma Working Group 2014 criteria) from April 2010 to June 2020 were reviewed after National Cancer Institute Institutional Review Board approval. Patients were assigned a risk score based on the 3 models, and concordance ratios were calculated among the models. Proportions of concordance, statistical significance values for comparisons using χ2 statistical values, and 95% CIs (modified Wald method) for these comparisons were calculated using GraphPad Prism, version 8.4.3 (GraphPad Software).
Results
The medical records of 145 patients were reviewed, and patients’ risk was stratified according to the 3 models. Baseline patient characteristics included mean (range) age of 66 (40-91) years, and 84 (57.9%) were male; 109 (75.2%) were White, 32 (22.1%) were Black, and 3 (2.1%) were Asian. The isotype was IgG, 103 (71.0%); IgA, 34 (23.4%); and light chain, 8 (5.5%); the median M protein, percent plasmacytosis, serum free light chain ratio, and percent aberrant plasma cells (flow cytometry) was 1.3 g/dL, 18%, 9.1, and 97%, respectively. A total of 101 patients (69.7%) had immunoparesis; 38 patients (26.2%) had 1 and 60 patients (41.4%) had 2 normal immunoglobulin isotypes depressed. Overall, 42 (29.0%) and 72 (49.7%) patients had agreement in risk stratification across LR, IR, and HR categories among the 2008 and 2018 Mayo Clinic models and the PETHEMA model, respectively (Table). The overall global rate of agreement across all 3 models for all 3 categories was 16.6%. Of the 145 patients evaluated, 24 patients were categorized in the same risk group using all 3 models. There was a significant difference in the rates of LR, IR, and HR among the 3 models (Figure, A). Stratifying HR patients is most important in terms of patient monitoring and enrollment into clinical trials. Therefore, discordance across models in detecting HR patients compared with all others (non-HR) was specifically examined. The ability of models to classify patients as HR vs non-HR was significantly different (Figure, B).
Table. Risk Stratification Models in Smoldering Multiple Myelomaa.
| Model/stratification | PETHEMA2 | |||
|---|---|---|---|---|
| No. (%) (n = 145) | Overall agreement, No./No. (%) [95% CI] | |||
| LR | IR | HR | ||
| Mayo 2008 | ||||
| LR | 13 (9.0) | 24 (16.6) | 23 (15.9) | 42/145 (29.0) [22.2-36.8] |
| IR | 8 (5.5) | 21 (14.5) | 47 (32.4) | |
| HR | 0 | 1 (0.7) | 8 (5.5) | |
| Mayo 20183 | ||||
| LR | 13 (9.0) | 17 (11.7) | 10 (6.9) | 72/145 (49.7) [41.6-57.7] |
| IR | 5 (3.4) | 13 (9.0) | 22 (15.2) | |
| HR | 3 (2.1) | 16 (11.0) | 46 (31.7) | |
| Risk agreement among PETHEMA, Mayo 2008, and Mayo 2018 | 11 (7.6) | 5 (3.5) | 8 (5.5) | 24/145 (16.6) [11.3-23.5] |
Abbreviations: HR, high risk; IR, intermediate risk; LR, low risk; Mayo 2008, Mayo Clinic Risk Stratification Model 2008; Mayo 2018, Mayo Clinic Risk Stratification Model 2018; PETHEMA, Programa para el Tratamiento de Hemopatias Malignas Model.
Rates of agreement among the 2008 and 2018 Mayo risk models and the PETHEMA model and global agreement across all 3 models.
Figure. Discordance Rates Among Risk Stratification Models.
