Abstract
This study examined the relationship between self-reported facial masking and quality of life (QoL) in people with Parkinson’s disease (PD), and tested experienced stigma as a mediator and gender as a moderator of this relationship. The strength of stigma as a mediator was compared against an alternative mediator, depression. Ninety people with PD (34 women) rated difficulty showing facial expression (masking), and completed the Stigma Scale for Chronic Illness, Geriatric Depression Scale (15-item), and Parkinson’s Disease Questionnaire-39. A conditional process model tested the indirect effect of facial masking on QoL through stigma, separately for women and men. A parallel indirect model included both stigma and depression to compare their statistical and clinical significance as mediators. Gender-moderated mediation of stigma reduced the association between facial masking and QoL to non-significance, suggesting stigma explained the association between facial masking and QoL. While facial masking was more stigmatizing for women than for men, stigma mediated the facial masking-QoL association for both women and men. Stigma (controlling for depression) reached a statistically and clinically significant level of mediation, whereas depression (controlling for stigma) reached a statistically yet not clinically significant level of mediation. People with PD who experience more severe facial masking feel more stigmatized, especially women. Regardless of gender, an increase in stigma from facial masking increases the likelihood of compromised QoL that reaches both statistical and clinical levels of significance.
Keywords: Parkinson’s disease, facial expression, quality of life, stigma, depression, moderated mediation, gender
Parkinson’s disease (PD) is a chronic neurodegenerative disorder which affects voluntary and spontaneous movement throughout the body, including the face (Bologna et al., 2013). Decreased ability to move facial musculature, termed facial masking or hypomimia, hinders the individual’s ability to express emotions, thoughts, and intentions to others. Qualitative research findings suggest that loss of facial expression may influence subjective well-being in people with PD, and that this may occur through a process of stigmatization (Nijhof, 1995; Caap-Ahlgren & Lannerheim, 2002). The inability to move facial musculature is a deviation from the norms of social competence, and people with PD report that difficulty with social interaction is one of their most severe psychosocial symptoms (Abundi et al., 1997). One’s subsequent feelings of stigma, including distress and embarrassment, may be further aggravated through difficulties with social interaction when encountering others’ negative reactions, such as staring or questioning about communication difficulties (Nijhof, 1995; Rao et al., 2009). Individuals may feel trapped in a mask, gradually altering their self-identity and isolating them from family, friends, and finally the outside world (Nijhof, 1995; Chiong-Rivero et al., 2011). It is thought that these problematic outcomes occur because the face is a primary medium of verbal and nonverbal communication, and people are able to perceive expressive individuals more accurately than inexpressive individuals (Ekman, 1989; Snodgrass, Hecht, & Ploutz-Snyder, 1998).
Indeed, observer-centered experimental and controlled cross-sectional studies have demonstrated that people with PD who have a moderate degree of facial masking, compared to those with minimal impairment, are perceived more negatively. Health care practitioners see those with more facial masking as more depressed, less sociable, and less cognitively competent than their actual attributes (Tickle-Degnen, Zebrowitz, & Ma, 2011). The detrimental effect of a higher degree of masking extends to first impressions formed by older adult observers, especially for emotional compared to instrumental social exchanges (Hemmesch, Tickle-Degnen, & Zebrowitz, 2009; Hemmesch, 2014). There appear to be consequences within families as well. One study found that the more that care partners perceive their spouses with PD to have difficulty showing facial expression, the less they report enjoying their interactions with their spouses (Gunnery, Habermann, Saint-Hilaire, Thomas, & Tickle-Degnen, 2016).
Although research findings suggest that facial masking creates negatively biased impressions of both women and men, this bias is greater in impressions of women (Hemmesch et al., 2009, Tickle-Degnen et al., 2011; Hemmesch, 2014). Gender norms would predict that facial masking leads to more severe stigmatization of women than of men (Hemmesch et al., 2009). Cross-culturally, society expects women to use more emotionally expressive and socially engaging nonverbal behavior than men. When these expectations are met, women are socially rewarded, and when not, they are subtly punished (Briton & Hall, 1995).
Quantitative research in this area focuses on the influence of observed measurement of masking on observers’ formation of negatively biased impressions. Very few controlled studies have extended this observer-centered stigmatization effect systematically to a person-centered effect by examining experienced difficulty of showing facial expression and consequences for feelings of stigmatization and QoL.
