Abstract
Two- and three-dimensional lead-halide perovskite (LHP) materials are novel semiconductors that have generated broad interest owing to their outstanding optical and electronic properties. Characterization and understanding of their atomic structure and structure–property relationships are often nontrivial as a result of the vast structural and compositional tunability of LHPs as well as the enhanced structure dynamics as compared with oxide perovskites or more conventional semiconductors. Nuclear magnetic resonance (NMR) spectroscopy contributes to this thrust through its unique capability of sampling chemical bonding element-specifically (1/2H, 13C, 14/15N, 35/37Cl, 39K, 79/81Br, 87Rb, 127I, 133Cs, and 207Pb nuclei) and locally and shedding light onto the connectivity, geometry, topology, and dynamics of bonding. NMR can therefore readily observe phase transitions, evaluate phase purity and compositional and structural disorder, and probe molecular dynamics and ionic motion in diverse forms of LHPs, in which they can be used practically, ranging from bulk single crystals (e.g., in gamma and X-ray detectors) to polycrystalline films (e.g., in photovoltaics, photodetectors, and light-emitting diodes) and colloidal nanocrystals (e.g., in liquid crystal displays and future quantum light sources). Herein we also outline the immense practical potential of nuclear quadrupolar resonance (NQR) spectroscopy for characterizing LHPs, owing to the strong quadrupole moments, good sensitivity, and high natural abundance of several halide nuclei (79/81Br and 127I) combined with the enhanced electric field gradients around these nuclei existing in LHPs as well as the instrumental simplicity. Strong quadrupole interactions, on one side, make 79/81Br and 127I NMR rather impractical but turn NQR into a high-resolution probe of the local structure around halide ions.
1. Introduction to Lead-Halide Perovskites
Lead-halide perovskites (LHPs) of an APbX3 composition, where A is Cs, methylammonium (MA), or formamidinium (FA) and X = Cl, Br, I, or mixtures thereof, are compounds isostructural to diverse ABO3-type oxide perovskites. LHPs have recently become a major class of optoelectronic materials owing to their exceptional electronic and optical characteristics.1−4 These materials are intensely pursued for applications in photovoltaics,5−10 LCD technologies,11−13 light-emitting diodes (LEDs),4,14−16 lasers,17−20 UV–vis–near-IR photodetectors,21−28 direct conversion X-ray and gamma detectors,9,29−36and scintillators37,38 and as emerging quantum light sources.39,40 In these applications, LHPs are used in their diverse forms, as single crystals, thick or thin films, and colloidal nanocrystals (NCs, Figure 1a–f), which are easy to produce by means of solution-phase chemistry or low-temperature melt-growth.31,41−43 The remarkable characteristics of these materials include long carrier lifetime–mobility products and low densities of electronic traps (on par with GaAs and CdTe)44−46 despite the large concentrations of structural defects and the enhanced structure dynamics, a seeming paradox often referred to as defect tolerance.4,47−52 Perovskite CsPbBr3 gamma detectors exhibit energy resolution better than commercial CdTe-based detectors.30,31,34,53 Perovskite X-ray detectors54 and UV–vis detectors21,55,56 exhibit high sensitivities on the order of 103 μC Gy1– cm–2 and detectivities up to 1014 Jones, respectively, which compare favorably with respective commercial technologies. Thin-film solar cells with perovskites as light absorbers exhibit power conversion efficiencies (25.2%)10,57 that surpass polycrystalline Si and essentially all emerging thin-film technologies and approach the single-crystalline Si world record (26.1%).10,58 Colloidal ligand-capped LHP NCs (Figure 1b,c) are the first examples of colloidal quantum dots (QDs) exhibiting near-unity photoluminescence quantum yields (PL QYs) across the entire visible spectral range without the need for epitaxial overcoating for electronic passivation.4,47 These NCs are also shown to exhibit long exciton coherence times and near-transform-limited emission line widths,39,59 which, along with stable single photon emission,60,61 makes them attractive for designing future sources of quantum light.39,40,62
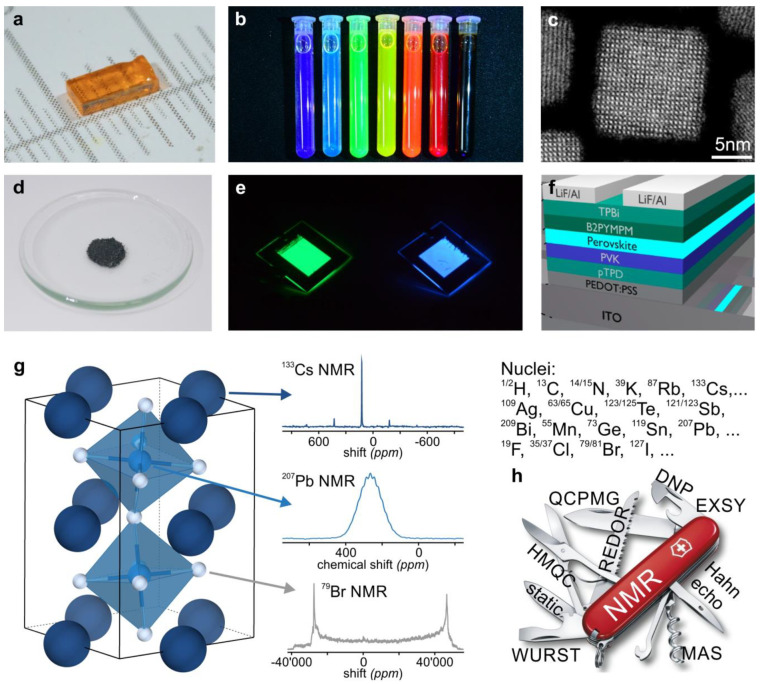
Figure 1.
LHP materials and the utility of NMR spectroscopy for their characterization. 3D and 2D LHPs are of practical interest in their various forms: (a) single crystals (Adapted with permission from ref (36). Copyright 2016 American Chemical Society) and (d) bulk powders, (b,c) colloidal NCs (Panel b Credit: Nadia Schwitz; Panel c Credit: Frank Krumeich), and (e,f) continuous films within optoelectronic devices (green and blue LEDs as examples) (Panel e Credit: Yevhen Shynkarenko; Panel f reprinted with permission from ref (83). Copyright 2019 American Chemical Society). (g) All elements of APbX3 LHPs possess NMR-active isotopes, as exemplified for CsPbBr3 using actual solid-state NMR spectra. (h) Modern NMR spectroscopy methods make for a versatile characterization toolbox. Depending on the probed spin interaction (Figure 2) and the structural aspect in question (Figure 3), a diverse range of pulse sequences and signal and resolution enhancing techniques are available (e.g., QCPMG, quadrupolar Carr–Purcell–Meiboom–Gill; REDOR, rotational-echo double resonance; DNP, dynamic nuclear polarization; EXSY, exchange spectroscopy; HMQC, heteronuclear multiple-quantum correlation; WURST, wideband, uniform rate, smooth truncation; MAS, magic-angle spinning). Image of Swiss Army Knife used with permission from Victorinox AG.
The crystal structure of APbX3 perovskites is characterized by the 3D corner-interconnection of PbX6 octahedra. Several polymorphs of such 3D LHPs exist as a result of the octahedral tilting and orientation of the A cation, thus reducing the symmetry from the archetypical cubic phase (to tetragonal, orthorhombic, or monoclinic) and deviating the Pb–X–Pb bond angles from the initial value of 180°. For instance, at room temperature (RT), the stable LHP polymorphs are cubic FAPbBr3, MAPbCl3, MAPbBr3, and FAPbCl3, tetragonal MAPbI3, orthorhombic CsPbBr3 (Figure 1d), and monoclinic CsPbCl3, whereas CsPbI3 and FAPbI3 crystallize in nonperovskite 1D polymorphs (with edge- and face-sharing connectivity, respectively).63−76 The structural instability of the perovskite phases for CsPbI3 and FAPbI3 stems from Cs and FA cations being, respectively, too small and too large to optimally fill the A site.77−82 Interestingly, stable perovskite phases can be obtained with mixed-ionic compositions (Cs/FA)PbI3 or (Cs/FA)Pb(Br/I)3.8,84−88 The substitutional A-site doping by other monovalent cations, such as Rb+, K+, guanidinium (G), azetidinium, or dimethylammonium (DMA), reportedly results in improved device performance or higher material stability.89−97 The incorporation of a larger ethylendiammonium cation requires the concomitant formation of lead and halide vacancies, yielding so-called hollow LHPs.98 The halides and the octahedral tilts define the band-gap energies of LHPs (ca. 1.5–3.0 eV).99−101 Perovskite lattices also form upon the substitution of Pb2+ with Sn2+ or Ge2+76,102−109 or by a combination of monovalent and trivalent cations (e.g., Ag+, Cu+, Bi3+, In3+, and Sb3+ in so-called double perovskites).109−114 These lead-free perovskites thus far fall behind in their optoelectronic quality due to both the different electronic structures (flat bands, indirect transitions in double perovskites, etc.)115 and the limited stability.84,115
Recent years have seen a surge of activities beyond APbX3, namely, in 2D LHPs, which are often reported to exhibit greater stability116,117 and wherein structural variety can be realized by disrupting the connectivity in one dimension, for example, by cutting 3D perovskites into 2D slabs of adjustable thickness.118−121 Most common are cuts along the <100> direction, as in Dion–Jacobson122 and Ruddlesden–Popper123 types, or structures with alternating cations in the interlayer space.124 2D slab thicknesses n (number of octahedral layers within the slab) are typically in the range of n = 1–7.121,125−128 The interlayer region comprises bulky spacers, such as long-chain alkyl-, aryl-,129 adamantantyl-,130 alkylphenyl-120 ammonium, and heterocyclic (e.g., benzimidazolium131 or quaterthiophene derivatives)132 cations. In general, owing to quantum and dielectric confinement, layered perovskites behave as multiple quantum-well structures,133,134 with band-gap and exciton binding energies decreasing for larger n.123,135,136 Larger exciton binding energies and cascade energy structures137 favor fast radiative recombination, as required in, for instance, LEDs.138,139 Smaller binding energies (n > 3) lead to efficient exciton dissociation into free carriers, as needed in photovoltaics119,140 and photodetectors.141,142 Octahedral distortions and tilts within 2D slabs give rise to piezo- and ferroelectric responses.129,143,144 The chirality of the organic cations allows for the emission or detection of polarized light145−148 and nonlinear optical responses.149−152 Static and dynamic structural distortions and defects have a profound impact on the PL line widths and PL QYs (energetic disorder, exciton–phonon coupling, etc.).117,153−155 Because of their strong spin–orbit coupling, 3D LHPs have long been considered for spintronic applications, and whereas the Rashba effect has been observed experimentally,156,157 its origin in these applications remains uncertain.158,159 The reduced dimensionality of 2D perovskites favors symmetry reduction, resulting in larger Rashba splitting.160,161 Moreover, the manipulation of spin polarization and spin funneling has been recently demonstrated in 2D perovskites.162−164
The in situ formation of 2D perovskites at the interfaces or grain boundaries or as individual grains is often invoked in explaining the favorable effects of the addition of bulkier cations into standard protocols for the deposition of 3D perovksite films in optoelectronics.165−170 Oftentimes, the exact structure of these concomitant phases remains unknown.
We also note that there exist nonperovskite lead halides with 1D corner-sharing or full disconnection of PbX6 octahedra or those formed by face- and edge-sharing of PbX6 octahedra as well as compounds with nonoctahedral coordination of Pb.155,171 These compounds comprise electronically isolated states and hence bound or self-trapped excitons with the characteristic broadband emission and a steep temperature-dependent PL QY.155,172 Such metal halides are also of great interest for optoelectronics and structural chemistry per se171,173,174 and will benefit from the development of the magnetic resonance methods.
LHPs represent a transitional case in between rigid crystalline materials and soft, dynamic, and disordered matter. It remains counterintuitive how the defect tolerance emerges, that is, how highly intrinsic electronic and optical characteristics are retained and not hampered by the structural defectiveness and structural dynamics, both being greatly enhanced in comparison with the structurally more rigid, conventional semiconductors. In fact, soft lattices of these halides are increasingly considered to be a favorable factor.178−181 In particular, the association of charge carriers with lattice vibrations—so-called polarons—may protect carriers from defect states and increase carrier lifetimes, can aid in exciton dissociation, and may slow carrier cooling. Polarons are also increasingly associated with the unusually high dielectric constants in LHPs. The broad compositional and structural engineerability of LHPs makes it possible to engineer their properties à la carte. There exists no perfect and universal structural characterization method for probing the atomic structure both statically and dynamically as well as locally and space-averaged. It is the entirety of observations from diverse methods that allows the complex structure–property relationships to be unveiled. Typically, detailed structural investigations into a new class of inorganic materials start with X-ray/electron/neutron diffraction methods and electron microscopy and may be complemented by vibrational spectroscopy. Time-resolved variants of these methods and their combinations with optical methods give an extra benefit of probing the structure dynamics or excited states.182,183 This Perspective emphasizes and exemplifies the immense utility of magnetic resonance methods for characterizing LHPs and other metal halide materials and outlines future avenues in this field.
2. Utility of NMR and NQR for Lead-Halide Perovskites
Solid-state nuclear magnetic resonance (NMR) spectroscopy is a versatile tool for sampling the chemical nature, geometry, and topology of the surrounding of atomic nuclei as well as the structure dynamics in materials without prerequisites for the crystallinity, size, or composition of the sample. All elements constituting LHPs possess NMR-active isotopes (i.e., nuclei with a spin), such as 1H, 13C, 14/15N, 19F, 35/37Cl, 39/41K, 55 Mn, 63/65Cu, 73Ge, 79/81Br, 85/87Rb, 107/109Ag, 119Sn, 121/123Sb, 123/125Te, 127I, 133Cs, 207Pb, and 209Bi (Figure 1g,h). NMR spectroscopy is highly chemical-element-specific, as each isotope has a different Larmor frequency (ω0) corresponding to the Zeeman splitting in the applied magnetic field and hence a very specific frequency range of the NMR signals. NMR spectroscopy is a high-resolution probe of the various interactions of the nuclear spin with other nuclear and electron spins or electromagnetic fields (Figure 2). Spin–spin interactions can provide, for instance, information about connectivity (J-coupling, HJ) or interatomic distance (dipolar coupling, HDD), whereas spectral features produced by the surrounding magnetic field (chemical shift, HCS) and electric field gradient (EFG, quadrupole interaction, HQ) contain information about the nature and the geometry of the chemical species. Low crystallinity, static and dynamic structural disorder, concomitant phases, and impurities, all being recurring matters in LHP research (Figure 3), are seen very differently by NMR as compared with diffraction-based methods. This important complementarity of NMR methods for LHPs is evident from the surge of research articles containing solid-state multinuclear NMR data. Several recent review articles may serve as initial guidance to the present stake of NMR in LHPs. Franssen et al. reviewed the structural and dynamical aspects,184 whereas Bernard et al. unified models and provided a perspective about the dynamics of MA in MAPbX3.185 In their perspective about the “ionics in hybrid halide perovskite”, Senocrate et al. summarized insights into the ion conduction in LHP obtained with NMR spectroscopy.186
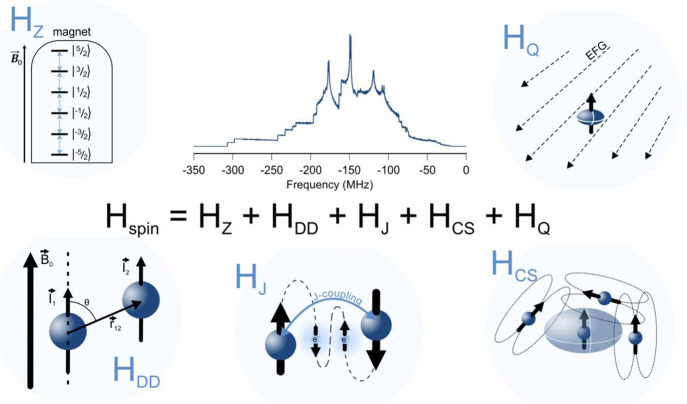
Figure 2.
Major interactions contributing to NMR spectra. In NMR spectroscopy, the spin energy transitions are studied. By applying an external magnetic field, the Zeeman interaction (HZ) lifts the degeneracy between nuclear spin states. A spin of I = 5/2 is shown, in the absence of other spin interactions. Spin–spin interactions such as the dipolar coupling (HDD) or the J-coupling (HJ) perturb the spin energies and so do deviations of the local magnetic field relative to the applied magnetic field by shielding and deshielding of neighboring spins (chemical shielding, HCS). Also, nearby charges affect the spin energies through the coupling of the quadrupole moments of spins of I > 1/2 with the electric field gradient (EFG) generated by these charges (quadrupole interaction, HQ). The NMR spectrum, which reflects the spin energy transitions, is affected by all of these interactions through the shift of the resonance frequency, signal broadening, shape distortion, generation of multiplets and tensor line shapes, and so on. Here a simulated 127I NMR spectrum of MAPbI3 at 16.4 T is shown as an illustration of the complexity that NMR spectra can exhibit. For a detailed discussion and mathematical descriptions of these interactions, the interested reader is referred to several NMR textbooks.175−177
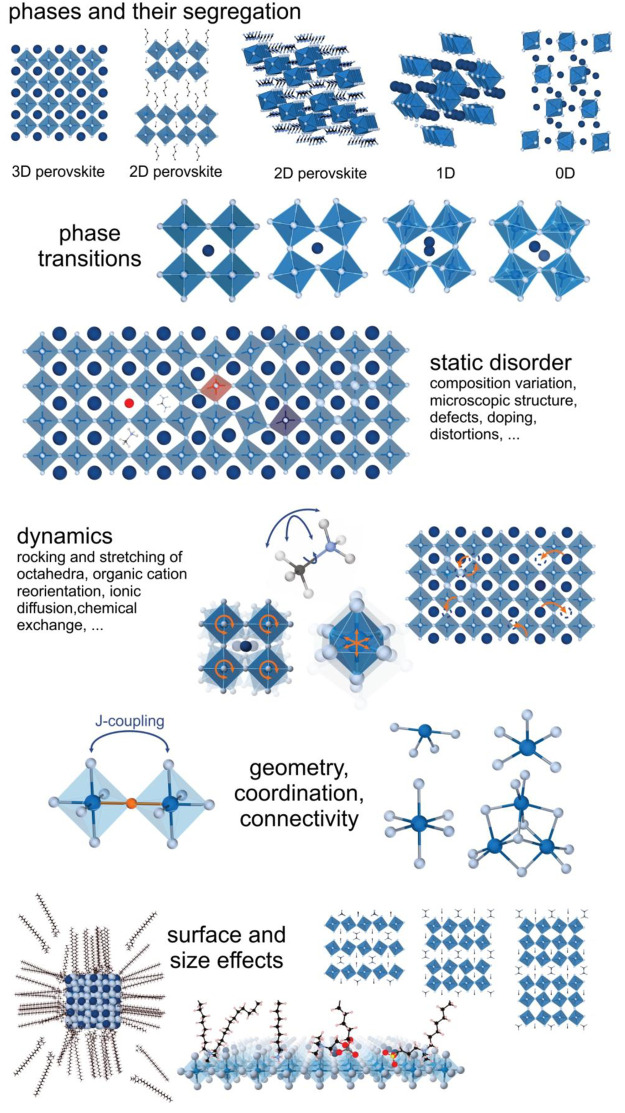
Figure 3.
Structural insights about LHPs accessible through NMR and NQR. The figure illustrates a nonexhaustive range of structural aspects that are within the reach of magnetic resonance methods. Besides these structural and chemistry insights, electronic effects of quantum size effects and dielectric confinement are addressable as well.
In this Perspective, we bring the reader’s attention to the vast insights that NMR spectroscopy yields about the structure and structure dynamics of diverse LHPs (2D and 3D compounds, from NCs to bulk single crystals). We then discuss prospects that highlight the complementarity of nuclear quadrupole resonance (NQR) for 79/81Br and 127I nuclei, possessing particularly large quadrupole moments and, in LHPs, exhibiting strong quadrupole interactions due to large EFGs. We also underline that a better understanding of the structure dynamics in LHPs should leverage rapid development in computational materials science (i.e., ab initio molecular dynamics (AIMD) and its combinations with classical molecular dynamics as well as density functional theory (DFT) calculations).187−189
Diverse structural and dynamic aspects of LHP materials (Figure 3) can be studied by both NMR and NQR methods. Various lead-halide compounds and, for an individual compound, also different polymorphs (phase transitions, coexistence of polymorphs) can be readily resolved as signals with different resonance frequencies (usually different chemical shifts in NMR).190−197 Static disorder in LHPs comprises (i) structural defects such as point defects (e.g., halide vacancies), interstitial atoms, stacking faults, and grain boundaries and (ii) compositional modulations such as the mixed-ionic occupation of lattice sites, ion segregation, and compositional gradients (typical for halide ions) as well as heteroatomic doping. Static disorder is seen as inhomogeneous broadening generated by the distribution of chemical surroundings, forming a continuum of resonance frequencies that define the signal shape. For certain types of defects (e.g., paramagnetic dopants), relaxation of the nuclear spins can be affected as well. The rich structural dynamics in LHPs ranges from the rotational and stretching freedom of the organic constituents or the various stretching and tilting modes of the lead-halide framework to rapid ionic motion. These dynamic processes can be probed through the temporal variation of spin interactions. Depending on the nature and the time scale of the dynamics, the relaxation behavior or the shape and position of the signal may be affected, oftentimes accompanied by significant homogeneous broadening of the respective signals. Surfaces become a chemically distinct feature in high-surface-area LHP materials, such as ligand-capped colloidal NCs or matrix-embedded NCs. NMR offers diverse handles to probe such surfaces by surface-selective excitation (e.g., through cross-polarization), signal enhancing methods (e.g., dynamic nuclear polarization (DNP)), as well as correlation and other types of 2D experiments. Finally, electronic effects of size quantization and dielectric confinement in colloidal LHP NCs or 2D LHPs are also within the reach of NMR spectroscopy. These can be investigated through, for instance, susceptibility effects or quadrupole and chemical shift interactions (paramagnetic shielding term).
3. A-Cations: Dynamics and Surfaces
There are only three monovalent cations known to form 3D APbX3—MA+, FA+, and Cs+—owing to their geometric fitness into the 12-coordinate A-site.198 Mixing these A-cations, concomitantly with mixing halides, is presently the most successful strategy for obtaining best-performing perovskite solar cells. For instance, the incorporation of MA or Cs stabilizes cubic FAPbI3 against spontaneous transition into a nonperovskite lattice. Examples of such heavily substituted multinary perovskites include FA0.83Cs0.17Pb(I0.6Br0.4)3,199 (FA0.3MA0.7)0.85Cs0.15PbI3,200 and FA0.79MA0.16Cs0.05Pb(I0.83Br0.17)2.97.201 Further cations (e.g., G and DMA), which are not perovskite formers when used alone, readily incorporate themselves as A-site dopants.92−95 Interestingly, the analogous substitutional incorporation of the smaller Rb+ and K+ onto A-sites was not corroborated by NMR studies, as will be discussed further later. Important pertinent questions that may be addressable with NMR are (i) to what extent different A-cations mix within the perovskite phase, (ii) how they are distributed between different grains, and (iii) the possibility of segregation at the surfaces and interfaces. For instance, the high PL QYs (60–70%) and carrier mobilities (40 cm2 V–1 s–1) of potassium-doped (Cs0.06FA0.79MA0.15)Pb(I0.85Br0.15)3 were attributed to surface and grain-boundary passivation with potassium halides.96 In MA-free Cs-FA LHP films, the graded A-cation composition of the crystalline grains was rationalized as an inhibiting factor for the transfer of electrons across grain-boundaries.202 NMR has been used to sample 1/2H, 13C, 14/15N, 39K, 87Rb, and 133Cs spins and has permitted us to identify the structural environments of monovalent cations,192−194,203−219 their dynamics,185,194,196,206,215,216,219−225 the composition within phases,98,193,222,226−242 and the distribution between phases of LHPs.192,193,215,217,222
Most of the early NMR studies on LHPs focused on MAPbX3 compositions. Starting with the 1985 paper by Wasylishen et al.,220 the dynamics of the MA cations was assessed through 1/2H, 13C, and 14/15N nuclei.194,196,206,219,221,222,224,225 The MA cation motion can be categorized into two kinds: the fast cone-shaped, wobbling libration (∼300 fs in MAPbI3) and the slower, jump-like rotation of the C–N axis of the MA molecule (∼3 ps in MAPbI3).243 The great interest in MA dynamics is attributed to its association with long carrier lifetimes244 and its contribution to the stabilization of the perovskite electronic structure via hydrogen bonding.244,245 In general, hydrogen bonding is little studied in LHPs, especially in 2D and lower dimensional derivatives.246,247 Typical structural characterization methods, that is, X-ray diffraction, are challenging due to the low atomic number of hydrogen. NMR does not experience this limitation. For example, Baikie et al. found, using 1H NMR, that only the amine moiety of MA interacts with the lead-halide framework and used their insights to refine their X-ray and neutron-diffraction data.221
The fastest dynamics resolvable with NMR are those affecting the T1 relaxation rates. Here the theoretical limit corresponds to the Larmor frequency range, which is several tens to hundreds of megahertz (depending on the gyromagnetic ratio of the nucleus and the applied magnetic field strength), corresponding to the time scale of a few picoseconds to several nanoseconds. Even faster dynamics is experienced by the nuclear spins as averaged electromagnetic fields, whereas slower dynamics from a few microseconds up to hundreds of milliseconds impacts T2 relaxation rates. Slower dynamics (seconds and slower) may also be resolvable, for instance, by the sequential acquisition of spectra. Hence, because of the fast dynamics of MA compared with the NMR time scale, 1H and 13C spectra of MAPbX3 exhibit only narrow lines with a very similar chemical shifts across different halide compositions,206,209,221,222 but proton T1 relaxation times change sharply at phase transitions.196,209,221,248 Quadrupolar couplings of the nuclear spins 14N and 2H (both spins of I = 1) were often used to investigate MA dynamics. 14N and 2H NMR spectra show no quadrupole splitting in the high-symmetry cubic phase due to isotropic spin surroundings and the fast reorientation of MA (Figure 4a).194,206,219,220,222,224,225 At lower temperatures, MAPbX3 crystallizes in tetragonal and orthorhombic crystal systems, and small quadrupole couplings arise due to the loss of symmetry from the slower dynamics of the MA molecule.194,206,219,220,224,225 On the basis of the values and the temperature dependence of the line width, the relaxation constants, and the quadrupole coupling strengths, the contribution of the different rotations of MA (correlated and uncorrelated C3 reorientation along the C–N axis, tunneling rotation, reorientation of the C–N axis, etc.) and their activation energies could be determined,194,196,206,219−222,224,225 as was comprehensively surveyed in ref (185). DFT calculations were used to determine the exact space group of MAPbI3 at RT.21914N quadrupole couplings constants (CQ) were calculated for four symmetrically identical orientations of MA in the crystallographic ab plane predicted by Weller et al. (top of Figure 4a).249 The simulated spectra agreed well with the experimentally observed temperature behavior of the static 14N solid-state NMR spectra (Figure 4a). The temperature-dependent experimental and calculated CQ values follow the ratio of the crystallographic c and a axes, thus permitting the space group of MAPbI3 to be determined to be I4/mcm.
