Abstract
Background
In late 2019, a cohort of patients presenting with pneumonia of unclear etiology in Wuhan, China, heralded the outbreak of coronavirus disease 19 (COVID-19). Previous severe acute respiratory syndrome (SARS) beta-coronavirus infections have been associated with tachyarrhythmias and signs and symptoms of heart failure. The emergence of SARS coronavirus 2 (SARS-CoV-2), which causes COVID-19, has rapidly developed into a pandemic, and a large number of infected patients have been reported to have underlying cardiovascular disease.
Objective
Since there are only scant published data regarding cardiovascular burden in the wake of viral epidemics, this study aimed to evaluate cardiac involvement in COVID-19.
Material and methods
This prospective cohort study included 40 adult inpatients at two centers in Germany. Adult patients diagnosed with COVID-19 in accordance with World Health Organization (WHO) interim guidance were included in the study, which focused on the potential cardiac involvement of SARS-CoV‑2. It was based on laboratory parameters as well as electro- and echocardiographic values to determine the impact of SARS-CoV‑2 virus on heart tissues.
Results
The conducted investigations confirmed the relationship between the presence of acute cardiac injury and COVID-19.
Conclusion
Myocardial injury and impaired myocardial function due to COVID-19 are common; however, no correlation was established between cardiac laboratory or echocardiographic values and mortality. Cardiovascular monitoring upon COVID-19 infection is crucial to determine the burden of cardiac involvement.
Keywords: COVID-19, Cardiovascular disease, SARS-CoV‑2, NT-proBNP, Cardiac troponin T
Abstract
Hintergrund
Ende 2019 markierte eine Kohorte von Patienten aus Wuhan, China, die sich mit Pneumonie unklarer Ätiologie vorstellten, den Ausbruch der „coronavirus disease 19“ (COVID-19). Frühere Severe-acute-respiratory-syndrome(SARS)-β-Coronavirus-Infektionen waren mit Tachyarrhythmien sowie Zeichen und Symptomen der Herzinsuffizienz assoziiert. Nach dem ersten Auftreten des SARS-Coronavirus 2 (SARS-CoV-2), das COVID-19 auslöst, entwickelte sich rasch eine Pandemie. Bei einer Großzahl infizierter Patienten wird über eine bestehende Herz-Kreislauf-Erkrankung berichtet.
Ziel
Da die publizierten Daten zur kardiovaskulären Last im Rahmen von Virusepidemien nur spärlich sind, wurde in der vorliegenden Studie die kardiale Beteiligung bei COVID-19 untersucht.
Material und Methoden
In die prospektive Kohortenstudie wurden 40 erwachsene stationäre Patienten aus zwei deutschen Zentren eingeschlossen. Einschluss fanden erwachsene Patienten mit COVID-19-Diagnose gemäß dem vorläufigen Leitfaden der Weltgesundheitsorganisation (WHO). Die Studie konzentrierte sich auf die potenzielle kardiale Beteiligung von SARS-CoV‑2. Auf Basis von Laborparametern sowie elektro- und echokardiographischen Daten wurde der Einfluss des SARS-CoV-2-Virus auf Herzgewebe bestimmt.
Ergebnisse
Die Untersuchungen bestätigten die Beziehung zwischen dem Vorliegen akuter kardialer Schäden und COVID-19.
Schlussfolgerung
COVID-19-bedingte Myokardschäden und Myokardfunktionsstörungen sind häufig, es fand sich jedoch keine Korrelation zwischen kardialen Labor- oder echokardiographischen Werten und der Mortalität. Eine kardiovaskuläre Überwachung bei COVID-19-Infektion ist von grundlegender Bedeutung, wenn die Last der kardialen Beteiligung bestimmt werden soll.
Schlüsselwörter: COVID-19, Herz-Kreislauf-Erkrankung, SARS-CoV‑2, NT-proBNP, Kardiales Troponin T
Introduction
In late 2019, a cohort of patients presenting with pneumonia of varying acuity and unclear etiology in Wuhan, China, heralded the outbreak of coronavirus disease 19 (COVID-19). Coronaviruses are known to cause respiratory or intestinal infections in humans and animals [1].
Previous severe acute respiratory syndrome (SARS) beta-coronavirus infections have been associated with tachyarrhythmias and signs and symptoms of heart failure [2].
Other acute respiratory infections, including influenza, respiratory syncytial virus, and bacterial pneumonias, are well-known triggers for cardiovascular diseases (CVD) [3, 4].
According to data from previous coronavirus epidemics (SARS and Middle East respiratory syndrome, MERS), these viral infections led mainly to pulmonary complications such as pneumonia and acute respiratory distress syndrome [5, 6]. Nevertheless, these viruses were reported to cause direct myocardial injury with subsequent myocarditis [7–9].
