Abstract
Background
Obstructive sleep apnea (OSA) is characterized by repetitive episodes of complete or partial obstruction of the upper airways during sleep. Conscious sedation for flexible bronchoscopy (FB) places patients in a sleep-like condition. We hypothesize that oxygen desaturation during flexible bronchoscopy may help to detect undiagnosed sleep apnea.
Methods
Single-centre, investigator-initiated and driven study including consecutive patients undergoing FB for clinical indication. Patients completed the Epworth Sleepiness Scale (ESS), Lausanne NoSAS score, STOP-BANG questionnaire and the Berlin questionnaire and underwent polygraphy within 7 days of FB. FB was performed under conscious sedation with propofol. Oxygen desaturation during bronchoscopy was measured with continuous monitoring of peripheral oxygen saturation with ixTrend (ixellence GmbH, Germany).
Results
145 patients were included in the study, 62% were male, and the average age was 65.8 ± 1.1 years. The vast majority of patients (n = 131, 90%) proved to fulfill OSA criteria based on polygraphy results: 52/131 patients (40%) had mild sleep apnea, 49/131 patients (37%) moderate sleep apnea and 30/131 patients (23%) severe sleep apnea. Patients with no oxygen desaturation had a significantly lower apnea–hypopnea index than patients with oxygen desaturation during bronchoscopy (AHI 11.94/h vs 21.02/h, p = 0.011). This association remained significant when adjusting for the duration of bronchoscopy and propofol dose (p = 0.023; 95% CI 1.382; 18.243) but did not hold when also adjusting for age and BMI.
Conclusion
The severity of sleep apnea was associated to oxygen desaturation during flexible bronchoscopy under conscious sedation. Patients with oxygen desaturation during bronchoscopy might be considered for sleep apnea screening.
Trial registration: The Study was approved by the Ethics Committee northwest/central Switzerland, EKNZ (EK 16/13) and was carried out according to the Declaration of Helsinki and Good Clinical Practice guidelines. Due to its observational character, the study did not require registration at a clinical trial registry.
Keywords: Bronchoscopy, Sleep apnea, Tonometry, Hypoxia
Introduction
Obstructive sleep apnea (OSA) is a condition caused by repetitive episodes of partial or complete airway collapse during sleep. The prevalence rates of OSA have increased substantially over the last decades, ranging from 14 to 55% depending on the age and gender of the patient population [1]. The significant relationship with cardiovascular and metabolic diseases forms the major health burden associated with OSA which leads to substantial morbidity and mortality [2–4].
Symptoms of OSA include increased daytime sleepiness, fatigue, irritability, inattention and a decrease in cognitive function resulting in a highly heterogeneous disease with multiple phenotypes [5–7]. Although OSA is a common problem, the fact that the respiratory events, (apnea and hypopnea) occur during sleep, results in an unawareness of- and underdiagnosed disease [8]. Various questionnaires including the Epworth Sleepiness scale (ESS), [9]; Berlin questionnaire [10]; the snoring, tiredness, observed apnea, high blood pressure, body mass index (BMI), age, neck circumference, and male gender (STOP-BANG) questionnaire [11] and scores Lausanne NoSAS score [12] and the Multivariable Apnea Prediction (MVAP) score [13] exist to aid in identifying patients with OSA.
Flexible bronchoscopy (FB) is a generally safe minimally invasive procedure used to assess, diagnose and treat patients with respiratory disease [14]. Transient hypoxemia due to upper airway obstruction is known to occur in patients undergoing flexible bronchoscopy [15–18]. However, the association between transient hypoxemia during FB and the presence of sleep apnea remains unexplored.
We hypothesize that transient hypoxemia during flexible bronchoscopy under conscious sedation might be associated with the apnea–hypopnea index (AHI) assessed by polygraphy and therefore could inform about the presence of sleep apnea.
Methods
Patient selection
This was a prospective, investigator-initiated and driven, single-centre cross-sectional study performed at the Clinic of Respiratory Medicine and Pulmonary Cell Research at the University Hospital of Basel between October 2018 and August 2019. Patients older than 18 years undergoing a diagnostic flexible bronchoscopy were sequentially recruited. Exclusion criteria were hypoxemia at rest defined as an oxygen saturation of < 90% in room air, rapid fatal disease, any disease or condition precluding the initiation of continuous positive airway pressure (CPAP) therapy within the next 6 months and a new onset of cardio-respiratory symptoms (“unstable state”) as defined by a deterioration of cardio-vital signs within the last 48 h. Patients who required intubation during the bronchoscopy were withdrawn from the study.
The Study was approved by the Ethics Committee northwest/central Switzerland, EKNZ (EK 16/13) and was carried out according to the Declaration of Helsinki and Good Clinical Practice guidelines. Due to its observational character, the study did not require registration at a clinical trial registry.
Collected data included patient demographic data, current pulmonary function test, arterial blood gas analysis, oxygen saturation during bronchoscopy and all information related to the bronchoscopy. A targeted physical examination was performed on all subjects including BMI, neck circumference and examination of the oral cavity to record the oropharyngeal Mallampati score. All patients completed the ESS, and Berlin Questionnaire. The NoSAS Score and STOP-BANG score were calculated from measurements and data collected from the patient. The ESS is a self-administered questionnaire that provides insight into the subject`s general level of daytime sleepiness. [9] The ESS is applied by rating the probability of falling asleep during eight different scenarios usually encountered in daily life. A meta-analysis found the sensitivity of the ESS questionnaire ranges between 47 and 52% depending on the severity of OSA [19]. The Berlin Questionnaire is a validated instrument for screening patients at risk for sleep apnea. In this questionnaire, snoring and observed apnea (first domain, five questions), daytime sleepiness (second domain, four questions) and BMI and hypertension (third domain, one question and information on height and weight) are assessed. The Berlin Questionnaire is positive when two of the three domains are positive. It has a sensitivity of 86% in predicting patients with a respiratory disturbance index (RDI) above 5/h [10]. The NoSAS score is a screening tool used to identify persons at risk for sleep apnea [12]. It uses information about neck circumference, BMI, snoring, age and gender to predict sleep apnea. A score of 8 points or higher is predictive of sleep apnea. The NoSAS score has a sensitivity of 79–85% [12]. The STOP-Bang questionnaire is a concise screening tool for OSA. It is self-reportable and includes four subjective features (Snoring, Tiredness, Observed apnea and presence of high blood pressure) and four demographic queries (BMI, Age, Neck circumference and Gender). Every item can be scored with zero or one point with a maximum score of eight points. Using a cutoff of ≥ 3, the STOP-BANG score has a sensitivity of 84% in detecting OSA [11].