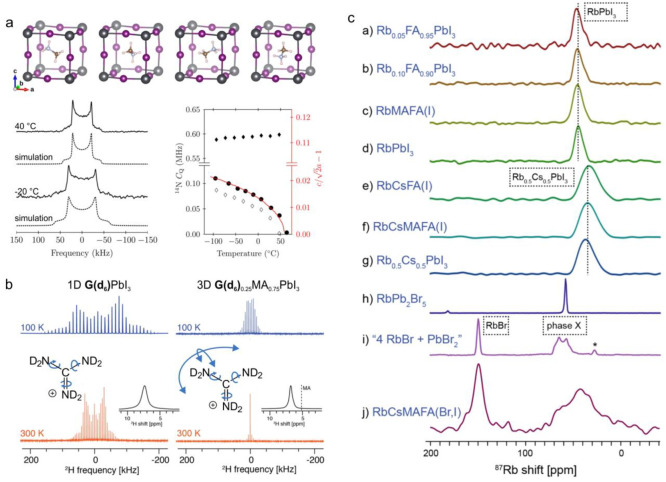
Figure 4.
NMR of A-cations in LHPs. (a) Top: Four MA orientations in the crystallographic ab plane predicted by Weller et al.249 Left: Experimental and simulated static 14N solid-state NMR spectra of MAPbI3 spectra. Right: 14N quadrupole coupling constants from the experiment (filled black circles) follow the ratio of the crystallographic c and a axes with varying temperatures (red, solid line). The filled and empty diamonds represent the individual and the averaged values calculated with DFT for the four MA orientations. Adapted with permission from ref (219). Copyright 2017 American Chemical Society. (b) 2H MAS NMR spectra of G(d6)0.25MA0.75PbI3 multinary perovskite and 1D G(d6)PbI3 at 100 and 300 K. Insets are zoom-ins of the center band at higher sample spinning speeds. The dashed line indicates the expected shift of CH3ND3. Adapted with permission from ref (215). Copyright 2018 American Chemical Society. (c) Substantial integration of Rb inside the 3D perovskite structure was ruled out, whereas the formation of a 1D structured phase containing Rb could be observed with the help of the 87Rb NMR spectra of APbX3 (A = Cs, FA, MA, Rb; X = Br, I) materials.192 Reprinted with permission from ref (192). Copyright 2017 American Chemical Society.
There are further studies investigating the dynamics of organic cations in pure-phase (FAPbBr3,250 FAPbI3)209,222 and increasingly mixed-ionic LHPs (FA0.67MA0.33PbI3;222 G0.25MA0.75PbI3,215Figure 4b; DMA1–xMAxPbI3).251 The capability to distinguish between dynamic and static disorder by separating homogeneous and inhomogeneous line width contributions is not unique to NMR spectroscopy, as, for instance, the PL line width as well as the PL QY and the radiative lifetime are also affected by temporal and spatial disorder.153−155 However, NMR holds the advantage that the involved chemical structures can be traced because the NMR signals of the concerned structural moieties are the ones exhibiting homogeneous or inhomogeneous line broadening, respectively. By sampling organic and inorganic A-cations in mixed LHPs, insights into the dynamics, the A-cation distribution, and their incorporation are available for a vast range of compositions involving organic (MA+, FA+, DMA+, and G+) or inorganic (Cs+, Rb+, and K+) monovalent cations.192,193,215,217,222,250,252 For instance, Kubicki et al. showed that K+ and Rb+ ions do not substitutionally dope the lattice of 3D LHPs, as can be seen in the absence of a continuously shifting signal with gradual compositional change (Figure 4c). The results rather point to the formation of new, structurally unidentified phases.192,193 The situation is different for the G-cation, which at low concentrations occupies the A-site positions.215 Noticeably, 2H NMR indicated that the reorientation of G+ in 3D structures is accelerated compared with the dynamics in a nonperovskite 1D GPbI3 (Figure 4b). The 2H quadrupole splitting of deuterated G+ reflects the reorientational dynamics of this cation within the structure. The broader splitting in nonperovskite G(d6)PbI3 indicates that the dynamics is restricted to rotations along the C–N axes, whereas isotropic reorientations on top of a fast C–N axial rotation are required to explain the narrow lines observed for G(d6)0.25MA0.75PbI3 perovskite materials. Kubicki et al. suggested that the reorientations of G-cations, which are faster than 106 s–1, could explain the improved performance of this material in solar cells,215,229 in a similar way as the fast MA reorientation prolongs charge-carrier lifetimes in MAPbI3.244 Grottel et al. have used 1H NMR relaxation as a probe for the G-cation dynamics and activation energies of the latter as well as phase transitions in 1D GPbI3 and 2D G2PbI4 structures.253
NMR spectroscopy is also increasingly instrumental for characterizing and elucidating the role of organic cations in 2D perovskites.130,253−257 For instance, the distinction between different molecules is straightforward, and their locations within slabs or interstitials can be clearly determined.130,253−255 Milić et al. could distinguish the FA cations in the slabs of 2D A2FAn–1PbnI3n+1 (A: (adamantan-1-yl)methanammonium spacers) from those incorporated into a concomitant 3D FAPbI3 using 2D 1H–1H spin-diffusion NMR spectra (Figure 5a).130 The dynamics of organic cations extracted from 1H relaxation times in different 2D LHP structures made it possible to draw conclusions about the crystal rigidity and, in turn, relate the softness of the lattice to the PL line width (exciton–phonon coupling) and other PL characteristics.257
Figure 5.
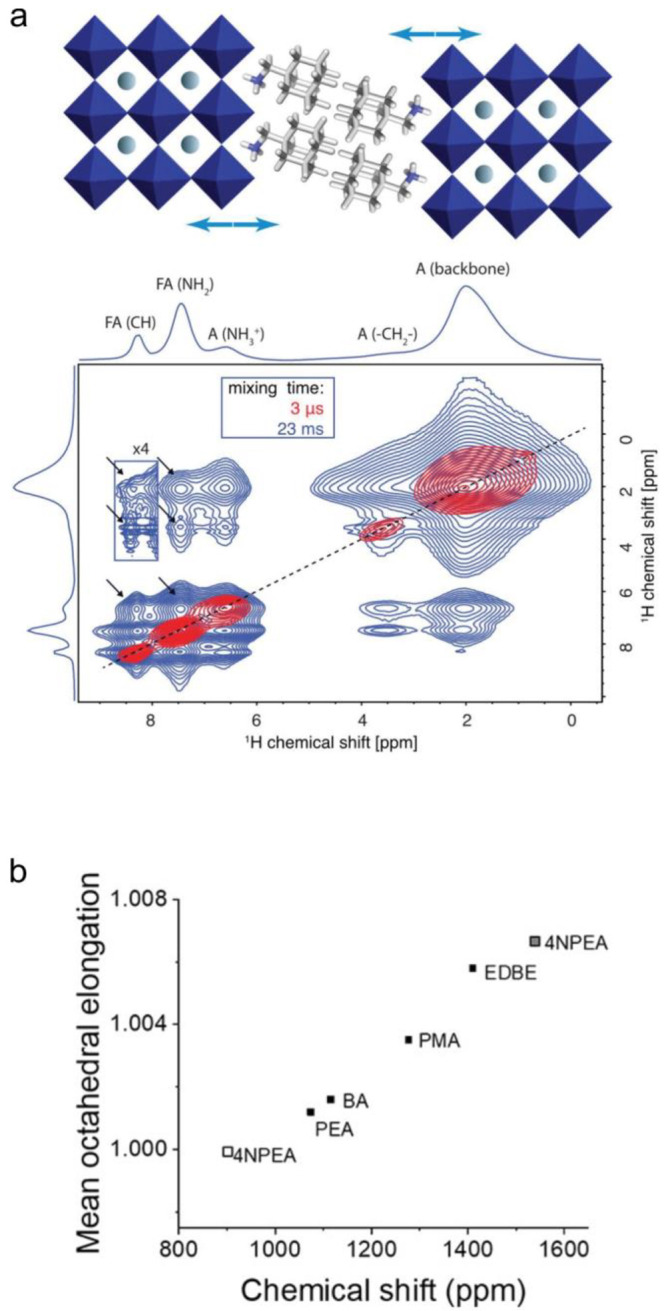
NMR of 2D LHPs. (a) Schematic of a 2D A2FA2Pb3I10 structure proposed by Milić et al., corroborated by 1H–1H spin-diffusion solid-state MAS NMR spectra recorded at different mixing times.130 The cross peaks highlighted by the arrows in the spectra with longer mixing times indicate the proximity between some FA cations and the spacer cation (adamantan-1-yl)methanammonium (blue arrows in the scheme) and support the proposed structure. Reprinted with permission from ref (130). Copyright 2019 by John Wiley and Sons. (b) Relationship between the mean elongation of lead-halide octahedra in 2D A2PbI4 structures with various A cations (4NPEA, 4-nitrophenylethylammonium (two 207Pb NMR signals); PEA, phenylethylammonium; BA, butylammonium; PMA, phenylmethylammonium; EDBE, NH3(CH2)2O(CH2)2O(CH2)2NH3) and their 207Pb NMR isotropic chemical shifts. Reprinted with permission from ref (255). Copyright 2019 American Chemical Society.
The known NMR studies on inorganic A-cations are mainly focused on phase transitions in CsPbX3 LHPs and the structural implications (tilting of lead-halide octahedra, symmetry loss/increase, increase in disorder, etc.) arising from temperature variations.203−205,258−260 In particular, 133Cs spins were found to be valuable probes to study ferroelastic, twinned domain structures in CsPbCl3.258,260−264
4. 207Pb NMR Spectroscopy of Lead-Halide Perovskites
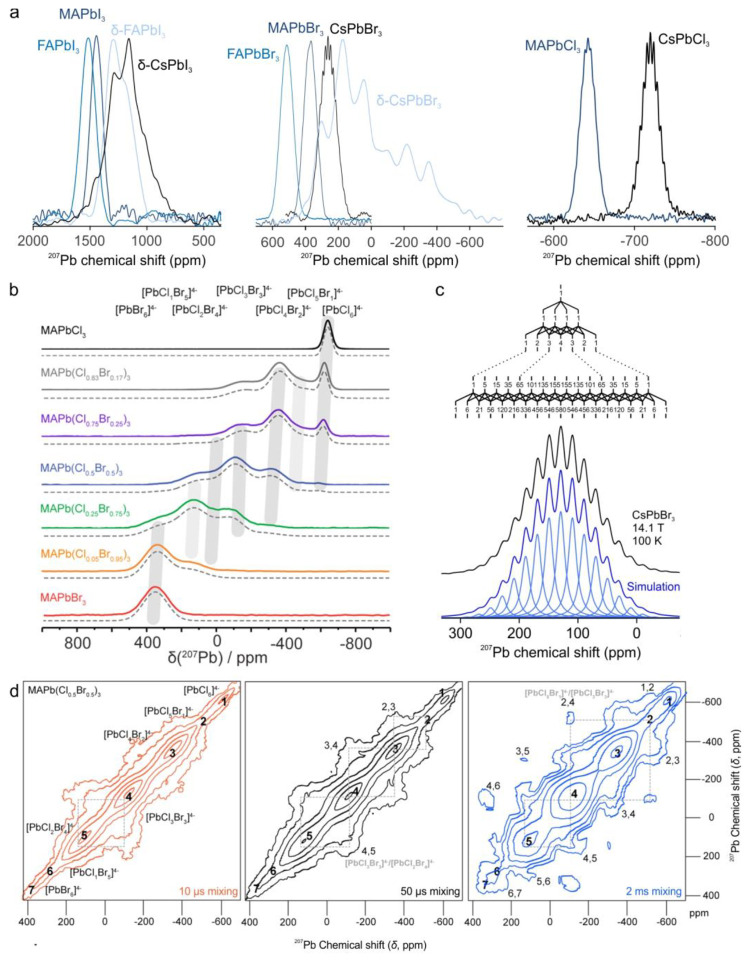
The large chemical shift range of 207Pb NMR (∼20 000 ppm) makes it a highly sensitive probe of major and subtle structural changes in the lead surroundings, including first, second, or third coordination shells. The 207Pb NMR signal of APbX3 compounds is more deshielded for heavier halides: δ(APbCl3) < δ(APbBr3) < δ(APbI3) (Figure 6a).206,265−270 In the mixed-halide perovskites APbClxBr3–x and APbBrxI3–x (A = MA, FA; x = 0–3), seven distinct lead environments PbXxX′6–x (x = 0–6) were found with chemical shift spanning the range between two monohalide counterparts (Figure 6b), and the 207Pb NMR chemical shifts evolved linearly with the composition.266−269 Any substantial dynamics of halide ions should manifest itself in 207Pb NMR, owing to the imparted changes in Pb–X bonding. 2D exchange spectroscopy (EXSY) captured the constantly changing Pb environment due to the exchange of halide atoms (Figure 6d),268,269 in agreement with the known high diffusivity of halide ions in these materials.271 The analysis of 207Pb NMR spectra from mixed-halide LHPs obtained with various synthetic procedures differentiates between solid solutions with different degrees of homogeneity and halide miscibility as well as crystallinity.266−269 In the 207Pb NMR spectrum of solution-grown MAPbI1.5Br1.5, two phases were observed, assigned as MAPbIBr2 and MAPbBr3 based on their chemical shifts.267 However, the MAPbBr3 phase was not seen in X-ray diffraction patterns, indicating its low crystallinity or localization as a thin layer around MAPbIBr2 grains.
Figure 6.
207Pb NMR of LHPs. (a) 207Pb solid-state NMR spectra of APbX3 compounds. Adapted with permission from ref (273) (Copyright 2020 by Wiley and Sons), ref (270) (Copyright 2019 Springer Nature), and ref (272) (Copyright 2018 American Chemical Society). (b) Gradual change of the halide composition, here from MAPbCl3 to MAPbBr3, results in lead environments with mixed-halide composition PbXxX′6–x (x = 0–6), whose positions in the spectra correspond almost exactly to the linear interpolation of the pure compounds APbX3 and APbX′3. Reprinted with permission from ref (269). Copyright 2018 American Chemical Society. (c) 207Pb LHP NMR of CsPbBr3 at 100 K. The signal splitting is a 19-fold multiplet generated by the J-coupling to six bromine nuclei. The spikelet conforms with Pascal’s triangle. Reprinted with permission from ref (270). Copyright 2019 Springer Nature. (d) MAPbCl1.5Br1.5207Pb EXSY NMR spectra with increasing exchange times from left to right. The exchange between two of the seven lead environments (1: [PbCl6]4–, 2: [PbCl5Br]4–, 3: [PbCl4Br2]4–, 4: [PbCl3Br3]4–, 5: [PbCl2Br4]4–, 6: [PbClBr5]4–, 7: [PbBr6]4–) produces a cross peak at their frequencies’ intersections, indicated by n,m for the exchange between environments n and m. With increasing mixing time, environments not only with one different halide atom (small squares) but also with two changing halides exchange (large squares). Adapted with permission from ref (269). Copyright 2018 American Chemical Society.
The influence of the composition of the halide coordination sphere of the lead atom on the lead isotropic chemical shifts (207Pb δiso) is stronger than the effect of connectivity between lead-halide octahedra or octahedral tilts. This is exemplified in Figure 6a, wherein different APbX3 compounds are grouped by composition.191,270,272,273,191 Of these, nonperovskite lattices are δ-CsPbBr3 and δ-CsPbI3 (1D chains of edge-sharing octahedra) and δ-FAPbI3 (face-sharing, 1D connectivity as well). A-cation substitution in 3D LHPs results in the 207Pb δiso trend CsPbX3 < MAPbX3 < FAPbX3 in monohalide series (Figure 6a). Besides the structural effect of A-cations (octahedral tilts), the different magnetic properties of the A-cations (different gyromagnetic ratios, polarizabilities, spin multiplicities, etc.) may also contribute to the observed trend. Different octahedral tilts and hence a change in the symmetry also occur upon temperature-induced phase transitions. For instance, MAPbI3 undergoes a transition from tetragonal to orthorhombic polymorphs upon cooling from 20 to −130 °C, seen as a reduction of 207Pb δiso from 1450 to ∼1100 ppm219 and accompanied by the gradual appearance of spinning sidebands (i.e., a chemical shift anisotropy (CSA) tensor) due to reduced structure symmetry. Because of the strong temperature dependence of the 207Pb chemical shifts, Bernard et al. proposed the use of MAPbCl3 as an internal thermometer for solid-state NMR experiments,274 alternative to the commonly used Pb(NO3)2 salt.275 In the 207Pb NMR spectra of MAPbCl3, there is no apparent CSA, unlike Pb(NO3)2, which facilitates the temperature calibration in static solid-state NMR experiments. Furthermore, the 13C NMR signal from MA could serve as an internal standard.
In general, the line width of 207Pb NMR signals is large, from a few kilohertz up to tens of kilohertz, and arises from several line-broadening effects. These include the CSA, the positional disorder leading to a distribution of isotropic chemical shifts, the dipole–dipole coupling (at least for static spectra), and the dispersion of J-coupling values due to variations of the bond lengths and geometries. For MAPbCl3, CsPbCl3, MAPbBr3, and CsPbBr3, it was shown that the signal width is dominated by the lead-halide J-coupling, which is on the order of ca. 400 Hz (1JPb–Cl) and 2.3 kHz (1JPb–Br).185,252,270 19-Fold multiplets were detected for these LHPs (only at low temperatures in the case of MAPbBr3) due to the (almost) identical couplings of 207Pb nuclei with the six coordinating halide atoms, which all possess spins of I = 3/2 (Figure 6c). The signal width of other APbX3 compounds is also likely to be defined by the lead-halide J-coupling patterns, but because of the increased inhomogeneous line width and lower structural symmetry, the lines of the pattern cannot be resolved.270 Although often overseen due to the inhomogeneous broadening of the signal or unsuited signal acquisition or processing, the lead-halide couplings had already been observed more than 20 years ago in the doctoral thesis of Holger Ulman (University Dortmund).276 A plethora of binary, ternary, and quaternary lead-halide materials were synthesized and characterized, and, for a large number of these compounds, the lead-halide coupling was recorded. 207Pb solid-state NMR can also be used to study decomposition products of MAPbI3 when exposed to humidity or thermal stress277 or to unravel side reactions. For instance, in situ DMA formation was captured as a result of the dimethylformamide (solvent) reaction with MA+ during the preparation of MAPbI3 thin films.236
The lower symmetry and the partially disrupted connectivity of the lead-halide octahedra in 2D LHPs affect the chemical shielding of 207Pb nuclei, leading to shifting of the isotropic value255,272 and the appearance of spinning sidebands due to an increased CSA, which differ for lead atoms within and at the edges of the slabs.255,278 Tremblay et al. delineated a linear dependence between the chemical shift and the elongation of the lead-halide octahedra produced by different slab-separating cations (Figure 5b).255 In 2D LHPs, the quantum confinement alters the energy levels of the conduction band (CB) and valence band (VB), resulting in different band-gap energies, whereas the dielectric effect does not alter the band -gap energy but equally shifts the CB and VB on an absolute energy scale. The isotropic and anisotropic chemical shifts may be highly potent probes of these confinement effects because changes in the electronic structure, affecting both the absolute energy values (isotropic chemical shift) as well as the electronic inhomogeneities (anisotropic chemical shift), impact the local magnetic field of 207Pb nuclei. The changes in the VB and CB energies might be compared with the HOMOs and LUMOs in Ramsey’s description of chemical shieldings in molecules,279 which was already applied to explain the observations in NMR spectra of quantized semiconductor materials (in particular, colloidal NCs and nanoplatelets).280−283 However, one should expect that for the elucidation of the relationship of confinement effects with the chemical shift in 2D perovskites, computational approaches, considering many-body interactions and spin–orbit couplings, are required.
The broad signal width of APbX3 materials, the modest gyromagnetic ratio (5.58 × 107 rad s–1 T–1; about five times smaller than that of a proton), and the low natural abundance (22.1%) of 207Pb make it a relatively unreceptive nucleus. Accordingly, recording conventional 207Pb NMR spectra of LHPs usually requires measurement times of several hours. Hanrahan et al. have therefore investigated and quantitatively compared several experimental procedures, such as magic-angle spinning (MAS), low temperatures, dynamic nuclear polarization (DNP), and proton detection, which improve the signal intensity and reduce the acquisition time of 207Pb NMR spectra to a few minutes.265 They noted that the low-temperature spectra must be interpreted with care because phase transitions can occur, and the dynamics is influenced by the temperature change. Although their DNP enhancement factors were moderate for MAPbCl3 (15–20) and MAPbBr3 (3 to 4) at the applied magnetic field strength (9.4 T) and negligible for MAPbI3, their results showcased a path to the efficient sampling of both LHP thin films and surface species in LHP NCs, which are morphologies with even lower lead concentrations than microcrystalline powders. Besides the experimentally demanding methods previously mentioned, another practically attractive avenue is the development of benchtop, low-magnetic field 207Pb NMR spectroscopy (with a permanent magnet) for the routine characterization of LHPs, as recently demonstrated for MAPbCl3.284
5. Halide NMR and NQR
All heavier halides possess a quadrupole moment because they have spins of I > 1/2 (35/37Cl I = 3/2, 79/81Br I = 3/2, 127I I = 5/2), whereas 19F has I = 1/2. Because fluorine is not a structure-former for LHPs, we focus on quadrupolar halide nuclei, yet we must note that 19F NMR is highly instrumental to the study of the fluorine-containing organic moieties of LHPs. For instance, Ruiz-Preciado et al. used it, in combination with DFT calculations, to probe halogen bonding at LHP surfaces.285 The occasional use of 19F NMR to characterize hybrid LHPs can also be found in refs (286 and 287). We also note that 19F solid-state NMR has been applied to numerous metal fluoride perovskites (ABF3)288−307 and 2D fluoride perovskites,308 where A = Li, Na, K, Cs, Rb, and ammonium (NH4+) and B = Mg, Ca, Ba, Sr, Mn, Co, Zn, and Ho, to study diverse materials aspects, such as fluoride ion mobility309 or the material’s paramagnetism.299,305
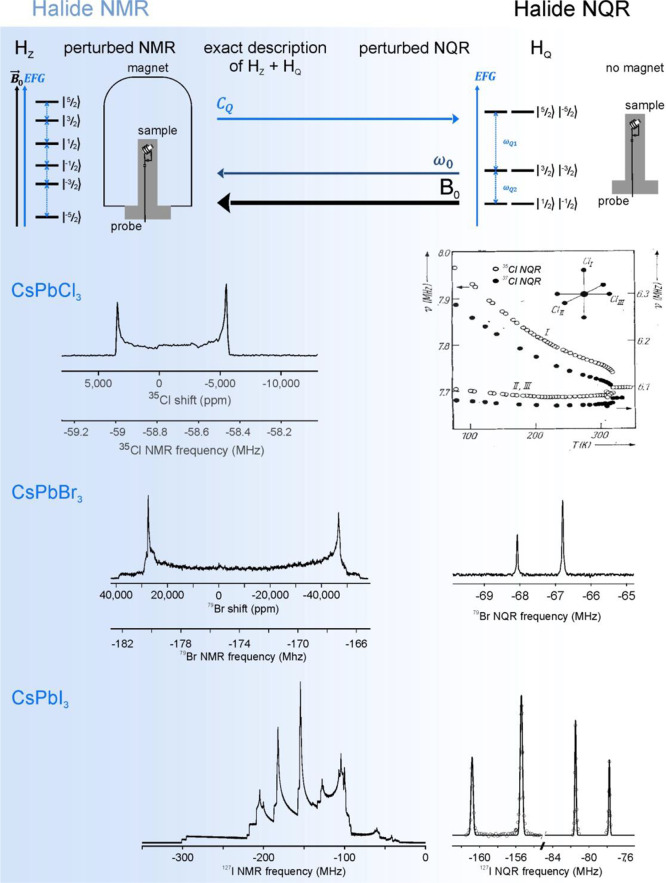
Quadrupolar halide nuclei are subject not only to the Zeeman interaction (HZ, Figures 2 and 7) but also to the quadrupole interaction (HQ) as their quadrupole moment couples to the EFG. The symmetry and the strength of the quadrupole interaction are described by, respectively, the asymmetry (ηQ) and the quadrupole coupling constant (CQ). The latter scales with the quadrupole moment of the nuclear spin. Together, CQ and ηQ parametrize the EFG, which contains structural, geometrical, and topological information about the nuclei’s surroundings, as they all reflect the charge distribution around the nuclear spin. With the continued advancements of higher magnetic fields, recently extending beyond 1 GHz for commercial devices, halide NMR spectroscopy of LHPs has a fruitful road ahead. For halide NMR to be feasible, the Zeeman interaction, which lifts the degeneracy of nuclear spin energies, must be at least of equal size as the quadrupole interaction. However, CQ constants reported for LHP are all quite large. Table 1 is a survey of known halide NQR and NMR signals of APbX3. The combination of the large quadrupole moments of the halide spins (Table 2) and the exceptionally large EFGs in LHPs leads to some of the largest CQ values reported for halide nuclei.310−312 Magnetic field strengths required to produce Larmor frequencies, ω0, that are comparable to or larger in size than halide CQ values in LHPs are at the limits of commercial spectrometers in the case of Cl and Br nuclei and are inaccessible for I nuclei. Therefore, NQR has been a method of choice for studying halide nuclear spin transitions (Figure 7). NQR is a zero-to-low-field sibling of NMR spectroscopy. In NQR, the EFG leads to the splitting of spin energy states, whose energy can be perturbed by a weak magnetic field, similarly to how NMR transitions are perturbed by the quadrupole interaction. A continuous transition from NQR to NMR is obtained with decreasing quadrupole coupling and by increasing the magnetic field strength (Figure 7). Theoretically, all of the material’s structure and dynamics aspects contained in the quadrupole interaction are equally accessible through NQR or NMR because the quadrupole parameters can be extracted either from the frequency of the NQR lines313−317 or from the width and the shape of NMR signals.318,319 Both kinds of nuclear resonance can be recorded on the same material; see, for instance, the chlorine and bromine NMR and NQR spectra of CsPbCl3 and CsPbBr3 in Figure 7. However, for very large quadrupole couplings, as in iodine-based LHPs, the presently available magnetic field strengths are low compared with the large CQ, and the acquisition of the complex and broad 127I NMR spectra (see the simulated spectrum in Figure 7) is impractical, whereas narrow-line 127I NQR spectra can be rapidly recorded (Figure 7). Finding the resonance frequency of the signal is far more difficult in NQR than in NMR, as a frequency range from a few hertz up to several hundreds of megahertz must be screened because the NQR resonances scale with the quadrupole coupling.