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes COVID-19, rapidly developed into a pandemic, and a large number of infected patients have been reported to have underlying CVD [10, 11].
Although COVID-19 appears to have greater infectivity and lower mortality than SARS and MERS, many uncertainties remain regarding, e.g., its viral evolution, appropriate anti-viral treatment, and strategies for disease control.
There are only scant published data regarding cardiovascular burden in the wake of viral epidemics. This study aimed to evaluate cardiac involvement in COVID-19.
Methods
Study design and participants
This prospective cohort study included 40 adult inpatients (≥18 years old) at the Braunschweig Municipal Hospital (21 patients) and the Bad Krozingen Heart Center (19 patients) in Germany. Adult patients diagnosed with COVID-19 in accordance with World Health Organization (WHO) interim guidance were included in the study.
The suspicion of COVID-19 infection was based on clinical presentation, contact to an active case with current infection, or having visited one of the known risk areas in China or Italy. Throat-swab specimens were obtained for detection of SARS-CoV‑2 polymerase chain reaction (PCR) examination at the emergency department. Asymptomatic positive patients were sent home to self-quarantine. Clinically stable positive patients with mild to moderate symptoms were admitted to isolation wards adapted for COVID-19 patients. Admission to an intensive care unit (ICU; arranged for infected patients) for initially unstable COVID-19 patients or upon clinical deterioration was available for further management.
The criteria for discharge included resolution of fever without the use of antipyretic medication, clinical improvement of signs and symptoms, pulmonary imaging showing obvious resolution of inflammation, and clinical remission of respiratory symptoms. Patients were discharged according to the evaluation of the treating physician. Discharge was followed by mandatory self-isolation at home or in a safe place for 8 days after the onset of symptoms in the event of mild cases or for 14 days in severe cases, in compliance with the European Centre for Disease Prevention and Control recommendations [12].
Surveillance visits by family physicians and local medical offices, as well as monitoring via telephone or other electronic devices for further check-ups were implemented.
Data collection
Routine blood examinations included complete blood count, coagulation profile, serum biochemical tests (including renal and liver function, creatine kinase [CK], lactate dehydrogenase, and electrolytes), myocardial enzymes, serum ferritin, procalcitonin, N‑terminal pro-brain natriuretic peptide (NT-proBNP), and immunoglobulin kappa and lambda. These parameters were recorded at hospital admission. Further follow-ups were performed based on clinical need. The authors only included the values at admission in their analysis. Chest radiographs or computed tomography (CT) scans were performed in all inpatients. A total of 21 patients with dyspnea or chest discomfort were examined with transthoracic echocardiography. Examinations were performed via a digital ultrasonic device system (Vivid 9 and Vivid 7, GE Vingmed Ultrasound, Horten, Norway) in harmonic mode 2.0/4.3 MHz, with a maximum frame per second (FPS) range of 64–112. Conventional echocardiography examination was performed including two-dimensional (2D) based M‑mode measures of cardiac chambers and ejection fraction (EF%), pulsed and continuous wave Doppler studies, color Doppler study, and calculation of EF% using Simpson’s method. The examinations were conducted on the isolation wards or in the ICU by two certified echocardiography specialists, observing the requirements and technical specifications of personal protective equipment (PPE) [13].
Fever was defined as a tympanic temperature of at least 37.3 °C. The level of suspicion of COVID-19 infection was graded according to CT findings from very low, or COVID-19 Reporting and Data System (CO-RADS) 1, to very high, or CO-RADS 5. CO-RADS is a categorical assessment scheme for pulmonary involvement of COVID-19 on non-enhanced chest CT developed by the Dutch Radiological Society [14]. All patients were categorized between CO-RADS 3 (unclear whether COVID-19 is present), CO-RADS 4 (CT abnormalities suspicious for COVID-19), or CO-RADS 5 (typical for COVID-19 infection).
Statistical analysis
Statistical analysis was performed using SPSS Statistics, version 25.0 (IBM Corporation, Armonk, NY). The categorical variables are expressed as numbers and percentages and were compared using the Chi-square test. Continuous data are expressed as mean ± standard deviation (SD) and compared using a one-way analysis of variance (ANOVA) and a post-hoc Tukey test. Independent predictors of mortality were determined using Cox proportional hazards regression models for all causes and cardiovascular disease. For all-cause mortality, age was forced into the model. P < 0.05 was considered significant. Logistic regression analysis was used to analyze correlations between the severity of lung involvement based on CO-RADS classification and cardiac biomarkers and was demonstrated with scatter plot curves.
