Most students in the biological and psychological sciences will be able to recall the unforgettable patient Henry Molaison (H.M.)—an amnesic patient whose characterization revolutionized our understanding of memory. Less well-known, however, is the impact of H.M.’s medial temporal lobectomy on food intake, where his ability to interpret interoceptive hunger signals was greatly diminished. In fact, after fully consuming one meal, when a second was presented to him a mere 60 s later, H.M. set about consuming it at an identical rate as the first (1). This consumption of the same meal in succession effectively increased overall meal size and may have reflected the bilateral resection of H.M.’s hippocampus. Thus, in addition to the hippocampus’ pivotal role in encoding memories, the study of H.M. revealed that the hippocampus also plays an indispensable role in coordinating the body’s energy needs with appropriate feeding behavior.
More recently, studies have begun to tease apart the role of the hippocampus in navigating interactions between endogenous energy signals and environmental cues that underlie the decision to eat (2). The body conveys energy status via circulating hormones including the gastric feeding peptide ghrelin that relays energy deficit, the adipokine leptin that signals energy surfeit and satiety signals such as via CCK (3). When received in the brain, these signals translate the energy needs of the body so that the brain can appropriately adjust behavior to address any energy imbalance (eat or stop eating). Most research has focused on understanding how these signals engage the hindbrain and hypothalamus to modify homeostatic feeding. Yet, actions in these “first responder” regions don’t explain all aspects of how peripheral signals contribute to feeding behavior. These same signals can also engage the hippocampus, but it remained unclear how the hippocampus uses these signals to modify feeding, and particularly, if the hippocampus “weighs” the input of the hormones to tune feeding responses.
In the current issue of Biological Psychiatry—Suarez et al. investigated how ghrelin acts in the ventral hippocampus (vHPC) amongst the milieu of peripheral satiety signals to modulate feeding (4). First, they fasted rats to make them hungry, and measured how much they ate when food was restored 24hr later. They verified that a low dose of ghrelin into the vHPC does not further accentuate re-feeding, indicating it is below the threshold to induce orexigenic effects. Peripheral treatment with a gastric preload (methycellulose) or satiety cues (CCK, the GLP1-receptor agonist Exendin-4, amylin) suppressed feeding, as expected. However, when these satiety cues were given along with the subthreshold dose of ghrelin in the vHPC it attenuated the rats feeding restraint. In other words, the satiety “brake” remained, but ghrelin acted in the vHPC to “lift the pedal” so that the rats ate a larger meal beyond what was required for satiation. But why? Did ghrelin decrease the ability of the satiety signals to communicate fullness; or, did it change the cognitive perception of fullness, and therefore how much rats chose to eat?
Testing the cognitive appreciation of fullness is tricky with rodent models since they can’t tell you if they feel hungry or full except via the action of eating (or not). To get around this, Suarez et al. creatively used a hippocampal-dependent Deprivation Intensity Discrimination task in which rats learned to associate their internal status (sated, hungry) with when they could receive sugar rewards (4). Separate groups of rats received rewards when they were either hungry or sated. Thus, this task forced the rat to recognize its interoceptive state (am I hungry or full?) and use it as an internal stimulus to determine when food will be made available (e.g., I can only get the reward if I feel full). Measuring how much time the rat spent in the magazine area where rewards were delivered indicated its perceived interoceptive state. Accordingly, rats who learned that hunger will yield sucrose rewards approached the magazine more when hungry than sated—the opposite was true for rats in which satiety predicted reward delivery. Fascinatingly, when rats were tested in a sated state, hippocampal ghrelin administration evoked a pattern of responding like that produced by hunger: enhancing the time spent in the magazine area for rats in which hunger signaled reward; and reducing this appetitive behavior in rats where hunger predicted the absence of food. The authors conclude that hippocampal ghrelin must convey the interoceptive state of hunger, which the rat interprets similar to the fasting signal to determine the likelihood of reward availability. This is the first study to demonstrate that ghrelin, even at a dose that doesn’t itself promote feeding, communicates to the vHPC the state of hunger that can shape the animal’s future eating.
