Abstract
Bitter taste is often associated with toxins, but accepting some bitter foods, such as green vegetables, can be an important part of maintaining a healthy diet. In rats and humans, repeated exposure to a bitter stimulus increases acceptance. Repeated exposure allows an individual the opportunity to learn about the food’s orosensory and postingestive effects. It also alters the salivary protein (SP) profile, which in turn alters taste signaling. We have hypothesized that altering the salivary proteome plays a role in the increased acceptance after repeated exposure. Here we test this and attempt to disentangle the contribution of learning during dietary exposure from the contribution of SPs in increased acceptance of bitter diet. Dietary exposure to quinine or tannic acid and injection of isoproterenol (IPR) result in similar salivary protein profiles. Here we used either the bitter stimulus tannic acid or IPR injection to upregulate a subset of SPs before exposing animals to a novel diet containing quinine (0.375%). Control animals received either a control diet before being exposed to quinine, or a diet containing sucrose octaacetate, a compound that the animals avoid but does not alter SP profiles. The treatments that alter SP expression increased rate of feeding on the quinine diet compared to the control treatments. Additionally, tannic acid exposure altered intake and meal size of the quinine diet. These data suggest that SPs, not just learning about bitter food, increase acceptance of the bitter diet.
Keywords: Saliva, Taste, Bitter, Diet choice
1. Introduction
A simple, non-invasive, and cost-effective intervention to prevent or mitigate the surge in obesity and its concurrent health risks (type 2 diabetes, chronic cardiovascular and respiratory diseases, depression, etc.) is to reduce overconsumption of fat- and sugar-abundant foods in favor of more nutritionally valuable food sources, like vegetables (Buckland, Bach, & Serra-Majem, 2008; Foster, et al., 2003; Larsen, et al., 2010). However, for many, healthy eating is difficult to employ or maintain; plant-based foods are often accompanied by an unpleasant taste, as the phytochemicals that plants use to discourage herbivory often activate bitter receptors (Barratt-Fornell & Drewnowski, 2002; Chung, Wong, Wei, Huang, & Lin, 1998; Drewnowski & Gomez-Carneros, 2000; Vasanthi, Mukherjee, & Das, 2009). Bitter taste is commonly described as the body’s first line of defense against toxins, for good reason; many of these bitter plant compounds are poisonous or anti-nutritional (Barratt-Fornell & Drewnowski, 2002). Bitter taste receptors are diverse, with ~32 unique receptors (Adler, et al., 2000; Chandrashekar, et al., 2000; Pronin, Tang, Connor, & Keung, 2004) that can respond to thousands of ligands (Bufe, et al., 2005; Bufe, Hofmann, Krautwurst, Raguse, & Meyerhof, 2002; Kuhn, et al., 2004; Meyerhof, et al., 2010; Meyerhof, Behrens, Brockhoff, Bufe, & Kuhn, 2005; Shi, Zhang, Yang, & Zhang, 2003; Soares, et al., 2013) and it is hypothesized that this diversity is beneficial to detect and avoid toxic plant compounds. However, bitter foods do not all result in negative health outcomes and are often valuable sources of nutrients.
Acceptance of bitter stimuli is subject to individual variation at multiple levels, including expression and distribution of receptor subtypes (Hayes, Feeney, & Allen, 2013; Hayes, et al., 2011; Nolden, McGeary, & Hayes, 2016; Roura, et al., 2015; Shi, et al., 2003), nerve responsivity (Clarke & Ossenkopp, 1998; Dahl, Erickson, & Simon, 1997; Inoue, Li, McCaughey, Beauchamp, & Bachmanov, 2001; Prutkin, et al.; St. John & Boughter, 2004), and representation in the brain (Di Lorenzo & Monroe, 1990; Di Lorenzo & Victor, 2003; Giza, McCaughey, Zhang, & Scott, 1996; Martin & Sollars, 2017; Roussin, Victor, Chen, & Di Lorenzo, 2008; Spector & Kopka, 2002; Wilson, Boughter, & Lemon, 2012). The most common method suggested for incorporating bitter foods into the diet is repeated exposure (Forestell & Mennella, 2007; Mennella & Beauchamp, 1993; Mennella, Nicklaus, Jagolino, & Yourshaw, 2008; Ventura & Mennella, 2011). One possible mechanism for increased intake after exposure is learning. Learning about the postingestive and orosensory properties of the food may be sufficient to increase intake once an animal has determined a particular food is safe. Additionally, we have postulated that repeated exposure to a safe bitter food may alter acceptance of the food to make it less aversive by altering salivary protein (SP) expression (Martin, et al., 2018).
Historically, work studying saliva and food consumption focused primarily on a group of SPs that are associated with an animal’s ability to consume tannins. Seventy percent of all SPs comprise a single class referred to as proline-rich proteins (Skopec, Hagerman, & Karasov, 2004). Proline-rich proteins interact with tannic acid (TA), a class of plant secondary compounds that is commonly found in foods (e.g., in beer, wine, tea, beans, nuts, etc.) and produces negative physiological effects at high concentrations (Mehansho, Butler, & Carlson, 1987). It has been hypothesized that proline-rich proteins bind to TA thereby preventing its negative physiological actions and perceived intensity, leading to an increase in its acceptability (Torregrossa, et al., 2014). This interaction does not appear to be atypical; mounting evidence has shown that SP expression is broadly related to bitter acceptance in human (Dsamou, et al., 2012; Morzel, et al., 2014; Morzel, et al., 2017; Neyraud, Sayd, Morzel, & Dransfield, 2006; Quintana, et al., 2009) and animal models (Glendinning, 1992; Martin, et al., 2018; Torregrossa, et al., 2014). Our lab has demonstrated that SPs are altered by TA or quinine exposure (Martin, et al., 2018; Torregrossa, et al., 2014) and that the suite of SPs upregulated in rats by these diets is similar (Martin, et al., 2018). The upregulation of these bitter-related SPs is concurrent with an increase in total intake, as well as increased rate of feeding, and meal size (Martin, et al., 2018; Torregrossa, et al., 2014). Additionally, animals that have bitter related SPs upregulated increase brief-access licking and the presence of SPs decreases taste nerve signaling (Martin, et al., 2018). These data provide converging evidence that SPs alter palatability of bitter foods.
Here we attempt to disentangle the role of previous experience with a bitter food from the role of SPs in altering bitter diet acceptability. To do this, we induced protein changes by feeding rats a bitter/astringent diet (TA) or by injecting them with isoproterenol (IPR, a non-specific beta-adrenergic agonist) before offering them a quinine diet. Control groups received either a bitter diet that does not alter SP profile (sucrose octaacetate, SOA) or a diet that contains no bitter compounds.
2. Materials and Methods
2.1. Subjects
Adult male Long Evans rats (Charles River Breeding Laboratory, Raleigh, NC), were used in all experiments. Colony rooms were maintained at 20 ± 2°C with a 12:12h light/dark cycle. All animal procedures were approved by Florida State University and/or University at Buffalo Animal Care and Use Committees.
2.2. Timeline
Four separate treatment groups were used in this study, across eight cohorts of animals. Animals were maintained on Purina 5001 or Envigo 2018 and tap water ad lib until study onset, after which all cohorts were given ad lib access to a custom control diet containing no bitter compounds (Skopec, et al., 2004) for two weeks. This allowed us to establish baseline intake measures and SP profiles. Animals then entered the pre-treatment phase. Cohorts with dietary pre-treatments received their assigned diet (TA or SOA, described in detail later) for 14 days. For graphical representation and statistical analysis, only the final three days of dietary pre-treatment were included. Cohorts with drug pre-treatments (IPR, described in detail later) received the drug for the final three days of the control diet. In each experiment, all treatment animals were matched with concurrent controls. In the first of the IPR experiments, the concurrent controls were allowed to eat control diet ad lib, while in the second, controls were restricted to the average amount consumed by the IPR-injected rats. All animals were then given 0.375% quinine diet for three days. Saliva samples were collected during the last three days the control phase (IPR) or the last three days of the pretreatment phase (TA, SOA) and during the quinine exposure.