Results
This prospective study included 40 patients from two centers in Germany (the Municipal Hospital of Braunschweig and the Bad Krozingen Heart Center). Women accounted for 37.5% of the study cohort (n = 15). A total of 19 patients (47.5%) had hypertension, while 11 had already been diagnosed with diabetes mellitus before admission. Of the studied group, 10 had already been previously diagnosed with cardiac disease (25%). The mean age in the study group was 67 ± 17 years. In all, 62.5% of patients complained of fever upon admission, while 12 patients had a sore throat. Half of the cohort presented with dyspnea (n = 20) and 11 patients had chest pain prior to admission. Eight patients in the present cohort died due to their infection with COVID-19 (Table 1).
Table 1.
Mean and standard deviation of troponin, N‑terminal pro-brain natriuretic peptide (NT-proBNP), creatine kinase, free light chain immunoglobulins (FLC Ig) lambda and kappa, and D‑dimer in relation to the laboratory reference range in patients with or without a history of known cardiac disease
| Mean | SD | No history of cardiac disease n. (%) |
Known history of cardiac disease n. (%) |
Reference range | |
|---|---|---|---|---|---|
| Troponin (pg/ml) | 27.75 | 20.7 | 18 (45) | 7 (17.5) | <14 |
| NT-proBNP (pg/ml) | 1847.5 |
2582.9 (median 682.5) |
20 (50) | 7 (17.5) | <486 |
| Creatinine kinase (U/l) | 242.9 |
254.3 (median 125.5) |
15 (37.5) | 2 (5) | <190 |
| FLC Ig lambda (mg/l) | 45.6 | 41.1 | 23 (57.5) | 9 (22.5) | 6.7–22.4 |
| FLC Ig kappa (mg/l) | 38.5 | 23.5 | 21 (52.5) | 8 (20) | 8.3–27.0 |
| D‑dimer (mg/l) | 2.3 |
3.7 (median 1.09) |
21 (52.5) | 12 (30) | <0.44 |
Cardiac manifestations
NT-proBNP was elevated in 27 patients at admission. The mean value of NT-proBNP was 1847.5 ± 2582.9 pg/ml (median 682.5). Moreover, troponin T was positive in 25 patients, revealing a mean value of 27.75 ± 20.7 pg/ml, 18 of which had no prior history of cardiac disease. At the same time, elevated creatine kinase was noted in 17 patients (mean value 242.9 ± 254.3 U/l, median 125.5), 15 of which had no past history of cardiac disease. The levels of free light chain immunoglobulins (FLC Ig) lambda were measured; 32 patients with a mean value of 45.6 ± 41.1 mg/l exhibited an elevation above the reference range (23 with no concomitant heart disease). FLC Ig kappa had a mean value of 38.5 ± 23.5 mg/l and was elevated in 29 patients (21 without known heart disease), with 33 patients showing increased D‑dimer levels at a mean value of 2.3 ± 3.7 mg/l (median 1.09). Of these patients, two had a history of thrombosis. One revealed a peripheral pulmonary embolism on thoracic CT and nine patients had a history of cardiac disease (Table 2).