The hippocampus is not a direct instigator of motoric behavior, but Suarez et al. identified a circuit by which ghrelin-responsive vHPC neurons could indirectly modify the initiation of feeding (4). Using a series of tract tracing techniques, they showed that vHPC neurons project to lateral hypothalamic orexin neurons, which are known to promote feeding in response to ghrelin (5). Hence, this indirect vHPC → orexin circuit may be important to coordinate cognitive interoceptive perception with the necessary arousal, motivation and motor actions for feeding—the latter of which are all governed by orexin. Suarez et al. also showed that orexin neurons project to the laterodorsal tegmental nucleus (LDTg) in the caudal midbrain, which has recently emerged as an important site to gate meal size (6). Practical limitations prevented the authors from labeling a complete vHPC → orexin → LTDg circuit, but they provided evidence that such a circuit functionally exists and contributes to ghrelin action and a brake on meal size (Figure 1). Indeed, hippocampal ghrelin treatment specifically increased meal size, not meal frequency, which could be blocked by an orexin receptor-1 inhibitor in the LDTg. Together these data are compelling evidence of an indirect circuit linking vHPC ghrelin “sensing” of interoceptive state with the orexin and LTDg regions implicated in modifying feeding behaviors.
Figure 1: Model of ghrelin-mediated vHPC circuit, based on (4).
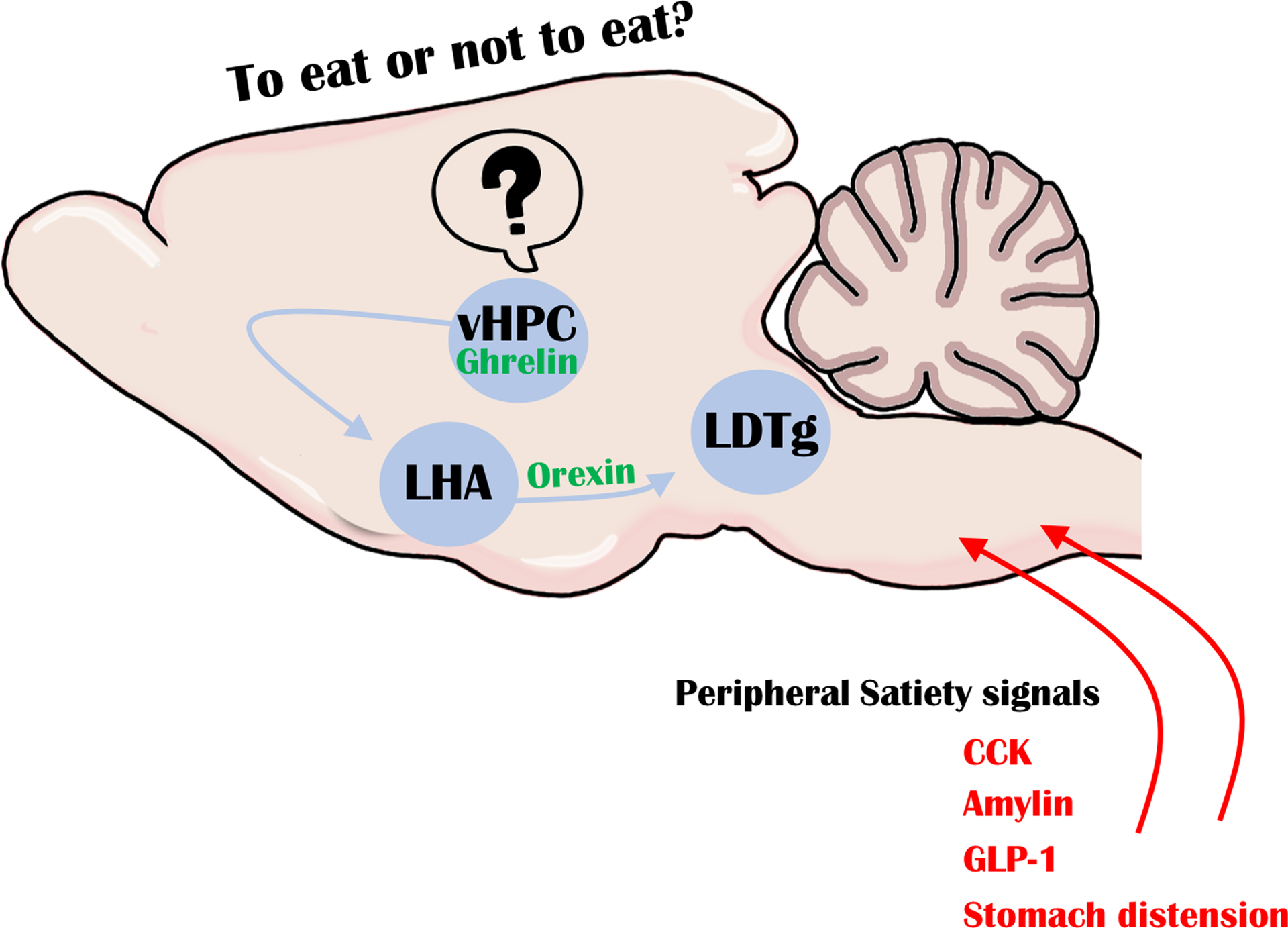
Peripheral satiety signals act in the brain to cease feeding, but the hormone ghrelin promotes feeding. Ghrelin acts specifically within the vHPC to increase the activation of LHA orexin-expressing neurons that project to the LDTg. Elevated ghrelin in the vHPC conveys the interoceptive state of hunger, and can “outweigh” satiety cues via the vHPC → orexin → LTDg circuit that induces animals to eat a larger meal.