2.3. Feeding and meal pattern analysis
Animals were individually housed in custom-designed Plexiglas shoebox cages with a feeding compartment that provided access to a spill-resistant food cup. An infrared light emitting diode (LED) and a photo detector were mounted in the food compartment, as previously described (Dotson, Colbert, Garcea, Smith, & Spector, 2012; Smith, 1989; Torregrossa, et al., 2014). Feeding events were detected when the rat’s head broke the photo beam. The time and duration of each beam break was recorded and used to estimate meal size and rate of feeding. Food cups were weighed daily to calculate 24h food intake. The start of a meal was defined as a minimum of 3s of activity in the food cup, and individual meals were considered terminated when there was no feeding activity for 5 minutes. Average daily meal size was defined as the average size of all meals in a 24h period (calculated as 24h food intake (g) divided by the number of meals consumed each day). Average rate of feeding was defined as 24h food intake divided by the total amount of feeding activity (min). Average meal number was defined as the number of meals/24h.
2.4. Saliva collection
Saliva was collected and processed as described in Torregrossa et al. 2014 (Torregrossa, et al., 2014). Briefly, saliva was collected from awake, trained animals. Training consisted of repeated pairings (up to 2mls/session, 14 sessions) of 30mM citric acid in 1M sucrose via a pipette tip placed under the tongue. This solution, which served as an unconditioned stimulus (US), was pipetted into the mouth in 200μl increments. The pipette tip, which served as a conditioned stimulus (CS), was inserted into the side of the mouth and was moved over and under the tongue during CS/US pairings. By the end of training, animals were conditioned to salivate by merely inserting the pipette into the mouth. Following the training period, saliva samples were collected from rats by inserting a 200μl pipette with wide orifice tip under the tongue and gently aspirating saliva. During saliva collection days, animals were given 2ml of the citric acid in sucrose solution to maintain training. After a sucrose /citric acid infusion, we waited at least 1-minute before collecting saliva. Saliva samples ranging from 50–100μl were immediately placed on ice in 10μl of 10x Halt protease/phosphatase inhibitor cocktail (Thermo Scientific). All samples were then frozen for later analysis. Saliva was collected from half of the rats on odd test days while the other half received only the citric acid/sucrose mixture and the reverse were collected on even days. Thus, saliva was collected representing each day of the trial.
2.5. Saliva processing and gel electrophoresis
Samples were processed and gels were run as previously described (Martin, et al., 2018). Briefly, saliva samples were defrosted and mixed with equal volumes of 0.2% trifluoroacetic acid. Samples were centrifuged at 2000 x g for 15 min at 4°C to remove cells and debris. The supernatant was then aspirated. Total protein concentration was determined by the bicinchoninic acid (BCA) protein assay method (Pierce Protein Biology Products).
For gel electrophoresis, equal volumes of whole saliva were mixed with 1/3rd volume of 4x Invitrogen sample buffer with reducing agent, heated at 80°C for 10 min and resolved on a 12% SDS-PAGE (Invitrogen) with MOPS buffer. Molecular mass markers (Bio-Rad, Ref#1610394) were run simultaneously with the samples in each gel to calibrate molecular masses of each protein band. Gels were fixed in 40% methanol in 10% acetic acid for 30min followed by staining with Coomassie Brilliant Blue R250 (Bio-Rad) for 1h, then destained in 10% acetic acid according to published protocols (Beeley, et al., 1991; Sarni-Manchado, Cheynier, & Moutounet, 1998). Bands were captured using Bio-Rad digital imager ChemiDoc™MP, or Azure c400 Gel Imaging System. Densitometric analysis was performed using Image Lab 5.0+ Software (Bio-Rad) or Azurespot Analysis Software (Azure Biosystems).
2.6. Dietary pre-treatments:
Animals were 200–250g at study onset. At the onset of the pre-treatment phase the control diet was adulterated with one of the following: SOA (4% by weight, n=8 control, n=8 SOA) or tannic acid (3% by weight (Torregrossa, et al., 2014), n=8 control, n=7 TA). These concentrations were chosen because our pilot testing suggested they resulted in similar decreases in food intake on the first day of exposure. In all cases, the bitter compound replaced the same amount of cellulose in the control diet; in this way the diets were kept isocaloric (Martin, et al., 2018). Rats were allowed ad lib access to all diets unless otherwise specified. Rats were tested in cohorts of eight animals, (four control and four experimental). One rat was dropped from the tannic acid diet for failing to use the food cup in the custom cage.
Previous reports have already confirmed the effects of IPR injection (Ann, Clements, Johnstone, & Carlson, 1987; Barka, Yagil, Van der Noen, & Naito, 1986; Bedi, 1991; Johnson, 1983), TA feeding, long term exposure to our control diet, and our collection techniques (Torregrossa, et al., 2014) on SP profiles. In order to separate the effect of dietary experience from the effects of SPs we wanted a control diet that interacts with a bitter receptor (Brockhoff, Behrens, Massarotti, Appendino, & Meyerhof, 2007), but did not alter salivary protein expression in the same way quinine and tannic acid did. We pilot-tested several compounds and chose to continue our studies with SOA, as our pilot data suggested that SOA did not alter SP profile. To establish the effects of dietary SOA on salivary protein expression, we examined the control (14d) and pre-treatment (14d) phases for the SOA cohorts in addition to the quinine test phase (3d). We monitored meal patterns (24h intake, rate of feeding, meal size, and number of meals), and protein expression.
2.7. Drug pre-treatment:
IPR is tolerated better by smaller animals, therefore for these studies rats were ranging from 125–175g (Balazs, Johnson, Joseph, Ehrreich, & Bloom, 1983). All rats in the drug trials were maintained on the control diet for two weeks prior to the quinine test diet. During the last three days of the control diet, rats were injected with 30mg/kg IPR (Barka, et al., 1986) (Ann, et al., 1987) IP and with 5ml of isotonic saline administered SC to counteract the dehydrating effects of the injection. A subset of the animals tested could not tolerate the injections and were unable to complete the trial, however, across four testing cohorts 15 rats completed the trial. Control rats were run across the same testing cohorts, were given a saline injection IP in addition to the 5ml of saline SC. Following the injection period, all rats were given ad lib access to the 0.375% quinine diet for three days. During two of the testing cohorts both the IPR injected and control animals were allowed ad lib access to the control diet during pre-treatment (IPR n=8, control n=8). However, because injection with IPR caused malaise and decreased food intake of the control diet (see results section), we ran the next cohorts such that control animals were pair-fed to the IPR animals during the pre-treatment phase (IPR n=7, control n=11). This ensured that any changes we saw on the quinine diet were due to the protein content of the saliva, not hyperphagia after reduced intake. Salivary proteins and microstructural variables of food intake were measured during all phases.
2.8. Statistical Analysis
To examine the SOA intake, behavioral data was compared using an ANOVA with diet as a factor (control vs SOA) and repeated measures across time (the 14 days of exposure). As we expected the effects of SOA to be in the first few days, we compared each of the first two nights of SOA intake with Bonferroni-corrected t-tests.
In the quinine trials, we first compared all of the groups in a single ANOVA with pre-treatment (control ad lib, control pair-fed, TA, SOA, and IPR) as a factor and day of quinine exposure as a repeated measure. Due to the high variation in body mass across our groups (IPR vs diet treatments), we converted 24h intake and meal size to intake per gram of body mass. We followed this with sub-ANOVAs to more carefully examine each treatment. In these cases, we did not correct intake and meal size by body weight, as the body weight variation within treatments was small. For the pre-treatment data, ANOVAs compared each pre-treatment condition (TA, SOA, IPR) to its paired control across the final three days of pre-treatment. In all cases, significant differences were followed by Bonferroni corrected t-tests.
Alterations in protein expression were analyzed to ensure they met the requirements for parametric analysis then compared in a series of two-sample Bonferroni corrected t-tests. Although each rat’s saliva was collected every other day, we wanted all animals to be present in the same repeated-measures analysis, therefore the final two days from the pre-treatment phase are collapsed into a single sampling period for the purposes of the statistics. ANOVAs and Bonferroni corrections were conducted using Systat 12. All statistical analyses were considered significant if p < 0.05.