Table 2.
Demographics and basic characteristics of patients, together with radiological and electrocardiographic results
| All patients (n = 40) |
No ICU admission (n = 27) |
ICU admission (n = 13) |
p-Value | |
|---|---|---|---|---|
| Clinical manifestations | ||||
| Age (years) | 67 ± 17 | 66 ± 19 | 69 ± 15 | 0.7 |
| Female gender, n (%) | 15 (37.5) | 14 (51.9) | 1 (7.7) | 0.013 |
| Hypertension, n (%) | 19 (47.5) | 14 (51.9) | 5 (38.5) | 0.511 |
| Diabetes, n (%) | 11 (27.5) | 4 (14. 8) | 7 (53.8) | 0.02 |
| History of cardiac disease, n (%) | 10 (25) | 0 (0) | 10 (25) | 0.016 |
| Family history of cardiac disease, n (%) | 4 (10) | 0 (0) | 4 (14.8) | 0.28 |
| Temperature, °C | 38.4 ± 1.6 | 38.5 ± 1.3 | 38.4 ± 1.5 | 0.73 |
| O2 (%) | 89 ± 5 | 91 ± 2 | 84 ± 7 | 0.001 |
| Catecholamine therapy, n (%) | 7 (17.5) | 0 (0) | 7 (53.8) | <0.01 |
| Mechanical ventilation, n (%) | 9 (22) | 0 (0) | 9 (60) | <0.01 |
| Mortality, n (%) | 8 (20) | 4 (14.8) | 4 (30.8) | 0.4 |
| Duration of hospital stay (days) | 14 ± 9 | 9 ± 6 | 21 ± 8 | <0.01 |
| Clinical symptoms | ||||
| Fever, n (%) | 25 (62.5) | 15 (55.6) | 10 (76.9) | 0.17 |
| Dyspnea, n (%) | 20 (50) | 12 (44.4) | 8 (61.5) | 0.5 |
| Sore throat, n (%) | 12 (30) | 6 (22.2) | 6 (46.2) | – |
| Chest pain, n (%) | 11 (52.5) | 7 (25.9) | 4 (30. 8) | 0.51 |
| Cough, n (%) | 17 (42.5) | 9 (33.3) | 8 (61.5 ) | 0.17 |
| CT staging (n = 40) | ||||
| CO-RADS 3, n (%) | 16 (40) | 16 (59.3) | 0 (0) | 0.001 |
| CO-RADS 4, n (%) | 10 (25) | 4 (14.8) | 6 (46.2) | |
| CO-RADS 5, n (%) | 14 (35) | 7 (25.9) | 7 (53.8) | |
| Laboratory findings | ||||
| CRP (mg/l) | 87.4 ± 61.6 | 58.6 ± 42.5 | 142.8 ± 55.2 | <0.01 |
| Leucocyte count (10e3/µl) | 7.9 ± 3.3 | 7.7 ± 3.9 | 8.1 ± 1.4 | 0.71 |
| Lymphocytes (10e3/µl) | 1.7 ± 1.8 | 2.1 ± 2.1 | 1 ± 0.5 | 0.06 |
| Thrombocytes (10e3/µl) | 299.9 ± 169.6 | 305.1 ± 168.5 | 289.2 ± 178.2 | 0.8 |
| Procalcitonin (µg/l) | 0.53 ± 0.98 | 0.49 ± 1.1 | 0.6 ± 0.67 | 0.7 |
| CK (U/l) |
242.9 ± 254.3 (median 125.5) |
143.8 ± 162.1 | 408.2 ± 297.2 | 0.001 |
| CKMB (U/l) | 24 ± 14.9 | 20.5 ± 8.9 | 31.2 ± 21.7 | <0.05 |
| Troponin (pg/ml) | 27.7 ± 20.7 | 20.8 ± 17.5 | 39.3 ± 21 | 0.005 |
| NT-ProBNP (pg/ml) |
1847.5 ± 2582.9 (median 682.5) |
738.4 ± 842.8 (median 590.0) |
3695.9 ± 3392.8 | <0.01 |
| D‑dimer (mg/l) |
2.3 ± 3.8 (median 1.09) |
1.6 ± 1.7 | 3.8 ± 6.0 | 0.08 |
| FLC Ig lambda (mg/l) | 45.6 ± 41.1 | 33.9 ± 16.6 | 69.9 ± 62.9 | 0.008 |
| FLC Ig kappa (mg/l) | 38.5 ± 23.7 | 31.2 ± 10.0 | 53.7 ± 34.8 | 0.003 |
| Ferritin (ng/ml) |
855.78 ± 1332.4 (median 601.00) |
619.3 ± 428.8 |
1345.6 ± 2233 (median 596.00) |
0.1 |
| ALT (U/l) | 63.9 ± 52.9 | 56.7 ± 39.8 | 78.8 ± 73 | 0.24 |