Figure 7.
Halide NMR versus NQR, exemplified for CsPbX3. Spins of I > 1/2 (I = 5/2 depicted example) possess a quadrupole moment, which interacts with the EFG generated by surrounding charges. This quadrupole interaction (HQ) can, depending on its strength relative to the Zeeman interaction (HZ), either perturb the NMR transitions (left part of the scheme) or, for strong quadrupole coupling strengths, be the dominant interaction. In the latter case, a perturbed NQR spectrum would be obtained, which would be further simplified by removing the magnetic field, producing a pure NQR spectrum, as shown on the right for CsPbBr3 and CsPbI3. For CsPbCl3, only the resonance frequencies (ν) are shown (at various temperatures, T). Reprinted with permission from ref (190). Copyright 1983 by John Wiley and Sons. For halide quadrupole couplings (CQ(35Cl, CsPbCl3) = 15.5 MHz, CQ(79Br, CsPbBr3) = 133.6, 136.4 MHz), which are smaller than the Larmor frequencies (ω0(35Cl) = −58.8 MHz @ 14.1 T and ω0(79Br) = 175.4 MHz @ 16.4 T inhere), halide NMR spectra can be recorded, but with involved experimental conditions. Iodine CQ values in LHPs are larger (ca. 500–600 MHz) than the 127I Larmor frequencies (e.g., 140.1 MHz @ 16.4 T). Hence 127I NMR is complex and too broad for practical acquisition. Only a simulation of the CsPbI3127I NMR spectrum at 16.4 T can be provided. Adapted with permission from ref (320). Copyright 2020 American Chemical Society.
Table 1. Summary of Reported Halide Quadrupole Parameters Obtained from Monohalide LHP NQR and NMR Spectra.
| compound | nucleus | NQR lines (MHz) | CQ (MHz) | ηQ | temperature | source |
|---|---|---|---|---|---|---|
| CsPbCl3 | 35Cl | 7.69–7.97 | 77–360 K | ref (190) | ||
| 15.48 (bulk) | 0 (bulk) | 100–273 K | ref (320) | |||
| 15.49–15.51 (NCs) | 0 (NCs) | 100–273 K | ref (320) | |||
| 37Cl | 7.66–7.89 | 77–360 K | ref (190) | |||
| MAPbCl3 | 35Cl | 8.128 | 298 K | ref (196) | ||
| CsPbBr3 | 79Br | 70.43, 67.155, 38.42, 37.11 | 77 K | ref (195) | ||
| 68.068, 66.781, 37.28 | 300 K | ref (195) | ||||
| 133.59, 136.36 | 0.006, 0.03 | RT | ref (320) | |||
| 81Br | 58.84, 56.14, 32.091, 30.975 | 77 K | ref (195) | |||
| 56.87, 55.782, 31.17 | 300 K | ref (195) | ||||
| MAPbBr3 | 79Br | 70.451 | 298 K | ref (196) | ||
| 69.701, 73.819 | 77.4 K | ref (196) | ||||
| 141.0185 | 0.0099 | RT | ref (320) | |||
| 81Br | 58.842 | 298 K | ref (196) | |||
| 58.239, 61.678 | 77.4 K | ref (196) | ||||
| FAPbBr3 | 79Br | 149.1034 | 0.0642 | RT | ref (320) | |
| 79Br | 74.6 | RT | ref (250) | |||
| 73.2, 74.4 | 160 K | ref (250) | ||||
| δ-CsPbI3 | 127I | 56.55, 94.84; 71.07, 126.10 | 325.4; 428.6 | 0.397; 0.319 | 77 K | ref (195) |
| 56.2, 92.3; 70.15, 125.2 | 317.9; 425.1 | 0.423; 0.311 | 300 K | ref (195) | ||
| 56.1744, 92.9890; 70.0794, 124.9071 | 319.7; 424.3 | 0.415; 0.311 | RT | ref (320) | ||
| γ-CsPbI3 | 127I | 517.98; 537.36.7 | 0.025; 0.101 | RT | ref (320) | |
| MAPbI3 | 127I | 83.430, 166.840; 82.062, 164.094 | 556.139; 573.063 | 0.010; 0.012 | 298 K | ref (196) |
| 85.973, 160.192; 84.895, 159.161 | 539.974; 536.132 | 0.241; 0.229 | 77.4 K | ref (196) | ||
| 82.057, 164.002; 83.449, 167.002 | –528.1; −558.6 | 0.29; 0.34 | 298 K | ref (194) | ||
| 83.430, 166.846; 82.073, 164.114 | 556.16; 547.06 | 0.00; 0.01 | 297.3 K | ref (197) | ||
| 82.13, 164.28 | 547.59 | 0.00 | 350.8 K | ref (197) | ||
| α-FAPbI3 | 127I | 87.294, 174.59; 85.205, 170.34 | 581.96; 567.84 | 0.00; 0.018 | 189.5 K | ref (197) |
| 86.61, 173.22 | 577.40 | 0.00 | 292.7 K | ref (197) | ||
| δ-FAPbI3 | 127I | 57.315, 87.935 | 306.50 | 0.508 | 300.0 K | ref (197) |
Table 2. NMR Properties of Quadrupolar Halide Nuclei.
| nucleus | spin321 | natural abundance (%)321 | gyromagnetic ratio γ (106 rad s–1 T–1)321 | quadrupole moment Q (e·fm–2)a 321 |
|---|---|---|---|---|
| 35Cl | 3/2 | 75.76 | 26.241991 | –8.165 |
| 37Cl | 3/2 | 24.24 | 21.843688 | –6.435 |
| 79Br | 3/2 | 50.69 | 67.25619 | 30.5 |
| 81Br | 3/2 | 49.31 | 72.49779 | 25.4 |
| 127I | 5/2 | 100 | 53.8957 | –71 |
e stands for the elementary charge 1.602·× 10–19 C, with which the tabulated value Q must be multiplied to obtain the quadrupole moment in C/fm2.
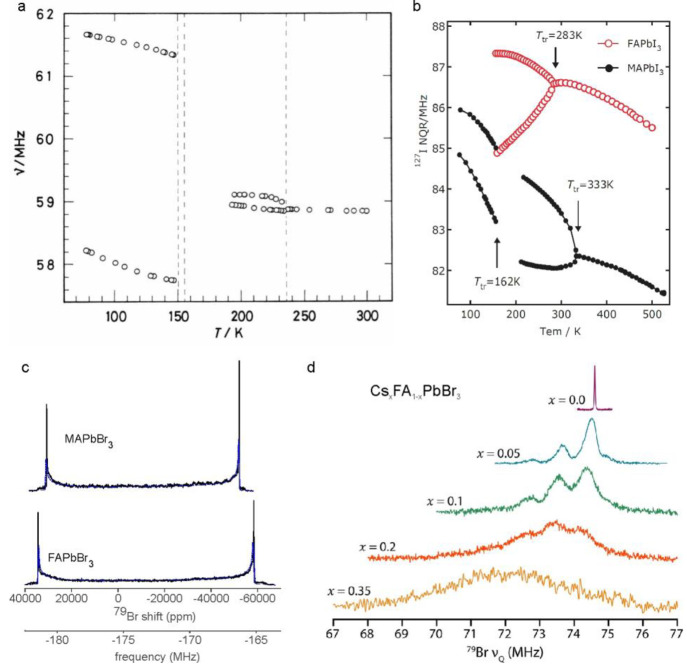
Unsurprisingly, halide NQR transitions in LHPs were investigated long before chloride and bromide NMR spectra, which were reported only recently.320 We have not found 127I NMR spectra attributable to LHPs in the literature and have not succeeded in obtaining them ourselves. The first halide NQR of CsPbX3 studies dates to the late 1960s and early 1970s,195,322,323 a decade after Møller reported the determination of a perovskite lattice for CsPbX3.324,32535Cl NQR was used to explore the phase transitions and structural properties of CsPbCl3.190,322,323,326 Possible crystal systems could be deduced from the number of NQR lines, their resonance frequencies, and their relaxation behavior. Furthermore, phase transitions and their order could be monitored and explained by the appearance of phonon vibrations. Some controversy existed about the temperatures of the phase transitions,327 possibly due to the various degrees of twinning in the CsPbCl3 structure258,259,261,262 and the influence of the thermal history of the sample on the spectroscopic results.323,326 Although fewer in number, similar 79/81Br NQR studies on CsPbBr3 were conducted in the past.195,205
After Weber reported the synthesis of MAPbX3 in 1978,328 the phase transitions in these materials were investigated using 35Cl, 79/81Br, and 127I NQR.196 Upon cooling, the signal from the single halide species in the cubic phase splits into two signals, corresponding to the inequivalent axial and equatorial halides of lead-halide octahedra in tetragonal and orthorhombic structures; see the 81Br NQR data of MAPbBr3 in Figure 8a. The advent of efficient perovskite photovoltaics also fueled the interest in MAPbI3 from the perspective of nuclear resonance studies. The recently reported 127I NQR spectra of MAPbI3194,197,219 correlated the line width to the powder quality219 as well as to the short-range dynamics (e.g., rotational/vibrational motion) of the iodine.194 The reported 127I quadrupole parameters of MAPbI3 vary in publications, but the NQR resonance frequencies are comparable (Table 1). Discrepancies could originate from slightly different experimental temperatures (Figure 8b), diverse synthetic procedures, and different approaches to determining CQ and ηQ.
Figure 8.
Halide NMR of organic–inorganic LHPs. (a) 81Br NQR frequencies of MAPbBr3 recorded at various temperatures, evidencing the continuous structural changes and sharp phase transitions (dashed lines). Between 150 and 180 K, the signals were too weak to be detected. Reprinted with permission from ref (196). Copyright 1991 De Gruyter. (b) Temperature dependence of low-frequency 127I NQR transitions of MAPbI3 and FAPbI3. Phase transitions are indicated by arrows. Upon cooling from the cubic (>333 K for MAPbI3 and >283 K for FAPbI3) to tetragonal crystal structure, the single 127I NQR signal splits into two signals with an intensity ratio of 2:1, corresponding to the unequal equatorial and axial iodine atoms. Reprinted with permission from ref (197). Copyright 2018 Chemical Society of Japan. (c) High magnetic field strengths (here 16.4 T) make the acquisition of bromine NMR spectra of LHP possible. 79Br NMR spectra of MAPbBr3 and FAPbBr3 are displayed, exhibiting the perfect axial symmetry of the bromine sites. Adapted with permission from ref (320). Copyright 2020 American Chemical Society. (d) 79Br NQR spectra of CsxFA1–xPbBr3 (x = 0.0 to 0.35). The three signals observed at low Cs concentrations were attributed to Br with zero, one, and two Cs atoms as nearest neighbors. Adapted with permission from ref (250). Copyright 2020 American Chemical Society.
Meanwhile, NQR spectra of other iodine-based LHPs are rare. Yamada et al. found the 127I NQR lines for two polymorphs of FAPbI3 (cubic 3D and nonperovskite 1D phases). They also observed a phase transition from the cubic to tetragonal perovskite lattice at 283 K,197 seen as the splitting of the single 127I NQR line (only the low-frequency one is shown in Figure 8b) of the cubic phase into two lines corresponding to the inequivalent iodine atoms in the tetragonal phase. A similar study by Volkov et al. reported the 127I NQR transitions and the corresponding quadrupole parameters of yellow nonperovskite δ-CsPbI3 at 77 and 300 K.195 Our group reproduced these findings recently and completed them with 127I NQR spectra of γ-CsPbI3 (Figure 7).320
The 35Cl NMR spectrum of CsPbCl3 and the 79Br NMR spectra of CsPbBr3, MAPbBr3, and FAPbBr3 are shown in Figures 7 and 8c. The large signal width requires the acquisition of multiple subspectra under static conditions with broadband excitation combined with the echo-train collection, as conventional signal averaging (e.g., MAS) brings no improvement. The acquisition of 127I NMR spectra is impractical, as can be seen from the simulated 127I NMR spectrum of orthorhombic γ-CsPbI3 at 16.4 T in Figure 7. The spectrum is >300 MHz broad and exhibits a complex shape, whose spectral features are not trivial to interpret.
Very recently, the impact of Cs incorporation on the inorganic lattice of FAPbBr3 was probed with 79Br NQR, showing signal broadening with increasing Cs content. For low Cs concentrations (up to 10%), the signal splits into three lines, which the authors assigned to Br with zero, one, and two Cs atoms as nearest neighbors (Figure 8d).250
6. Lead-Halide Perovskite Colloidal Nanocrystals and Thin Films
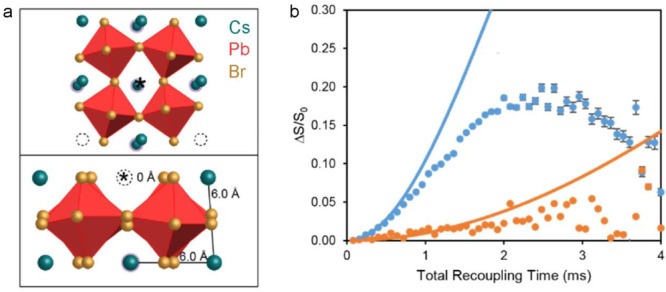
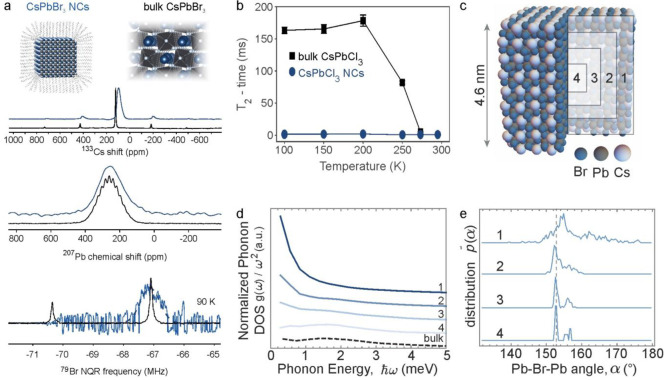
LHP NCs are the latest generation of colloidal inorganic semiconductors.69,329 Colloidal NCs comprise a crystalline core of typically 3–20 nm in diameter. When the NC size is comparable to or smaller than the Bohr diameter of a photogenerated electron–hole pair (exciton), the NC’s electronic structure is altered; in particular, the band-gap energy increases with decreasing NC size (quantum-size effect).40,330 There exists an interface between the inorganic crystalline core and the long-hydrocarbon-chain capping ligands anchored to the surface via a suitable binding group. These ligands ensure the structural integrity of NCs and impart colloidal stability in apolar solvents. The surface state is also of paramount importance for the optical and electronic characteristics of semiconductor NCs.331 Understanding and characterizing the NC surfaces has always been nontrivial. NMR spectroscopy is a rare analytical method that is (nearly) fully forgiving yet is highly sensitive to the degree and disruption of atomic order at the surface. For the investigation of the organic–inorganic interface, it is most desired to study the colloidal NCs in their native colloidal state. Ligands’ binding motifs and binding dynamics can be deduced from the line width and position of signals in 1D NMR spectra as well as from the information about internuclear distances and mobility from 2D NMR experiments (nuclear Overhauser effect spectroscopy (NOESY), diffusion ordered spectroscopy (DOSY), etc.). This suite of solution-state NMR methods for characterizing ligand shells has been well established for conventional colloidal semiconductor NCs332,333 and recently extended to LHP NCs.334−336 Also, solid-state NMR was used to probe the inorganic surface termination of CsPbBr3 NCs.337 In ref (337), the distance dependence of the dipolar coupling (Figure 2) was utilized to probe the outmost Cs and Pb atoms by performing 1H{133Cs} RESPDOR (resonance-echo saturation-pulse double-resonance)and 1H{207Pb} S-REDOR (symmetry-based resonance-echo double-resonance) experiments. These experiments probed the distance between the ammonium protons of the oleylammonium ligands and the closest 133Cs and 207Pb nuclei. The dipolar coupling leads to a decrease in the intensity of the oleylammonium proton signal with increasing duration of the total recoupling time (the time during which the dipolar coupling is reintroduced). Strong couplings lead to a fast decrease in the signal intensity, and hence the behavior of the signal intensity over different recoupling times contains information about the number of, and the distance to, neighboring heteronuclei. The experimental initial rates were compared with the simulated curves obtained for four possible surface terminations. From this comparison, it was concluded that the studied CsPbBr3 NCs exhibit Cs termination, and some of the surface Cs ions are replaced by oleylammonium ligands (Figure 9). In general, both the surface and the inorganic core of LHP NCs are addressable by NMR methods. The 207Pb NMR spectra of MAPbX3 NCs265,266 and CsPbBr3 NCs270,337 exhibit rather featureless signals at similar chemical shifts as their bulk counterparts (Figure 10a) but with much greater signal line width due to chemical and electronic disorder (inhomogeneous broadening). The 35Cl NMR spectra of CsPbCl3 NCs and the 79Br and 127I NQR lines of CsPbBr3 and CsPbI3 NCs are also closely matching those of the bulk counterparts in terms of the resonance frequencies and the spectral shape.320 However, the 79Br NQR lines of CsPbBr3 NCs are six times broader than those of the bulk due to inhomogeneous contributions. These NCs’ 79Br NQR signals are also much less intense, such that the higher frequency line could not be detected (Figure 10a). A characteristic difference between the bulk and the NCs is the accelerated spin–spin relaxation (T2) of halide nuclei in NCs.320 For instance, 35Cl NMR signals relax faster in CsPbCl3 NCs than in the bulk (Figure 10b), and the discrepancy between the T1 and T2 relaxation behaviors in the two sample morphologies is a strong indication of the accelerated structure dynamics in NCs, which was corroborated by AIMD calculations. These simulations indicated that surface and near-surface regions in NCs exhibit a much higher density of low-frequency phonon states (Figure 10c,d) and a larger distribution of lead-halide bond angles (Figure 10e). An alternative or complementary explanation to this anharmonic dynamics driven by low-energy modes could be the existence of a new pathway for ionic motion, for example, surface ionic mobility with extremely low activation energies, which could also account for the observed disparity in the T2 behavior between NCs and bulk LHPs. A further computational analysis involving AIMD and DFT calculations would be needed to explore this and other structural hypotheses. We expect that embarking on the combination of magnetic resonance methods and increasingly powerful atomistic computational tools for materials is a strategy that will be indispensable for rationalizing the observed T1 and T2 behaviors in diverse solid-state materials.
Figure 9.
NMR of the NC surface. (a) Structural models of the orthorhombic (010) CsPbBr3 surface. The position of an ammonium proton is indicated by an asterisk. Its simulated 1H{133Cs}/1H{207Pb} multispin RESPDOR/S-REDOR curves in panel b (blue/orange lines) match the best experimental data of oleylamine- and oleic-acid-terminated CsPbBr3 NCs. The curves correspond to the evolution of the 1H signal intensity, ΔS/S0 = (S – S0)/S0, where S is the signal at a given recoupling time and S0 is the initial signal intensity over the exposure time (total recoupling time) of the 1H spins to the dipolar coupling with 133Cs/207Pb nuclei, respectively. Adapted with permission from ref (337). Copyright 2020 American Chemical Society.
Figure 10.
Structure disorder and dynamics in LHP NCs. (a) Both in bulk (black) and in NCs (blue), all atoms are accessible with magnetic resonance, as exemplified by CsPbBr3 (A-cation: 133Cs NMR spectra, unpublished; Pb(II) cation;270 halides: 79Br NQR spectra at 90 K).320 (b) Temperature-dependent 35Cl NMR T2 relaxation times of bulk and nanocrystalline CsPbCl3. (c) Regions 1–4 within a CsPbBr3 NCs. (d) Plot of the partial phonon density of states in each of the four NC regions, normalized to the phonon frequency squared to accentuate the low-energy region. (e) Plots of the statistical distribution of Pb–Cl–Pb angles, α, within each NC region. Adapted from ref (270) (Copyright 2020 Springer Nature) and ref (320) (Copyright 2020 American Chemical Society).
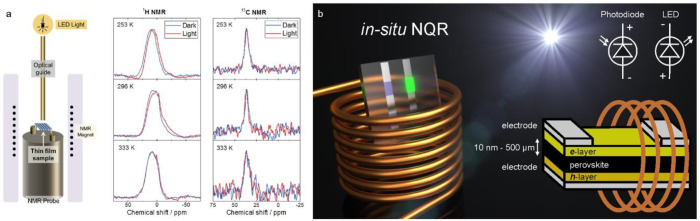
Thin films of LHPs find use in LEDs (thicknesses down to few nanometers),14 photodetectors, and solar cells (hundreds of nanometers)5,6 as well as in lasers.17,338,339 Thicker films of up to and above 50 μm are required in X-ray and gamma detectors.29 These films may greatly vary in their crystallinity41,340 Furthermore, such films can be fully planar or may conformally coat diverse objects and shapes, such as in the case of LHP-coated microbeads for lasing.341 Arguably, it is most desired to characterize such forms of LHPs intact, without, for instance, scraping the material of the film into a powder. Any such sample preparation is invasive by nature, especially for such soft materials as LHPs. There exist scarce examples of NMR measurements on thin films of LHPs.192,215,342 Senocrate et al. have investigated the effect of light absorption on MAPbI3 thin films by illuminating them in situ during the acquisition of NMR signals from MA cations (Figure 11a). They found no apparent difference between dark and illuminated samples, which corroborates the absence of the anticipated translational motion of MA cations.223 As discussed previously, the detection of 207Pb NMR LHP signals is time-demanding, and small sample quantities exacerbate the sensitivity issue. The sensitivity-enhancement experiments, however, have showcased the feasibility of the direct acquisition of the 207Pb NMR signal in thin films.265 As for halide nuclei, there have been no reports on NMR (or NQR) nuclear halide signals from LHP thin films. To this end, we would like to emphasize the practical feasibility of halide NQR studies of LHP thin films due to the high sensitivity of 79/81Br and 127I NQR. As an illustration, extrapolating from the 127I NQR of the α-FAPbI3 bulk signal, a thin film with a thickness of 10 nm and an area of 1 cm2 (ca. 4 μg) should yield a detectable signal within several minutes (ca. 10 min for S/N > 3). The experimental simplicity and the low costs of the NQR setup as well as its noninvasive nature predispose the NQR for the direct probing of LHPs incorporated within the devices, possibly even in situ during device operation or upon external stimuli (Figure 11b). Most electron- or hole-conducting, metallic, or other layers will not affect the NQR experiment, and integrating simultaneous irradiation or optical spectroscopy is easier to implement compared with NMR spectroscopy. Such direct application of the NQR of devices eliminates the sample-altering manipulations for the removal of perovskite layers from the devices
Figure 11.