Sleep apnea evaluation
Polygraphy, was used to screen the patients for sleep apnea (WatchPAT™200 Unified, Itamar medical) and was performed in an in-patient setting within 7 days before the bronchoscopy. The following parameters were measured via three points of contact: peripheral arterial tonometry signal, heart rate, oximetry, actigraphy, body position, snoring and chest motion. AHI, RDI and oxygen desaturation index (ODI) based on true sleep time and sleep staging were assessed. Mild sleep apnea was defined as 5 ≤ AHI ≤ 15, moderate sleep apnea as 15 < AHI ≤ 30 and severe sleep apnea as AHI > 30. Sleep apnea syndrome is defined as AHI ≥ 5/h + symptoms of daytime sleepiness [8]. We calculated sleep apnea syndrome using the ESS, Berlin score, NoSAS score and STOP-BANG score.
Bronchoscopy
All patients were assessed prior to the bronchoscopy by a physician or a member of the nursing team trained in anesthesiology and graded according to the American Society of Anesthesiologists (ASA) criteria. The bronchoscopy was performed trans-nasally or trans-orally with the patients in a semi-recumbent position. Pulse oximetry and electrocardiography were recorded continuously and blood pressure was monitored automatically and non-invasively every 5 min. These readings were recorded with the patient monitor, ixTrend Express (ixitos GmbH, Berlin Germany). Supplemental oxygen at 4 l/min was supplied through a nasal cannula to all patients, and oxygen delivery was increased gradually up to 12 L/min when oxygen saturation dropped below 90%. Lidocaine 2% gel was applied as a nasal anesthesia. The bronchoscopist introduced 5-ml aliquots of 1% lidocaine over the vocal cords, the trachea and both left and right main bronchi. In certain patients, up to 4 mg hydrocodone was administered prior to the procedure [20]. Nurses trained in endoscopy performed the conscious sedation with propofol, which was applied intravenously through an infusion-pump and in addition, propofol-boluses were applied and titrated to achieve adequate sedation (onset of ptosis) for the bronchoscopy. The amount of propofol applied was further titrated during the bronchoscopy in order to maintain conscious sedation, defined as a decreased state of consciousness that minimizes discomfort [21–25]. Hydrocodone was usually applied as a cough-suppressant. [26, 27]
The event of a relevant oxygen desaturation during conscious sedation for flexible bronchoscopy was characterized as a minimal transcutaneous oxygen saturation < 90% for ≥ 5 s. For additional explorative analyses we also analysed oxygen desaturations < 90% for ≥ 1 min, desaturations ≤ 88% for 5 s and a ≥ 4% decrease in oxygen saturation from baseline. During the bronchoscopy the following additional parameters were noted: audible snoring for ≥ 10 s, non-invasive ventilation, chin support and witnessed apnea longer than 10 s.
Bronchoscopy complications were defined as bleeding requiring intervention, need for non-invasive ventilation, need to abort the examination, transfer to the intensive care unit, pneumothorax or death. The decision to discontinue the bronchoscopy, to provide chin support or non-invasive ventilation was taken by the bronchoscopist. [24, 27]
Statistical analysis
Differences in dichotomous variables were evaluated using the Chi-squared test or Fisher exact test, as appropriate. Normally distributed parameters were analyzed using the Student t-test for equality of means. All other continuously non-normally distributed parameters were evaluated using the non-parametric Mann–Whitney U test or Kruskal–Wallis test, as appropriate. The association between oxygen desaturation and a diagnosis of sleep apnea was evaluated by linear regression using a univariate model and a multivariate model adjusting for the duration of the bronchoscopy and amount of propofol used. The Statistical Package for Social Sciences Program (SPSS Inc, version 22 for Windows) was used. All tests are two-tailed, a P-value < 0.05 was considered significant. Results are expressed as mean (SEM) or median (interquartile range) unless stated otherwise.
Sample size calculation
We assumed that patients without desaturation below 90% during flexible bronchoscopy under conscious sedation would depict an AHI of 5/h ± 18 during polygraphy whereas patients with oxygen desaturation below 90% during FB would depict an AHI of 15/h ± 18. We considered an uneven distribution of desaturation during bronchoscopy of 70–30%. A total of 131 patients (77 + 54) would need to be included in the study to achieve a level of significance of 5% and a power of 80%. Considering a lost-to-follow-up of 5%, a total of 145 patients (85 + 60) was planned for inclusion in the study.
Results
Baseline characteristics
The number of patients screened for the study totaled 178. Of these 178 patients, 33 patients were excluded and 145 patients were included in the study (Fig. 1). The included population consisted of 90 (62%) males, the mean BMI was 25.6 ± 0.4 kg/m2 and a third of the patients were current smokers (n = 48; Table 1). The majority of the patients had an ASA-Score III. A Mallampati score of 3–4 was present in 105/145 (72%) of the patients. The total number of patients with observed snoring during the bronchoscopy (n = 107; 74%) was higher than the number of patients with self-reported snoring (n = 74; 51%) and lower than the number of patients recorded snoring using polygraphy (n = 121; 83%). The most prevalent comorbidity was chronic obstructive pulmonary disease (COPD) followed by renal disease and coronary artery disease (Table 1).
Fig. 1.
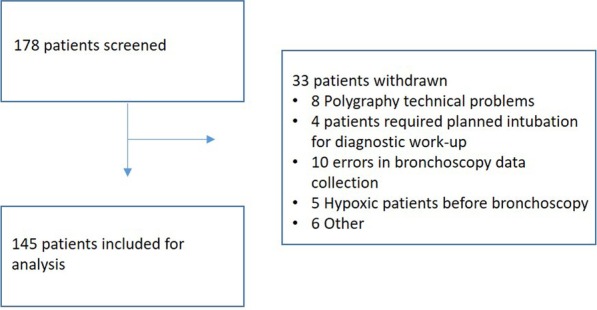
A schematic diagram according to the CONSORT recommendations depicting patient inclusion in the study
Table 1.
Basic characteristics of the patients included in the study
| N(%), mean ± SEM | |
|---|---|
| Age, years | 65.8 ± 1.1 |
| Male | 90 (62) |
| Smoking status | |
| Current smoker | 48 (33) |
| Past smoker | 64 (44) |
| Never smoker | 33 (23) |
| ASA class | |
| I–II | 38 (26) |
| III–V | 107 (74) |
| BMI | 25.6 ± 0.4 |
| Mallampati score | |
| 1–2 | 40 (27.6) |
| 3–4 | 105 (72.4) |
| Neck circumference, cm | 39.7 ± 0.37 |
| Snore | |
| According to patient | 74 (51) |
| According to sleep report | 121 (83) |
| Comorbidities | |
| Alcoholism | 18 (12) |
| Cerebral vascular disease | 18 (12) |
| Chronic obstructive pulmonary disease | 71 (49) |
| Congestive heart failure | 11 (8) |
| Coronary artery disease | 27 (19) |
| Depression | 21 (14) |
| Diabetes mellitus | 21 (14) |
| Liver disease | 20 (14) |
| Malignant solid tumour | 7 (5) |
| Pulmonary neoplasia | 3 (2) |
| Renal disease | 37 (26) |
| Rheumatological disease | 10 (7) |
| Other* | 16 (11) |
*Other includes drug abuse, hematologic malignancy, HIV and immunosuppression
Twenty-eight patients (19%) had an elevated ESS whereas the STOP-BANG was suggestive of 93/145 (64%) high-risk patients (Table 2). Nineteen/28 (68%), 18/28 (64%) and 22/28 (79%) patients with an elevated ESS were concomitantly ranked as high-risk by the Berlin score, the Lausanne No-SAS and the STOP-BANG score respectively.
