This cohort study evaluates whether patients with COVID-19 have a higher incidence of full-thickness tracheal lesions and tracheoesophageal fistulas than matched controls and investigates potential mechanisms.
Key Points
Question
Are tracheal complications of invasive mechanical ventilation more frequent in patients with coronavirus disease 2019 (COVID-19)?
Findings
In this cohort study of 98 patients with COVID-19 and severe respiratory failure, the incidence of full-thickness tracheal lesions or tracheoesophageal fistulas after prolonged (≥14 days) invasive mechanical ventilation was significantly higher in patients with COVID-19 (46.7%) than matched controls (2.2%).
Meaning
Among patients with COVID-19, treatment with prolonged invasive mechanical ventilation may be associated with increased risk of full-thickness tracheal lesion and/or tracheoesophageal fistula.
Abstract
Importance
Full-thickness tracheal lesions and tracheoesophageal fistulas are severe complications of invasive mechanical ventilation. The incidence of tracheal complications in ventilated patients with coronavirus disease 2019 (COVID-19) is unknown.
Objective
To evaluate whether patients with COVID-19 have a higher incidence of full-thickness tracheal lesions and tracheoesophageal fistulas than matched controls and to investigate potential mechanisms.
Design, Setting, and Participants
This is a retrospective cohort study in patients admitted to the intensive care unit in a tertiary referral hospital. Among 98 consecutive patients with COVID-19 with severe respiratory failure, 30 underwent prolonged (≥14 days) invasive mechanical ventilation and were included in the COVID-19 group. The control group included 45 patients without COVID-19. Patients with COVID-19 were selected from March 1 to May 31, 2020, while the control group was selected from March 1 to May 31, 2019.
Exposures
Patients with COVID-19 had severe acute respiratory syndrome coronavirus 2 infection diagnosed by nasopharyngeal/oropharyngeal swabs and were treated according to local therapeutic procedures.
Main Outcomes and Measures
The primary study outcome was the incidence of full-thickness tracheal lesions or tracheoesophageal fistulas in patients with prolonged invasive mechanical ventilation.
Results
The mean (SD) age was 68.8 (9.0) years in the COVID-19 group and 68.5 (14.1) years in the control group (effect size, 0.3; 95% CI, −5.0 to 5.6). Eight (27%) and 15 (33%) women were enrolled in the COVID-19 group and the control group, respectively. Fourteen patients (47%) in the COVID-19 group had full-thickness tracheal lesions (n = 10, 33%) or tracheoesophageal fistulas (n = 4, 13%), while 1 patient (2.2%) in the control group had a full-thickness tracheal lesion (odds ratio, 38.4; 95% CI, 4.7 to 316.9). Clinical and radiological presentations of tracheal lesions were pneumomediastinum (n = 10, 71%), pneumothorax (n = 6, 43%), and/or subcutaneous emphysema (n = 13, 93%).
Conclusions and Relevance
In this cohort study, almost half of patients with COVID-19 developed full-thickness tracheal lesions and/or tracheoesophageal fistulas after prolonged invasive mechanical ventilation. Attempts to prevent these lesions should be made and quickly recognized when they occur to avoid potentially life-threatening complications in ventilated patients with COVID-19.
Introduction
Full-thickness tracheal lesions (FTTLs) and tracheoesophageal fistulas (TEFs) are serious but rare (0.3%-3%) complications of prolonged invasive mechanical ventilation (MV).1,2,3 With the coronavirus disease 2019 (COVID-19) outbreak, however, we experienced an unprecedented increase in the incidence of FTTLs and TEFs in ventilated patients with COVID-19, the reasons for which are unknown. Additionally, a growing number of reports in these patients highlighted an increased incidence of pneumomediastinum, pneumothorax, and/or subcutaneous emphysema, which are potentially life-threatening complications of undiagnosed FTTLs.4,5,6,7 Therefore, this study evaluated the incidence and features of patients with COVID-19 with tracheal complications who underwent prolonged invasive MV.