Magnetic resonance of LHP thin films and devices. (a) In situ NMR setup and 1H and 13C NMR in situ spectra of a 13C-enriched MAPbI3 thin film at various temperatures under irradiation (light) and under identical conditions in the dark. Adapted with permission from ref (223). Copyright 2018 American Chemical Society. (b) We outline NQR as a highly suitable method for acquiring spectra from LHP optoelectronic devices, including in situ and in operando measurements. “e-layer” and “h-layer” denote electron- and hole-conducting or injecting layer(s), respectively. The device structure may employ electron- and hole-blocking layers. Electrodes are commonly metallic or transparent conductive oxides of a suitable workfunction for injecting electrons and holes. Credit: Yevhen Shynkarenko.
7. Concluding Remarks
LHPs and other metal halides comprise chemical elements, most of which are easily accessible with magnetic resonance methods, and element specificity reduces the interference from other materials present in the sample. Magnetic resonance methods are applicable to crystalline and amorphous materials, including large single crystals, NCs, thin films, and so on. NMR and NQR probe both static and dynamic structural disorder, peculiarities of chemical bonding, and electronic effects. Highly porous materials, surfaces, and interfaces can be discriminatively probed with suitable adaptations of the methods. On top of reduced/disrupted crystallinity, as in LHP NCs and polycrystalline thin films, anomalous distributions and gradients of elements may emerge as well. NC surfaces and surface-bound ligands are structurally distinct regions, too. These effects are all within the reach of NMR and NQR techniques.
The strong quadrupole interaction imparts further potential to probe the material’s structure with accuracies comparable to those of synchrotron X-ray diffraction. For instance, the expansion of the crystal unit cell of CsPbBr3 by just 0.001 Å produces, according to DFT calculations, a change of tens of kilohertz in the 79Br CQ.320 The effect on the 127I CQ values will be even more extreme due to the larger quadrupole moment of iodine nuclei (Table 2).
NQR spectra, while containing similar information on the structure and dynamics, can be easier to acquire for nuclei with stronger quadrupole coupling, such as those of halides. NQR also has a very useful practical twist—it does not require high-field magnets. Hence NQR can be more easily adapted for in situ and in operando studies. It does not require special sample preparation or separation from other components (e.g., conducting or protective layers). For instance, 14N and 35Cl NQR are commonplace for drug, landmine, and explosive detection343 as well as for chemical quality and quantity control in the pharmaceutical industry.344−346 In both cases, opening of containers or moving of the sample is either not desired or not possible (too heavy, consecutive degradation, explosion, destruction, etc.), a problem that is also faced with thin-film LHP devices. Hence, NQR spectroscopy has the potential to become a highly sensitive fingerprint quality-control tool for LHP devices or may give unique insight into the LHP material structure in relation to the operation conditions or the device fabrication methods.
Acknowledgments
M.K. acknowledges financial support from the European Union through the FP7 (ERC Starting Grant NANOSOLID, grant agreement no. [306733]) and through Horizon 2020 (ERC Consolidator Grant SCALE-HALO grant agreement no. [819740]). L.P. acknowledges financial support from the Scholarship Fund of the Swiss Chemical Industry (SSCI Award 2015) and from the Swiss National Science Foundation (Early Postdoc Mobility scholarship, P2EYP2_188002). Marcel Aebli is acknowledged for fruitful discussions. Kyle McCall is acknowledged for reading the manuscript.
Author Contributions
L.P., V.M., and M.K. prepared the manuscript.
The authors declare no competing financial interest.
References
- Pazos-Outón L. M.; Szumilo M.; Lamboll R.; Richter J. M.; Crespo-Quesada M.; Abdi-Jalebi M.; Beeson H. J.; Vrućinić M.; Alsari M.; Snaith H. J.; Ehrler B.; Friend R. H.; Deschler F. Photon recycling in lead iodide perovskite solar cells. Science 2016, 351 (6280), 1430–1433. 10.1126/science.aaf1168. [DOI] [PubMed] [Google Scholar]
- Xing G.; Mathews N.; Sun S.; Lim S. S.; Lam Y. M.; Grätzel M.; Mhaisalkar S.; Sum T. C. Long-Range Balanced Electron- and Hole-Transport Lengths in Organic-Inorganic CH3NH3PbI3. Science 2013, 342 (6156), 344. 10.1126/science.1243167. [DOI] [PubMed] [Google Scholar]
- Shi D.; Adinolfi V.; Comin R.; Yuan M.; Alarousu E.; Buin A.; Chen Y.; Hoogland S.; Rothenberger A.; Katsiev K.; Losovyj Y.; Zhang X.; Dowben P. A.; Mohammed O. F.; Sargent E. H.; Bakr O. M. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347 (6221), 519–522. 10.1126/science.aaa2725. [DOI] [PubMed] [Google Scholar]
- Kovalenko M. V.; Protesescu L.; Bodnarchuk M. I. Properties and Potential Optoelectronic Applications of Lead Halide Perovskite Nanocrystals. Science 2017, 358 (6364), 745–750. 10.1126/science.aam7093. [DOI] [PubMed] [Google Scholar]
- Kim H.-S.; Lee C.-R.; Im J.-H.; Lee K.-B.; Moehl T.; Marchioro A.; Moon S.-J.; Humphry-Baker R.; Yum J.-H.; Moser J. E.; Grätzel M.; Park N.-G. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2 (1), 591. 10.1038/srep00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. M.; Teuscher J.; Miyasaka T.; Murakami T. N.; Snaith H. J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338 (6107), 643–647. 10.1126/science.1228604. [DOI] [PubMed] [Google Scholar]
- Kojima A.; Teshima K.; Shirai Y.; Miyasaka T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131 (17), 6050–6051. 10.1021/ja809598r. [DOI] [PubMed] [Google Scholar]
- Saliba M.; Matsui T.; Seo J.-Y.; Domanski K.; Correa-Baena J.-P.; Nazeeruddin M. K.; Zakeeruddin S. M.; Tress W.; Abate A.; Hagfeldt A.; Grätzel M. Cesium-Containing Triple Cation Perovskite Solar Cells: Improved Stability, Reproducibility and High Efficiency. Energy Environ. Sci. 2016, 9 (6), 1989–1997. 10.1039/C5EE03874J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z.; Jayatissa A. H. Perovskites-Based Solar Cells: A Review of Recent Progress, Materials and Processing Methods. Materials 2018, 11 (5), 729. 10.3390/ma11050729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NREL , Best Research-Cell Efficiencies, 2020. https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.20200406.pdf.
- Yoon H. C.; Lee H.; Kang H.; Oh J. H.; Do Y. R. Highly efficient wide-color-gamut QD-emissive LCD□s using red and green perovskite core/shell QDs. J. Mater. Chem. C 2018, 6 (47), 13023–13033. 10.1039/C8TC04537B. [DOI] [Google Scholar]
- Chen N.; Bai Z.; Wang Z.; Ji H.; Liu R.; Cao C.; Wang H.; Jiang F.; Zhong H. P-119: Low Cost Perovskite Quantum Dots Film Based Wide Color Gamut Backlight Unit for LCD TVs. Dig. Tech. Pap. - Soc. Inf. Disp. Int. Symp. 2018, 49 (1), 1657–1659. 10.1002/sdtp.12303. [DOI] [Google Scholar]
- He Z.; Zhang C.; Dong Y.; Wu S.-T. Emerging Perovskite Nanocrystals-Enhanced Solid-State Lighting and Liquid-Crystal Displays. Crystals 2019, 9, 59. 10.3390/cryst9020059. [DOI] [Google Scholar]
- Wang Q.; Wang X.; Yang Z.; Zhou N.; Deng Y.; Zhao J.; Xiao X.; Rudd P.; Moran A.; Yan Y.; Huang J. Efficient sky-blue perovskite light-emitting diodes via photoluminescence enhancement. Nat. Commun. 2019, 10 (1), 5633. 10.1038/s41467-019-13580-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumawat N. K.; Liu X.-K.; Kabra D.; Gao F. Blue perovskite light-emitting diodes: progress, challenges and future directions. Nanoscale 2019, 11 (5), 2109–2120. 10.1039/C8NR09885A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W.; Hu Q.; Bai S.; Bao C.; Miao Y.; Yuan Z.; Borzda T.; Barker A. J.; Tyukalova E.; Hu Z.; Kawecki M.; Wang H.; Yan Z.; Liu X.; Shi X.; Uvdal K.; Fahlman M.; Zhang W.; Duchamp M.; Liu J.-M.; Petrozza A.; Wang J.; Liu L.-M.; Huang W.; Gao F. Rational molecular passivation for high-performance perovskite light-emitting diodes. Nat. Photonics 2019, 13 (6), 418–424. 10.1038/s41566-019-0390-x. [DOI] [Google Scholar]
- Jia Y.; Kerner R. A.; Grede A. J.; Rand B. P.; Giebink N. C. Continuous-wave lasing in an organic–inorganic lead halide perovskite semiconductor. Nat. Photonics 2017, 11 (12), 784–788. 10.1038/s41566-017-0047-6. [DOI] [Google Scholar]
- Xing G.; Mathews N.; Lim S. S.; Yantara N.; Liu X.; Sabba D.; Grätzel M.; Mhaisalkar S.; Sum T. C. Low-Temperature Solution-Processed Wavelength-Tunable Perovskites for Lasing. Nat. Mater. 2014, 13 (5), 476–480. 10.1038/nmat3911. [DOI] [PubMed] [Google Scholar]
- Zhu H.; Fu Y.; Meng F.; Wu X.; Gong Z.; Ding Q.; Gustafsson M. V.; Trinh M. T.; Jin S.; Zhu X. Y. Lead Halide Perovskite Nanowire Lasers with Low Lasing Thresholds and High Quality Factors. Nat. Mater. 2015, 14 (6), 636–642. 10.1038/nmat4271. [DOI] [PubMed] [Google Scholar]
- Yakunin S.; Protesescu L.; Krieg F.; Bodnarchuk M. I.; Nedelcu G.; Humer M.; De Luca G.; Fiebig M.; Heiss W.; Kovalenko M. V. Low-Threshold Amplified Spontaneous Emission and Lasing from Colloidal Nanocrystals of Caesium Lead Halide Perovskites. Nat. Commun. 2015, 6 (1), 8056. 10.1038/ncomms9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou L.; Yang Y.; You J.; Hong Z.; Chang W.-H.; Li G.; Yang Y. Solution-Processed Hybrid Perovskite Photodetectors with High Detectivity. Nat. Commun. 2014, 5 (1), 5404. 10.1038/ncomms6404. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Zhang F.; Wang Y.; Zhu L.; Hu Y.; Lou Z.; Hou Y.; Teng F. High-Performance Photodiode-Type Photodetectors Based on Polycrystalline Formamidinium Lead Iodide Perovskite Thin Films. Sci. Rep. 2018, 8 (1), 11157. 10.1038/s41598-018-29147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidaminov M. I.; Adinolfi V.; Comin R.; Abdelhady A. L.; Peng W.; Dursun I.; Yuan M.; Hoogland S.; Sargent E. H.; Bakr O. M. Planar-Integrated Single-Crystalline Perovskite Photodetectors. Nat. Commun. 2015, 6 (1), 8724. 10.1038/ncomms9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y.; Dong Q.; Shao Y.; Yuan Y.; Huang J. Highly Narrowband Perovskite Single-Crystal Photodetectors Enabled by Surface-Charge Recombination. Nat. Photonics 2015, 9 (10), 679–686. 10.1038/nphoton.2015.156. [DOI] [Google Scholar]
- Wang X.; Li M.; Zhang B.; Wang H.; Zhao Y.; Wang B. Recent Progress in Organometal Halide Perovskite Photodetectors. Org. Electron. 2018, 52, 172–183. 10.1016/j.orgel.2017.10.027. [DOI] [Google Scholar]
- Ahmadi M.; Wu T.; Hu B. A Review on Organic–Inorganic Halide Perovskite Photodetectors: Device Engineering and Fundamental Physics. Adv. Mater. 2017, 29 (41), 1605242. 10.1002/adma.201605242. [DOI] [PubMed] [Google Scholar]
- Feng J.; Gong C.; Gao H.; Wen W.; Gong Y.; Jiang X.; Zhang B.; Wu Y.; Wu Y.; Fu H.; Jiang L.; Zhang X. Single-Crystalline Layered Metal-Halide Perovskite Nanowires for Ultrasensitive Photodetectors. Nat. Electron. 2018, 1 (7), 404–410. 10.1038/s41928-018-0101-5. [DOI] [Google Scholar]
- García de Arquer F. P.; Armin A.; Meredith P.; Sargent E. H. Solution-processed semiconductors for next-generation photodetectors. Nat. Rev. Mater. 2017, 2 (3), 16100. 10.1038/natrevmats.2016.100. [DOI] [Google Scholar]
- Wei H.; Huang J. Halide lead perovskites for ionizing radiation detection. Nat. Commun. 2019, 10 (1), 1066. 10.1038/s41467-019-08981-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. C.; Kim K. H.; Son D.-Y.; Jeong D.-N.; Seo J.-Y.; Choi Y. S.; Han I. T.; Lee S. Y.; Park N.-G. Printable Organometallic Perovskite Enables Large-Area, Low-Dose X-ray Imaging. Nature 2017, 550 (7674), 87–91. 10.1038/nature24032. [DOI] [PubMed] [Google Scholar]
- He Y.; Matei L.; Jung H. J.; McCall K. M.; Chen M.; Stoumpos C. C.; Liu Z.; Peters J. A.; Chung D. Y.; Wessels B. W.; Wasielewski M. R.; Dravid V. P.; Burger A.; Kanatzidis M. G. High spectral resolution of gamma-rays at room temperature by perovskite CsPbBr3 single crystals. Nat. Commun. 2018, 9 (1), 1609. 10.1038/s41467-018-04073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q.; Fang Y.; Shao Y.; Mulligan P.; Qiu J.; Cao L.; Huang J. Electron-Hole Diffusion Lengths > 175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347 (6225), 967–970. 10.1126/science.aaa5760. [DOI] [PubMed] [Google Scholar]
- Yakunin S.; Dirin D. N.; Protesescu L.; Sytnyk M.; Tollabimazraehno S.; Humer M.; Hackl F.; Fromherz T.; Bodnarchuk M. I.; Kovalenko M. V.; Heiss W. High Infrared Photoconductivity in Films of Arsenic-Sulfide-Encapsulated Lead-Sulfide Nanocrystals. ACS Nano 2014, 8 (12), 12883–12894. 10.1021/nn5067478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H.; DeSantis D.; Wei W.; Deng Y.; Guo D.; Savenije T. J.; Cao L.; Huang J. Dopant Compensation in Alloyed CH3NH3PbBr3–xClx Perovskite Single Crystals for Gamma-Ray Spectroscopy. Nat. Mater. 2017, 16 (8), 826–833. 10.1038/nmat4927. [DOI] [PubMed] [Google Scholar]
- Yakunin S.; Dirin D. N.; Shynkarenko Y.; Morad V.; Cherniukh I.; Nazarenko O.; Kreil D.; Nauser T.; Kovalenko M. V. Detection of gamma photons using solution-grown single crystals of hybrid lead halide perovskites. Nat. Photonics 2016, 10 (9), 585–589. 10.1038/nphoton.2016.139. [DOI] [Google Scholar]
- Dirin D. N.; Cherniukh I.; Yakunin S.; Shynkarenko Y.; Kovalenko M. V. Solution-Grown CsPbBr3 Perovskite Single Crystals for Photon Detection. Chem. Mater. 2016, 28 (23), 8470–8474. 10.1021/acs.chemmater.6b04298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birowosuto M. D.; Cortecchia D.; Drozdowski W.; Brylew K.; Lachmanski W.; Bruno A.; Soci C. X-Ray Scintillation in Lead Halide Perovskite Crystals. Sci. Rep. 2016, 6 (1), 37254. 10.1038/srep37254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q.; Wu J.; Ou X.; Huang B.; Almutlaq J.; Zhumekenov A. A.; Guan X.; Han S.; Liang L.; Yi Z.; Li J.; Xie X.; Wang Y.; Li Y.; Fan D.; Teh D. B. L.; All A. H.; Mohammed O. F.; Bakr O. M.; Wu T.; Bettinelli M.; Yang H.; Huang W.; Liu X. All-inorganic perovskite nanocrystal scintillators. Nature 2018, 561 (7721), 88–93. 10.1038/s41586-018-0451-1. [DOI] [PubMed] [Google Scholar]
- Utzat H.; Sun W.; Kaplan A. E. K.; Krieg F.; Ginterseder M.; Spokoyny B.; Klein N. D.; Shulenberger K. E.; Perkinson C. F.; Kovalenko M. V.; Bawendi M. G. Coherent single-photon emission from colloidal lead halide perovskite quantum dots. Science 2019, 363 (6431), 1068. 10.1126/science.aau7392. [DOI] [PubMed] [Google Scholar]
- Rainò G.; Becker M. A.; Bodnarchuk M. I.; Mahrt R. F.; Kovalenko M. V.; Stöferle T. Superfluorescence from lead halide perovskite quantum dot superlattices. Nature 2018, 563 (7733), 671–675. 10.1038/s41586-018-0683-0. [DOI] [PubMed] [Google Scholar]
- Matt G. J.; Levchuk I.; Knüttel J.; Dallmann J.; Osvet A.; Sytnyk M.; Tang X.; Elia J.; Hock R.; Heiss W.; Brabec C. J. Sensitive Direct Converting X-Ray Detectors Utilizing Crystalline CsPbBr3 Perovskite Films Fabricated via Scalable Melt Processing. Adv. Mater. Interfaces 2020, 7 (4), 1901575. 10.1002/admi.201901575. [DOI] [Google Scholar]
- Pan W.; Yang B.; Niu G.; Xue K.-H.; Du X.; Yin L.; Zhang M.; Wu H.; Miao X.-S.; Tang J. Hot-Pressed CsPbBr3 Quasi-Monocrystalline Film for Sensitive Direct X-ray Detection. Adv. Mater. 2019, 31 (44), 1904405. 10.1002/adma.201904405. [DOI] [PubMed] [Google Scholar]
- Zhang P.; Sun Q.; Xu Y.; Li X.; Liu L.; Zhang G.; Tao X. Enhancing Carrier Transport Properties of Melt-grown CsPbBr3 Single Crystals by Eliminating Inclusions. Cryst. Growth Des. 2020, 20 (4), 2424–2431. 10.1021/acs.cgd.9b01616. [DOI] [Google Scholar]
- Jiang J.; Sun X.; Chen X.; Wang B.; Chen Z.; Hu Y.; Guo Y.; Zhang L.; Ma Y.; Gao L.; Zheng F.; Jin L.; Chen M.; Ma Z.; Zhou Y.; Padture N. P.; Beach K.; Terrones H.; Shi Y.; Gall D.; Lu T.-M.; Wertz E.; Feng J.; Shi J. Carrier lifetime enhancement in halide perovskite via remote epitaxy. Nat. Commun. 2019, 10 (1), 4145. 10.1038/s41467-019-12056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz L. M. Charge-Carrier Mobilities in Metal Halide Perovskites: Fundamental Mechanisms and Limits. ACS Energy Lett. 2017, 2 (7), 1539–1548. 10.1021/acsenergylett.7b00276. [DOI] [Google Scholar]
- Zhang Z.; Yang G. Recent advancements in using perovskite single crystals for gamma-ray detection. J. Mater. Sci.: Mater. Electron. 2020, 10.1007/s10854-020-03519-z. [DOI] [Google Scholar]
- Akkerman Q. A.; Rainò G.; Kovalenko M. V.; Manna L. Genesis, Challenges and Opportunities for Colloidal Lead Halide Perovskite Nanocrystals. Nat. Mater. 2018, 17 (5), 394–405. 10.1038/s41563-018-0018-4. [DOI] [PubMed] [Google Scholar]
- Huang H.; Bodnarchuk M. I.; Kershaw S. V.; Kovalenko M. V.; Rogach A. L. Lead Halide Perovskite Nanocrystals in the Research Spotlight: Stability and Defect Tolerance. ACS Energy Lett. 2017, 2 (9), 2071–2083. 10.1021/acsenergylett.7b00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.; Wang Z.; Deschler F.; Gao S.; Friend R. H.; Cheetham A. K. Chemically Diverse and Multifunctional Hybrid Organic–Inorganic Perovskites. Nat. Rev. Mater. 2017, 2 (3), 16099. 10.1038/natrevmats.2016.99. [DOI] [Google Scholar]
- Ball J. M.; Petrozza A. Defects in Perovskite-Halides and their Effects in Solar Cells. Nat. Energy 2016, 1 (11), 16149. 10.1038/nenergy.2016.149. [DOI] [Google Scholar]
- Wang F.; Bai S.; Tress W.; Hagfeldt A.; Gao F. Defects Engineering for High-Performance Perovskite Solar Cells. npj Flex. Electron. 2018, 2 (1), 22. 10.1038/s41528-018-0035-z. [DOI] [Google Scholar]
- Huang J.; Yuan Y.; Shao Y.; Yan Y. Understanding the Physical Properties of Hybrid Perovskites for Photovoltaic Applications. Nat. Rev. Mater. 2017, 2 (7), 17042. 10.1038/natrevmats.2017.42. [DOI] [Google Scholar]
- Takahashi T.; Watanabe S. Recent progress in CdTe and CdZnTe detectors. IEEE Trans. Nucl. Sci. 2001, 48 (4), 950–959. 10.1109/23.958705. [DOI] [Google Scholar]
- Zhang H.; Wang F.; Lu Y.; Sun Q.; Xu Y.; Zhang B.-B.; Jie W.; Kanatzidis M. G. High-sensitivity X-ray detectors based on solution-grown caesium lead bromide single crystals. J. Mater. Chem. C 2020, 8 (4), 1248–1256. 10.1039/C9TC05490A. [DOI] [Google Scholar]
- Sutherland B. R.; Johnston A. K.; Ip A. H.; Xu J.; Adinolfi V.; Kanjanaboos P.; Sargent E. H. Sensitive, Fast, and Stable Perovskite Photodetectors Exploiting Interface Engineering. ACS Photonics 2015, 2 (8), 1117–1123. 10.1021/acsphotonics.5b00164. [DOI] [Google Scholar]
- Zhao Y.; Li C.; Shen L. Recent advances on organic-inorganic hybrid perovskite photodetectors with fast response. InfoMat 2019, 1 (2), 164–182. 10.1002/inf2.12010. [DOI] [Google Scholar]
- Green M. A.; Dunlop E. D.; Hohl-Ebinger J.; Yoshita M.; Kopidakis N.; Ho-Baillie A. W. Y. Solar cell efficiency tables (Version 55). Prog. Photovoltaics 2020, 28 (1), 3–15. 10.1002/pip.3228. [DOI] [Google Scholar]