Within the baseline sample of the Emergence and Evolution of Social Self-Management of Parkinson’s Disease study (SocM-PD; Tickle-Degnen et al., 2014), an in progress 3-year prospective cohort study, we have begun to address this gap. Recent work conducted with subsets of the sample investigated the relationship between self-reported facial masking and social wellbeing (Gunnery et al., 2016) and between stigma and QoL (Ma, Saint-Hilaire, Thomas, & Tickle-Degnen, 2016b). In 40 participants and their care partners, we found a relationship between how much difficulty people thought they had showing expression in their face and how much social rejection (a combination of experienced stigma and social isolation) they experienced, but this relationship was no longer significant when depression was included in the model (Gunnery et al., 2016). In a study of a larger portion of the same sample (N = 73), we demonstrated that experienced stigma was a determinant of QoL, after controlling for depression and motor experiences of daily living (Ma et al., 2016). These two studies show a connection between self-reported facial masking and negative social wellbeing, and between stigma and quality of life in people with PD, while both addressing the role of depression as a possible contributor.
To build on this recent work and develop a person-centered perspective of the pathway from facial masking and stigmatization to QoL outcomes, the present study utilized the full baseline sample of 90 participants with PD of the Soc-M PD study. Our first aim was to investigate the influence of one’s experienced difficulty in producing facial expression on QoL outcomes, and the role of experienced stigmatization as a mediator of these outcomes. Second, we examined whether the mediating role of such stigmatization varies as a function of gender. Our mediation and moderated mediation models are shown in Figures 1 and 2 (depression is shown in parentheses to represent the models where it is the mediator of interest).
Figure 1.
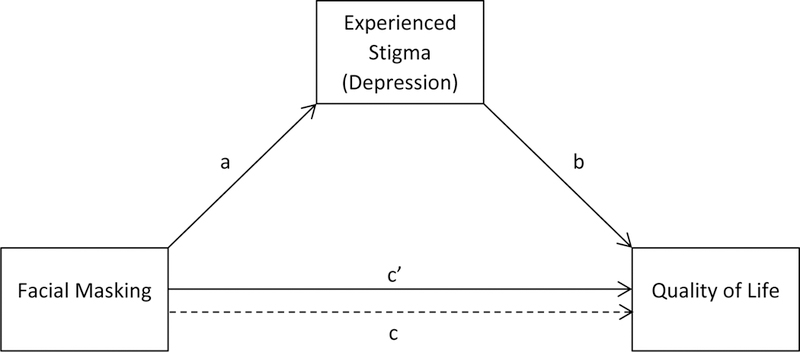
Statistical diagram of the mediation model
Figure 2.
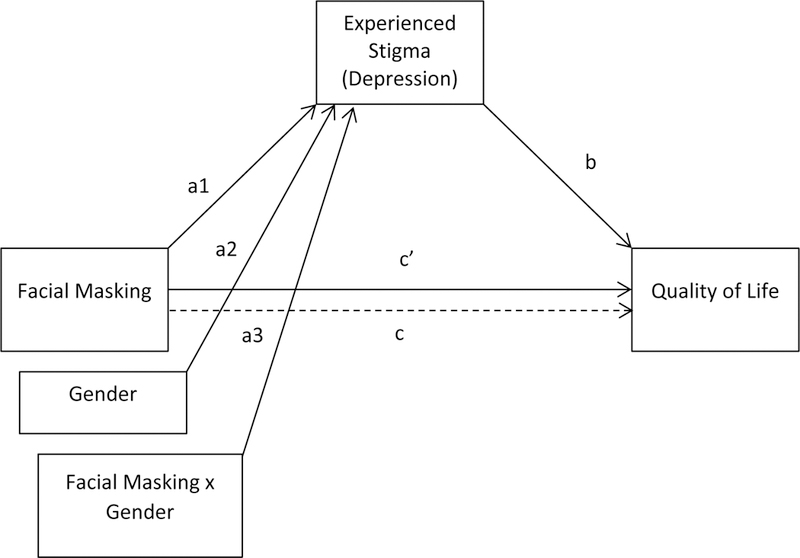
Statistical diagram of the conditional process model
Based on this model, we hypothesized that people who experienced higher levels of facial masking severity would have more problematic quality of life outcomes (Hypothesis 1). We also hypothesized that the relationship between facial masking and problematic QoL would be mediated by feelings of stigma (Hypothesis 2). Additionally, we hypothesized that facial masking would create more feelings of stigmatization in women than in men, and that this would carry through to QoL outcomes, indicating that gender moderates the mediation effect of Hypothesis 2 (Hypothesis 3).