Going forward, it will be essential to parse the physiological role of ghrelin via the vHPC → orexin → LTDg circuit described here in relation to ghrelin actions mediated elsewhere. Indeed, ghrelin receptors are expressed widely throughout the brain, including within the LDTg (7), and likely impact feeding via multiple neural mechanisms. Presumably ghrelin acts directly within the LDTg as well, but the consequence of this has yet to be investigated. Ghrelin also acts directly upon the lateral hypothalamus, where it has been shown to modulate orexin neurons that project to ventral tegmental area (VTA) dopamine neurons to modulate feeding (5). One tantalizing question is whether the ghrelin actions via the vHPC to increase meal size converge upon the same orexin neurons that engage the VTA to modify motivation to eat. Since orexin neurons are heterogenous in function and connectivity (8), it is possible that different orexin neurons mediate the indirect (vHPC) and local (lateral hypothalamic area) effects of ghrelin, and hence, coordinate different aspects of feeding. Alternatively, if both ghrelin-mediated pathways converge upon the same orexin neurons it could serve to coordinate the motivation to eat beyond satiety, and could yield insight into the possible causes of overeating that lead to overweight and obesity. On the other end of the spectrum, ghrelin levels are elevated in patients with anorexia nervosa (9). Accordingly, how might ghrelin’s action in this forebrain to hindbrain circuit compensate for chronic periods of energy depletion? More broadly, these studies offer insight into the neurobiological mechanisms of interoception, placing the vHPC as a key player mediating the detection of interoceptive signals from the viscera that are critical for maintaining appropriate homeostatic control. Since the Suarez et al. studies were only done in male rats (4), it also remains to be seen whether the indirect vHPC → orexin circuit exerts sexually dimorphic regulation of feeding, as has been demonstrated for direct ghrelin treatment within the lateral hypothalamus (10). Thus, while there is much yet to be understood about the extent of ghrelin action in the brain, Suarez et al.’s elegant work herein (4) identifies a sizable role for the hippocampus that has only whetted our appetite to learn more about this system.
Acknowledgements
This work was supported by the National Institute of Diabetes and Digestive and Kidney Disease of the National Institutes of Health under award number R01-DK111475 to AWJ and R01-DK103808 to GML.
Footnotes
Disclosures
AWJ and GML have no conflicts of interest to disclose.
References
- 1.Hebben N, et al. , Diminished ability to interpret and report internal states after bilateral medial temporal resection: case H.M. Behav Neurosci, 1985. 99(6): 1031–9. [DOI] [PubMed] [Google Scholar]
- 2.Davidson TL, et al. , The Cognitive Control of Eating and Body Weight: It’s More Than What You “Think”. Front Psychol, 2019. 10: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams KW and Elmquist JK, From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci, 2012. 15(10): 1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suarez AN, et al. , Ghrelin and Orexin Interact to Increase Meal Size Through a Descending Hippocampus to Hindbrain Signaling Pathway. Biol Psychiatry, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cone JJ, McCutcheon JE, and Roitman MF, Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J Neurosci, 2014. 34(14): 4905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reiner DJ, et al. , Glucagon-Like Peptide-1 Receptor Signaling in the Lateral Dorsal Tegmental Nucleus Regulates Energy Balance. Neuropsychopharmacology, 2018. 43(3): 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zigman JM, et al. , Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol, 2006. 494(3): 528–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyer M, et al. , Identification of discrete, intermingled hypocretin neuronal populations. J Comp Neurol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiiya T, et al. , Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab, 2002. 87(1): 240–4. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Ferreras L, et al. , Ghrelin’s control of food reward and body weight in the lateral hypothalamic area is sexually dimorphic. Physiol Behav, 2017. 176: 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]