- Hollemann C.; Haase F.; Rienäcker M.; Barnscheidt V.; Krügener J.; Folchert N.; Brendel R.; Richter S.; Großer S.; Sauter E.; Hübner J.; Oestreich M.; Peibst R. Separating the two polarities of the POLO contacts of an 26.1%-efficient IBC solar cell. Sci. Rep. 2020, 10 (1), 658. 10.1038/s41598-019-57310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M. A.; Scarpelli L.; Nedelcu G.; Rainò G.; Masia F.; Borri P.; Stöferle T.; Kovalenko M. V.; Langbein W.; Mahrt R. F. Long Exciton Dephasing Time and Coherent Phonon Coupling in CsPbBr2Cl Perovskite Nanocrystals. Nano Lett. 2018, 18 (12), 7546–7551. 10.1021/acs.nanolett.8b03027. [DOI] [PubMed] [Google Scholar]
- Rainò G.; Nedelcu G.; Protesescu L.; Bodnarchuk M. I.; Kovalenko M. V.; Mahrt R. F.; Stöferle T. Single Cesium Lead Halide Perovskite Nanocrystals at Low Temperature: Fast Single-Photon Emission, Reduced Blinking, and Exciton Fine Structure. ACS Nano 2016, 10 (2), 2485–2490. 10.1021/acsnano.5b07328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamarat P.; Bodnarchuk M. I.; Trebbia J.-B.; Erni R.; Kovalenko M. V.; Even J.; Lounis B. The ground exciton state of formamidinium lead bromide perovskite nanocrystals is a singlet dark state. Nat. Mater. 2019, 18 (7), 717–724. 10.1038/s41563-019-0364-x. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Ng J. D. A.; Friend R. H.; Tan Z.-K. Opportunities and challenges in perovskite light-emitting devices. ACS Photonics 2018, 5 (10), 3866–3875. 10.1021/acsphotonics.8b00745. [DOI] [Google Scholar]
- Imran M.; Caligiuri V.; Wang M.; Goldoni L.; Prato M.; Krahne R.; De Trizio L.; Manna L. Benzoyl Halides as Alternative Precursors for the Colloidal Synthesis of Lead-Based Halide Perovskite Nanocrystals. J. Am. Chem. Soc. 2018, 140 (7), 2656–2664. 10.1021/jacs.7b13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levchuk I.; Osvet A.; Tang X.; Brandl M.; Perea J. D.; Hoegl F.; Matt G. J.; Hock R.; Batentschuk M.; Brabec C. J. Brightly Luminescent and Color-Tunable Formamidinium Lead Halide Perovskite FAPbX3 (X = Cl, Br, I) Colloidal Nanocrystals. Nano Lett. 2017, 17 (5), 2765–2770. 10.1021/acs.nanolett.6b04781. [DOI] [PubMed] [Google Scholar]
- Chen C.; Hu X.; Lu W.; Chang S.; Shi L.; Li L.; Zhong H.; Han J.-B. Elucidating the phase transitions and temperature-dependent photoluminescence of MAPbBr3 single crystal. J. Phys. D: Appl. Phys. 2018, 51 (4), 045105 10.1088/1361-6463/aaa0ed. [DOI] [Google Scholar]
- Whitfield P. S.; Herron N.; Guise W. E.; Page K.; Cheng Y. Q.; Milas I.; Crawford M. K. Structures, Phase Transitions and Tricritical Behavior of the Hybrid Perovskite Methyl Ammonium Lead Iodide. Sci. Rep. 2016, 6 (1), 35685. 10.1038/srep35685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu S.; Harada J.; Iizumi M.; Gesi K. Structural Phase Transitions in CsPbBr3. J. Phys. Soc. Jpn. 1974, 37 (5), 1393–1398. 10.1143/JPSJ.37.1393. [DOI] [Google Scholar]
- Schueller E. C.; Laurita G.; Fabini D. H.; Stoumpos C. C.; Kanatzidis M. G.; Seshadri R. Crystal Structure Evolution and Notable Thermal Expansion in Hybrid Perovskites Formamidinium Tin Iodide and Formamidinium Lead Bromide. Inorg. Chem. 2018, 57 (2), 695–701. 10.1021/acs.inorgchem.7b02576. [DOI] [PubMed] [Google Scholar]
- Protesescu L.; Yakunin S.; Bodnarchuk M. I.; Bertolotti F.; Masciocchi N.; Guagliardi A.; Kovalenko M. V. Monodisperse Formamidinium Lead Bromide Nanocrystals with Bright and Stable Green Photoluminescence. J. Am. Chem. Soc. 2016, 138 (43), 14202–14205. 10.1021/jacs.6b08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y.; Hoshino S.; Yamada Y.; Shirane G. Neutron-Scattering Study on Phase Transitions of CsPbCl3. Phys. Rev. B 1974, 9 (10), 4549–4559. 10.1103/PhysRevB.9.4549. [DOI] [Google Scholar]
- Binek A.; Hanusch F. C.; Docampo P.; Bein T. Stabilization of the Trigonal High-Temperature Phase of Formamidinium Lead Iodide. J. Phys. Chem. Lett. 2015, 6 (7), 1249–1253. 10.1021/acs.jpclett.5b00380. [DOI] [PubMed] [Google Scholar]
- Weber O. J.; Ghosh D.; Gaines S.; Henry P. F.; Walker A. B.; Islam M. S.; Weller M. T. Phase behavior and polymorphism of formamidinium lead iodide. Chem. Mater. 2018, 30 (11), 3768–3778. 10.1021/acs.chemmater.8b00862. [DOI] [Google Scholar]
- Poglitsch A.; Weber D. Dynamic Disorder in Methylammoniumtrihalogenoplumbates (II) observed by Millimeter-Wave Spectroscopy. J. Chem. Phys. 1987, 87 (11), 6373–6378. 10.1063/1.453467. [DOI] [Google Scholar]
- Chi L.; Swainson I.; Cranswick L.; Her J.-H.; Stephens P.; Knop O. The ordered phase of methylammonium lead chloride CH3ND3PbCl3. J. Solid State Chem. 2005, 178 (5), 1376–1385. 10.1016/j.jssc.2004.12.037. [DOI] [Google Scholar]
- Sutton R. J.; Filip M. R.; Haghighirad A. A.; Sakai N.; Wenger B.; Giustino F.; Snaith H. J. Cubic or Orthorhombic? Revealing the Crystal Structure of Metastable Black-Phase CsPbI3 by Theory and Experiment. ACS Energy Lett. 2018, 3 (8), 1787–1794. 10.1021/acsenergylett.8b00672. [DOI] [Google Scholar]
- Stoumpos C. C.; Malliakas C. D.; Kanatzidis M. G. Semiconducting Tin and Lead Iodide Perovskites with Organic Cations: Phase Transitions, High Mobilities, and Near-Infrared Photoluminescent Properties. Inorg. Chem. 2013, 52 (15), 9019–9038. 10.1021/ic401215x. [DOI] [PubMed] [Google Scholar]
- Goldschmidt V. M. Die Gesetze der Krystallochemie. Naturwissenschaften 1926, 14 (21), 477–485. 10.1007/BF01507527. [DOI] [Google Scholar]
- Kieslich G.; Sun S.; Cheetham A. K. An Extended Tolerance Factor Approach for Organic–Inorganic Perovskites. Chem. Sci. 2015, 6 (6), 3430–3433. 10.1039/C5SC00961H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieslich G.; Sun S.; Cheetham A. K. Solid-State Principles Applied to Organic–Inorganic Perovskites: New Tricks for an Old Dog. Chem. Sci. 2014, 5 (12), 4712–4715. 10.1039/C4SC02211D. [DOI] [Google Scholar]
- Li Z.; Yang M.; Park J.-S.; Wei S.-H.; Berry J. J.; Zhu K. Stabilizing Perovskite Structures by Tuning Tolerance Factor: Formation of Formamidinium and Cesium Lead Iodide Solid-State Alloys. Chem. Mater. 2016, 28 (1), 284–292. 10.1021/acs.chemmater.5b04107. [DOI] [Google Scholar]
- Travis W.; Glover E. N. K.; Bronstein H.; Scanlon D. O.; Palgrave R. G. On the Application of the Tolerance Factor to Inorganic and Hybrid Halide Perovskites: a Revised System. Chem. Sci. 2016, 7 (7), 4548–4556. 10.1039/C5SC04845A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M. R.; Eperon G. E.; Snaith H. J.; Giustino F. Steric Engineering of Metal-Halide Perovskites with Tunable Optical Band Gaps. Nat. Commun. 2014, 5, 5757. 10.1038/ncomms6757. [DOI] [PubMed] [Google Scholar]
- Shynkarenko Y.; Bodnarchuk M. I.; Bernasconi C.; Berezovska Y.; Verteletskyi V.; Ochsenbein S. T.; Kovalenko M. V. Direct Synthesis of Quaternary Alkylammonium-Capped Perovskite Nanocrystals for Efficient Blue and Green Light-Emitting Diodes. ACS Energy Letters 2019, 4 (11), 2703–2711. 10.1021/acsenergylett.9b01915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijtens T.; Eperon G. E.; Noel N. K.; Habisreutinger S. N.; Petrozza A.; Snaith H. J. Stability of Metal Halide Perovskite Solar Cells. Adv. Energy Mater. 2015, 5 (20), 1500963. 10.1002/aenm.201500963. [DOI] [Google Scholar]
- Bella F.; Renzi P.; Cavallo C.; Gerbaldi C. Caesium for Perovskite Solar Cells: An Overview. Chem. - Eur. J. 2018, 24 (47), 12183–12205. 10.1002/chem.201801096. [DOI] [PubMed] [Google Scholar]
- Gong J.; Guo P.; Benjamin S. E.; Van Patten P. G.; Schaller R. D.; Xu T. Cation Engineering on Lead Iodide Perovskites for Stable and High-Performance Photovoltaic Applications. J. Energy Chem. 2018, 27 (4), 1017–1039. 10.1016/j.jechem.2017.12.005. [DOI] [Google Scholar]
- Ono L. K.; Juarez-Perez E. J.; Qi Y. Progress on Perovskite Materials and Solar Cells with Mixed Cations and Halide Anions. ACS Appl. Mater. Interfaces 2017, 9 (36), 30197–30246. 10.1021/acsami.7b06001. [DOI] [PubMed] [Google Scholar]
- Xu F.; Zhang T.; Li G.; Zhao Y. Mixed Cation Hybrid Lead Halide Perovskites with Enhanced Performance and Stability. J. Mater. Chem. A 2017, 5 (23), 11450–11461. 10.1039/C7TA00042A. [DOI] [Google Scholar]
- Zhao P.; Yin W.; Kim M.; Han M.; Song Y. J.; Ahn T. K.; Jung H. S. Improved carriers injection capacity in perovskite solar cells by introducing A-site interstitial defects. J. Mater. Chem. A 2017, 5 (17), 7905–7911. 10.1039/C7TA01203A. [DOI] [Google Scholar]
- Saliba M.; Matsui T.; Domanski K.; Seo J.-Y.; Ummadisingu A.; Zakeeruddin S. M.; Correa-Baena J.-P.; Tress W. R.; Abate A.; Hagfeldt A.; Grätzel M. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 2016, 354 (6309), 206–209. 10.1126/science.aah5557. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Chen J.; Bakr O. M.; Sun H.-T. Metal-Doped Lead Halide Perovskites: Synthesis, Properties, and Optoelectronic Applications. Chem. Mater. 2018, 30 (19), 6589–6613. 10.1021/acs.chemmater.8b02989. [DOI] [Google Scholar]
- De Marco N.; Zhou H.; Chen Q.; Sun P.; Liu Z.; Meng L.; Yao E.-P.; Liu Y.; Schiffer A.; Yang Y. Guanidinium: A Route to Enhanced Carrier Lifetime and Open-Circuit Voltage in Hybrid Perovskite Solar Cells. Nano Lett. 2016, 16 (2), 1009–1016. 10.1021/acs.nanolett.5b04060. [DOI] [PubMed] [Google Scholar]
- Hou X.; Hu Y.; Liu H.; Mei A.; Li X.; Duan M.; Zhang G.; Rong Y.; Han H. Effect of guanidinium on mesoscopic perovskite solar cells. J. Mater. Chem. A 2017, 5 (1), 73–78. 10.1039/C6TA08418D. [DOI] [Google Scholar]
- Ke W.; Spanopoulos I.; Stoumpos C. C.; Kanatzidis M. G. Myths and reality of HPbI3 in halide perovskite solar cells. Nat. Commun. 2018, 9 (1), 4785. 10.1038/s41467-018-07204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eperon G. E.; Stone K. H.; Mundt L. E.; Schloemer T. H.; Habisreutinger S. N.; Dunfield S. P.; Schelhas L. T.; Berry J. J.; Moore D. T. The Role of Dimethylammonium in Bandgap Modulation for Stable Halide Perovskites. ACS Energy Lett. 2020, 5 (6), 1856–1864. 10.1021/acsenergylett.0c00872. [DOI] [Google Scholar]
- Abdi-Jalebi M.; Andaji-Garmaroudi Z.; Cacovich S.; Stavrakas C.; Philippe B.; Richter J. M.; Alsari M.; Booker E. P.; Hutter E. M.; Pearson A. J.; Lilliu S.; Savenije T. J.; Rensmo H.; Divitini G.; Ducati C.; Friend R. H.; Stranks S. D. Maximizing and stabilizing luminescence from halide perovskites with potassium passivation. Nature 2018, 555 (7697), 497–501. 10.1038/nature25989. [DOI] [PubMed] [Google Scholar]
- Pering S. R.; Deng W.; Troughton J. R.; Kubiak P. S.; Ghosh D.; Niemann R. G.; Brivio F.; Jeffrey F. E.; Walker A. B.; Islam M. S.; Watson T. M.; Raithby P. R.; Johnson A. L.; Lewis S. E.; Cameron P. J. Azetidinium Lead Iodide for Perovskite Solar Cells. J. Mater. Chem. A 2017, 5 (39), 20658–20665. 10.1039/C7TA07545F. [DOI] [Google Scholar]
- Spanopoulos I.; Ke W.; Stoumpos C. C.; Schueller E. C.; Kontsevoi O. Y.; Seshadri R.; Kanatzidis M. G. Unraveling the Chemical Nature of the 3D “Hollow” Hybrid Halide Perovskites. J. Am. Chem. Soc. 2018, 140 (17), 5728–5742. 10.1021/jacs.8b01034. [DOI] [PubMed] [Google Scholar]
- Zarick H. F.; Soetan N.; Erwin W. R.; Bardhan R. Mixed Halide Hybrid Perovskites: A Paradigm Shift in Photovoltaics. J. Mater. Chem. A 2018, 6 (14), 5507–5537. 10.1039/C7TA09122B. [DOI] [Google Scholar]
- Prasanna R.; Gold-Parker A.; Leijtens T.; Conings B.; Babayigit A.; Boyen H.-G.; Toney M. F.; McGehee M. D. Band Gap Tuning via Lattice Contraction and Octahedral Tilting in Perovskite Materials for Photovoltaics. J. Am. Chem. Soc. 2017, 139 (32), 11117–11124. 10.1021/jacs.7b04981. [DOI] [PubMed] [Google Scholar]
- Tao S.; Schmidt I.; Brocks G.; Jiang J.; Tranca I.; Meerholz K.; Olthof S. Absolute energy level positions in tin- and lead-based halide perovskites. Nat. Commun. 2019, 10 (1), 2560. 10.1038/s41467-019-10468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao F.; Stoumpos C. C.; Cao D. H.; Chang R. P. H.; Kanatzidis M. G. Lead-free solid-state organic–inorganic halide perovskite solar cells. Nat. Photonics 2014, 8 (6), 489–494. 10.1038/nphoton.2014.82. [DOI] [Google Scholar]
- Noel N. K.; Stranks S. D.; Abate A.; Wehrenfennig C.; Guarnera S.; Haghighirad A.-A.; Sadhanala A.; Eperon G. E.; Pathak S. K.; Johnston M. B.; Petrozza A.; Herz L. M.; Snaith H. J. Lead-free organic–inorganic tin halide perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7 (9), 3061–3068. 10.1039/C4EE01076K. [DOI] [Google Scholar]
- Liang L.; Gao P. Lead-Free Hybrid Perovskite Absorbers for Viable Application: Can We Eat the Cake and Have It too?. Adv. Sci. 2018, 5 (2), 1700331. 10.1002/advs.201700331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eperon G. E.; Ginger D. S. B-Site Metal Cation Exchange in Halide Perovskites. ACS Energy Lett. 2017, 2 (5), 1190–1196. 10.1021/acsenergylett.7b00290. [DOI] [Google Scholar]
- Eperon G. E.; Leijtens T.; Bush K. A.; Prasanna R.; Green T.; Wang J. T.-W.; McMeekin D. P.; Volonakis G.; Milot R. L.; May R.; Palmstrom A.; Slotcavage D. J.; Belisle R. A.; Patel J. B.; Parrott E. S.; Sutton R. J.; Ma W.; Moghadam F.; Conings B.; Babayigit A.; Boyen H.-G.; Bent S.; Giustino F.; Herz L. M.; Johnston M. B.; McGehee M. D.; Snaith H. J. Perovskite-perovskite tandem photovoltaics with optimized band gaps. Science 2016, 354 (6314), 861–865. 10.1126/science.aaf9717. [DOI] [PubMed] [Google Scholar]
- Zhao B.; Abdi-Jalebi M.; Tabachnyk M.; Glass H.; Kamboj V. S.; Nie W.; Pearson A. J.; Puttisong Y.; Gödel K. C.; Beere H. E.; Ritchie D. A.; Mohite A. D.; Dutton S. E.; Friend R. H.; Sadhanala A. High Open-Circuit Voltages in Tin-Rich Low-Bandgap Perovskite-Based Planar Heterojunction Photovoltaics. Adv. Mater. 2017, 29 (2), 1604744. 10.1002/adma.201604744. [DOI] [PubMed] [Google Scholar]
- Leijtens T.; Prasanna R.; Bush K. A.; Eperon G. E.; Raiford J. A.; Gold-Parker A.; Wolf E. J.; Swifter S. A.; Boyd C. C.; Wang H.-P.; Toney M. F.; Bent S. F.; McGehee M. D. Tin–lead halide perovskites with improved thermal and air stability for efficient all-perovskite tandem solar cells. Sustainable Energy Fuels 2018, 2 (11), 2450–2459. 10.1039/C8SE00314A. [DOI] [Google Scholar]
- Ke W.; Kanatzidis M. G. Prospects for low-toxicity lead-free perovskite solar cells. Nat. Commun. 2019, 10 (1), 965. 10.1038/s41467-019-08918-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavney A. H.; Leppert L.; Saldivar Valdes A.; Bartesaghi D.; Savenije T. J.; Neaton J. B.; Karunadasa H. I. Small-Band-Gap Halide Double Perovskites. Angew. Chem., Int. Ed. 2018, 57 (39), 12765–12770. 10.1002/anie.201807421. [DOI] [PubMed] [Google Scholar]
- Du K.-z.; Meng W.; Wang X.; Yan Y.; Mitzi D. B. Bandgap Engineering of Lead-Free Double Perovskite Cs2AgBiBr6 through Trivalent Metal Alloying. Angew. Chem., Int. Ed. 2017, 56 (28), 8158–8162. 10.1002/anie.201703970. [DOI] [PubMed] [Google Scholar]
- Volonakis G.; Haghighirad A. A.; Milot R. L.; Sio W. H.; Filip M. R.; Wenger B.; Johnston M. B.; Herz L. M.; Snaith H. J.; Giustino F. Cs2InAgCl6: A New Lead-Free Halide Double Perovskite with Direct Band Gap. J. Phys. Chem. Lett. 2017, 8 (4), 772–778. 10.1021/acs.jpclett.6b02682. [DOI] [PubMed] [Google Scholar]
- Luo J.; Li S.; Wu H.; Zhou Y.; Li Y.; Liu J.; Li J.; Li K.; Yi F.; Niu G.; Tang J. Cs2AgInCl6 Double Perovskite Single Crystals: Parity Forbidden Transitions and Their Application For Sensitive and Fast UV Photodetectors. ACS Photonics 2018, 5 (2), 398–405. 10.1021/acsphotonics.7b00837. [DOI] [Google Scholar]
- Zhou J.; Xia Z.; Molokeev M. S.; Zhang X.; Peng D.; Liu Q. Composition design, optical gap and stability investigations of lead-free halide double perovskite Cs2AgInCl6. J. Mater. Chem. A 2017, 5 (29), 15031–15037. 10.1039/C7TA04690A. [DOI] [Google Scholar]
- Shi Z.; Guo J.; Chen Y.; Li Q.; Pan Y.; Zhang H.; Xia Y.; Huang W. Lead-Free Organic–Inorganic Hybrid Perovskites for Photovoltaic Applications: Recent Advances and Perspectives. Adv. Mater. 2017, 29 (16), 1605005. 10.1002/adma.201605005. [DOI] [PubMed] [Google Scholar]
- Etgar L. The merit of perovskite’s dimensionality; can this replace the 3D halide perovskite?. Energy Environ. Sci. 2018, 11 (2), 234–242. 10.1039/C7EE03397D. [DOI] [Google Scholar]
- Grancini G.; Nazeeruddin M. K. Dimensional tailoring of hybrid perovskites for photovoltaics. Nat. Rev. Mater. 2019, 4 (1), 4–22. 10.1038/s41578-018-0065-0. [DOI] [Google Scholar]
- Cao D. H.; Stoumpos C. C.; Farha O. K.; Hupp J. T.; Kanatzidis M. G. 2D Homologous Perovskites as Light-Absorbing Materials for Solar Cell Applications. J. Am. Chem. Soc. 2015, 137 (24), 7843–7850. 10.1021/jacs.5b03796. [DOI] [PubMed] [Google Scholar]
- Tsai H.; Nie W.; Blancon J.-C.; Stoumpos C. C.; Asadpour R.; Harutyunyan B.; Neukirch A. J.; Verduzco R.; Crochet J. J.; Tretiak S.; Pedesseau L.; Even J.; Alam M. A.; Gupta G.; Lou J.; Ajayan P. M.; Bedzyk M. J.; Kanatzidis M. G.; Mohite A. D. High-efficiency two-dimensional Ruddlesden–Popper perovskite solar cells. Nature 2016, 536 (7616), 312–316. 10.1038/nature18306. [DOI] [PubMed] [Google Scholar]
- Mao L.; Stoumpos C. C.; Kanatzidis M. G. Two-Dimensional Hybrid Halide Perovskites: Principles and Promises. J. Am. Chem. Soc. 2019, 141 (3), 1171–1190. 10.1021/jacs.8b10851. [DOI] [PubMed] [Google Scholar]
- Smith M. D.; Crace E. J.; Jaffe A.; Karunadasa H. I. The Diversity of Layered Halide Perovskites. Annu. Rev. Mater. Res. 2018, 48 (1), 111–136. 10.1146/annurev-matsci-070317-124406. [DOI] [Google Scholar]
- Mao L.; Ke W.; Pedesseau L.; Wu Y.; Katan C.; Even J.; Wasielewski M. R.; Stoumpos C. C.; Kanatzidis M. G. Hybrid Dion–Jacobson 2D Lead Iodide Perovskites. J. Am. Chem. Soc. 2018, 140 (10), 3775–3783. 10.1021/jacs.8b00542. [DOI] [PubMed] [Google Scholar]
- Stoumpos C. C.; Cao D. H.; Clark D. J.; Young J.; Rondinelli J. M.; Jang J. I.; Hupp J. T.; Kanatzidis M. G. Ruddlesden–Popper Hybrid Lead Iodide Perovskite 2D Homologous Semiconductors. Chem. Mater. 2016, 28 (8), 2852–2867. 10.1021/acs.chemmater.6b00847. [DOI] [Google Scholar]
- Soe C. M. M.; Stoumpos C. C.; Kepenekian M.; Traoré B.; Tsai H.; Nie W.; Wang B.; Katan C.; Seshadri R.; Mohite A. D.; Even J.; Marks T. J.; Kanatzidis M. G. New Type of 2D Perovskites with Alternating Cations in the Interlayer Space, (C(NH2)3)(CH3NH3)nPbnI3n+1: Structure, Properties, and Photovoltaic Performance. J. Am. Chem. Soc. 2017, 139 (45), 16297–16309. 10.1021/jacs.7b09096. [DOI] [PubMed] [Google Scholar]
- Soe C. M. M.; Nagabhushana G. P.; Shivaramaiah R.; Tsai H.; Nie W.; Blancon J.-C.; Melkonyan F.; Cao D. H.; Traoré B.; Pedesseau L.; Kepenekian M.; Katan C.; Even J.; Marks T. J.; Navrotsky A.; Mohite A. D.; Stoumpos C. C.; Kanatzidis M. G. Structural and thermodynamic limits of layer thickness in 2D halide perovskites. Proc. Natl. Acad. Sci. U. S. A. 2019, 116 (1), 58–66. 10.1073/pnas.1811006115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.; Sun Z.; Wang P.; Hu W.; Wang S.; Ji C.; Hong M.; Luo J. Tailored Engineering of an Unusual (C4H9NH3)2(CH3NH3)2Pb3Br10 Two-Dimensional Multilayered Perovskite Ferroelectric for a High-Performance Photodetector. Angew. Chem., Int. Ed. 2017, 56 (40), 12150–12154. 10.1002/anie.201705836. [DOI] [PubMed] [Google Scholar]
- Leng K.; Fu W.; Liu Y.; Chhowalla M.; Loh K. P. From bulk to molecularly thin hybrid perovskites. Nat. Rev. Mater. 2020, 5 (7), 482–500. 10.1038/s41578-020-0185-1. [DOI] [Google Scholar]