3. Results
3.1. Salivary protein induction
We quantified the protein level of seven protein bands previously reported to be altered with TA or quinine exposure (37 kDa, 35 kDa, 25 kDa, 23 kDa, 19 kDa, 18.5 kDa and 14 kDa) (Martin, et al., 2018; Torregrossa, et al., 2014). All protein data were analyzed to verify that they met the requirements for parametric statistics. Kurtosis and skewness values for protein data did not exceed the range of −2 to +2, with average skewness of 0.55 (±0.13) and kurtosis of 0.16 (±0.20). Pre-treatment with TA or IPR increased expression of proteins previously associated with quinine acceptance (Table 1), relative to controls. IPR injections upregulated bands at 37, 25, and 14 kDa to a greater degree than dietary TA (37 kDa and 25 kDa bands are not altered by TA exposure). TA treatment and IPR injections upregulated bands at 35, 23, 19, and 18.5 kDa to a similar degree (Table 2). SOA pre-treatment did not increase or decrease the density of any band quantified (Table 1), confirming that SOA does not alter the SP content of saliva in a way similar to quinine, TA, and IPR injection.
Table 1.
Summary of Bonferroni corrected two-sample t-tests comparing normalized densitometry units of protein bands at each of the listed masses (kDa).
| kDa | t(df) | p | |
|---|---|---|---|
| TA pre-treatment | 37 | 0.264(14) | 0.796 |
| 35 | 4.262(14) | 0.006* | |
| 25 | 0.476(14) | 0.642 | |
| 23 | 2.224(14) | 0.043* | |
| 19 | 2.421(14) | 0.030* | |
| 18.5 | 2.507(14) | 0.026* | |
| 14 | 2.602(14) | 0.021* | |
| IPR injection ad lib feeding | 37 | 3.647(9) | 0.005* |
| 35 | 3.102(9) | 0.013* | |
| 25 | 4.644(9) | 0.001* | |
| 23 | 4.327(9) | 0.002* | |
| 19 | 2.906(9) | 0.033* | |
| 18.5 | 8.140(9) | < 0.001* | |
| 14 | 2.273(9) | 0.049* | |
| IPR injection pair-fed feeding | 37 | 3.879(13) | 0.002* |
| 35 | 3.035(12) | 0.010* | |
| 25 | 3.625(13) | 0.003* | |
| 23 | 3.809(10) | 0.003* | |
| 19 | 5.737(13) | < 0.001* | |
| 18.5 | 7.373(10) | < 0.001* | |
| 14 | 2.734(13) | 0.017* | |
| SOA pre-treatment | 37 | 0.377(14) | 0.712 |
| 35 | 0.099(14) | 0.923 | |
| 25 | 0.086(14) | 0.933 | |
| 23 | 0.412(14) | 0.687 | |
| 19 | 0.641(14) | 0.532 | |
| 18.5 | 0.077(14) | 0.940 | |
| 14 | 0.357(14) | 0.726 | |
Table 2.
Data are densitometry units presented as difference from average control diet protein expressions (set to 1). Saliva collected after 14 days on the 3% tannic acid diet and 4 days of IPR injection (IPR pair-fed group) were compared.
| kDa | 3% tannic acid | IPR injection | Statistical comparison |
|---|---|---|---|
| 37 | −0.21 ± 0.14 | 1.56 ± 0.34 | F(1,12) = 29.074, p < 0.001* |
| 35 | 0.58 ± 0.30 | 1.43 ± 0.34 | F(1,12) = 3.489, p = 0.086 |
| 25 | −0.24 ± 0.19 | 2.03 ± 0.55 | F(1,12) = 19.154, p = 0.001* |
| 23 | 0.91 ± 0.29 | 1.18 ± 0.23 | F(1,12) = 0.497, p = 0.494 |
| 19 | 0.87 ± 0.63 | 1.26 ± 0.19 | F(1,12) = 0.256, p = 0.622 |
| 18.5 | 1.20 ± 0.68 | 2.12 ± 0.57 | F(1,12) = 0.969, p = 0.344 |
| 14 | 0.39 ± 0.10 | 2.34 ± 0.88 | F(1,12) = 6.533, p = 0.025* |
3.2. SOA
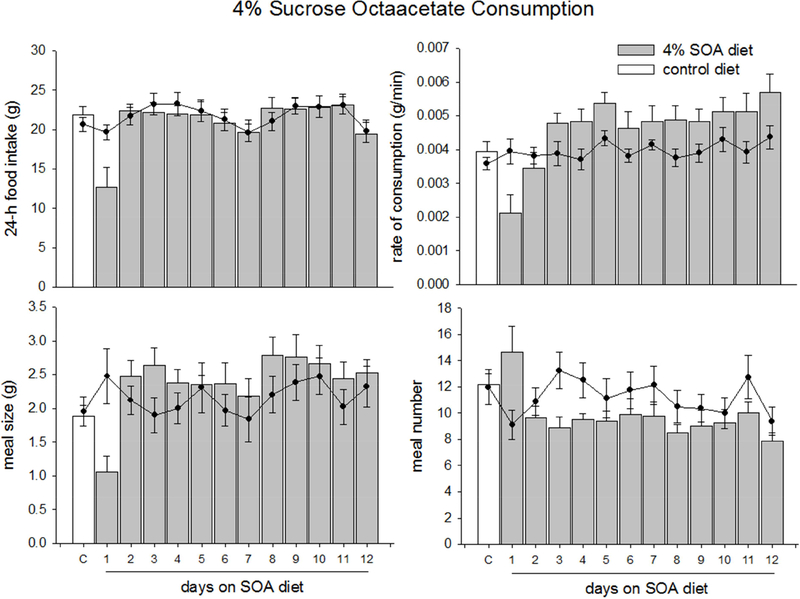
On the first night of SOA exposure, experimental animals ate less than controls (Fig. 1A, t(14) = 2.600, p = 0.042). SOA-exposed animals also ate slower (Fig. 1B, t(14) = 2.747, p = 0.016) and ate smaller meals (Fig. 1C, t(14) = 2.997, p = 0.010) than controls. Possibly to compensate for the small meal sizes, experimental animals initiated more meals than controls (Fig. 1D, t(14) = 2.389, p = 0.032). On the second night of SOA exposure, animals increase all measures to baseline levels (t(14) = 0.507–1.089, p > 0.3) and there was no change in intake for the remainder of the experiment (F(1,14) = 0.129–2.415, p >0.12, Fig. 1).
Figure 1.
Data are average food intake, rate of feeding, meal size, or number of meals ±SEM of rats fed a control and sucrose octaaceetate (SOA) diet. A-D White bars represent feeding behaviors of animals while on a control diet; gray bars represent the feeding behaviors of animals while on the 4% sucrose octaacetate (SOA) diet. The filled circles overlaid on the graph represent the data collected from animals who consumed only the control diet. The data labeled ‘C’ (for control) represents the 3-day average of the behavioral measures before the test diet. Food intake (A) meal size (B) and rate of feeding (D) decreased in the first day of diet exposure, and returned to baseline levels on the second day. Meal number (C), increased during the first night but fell on the second day.
3.3. Quinine acceptance
3.3.1. 24h intake
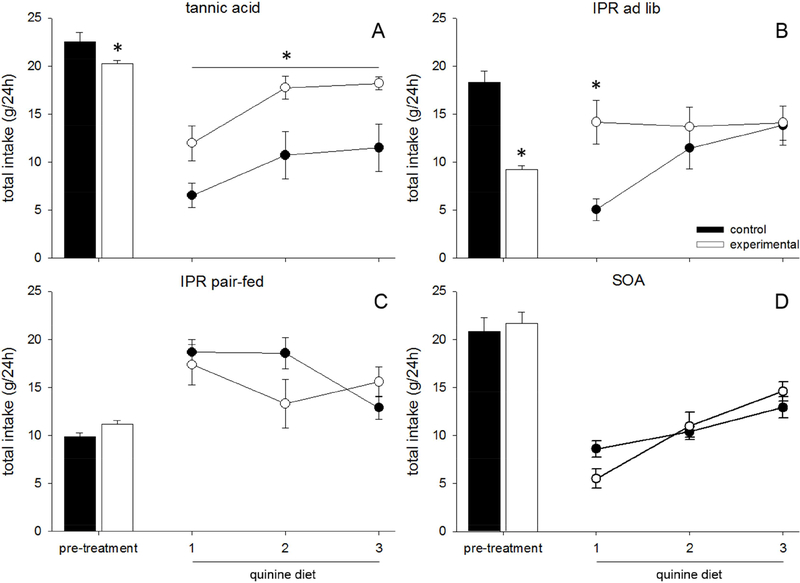
TA and IPR animals ate less than their paired controls during the last 3 days of pre-treatment (TA, main effect of diet: F(1,14) = 6.273, p = 0.025, Fig 2A, IPR ad lib, main effect of drug: F(1,14) = 51.428, p < 0.001, Fig 2B, IPR pair-fed, main effect of drug: F(1,16) = 6.779, p = 0.019, Fig 2C). SOA animals did not eat differently from their paired controls during the final 3 days (no effect, F(1,14) = 0.947, p = 0.347, Fig 2D). All pre-treatment animals maintained comparable body weights to their paired controls during the pre-treatment phase (TA: F(1,14) = 0.949, p = 0.346, SOA: F(1,14) = 0.221, p = 0.645, IPR pair-fed: F(1,16) = 3.218, p = 0.092, IPR ad lib: F(1,14) = 0.008, p = 0.931).