Table 2.
Data regarding the flexible bronchoscopy and questionnaire scores
| N(%) | |
|---|---|
| Bronchoscopy | |
| Chin support | 116 (80) |
| Non-invasive ventilation | 4 (3) |
| Witnessed apnea | 7 (5) |
| Complications | 7 (5) |
| Procedures | |
| Bronchoalveolar lavage | 135 (93) |
| Bronchial washing | 15 (10) |
| Endobronchial biopsy | 60 (41) |
| Transbronchial lung biopsy | 32 (22) |
| Endobronchial ultrasound needle aspiration | 54 (37) |
| Bronchial brushing | 31 (21) |
| Transbronchial needle aspiration | 14 (10) |
| Endoscopic lung volume reduction | 5 (3) |
| Bronchial thermoplasty | 2 (1) |
| Radial probe endobronchial ultrasound | 6 (4) |
| Questionnaires and Scores | |
| Epworth score ≥ 10 | 28 (19) |
| Berlin score – high risk | 85 (59) |
| NoSAS ≥ 9 | 81 (56) |
| STOP-BANG – high risk | 93 (64) |
The mean recorded time during the polygraphy was 7.2 ± 0.12 h with an average sleep time of 5.3 ± 0.12 h. The snore volume was on average 44 ± 0.76 dB and the average AHI was 20.3 ± 1.2. Information regarding sleep parameters can be found in Additional file 1: Table 1.
A normal AHI was measured in 14/145 (10%) of the patients. We detected mild sleep apnea in 52/145 patients (36%), moderate sleep apnea was observed in 49/145 patients (34%) and severe sleep apnea was seen in 30/145 patients (20%; Fig. 2).
Fig. 2.
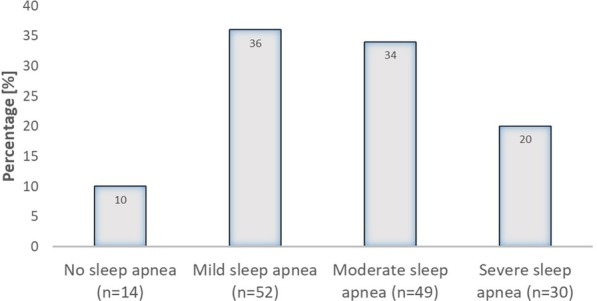
Incidence of sleep apnea in our cohort. Mild sleep apnea was defined as 5/h < AHI ≤ 15/h; moderate sleep apnea was defined as 15/h < AHI ≤ 30/h and severe sleep apnea was defined as AHI > 30/h
There was a significant association between gender and incidence of sleep apnea (p = 0.034) with a higher incidence in males (94%) than females (84%). Oxygen desaturation during bronchoscopy, however, was not associated with gender (p = 0.56). When adjusting for Mallampati score and duration of bronchoscopy, oxygen desaturation during the bronchoscopy still had a significant association with AHI (p = 0.035). Logistic regression analyzing Mallampati score and its association with sleep apnea or no sleep apnea, showed no association whether as a discrete, categorical variable (p = 0.832) or when grouped according to severity (p = 0.477).
There was no association between the prevalence of sleep apnea and snoring as measured during the polygraphy (Pearson Chi-square, p = 0.729). Conversely, there was a significant correlation between AHI and age (Spearman Rho correlation coefficient = 0.254; p = 0.002), BMI (Rho = 0.347; p < 0.001), forced vital capacity (FVC) % predicted (Rho = − 0.188; p = 0.023); and total lung capacity (TLC) %predicted (Rho = − 0.277; p < 0.001). There was no correlation between AHI and pre or post bronchodilator forced expiratory volume in 1 s (FEV1).
Bronchoscopy was performed on average 1.5 ± 0.08 days after polygraphy and had a mean duration of 23.5 ± 1.3 min. The average dosage of propofol needed for sedation was 323 ± 16.5 mg and 105/145 patients (72%) also received hydrocodone as a cough suppressant. There was no association (p = 0.35) between the administration of hydrocodone and sleep apnea. Of the patients receiving hydrocodone, 93/105 (89%) had sleep apnea. Of the patients not administered hydrocodone, 38/40 (95%) had sleep apnea. Hydrocodone had no effect on the incidence of oxygen desaturations (Pearson Chi-square, p = 0.75). Chin support was performed on 116/145 patients (80%), and 4/145 patients (3%) required non-invasive ventilation.
During bronchoscopy, an oxygen saturation of < 90% for ≥ 5 s was measured in 132/145 patients (91%; Fig. 3), an oxygen saturation < 88% for ≥ 5 s was measured in 123/145 patients (85%) and a decrease in oxygen saturation of ≥ 4% of the baseline oxygen saturation was measured in 132/145 patients (91%).
Fig. 3.
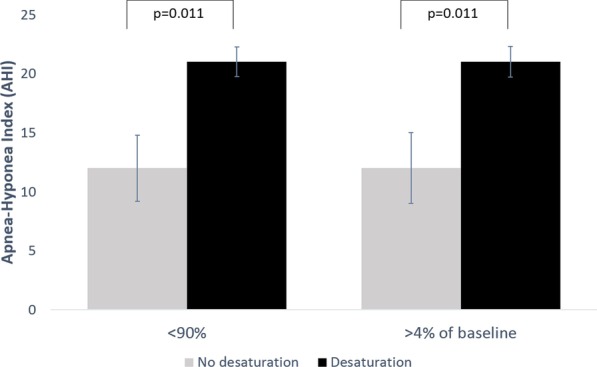
Apnea–Hypopnea Index (AHI) was significantly higher in patients who had any SaO2 < 90% and in patients with a drop in SaO2 of ≥ 4% from baseline compared to patients who did not develop hypoxemia
There was a significant difference in age between the patients who remained normoxemic during the bronchoscopy and the patients who had an oxygen saturation < 90% (57.9 ± 3.5 vs. 66.6 ± 1.1 years; p = 0.017), < 88% (56.0 ± 3.1 vs. 67.5 ± 1.1 years; p = 0.001) and ≥ 4% decrease from baseline (57.9 ± 3.5 vs. 66.6 ± 1.2 years; p = 0.017). There was also a difference in BMI between the patients who remained normoxemic and the patients who had an oxygen saturation < 90% (23.5 ± 1.3 vs 25.8 ± 0.42 kg/m2; p = 0.055), and those who had a ≥ 4% decrease from baseline (23.5 ± 1.3 vs. 25.8 ± 0.4 kg/m2; p = 0.055). Remarkably, lung function parameters such as post-bronchodilator FEV1 (2.1 ± 0.3 vs. 2.3 ± 0.5 L; p = 0.248), post-bronchodilator FVC (3.4 ± 0.4 vs. 3.0 ± 0.1 L; p = 0.262), post-bronchodilator TLC (100 ± 8.5 vs. 107 ± 2.0%; p = 0.355) and post-bronchodilator vital capacity (VC) (3.6 ± 0.35 vs. 3.3 ± 0.1 L; p = 0.408) did not differ significantly between the normoxemic and the hypoxemic patients. Patients with oxygen desaturation during the bronchoscopy had significantly less sleep time during the polygraphy (Table 3).