Methods
We analyzed data from the electronic medical records of our tertiary referral hospital. We screened patients admitted to the intensive care unit (ICU) from March 1 to May 31 in 2020 (COVID-19 group) and in 2019 (control group). All patients who underwent prolonged (≥14 days) invasive MV within these time periods were included. Patients with COVID-19 had severe acute respiratory syndrome coronavirus 2 detected in nasopharyngeal/oropharyngeal swabs. They were treated according to local procedures and underwent chest computed tomography (CT) scans at admission and in case of worsening of their clinical status. The FTTLs and TEFs were detected either directly with a bronchoscopy before performing a tracheostomy, or clinically and/or with CT scans in case of onset of other complications, such as pneumothorax, pneumomediastinum, or subcutaneous emphysema, and then confirmed with bronchoscopy. All tracheostomies were performed with percutaneous technique under bronchoscopy control by experienced physicians. Data on comorbidities at the time of admission were collected using the Adult Comorbidity Evaluation 27 index (ACE-27).8 This study was approved by the Local Ethics Committee on June 19, 2020. Written informed consent to collect deidentified data was obtained from all patients. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Descriptive statistics were determined for all variables. Effect size metrics were reported as differences between means or proportions for continuous and categorical variables, respectively, along with their 95% CIs. Logistic regression was used to calculate odds ratios (ORs) and 95% CIs. Analyses were performed using JMP Pro, version 13.2.1 (SAS Institute).
Results
The mean (SD) age was 68.8 (9.0) years in the COVID-19 group and 68.5 (14.1) years in the control group (effect size, 0.3; 95% CI, −5.0 to 5.6). Eight (27%) and 15 (33%) women were enrolled in the COVID-19 group and the control group, respectively. Among 98 consecutive patients with COVID-19 with severe respiratory failure admitted in 2020, 30 underwent prolonged invasive MV (Table 1). Forty-five consecutive control patients matched for age and sex were selected according to our inclusion and exclusion criteria in the aforementioned time frame (Table 1). Fourteen patients (47%) in the COVID-19 group had FTTLs (n = 10, 33%) or TEFs (n = 4, 13%) (Figure 1), while 1 patient (2%) in the control group had FTTLs (OR, 38.4, 95% CI, 4.7-316.9). The latter had a tracheal ring rupture diagnosed during the tracheostomy procedure. All 30 patients with COVID-19 underwent pronation maneuvers and received steroid therapy, compared with 5 (11%) and 14 (31%) patients in the control group, respectively. Furthermore, the COVID-19 group was treated with higher steroid doses (intravenous methylprednisolone, 80 mg vs 40 mg) and had lower partial pressure of arterial oxygen to fraction of inspired oxygen (Pao2 /Fio2) ratio during the second week of invasive MV. No further differences emerged between these 2 groups.