Figure 2.
Data are averaged food intake over 24h (g) ±SEM in rats fed tannic acid (TA), sucrose octaacetate (SOA), or control diet. (A-D) Closed bars represent the 3-day average of intake of control animals during the pre-treatment phase, during which they consumed control diet, while open bars represent experimental animals who were exposed to either TA (A), IPR injections (B, C), or SOA (D). Closed circles represent quinine intake of control animals, while open circles represent quinine intake of pretreated animals. TA (A) and IPR pre-treated animals (B) ate more quinine than the ad lib controls.
There was an effect of pre-treatment on intake/gram of quinine (main effect of pre-treatment: F(4,61) = 20.017, p < 0.001). There was an additional effect of time (F(2,122) = 3.927, p = 0.022), indicating that quinine intake generally rose from day 1 to day 3, and an interaction (F(8,122) = 13.789, p < 0.001).
Further ANOVAs compared each pre-treatment to its paired control, to clarify each effect. TA and IPR pre-treated animals ate more quinine diet than ad lib fed control rats (TA, main effect of diet: F(1,14) = 9.306, p = 0.009, Fig 2A, IPR ad lib, main effect of drug: F(1,14) = 17.14, p = 0.021, Fig 2B). There was an interaction between pre-treatment (IPR) and day of quinine diet (F(2,28) = 15.579, p < 0.001), such that IPR pretreated animals ate more on the first day of quinine exposure (p = 0.003). There was no effect of IPR pre-treatment on quinine intake when the saline-injected rats were pair-fed (F(1,16) = 0.400, p = 0.536, Fig 2C), and no effect of SOA on quinine diet acceptance (F(1,14) = 0.080, p = 0.782, Fig 2D).
3.3.2. Rate of feeding
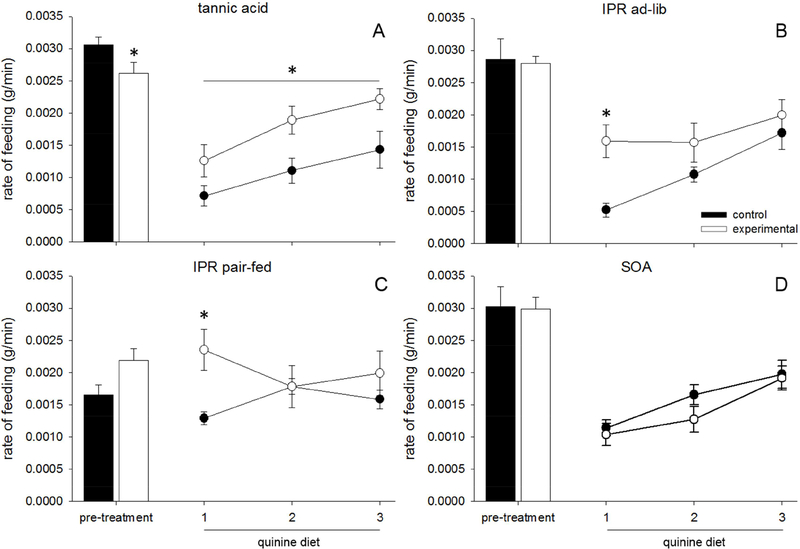
Animals consumed TA more slowly than animals consuming control diets during the pre-treatment phase (TA, main effect of diet: F(1,14) = 13.511, p = 0.002, Fig 3A). There were no effects of the IPR injection or SOA on rate of feeding (IPR ad lib: F(1,14) = 0.038, p = 0.849, Fig 3B, IPR pair-fed: F(1,16) = 2.013, p = 0.175, Fig 3C, SOA: F(1,14) = 2.683, p = 0.124, Fig 3D).
Figure 3.
Data are average rates of feeding (g/min) ±SEM in rats fed tannic acid (TA), sucrose octaacetate (SOA), or control diet. (A-D) Closed bars represent the 3-day average of rate of feeding for control animals during the pre-treatment phase, during which they consumed control diet, while open bars represent experimental animals who were exposed to either TA (A), IPR injections (B, C), or SOA (D). Closed circles represent quinine intake of control animals, while open circles represent quinine intake of pretreated animals. TA (A) and IPR pre-treated (C, D) animals ate quinine faster than their controls.
There was an effect of pre-treatment (IPR, SOA, TA, control etc.) on average rate of feeding on quinine (main effect of pretreatment: F(4,62) = 5.053, p = 0.003). There was also an effect of time (F(2,122) = 21.430, p < 0.001) and an interaction between pre-treatment and time (F(8,122) = 3.274, p = 0.005), indicating that rate of feeding increased between day one and three of the quinine diet, and this increase was affected by pre-treatment.
Further ANOVAs suggested that TA and IPR increased the rate of feeding on the quinine diet over the control animals (TA, main effect of diet: F(1,14) = 7.378, p = 0.017, Fig 3A, IPR ad lib, main effect of drug: F(1,14) = 4.600, p = 0.050, Fig 3B, IPR pair fed, main effect of drug: F(1,16) = 4.555, p = 0.049, Fig 3C). There was a significant interaction between time and drug in both IPR groups (IPR ad lib: F(2,28) = 5.607, p = 0.009, Fig 3B, IPR pair-fed: F(2,32) = 0.010, p = 0.010, Fig 3C) such that IPR pretreated rats eat faster only on the first day (p = 0.002). Rats pretreated with SOA did not differ from control rats (no effect, F(1,14) = 0.574, p = 0.461, Fig 3D) on the quinine diet.
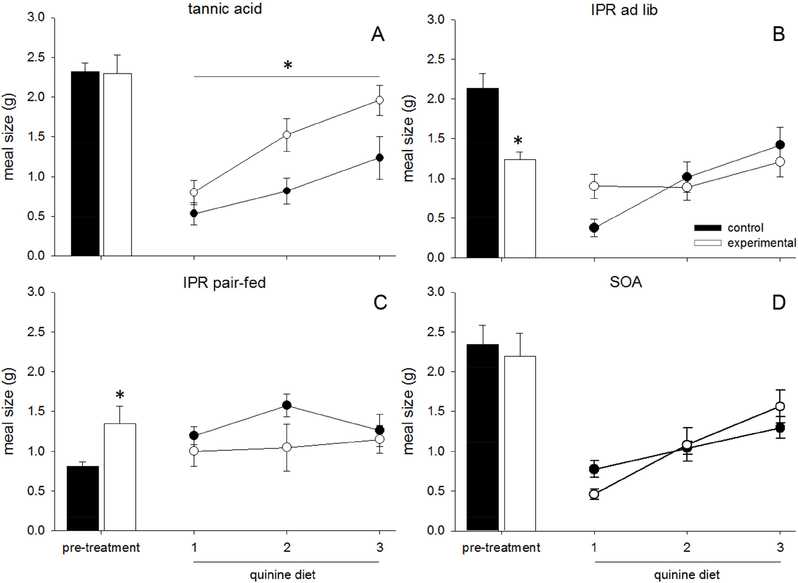
3.3.3. Meal size
Meal sizes during the pre-treatment period were not different between the dietary pre-treatments (TA, SOA) and their control animals (TA, no effect: F(1,14) = 0.072, p = 0.792, Fig 4A, SOA, no effect: F(1,14) = 0.031, p = 0.862, Fig 4D). IPR-injected rats ate smaller meals than the ad lib fed saline-treated rats (main effect of drug: F(1,14) = 18.461, p = 0.001, Fig 4B). However the opposite was true when saline rats were pair-fed, as pair-fed saline animals ate smaller meals than IPR-injected rats (main effect of drug: F(1,16) = 4.948, p = 0.041, Fig 4C).