Table 3.
Baseline characteristics stratified according to whether patients depicted at least one desaturation during bronchoscopy
| No desaturation n = 13 |
Desaturation n = 132 |
p Value | |
|---|---|---|---|
| Sleep time during polygraphy (h) | 6.0 ± 0.3 | 5.2 ± 0.1 | 0.045 |
| Snore | |||
| According to patient | 3 (23) | 71 (54) | 0.035 |
| According to sleep report | 8 (62) | 113 (86) | 0.790 |
| Time between polygraphy/bronchoscopy (days) | 1.5 ± 0.2 | 1.5 ± 0.08 | 0.775 |
| Bronchoscopy procedures | |||
| Bronchoalveolar lavage | 15 (11) | 120 (89) | 0.266 |
| Bronchial washing | 1 (7) | 14 (93) | 0.621 |
| Endobronchial biopsy | 8 (13) | 52 (87) | 0.321 |
| Transbronchial lung biopsy | 1 (3) | 31 (97) | 0.129 |
| Endobronchial ultrasound needle aspiration | 3 (6) | 51 (94) | 0.145 |
| Bronchial brushing | 2 (6) | 29 (94) | 0.422 |
| Transbronchial needle aspiration | 1 (7) | 13 (93) | 0.679 |
| Endoscopic lung volume reduction | 0 (0) | 5 (100) | 0.440 |
| Bronchial thermoplasty | 0 (0) | 2 (100) | 0.629 |
| Radial probe endobroncial ultrasound | 0 (0) | 6 (100) | 0.395 |
| Bronchoscopy duration (min) | 11.05 ± 1.99 | 24.7 ± 81.37 | 0.001 |
| Total Propofol dose (mg) | 210.9 ± 31.4 | 334 ± 17.6 | 0.019 |
| Questionnaires and Scores | |||
| Epworth score ≥ 10 | 4 (31) | 24 (18) | 0.273 |
| Berlin score – high risk | 6 (46) | 79 (60) | 0.339 |
| NoSAS ≥ 9 | 4 (31) | 77 (58) | 0.056 |
| STOP-BANG-high risk** | 5 (38) | 88 (67) | 0.043 |
P-values < 0.05 are shown in bold
**STOP-BANG snoring, tired, observed, pressure, body mass index, age, neck size, gender
Patients with oxygen desaturation < 90% had significantly higher AHI values compared to patients with no oxygen desaturation (Fig. 3). Thus, there was a significant association between AHI and oxygen desaturation < 90% (β 9.082 CI 0.982–17.182, p = 0.028) suggesting that patients presenting oxygen desaturation < 90% during bronchoscopy had an AHI 9.1/h higher than patients with no oxygen desaturation during bronchoscopy. This association remained significant after adjusting for the duration of the procedure and for the administered propofol dose (β 9.813 CI 1.382–18.243 p = 0.023) and also held true when examining desaturation < 90% for ≥ 1 min (Additional file 1: Fig. 1). The association disappeared when adjusting for gender, age and BMI together (β 5.729 CI − 2.261–13.719; p = 0.159).
When using polygraphy as a reference standard, oxygen desaturation during bronchoscopy had a sensitivity of 92% and a specificity of 14% to diagnose sleep apnea. The average score for the Epworth sleepiness questionnaire and the NoSAS score according to oxygen desaturation or no oxygen desaturation is depicted in Additional file 1: Table 2. STOP-BANG had the highest sensitivity and ESS the lowest sensitivity to diagnose sleep apnea when using either AHI or oxygen desaturation during bronchoscopy as reference standards (Table 4).
Table 4.
Sensitivity, specificity, predictive values and likelihood ratios of the various questionnaires in relation to AHI and desaturation during bronchoscopy
| Berlin Questionnaire | Epworth Sleepiness Scale | Lausanne NoSAS | STOP-BANG | |
|---|---|---|---|---|
| Using sleep apnea assessed by polygraphy as reference standard | ||||
| Sensitivity | 59 | 20 | 58 | 69 |
| Specificity | 43 | 86 | 64 | 79 |
| Positive predictive value | 91 | 93 | 94 | 97 |
| Negative predictive value | 10 | 10 | 14 | 21 |
| Positive likelihood ratio | 1.0 | 1.6 | 1.6 | 3.2 |
| Negative likelihood ratio | 0.96 | 0.94 | 0.66 | 0.4 |
| Using desaturation during bronchoscopy as reference standard | ||||
| Sensitivity | 60 | 18 | 58 | 67 |
| Specificity | 54 | 69 | 69 | 62 |
| Positive predictive value | 93 | 86 | 95 | 95 |
| Negative predictive value | 12 | 7.7 | 14 | 15 |
| Positive likelihood ratio | 1.3 | 0.59 | 1.9 | 1.8 |
| Negative likelihood ratio | 0.74 | 1.2 | 0.61 | 0.54 |
The sensitivity and specificity of oxygen desaturation < 90% during bronchoscopy to diagnose obstructive sleep apnea syndrome (OSAS) using AHI > 5/h and a positive symptom score as a reference standard is depicted in Table 5.
Table 5.
The sensitivity and specificity of desaturation during bronchoscopy for determining OSAS using AHI > 5/h and a positive symptom score using the Berlin questionnaire, Epworth sleepiness scale, Lausanne NoSAS and STOP-BANG as a reference standard
| OSAS using Berlin Questionnaire | OSAS using Epworth Sleepiness Scale | OSAS using Lausanne NoSAS | OSAS using STOP-BANG | |
|---|---|---|---|---|
| Sensitivity | 92 | 85 | 95 | 94 |
| Specificity | 10 | 7.6 | 13 | 15 |
| Positive predictive value | 54 | 17 | 55 | 64 |
| Negative predictive value | 54 | 69 | 69 | 62 |
| Positive likelihood ratio | 1.0 | 0.9 | 1.1 | 1.1 |
| Negative likelihood ratio | 0.8 | 1.97 | 0.38 | 0.40 |
The values changed when taking into consideration sleep apnea syndrome with a pAHI ≥ 15 (Additional file 1: Table 3).
Discussion
Transient hypoxemia is known to occur in patients undergoing FB, however, the association between transient hypoxemia during FB and OSA is unknown. We found that patients who experienced hypoxemia during FB under conscious sedation had increased AHI in the polygraphy. The association between oxygen desaturation < 90% during bronchoscopy and AHI remained significant after adjustment for duration of the procedure and propofol dose. Accordingly, patients presenting oxygen desaturation < 90% during bronchoscopy had a 9.1/h higher AHI than those not presenting oxygen desaturation during the procedure.