| Creatinine (mg/dl) | 0.8 ± 0.2 | 0.73 ± 0.15 | 0.89 ± 0.23 | 0.02 |
| Electrocardiographic parameters | ||||
| Diffuse ST-segment elevation, n (%) | 2 (5) | 1 (4) | 1 (6.7) | 1 |
| Prolonged PR time, n (%) | 8 (20) | 4 (16) | 4 (26.7) | 0.44 |
| PR time (ms) | 181 ± 25 | 180 ± 25 | 182 ± 26 | 0.34 |
| Prolonged QTc time, n (%) | 2 (5) | 0 (0) | 2 (13.3) | 0.14 |
| QTc time (ms) | 436 ± 31 | 434 ± 32 | 438 ± 28 | 0.43 |
| Right bundle branch block, n (%) | 2 (5) | 0 (0) | 2 (13.3) | 0.13 |
| Left bundle branch block, n (%) | 1 (2.5) | 1 (4) | 0 (0) | 1 |
| New-onset atrial fibrillation, n (%) | 2 (5) | 1 (4) | 1 (6.7) | 1 |
O2 oxygen saturation, CO-RADS COVID-19 Reporting and Data System, CRP C-reactive protein, CK creatine kinase, CKMB creatine kinase mb-fraction, NT-ProBNP N-terminal pro brain natriuretic peptide, FLC Ig free light chain immunoglobulin, ALT alanine aminotransferase, IL6 interleukin 6, SD standard deviation
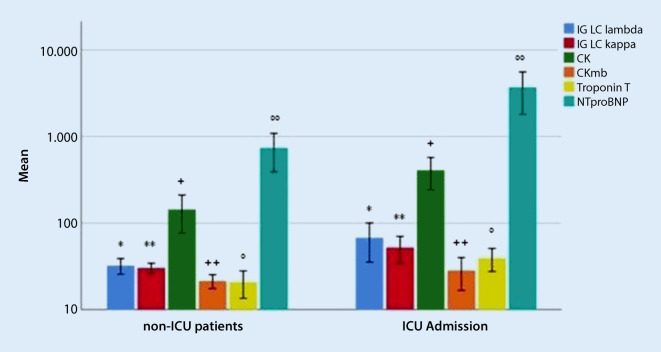
Cardiac troponin T was significantly higher in patients that were admitted to an ICU (39.3 ± 21.0 pg/ml) in comparison to the patients that did not need intensive care admission (20.8 ± 17.5 pg/ml; p-value 0.005). Correspondingly, the ICU patients showed statistically significant elevation of NT-proBNP and creatine kinase (3695.9 ± 3392.8 pg/ml and 408.2 ± 297.2 U/l) compared to non-ICU patients (738.4 ± 842.8 pg/ml and 143.8 ± 162.1 U/l; p-values <0.01 and 0.001, respectively). Similarly, the FLC Ig lambda and kappa were significantly higher among the patients that required ICU management (69.9 ± 62.9 mg/l and 53.7 ± 34.8 mg/l), in contrast to patients admitted to normal wards (33.9 ± 16.6 mg/l and 31.2 ± 10.0 mg/l; p-values 0.008 and 0.003 respectively) (Fig. 1; Table 1).
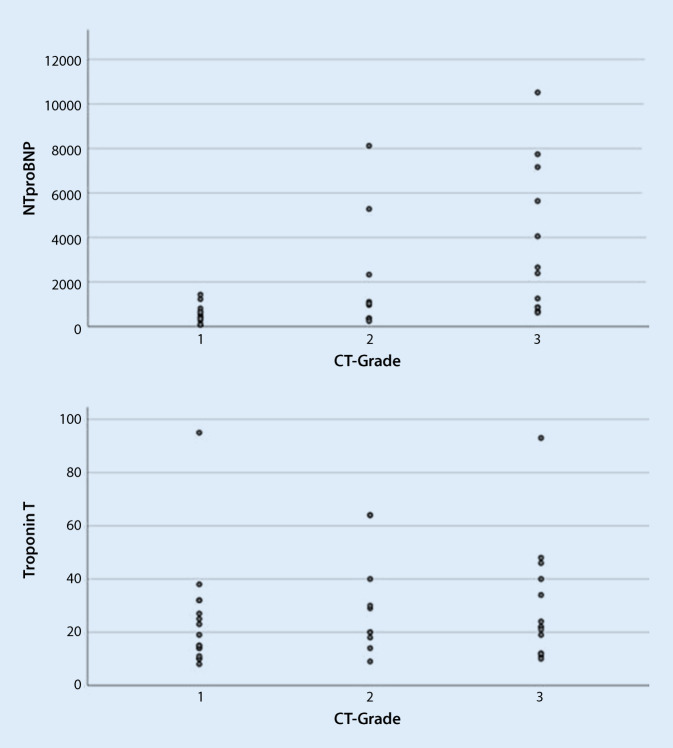
Fig. 2.
Correlation of N‑terminal pro-brain natriuretic peptide (NTproBNP) and troponin T to computed tomography (CT) grading using sinple scatter
Fig. 1.
Comparison of mean values and standard deviation of cardiac laboratory parameters in relation to intensive care unit (ICU) admission. * p-Value 0.008, ** p-value 0.003, + p-value 0.001, ++ p-value <0.05, ° p-value 0.005, °° p-value <0.01
Additionally, a significant correlation between the severity of pulmonary involvement, based on CO-RAD classification, and NT-proBNP was revealed (r = 0.47, p = 0.002). The correlation between the CT findings and troponin was less significant (r = 0.11, p = 0.048). A further correlation was revealed between creatine kinase and lung CT (r = 0.46, p 0.003). Moreover, a weak correlation with FLC Ig kappa (r 0.14, p = 0.40) was established, while no significant correlation with FLC Ig lambda was documented (r = 0.06, p = 0.71).