- Saparov B.; Mitzi D. B. Organic–Inorganic Perovskites: Structural Versatility for Functional Materials Design. Chem. Rev. 2016, 116 (7), 4558–4596. 10.1021/acs.chemrev.5b00715. [DOI] [PubMed] [Google Scholar]
- Du K.-z.; Tu Q.; Zhang X.; Han Q.; Liu J.; Zauscher S.; Mitzi D. B. Two-Dimensional Lead(II) Halide-Based Hybrid Perovskites Templated by Acene Alkylamines: Crystal Structures, Optical Properties, and Piezoelectricity. Inorg. Chem. 2017, 56 (15), 9291–9302. 10.1021/acs.inorgchem.7b01094. [DOI] [PubMed] [Google Scholar]
- Milić J. V.; Im J.-H.; Kubicki D. J.; Ummadisingu A.; Seo J.-Y.; Li Y.; Ruiz-Preciado M. A.; Dar M. I.; Zakeeruddin S. M.; Emsley L.; Grätzel M. Supramolecular Engineering for Formamidinium-Based Layered 2D Perovskite Solar Cells: Structural Complexity and Dynamics Revealed by Solid-State NMR Spectroscopy. Adv. Energy Mater. 2019, 9 (20), 1900284. 10.1002/aenm.201900284. [DOI] [Google Scholar]
- Lermer C.; Harm S. P.; Birkhold S. T.; Jaser J. A.; Kutz C. M.; Mayer P.; Schmidt-Mende L.; Lotsch B. V. Benzimidazolium Lead Halide Perovskites: Effects of Anion Substitution and Dimensionality on the Bandgap. Z. Anorg. Allg. Chem. 2016, 642 (23), 1369–1376. 10.1002/zaac.201600371. [DOI] [Google Scholar]
- Mitzi D. B.; Chondroudis K.; Kagan C. R. Design, Structure, and Optical Properties of Organic–Inorganic Perovskites Containing an Oligothiophene Chromophore. Inorg. Chem. 1999, 38 (26), 6246–6256. 10.1021/ic991048k. [DOI] [PubMed] [Google Scholar]
- Guo Z.; Wu X.; Zhu T.; Zhu X.; Huang L. Electron–Phonon Scattering in Atomically Thin 2D Perovskites. ACS Nano 2016, 10 (11), 9992–9998. 10.1021/acsnano.6b04265. [DOI] [PubMed] [Google Scholar]
- Hong X.; Ishihara T.; Nurmikko A. V. Dielectric confinement effect on excitons in PbI4-based layered semiconductors. Phys. Rev. B: Condens. Matter Mater. Phys. 1992, 45 (12), 6961–6964. 10.1103/PhysRevB.45.6961. [DOI] [PubMed] [Google Scholar]
- Tanaka K.; Kondo T. Bandgap and exciton binding energies in lead-iodide-based natural quantum-well crystals. Sci. Technol. Adv. Mater. 2003, 4 (6), 599–604. 10.1016/j.stam.2003.09.019. [DOI] [Google Scholar]
- Dou L.; Wong A. B.; Yu Y.; Lai M.; Kornienko N.; Eaton S. W.; Fu A.; Bischak C. G.; Ma J.; Ding T.; Ginsberg N. S.; Wang L.-W.; Alivisatos A. P.; Yang P. Atomically thin two-dimensional organic-inorganic hybrid perovskites. Science 2015, 349 (6255), 1518–1521. 10.1126/science.aac7660. [DOI] [PubMed] [Google Scholar]
- Chin X. Y.; Perumal A.; Bruno A.; Yantara N.; Veldhuis S. A.; Martínez-Sarti L.; Chandran B.; Chirvony V.; Lo A. S.-Z.; So J.; Soci C.; Grätzel M.; Bolink H. J.; Mathews N.; Mhaisalkar S. G. Self-assembled hierarchical nanostructured perovskites enable highly efficient LEDs via an energy cascade. Energy Environ. Sci. 2018, 11 (7), 1770–1778. 10.1039/C8EE00293B. [DOI] [Google Scholar]
- Cho H.; Jeong S.-H.; Park M.-H.; Kim Y.-H.; Wolf C.; Lee C.-L.; Heo J. H.; Sadhanala A.; Myoung N.; Yoo S.; Im S. H.; Friend R. H.; Lee T.-W. Overcoming the electroluminescence efficiency limitations of perovskite light-emitting diodes. Science 2015, 350 (6265), 1222–1225. 10.1126/science.aad1818. [DOI] [PubMed] [Google Scholar]
- Yuan M.; Quan L. N.; Comin R.; Walters G.; Sabatini R.; Voznyy O.; Hoogland S.; Zhao Y.; Beauregard E. M.; Kanjanaboos P.; Lu Z.; Kim D. H.; Sargent E. H. Perovskite energy funnels for efficient light-emitting diodes. Nat. Nanotechnol. 2016, 11 (10), 872–877. 10.1038/nnano.2016.110. [DOI] [PubMed] [Google Scholar]
- Grätzel M. The Rise of Highly Efficient and Stable Perovskite Solar Cells. Acc. Chem. Res. 2017, 50 (3), 487–491. 10.1021/acs.accounts.6b00492. [DOI] [PubMed] [Google Scholar]
- Li J.; Wang J.; Ma J.; Shen H.; Li L.; Duan X.; Li D. Self-trapped state enabled filterless narrowband photodetections in 2D layered perovskite single crystals. Nat. Commun. 2019, 10 (1), 806. 10.1038/s41467-019-08768-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H.; Liu F.; Shrestha S.; Fernando K.; Tretiak S.; Scott B.; Vo D. T.; Strzalka J.; Nie W. A sensitive and robust thin-film x-ray detector using 2D layered perovskite diodes. Sci. Adv. 2020, 6 (15), eaay0815 10.1126/sciadv.aay0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.; Shang X.; Wang S.; Dong N.; Ji C.; Chen X.; Zhao S.; Wang J.; Sun Z.; Hong M.; Luo J. Bilayered Hybrid Perovskite Ferroelectric with Giant Two-Photon Absorption. J. Am. Chem. Soc. 2018, 140 (22), 6806–6809. 10.1021/jacs.8b04014. [DOI] [PubMed] [Google Scholar]
- Shahrokhi S.; Gao W.; Wang Y.; Anandan P. R.; Rahaman M. Z.; Singh S.; Wang D.; Cazorla C.; Yuan G.; Liu J.-M.; Wu T. Emergence of Ferroelectricity in Halide Perovskites. Small Methods 2020, 4, 2000149. 10.1002/smtd.202000149. [DOI] [Google Scholar]
- Lu H.; Xiao C.; Song R.; Li T.; Maughan A. E.; Levin A.; Brunecky R.; Berry J. J.; Mitzi D. B.; Blum V.; Beard M. C. Highly Distorted Chiral Two-Dimensional Tin Iodide Perovskites for Spin Polarized Charge Transport. J. Am. Chem. Soc. 2020, 142 (30), 13030–13040. 10.1021/jacs.0c03899. [DOI] [PubMed] [Google Scholar]
- Ma J.; Fang C.; Chen C.; Jin L.; Wang J.; Wang S.; Tang J.; Li D. Chiral 2D Perovskites with a High Degree of Circularly Polarized Photoluminescence. ACS Nano 2019, 13 (3), 3659–3665. 10.1021/acsnano.9b00302. [DOI] [PubMed] [Google Scholar]
- Chen C.; Gao L.; Gao W.; Ge C.; Du X.; Li Z.; Yang Y.; Niu G.; Tang J. Circularly polarized light detection using chiral hybrid perovskite. Nat. Commun. 2019, 10 (1), 1927. 10.1038/s41467-019-09942-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long G.; Sabatini R.; Saidaminov M. I.; Lakhwani G.; Rasmita A.; Liu X.; Sargent E. H.; Gao W. Chiral-perovskite optoelectronics. Nat. Rev. Mater. 2020, 5 (6), 423–439. 10.1038/s41578-020-0181-5. [DOI] [Google Scholar]
- Saouma F. O.; Stoumpos C. C.; Wong J.; Kanatzidis M. G.; Jang J. I. Selective enhancement of optical nonlinearity in two-dimensional organic-inorganic lead iodide perovskites. Nat. Commun. 2017, 8 (1), 742. 10.1038/s41467-017-00788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T.; Iwamoto S.; Hayase S.; Tanaka K.; Ishi J.; Mizuno M.; Ema K.; Ito R. Resonant third-order optical nonlinearity in the layered perovskite-type material (C6H13NH3)2PbI4. Solid State Commun. 1998, 105 (8), 503–506. 10.1016/S0038-1098(97)10166-1. [DOI] [Google Scholar]
- Wang J.; Mi Y.; Gao X.; Li J.; Li J.; Lan S.; Fang C.; Shen H.; Wen X.; Chen R.; Liu X.; He T.; Li D. Giant Nonlinear Optical Response in 2D Perovskite Heterostructures. Adv. Opt. Mater. 2019, 7 (15), 1900398. 10.1002/adom.201900398. [DOI] [Google Scholar]
- Walters G.; Haeberlé L.; Quintero-Bermudez R.; Brodeur J.; Kéna-Cohen S.; Sargent E. H. Directional Light Emission from Layered Metal Halide Perovskite Crystals. J. Phys. Chem. Lett. 2020, 11 (9), 3458–3465. 10.1021/acs.jpclett.0c00901. [DOI] [PubMed] [Google Scholar]
- Lanty G.; Jemli K.; Wei Y.; Leymarie J.; Even J.; Lauret J.-S.; Deleporte E. Room-Temperature Optical Tunability and Inhomogeneous Broadening in 2D-Layered Organic–Inorganic Perovskite Pseudobinary Alloys. J. Phys. Chem. Lett. 2014, 5 (22), 3958–3963. 10.1021/jz502086e. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Lu H.; Tong J.; Berry J. J.; Beard M. C.; Zhu K. Advances in two-dimensional organic–inorganic hybrid perovskites. Energy Environ. Sci. 2020, 13 (4), 1154–1186. 10.1039/C9EE03757H. [DOI] [Google Scholar]
- Smith M. D.; Connor B. A.; Karunadasa H. I. Tuning the Luminescence of Layered Halide Perovskites. Chem. Rev. 2019, 119 (5), 3104–3139. 10.1021/acs.chemrev.8b00477. [DOI] [PubMed] [Google Scholar]
- Niesner D.; Wilhelm M.; Levchuk I.; Osvet A.; Shrestha S.; Batentschuk M.; Brabec C.; Fauster T. Giant Rashba Splitting in CH3NH3PbBr3 Organic-Inorganic Perovskite. Phys. Rev. Lett. 2016, 117 (12), 126401. 10.1103/PhysRevLett.117.126401. [DOI] [PubMed] [Google Scholar]
- Isarov M.; Tan L. Z.; Bodnarchuk M. I.; Kovalenko M. V.; Rappe A. M.; Lifshitz E. Rashba Effect in a Single Colloidal CsPbBr3 Perovskite Nanocrystal Detected by Magneto-Optical Measurements. Nano Lett. 2017, 17 (8), 5020–5026. 10.1021/acs.nanolett.7b02248. [DOI] [PubMed] [Google Scholar]
- Mosconi E.; Etienne T.; De Angelis F. Rashba Band Splitting in Organohalide Lead Perovskites: Bulk and Surface Effects. J. Phys. Chem. Lett. 2017, 8 (10), 2247–2252. 10.1021/acs.jpclett.7b00328. [DOI] [PubMed] [Google Scholar]
- Che X.; Traore B.; Katan C.; Kepenekian M.; Even J. Does Rashba splitting in CH3NH3PbBr3 arise from 2 × 2 surface reconstruction?. Phys. Chem. Chem. Phys. 2018, 20 (14), 9638–9643. 10.1039/C8CP00745D. [DOI] [PubMed] [Google Scholar]
- Zhai Y.; Baniya S.; Zhang C.; Li J.; Haney P.; Sheng C.-X.; Ehrenfreund E.; Vardeny Z. V. Giant Rashba splitting in 2D organic-inorganic halide perovskites measured by transient spectroscopies. Sci. Adv. 2017, 3 (7), e1700704 10.1126/sciadv.1700704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd S. B.; Riley D. B.; Binai-Motlagh A.; Clegg C.; Ramachandran A.; March S. A.; Hoffman J. M.; Hill I. G.; Stoumpos C. C.; Kanatzidis M. G.; Yu Z.-G.; Hall K. C. Detection of Rashba spin splitting in 2D organic-inorganic perovskite via precessional carrier spin relaxation. APL Mater. 2019, 7 (8), 081116 10.1063/1.5099352. [DOI] [Google Scholar]
- Giovanni D.; Chong W. K.; Dewi H. A.; Thirumal K.; Neogi I.; Ramesh R.; Mhaisalkar S.; Mathews N.; Sum T. C. Tunable room-temperature spin-selective optical Stark effect in solution-processed layered halide perovskites. Sci. Adv. 2016, 2 (6), e1600477 10.1126/sciadv.1600477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long G.; Jiang C.; Sabatini R.; Yang Z.; Wei M.; Quan L. N.; Liang Q.; Rasmita A.; Askerka M.; Walters G.; Gong X.; Xing J.; Wen X.; Quintero-Bermudez R.; Yuan H.; Xing G.; Wang X. R.; Song D.; Voznyy O.; Zhang M.; Hoogland S.; Gao W.; Xiong Q.; Sargent E. H. Spin control in reduced-dimensional chiral perovskites. Nat. Photonics 2018, 12 (9), 528–533. 10.1038/s41566-018-0220-6. [DOI] [Google Scholar]
- Giovanni D.; Lim J. W. M.; Yuan Z.; Lim S. S.; Righetto M.; Qing J.; Zhang Q.; Dewi H. A.; Gao F.; Mhaisalkar S. G.; Mathews N.; Sum T. C. Ultrafast long-range spin-funneling in solution-processed Ruddlesden–Popper halide perovskites. Nat. Commun. 2019, 10 (1), 3456. 10.1038/s41467-019-11251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grancini G.; Roldán-Carmona C.; Zimmermann I.; Mosconi E.; Lee X.; Martineau D.; Narbey S.; Oswald F.; De Angelis F.; Graetzel M.; Nazeeruddin M. K. One-Year stable perovskite solar cells by 2D/3D interface engineering. Nat. Commun. 2017, 8 (1), 15684. 10.1038/ncomms15684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P.; Bin Mohd Yusoff A. R.; Nazeeruddin M. K. Dimensionality engineering of hybrid halide perovskite light absorbers. Nat. Commun. 2018, 9 (1), 5028. 10.1038/s41467-018-07382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schileo G.; Grancini G. Halide perovskites: current issues and new strategies to push material and device stability. J. Phys. Energy 2020, 2 (2), 021005 10.1088/2515-7655/ab6cc4. [DOI] [Google Scholar]
- Na Quan L.; Ma D.; Zhao Y.; Voznyy O.; Yuan H.; Bladt E.; Pan J.; García de Arquer F. P.; Sabatini R.; Piontkowski Z.; Emwas A.-H.; Todorović P.; Quintero-Bermudez R.; Walters G.; Fan J. Z.; Liu M.; Tan H.; Saidaminov M. I.; Gao L.; Li Y.; Anjum D. H.; Wei N.; Tang J.; McCamant D. W.; Roeffaers M. B. J.; Bals S.; Hofkens J.; Bakr O. M.; Lu Z.-H.; Sargent E. H. Edge stabilization in reduced-dimensional perovskites. Nat. Commun. 2020, 11 (1), 170. 10.1038/s41467-019-13944-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M.-H.; Kim J. S.; Heo J.-M.; Ahn S.; Jeong S.-H.; Lee T.-W. Boosting Efficiency in Polycrystalline Metal Halide Perovskite Light-Emitting Diodes. ACS Energy Lett. 2019, 4 (5), 1134–1149. 10.1021/acsenergylett.9b00518. [DOI] [Google Scholar]
- Xiao Z.; Kerner R. A.; Zhao L.; Tran N. L.; Lee K. M.; Koh T.-W.; Scholes G. D.; Rand B. P. Efficient perovskite light-emitting diodes featuring nanometre-sized crystallites. Nat. Photonics 2017, 11 (2), 108–115. 10.1038/nphoton.2016.269. [DOI] [Google Scholar]
- Lin H.; Zhou C.; Tian Y.; Siegrist T.; Ma B. Low-Dimensional Organometal Halide Perovskites. ACS Energy Lett. 2018, 3 (1), 54–62. 10.1021/acsenergylett.7b00926. [DOI] [Google Scholar]
- Dohner E. R.; Hoke E. T.; Karunadasa H. I. Self-Assembly of Broadband White-Light Emitters. J. Am. Chem. Soc. 2014, 136 (5), 1718–1721. 10.1021/ja411045r. [DOI] [PubMed] [Google Scholar]
- Katan C.; Mercier N.; Even J. Quantum and Dielectric Confinement Effects in Lower-Dimensional Hybrid Perovskite Semiconductors. Chem. Rev. 2019, 119 (5), 3140–3192. 10.1021/acs.chemrev.8b00417. [DOI] [PubMed] [Google Scholar]
- McCall K. M.; Morad V.; Benin B. M.; Kovalenko M. V. Efficient Lone Pair-Driven Luminescence: Structure-Property Relationships in Emissive 5s2 Metal Halides. ACS Mater. Lett. 2020, 2 (9), 1218–1232. 10.1021/acsmaterialslett.0c00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt M. H.Spin Dynamics. Basics of Nuclear Magnetic Resonance; John Wiley & Sons, Ltd.: Chichester, U.K., 2008. [Google Scholar]
- Keeler J.Understanding NMR Spectroscopy; Wiley: Chichester, U.K., 2005. [Google Scholar]
- Duer M. J.Introduction to Solid-State NMR Spectroscopy; Blackwell Publishing: Oxford, U.K., 2004. [Google Scholar]
- Anusca I.; Balčiu̅nas S.; Gemeiner P.; Svirskas Š.; Sanlialp M.; Lackner G.; Fettkenhauer C.; Belovickis J.; Samulionis V.; Ivanov M.; Dkhil B.; Banys J.; Shvartsman V. V.; Lupascu D. C. Dielectric Response: Answer to Many Questions in the Methylammonium Lead Halide Solar Cell Absorbers. Adv. Energy Mater. 2017, 7 (19), 1700600. 10.1002/aenm.201700600. [DOI] [Google Scholar]
- Miyata K.; Atallah T. L.; Zhu X.-Y. Lead halide perovskites: Crystal-liquid duality, phonon glass electron crystals, and large polaron formation. Sci. Adv. 2017, 3 (10), e1701469 10.1126/sciadv.1701469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.; Yaffe O.; Hull T. D.; Owen J. S.; Reichman D. R.; Brus L. E. Dynamic emission Stokes shift and liquid-like dielectric solvation of band edge carriers in lead-halide perovskites. Nat. Commun. 2019, 10 (1), 1175. 10.1038/s41467-019-09057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu W.; Zheng Q.; Prezhdo O. V.; Zhao J.; Saidi W. A. Low-frequency lattice phonons in halide perovskites explain high defect tolerance toward electron-hole recombination. Sci. Adv. 2020, 6 (7), eaaw7453 10.1126/sciadv.aaw7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi P. P.; Maehrlein S. F.; Zhu X. Dynamic Screening and Slow Cooling of Hot Carriers in Lead Halide Perovskites. Adv. Mater. 2019, 31 (47), 1803054. 10.1002/adma.201803054. [DOI] [PubMed] [Google Scholar]
- Palmieri T.; Baldini E.; Steinhoff A.; Akrap A.; Kollár M.; Horváth E.; Forró L.; Jahnke F.; Chergui M. Mahan excitons in room-temperature methylammonium lead bromide perovskites. Nat. Commun. 2020, 11 (1), 850. 10.1038/s41467-020-14683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen W. M. J.; Kentgens A. P. M. Solid–state NMR of hybrid halide perovskites. Solid State Nucl. Magn. Reson. 2019, 100, 36–44. 10.1016/j.ssnmr.2019.03.005. [DOI] [PubMed] [Google Scholar]
- Bernard G. M.; Wasylishen R. E.; Ratcliffe C. I.; Terskikh V.; Wu Q.; Buriak J. M.; Hauger T. Methylammonium Cation Dynamics in Methylammonium Lead Halide Perovskites: A Solid-State NMR Perspective. J. Phys. Chem. A 2018, 122 (6), 1560–1573. 10.1021/acs.jpca.7b11558. [DOI] [PubMed] [Google Scholar]
- Senocrate A.; Maier J. Solid state ionics of hybrid halide perovskites. J. Am. Chem. Soc. 2019, 141 (21), 8382–8396. 10.1021/jacs.8b13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhomme C.; Gervais C.; Babonneau F.; Coelho C.; Pourpoint F.; Azaïs T.; Ashbrook S. E.; Griffin J. M.; Yates J. R.; Mauri F.; Pickard C. J. First-Principles Calculation of NMR Parameters Using the Gauge Including Projector Augmented Wave Method: A Chemist’s Point of View. Chem. Rev. 2012, 112 (11), 5733–5779. 10.1021/cr300108a. [DOI] [PubMed] [Google Scholar]
- Charpentier T.; Menziani M. C.; Pedone A. Computational simulations of solid state NMR spectra: a new era in structure determination of oxide glasses. RSC Adv. 2013, 3 (27), 10550–10578. 10.1039/c3ra40627j. [DOI] [Google Scholar]
- Ashbrook S. E.; Hodgkinson P. Perspective: Current advances in solid-state NMR spectroscopy. J. Chem. Phys. 2018, 149 (4), 040901 10.1063/1.5038547. [DOI] [PubMed] [Google Scholar]
- Hidaka M.; Okamoto Y.; Zikumaru Y. Structural Phase Transition of CsPbCl3 below Room Temperature. Phys. Status Solidi A 1983, 79 (1), 263–269. 10.1002/pssa.2210790129. [DOI] [Google Scholar]
- Karmakar A.; Dodd M. S.; Zhang X.; Oakley M. S.; Klobukowski M.; Michaelis V. K. Mechanochemical synthesis of 0D and 3D cesium lead mixed halide perovskites. Chem. Commun. 2019, 55 (35), 5079–5082. 10.1039/C8CC09622H. [DOI] [PubMed] [Google Scholar]
- Kubicki D. J.; Prochowicz D.; Hofstetter A.; Zakeeruddin S. M.; Grätzel M.; Emsley L. Phase Segregation in Cs-, Rb- and K-Doped Mixed-Cation (MA)x(FA)1–xPbI3 Hybrid Perovskites from Solid-State NMR. J. Am. Chem. Soc. 2017, 139 (40), 14173–14180. 10.1021/jacs.7b07223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki D. J.; Prochowicz D.; Hofstetter A.; Zakeeruddin S. M.; Grätzel M.; Emsley L. Phase Segregation in Potassium-Doped Lead Halide Perovskites from 39K solid-state NMR at 21.1 T. J. Am. Chem. Soc. 2018, 140 (23), 7232–7238. 10.1021/jacs.8b03191. [DOI] [PubMed] [Google Scholar]
- Senocrate A.; Moudrakovski I.; Kim G. Y.; Yang T.-Y.; Gregori G.; Grätzel M.; Maier J. The Nature of Ion Conduction in Methylammonium Lead Iodide: A Multimethod Approach. Angew. Chem., Int. Ed. 2017, 56 (27), 7755–7759. 10.1002/anie.201701724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov A. F.; Venevtsev Y. N.; Semin G. K. Nuclear Quadrupole Resonance (NQR) of 79Br and 81Br in Perovskite and Orthorhombic Forms of CsPbBr3 and CsPbJ3. Phys. Status Solidi B 1969, 35 (2), K167–K169. 10.1002/pssb.19690350277. [DOI] [Google Scholar]
- Xu Q.; Eguchi T.; Nakayama H.; Nakamura N.; Kishita M. Molecular Motions and Phase Transitions in Solid CH3NH3PbX3 (X = Cl, Br, I) as Studied by NMR and NQR. Z. Naturforsch., A: Phys. Sci. 1991, 46, 240–246. 10.1515/zna-1991-0305. [DOI] [Google Scholar]
- Yamada K.; Hino S.; Hirose S.; Yamane Y.; Turkevych I.; Urano T.; Tomiyasu H.; Yamagishi H.; Aramaki S. Static and Dynamic Structures of Perovskite Halides ABX3 (B = Pb, Sn) and Their Characteristic Semiconducting Properties by a Hückel Analytical Calculation. Bull. Chem. Soc. Jpn. 2018, 91 (8), 1196–1204. 10.1246/bcsj.20180068. [DOI] [Google Scholar]
- Stoumpos C. C.; Kanatzidis M. G. The Renaissance of Halide Perovskites and Their Evolution as Emerging Semiconductors. Acc. Chem. Res. 2015, 48 (10), 2791–2802. 10.1021/acs.accounts.5b00229. [DOI] [PubMed] [Google Scholar]
- Saliba M.; Matsui T.; Domanski K.; Seo J.; Zakeeruddin S. M.; Tress W. R.; Graetzel M.. Mixed Cation Perovskite Solid State Solar Cell and Fabrication Thereof. European Patent 3,272,757, 2018.