Hypoxia is the most commonly cited adverse event in patients undergoing bronchoscopy [16, 17, 28]. The occurrence of hypoxemia was highly prevalent during FB in our study, with an oxygen saturation of < 90% for ≥ 5 s measured in 132 patients (91%). This reflects the severity and multi-morbidity of the patient population included in the study with almost 50% of the participants suffering from COPD and 74% classified as ASA Class III or higher. In a recent study by Cho et al. [18] they had an incidence of hypoxemia of 35% during moderate sedation bronchoscopy. Their population consisted of 35% ASA III patients and 65% ASA II patients. Although ASA classification was introduced as a subjectively determined marker of general health used to evaluate perioperative morbidity [29], it was also reported as a predictor for occurrence of adverse events, including hypoxemia, during endoscopic procedures [30–32]. Nonetheless, the occurrence of hypoxemia during flexible bronchoscopy with sedation is similar in studies with and without supplemental oxygen [16, 33–35]. A recent study in Norway has shown that when patients chose to have no sedation, it significantly increased unplanned interventions during the bronchoscopy [17].
Although the safety and efficacy of propofol used as a sedative during bronchoscopy has been explored [21–23, 25], airway collapsibility is affected by propofol in a dose dependent manner making proper titration a decisive factor in avoiding overestimating the severity of airway obstruction and implicitly OSA [36]. The mean dose of propofol required to achieve proper sedation in the present study was slightly higher than previously reported [21, 22, 37]. However, the number of complex procedures performed during bronchoscopy has increased compared to earlier studies, thus underpinning a higher sedative requirement. In addition the intravenous continuous infusion of propofol, which is as safe as bolus administration, is known to be associated with an increased dosage during FB [25]. Endoscopic procedures known as drug-induced sleep endoscopy (DISE) are widely recognized as diagnostic instruments for OSA [38] due to induction of airway obstruction and collapse during sedation [39]. Propofol is the recommended pharmacologic agent for DISE [38]. The use of propofol to induce a sleep-like state, mimics the drop in oxygen saturation seen during polysomnography and the respiratory events are comparable to the respiratory events seen during polysomnography [40, 41].
We found that the association between oxygen desaturation during bronchoscopy and higher AHI remained significant after adjusting for propofol dose even though patients do not enter REM sleep during the bronchoscopy. It has been shown that most of the respiratory events in sleep apnea occur during N2 sleep [40, 42–44].
Sleep apnea assessed using polygraphy, was highly prevalent in our population with 90% of patients having an AHI above 5/h, even though BMI and neck circumference were normal. Similar results were found by Cho et al. [18] who, using the STOP-BANG score ≥ 3 found that 67% of their patients were at high risk of sleep apnea even though the average neck circumference and BMI were in the normal range. The high prevalence of sleep apnea found in the present study is similar to values previously reported [45]. Although the subjects enrolled in the other study had similar BMI (25.6 vs 25.6) and neck circumference (36.9 vs 39.7) the patients in our study were older (57 vs 66 years) [45]. In addition, a moderate to severe sleep apnea was observed in 54% of our patients and a gender difference in prevalence was evident. These results are similar to previously published rates of sleep apnea in the general population [45–47]. The difference in sleep apnea incidence between males and females may be due to differences in upper airway mechanical or neuromuscular properties, chemoreflex control of breathing or sex hormone levels [46]. There was, however, no difference in the occurrence of oxygen desaturation during bronchoscopy when comparing males and females.
The Mallampati index is a quick instrument for assessing airway patency before intubation [48]. There is growing data pointing out the association of a higher Mallampati index to severity of OSA [49, 50]. Most of the patients enrolled in our study (72%) had a Mallampati index of three or more. In the study by Wang et al., they found that 85% of patients with sleep apnea had a Mallampati score of three or higher [51]. In addition, Sharara et al. [52] also found a Mallampati score of at least three with a prevalence of 83% in a population of snorers. Furthermore, in our study the Mallampati score was assessed in the supine position, which may have led to an increased number of patients being graded with a Mallampati score of three or four. In a study by Bindra et al. [53] the number of patients with a Mallampati score of three or four, more than doubled when assessed in the supine position. We, however, found no association between Mallampati index and sleep apnea or no sleep apnea.
The incidence of OSAS ranged between 15 and 59% depending on the questionnaire or score used. This was much higher than the incidence reported in the literature [54–56] and could be due to the population studied, as our population was older and had more severe disease.
In our study, patients who had hypoxemia during flexible bronchoscopy under conscious sedation had increased AHI as measured by polygraphy. Harvin et al. found that patients with a high risk of sleep apnea as assessed by the Berlin score had a higher rate of hypoxemia during conscious sedation for colonoscopy [28]. It is unclear whether this association remained true after adjusting for confounding variables. Cho et al. found an association between a high risk for sleep apnea, assessed using the STOP-BANG score, and cardiopulmonary events, such as hypoxia, during bronchoscopy [18]. As with male gender, there is also a known association between sleep apnea, age and BMI [47]. Thus both characteristics are also included in sleep apnea screening tools like Lausanne NoSAS [12] and the STOP-Bang questionnaire [11]. The association between AHI and oxygen desaturation disappeared in a multivariate analysis when adjusting for age and BMI and duration of bronchoscopy. Whereas age might be a confounder, BMI is probably related to effect modification, as risks tend to increase as a certain cut-off is reached.
In our non-selected group of patients, we had an incidence of overlap syndrome (COPD + sleep apnea) in 66/145 patients (46%). This is similar to incidence rates observed by Wang et al. [57] and Zhang et al. [58].
In our study, the Berlin Questionnaire had a 59% sensitivity and 43% specificity to identify sleep apnea as assessed by polygraphy. Only the STOP-BANG score was associated with oxygen desaturation during flexible bronchoscopy. This corroborates the findings of Cho et al. [18]. The sensitivity and specificity of ESS was low and no association was seen with AHI. Conversely, Johns MW [9] found that a higher ESS score correlates with the respiratory index measured during polysomnography and with the minimum oxygen saturation measured during the night.
The key message of the present study is that oxygen desaturation during endoscopic procedures, in particular flexible bronchoscopy, should prompt the treating physician to screen for sleep apnea. This could prove to be essential especially if we consider the prevalence of sleep apnea in this study, which is higher than in other epidemiologic studies. It also underlines that foremost in patient populations with high number of comorbidities, some of the screening tools, as for example the questionnaires evaluated in our study (NoSAS, STOP-BANG and ESS), could underestimate the prevalence of sleep-disordered breathing.
Limitations of this study include the fact that the sedation was applied by a trained nurse on the basis of clinical evaluation of sedation level (i.e. to achieve ptosis). This may result in an excessive sedation. Also, this was a single-center study in a tertiary hospital with patients with high ASA scores, therefore, the results may not be applicable to the general population.