A total of 21 patients were examined by echocardiography as indicated from their clinical presentation. Five of 21 patients demonstrated reduced left ventricular EF (<50%), four patients had elevated pulmonary artery systolic pressure (>25 mm Hg), and five patients revealed diastolic dysfunction with preserved systolic function (E/e’ ratio >15; Table 3). The electrocardiogram noted diffuse ST elevation in two patients without concomitant coronary artery stenosis (exclusion based on coronary angiography). First degree atrioventricular (AV) block with prolonged PR time was documented in eight patients (20%) and prolonged QTc time in two patients before initiating antiviral therapy. No statistically significant difference was found between the mean values of PR time and QTc time in relation to ICU admission. The electrocardiographic and echocardiographic findings are shown in Table 2.
Table 3.
Echocardiographic values in patients with positive coronavirus disease 19
| Echocardiographic parameters | All patients (n = 21) |
No ICU admission | ICU admission | |
|---|---|---|---|---|
| Normal LV ejection fraction, n (%) | 16 (40) | 10 (40) | 6 (40) | 0.09 |
| Reduced LV ejection fraction, n (%) | 5 (12.5) | 1 (4) | 4 (26.7) | 0.09 |
| New significant valve lesions, n (%) | 0 (0) | 0 (0) | 0 (0) | – |
| Elevated pulmonary systolic pressure, n (%) | 4 (10) | 1 (4) | 3 (20) | 0.14 |
LV left ventricular
Discussion
This study focused on the potential cardiac involvement of SARS-CoV‑2 virus. It was based on laboratory parameters as well as electro- and echocardiographic values to determine the impact of SARS-CoV‑2 virus on heart tissues. The conducted investigations confirmed the relationship between the presence of acute cardiac injury and COVID-19.
The authorsʼ patients experienced myocardial injuries (45% of the study group): myocardial necrosis was suggested by increased troponin T levels, and myocardial functional disturbance by elevated NT-proBNP, as well as disturbed left ventricular systolic and diastolic function. In addition to these findings, the existence of enhanced inflammatory biomarkers such as CRP, ferritin, and FLCs suggested that myocardial injury may be caused by inflammatory myocardial processes. NT-proBNP showed significant correlation with the length of hospital management and the severity of pulmonary CT findings.
The electrocardiographic findings in these patients, such as ST elevation without reciprocal ST depression in the absence of acute coronary artery insult, and the conduction disturbances suggest further evidence of myocarditis, most likely due to direct viral invasion or immune-mediated myocardial injury. The presence of myocardial interstitial oedema in acute viral myocarditis can lead to disturbance of both systolic and diastolic function, which was detected in 25% of the patients. Patients with myocarditis can show pulmonary hypertension in the acute course. Other compensatory mechanisms, such as right ventricular hypertrophy, need weeks or months to develop. The thrombogenic nature of COVID-19 may have led to peripheral pulmonary vasculature insults with acute elevated pulmonary pressure. This theory cannot be excluded, even though no central pulmonary embolisms were detected on thorax CT.
The results of univariate Cox proportional hazards analysis for laboratory biomarkers or echocardiographic parameters, possibly due to relatively small sample size, showed no significant independent prognostic value for mortality in the present COVID patients.
The myocardium may be infected by a wide variety of viruses [15, 16]. In patients with moderate to severe heart failure (EF <45%) and inflammation in the Marburg registry, 42.1% were virus-positive [17].
In 2006, a study of patients diagnosed with SARS revealed that tachycardia was the most common finding (72%) beside hypotension (50%), bradycardia (15%), transient cardiomegaly (11%), and transient paroxysmal atrial fibrillation in only one patient (0.8%) as a result of direct cardiac injury in the absence of underlying heart disease [2]. Another group aimed to characterize cardiac manifestations in the 2009 influenza pandemic (H1N1). In all, 46% of patients showed evidence of myocardial injury. Of 28 patients in whom an echocardiogram was clinically indicated, 20 had left ventricular systolic dysfunction. Of these, 14 patients were diagnosed as having myocarditis, with most (12 patients) developing this early on [18]. Fulminant myocarditis caused by the H1N1 strain of influenza was also reported [19]. Various studies described myocarditis related to other influenza forms [20–22]. Further reports linked MERS coronavirus (MERS-CoV) to myocarditis and severe left ventricular systolic dysfunction [9]. Furthermore, an animal model study clearly stated that viral RNA could be seen in cardiac tissue, implying direct cardiac pathology [23].