Figure 4.
Data are average meal sizes (g) ±SEM in rats fed tannic acid (TA), sucrose octaacetate (SOA), or control diet. (A-D) Closed bars represent the 3-day average of meal size for control animals during the pre-treatment phase, during which they consumed control diet, while open bars represent experimental animals who were exposed to either TA (A), IPR injections (B, C), or SOA (D). Closed circles represent quinine intake of control animals, while open circles represent quinine intake of pretreated animals. Only TA (A) increased meal size on a quinine diet.
There was a significant effect of pre-treatment on quinine meal size/g (main effect of pre-treatment: F(4,61) = 12.971, p < 0.001). There was also an effect of time on meal size/g (F(2,122) = 18.750, p < 0.001), indicating that the meal size/g generally increased from day one of quinine to day three, and a significant interaction (F(8,122) = 4.656, p < 0.001), indicating that the increase in meal size was affected by pre-treatment.
Further ANOVAs compared each pre-treatment to its paired control, to clarify the effects seen in the larger ANOVA. Animals pre-treated with TA ate larger meals when given the quinine diet (main effect of diet: F(1,14) = 7.353, p = 0.017, Fig 4A), but no other treatment resulted in an increased meal size upon initial exposure to quinine (IPR ad lib, no effect: F(1,14) = 0.092, p = 0.766, Fig 4B, IPR pair-fed, no effect: F(1,16) = 1.613, p = 0.222, Fig 4C, SOA, no effect: F(1,14) = 0.095, p = 0.762, Fig 4D).
3.3.4. Meal number
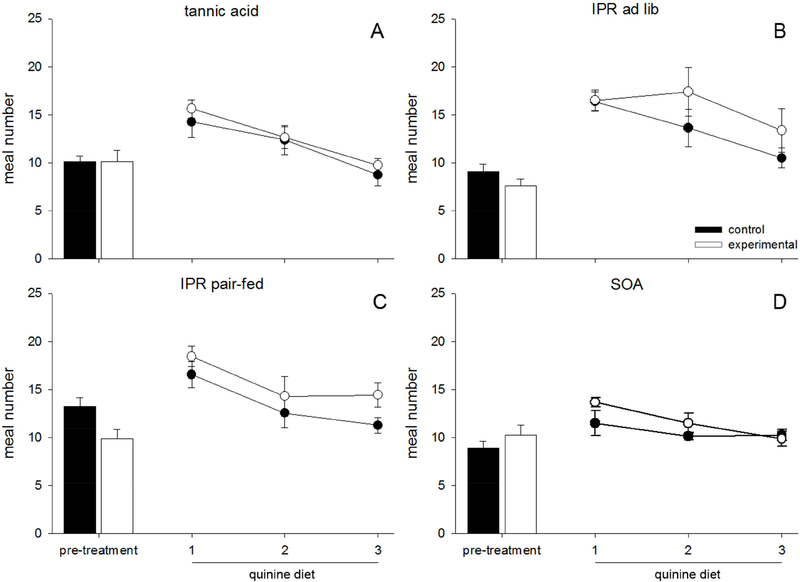
There was a trending effect of pre-treatment on the number of meals (F(4,61) = 2.287, p = 0.070, Fig 5). There was an effect of time on meals initiated on the quinine diet (F(2,122) = 36.710, p < 0.001), indicating that the number of meals initiated decreased from day one of quinine to day three, and no interaction. As there was no effect of pre-treatment on meal number, we did not follow this finding with any further statistical tests.
Figure 5.
Data are average number of meals ±SEM consumed in 24h in rats fed tannic acid (TA), sucrose octaacetate (SOA), or control diet. (A-D) Closed bars represent the 3-day average of meal number for control animals during the pre-treatment phase, during which they consumed control diet, while open bars represent experimental animals who were exposed to either TA (A), IPR injections (B, C), or SOA (D). Closed circles represent quinine intake of control animals, while open circles represent quinine intake of pretreated animals. (A-D) No group of animals differed in meal number at any point during either the pre-treatment or the quinine phases.
4. Discussion
There is growing evidence that saliva, a complex solution that under normal conditions contacts taste stimuli, has both the opportunity and ability to alter diet acceptance. Work in humans has implicated SP profiles in the perception of bitter taste and preference for bitter foods (Dsamou, et al., 2012; Morzel, et al., 2014; Quintana, et al., 2009). In rats, previous research has implicated specific salivary proteins in increased TA acceptance (Torregrossa, et al., 2014), and identified a link between protein upregulation and increased quinine diet acceptance (Martin, et al., 2018). Here we used dietary pre-exposure and pharmacological stimulation to confirm that salivary protein composition, separate from past bitter diet experience, can drive the increased acceptance of quinine - introducing saliva as a part of a possible mechanism for learned acceptance of bitter diets.
To address this question, we recorded the behavior of rats under one of five conditions. Rats were either allowed ad lib access to a control diet, had restricted access to a control diet, or had ad lib access to one of three experimental conditions. Rats were treated with 1) TA, a bitter diet that upregulated SPs similar to those upregulated by quinine (Martin, et al., 2018), 2) IPR, injections of a drug that has been demonstrated to increase SP expression, while the animals were maintained on a control diet (Barka, et al., 1986), 3) or SOA, a bitter diet that did not significantly alter SP expression. Once the animals were pre-treated, they were given a 0.375% quinine diet for three days, while several measures of diet acceptance (24h intake, average rate of feeding, meal size, and number of meals) were collected.
Since increased quinine acceptance could have more than one cause, we considered the three most likely explanations: defense of body mass, experience-dependent bitter habituation, and a shift in salivary proteins, as altering their experience with the diet (Table 3). We hypothesized that if defense of body mass drove quinine diet acceptance, the pre-treatments that reduced intake (TA diet, IPR injections, and restricted control feeding) would cause increases in quinine acceptance, while the pre-treatments that did not reduce intake (ad lib control consumption, SOA diet) would not have altered quinine consumption. If bitter experience was driving quinine diet acceptance, the pre-treatments that offered experience with a bitter diet (SOA and TA diets) would increase quinine acceptance. Finally, if SP profile changes increased quinine acceptance, then pre-treatments that altered SP profile (TA diet and IPR injections) would increase measures of quinine acceptance, while treatments that did not alter SP profile (SOA, control ad lib and restricted control feeding) would not.
Table 3.
Summary of hypothesized influences on increased quinine acceptance, the pattern of results that would support these hypotheses, and measures of acceptance that match these patterns (bold type).
| pre-treatment | Hypothesized patterns of quinine acceptance |
||
|---|---|---|---|
| defense of body mass | salivary proteins | bitter habituation | |
| control (ad lib) | = | = | = |
| control (restricted) | ↑ | = | = |
| IPR | ↑ | ↑ | = |
| SOA | = | = | ↑ |
| TA | ↑ | ↑ | ↑ |
| Measures of acceptance that match the hypothesized pattern | total intake | rate of feeding | none |
Although the animals did not lose mass in any treatment, several days of reduced intake could lead an animal to become hyperphagic to defend their body mass. Defense of body mass, rather than SP profile, appeared to dictate changes in total intake of quinine diet. Although TA pre-treatment and IPR injection increased quinine intake, when an animal’s control diet consumption was restricted during the pre-treatment by pair-feeding, the animals ate more quinine than the other control conditions. We have previously reported that decreased intake alone was not sufficient to increase SPs (Torregrossa, et al., 2014). We have replicated that finding here therefore it is unlikely that SPs are the mediator of the increase in quinine intake. Furthermore, animals who consumed the same amount as ad lib controls (SOA) exhibited the typical decreases in quinine intake. Together these data suggest that hyperphagia after restricted intake may be driving the overall increase in quinine consumption. Although total intake is a common measure of acceptance, it is informed by both the animal’s motivational state and its physiology, so the role of SPs and the role of body mass defense cannot be pulled apart here, and SPs are likely not strong enough to outweigh the motivation resulting from food restriction.