Table 1. Characteristics of Study Participants.
| Characteristic | No. (%) | Effect size (95% CI)a | |
|---|---|---|---|
| COVID-19 (n = 30) | Controls (n = 45) | ||
| Baseline characteristics | |||
| Age, mean (SD), y | 68.8 (9.0) | 68.5 (14.1) | 0.3 (−5.0 to 5.6) |
| Women | 8 (27) | 15 (33) | −6.7 (−26.6 to 14.7) |
| Blood pressure, mean (SD), mm Hg | |||
| Systolic | 126 (26) | 120 (16) | 5 (−5 to 16) |
| Diastolic | 67 (13) | 62 (9) | 5 (−0.3 to 10) |
| Heart rate, mean (SD), bpm | 91 (22) | 80 (14) | 10 (1 to 19) |
| Overall burden of comorbiditiesb | |||
| None | 15 (50) | 20 (44) | 5.5 (−17.1 to 27.7) |
| Mild | 14 (47) | 17 (38) | 8.9 (−13.6 to 30.8) |
| Moderate | 1 (3) | 5 (11) | −7.8 (−19.2 to 6.2) |
| Severe | 0 | 3 (7) | −6.7 (−15.4 to 4.6) |
| Treatments | |||
| MV duration, median (IQR), d | 22 (11) | 29 (19) | −7 (−11 to 1) |
| Orotracheal intubation | 10 (2) | 8 (2) | 2 (0.5 to 3) |
| Tracheostomy cannulas | 11 (8) | 21 (16) | −10 (−13 to −2.5) |
| Pronation maneuvers | 30 (100) | 5 (11) | 88.9 (72.8 to 95.4) |
| PEEP, median (IQR), cm H2O | |||
| Day 1 | 12 (2) | 12 (2) | 0 (−2 to 1) |
| Day 7 | 12 (2) | 12 (2) | 0 (−3 to 2) |
| Day 14 | 11 (2) | 11 (2) | 0 (−1 to 1) |
| Average | 11 (2) | 12 (2) | −1 (−2 to 1) |
| Medications | |||
| Antibiotics | 30 (100) | 45 (100) | NA |
| Steroids | 30 (100) | 14 (31) | 62.2 (42.0 to 75.4) |
| Dose, median (IQR), mgc | 80 (20) | 40 (20) | 40 (20 to 40) |
| Anticoagulantsd | 30 (100) | 45 (100) | NA |
| Prophylactic dosage | 14 (47) | 39 (87) | −40.0 (−58 to −18) |
| Therapeutic dosage | 16 (53) | 6 (13) | 40.0 (18 to 58) |
| Outcomes | |||
| Tracheal complications | 14 (47) | 1 (2) | 44.4 (24.4 to 60.8) |
| Full-thickness tracheal lesions | 10 (33) | 1 (2) | 31.1 (12.7 to 47.6) |
| Tracheoesophageal fistulas | 4 (13) | 0 | 13.3 (0.3 to 26.7) |
| Thoracic complications | 14 (47) | 0 | 46.7 (27.0 to 62.5) |
| Pneumothorax | 6 (20) | 0 | 20.0 (4.8 to 34.7) |
| Pneumomediastinum | 10 (33) | 0 | 33.3 (15.3 to 49.2) |
| Subcutaneous emphysema | 13 (43) | 0 | 43.3 (23.9 to 59.3) |
| Pao2/Fio2 ratio, median (IQR)e | |||
| Day 1 | 123 (70) | 144 (38) | −21 (−32 to 18) |
| Day 7 | 151 (82) | 156 (44) | −5 (−32 to 46) |
| Day 14 | 118 (59) | 176 (61) | −58 (−75 to −12) |
| Average | 149 (40) | 156 (36) | −7 (−30 to 5) |
| Deaths | 8 (27) | 14 (31) | −4.4 (−24.3 to 16.7) |
Abbreviations: bpm, beats per minute; COVID-19, coronavirus disease 2019; Fio2, fraction of inspired oxygen; IQR, interquartile range; MV, mechanical ventilation; NA, not applicable; Pao2, partial pressure of arterial oxygen; PEEP, positive end-expiratory pressure.
Estimated difference in medians or percentage between groups and associated 95% CI.
The overall burden of comorbid ailments in each patient was assessed using the Adult Comorbidity Evaluation 27 (ACE-27) instrument.
Methylprednisolone, administered intravenously, in those receiving steroid therapy.
Low-molecular-weight heparin or fondaparinux.
Ratio of Pao2 (in mm Hg) to Fio2 (expressed as a fraction).