Shi et al. conducted a single-center cohort study at Wuhan University, China, and retrospectively included a total of 416 hospitalized patients with COVID-19. Approximately 82 patients (19.7%) were reported to have cardiac injury. A higher mortality rate (p < 0.001) was noticed in patients with cardiac injury (51.2%) than those without cardiac injury (4.5%) [24]. To evaluate the association of underlying CVD and myocardial injury with fatal outcomes in COVID-19 patients, Guo et al. conducted a retrospective single-center study. Of a total of 187 patients with confirmed COVID-19, 52 (27.8%) patients exhibited myocardial injury as indicated by elevated troponin T levels [25]. Likewise, myocardial injury proved by troponin T elevation was determined in 45% of the present cohort. Similar to the current study, the authors did not observe any significant correlation between troponin T and NT-proBNP in relation to mortality [25].
Case reports have depicted the cardiac involvement of COVID-19 infection. The diagnosis of myocarditis was established upon troponin T and NT-proBNP elevation and was confirmed in one case through echocardiography and in the other with cardiac MRI [26, 27]. Another group from New York, USA, identified ST-segment elevation in 18 patients infected with COVID-19. Noncoronary myocardial injury was noted in 10 patients indicating myo/pericarditis [28].
The authors did not detect any ventricular arrhythmias or sudden cardiac death (SCD) in patients under monitor surveillance. The proportion of SCD caused by myocarditis has been reported as ranging from 1% to 14% of all SCD [29, 30].
Elevation of FLC kappa and lambda in the current patient cohort suggests that the clones of B lymphocytes and plasma cells that produce FLCs may be activated in COVID-19 patients, although the number of lymphocytes were decreased. However, there were contradictory observations of FLC in heart failure, which may partly reflect the different FLC assays used [31, 32].
Limitation
The number of patients included in this study was relatively small, which did not allow proper monitoring of the prognostic value of various cardiac parameters for mortality risk. Given the difficulties associated with performing myocardial biopsy in COVID-19 patients, pathological diagnosis remains to be clarified. The authors cannot exclude a possible sample selection bias due to a discrepancy between the two recruiting centers (a heart center and a municipal hospital).
Conclusion
Myocardial injury and impaired myocardial function due to COVID-19 are common. Patients with elevated cardiac parameters such as NT-proBNP and cardiac troponin in the absence of a known history of heart disease are at higher risk for ICU admission. No correlation was established between cardiac laboratory or echocardiographic values and mortality. Cardiovascular monitoring upon COVID-19 infection is crucial to determine the burden of cardiac involvement.
Compliance with ethical guidelines
Conflict of interest
A. Saleh, A. Matsumori, S. Abdelrazek, S. Eltaweel, A. Salous, F.-J. Neumann, and M. Antz declare that they have no competing interests.
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
References
- 1.Cheng VCC, Lau SKP, Woo PCY, Yuen KY. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20(4):660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu CM, Wong RS, Wu EB, et al. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J. 2006;82(964):140–144. doi: 10.1136/pgmj.2005.037515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cowan LT, Lutsey PL, Pankow JS, Matsushita K, Ishigami J, Lakshminarayan K. Inpatient and outpatient infection as a trigger of cardiovascular disease: the ARIC study. J Am Heart Assoc. 2018;7(22):e009683–e009683. doi: 10.1161/JAHA.118.009683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madjid M, Miller CC, Zarubaev VV, et al. Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy-confirmed coronary heart disease death: results from 8 years of autopsies in 34,892 subjects. Eur Heart J. 2007;28(10):1205–1210. doi: 10.1093/eurheartj/ehm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ksiazek TG, Erdman D, Goldsmith CS. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;15(348):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 6.Van Boheemen ZAM, Bestebroer STM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 7.Ukimura A, Satomi H, Ooi Y. Myocarditis associated with influenza A H1N1pdm2009. Influenza Res Treat. 2012;2012:1–8. doi: 10.1155/2012/351979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao S, Sasser W, Diaz F. Coronavirus associated fulminant myocarditis successfully treated with intravenous immunoglobulin and extracorporeal membrane oxygenation. Chest. 2014;146(4):336A. doi: 10.1378/chest.1992018. [DOI] [Google Scholar]
- 9.Alhogbani T. Acute myocarditis associated with novel Middle East respiratory syndrome coronavirus. Ann Saudi Med. 2016;36:78–80. doi: 10.5144/0256-4947.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhainaut J-F, Claessens Y-E, Janes J, Nelson DR. Underlying disorders and their impact on the host response to infection. Clin Infect Dis. 2005;41(suppl 7):S481–S489. doi: 10.1086/432001. [DOI] [PubMed] [Google Scholar]
- 11.Fauci AS, Lane HC, Redfield RR. Covid-19: navigating the uncharted. N Engl J Med. 2020 doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Centre for Disease Prevention and Control . Guidance for discharge and ending isolation in the context of widespread community transmission of COVID-19, 8 April 2020. Stockholm: ECDC; 2020. [Google Scholar]
- 13.Advice on the use of masks in the community, during home care, and in health care settings in the context of COVID-19: WHO interim guidance (19 March 2020).