In addition to total intake, we calculated the average rate of feeding. It has been demonstrated that as animals feed on more hedonically positive foods the rate of feeding increases, and likewise, it decreases as the food becomes less desirable (Davis, 1973; Smith, 2000). While averaged rate data do not capture the real-time changes in orosensory and postingestive feedback seen within a meal, they have been used as a proxy for general diet acceptability (Davis, 1989; Davis, 1999; Davis, Kung, & Rosenak, 1995; Davis & Perez, 1993; Dotson, et al., 2012; Gannon, Smith, Henderson, & Hendrick, 1992; Markison, Thompson, Smith, & Spector, 2000; Smith, 1989; Smith, 2000). We found that animals with upregulated salivary proteins (TA diet, IPR injection) ate quinine diet at a higher average rate than the animals without SPs, which suggests that SP expression influenced this measure of diet acceptance. The change in this measure of acceptance was likely not driven by previous bitter experience, as animals with only experience and no SP alteration (SOA) did not eat the quinine diet more quickly. Additionally, the IPR-injected rats were bitter-naïve at first presentation of quinine.
These data do not allow us to separate the contributions of orosensory and postingestive feedback here. Our previous work has shown that, in a brief-access paradigm, rats were less avoidant of intermediate concentrations of quinine if they had SPs previously upregulated by TA exposure (Torregrossa, et al., 2014). Together these findings suggest that SPs may act to reduce the salience of bitter stimuli, and this reduction in negative perception may facilitate acceptance of the diet, although more work is necessary to determine this conclusively.
It is interesting that while TA pre-treated rats had higher rates of feeding than controls on day one of the quinine diet, they were not totally “prepared” for quinine, as rate of feeding increased from day one to day three. This may be due to differences in SP profile. We have previously noted that quinine and TA upregulated SP profiles that are similar, but did not completely overlap (Martin, et al., 2018; Torregrossa, et al., 2014). Perhaps there are parts of the SP profile that are important in designating diets as safe. It is possible that the profile generated by TA is ideal for increasing acceptance of TA via binding to the stimulus or necessary receptors, while that same profile may be less suited for quinine. Additional work is necessary to identify the proteins responsible for increased acceptance and the mechanism by which these proteins alter acceptance.
Intake within a 24h period (total intake) can be broken down into feeding events, or meals. The size of these meals is hypothesized to be driven by both positive orosensory feedback, which maintains feeding during the meal, and negative post-ingestive feedback, which terminates a meal (Davis & Smith, 1990). Changes in meal size have been correlated with SP expression in our previous work (Martin, et al., 2018; Torregrossa, et al., 2014) and we hypothesized that SPs may bind to the taste stimulus and keep it from interacting with oral and gut bitter receptors. In the current study, TA pretreated rats showed an increased meal size when consuming quinine; however, neither SP expression (IPR pre-treatment) nor bitter exposure (SOA pre-treatment) alone altered meal size. TA pre-treatment may have altered quinine meal size through a combination of upregulated SPs and a conferred tolerance to the toxic post-ingestive effects of quinine (Kratz, Levitsky, & Lustick, 1978). These findings suggest that alteration of meal size may be due to the combined effects of learning (to accept the bitter, but ultimately safe, diet) and increased salivary protein expression.
4.1. Limitations and future directions
There are limitations to using diet to upregulate SPs. The first of these is that the bitters we chose likely do not offer identical experiences to quinine. Although SOA has been demonstrated to bind to bitter receptors (Brockhoff, et al., 2007), the literature is in agreement with our findings that that a rat’s acceptance of SOA and quinine are not identical. At levels that were equally rejected in a short term tests (20 min preference test), rats avoided a quinine-adulterated diet far longer than an SOA adulterated diet (Kratz, et al., 1978) in 24h tests. Consistent with our findings, it has been previously shown that when animals were first exposed to an SOA diet, then a quinine diet, they maintained their avoidance of quinine. However, when animals were first exposed to a quinine diet, then switched to SOA diet, they exhibited no avoidance (Aravich & Sclafani, 1980; Sclafani, Aravich, & Schwartz, 1979). In the current study, we fed animals an SOA-containing diet while monitoring their feeding behavior and confirmed that rats initially rejected the diet, but they accepted the diet more quickly than what we have reported for quinine and TA diets (Martin, et al., 2018; Torregrossa, et al., 2014). Furthermore, unlike rats exposed to dietary quinine and TA, these animals did not show a concurrent change in SP profiles. The mechanism of SP upregulation is yet unknown, therefore we can only speculate why SOA was unsuccessful in driving upregulation. Perhaps the activation of particular T2Rs is necessary, or there is a metabolite of these compounds that alters protein expression (Glendinning, 1992; Martin, et al., 2018). Just as SOA and quinine diets appeared not to offer the same bitter experience, TA diet was also likely quite different from the quinine diet, as tannin is astringent in addition to bitter (Simons, Boucher, Carstens, & Carstens, 2003), while quinine (and SOA) are not typically described as astringent. Lastly, the experience of intensity may also vary between the diets, the concentrations of bitter in these diets were chosen on the basis of similar first day intake (i.e., rats reduced intake of 3% TA, 0.375% quinine, and 4% SOA diets to the same degree on first presentation), but it must be acknowledged this is not a perfect control for the perceptual experience of the diet. These differences in diet mean that we cannot entirely rule out a role for bitter experience in bitter acceptance. However, these data do suggest that SPs likely contributed to some measures of diet acceptance.
We have previously shown that the presence of SPs altered measures of taste and taste nerve signaling (Martin, et al., 2018; Torregrossa, et al., 2014) suggesting that taste could play a role in the increased acceptance. However, this does not rule out a role for postingestive feedback. Our measures of acceptance represent both orosensory and postingestive feedback, so it is possible that altering the negative postingestive consequences of TA and quinine are contributing to or mediating the changes we have seen. More work is needed to disentangle the relative contributions of taste and gut feedback to changes in acceptance.
5. Conclusions
Preference for bitter foods is largely considered a consequence of learning (Forestell & Mennella, 2007). Animals’ innate avoidance to bitter-tasting foods is often framed as an evolutionary adaptation; in nature, bitter regularly signals toxicity. Likewise, it is advantageous for animals to increase acceptance of certain bitter foods, once they are determined to be safe, and if they are valuable food sources (Provenza, 1995). Repeated exposure increases the acceptability of initially rejected foods, an effect that is almost always explained using some form of learning theory (e.g., learned safety, mere exposure, flavor-consequence learning) (Forestell & Mennella, 2007; Mennella & Beauchamp, 1993; Yeomans, 2006). Here we attempted to disentangle previous experience with a bitter food from the role of SPs in altering bitter acceptance. We have shown that, by some measures, acceptance of bitter foods is, at least in part, subject to an animal’s SP profile, indicating that as an animal learns about the nature of a bitter food, it also undergoes a physiological change to make that food more acceptable. These changes enable the animal incorporate the food into its diet. Future work should examine how SPs alter perception, i.e., whether they make food taste better/worse, or they decrease/increase sensitivity to the taste. This represents an ideal therapeutic target, as SPs can alter taste independently from diet experience. Once individual proteins are identified as responsible for the change in taste, they could be used to increase consumption of healthy foods. Our data support that SP profiles should be considered a significant part of experience-driven changes in food perception, and by extension, food preference.
Acknowledgements
We thank James C. Smith for donating the behavioral equipment to the Torregrossa lab, Holly Annunziato, Liubava Yermakova, and Gregory Loney for technical assistance and Kathleen Harper for assistance with the drug preparation. We also thank the anonymous reviewers for their thoughtful contributions to this work.