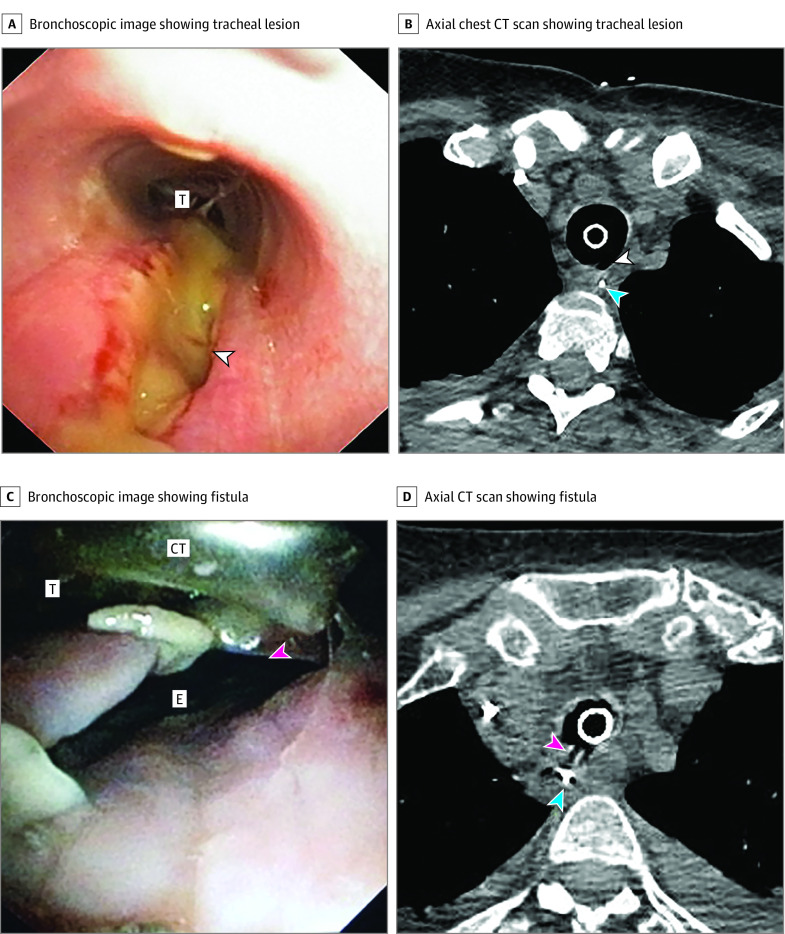
Figure 1. Early Tracheal Complications.
A, Bronchoscopic image showing a full-thickness tracheal lesion of the pars membranacea. B, Axial chest computed tomography (CT) scan showing a full-thickness tracheal lesion of the pars membranacea. C, Bronchoscopic image showing a tracheoesophageal fistula. D, Axial chest CT scan showing a tracheoesophageal fistula. The white arrowhead indicates full-thickness tracheal lesion; pink arrowhead, tracheoesophageal fistula; blue arrowhead, nasogastric tube. CT indicates cuff tube; E, esophagus; T, trachea.
Patients with COVID-19 with and without tracheal complications had similar demographic characteristics, comorbidities, ventilation parameters and duration, and therapeutic management (Table 2). Ten (71%) patients with tracheal damage had pneumomediastinum, 6 (43%) had pneumothorax, and 13 (93%) had subcutaneous emphysema, while these complications were not observed in patients with COVID-19 without tracheal damage.
Table 2. Characteristics of Patients With COVID-19 According to Presence of Tracheal Complications.
| Characteristic | Complicated, No. (%) | Effect size (95% CI)a | |
|---|---|---|---|
| Yes (n = 14) | No (n = 16) | ||
| Baseline characteristics | |||
| Age, mean (SD), y | 68.6 (9.6) | 68.9 (8.8) | −0.4 (−7.3 to 6.6) |
| Women | 5 (36) | 3 (19) | 17.0 (−15.2 to 46.0) |
| Blood pressure, mean (SD), mm Hg | |||
| Systolic | 124 (24) | 128 (28) | 4 (−15 to 24) |
| Diastolic | 65 (13) | 68 (13) | 3 (−6 to 13) |
| Heart rate, mean (SD), bpm | 94 (25) | 87 (19) | −7 (−24 to 10) |
| Overall burden of comorbiditiesb | |||
| None | 7 (50) | 8 (5) | 0 (−33.7 to 33.7) |
| Mild | 7 (50) | 7 (44) | 6.3 (−28.0 to 39.1) |
| Moderate | 0 | 1 (6) | −6.3 (−23.6 to 13.9) |
| Treatments | |||
| MV duration, median (IQR), d | 22 (15) | 22 (10) | 0 (−11 to 5) |
| Orotracheal intubation | 10 (3) | 10 (5) | 0 (−1 to 6) |
| Tracheostomy cannulas | 12 (15) | 9 (8) | 3 (−2 to 16) |
| PEEP, median (IQR), cm H2O | |||
| Day 1 | 12 (3) | 12 (2) | 0 (0 to 4) |
| Day 7 | 11 (2) | 12 (4) | −1 (−2 to 1) |
| Day 14 | 11 (2) | 11 (3) | 0 (−2 to 4) |
| Average | 11 (3) | 11 (2) | 0 (−2 to 1) |
| Pronation maneuvers | 14 (100) | 16 (100) | NA |
| Medications | |||
| Antivirals | 13 (93) | 13 (81) | 11.6 (−15.4 to 34.9) |
| Lopinavir/ritonavir | 3 (21) | 8 (50) | −28.6 (−56.4 to 6.4) |
| Remdesivir | 2 (14) | 1 (6) | 8.0 (−16.4 to 31.7) |
| Lopinavir/ritonavir + remdesivir | 8 (57) | 4 (25) | 32.1 (−3.4 to 60.4) |
| Hydroxychloroquine | 14 (100) | 16 (100) | NA |
| Tocilizumab | 0 | 1 (6) | −6.3 (−23.6 to 13.9) |