- Bing J.; Lee D. S.; Zheng J.; Zhang M.; Li Y.; Kim J.; Lau C. F. J.; Cho Y.; Green M. A.; Huang S.; Ho-Baillie A. W. Y. Deconstruction-assisted perovskite formation for sequential solution processing of Cs0.15(MA0.7FA0.3)0.85PbI3 solar cells. Sol. Energy Mater. Sol. Cells 2019, 203, 110200. 10.1016/j.solmat.2019.110200. [DOI] [Google Scholar]
- Christians J. A.; Schulz P.; Tinkham J. S.; Schloemer T. H.; Harvey S. P.; Tremolet de Villers B. J.; Sellinger A.; Berry J. J.; Luther J. M. Tailored interfaces of unencapsulated perovskite solar cells for > 1,000 h operational stability. Nat. Energy 2018, 3 (1), 68–74. 10.1038/s41560-017-0067-y. [DOI] [Google Scholar]
- Saidaminov M. I.; Williams K.; Wei M.; Johnston A.; Quintero-Bermudez R.; Vafaie M.; Pina J. M.; Proppe A. H.; Hou Y.; Walters G.; Kelley S. O.; Tisdale W. A.; Sargent E. H. Multi-cation perovskites prevent carrier reflection from grain surfaces. Nat. Mater. 2020, 19 (4), 412–418. 10.1038/s41563-019-0602-2. [DOI] [PubMed] [Google Scholar]
- Armstrong R. L.; Lourens J. A. J.; Stroud J. D. 133Cs spin-Lattice Relaxation Study of Phase Transitions in CsPbCl3. Phys. Rev. B 1976, 13 (11), 5099–5101. 10.1103/PhysRevB.13.5099. [DOI] [Google Scholar]
- Plesko S.; Kind R.; Roos J. Structural Phase Transitions in CsPbCl3 and RbCdCl3. J. Phys. Soc. Jpn. 1978, 45 (2), 553–557. 10.1143/JPSJ.45.553. [DOI] [Google Scholar]
- Sharma S.; Weiden N.; Weiss A. Phase Transitions in CsSnCl3 and CsPbBr3 An NMR and NQR Study. Z. Naturforsch., A: Phys. Sci. 1991, 46, 329–336. 10.1515/zna-1991-0406. [DOI] [Google Scholar]
- Roiland C.; Trippe-Allard G.; Jemli K.; Alonso B.; Ameline J.-C.; Gautier R.; Bataille T.; Le Polles L.; Deleporte E.; Even J.; Katan C. Multinuclear NMR as a Tool for Studying Local Order and Dynamics in CH3NH3PbX3 (X = Cl, Br, I) Hybrid Perovskites. Phys. Chem. Chem. Phys. 2016, 18 (39), 27133–27142. 10.1039/C6CP02947G. [DOI] [PubMed] [Google Scholar]
- Acik M.; Alam T. M.; Guo F.; Ren Y.; Lee B.; Rosenberg R. A.; Mitchell J. F.; Park I. K.; Lee G.; Darling S. B. Substitutional Growth of Methylammonium Lead Iodide Perovskites in Alcohols. Adv. Energy Mater. 2018, 8 (5), 1701726. 10.1002/aenm.201701726. [DOI] [Google Scholar]
- Chen Y.-F.; Tsai Y.-T.; Hirsch L.; Bassani D. M. Kinetic Isotope Effects Provide Experimental Evidence for Proton Tunneling in Methylammonium Lead Triiodide Perovskites. J. Am. Chem. Soc. 2017, 139 (45), 16359–16364. 10.1021/jacs.7b09526. [DOI] [PubMed] [Google Scholar]
- Fabini D. H.; Siaw T. A.; Stoumpos C. C.; Laurita G.; Olds D.; Page K.; Hu J. G.; Kanatzidis M. G.; Han S.; Seshadri R. Universal Dynamics of Molecular Reorientation in Hybrid Lead Iodide Perovskites. J. Am. Chem. Soc. 2017, 139 (46), 16875–16884. 10.1021/jacs.7b09536. [DOI] [PubMed] [Google Scholar]
- Lu J.; Jiang L.; Li W.; Li F.; Pai N. K.; Scully A. D.; Tsai C.-M.; Bach U.; Simonov A. N.; Cheng Y.-B.; Spiccia L. Diammonium and Monoammonium Mixed-Organic-Cation Perovskites for High Performance Solar Cells with Improved Stability. Adv. Energy Mater. 2017, 7 (18), 1700444. 10.1002/aenm.201700444. [DOI] [Google Scholar]
- Si H.; Liao Q.; Kang Z.; Ou Y.; Meng J.; Liu Y.; Zhang Z.; Zhang Y. Deciphering the NH4PbI3 Intermediate Phase for Simultaneous Improvement on Nucleation and Crystal Growth of Perovskite. Adv. Funct. Mater. 2017, 27 (30), 1701804. 10.1002/adfm.201701804. [DOI] [Google Scholar]
- Ban M.; Zou Y.; Rivett J. P. H.; Yang Y.; Thomas T. H.; Tan Y.; Song T.; Gao X.; Credgington D.; Deschler F.; Sirringhaus H.; Sun B. Solution-Processed Perovskite Light Emitting Diodes with Efficiency Exceeding 15% through Additive-Controlled Nanostructure Tailoring. Nat. Commun. 2018, 9 (1), 3892. 10.1038/s41467-018-06425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella S.; Todaro M.; Masi S.; Listorti A.; Altamura D.; Caliandro R.; Giannini C.; Carignani E.; Geppi M.; Meggiolaro D.; Buscarino G.; De Angelis F.; Rizzo A. Light-Induced Formation of Pb3+ Paramagnetic Species in Lead Halide Perovskites. ACS Energy Lett. 2018, 3 (8), 1840–1847. 10.1021/acsenergylett.8b00944. [DOI] [Google Scholar]
- Kerner R.; Schloemer T. H.; Schulz P.; Berry J. J.; Schwartz J.; Sellinger A.; Rand B. P. Amine Additive Reactions Induced by the Soft Lewis Acidity of Pb2+ in Halide Perovskites. Part I: Evidence for Pb-Alkylmide Formation. J. Mater. Chem. C 2019, 7, 5251–5259. 10.1039/C8TC04871A. [DOI] [Google Scholar]
- Kubicki D. J.; Prochowicz D.; Hofstetter A.; Saski M.; Yadav P.; Bi D.; Pellet N.; Lewiński J.; Zakeeruddin S. M.; Grätzel M.; Emsley L. Formation of Stable Mixed Guanidinium–Methylammonium Phases with Exceptionally Long Carrier Lifetimes for High-Efficiency Lead Iodide-Based Perovskite Photovoltaics. J. Am. Chem. Soc. 2018, 140 (9), 3345–3351. 10.1021/jacs.7b12860. [DOI] [PubMed] [Google Scholar]
- Mencel K.; Durlak P.; Rok M.; Jakubas R.; Baran J.; Medycki W.; Ciżman A.; Piecha-Bisiorek A. Widely Used Hardly Known. An Insight into Electric and Dynamic Properties of Formamidinium Iodide. RSC Adv. 2018, 8 (47), 26506–26516. 10.1039/C8RA03871F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochowicz D.; Yadav P.; Saliba M.; Kubicki D. J.; Tavakoli M. M.; Zakeeruddin S. M.; Lewiński J.; Emsley L.; Grätzel M. One-Step Mechanochemical Incorporation of an Insoluble Cesium Additive for High Performance Planar Heterojunction Solar Cells. Nano Energy 2018, 49, 523–528. 10.1016/j.nanoen.2018.05.010. [DOI] [Google Scholar]
- Yong Z.-J.; Guo S.-Q.; Ma J.-P.; Zhang J.-Y.; Li Z.-Y.; Chen Y.-M.; Zhang B.-B.; Zhou Y.; Shu J.; Gu J.-L.; Zheng L.-R.; Bakr O. M.; Sun H.-T. Doping-Enhanced Short-Range Order of Perovskite Nanocrystals for Near-Unity Violet Luminescence Quantum Yield. J. Am. Chem. Soc. 2018, 140 (31), 9942–9951. 10.1021/jacs.8b04763. [DOI] [PubMed] [Google Scholar]
- Franssen W. M. J.; van Es S. G. D.; Dervişoǧlu R.; de Wijs G. A.; Kentgens A. P. M. Symmetry, Dynamics, and Defects in Methylammonium Lead Halide Perovskites. J. Phys. Chem. Lett. 2017, 8 (1), 61–66. 10.1021/acs.jpclett.6b02542. [DOI] [PubMed] [Google Scholar]
- Wasylishen R. E.; Knop O.; Macdonald J. B. Cation Rotation in Methylammonium Lead Halides. Solid State Commun. 1985, 56 (7), 581–582. 10.1016/0038-1098(85)90959-7. [DOI] [Google Scholar]
- Baikie T.; Barrow N. S.; Fang Y.; Keenan P. J.; Slater P. R.; Piltz R. O.; Gutmann M.; Mhaisalkar S. G.; White T. J. A Combined Single Crystal Neutron/X-ray Diffraction and Solid-State Nuclear Magnetic Resonance Study of the Hybrid Perovskites CH3NH3PbX3 (X = I, Br and Cl). J. Mater. Chem. A 2015, 3 (17), 9298–9307. 10.1039/C5TA01125F. [DOI] [Google Scholar]
- Kubicki D. J.; Prochowicz D.; Hofstetter A.; Péchy P.; Zakeeruddin S. M.; Grätzel M.; Emsley L. Cation Dynamics in Mixed-Cation (MA)x(FA)1–xPbI3 Hybrid Perovskites from Solid-State NMR. J. Am. Chem. Soc. 2017, 139 (29), 10055–10061. 10.1021/jacs.7b04930. [DOI] [PubMed] [Google Scholar]
- Senocrate A.; Moudrakovski I.; Acartürk T.; Merkle R.; Kim G. Y.; Starke U.; Grätzel M.; Maier J. Slow CH3NH3+ Diffusion in CH3NH3PbI3 under Light Measured by Solid-State NMR and Tracer Diffusion. J. Phys. Chem. C 2018, 122 (38), 21803–21806. 10.1021/acs.jpcc.8b06814. [DOI] [Google Scholar]
- Senocrate A.; Moudrakovski I.; Maier J. Short-Range Ion Dynamics in Methylammonium Lead Iodide by Multinuclear Solid State NMR and 127I NQR. Phys. Chem. Chem. Phys. 2018, 20 (30), 20043–20055. 10.1039/C8CP01535J. [DOI] [PubMed] [Google Scholar]
- Knop O.; Wasylishen R. E.; White M. A.; Cameron T. S.; Oort M. J. M. V. Alkylammonium Lead Halides. Part 2. CH3NH3PbX3 (X = Cl, Br, I) Perovskites: Cuboctahedral Halide Cages with Isotropic Cation Reorientation. Can. J. Chem. 1990, 68 (3), 412–422. 10.1139/v90-063. [DOI] [Google Scholar]
- Dai J.; Fu Y.; Manger L. H.; Rea M. T.; Hwang L.; Goldsmith R. H.; Jin S. Carrier Decay Properties of Mixed Cation Formamidinium–Methylammonium Lead Iodide Perovskite [HC(NH2)2]1–x[CH3NH3]xPbI3 Nanorods. J. Phys. Chem. Lett. 2016, 7 (24), 5036–5043. 10.1021/acs.jpclett.6b01958. [DOI] [PubMed] [Google Scholar]
- Chen B.-X.; Li W.-G.; Rao H.-S.; Xu Y.-F.; Kuang D.-B.; Su C.-Y. Large-Grained Perovskite Films via FAxMA1–xPb(IxBr1–x)3 Single Crystal Precursor for Efficient Solar Cells. Nano Energy 2017, 34, 264–270. 10.1016/j.nanoen.2017.02.034. [DOI] [Google Scholar]
- Huang Y.; Li L.; Liu Z.; Jiao H.; He Y.; Wang X.; Zhu R.; Wang D.; Sun J.; Chen Q.; Zhou H. The Intrinsic Properties of FA(1–x)MAxPbI3 Perovskite Single Crystals. J. Mater. Chem. A 2017, 5 (18), 8537–8544. 10.1039/C7TA01441D. [DOI] [Google Scholar]
- Jodlowski A. D.; Roldán-Carmona C.; Grancini G.; Salado M.; Ralaiarisoa M.; Ahmad S.; Koch N.; Camacho L.; de Miguel G.; Nazeeruddin M. K. Large Guanidinium Cation Mixed with Methylammonium in Lead Iodide Perovskites for 19% Efficient Solar Cells. Nat. Energy 2017, 2 (12), 972–979. 10.1038/s41560-017-0054-3. [DOI] [Google Scholar]
- Li C.; Zhou Y.; Wang L.; Chang Y.; Zong Y.; Etgar L.; Cui G.; Padture N. P.; Pang S. Methylammonium-Mediated Evolution of Mixed-Organic-Cation Perovskite Thin Films: A Dynamic Composition-Tuning Process. Angew. Chem. 2017, 129 (26), 7782–7786. 10.1002/ange.201704188. [DOI] [PubMed] [Google Scholar]
- Li W.-G.; Rao H.-S.; Chen B.-X.; Wang X.-D.; Kuang D.-B. A Formamidinium–Methylammonium Lead Iodide Perovskite Single Crystal Exhibiting Exceptional Optoelectronic Properties and Long-Term Stability. J. Mater. Chem. A 2017, 5 (36), 19431–19438. 10.1039/C7TA04608A. [DOI] [Google Scholar]
- Longo G.; Wong A.; Sessolo M.; Bolink H. J. Effect of the Precursor’s Stoichiometry on the Optoelectronic Properties of Methylammonium Lead Bromide Perovskites. J. Lumin. 2017, 189, 120–125. 10.1016/j.jlumin.2017.03.048. [DOI] [Google Scholar]
- Pont S.; Bryant D.; Lin C.-T.; Aristidou N.; Wheeler S.; Ma X.; Godin R.; Haque S. A.; Durrant J. R. Tuning CH3NH3Pb(I1–xBrx)3 Perovskite Oxygen Stability in Thin Films and Solar Cells. J. Mater. Chem. A 2017, 5 (20), 9553–9560. 10.1039/C7TA00058H. [DOI] [Google Scholar]
- Wang C.; Yang S.; Chen X.; Wen T.; Yang H. G. Surface-Functionalized Perovskite Films for Stable Photoelectrochemical Water Splitting. J. Mater. Chem. A 2017, 5 (3), 910–913. 10.1039/C6TA08812K. [DOI] [Google Scholar]
- Daub M.; Hillebrecht H. Tailoring the Band Gap in 3D Hybrid Perovskites by Substitution of the Organic Cations: (CH3NH3)1–2y(NH3(CH2)2NH3)2yPb1–yI3 (0 ≤ y ≤ 0.25). Chem. - Eur. J. 2018, 24 (36), 9075–9082. 10.1002/chem.201800244. [DOI] [PubMed] [Google Scholar]
- Franssen W.; Bruijnaers B.; Portengen V.; Kentgens A. Dimethylammonium Incorporation in Lead Acetate based MAPbI3 Perovskite Solar Cells. ChemPhysChem 2018, 19, 3107. 10.1002/cphc.201800732. [DOI] [PubMed] [Google Scholar]
- Nayak P. K.; Sendner M.; Wenger B.; Wang Z.; Sharma K.; Ramadan A. J.; Lovrinčić R.; Pucci A.; Madhu P. K.; Snaith H. J. Impact of Bi3+ Heterovalent Doping in Organic–Inorganic Metal Halide Perovskite Crystals. J. Am. Chem. Soc. 2018, 140 (2), 574–577. 10.1021/jacs.7b11125. [DOI] [PubMed] [Google Scholar]
- Pareja-Rivera C.; Solís-Cambero A. L.; Sánchez-Torres M.; Lima E.; Solis-Ibarra D. On the True Composition of Mixed-Cation Perovskite Films. ACS Energy Lett. 2018, 3 (10), 2366–2367. 10.1021/acsenergylett.8b01577. [DOI] [Google Scholar]
- Saidaminov M. I.; Kim J.; Jain A.; Quintero-Bermudez R.; Tan H.; Long G.; Tan F.; Johnston A.; Zhao Y.; Voznyy O.; Sargent E. H. Suppression of Atomic Vacancies via Incorporation of Isovalent Small Ions to Increase the Stability of Halide Perovskite Solar Cells in Ambient Air. Nat. Energy 2018, 3 (8), 648–654. 10.1038/s41560-018-0192-2. [DOI] [Google Scholar]
- Sun M.; Liang C.; Zhang H.; Ji C.; Sun F.; You F.; Jing X.; He Z. Tailoring a Dynamic Crystalline Process during the Conversion of Lead-Halide Perovskite Layer to Achieve High Performance Solar Cells. J. Mater. Chem. A 2018, 6, 24793–24804. 10.1039/C8TA07462C. [DOI] [Google Scholar]
- Tan H.; Che F.; Wei M.; Zhao Y.; Saidaminov M. I.; Todorović P.; Broberg D.; Walters G.; Tan F.; Zhuang T.; Sun B.; Liang Z.; Yuan H.; Fron E.; Kim J.; Yang Z.; Voznyy O.; Asta M.; Sargent E. H. Dipolar Cations Confer Defect Tolerance in Wide-Bandgap Metal Halide Perovskites. Nat. Commun. 2018, 9 (1), 3100. 10.1038/s41467-018-05531-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gompel W. T. M.; Herckens R.; Reekmans G.; Ruttens B.; D’Haen J.; Adriaensens P.; Lutsen L.; Vanderzande D. Degradation of the Formamidinium Cation and the Quantification of the Formamidinium–Methylammonium Ratio in Lead Iodide Hybrid Perovskites by Nuclear Magnetic Resonance Spectroscopy. J. Phys. Chem. C 2018, 122 (8), 4117–4124. 10.1021/acs.jpcc.7b09805. [DOI] [Google Scholar]
- Bakulin A. A.; Selig O.; Bakker H. J.; Rezus Y. L. A.; Müller C.; Glaser T.; Lovrincic R.; Sun Z.; Chen Z.; Walsh A.; Frost J. M.; Jansen T. L. C. Real-Time Observation of Organic Cation Reorientation in Methylammonium Lead Iodide Perovskites. J. Phys. Chem. Lett. 2015, 6 (18), 3663–3669. 10.1021/acs.jpclett.5b01555. [DOI] [PubMed] [Google Scholar]
- Gong J.; Yang M.; Ma X.; Schaller R. D.; Liu G.; Kong L.; Yang Y.; Beard M. C.; Lesslie M.; Dai Y.; Huang B.; Zhu K.; Xu T. Electron–Rotor Interaction in Organic–Inorganic Lead Iodide Perovskites Discovered by Isotope Effects. J. Phys. Chem. Lett. 2016, 7 (15), 2879–2887. 10.1021/acs.jpclett.6b01199. [DOI] [PubMed] [Google Scholar]
- Motta C.; El-Mellouhi F.; Kais S.; Tabet N.; Alharbi F.; Sanvito S. Revealing the Role of Organic Cations in Hybrid Halide Perovskite CH3NH3PbI3. Nat. Commun. 2015, 6 (1), 7026. 10.1038/ncomms8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadwaj P. R.; Varadwaj A.; Marques H. M.; Yamashita K. Significance of hydrogen bonding and other noncovalent interactions in determining octahedral tilting in the CH3NH3PbI3 hybrid organic-inorganic halide perovskite solar cell semiconductor. Sci. Rep. 2019, 9 (1), 50. 10.1038/s41598-018-36218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui B.-B.; Han Y.; Huang B.; Zhao Y.; Wu X.; Liu L.; Cao G.; Du Q.; Liu N.; Zou W.; Sun M.; Wang L.; Liu X.; Wang J.; Zhou H.; Chen Q. Locally collective hydrogen bonding isolates lead octahedra for white emission improvement. Nat. Commun. 2019, 10 (1), 5190. 10.1038/s41467-019-13264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa Y.; Nakamura D. Cationic Dynamics in the Crystalline Phases of (CH3NH3)PbX3 (X: Cl, Br) as Studied by Proton Magnetic Resonance Techniques. Z. Naturforsch., A: Phys. Sci. 1989, 44, 1122–1126. 10.1515/zna-1989-1115. [DOI] [Google Scholar]
- Weller M. T.; Weber O. J.; Henry P. F.; Di Pumpo A. M.; Hansen T. C. Complete structure and cation orientation in the perovskite photovoltaic methylammonium lead iodide between 100 and 352 K. Chem. Commun. 2015, 51 (20), 4180–4183. 10.1039/C4CC09944C. [DOI] [PubMed] [Google Scholar]
- Mozur E. M.; Hope M. A.; Trowbridge J. C.; Halat D. M.; Daemen L. L.; Maughan A. E.; Prisk T. R.; Grey C. P.; Neilson J. R. Cesium Substitution Disrupts Concerted Cation Dynamics in Formamidinium Hybrid Perovskites. Chem. Mater. 2020, 32 (14), 6266–6277. 10.1021/acs.chemmater.0c01862. [DOI] [Google Scholar]
- Franssen W. M. J.; van Heumen C. M. M.; Kentgens A. P. M. Structural Investigations of MA1–xDMAxPbI3 Mixed-Cation Perovskites. Inorg. Chem. 2020, 59 (6), 3730–3739. 10.1021/acs.inorgchem.9b03380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwat A.; Yantara N.; Ng Y. F.; Hooper T. J. N.; rana p. j. s.; Febriansyah B.; Harikesh P. C.; Salim T.; Vashishtha P.; Mhaisalkar S. G.; Mathews N. Stabilizing the Electroluminescence of Halide Perovskites with Potassium Passivation. ACS Energy Lett. 2020, 5 (6), 1804–1813. 10.1021/acsenergylett.0c00553. [DOI] [Google Scholar]
- Grottel M.; Szafrański M.; Paja̧k Z. NMR Study of Cation Motion in Guanidinium Iodoplumbates. Z. Naturforsch., A: Phys. Sci. 1997, 52, 783–788. 10.1515/zna-1997-1105. [DOI] [Google Scholar]
- Ueda T.; Shimizu K.; Ohki H.; Okuda T. 13C CP/MAS NMR Study of the Layered Compounds [C6H5CH2CH2NH3]2[CH3NH3]n-1Pbnl3n+1 (n = 1, 2). Z. Naturforsch., A: Phys. Sci. 1996, 51, 910–914. 10.1515/zna-1996-0805. [DOI] [Google Scholar]
- Tremblay M.-H.; Thouin F.; Leisen J.; Bacsa J.; Srimath Kandada A. R.; Hoffman J. M.; Kanatzidis M. G.; Mohite A. D.; Silva C.; Barlow S.; Marder S. R. (4NPEA)2PbI4 (4NPEA = 4-Nitrophenylethylammonium): Structural, NMR, and Optical Properties of a 3 × 3 Corrugated 2D Hybrid Perovskite. J. Am. Chem. Soc. 2019, 141 (11), 4521–4525. 10.1021/jacs.8b13207. [DOI] [PubMed] [Google Scholar]
- Hong L.; Milić J. V.; Ahlawat P.; Mladenović M.; Kubicki D. J.; Jahanabkhshi F.; Ren D.; Gélvez-Rueda M. C.; Ruiz-Preciado M. A.; Ummadisingu A.; Liu Y.; Tian C.; Pan L.; Zakeeruddin S. M.; Hagfeldt A.; Grozema F. C.; Rothlisberger U.; Emsley L.; Han H.; Graetzel M. Guanine-Stabilized Formamidinium Lead Iodide Perovskites. Angew. Chem., Int. Ed. 2020, 59 (12), 4691–4697. 10.1002/anie.201912051. [DOI] [PubMed] [Google Scholar]
- Gong X.; Voznyy O.; Jain A.; Liu W.; Sabatini R.; Piontkowski Z.; Walters G.; Bappi G.; Nokhrin S.; Bushuyev O.; Yuan M.; Comin R.; McCamant D.; Kelley S. O.; Sargent E. H. Electron–phonon interaction in efficient perovskite blue emitters. Nat. Mater. 2018, 17 (6), 550–556. 10.1038/s41563-018-0081-x. [DOI] [PubMed] [Google Scholar]
- Lim A. R.; Jeong S. Y. Twin Structure by 133Cs NMR in Ferroelastic CsPbCl3 Crystal. Solid State Commun. 1999, 110 (3), 131–136. 10.1016/S0038-1098(99)00052-6. [DOI] [Google Scholar]
- Lim A. R.; Jeong S.-Y. Ferroelastic Phase Transition and Twin Structure by 133Cs NMR in a CsPbCl3 Single Crystal. Phys. B 2001, 304 (1), 79–85. 10.1016/S0921-4526(01)00485-9. [DOI] [Google Scholar]
- Ran Lim A.; Gyoo Kim I. Phase Transition Study by using 133Cs and 207Pb Nuclear Magnetic Resonance in a CsPbCl3 Single Crystal. J. Phys. Soc. Jpn. 2004, 73 (2), 475–479. 10.1143/JPSJ.73.475. [DOI] [Google Scholar]
- Chabin M.; Gilletta F. Theoretical Investigation of the Ferroelastic Domain Structure in Cesium Lead Chloride in the Monoclinic Phase. J. Appl. Crystallogr. 1980, 13 (6), 533–538. 10.1107/S0021889880012733. [DOI] [Google Scholar]
- Chabin M.; Gilletta F. Experiment Investigation of the Ferroelastic Domain Structure in Cesium Lead Chloride in the Monoclinic Phase. J. Appl. Crystallogr. 1980, 13 (6), 539–543. 10.1107/S0021889880012745. [DOI] [Google Scholar]
- Shin E. J.; Jeong H. K.; Kim S. Y.; Jeong J. The Orientation of Ferroelastic Domain in Single Crystal, CsPbCl3. J. Korean Assoc. Cryst. Growth 1997, 7 (1), 117–225. [Google Scholar]
- Ran Lim A.; Jeong S.-Y. Ferroelastic Phase Transition of CsPbCl3 Single Crystals Studied by External Stress. Phys. B 1998, 245 (3), 277–281. 10.1016/S0921-4526(97)00883-1. [DOI] [Google Scholar]
- Hanrahan M. P.; Men L.; Rosales B. A.; Vela J.; Rossini A. J. Sensitivity-Enhanced 207Pb Solid-State NMR Spectroscopy for the Rapid, Non-Destructive Characterization of Organolead Halide Perovskites. Chem. Mater. 2018, 30 (20), 7005–7015. 10.1021/acs.chemmater.8b01899. [DOI] [Google Scholar]
- Rosales B. A.; Hanrahan M. P.; Boote B. W.; Rossini A. J.; Smith E. A.; Vela J. Lead Halide Perovskites: Challenges and Opportunities in Advanced Synthesis and Spectroscopy. ACS Energy Lett. 2017, 2 (4), 906–914. 10.1021/acsenergylett.6b00674. [DOI] [Google Scholar]