Funding
This work was supported by NIDCD grant R03DC012632 and the University at Buffalo. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJP, & Zuker CS (2000). A Novel Family of Mammalian Taste Receptors. Cell, 100, 693–702. [DOI] [PubMed] [Google Scholar]
- Ann DK, Clements S, Johnstone EM, & Carlson DM (1987). Induction of tissue-specific proline-rich protein multigene families in rat and mouse parotid glands by isoproterenol. Unusual strain differences of proline-rich protein mRNAs. Journal of Biological Chemistry, 262, 899–904. [PubMed] [Google Scholar]
- Aravich PF, & Sclafani A (1980). Dietary preference behavior in rats fed bitter tasting quinine and sucrose octa acetate adulterated diets. Physiology & Behavior, 25, 157–160. [DOI] [PubMed] [Google Scholar]
- Balazs T, Johnson G, Joseph X, Ehrreich S, & Bloom S (1983). Sensitivity and resistance of the myocardium to the toxicity of isoproterenol in rats In Myocardial Injury (pp. 563–577): Springer. [DOI] [PubMed] [Google Scholar]
- Barka T, Yagil C, Van der Noen H, & Naito Y (1986). Induction of the synthesis of a specific protein in rat submandibular gland by isoproterenol. Lab Invest, 54, 165–171. [PubMed] [Google Scholar]
- Barratt-Fornell A, & Drewnowski A (2002). The Taste of Health: Nature’s Bitter Gifts. Nutrition Today, 37, 144–150. [DOI] [PubMed] [Google Scholar]
- Bedi GS (1991). The effect of adrenergic agonists and antagonists on cysteine-proteinase inhibitor (cystatin) in rat saliva. Archives of Oral Biology, 36, 611–618. [DOI] [PubMed] [Google Scholar]
- Beeley JA, Sweeney D, Lindsay JCB, Buchanan ML, Sarna L, & Khoo KS (1991). Sodium dodecyl sulphate-polyacrylamide gel electrophoresis of human parotid salivary proteins. ELECTROPHORESIS, 12, 1032–1041. [DOI] [PubMed] [Google Scholar]
- Brockhoff A, Behrens M, Massarotti A, Appendino G, & Meyerhof W (2007). Broad tuning of the human bitter taste receptor hTAS2R46 to various sesquiterpene lactones, clerodane and labdane diterpenoids, strychnine, and denatonium. Journal of Agricultural and Food Chemistry, 55, 6236–6243. [DOI] [PubMed] [Google Scholar]
- Buckland G, Bach A, & Serra-Majem L (2008). Obesity and the Mediterranean diet: a systematic review of observational and intervention studies. Obesity reviews, 9, 582–593. [DOI] [PubMed] [Google Scholar]
- Bufe B, Breslin PA, Kuhn C, Reed DR, Tharp CD, Slack JP, Kim U-K, Drayna D, & Meyerhof W (2005). The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Current Biology, 15, 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufe B, Hofmann T, Krautwurst D, Raguse J-D, & Meyerhof W (2002). The human TAS2R16 receptor mediates bitter taste in response to [beta]-glucopyranosides. Nat Genet, 32, 397–401. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Mueller K, Hoon M, Adler E, Feng L, Guo W, Zuker C, & Ryba N (2000). T2Rs function as bitter taste receptors. Cell, 100, 703–711. [DOI] [PubMed] [Google Scholar]
- Chung K-T, Wong TY, Wei C-I, Huang Y-W, & Lin Y (1998). Tannins and Human Health: A Review. Critical Reviews in Food Science and Nutrition, 38, 421–464. [DOI] [PubMed] [Google Scholar]
- Clarke SN, & Ossenkopp K-P (1998). Taste reactivity responses in rats: influence of sex and the estrous cycle. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 274, R718–R724. [DOI] [PubMed] [Google Scholar]
- Dahl M, Erickson R, & Simon S (1997). Neural responses to bitter compounds in rats. Brain research, 756, 22–34. [DOI] [PubMed] [Google Scholar]
- Davis JD (1973). The effectiveness of some sugars in stimulating licking behavior in the rat. Physiology & Behavior, 11, 39–45. [DOI] [PubMed] [Google Scholar]
- Davis JD (1989). The microstructure of ingestive behavior. Annals of the New York Academy of Sciences, 575, 106–121. [DOI] [PubMed] [Google Scholar]
- Davis JD (1999). Some new developments in the understanding of oropharyngeal and postingestional controls of meal size. Nutrition, 15, 32–39. [DOI] [PubMed] [Google Scholar]
- Davis JD, Kung TM, & Rosenak R (1995). Interaction between orosensory and postingestional stimulation in the control of corn oil intake by rats. Physiology & Behavior, 57, 1081–1087. [DOI] [PubMed] [Google Scholar]
- Davis JD, & Perez MC (1993). Food deprivation-and palatability-induced microstructural changes in ingestive behavior. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 264, R97–R103. [DOI] [PubMed] [Google Scholar]
- Davis JD, & Smith GP (1990). Learning to sham feed: behavioral adjustments to loss of physiological postingestional stimuli. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 259, R1228–R1235. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, & Monroe S (1990). Taste responses in the parabrachial pons of ovariectomized rats. Brain research bulletin, 25, 741–748. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, & Victor JD (2003). Taste Response Variability and Temporal Coding in the Nucleus of the Solitary Tract of the Rat. Journal of Neurophysiology, 90, 1418. [DOI] [PubMed] [Google Scholar]
- Dotson CD, Colbert CL, Garcea M, Smith JC, & Spector AC (2012). The consequences of gustatory deafferentation on body mass and feeding patterns in the rat. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 303, R611–R623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewnowski A, & Gomez-Carneros C (2000). Bitter taste, phytonutrients, and the consumer: a review. The American journal of clinical nutrition, 72, 1424–1435. [DOI] [PubMed] [Google Scholar]
- Dsamou M, Palicki O, Septier C, Chabanet C, Lucchi G, Ducoroy P, Chagnon M-C, & Morzel M (2012). Salivary Protein Profiles and Sensitivity to the Bitter Taste of Caffeine. Chemical Senses, 37, 87–95. [DOI] [PubMed] [Google Scholar]
- Forestell CA, & Mennella JA (2007). Early determinants of fruit and vegetable acceptance. Pediatrics, 120, 1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, & Klein S (2003). A randomized trial of a low-carbohydrate diet for obesity. New England Journal of Medicine, 348, 2082–2090. [DOI] [PubMed] [Google Scholar]
- Gannon KS, Smith JC, Henderson R, & Hendrick P (1992). A system for studying the microstructure of ingestive behavior in mice. Physiology & Behavior, 51, 515–521. [DOI] [PubMed] [Google Scholar]
- Giza BK, McCaughey SA, Zhang L, & Scott TR (1996). Taste Responses in the Nucleus of the Solitary Tract in Saccharin-preferring and Saccharin-averse Rats. Chemical Senses, 21, 147–157. [DOI] [PubMed] [Google Scholar]
- Glendinning JI (1992). Effect of salivary proline-rich proteins on ingestive responses to tannic acid in mice. Chemical Senses, 17, 1–12. [Google Scholar]
- Hayes JE, Feeney EL, & Allen AL (2013). Do polymorphisms in chemosensory genes matter for human ingestive behavior? Food Quality and Preference, 30, 202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JE, Wallace MR, Knopik VS, Herbstman DM, Bartoshuk LM, & Duffy VB (2011). Allelic Variation in TAS2R Bitter Receptor Genes Associates with Variation in Sensations from and Ingestive Behaviors toward Common Bitter Beverages in Adults. Chemical Senses, 36, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Li X, McCaughey S, Beauchamp G, & Bachmanov A (2001). Soa genotype selectively affects mouse gustatory neural responses to sucrose acetate. Physiol Genomics, 5, 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DA (1983). Differences in basic proline-rich proteins in rat parotid saliva following chronic isoproterenol treatment or maintenance on a liquid diet. Archives of Oral Biology, 28, 549–554. [DOI] [PubMed] [Google Scholar]
- Kratz CM, Levitsky DA, & Lustick S (1978). Differential effects of quinine and sucrose octa acetate on food intake in the rat. Physiology & Behavior, 20, 665–667. [DOI] [PubMed] [Google Scholar]