- 14.Prokop M, van Rees Vellinga T, The CO-RADS—A categorical CT assessment scheme for patients with suspected COVID-19: definition and evaluation. Radiology. 2020;296(2):E97–E104. doi: 10.1148/radiol.2020201473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumori A. Cardiomyopathies and heart failure. Biomolecular, infectious and immune mechanisms. In: Matsumori A, editor. Cardiomyopathy and heart failure. Boston, Dordrecht, London: Kluwer Academic Publishes; 2003. pp. 1–15. [Google Scholar]
- 16.Saleh A, Matsumori A, Negm H, Fouad H, Hamdy E. Assessment of cardiac involvement of hepatitis C virus; tissue Doppler imaging and NTproBNP study. J Saudi Heart Assoc. 2011;23:217–223. doi: 10.1016/j.jsha.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhl U, Pauschinger M, Noutsias M, et al. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation. 2005;111(7):887–893. doi: 10.1161/01.CIR.0000155616.07901.35. [DOI] [PubMed] [Google Scholar]
- 18.Binila Chacko JVP, Pichamuthu K, Ramakrishna K, Moorthy M, Karthik R, John G. Cardiac manifestations in patients with pandemic (H1N1) 2009 virus infection needing intensive care. J Crit Care. 2012;27(1):106.e1–106.e6. doi: 10.1016/j.jcrc.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Al-Amoodi M, Kavitha Rao, Seshu Rao, Brewer JH, Magalski A, Chhatriwalla AK. Fulminant myocarditis due to H1N1 influenza. Circ Heart Fail. 2010;3:e7–e9. doi: 10.1161/CIRCHEARTFAILURE.110.938506. [DOI] [PubMed] [Google Scholar]
- 20.Greaves K, Oxford JS, Price CP. The prevalence of myocarditis and skeletal muscle injury during acute viral infection in adults: measurement of cardiac troponins I and T in 152 patients with acute influenza infection. Arch Intern Med. 2003;163:165–168. doi: 10.1001/archinte.163.2.165. [DOI] [PubMed] [Google Scholar]
- 21.Connolly AM, Salmon RL, Lervy B. What are the complications of influenza and can they be prevented? Experience from the 1989 epidemic of H3N2 influenza A in general practice. BMJ. 1993;306:1452–1454. doi: 10.1136/bmj.306.6890.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ison MG, Campbell V, Rembold C. Cardiac findings during uncomplicated acute influenza in ambulatory adults. Clin Infect Dis. 2005;40:415–422. doi: 10.1086/427282. [DOI] [PubMed] [Google Scholar]
- 23.Agrawal AS, Garron T, Tao X, Peng BH, Wakamiya M, Chan TS, et al. Generation of a transgenic mouse model of Middle East respiratory syndrome coronavirus infection and disease. J Virol. 2015;89:3659–3670. doi: 10.1128/JVI.03427-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan. China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng JH, Liu YX, Yuan J, et al. First case of COVID-19 infection with fulminant myocarditis complication: case report and insights. Infection. 2020 doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bangalore S, Sharma A, Slotwiner A. ST-segment elevation in patients with Covid-19—A case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wisten A, Krantz P, Stattin E-L. Sudden cardiac death among the young in Sweden from 2000 to 2010: an autopsy-based study. Europace. 2017;19:1327–1334. doi: 10.1093/europace/euw249. [DOI] [PubMed] [Google Scholar]
- 30.Bagnall RD, Weintraub RG, Ingles J. A prospective study of sudden cardiac death among children and young adults. N Engl J Med. 2016;374:2441–2452. doi: 10.1056/NEJMoa1510687. [DOI] [PubMed] [Google Scholar]
- 31.Matsumori A, Shimada T, Nakatani E, Shimada M, Tracy S, Chapman NM, Drayson MT, Hartz VL, Mason JW. Immunoglobulin free light chains as an inflammatory biomarker of heart failure with myocarditis. Clin Immunol. 2020;217:108455. doi: 10.1016/j.clim.2020.108455. [DOI] [PubMed] [Google Scholar]
- 32.Jackson CE, Haig C, Welsh P, Dalzell JR, Tsorlalis IK, McConnachie A, Preiss D, McInnes IB, Sattar N, Petrie MC, Gardner RS, McMurray JJ. Combined free light chains are novel predictors of prognosis in heart failure. JACC Heart Fail. 2015;3:618–625. doi: 10.1016/j.jchf.2015.03.014. [DOI] [PubMed] [Google Scholar]