- Rosales B. A.; Men L.; Cady S. D.; Hanrahan M. P.; Rossini A. J.; Vela J. Persistent Dopants and Phase Segregation in Organolead Mixed-Halide Perovskites. Chem. Mater. 2016, 28 (19), 6848–6859. 10.1021/acs.chemmater.6b01874. [DOI] [Google Scholar]
- Askar A. M.; Karmakar A.; Bernard G. M.; Ha M.; Terskikh V. V.; Wiltshire B. D.; Patel S.; Fleet J.; Shankar K.; Michaelis V. K. Composition-Tunable Formamidinium Lead Mixed Halide Perovskites via Solvent-Free Mechanochemical Synthesis: Decoding the Pb Environments Using Solid-State NMR Spectroscopy. J. Phys. Chem. Lett. 2018, 9 (10), 2671–2677. 10.1021/acs.jpclett.8b01084. [DOI] [PubMed] [Google Scholar]
- Karmakar A.; Askar A. M.; Bernard G. M.; Terskikh V. V.; Ha M.; Patel S.; Shankar K.; Michaelis V. K. Mechanochemical Synthesis of Methylammonium Lead Mixed–Halide Perovskites: Unraveling the Solid-Solution Behavior Using Solid-State NMR. Chem. Mater. 2018, 30 (7), 2309–2321. 10.1021/acs.chemmater.7b05209. [DOI] [Google Scholar]
- Aebli M.; Piveteau L.; Nazarenko O.; Benin B. M.; Krieg F.; Verel R.; Kovalenko M. V. Lead-Halide Scalar Couplings in 207Pb NMR of APbX3 Perovskites (A = Cs, Methylammonium, Formamidinium; X = Cl, Br, I). Sci. Rep. 2020, 10 (1), 8229. 10.1038/s41598-020-65071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh A.; Stranks S. D. Taking control of ion transport in halide perovskite solar cells. ACS Energy Lett. 2018, 3 (8), 1983–1990. 10.1021/acsenergylett.8b00764. [DOI] [Google Scholar]
- Nazarenko O.; Kotyrba M. R.; Yakunin S.; Aebli M.; Rainò G.; Benin B. M.; Wörle M.; Kovalenko M. V. Guanidinium-Formamidinium Lead Iodide: A Layered Perovskite-Related Compound with Red Luminescence at Room Temperature. J. Am. Chem. Soc. 2018, 140 (11), 3850–3853. 10.1021/jacs.8b00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebli M.; Benin B.; McCall K. M.; Morad V.; Thöny D.; Grützmacher H.; Kovalenko M. V. White CsPbBr3: Characterizing the One-Dimensional Cesium Lead Bromide Polymorph. Helv. Chim. Acta 2020, 103, e2000080 10.1002/hlca.202000080. [DOI] [Google Scholar]
- Bernard G. M.; Goyal A.; Miskolzie M.; McKay R.; Wu Q.; Wasylishen R. E.; Michaelis V. K. Methylammonium Lead Chloride: A Sensitive Sample for an Accurate NMR Thermometer. J. Magn. Reson. 2017, 283, 14–21. 10.1016/j.jmr.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Takahashi T.; Kawashima H.; Sugisawa H.; Toshihide B. 207Pb Chemical Shift Thermometer at High Temperature for Magic Angle Spinning Experiments. Solid State Nucl. Magn. Reson. 1999, 15 (2), 119–123. 10.1016/S0926-2040(99)00039-9. [DOI] [PubMed] [Google Scholar]
- Ullmann H.Strukturchemische und MAS-NMR-Spektroskopische Untersuchungen mit Quantenchemischen Berechnungen von Binären, Ternären und Quaternären Blei(II)-Halogeniden; Herbert Utz Verlag Wissenschaft: Munich, 1998. [Google Scholar]
- Askar A. M.; Bernard G. M.; Wiltshire B.; Shankar K.; Michaelis V. K. Multinuclear Magnetic Resonance Tracking of Hydro, Thermal, and Hydrothermal Decomposition of CH3NH3PbI3. J. Phys. Chem. C 2017, 121 (2), 1013–1024. 10.1021/acs.jpcc.6b10865. [DOI] [Google Scholar]
- Lermer C.; Birkhold S. T.; Moudrakovski I. L.; Mayer P.; Schoop L. M.; Schmidt-Mende L.; Lotsch B. V. Toward Fluorinated Spacers for MAPI-Derived Hybrid Perovskites: Synthesis, Characterization, and Phase Transitions of (FC2H4NH3)2PbCl4. Chem. Mater. 2016, 28 (18), 6560–6566. 10.1021/acs.chemmater.6b02151. [DOI] [Google Scholar]
- Ramsey N. F. Magnetic Shielding of Nuclei in Molecules. Phys. Rev. 1950, 78 (6), 699–703. 10.1103/PhysRev.78.699. [DOI] [Google Scholar]
- Tomaselli M.; Yarger J. L.; Bruchez M.; Havlin R. H.; deGraw D.; Pines A.; Alivisatos A. P. NMR Study of InP Quantum Dots: Surface Structure and Size Effects. J. Chem. Phys. 1999, 110 (18), 8861–8864. 10.1063/1.478858. [DOI] [Google Scholar]
- Piveteau L.; Dirin D. N.; Gordon C. P.; Walder B. J.; Ong T.-C.; Emsley L.; Copéret C.; Kovalenko M. V. Colloidal-ALD-Grown Core/Shell CdSe/CdS Nanoplatelets as Seen by DNP Enhanced PASS–PIETA NMR Spectroscopy. Nano Lett. 2020, 20 (5), 3003–3018. 10.1021/acs.nanolett.9b04870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer A. M.; Steigerwald M. L.; Duncan T. M.; Douglass D. C. NMR Study of Semiconductor Molecular Clusters. Phys. Rev. Lett. 1988, 60 (25), 2673–2676. 10.1103/PhysRevLett.60.2673. [DOI] [PubMed] [Google Scholar]
- Piveteau L.; Ong T.-C.; Walder B. J.; Dirin D. N.; Moscheni D.; Schneider B.; Bär J.; Protesescu L.; Masciocchi N.; Guagliardi A.; Emsley L.; Copéret C.; Kovalenko M. V. Resolving the Core and the Surface of CdSe Quantum Dots and Nanoplatelets Using Dynamic Nuclear Polarization Enhanced PASS–PIETA NMR Spectroscopy. ACS Cent. Sci. 2018, 4 (9), 1113–1125. 10.1021/acscentsci.8b00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard G. M.; Michaelis V. K. Lead-207 NMR Spectroscopy at 1.4 T: Application of Benchtop Instrumentation to a Challenging I = 1/2 Nucleus. Magn. Reson. Chem. 2020, 1–10. 10.1002/mrc.5036. [DOI] [PubMed] [Google Scholar]
- Ruiz-Preciado M. A.; Kubicki D. J.; Hofstetter A.; McGovern L.; Futscher M. H.; Ummadisingu A.; Gershoni-Poranne R.; Zakeeruddin S. M.; Ehrler B.; Emsley L.; Milić J. V.; Grätzel M. Supramolecular Modulation of Hybrid Perovskite Solar Cells via Bifunctional Halogen Bonding Revealed by Two-Dimensional 19F Solid-State NMR Spectroscopy. J. Am. Chem. Soc. 2020, 142 (3), 1645–1654. 10.1021/jacs.9b13701. [DOI] [PubMed] [Google Scholar]
- Abate A.; Saliba M.; Hollman D. J.; Stranks S. D.; Wojciechowski K.; Avolio R.; Grancini G.; Petrozza A.; Snaith H. J. Supramolecular Halogen Bond Passivation of Organic–Inorganic Halide Perovskite Solar Cells. Nano Lett. 2014, 14 (6), 3247–3254. 10.1021/nl500627x. [DOI] [PubMed] [Google Scholar]
- Bi S.; Wang H.; Zhou J.; You S.; Zhang Y.; Shi X.; Tang Z.; Zhou H. Halogen bonding reduces intrinsic traps and enhances charge mobilities in halide perovskite solar cells. J. Mater. Chem. A 2019, 7 (12), 6840–6848. 10.1039/C8TA11835C. [DOI] [Google Scholar]
- Kaliaperumal R.; Sears R. E. J.; Finch C. B. 19F shielding anisotropy in RbCaF3. J. Chem. Phys. 1987, 87 (1), 68–72. 10.1063/1.453565. [DOI] [Google Scholar]
- Kaliaperumal R.; Sears R. E. J. 19F shielding anisotropy in KMgF3. J. Chem. Phys. 1988, 88 (2), 1468–1469. 10.1063/1.454220. [DOI] [Google Scholar]
- Okuda T.; Fukutonh S.; Takesako K.; Ohki H.; Yamada K. Fluoride Ion Conductors RPbF3 (R = Rb, Cs) Studied by X-ray Diffraction and NMR. Solid State Ionics 2002, 569–574. 10.1142/9789812776259_0066. [DOI] [Google Scholar]
- Payne R. E.; Forman R. A.; Kahn A. H. Nuclear Magnetic Resonance in RbMnF3. J. Chem. Phys. 1965, 42 (11), 3806–3808. 10.1063/1.1695841. [DOI] [Google Scholar]
- Grosescu R.; Haeberlen U. The Nuclear Magnetic Shielding of the 19F-Nuclei in KZnF3. Z. Naturforsch., A: Phys. Sci. 1985, 40 (3), 283–293. 10.1515/zna-1985-0314. [DOI] [Google Scholar]
- Petrov M. P.; Nedlin G. M. Spin-Density Space Oscillations and Hyperfine Interaction in RbCoF3. J. Appl. Phys. 1968, 39 (2), 1012–1014. 10.1063/1.1656148. [DOI] [Google Scholar]
- Smolenskii G. A.; Petrov M. P.; Pisarev R. V. Spin-Density Distribution and Electronic Structure in Fluorides with the Perovskite Structure (ABF3, where A = Na, Rb; B = Mn, Ni, Co). J. Appl. Phys. 1967, 38 (3), 1269–1271. 10.1063/1.1709573. [DOI] [Google Scholar]
- Engelsberg M.; Rezende S. M.; Soares E. A. 19F spin-lattice relaxation in antiferromagnets with the perovskite structure. J. Appl. Phys. 1979, 50 (B3), 1929–1931. 10.1063/1.327168. [DOI] [Google Scholar]
- Cai S.-H.; Yu X.-Y.; Chen Z.; Wan H.-L. Theoretical study on 19F magnetic shielding constants of some metal fluorides. Magn. Reson. Chem. 2003, 41 (11), 902–907. 10.1002/mrc.1274. [DOI] [Google Scholar]
- Bureau B.; Silly G.; Buzaré J. Y.; Emery J. Superposition model for 19F isotropic chemical shift in ionic fluorides: from basic metal fluorides to transition metal fluoride glasses. Chem. Phys. 1999, 249 (1), 89–104. 10.1016/S0301-0104(99)00253-0. [DOI] [Google Scholar]
- Walker M. B.; Stevenson R. W. H. Nuclear magnetic resonance in RbMnF3. Proc. Phys. Soc., London 1966, 87 (1), 35–43. 10.1088/0370-1328/87/1/303. [DOI] [Google Scholar]
- Elwell D. 19F nuclear magnetic resonance in some paramagnetic fluorides. Proc. Phys. Soc., London 1964, 84 (3), 409–415. 10.1088/0370-1328/84/3/310. [DOI] [Google Scholar]
- Bouznik V. M.; Gabuda S. P. Magnetic Screening of 19F Nuclei in Perovskite KMgF3. Spectrosc. Lett. 1969, 2 (6), 185–189. 10.1080/00387016908050039. [DOI] [Google Scholar]
- Moskvin O. I.; Voronov V. N.; Vopilov E. A.; Buznik V. M. A study of the 19F NMR chemical shifts in perovskites ABF3. J. Struct. Chem. 1979, 20 (3), 457–459. 10.1007/BF00746825. [DOI] [Google Scholar]
- Schütz F.; Lange L.; Scheurell K.; Scholz G.; Kemnitz E. Synthesis and Characterization of Perovskite-Type [K1–xNax]MgF3 Mixed Phases via the Fluorolytic Sol-Gel Synthesis. Crystals 2018, 8 (2), 66. 10.3390/cryst8020066. [DOI] [Google Scholar]
- Martin C. D.; Chaudhuri S.; Grey C. P.; Parise J. B. Effect of A-site cation radius on ordering of BX6 octahedra in (K,Na)MgF3 perovskite. Am. Mineral. 2005, 90 (10), 1522–1533. 10.2138/am.2005.1693. [DOI] [Google Scholar]
- Düvel A.; Wegner S.; Efimov K.; Feldhoff A.; Heitjans P.; Wilkening M. Access to metastable complex ion conductors viamechanosynthesis: preparation, microstructure and conductivity of (Ba,Sr)LiF3 with inverse perovskite structure. J. Mater. Chem. 2011, 21 (17), 6238–6250. 10.1039/c0jm03439h. [DOI] [Google Scholar]
- Kapturczak J.; Pajak Z.; Wachowski L. 1H and 19F NMR in paramagnetic fluoroperovskite structures of NH4CoxMg1-xF3. J. Phys. C: Solid State Phys. 1986, 19 (18), 3433–3441. 10.1088/0022-3719/19/18/015. [DOI] [Google Scholar]
- Ahrens M.; Scholz G.; Kemnitz E. Synthesis and Crystal Structure of RbKLiAlF6 – the First Al-Elpasolite with Three Different Alkali Metals. Z. Anorg. Allg. Chem. 2008, 634 (15), 2978–2981. 10.1002/zaac.200800328. [DOI] [Google Scholar]
- Veenendaal E. J.; Brom H. B. Hyperfine splitting in the elpasolite Cs2NaHoF6, measured by enhanced 165Ho NMR. Physica B+C 1982, 113 (1), 118–120. 10.1016/0378-4363(82)90115-2. [DOI] [Google Scholar]
- Choy J.-H.; Kim J.-Y.; Kim S.-J.; Sohn J.-S.; Han O. H. New Dion–Jacobson-Type Layered Perovskite Oxyfluorides, ASrNb2O6F (A = Li, Na, and Rb). Chem. Mater. 2001, 13 (3), 906–912. 10.1021/cm000673g. [DOI] [Google Scholar]
- Chadwick A. V.; Strange J. H.; Ranieri G. A.; Terenzi M. Studies of ionic motion in perovskite fluorides. Solid State Ionics 1983, 9–10, 555–558. 10.1016/0167-2738(83)90294-1. [DOI] [Google Scholar]
- Bryce D. L.; Widdifield C. M.; Chapman R. P.; Attrell R. J. Chlorine, Bromine, and Iodine Solid-State NMR. eMagRes. 2007, 10.1002/9780470034590.emrstm1214. [DOI] [Google Scholar]
- Widdifield C. M.; Chapman R. P.; Bryce D. L. Chapter 5 Chlorine, Bromine, and Iodine Solid-State NMR Spectroscopy. Annu. Rep. NMR Spectrosc. 2009, 66, 195–326. 10.1016/S0066-4103(08)00405-5. [DOI] [Google Scholar]
- Szell P. M. J.; Bryce D. L. Chapter Three - Recent Advances in Chlorine, Bromine, and Iodine Solid-State NMR Spectroscopy. Annu. Rep. NMR Spectrosc. 2015, 84, 115–162. 10.1016/bs.arnmr.2014.10.002. [DOI] [Google Scholar]
- Das T. P.; Hahn E. L.. Nuclear Quadrupole Resonance Spectroscopy; Academic Press, Inc.: London, 1958. [Google Scholar]
- Dean C. Zeeman Splitting of Nuclear Quadrupole Resonances. Phys. Rev. 1954, 96 (4), 1053–1059. 10.1103/PhysRev.96.1053. [DOI] [Google Scholar]
- Bersohn R. Nuclear Electric Quadrupole Spectra in Solids. J. Chem. Phys. 1952, 20 (10), 1505–1509. 10.1063/1.1700203. [DOI] [Google Scholar]
- Cohen M. H. Nuclear Quadrupole Spectra in Solids. Phys. Rev. 1954, 96 (5), 1278–1284. 10.1103/PhysRev.96.1278. [DOI] [Google Scholar]
- Semin G. K. On Solving Secular Equations for Half-Integer Spins (I = 5/2, 7/2, and 9/2) in NQR Spectroscopy. Russ. J. Phys. Chem. 2007, 81 (1), 38–46. 10.1134/S0036024407010104. [DOI] [Google Scholar]
- Man P. P. Quadrupolar Interactions. eMagRes. 2007, 10.1002/9780470034590.emrstm0429.pub2. [DOI] [Google Scholar]
- Vega A. J. Quadrupolar Nuclei in Solids. eMagRes. 2007, 10.1002/9780470034590.emrstm0431.pub2. [DOI] [Google Scholar]
- Piveteau L.; Aebli M.; Yazdani N.; Millen M.; Korosec L.; Krieg F.; Benin B. M.; Morad V.; Piveteau C.; Shiroka T.; Comas-Vives A.; Copéret C.; Lindenberg A. M.; Wood V.; Verel R.; Kovalenko M. V. Bulk and Nanocrystalline Cesium Lead-Halide Perovskites as Seen by Halide Magnetic Resonance. ACS Cent. Sci. 2020, 6 (7), 1138–1149. 10.1021/acscentsci.0c00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. K.; Becker E. D.; Cabral de Menezes S. M.; Goodfellow R.; Granger P. NMR Nomenclature: Nuclear Spin Properties and Conventions for Chemical Shifts: IUPAC Recommendations 2001. Solid State Nucl. Magn. Reson. 2002, 22 (4), 458–483. 10.1006/snmr.2002.0063. [DOI] [PubMed] [Google Scholar]
- Tovborg-Jensen N. NQR Investigation of Phase Transitions in Cesium Plumbochloride. J. Chem. Phys. 1969, 50 (1), 559–560. 10.1063/1.1670853. [DOI] [Google Scholar]
- van Driel H. M.; Armstrong R. L. 35Cl Spin-Lattice Relaxation Study of Phase Tansitions in CsPbCl3. Phys. Rev. B 1975, 12 (3), 839–841. 10.1103/PhysRevB.12.839. [DOI] [Google Scholar]
- Møller C. K. A Phase Transition in Caesium Plumbochloride. Nature 1957, 180 (4593), 981–982. 10.1038/180981a0. [DOI] [Google Scholar]
- Møller C. K. Crystal Structure and Photoconductivity of Caesium Plumbohalides. Nature 1958, 182 (4647), 1436–1436. 10.1038/1821436a0. [DOI] [Google Scholar]
- Armstrong R. L. Pure Nuclear Quadrupole Resonance Studies of Structural Phase Transitions. J. Magn. Reson. 1975, 20 (2), 214–231. 10.1016/0022-2364(75)90245-0. [DOI] [Google Scholar]
- Cohen M. I.; Young K. F.; Chang T. T.; Brower W. S. Jr. Phase Transitions in CsPbCl3. J. Appl. Phys. 1971, 42 (13), 5267–5272. 10.1063/1.1659935. [DOI] [Google Scholar]
- Weber D. CH3NH3PbX3, a Pb(II)-System with Cubic Perovskite Structure. Z. Naturforsch., B: J. Chem. Sci. 1978, 33b, 1443–1445. 10.1515/znb-1978-1214. [DOI] [Google Scholar]
- Protesescu L.; Yakunin S.; Bodnarchuk M. I.; Krieg F.; Caputo R.; Hendon C. H.; Yang R. X.; Walsh A.; Kovalenko M. V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15 (6), 3692–3696. 10.1021/nl5048779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M. A.; Vaxenburg R.; Nedelcu G.; Sercel P. C.; Shabaev A.; Mehl M. J.; Michopoulos J. G.; Lambrakos S. G.; Bernstein N.; Lyons J. L.; Stöferle T.; Mahrt R. F.; Kovalenko M. V.; Norris D. J.; Rainò G.; Efros A. L. Bright triplet excitons in caesium lead halide perovskites. Nature 2018, 553 (7687), 189–193. 10.1038/nature25147. [DOI] [PubMed] [Google Scholar]
- Boles M. A.; Ling D.; Hyeon T.; Talapin D. V. The Surface Science of Nanocrystals. Nat. Mater. 2016, 15 (2), 141–153. 10.1038/nmat4526. [DOI] [PubMed] [Google Scholar]
- Kovalenko M. V.; Manna L.; Cabot A.; Hens Z.; Talapin D. V.; Kagan C. R.; Klimov V. I.; Rogach A. L.; Reiss P.; Milliron D. J.; Guyot-Sionnnest P.; Konstantatos G.; Parak W. J.; Hyeon T.; Korgel B. A.; Murray C. B.; Heiss W. Prospects of Nanoscience with Nanocrystals. ACS Nano 2015, 9 (2), 1012–1057. 10.1021/nn506223h. [DOI] [PubMed] [Google Scholar]
- Hens Z.; Martins J. C. A Solution NMR Toolbox for Characterizing the Surface Chemistry of Colloidal Nanocrystals. Chem. Mater. 2013, 25 (8), 1211–1221. 10.1021/cm303361s. [DOI] [Google Scholar]
- De Roo J.; Ibáñez M.; Geiregat P.; Nedelcu G.; Walravens W.; Maes J.; Martins J. C.; Van Driessche I.; Kovalenko M. V.; Hens Z. Highly Dynamic Ligand Binding and Light Absorption Coefficient of Cesium Lead Bromide Perovskite Nanocrystals. ACS Nano 2016, 10 (2), 2071–2081. 10.1021/acsnano.5b06295. [DOI] [PubMed] [Google Scholar]
- Grisorio R.; Di Clemente M. E.; Fanizza E.; allegretta i.; Altamura D.; Striccoli M.; Terzano R.; Giannini C.; Irimia-Vladu M.; Suranna G. P. Exploring the Surface Chemistry of Cesium Lead Halide Perovskite Nanocrystals. Nanoscale 2019, 11, 986–999. 10.1039/C8NR08011A. [DOI] [PubMed] [Google Scholar]
- Smock S. R.; Williams T. J.; Brutchey R. L. Quantifying the Thermodynamics of Ligand Binding to CsPbBr3 Quantum Dots. Angew. Chem., Int. Ed. 2018, 57 (36), 11711–11715. 10.1002/anie.201806916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Smock S. R.; Flintgruber A. H.; Perras F. A.; Brutchey R. L.; Rossini A. J. The Surface Termination of CsPbBr3 Perovskite Quantum Dots Determined by Solid-State NMR Spectroscopy. J. Am. Chem. Soc. 2020, 142 (13), 6117–6127. 10.1021/jacs.9b13396. [DOI] [PubMed] [Google Scholar]
- Brenner P.; Bar-On O.; Jakoby M.; Allegro I.; Richards B. S.; Paetzold U. W.; Howard I. A.; Scheuer J.; Lemmer U. Continuous wave amplified spontaneous emission in phase-stable lead halide perovskites. Nat. Commun. 2019, 10 (1), 988. 10.1038/s41467-019-08929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Meng L.; Chen L.; Huang S.; Wu X.; Dai G.; Deng L.; Han J.; Zou B.; Zhang C.; Zhong H. Ultralow-Threshold and Color-Tunable Continuous-Wave Lasing at Room-Temperature from In Situ Fabricated Perovskite Quantum Dots. J. Phys. Chem. Lett. 2019, 10 (12), 3248–3253. 10.1021/acs.jpclett.9b00658. [DOI] [PubMed] [Google Scholar]
- Shrestha S.; Fischer R.; Matt G. J.; Feldner P.; Michel T.; Osvet A.; Levchuk I.; Merle B.; Golkar S.; Chen H.; Tedde S. F.; Schmidt O.; Hock R.; Rührig M.; Göken M.; Heiss W.; Anton G.; Brabec C. J. High-performance direct conversion X-ray detectors based on sintered hybrid lead triiodide perovskite wafers. Nat. Photonics 2017, 11 (7), 436–440. 10.1038/nphoton.2017.94. [DOI] [Google Scholar]
- Sutherland B. R.; Hoogland S.; Adachi M. M.; Wong C. T. O.; Sargent E. H. Conformal Organohalide Perovskites Enable Lasing on Spherical Resonators. ACS Nano 2014, 8 (10), 10947–10952. 10.1021/nn504856g. [DOI] [PubMed] [Google Scholar]
- Xiang W.; Wang Z.; Kubicki D. J.; Tress W.; Luo J.; Prochowicz D.; Akin S.; Emsley L.; Zhou J.; Dietler G.; Grätzel M.; Hagfeldt A. Europium-Doped CsPbI2Br for Stable and Highly Efficient Inorganic Perovskite Solar Cells. Joule 2019, 3 (1), 205–214. 10.1016/j.joule.2018.10.008. [DOI] [Google Scholar]
- Miller J. B.; Barrall G. A. Explosives Detection with Nuclear Quadrupole Resonance: An Emerging Technology will Help to Uncover Land Mines and Terrorist Bombs. Am. Sci. 2005, 93 (1), 50–57. 10.1511/2005.51.953. [DOI] [Google Scholar]
- Balchin E.; Malcolme-Lawes D. J.; Poplett I. J. F.; Rowe M. D.; Smith J. A. S.; Pearce G. E. S.; Wren S. A. C. Potential of Nuclear Quadrupole Resonance in Pharmaceutical Analysis. Anal. Chem. 2005, 77 (13), 3925–3930. 10.1021/ac0503658. [DOI] [PubMed] [Google Scholar]
- Pérez S. C.; Cerioni L.; Wolfenson A. E.; Faudone S.; Cuffini S. L. Utilization of pure nuclear quadrupole resonance spectroscopy for the study of pharmaceutical crystal forms. Int. J. Pharm. 2005, 298 (1), 143–152. 10.1016/j.ijpharm.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Trontelj Z.; Pirnat J.; Jazbinšek V.; Lužnik J.; Srčič S.; Lavrič Z.; Beguš S.; Apih T.; Žagar V.; Seliger J. Nuclear Quadrupole Resonance (NQR)—A Useful Spectroscopic Tool in Pharmacy for the Study of Polymorphism. Crystals 2020, 10 (6), 450. 10.3390/cryst10060450. [DOI] [Google Scholar]