| Baricitinib | 0 | 3 (19) | −18.8 (−38.5 to 6.6) |
| Antibiotics | 14 (100) | 16 (100) | NA |
| Steroids | 14 (100) | 16 (100) | NA |
| Dose, median (IQR), mgc | 80 (20) | 80 (15) | 0 (0 to 20) |
| Anticoagulantsd | 14 (100) | 16 (100) | NA |
| Prophylactic dosage | 6 (43) | 8 (50) | −7.1 (−39.8 to 27.3) |
| Therapeutic dosage | 8 (57) | 8 (50) | 7.1 (−27.3 to 39.8) |
| Outcomes | |||
| Full-thickness tracheal lesions | 10 (71) | 0 | 71.4 (38.1 to 88.3) |
| Tracheoesophageal fistulas | 4 (29) | 0 | 28.6 (0.6 to 50.8) |
| Pneumothorax | 6 (43) | 0 | 42.9 (11.7 to 64.7) |
| Pneumomediastinum | 10 (71) | 0 | 71.4 (38.1 to 88.3) |
| Subcutaneous emphysema | 13 (93) | 0 | 92.9 (62.6 to 100) |
| Pao2/Fio2 ratio, median (IQR)e | |||
| Day 1 | 139 (88) | 120 (64) | 19 (−31 to 64) |
| Day 7 | 141 (83) | 158 (85) | −17 (−83 to 50) |
| Day 14 | 122 (66) | 107 (50) | 15 (−30 to 43) |
| Average | 151 (53) | 140 (27) | 11 (−22 to 35) |
| Deaths | 3 (21) | 5 (31) | −9.8 (−38.7 to 22.1) |
Abbreviations: bpm, beats per minute; COVID-19, coronavirus disease 2019; Fio2, fraction of inspired oxygen; IQR, interquartile range; MV, mechanical ventilation; NA, not applicable; Pao2, partial pressure of arterial oxygen; PEEP, positive end-expiratory pressure.
Estimated difference in medians/means or percentage between groups and associated 95% CI.
The overall burden of comorbid ailments in each patient was assessed using the Adult Comorbidity Evaluation 27 (ACE-27) instrument.
Methylprednisolone, administered intravenously, in those receiving steroid therapy.
Low-molecular-weight heparin or fondaparinux.
Ratio of Pao2 (in mm Hg) to Fio2 (expressed as a fraction).
Discussion
Tracheal damages associated with life-threatening complications occurred in almost half of patients with COVID-19 treated with prolonged invasive MV, with a marked increase in incidence compared with non-COVID-19 matched controls and previous case series.1,2,3 Several mechanisms specifically related to COVID-19 treatment and pathophysiology may explain the greater incidence of tracheal complications in mechanically ventilated patients with COVID-19:
Early implementation of pronation maneuvers, which increase the cuff pressure on the tracheal walls. The pathogenetic role of pronation maneuvers in the development of tracheal complications is supported by our findings, but previous results in different settings were contradictory and cannot lead us to draw definite conclusions.9,10
Prothrombotic and antifibrinolytic state of patients with COVID-19,11,12 which may cause microvascular injury and necrosis of tracheal and esophageal mucosa.