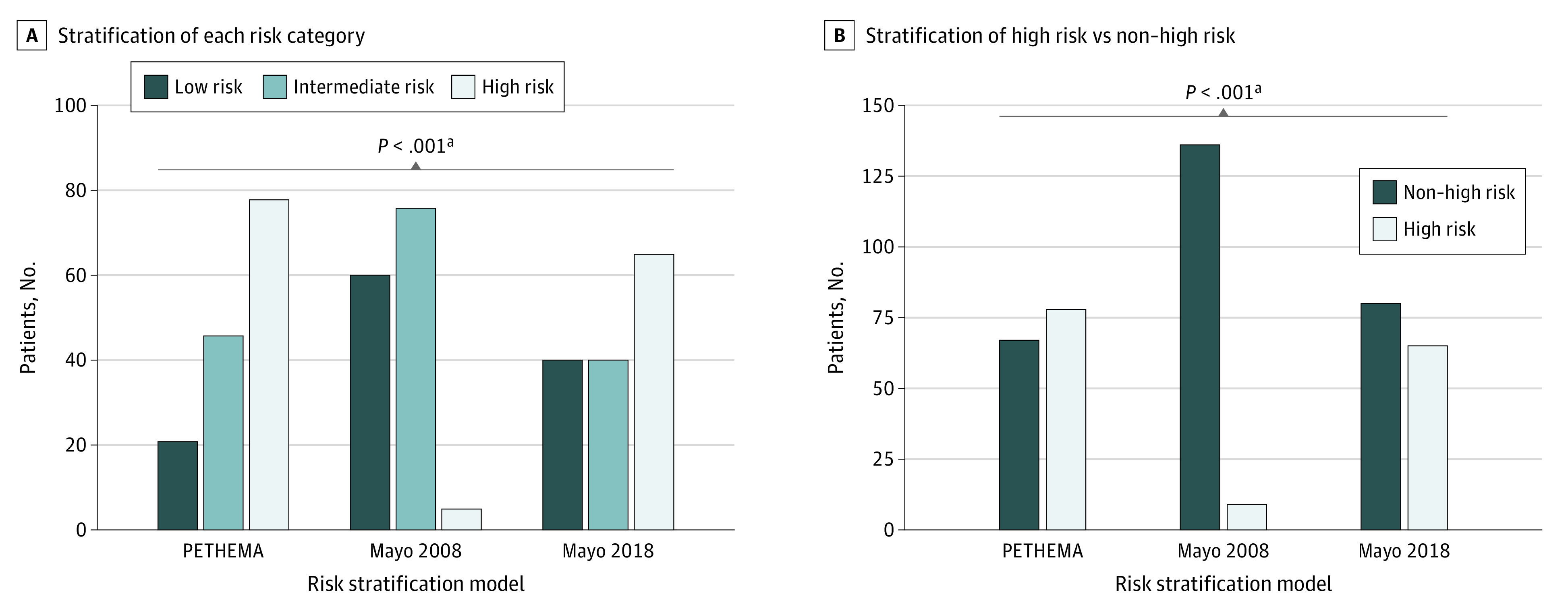
A, The numbers of patients classified in each risk category for each of the 3 risk stratification models are significantly discordant. B, Risks remain significantly discordant when stratifying patients to either high risk or non–high risk.
Mayo 2008 indicates Mayo Clinic Risk Stratification Model 2008; Mayo 2018, Mayo Clinic Risk Stratification Model 2018; PETHEMA, Programa para el Tratamiento de Hemopatias Malignas Model.
aχ2 test.
Discussion
The accurate identification of patients with SMM at highest risk of developing MM remains difficult. While the 2018 Mayo Clinic model has a higher concordance rate with the PETHEMA model, it remains significantly discordant. Furthermore, these models are limited because they do not incorporate sensitive imaging techniques, such as positron emission tomography–integrated computed tomography, which can aid in risk stratifying patients who are at highest risk of developing symptomatic MM.5 These results suggest that the current clinical variables used to determine risk are not reliable, most likely because they are markers of disease burden, subject to increase over time, and not true markers of biological disease characteristics.
Our study does have a number of limitations. First, as it was conducted at a national MM referral center, the patient population seen might not completely represent the average patient population. Furthermore, because a subset of patients in this study were referred with a previsit diagnosis of HR SMM, the distribution of the 3 risk categories might have been skewed.
As recently outlined by Maura et al,6 the incorporation of genomic signatures has the potential to significantly improve SMM risk stratification and is urgently needed. Until that time, it remains unclear which patients warrant early intervention, and we would caution against treatment of patients with SMM outside of a clinical trial.
References
- 1.Landgren O, Kyle RA, Pfeiffer RM, et al. . Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113(22):5412-5417. doi: 10.1182/blood-2008-12-194241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pérez-Persona E, Vidriales MB, Mateo G, et al. . New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood. 2007;110(7):2586-2592. doi: 10.1182/blood-2007-05-088443 [DOI] [PubMed] [Google Scholar]
- 3.Lakshman A, Rajkumar SV, Buadi FK, et al. . Risk stratification of smoldering multiple myeloma incorporating revised IMWG diagnostic criteria. Blood Cancer J. 2018;8(6):59-59. doi: 10.1038/s41408-018-0077-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill E, Dew A, Kazandjian D. State of the science in smoldering myeloma: should we be treating in the clinic? Semin Oncol. 2019;46(2):112-120. doi: 10.1053/j.seminoncol.2019.03.001 [DOI] [PubMed] [Google Scholar]
- 5.Zamagni E, Nanni C, Gay F, et al. . 18F-FDG PET/CT focal, but not osteolytic, lesions predict the progression of smoldering myeloma to active disease. Leukemia. 2016;30(2):417-422. doi: 10.1038/leu.2015.291 [DOI] [PubMed] [Google Scholar]
- 6.Maura F, Bolli N, Rustad EH, Hultcrantz M, Munshi N, Landgren O. Moving from cancer burden to cancer genomics for smoldering myeloma: a review. JAMA Oncol. 2020;6(3):425-432. doi: 10.1001/jamaoncol.2019.4659 [DOI] [PMC free article] [PubMed] [Google Scholar]