Sleep apnea was determined by polygraphy and not polysomnography. However, polygraphy is confirmed as a viable diagnostic tool for sleep apnea and is included in the clinical algorithm for implementation of clinical practice guidelines by the American Academy of Sleep Medicine [59]. The high agreement ratio between polygraphy and polysomnography [60] may be attributable to the development of the peripheral arterial tonometry signal (PAT) which addresses the inability of other home sleep apnea tests to record and stage sleep. The algorithm used for sleep/wake detection and categorizing sleep stages REM/NREM have been described [60, 61]. The overall agreement in detecting REM/NREM sleep was 88.7%/88.6%, respectively when compared to polysomnography [62]. The limitation in using WatchPAT is that it is unable to reliably differentiate sleep apnea into obstructive, central or obstructive/central.
Another possible limitation is that since the exclusion criteria were few and the possibility to participate in the study was open, prevalently patients concerned about having a sleep disorder would participate. We don’t feel that this bias existed in our population. According to the Epworth Sleepiness Scale results, which is the reference standard used to determine the symptoms experienced by the patient, only 28/145 patients in the study actually had symptoms of a sleep disorder. Most of the patients had no symptoms even though they were at high risk of developing sleep apnea as shown with the STOP-BANG score, in which 93/145 (64%) patients had a score ≥ 3. In addition, 5.6% of the screened patients refused participation.
The strength of our study is the objective assessment of sleep apnea before undergoing bronchoscopy and the clinical applicability of study results.
In conclusion, oxygen desaturations during FB are associated with more severe sleep apnea. This association remains significant after adjusting for sedative dose and duration of procedure. It appears justifiable to consider sleep apnea screening for patients with oxygen desaturation during bronchoscopy.
Supplementary information
Additional file 1: Table 1. Variables related to sleep apnea measured during REM sleep and non-REM sleep. Table 2. Epworth sleepiness score and Lausanne NoSAS were not associated with oxygen desaturation during conscious bronchoscopy. Table 3. The sensitivity and specificity of OSAS as calculated from AHI > 15/h and a positive symptom score using the Berlin questionnaire, Epworth sleepiness scale, Lausanne NoSAS and STOP-BANG and using desaturation as a reference standard. Figure 1. Apnea-Hypopnea Index (AHI) was significantly higher in patients who had any SaO2 < 90% for ≥ 1min compared to patients who did not develop hypoxemia.
Acknowledgements
Not applicable.
Abbreviations
- AHI
Apnea–hypopnea index
- ASA
American Society of Anesthesiologists
- BMI
Body mass index
- COPD
Chronic obstructive pulmonary disease
- CPAP
Continuous positive airway pressure
- DISE
Drug-induced sleep endoscopy
- ESS
Epworth sleepiness scale
- FB
Flexible bronchoscopy
- FEV1
Forced expiratory volume in 1 second
- FVC
Forced vital capacity
- OSA
Obstructive sleep apnea
- OSAS
Obstructive sleep apnea syndrome
- STOP-BANG
Snoring, tired, observed, pressure, body mass index, age, neck circumference, gender
Authors’ contributions
All authors listed have substantial contributions to the conception or design of the work, or the acquisition, analysis or interpretation of data. All authors listed have been involved in drafting the work or revising it critically for important intellectual content. All authors listed have provided final approval of the version published. All authors listed have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Funding
There is no funding to report for this submission.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The Study was approved by the Ethics Committee northwest/central Switzerland, EKNZ (EK 16/13) and was carried out according to the Declaration of Helsinki and Good Clinical Practice guidelines.
Consent for publication
Not applicable.
Competing interests
The authors of this manuscript have no conflicts of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12931-020-01573-z.
References
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNicholas WT., Bonsigore MR., Management Committee of EU Cost Action B26 Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29:156–178. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 3.Kent BD, Grote L, Ryan S, Pepin JL, Bonsignore MR, Tkacova R, Saaresranta T, Verbraecken J, Levy P, Hedner J, McNicholas WT. Diabetes mellitus prevalence and control in sleep-disordered breathing: the European Sleep Apnea Cohort (ESADA) study. Chest. 2014;146:982–990. doi: 10.1378/chest.13-2403. [DOI] [PubMed] [Google Scholar]
- 4.Oh MS, Bliwise DL, Smith AL, Collop NA, Quyyumi AA, Dedhia RC. Obstructive sleep apnea, sleep symptoms, and their association with cardiovascular disease. Laryngoscope. 2019;130:1595–1602. doi: 10.1002/lary.28293. [DOI] [PubMed] [Google Scholar]
- 5.Mulgrew AT, Nasvadi G, Butt A, Cheema R, Fox N, Fleetham JA, Ryan CF, Cooper P, Ayas NT. Risk and severity of motor vehicle crashes in patients with obstructive sleep apnea/hypopnoea. Thorax. 2008;63:536–541. doi: 10.1136/thx.2007.085464. [DOI] [PubMed] [Google Scholar]
- 6.Jackson ML, Tolson J, Bartlett D, Berlowitz DJ, Varma P, Barnes M. Clinical depression in untreated obstructive sleep apnea: examining predictors and a meta-analysis of prevalence rates. Sleep Med. 2019;27:22–28. doi: 10.1016/j.sleep.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Zheng D, Xu Y, You S, Hackett ML, Woodman RJ, Li Q, Woodward M, Loffler KA, Rodgers A, Drager LF, et al. Effects of continuous positive airway pressure on depression and anxiety symptoms in patients with obstructive sleep apnoea: results from the sleep apnoea cardiovascular Endpoint randomised trial and meta-analysis. EClinicalMedicine. 2019;11:89–96. doi: 10.1016/j.eclinm.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–706. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 9.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 10.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 11.Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149:631–638. doi: 10.1378/chest.15-0903. [DOI] [PubMed] [Google Scholar]
- 12.Marti-Soler H, Hirotsu C, Marques-Vidal P, Vollenweider P, Waeber G, Preisig M, Tafti M, Tufik SB, Bittencourt L, Tufik S, et al. The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med. 2016;4:742–748. doi: 10.1016/S2213-2600(16)30075-3. [DOI] [PubMed] [Google Scholar]