High viral replication within the tracheal epithelium that weakens the mucosa. In fact, Bradley et al13 reported the observation of viral particles in tracheal epithelial cells and within the extracellular mucus in the tracheal lumen of 12 autopsies.
High dose of systemic steroids and their chronic use that may cause mucosal atrophy and alter normal healing of tracheal wall microwounds caused by intubation, cuff pressure, or tracheostomy.14 Accumulating evidence suggests that administration of corticosteroids is associated with lower all-cause mortality in patients with COVID-19.15,16 Nonetheless, whether high-dose corticosteroids provide additional benefits compared with low-dose corticosteroids is debated.15,16
The hypoxic damage to the tracheal mucosa witnessed by a lower Pao2/Fio2 ratio in the second week of invasive MV compared with the control group.
Emotional and physical exhaustion of health care professionals involved in the management of patients with COVID-19, increasing the risk of unreported accidents or mistakes.
The greater incidence of tracheal complications is unlikely to be attributable to the duration of invasive MV. Known etiopathogenetic mechanisms of FTTLs and TEFs include use of nasogastric tubes, high cuff pressure (>40 cm H2O), poor health conditions, history of diabetes, and steroid use.3 In our intensive care unit, however, cuff pressure is periodically monitored with dedicated manometer and kept below 25 cm H2O. Furthermore, patients with COVID-19 and control patients had similar comorbidities, and nasogastric tubes were positioned early in both groups to allow enteral feeding. The main differences between the 2 groups that could justify a higher incidence of tracheal complications are pronation maneuvers, the use of higher doses of steroids, and lower Pao2/Fio2 ratio.
Limitations
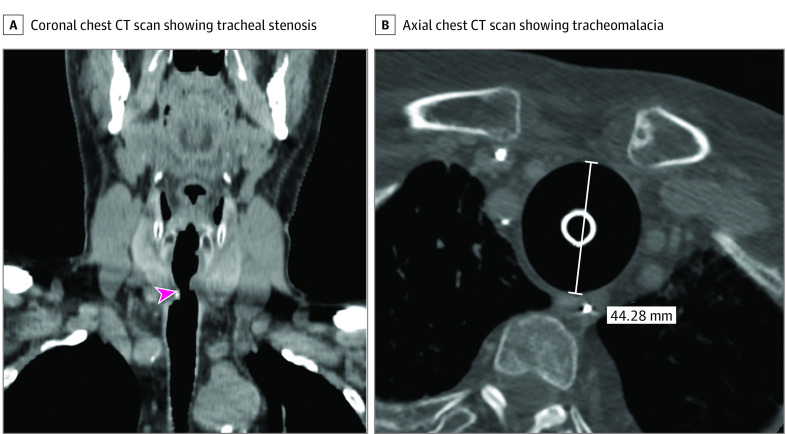
This study has several limitations. The first is its retrospective nature. Second, the follow-up time is relatively short, and, at the time of writing, we are beginning to see the first cases of late tracheal complications, such as tracheal stenosis and tracheomalacia (Figure 2). Third, we could not take and analyze tracheal tissue samples because it was not allowed by the Italian government during the pandemic. Fourth, we cannot exclude that some complications may have been caused or favored by the percutaneous tracheostomy because we do not know the exact time of their onset. For those reasons, prospective case-control and histological studies are warranted.
Figure 2. Late Tracheal Complications.
A, Coronal chest computed tomography (CT) scan showing a tracheal stenosis. B, Axial chest CT scan showing a tracheomalacia. See the loss of the tracheal rings and the large size of the tracheal lumen, which is kept patent by the tracheostomy tube. The pink arrowhead indicates tracheal stenosis.