- Kuhn C, Bufe B, Winnig M, Hofmann T, Frank O, Behrens M, Lewtschenko T, Slack JP, Ward CD, & Meyerhof W (2004). Bitter taste receptors for saccharin and acesulfame K. Journal of Neuroscience, 24, 10260–10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen TM, Dalskov S, Van Baak M, Jebb S, Kafatos A, Pfeiffer A, Martinez J, Handjieva-Darlenska T, Kunešová M, & Holst C (2010). The Diet, Obesity and Genes (Diogenes) Dietary Study in eight European countries–a comprehensive design for long-term intervention. Obesity reviews, 11, 76–91. [DOI] [PubMed] [Google Scholar]
- Markison S, Thompson BL, Smith JC, & Spector AC (2000). Time Course and Pattern of Compensatory Ingestive Behavioral Adjustments to Lysine Deficiency in Rats. The Journal of nutrition, 130, 1320–1328. [DOI] [PubMed] [Google Scholar]
- Martin LE, Nikonova LV, Kay K, Paedae AB, Contreras RJ, & Torregrossa A-M (2018). Salivary proteins alter taste-guided behaviors and taste nerve signaling in rat. Physiology & Behavior, 184, 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, & Sollars SI (2017). Contributory role of sex differences in the variations of gustatory function. Journal of neuroscience research, 95, 594–603. [DOI] [PubMed] [Google Scholar]
- Mehansho H, Butler LG, & Carlson DM (1987). Dietary Tannins and Salivary Proline-Rich Proteins: Interactions, Induction, and Defense Mechanisms. Annual Review of Nutrition, 7, 423–440. [DOI] [PubMed] [Google Scholar]
- Mennella JA, & Beauchamp GK (1993). The effects of repeated exposure to garlic-flavored milk on the nursling’s behavior. Pediatric Research, 34, 805. [DOI] [PubMed] [Google Scholar]
- Mennella JA, Nicklaus S, Jagolino AL, & Yourshaw LM (2008). Variety is the spice of life: strategies for promoting fruit and vegetable acceptance during infancy. Physiology & Behavior, 94, 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, & Behrens M (2010). The molecular receptive ranges of human TAS2R bitter taste receptors. Chemical Senses, 35, 157–170. [DOI] [PubMed] [Google Scholar]
- Meyerhof W, Behrens M, Brockhoff A, Bufe B, & Kuhn C (2005). Human bitter taste perception. Chem Senses, 30 Suppl 1, i14–15. [DOI] [PubMed] [Google Scholar]
- Morzel, Chabanet C, Schwartz C, Lucchi G, Ducoroy P, & Nicklaus S (2014). Salivary protein profiles are linked to bitter taste acceptance in infants. European journal of pediatrics, 173, 575–582. [DOI] [PubMed] [Google Scholar]
- Morzel M, Truntzer C, Neyraud E, Brignot H, Ducoroy P, Lucchi G, Canlet C, Gaillard S, Nicod F, Nicklaus S, Peretti N, & Feron G (2017). Associations between food consumption patterns and saliva composition: Specificities of eating difficulties children. Physiology & Behavior, 173, 116–123. [DOI] [PubMed] [Google Scholar]
- Neyraud E, Sayd T, Morzel M, & Dransfield E (2006). Proteomic Analysis of Human Whole and Parotid Salivas Following Stimulation by Different Tastes. Journal of Proteome Research, 5, 2474–2480. [DOI] [PubMed] [Google Scholar]
- Nolden AA, McGeary JE, & Hayes JE (2016). Differential bitterness in capsaicin, piperine, and ethanol associates with polymorphisms in multiple bitter taste receptor genes. Physiology & Behavior, 156, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronin AN, Tang H, Connor J, & Keung W (2004). Identification of Ligands for Two Human Bitter T2R Receptors. Chemical Senses, 29, 583–593. [DOI] [PubMed] [Google Scholar]
- Provenza FD (1995). Postingestive feedback as an elementary determinant of food preference and intake in ruminants. Rangeland Ecology & Management/Journal of Range Management Archives, 48, 2–17. [Google Scholar]
- Prutkin J, Duffy VB, Etter L, Fast K, Gardner E, Lucchina LA, Snyder DJ, Tie K, Weiffenbach J, & Bartoshuk LM Genetic variation and inferences about perceived taste intensity in mice and men. Physiology & Behavior, 69, 161–173. [DOI] [PubMed] [Google Scholar]
- Quintana M, Palicki O, Lucchi G, Ducoroy P, Chambon C, Salles C, & Morzel M (2009). Short-Term Modification of Human Salivary Proteome Induced by Two Bitter Tastants, Urea and Quinine. Chemosensory Perception, 2, 133–142. [Google Scholar]
- Roura E, Aldayyani A, Thavaraj P, Prakash S, Greenway D, Thomas WG, Meyerhof W, Roudnitzky N, & Foster SR (2015). Variability in Human Bitter Taste Sensitivity to Chemically Diverse Compounds Can Be Accounted for by Differential TAS2R Activation. Chemical Senses, 40, 427–435. [DOI] [PubMed] [Google Scholar]
- Roussin AT, Victor JD, Chen J-Y, & Di Lorenzo PM (2008). Variability in Responses and Temporal Coding of Tastants of Similar Quality in the Nucleus of the Solitary Tract of the Rat. Journal of Neurophysiology, 99, 644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarni-Manchado P, Cheynier V, & Moutounet M (1998). Interactions of Grape Seed Tannins with Salivary Proteins. Journal of Agricultural and Food Chemistry, 47, 42–47. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Aravich PF, & Schwartz J (1979). Hypothalamic hyperphagic rats overeat bitter sucrose octa acetate diets but not quinine diets. Physiology & Behavior, 22, 759–766. [DOI] [PubMed] [Google Scholar]
- Shi P, Zhang J, Yang H, & Zhang Y. p. (2003). Adaptive Diversification of Bitter Taste Receptor Genes in Mammalian Evolution. Molecular biology and evolution, 20, 805–814. [DOI] [PubMed] [Google Scholar]
- Simons CT, Boucher Y, Carstens MI, & Carstens E (2003). Lack of Quinine-evoked Activity in Rat Trigeminal Subnucleus Caudalis. Chemical Senses, 28, 253–259. [DOI] [PubMed] [Google Scholar]
- Skopec MM, Hagerman AE, & Karasov WH (2004). Do Salivary Proline-Rich Proteins Counteract Dietary Hydrolyzable Tannin in Laboratory Rats? Journal of Chemical Ecology, 30, 1679–1692. [DOI] [PubMed] [Google Scholar]
- Smith JC (1989). Apparatus for the Long-Term Study of Ingestive Behavior and Taste in the Rat and Mouse a. Annals of the New York Academy of Sciences, 561, 329–329. [Google Scholar]
- Smith JC (2000). Microstructure of the rat’s intake of food, sucrose and saccharin in 24-hour tests. Neuroscience & Biobehavioral Reviews, 24, 199–212. [DOI] [PubMed] [Google Scholar]
- Soares S, Kohl S, Thalmann S, Mateus N, Meyerhof W, & De Freitas V (2013). Different Phenolic Compounds Activate Distinct Human Bitter Taste Receptors. Journal of Agricultural and Food Chemistry, 61, 1525–1533. [DOI] [PubMed] [Google Scholar]
- Spector AC, & Kopka SL (2002). Rats Fail to Discriminate Quinine from Denatonium: Implications for the Neural Coding of Bitter-Tasting Compounds. The Journal of Neuroscience, 22, 1937–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John SJ, & Boughter JD (2004). The Contribution of Taste Bud Populations to Bitter Avoidance in Mouse Strains Differentially Sensitive to Sucrose Octa-acetate and Quinine. Chemical Senses, 29, 775–795. [DOI] [PubMed] [Google Scholar]
- Torregrossa A-M, Nikonova L, Bales MB, Villalobos Leal M, Smith JC, Contreras RJ, & Eckel LA (2014). Induction of Salivary Proteins Modifies Measures of Both Orosensory and Postingestive Feedback during Exposure to a Tannic Acid Diet. PLoS One, 9, e105232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasanthi HR, Mukherjee S, & Das DK (2009). Potential Health Benefits of Broccoli- A Chemico-Biological Overview. Mini Reviews in Medicinal Chemistry, 9, 749–759. [DOI] [PubMed] [Google Scholar]
- Ventura AK, & Mennella JA (2011). Innate and learned preferences for sweet taste during childhood. Current Opinion in Clinical Nutrition & Metabolic Care, 14, 379–384. [DOI] [PubMed] [Google Scholar]
- Wilson DM, Boughter JD Jr., & Lemon CH (2012). Bitter Taste Stimuli Induce Differential Neural Codes in Mouse Brain. PLoS One, 7, e41597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans MR (2006). The role of learning in development of food preferences. Frontiers in Nutritional Science, 3, 93. [Google Scholar]