- 13.Maislin G, Pack AI, Kribbs NB, Smith PL, Schwartz AR, Kline LR, Schwab RJ, Dinges DF. A survey screen for prediction of apnea. Sleep. 1995;18:158–166. doi: 10.1093/sleep/18.3.158. [DOI] [PubMed] [Google Scholar]
- 14.British Thoracic Society Bronchoscopy Guidelines Committee British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax. 2001;56(Suppl 1):i1–21. doi: 10.1136/thorax.56.suppl_1.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chhajed PN, Aboyoun C, Malouf MA, Hopkins PM, Plit M, Grunstein RR, Glanville AR. Prophylactic nasopharyngeal tube insertion prevents acute hypoxaemia due to upper-airway obstruction during flexible bronchoscopy. Intern Med J. 2003;33:317–318. doi: 10.1046/j.1445-5994.2003.00403.x. [DOI] [PubMed] [Google Scholar]
- 16.Leiten EO, Martinsen EM, Bakke PS, Eagan TM, Grønseth R. Complications and discomfort of bronchoscopy: a systematic review. Eur Clin Respir J. 2016;3:33324. doi: 10.3402/ecrj.v3.33324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leiten EO, Eagan TML, Martinsen EMH, Nordeide E, Husebø GR, Knudsen KS, Lehmann S, Svanes Ø, Bakke PS, Nielsen R. Complications and discomfort after research bronchoscopy in the MicroCOPD study. BMJ Open Respir Res. 2020;7:e000449. doi: 10.1136/bmjresp-2019-000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho J, Choi SM, Park YS, Lee CH, Lee SM, Yoo CG, Kim YW, Lee J. Prediction of cardiopulmonary events using the STOP-Bang questionnaire in patients undergoing bronchoscopy with moderate sedation. Sci Rep. 2020;10:14471. doi: 10.1038/s41598-020-71314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu HY, Chen PY, Chuang LP, Chen NH, Tu YK, Hsieh YJ, Wang YC, Guilleminault C. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57–70. doi: 10.1016/j.smrv.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Stolz D, Chhajed PN, Leuppi J, Pflimlin E, Tamm M. Nebulized lidocaine for flexible bronchoscopy: a randomized, double-blind, placebo-controlled trial. Chest. 2005;128:1756–1760. doi: 10.1016/S0012-3692(15)52214-9. [DOI] [PubMed] [Google Scholar]
- 21.Stolz D, Kurer G, Meyer A, Chhajed PN, Pflimlin E, Strobel W, Tamm M. Propofol versus combined sedation in flexible bronchoscopy: a randomised non-inferiority trial. Eur Respir J. 2009;34:1024–1030. doi: 10.1183/09031936.00180808. [DOI] [PubMed] [Google Scholar]
- 22.Schlatter L, Pflimlin E, Fehrke B, Meyer A, Tamm M, Stolz D. Propofol versus propofol plus hydrocodone for flexible bronchoscopy: a randomised study. Eur Respir J. 2011;38:529–537. doi: 10.1183/09031936.00121610. [DOI] [PubMed] [Google Scholar]
- 23.Grendelmeier P, Kurer G, Pflimlin E, Tamm M, Stolz D. Feasibility and safety of propofol sedation in flexible bronchoscopy. Swiss Med Wkly. 2011;141:w13248. doi: 10.4414/smw.2011.13248. [DOI] [PubMed] [Google Scholar]
- 24.Grendelmeier P, Tamm M, Jahn K, Pflimlin E, Stolz D. Flexible bronchoscopy with moderate sedation in COPD: a case-control study. Int J Chron Obstruct Pulmon Dis. 2017;12:177–187. doi: 10.2147/COPD.S119575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grendelmeier P, Tamm M, Pflimlin E, Stolz D. Propofol sedation for flexible bronchoscopy: a randomised, noninferiority trial. Eur Respir J. 2014;43:591–601. doi: 10.1183/09031936.00200412. [DOI] [PubMed] [Google Scholar]
- 26.Chhajed PN, Wallner J, Stolz D, Baty F, Strobel W, Brutsche MH, Tamm M. Sedative drug requirements during flexible bronchoscopy. Respiration. 2005;72:617–621. doi: 10.1159/000089577. [DOI] [PubMed] [Google Scholar]
- 27.Stolz D, Chhajed PN, Leuppi JD, Brutsche M, Pflimlin E, Tamm M. Cough suppression during flexible bronchoscopy using combined sedation with midazolam and hydrocodone: a randomised, double blind, placebo controlled trial. Thorax. 2004;59:773–776. doi: 10.1136/thx.2003.019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harvin G, Ali E, Raina A, Leland W, Abid S, Vahora Z, Movahed H, Kachru S, Tee R. Patients presenting for colonoscopy: a great opportunity to screen for sleep apnea. World J Gastrointest Endosc. 2016;8:697–700. doi: 10.4253/wjge.v8.i19.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sidi A, Lobato EB, Cohen JA. The American Society of Anesthesiologists' Physical Status: category V revisited. J Clin Anesth. 2000;12:328–334. doi: 10.1016/S0952-8180(00)00168-9. [DOI] [PubMed] [Google Scholar]
- 30.Müller S, Prolla JC, Maguilnik I, Breyer HP. Predictive factors of oxygen desaturation of patients submitted to endoscopic retrograde cholangiopancreatography under conscious sedation. Arq Gastroenterol. 2004;41:162–166. doi: 10.1590/S0004-28032004000300005. [DOI] [PubMed] [Google Scholar]
- 31.Vargo JJ, Holub JL, Faigel DO, Lieberman DA, Eisen GM. Risk factors for cardiopulmonary events during propofol-mediated upper endoscopy and colonoscopy. Aliment Pharmacol Ther. 2006;24:955–963. doi: 10.1111/j.1365-2036.2006.03099.x. [DOI] [PubMed] [Google Scholar]
- 32.Sharma VK, Nguyen CC, Crowell MD, Lieberman DA, de Garmo P, Fleischer DE. A national study of cardiopulmonary unplanned events after GI endoscopy. Gastrointest Endosc. 2007;66:27–34. doi: 10.1016/j.gie.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 33.Milman N, Faurschou P, Grode G, Jorgensen A. Pulse oximetry during fibreoptic bronchoscopy in local anaesthesia: frequency of hypoxaemia and effect of oxygen supplementation. Respiration. 1994;61:342–347. doi: 10.1159/000196366. [DOI] [PubMed] [Google Scholar]
- 34.Fang WF, Chen YC, Chung YH, Woon WT, Tseng CC, Chang HW, Lin MC. Predictors of oxygen desaturation in patients undergoing diagnostic bronchoscopy. Chang Gung Med J. 2006;29:306–312. [PubMed] [Google Scholar]
- 35.Jones AM, O'Driscoll R. Do all patients require supplemental oxygen during flexible bronchoscopy? Chest. 2001;119:1906–1909. doi: 10.1378/chest.119.6.1906. [DOI] [PubMed] [Google Scholar]
- 36.Eastwood PR, Platt PR, Shepherd K, Maddison K, Hillman DR. Collapsibility of the upper airway at different concentrations of propofol anesthesia. Anesthesiology. 2005;103:470–477. doi: 10.1097/00000542-200509000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Chhajed PN, Aboyoun C, Chhajed TP, Malouf MA, Harrison GA, Tamm M, Leuppi JD, Glanville AR. Sedative drug requirements during bronchoscopy are higher in cystic fibrosis after lung transplantation. Transplantation. 2005;80:1081–1085. doi: 10.1097/01.tp.0000176925.13074.90. [DOI] [PubMed] [Google Scholar]