Conclusions
Although the exact mechanisms and long-term outcomes of tracheal complications should be further investigated, 3 important recommendations for the management of patients with COVID-19 can be extrapolated from these findings. First, a bronchoscopy is indicated periodically (eg, weekly) to detect any signs of tracheal and endobronchial lesions early. This could lead to the identification of a greater number of tracheal lesions still in the subclinical phase (mucosal hyperemia, mucosal ischemia, and/or ulcer).17 Second, high steroid doses (intravenous methylprednisolone, 80 mg) should be used with caution, and the cuff pressure should be monitored to avoid hypoperfusion and pressure sores of the tracheal mucosa, particularly when a nasogastric tube is positioned. Third, an adequate clinical and radiological follow-up should be performed in patients treated with prolonged invasive MV to allow early identification and management of FTTLs, TEFs, and their complications.
References
- 1.Couraud L, Ballester MJ, Delaisement C. Acquired tracheoesophageal fistula and its management. Semin Thorac Cardiovasc Surg. 1996;8(4):392-399. [PubMed] [Google Scholar]
- 2.Bibas BJ, Cardoso PFG, Minamoto H, Pêgo-Fernandes PM. Surgery for intrathoracic tracheoesophageal and bronchoesophageal fistula. Ann Transl Med. 2018;6(11):210-210. doi: 10.21037/atm.2018.05.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green MS, J Mathew J, J Michos L, Green P, M Aman M. Using bronchoscopy to detect acquired tracheoesophageal fistula in mechanically ventilated patients. Anesth Pain Med. 2017;7(4):e57801. doi: 10.5812/aapm.57801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wali A, Rizzo V, Bille A, Routledge T, Chambers AJ. Pneumomediastinum following intubation in COVID-19 patients: a case series. Anaesthesia. 2020;75(8):1076-1081. doi: 10.1111/anae.15113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou C, Gao C, Xie Y, Xu M. COVID-19 with spontaneous pneumomediastinum. Lancet Infect Dis. 2020;20(4):510. doi: 10.1016/S1473-3099(20)30156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun R, Liu H, Wang X. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J Radiol. 2020;21(5):541-544. doi: 10.3348/kjr.2020.0180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Su X, Zhang T, Zheng C. Spontaneous pneumomediastinum: a probable unusual complication of coronavirus disease 2019 (COVID-19) pneumonia. Korean J Radiol. 2020;21(5):627-628. doi: 10.3348/kjr.2020.0281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441-2447. doi: 10.1001/jama.291.20.2441 [DOI] [PubMed] [Google Scholar]
- 9.Minonishi T, Kinoshita H, Hirayama M, et al. The supine-to-prone position change induces modification of endotracheal tube cuff pressure accompanied by tube displacement. J Clin Anesth. 2013;25(1):28-31. doi: 10.1016/j.jclinane.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 10.Kim D, Jeon B, Son J-S, Lee J-R, Ko S, Lim H. The changes of endotracheal tube cuff pressure by the position changes from supine to prone and the flexion and extension of head. Korean J Anesthesiol. 2015;68(1):27-31. doi: 10.4097/kjae.2015.68.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi: 10.1016/j.trsl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright FL, Vogler TO, Moore EE, et al. Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J Am Coll Surg. 2020;231(2):193-203.e1. doi: 10.1016/j.jamcollsurg.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley BT, Maioli H, Johnston R, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396(10247):320-332. doi: 10.1101/2020.04.17.20058545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711-1723. doi: 10.1056/NEJMra050541 [DOI] [PubMed] [Google Scholar]
- 15.The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330-1341. doi: 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prescott HC, Rice TW. Corticosteroids in COVID-19 ARDS: evidence and hope during the pandemic. JAMA. 2020;324(13):1292-1295. doi: 10.1001/jama.2020.16747 [DOI] [PubMed] [Google Scholar]
- 17.Touat L, Fournier C, Ramon P, Salleron J, Durocher A, Nseir S. Intubation-related tracheal ischemic lesions: incidence, risk factors, and outcome. Intensive Care Med. 2013;39(4):575-582. doi: 10.1007/s00134-012-2750-6 [DOI] [PubMed] [Google Scholar]