- 38.De Vito A, Carrasco Llatas M, Ravesloot MJ, Kotecha B, De Vries N, Hamans E, Maurer J, Bosi M, Blumen M, Heiser C, et al. European position paper on drug-induced sleep endoscopy: 2017 update. Clin Otolaryngol. 2018;43:1541–1552. doi: 10.1111/coa.13213. [DOI] [PubMed] [Google Scholar]
- 39.Ehsan Z, Mahmoud M, Shott SR, Amin RS, Ishman SL. The effects of anesthesia and opioids on the upper airway: a systematic review. Laryngoscope. 2016;126:270–284. doi: 10.1002/lary.25399. [DOI] [PubMed] [Google Scholar]
- 40.Ghorbani J, Adimi Naghan P, Safavi Naeini A, Sadeghi HK. Can be compared obstructive respiratory events during drug induced sleep endoscopy (DISE) and nocturnal polysomnography. Eur Arch Otorhinolaryngol. 2020;277:1379–1384. doi: 10.1007/s00405-020-05848-5. [DOI] [PubMed] [Google Scholar]
- 41.Viana A, Zhao C, Rosa T, Couto A, Neves DD, Araújo-Melo MH, Capasso R. The effect of sedating agents on drug-induced sleep endoscopy findings. Laryngoscope. 2019;129:506–513. doi: 10.1002/lary.27298. [DOI] [PubMed] [Google Scholar]
- 42.Carrasco Llatas M, Agostini Porras G, Cuesta González MT, Rodrigo Sanbartolomé A, Giner Bayarri P, Gómez-Pajares F, Dalmau GJ. Drug-induced sleep endoscopy: a two drug comparison and simultaneous polysomnography. Eur Arch Otorhinolaryngol. 2014;271:181–187. doi: 10.1007/s00405-013-2548-3. [DOI] [PubMed] [Google Scholar]
- 43.Alzoubaidi M, Mokhlesi B. Obstructive sleep apnea during rapid eye movement sleep: clinical relevance and therapeutic implications. Curr Opin Pulm Med. 2016;22:545–554. doi: 10.1097/MCP.0000000000000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al Oweidat K, AlRyalat SA, Al-Essa M, Obeidat N. Comparing REM- and NREM-related obstructive sleep apnea in Jordan: a cross-sectional study. Can Respir J. 2018;2018:9270329. doi: 10.1155/2018/9270329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, Mooser V, Preisig M, Malhotra A, Waeber G, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonsignore MR, Saaresranta T, Riha RL. Sex differences in obstructive sleep apnoea. Eur Respir Rev. 2019 doi: 10.1183/16000617.0030-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, Hamilton GS, Dharmage SC. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Mallampati SR, Gatt SP, Gugino LD, Desai SP, Waraksa B, Freiberger D, Liu PL. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32:429–434. doi: 10.1007/BF03011357. [DOI] [PubMed] [Google Scholar]
- 49.Amra B, Pirpiran M, Soltaninejad F, Penzel T, Fietze I, Schoebel C. The prediction of obstructive sleep apnea severity based on anthropometric and Mallampati indices. J Res Med Sci. 2019;24:66. doi: 10.4103/jrms.JRMS_653_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davidson TM, Patel MR. Waist circumference and sleep disordered breathing. Laryngoscope. 2008;118:339–347. doi: 10.1097/MLG.0b013e3181587d7c. [DOI] [PubMed] [Google Scholar]
- 51.Wang WM, Hsu YB, Lan MY, Yang MC, Huang TT, Liu CJ, Lan MC. The relationship between modified Mallampati Score, Müller's Maneuver and drug-induced sleep endoscopy regarding retrolingual obstruction. Ann Otol Rhinol Laryngol. 2018;127:463–469. doi: 10.1177/0003489418778302. [DOI] [PubMed] [Google Scholar]
- 52.Sharara AI, El Zahabi L, Maasri K, Hashash JG, Mansour N, Skoury A, Kanafani Z, Bou-Khalil P, Husari A. Persistent snoring under conscious sedation during colonoscopy is a predictor of obstructive sleep apnea. Gastrointest Endosc. 2010;71:1224–1230. doi: 10.1016/j.gie.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 53.Bindra A, Prabhakar H, Singh GP, Ali Z, Singhal V. Is the modified Mallampati test performed in supine position a reliable predictor of difficult tracheal intubation? J Anesth. 2010;24:482–485. doi: 10.1007/s00540-010-0905-6. [DOI] [PubMed] [Google Scholar]
- 54.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11:441–446. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 55.Bednarek M, Plywaczewski R, Jonczak L, Zielinski J. There is no relationship between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome: a population study. Respiration. 2005;72:142–149. doi: 10.1159/000084044. [DOI] [PubMed] [Google Scholar]
- 56.Taj F, Aly Z, Kassi M, Ahmed M. Identifying people at high risk for developing sleep apnea syndrome (SAS): a cross-sectional study in a Pakistani population. BMC Neurol. 2008;8:50. doi: 10.1186/1471-2377-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Li B, Li P, Gong T, Wu M, Fu J, Nie M, Dong Y, Hu K. Severe obstructive sleep apnea in patients with chronic obstructive pulmonary disease is associated with an increased prevalence of mild cognitive impairment. Sleep Med. 2020;75:522–530. doi: 10.1016/j.sleep.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 58.Zhang XL, Gao B, Han T, Xiang BY, Liu X. Moderate-to-severe obstructive sleep apnea and cognitive function impairment in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:1813–1822. doi: 10.2147/COPD.S257796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, Harrod CG. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13:479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pittman SD, Ayas NT, MacDonald MM, Malhotra A, Fogel RB, White DP. Using a wrist-worn device based on peripheral arterial tonometry to diagnose obstructive sleep apnea: in-laboratory and ambulatory validation. Sleep. 2004;27:923–933. doi: 10.1093/sleep/27.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herscovici S, Pe'er A, Papyan S, Lavie P. Detecting REM sleep from the finger: an automatic REM sleep algorithm based on peripheral arterial tone (PAT) and actigraphy. Physiol Meas. 2007;28:129–140. doi: 10.1088/0967-3334/28/2/002. [DOI] [PubMed] [Google Scholar]
- 62.Hedner J, White DP, Malhotra A, Herscovici S, Pittman SD, Zou D, Grote L, Pillar G. Sleep staging based on autonomic signals: a multi-center validation study. J Clin Sleep Med. 2011;7:301–306. doi: 10.5664/JCSM.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table 1. Variables related to sleep apnea measured during REM sleep and non-REM sleep. Table 2. Epworth sleepiness score and Lausanne NoSAS were not associated with oxygen desaturation during conscious bronchoscopy. Table 3. The sensitivity and specificity of OSAS as calculated from AHI > 15/h and a positive symptom score using the Berlin questionnaire, Epworth sleepiness scale, Lausanne NoSAS and STOP-BANG and using desaturation as a reference standard. Figure 1. Apnea-Hypopnea Index (AHI) was significantly higher in patients who had any SaO2 < 90% for ≥ 1min compared to patients who did not develop hypoxemia.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