This being the first study, to our knowledge, to investigate quantitatively the relationship between self-reported facial masking, stigma, gender, and QoL in people with PD, it was important to rule out bias in self-report as a confounding factor. To test if the potential mediating effect of stigma on the relationship between facial masking and QoL was driven by a negative bias in self-report, we examined a similarly-valenced self-report measure, depression, in our model. Depression is a separate construct from stigma but similar in recording negativity of experience. Preliminary studies from this database, described above, have shown that depression is related to self-reported facial masking (Gunnery et al., 2016) and experienced stigma and quality of life (Ma et al., 2016). Furthermore, depression is conceptually linked with the ability to show expression in the face, stigma, and quality of life (Girard et al., 2014; Earnshaw & Quinn, 2012), and so an additional aim was to explore depression as a potential parallel mediator, along with experienced stigma. We did not have a priori hypotheses about whether depression would mediate the relationship between facial masking and QoL because the empirical evidence for this mediation is not as clear as that for stigma. Our exploratory hypothesis for this aim was that the mediating effect of stigma would remain when controlling for depression as a second mediator in the model.
Methods
Participants
This study analyzed baseline data from the SocM-PD study (Tickle-Degnen et al., 2014). Inclusion criteria were (a) diagnosis of idiopathic PD utilizing the United Kingdom Parkinson’s Disease Society Brain Bank clinical diagnostic criteria, (b) modified Hoehn and Yahr stage 1 (mild, unilateral involvement only, no need for assistance) through 4 (severe, still able to stand or walk unassisted), (c) score ≥ 26 on the Mini-Mental Status Exam, (d) home setting within travel distance to study locations, (e) ability to communicate clearly and in English with research staff, (f) interest in participating and willingness and ability to provide informed consent. All but 9 participants (2 women) reported being on antiparkinsonian medications. Protocols were approved by the institutional review boards of Tufts University and Boston University Medical Center. All participants provided written informed consent before the testing and interview began.
Measures
Parkinson’s disease characteristics
Disease symptom severity and its impact on daily life functioning was measured with the Movement Disorder Society’s Unified Parkinson’s Disease Rating Scales (MDS-UPDRS, Goetz et al., 2008). The MDS-UPDRS consists of four parts: Part I (13 items), self-reported non-motor experiences of daily living; Part II (13 items), self-reported motor experiences of daily living; Part III (33 items), motor examination; Part IV (6 items), motor complications. Items are assessed on a 5-point Likert scale from 0 (normal) to 4 (severe). Four scores are calculated by summing the items within each part. Hoehn and Yahr staging was assessed after the administration of Part III of the MDS-UPDRS.
Facial masking
The severity of facial masking was self-reported using the single item, “How severe is your difficulty in showing expressions (emotions) in your face?” Participants answered on a 5-point Likert scale from 1 (no difficulty) to 5 (very severe difficulty). Validity of similar symptom self-ratings of PD has been supported by testing against QoL assessment and observed ratings of behaviors (Peto et al., 1995; Lyons & Tickle-Degnen, 2011; Gunnery et al. 2016). In our sample, self-reported facial masking is significantly correlated with clinician-rated facial masking in the MDS-UPDRS, r = .22, p < .05.
Stigma
Stigma was measured with the 24-item Stigma Scale for Chronic Illness (SSCI) (Rao et al., 2009). It contains questions about felt stigma, such as feelings of embarrassment, worry, and self-blame, and enacted stigma, the perceived behavior of others toward the respondent, such as avoiding contact, staring, and being unkind. Participants self-reported the frequency of experiencing stigma on a 5-point Likert scale from 1 (Never) to 5 (Always). For this study the 24 items were averaged (possible range of 1 to 5). The SSCI has good content validity and fair internal consistency (Stevelink et al., 2012).
Depression
The 15-item Geriatric Depression Scale (GDS) detects depressive symptoms in adults (Almeida & Almeida, 1999). Each item has a dichotomous yes/no answer, with one-point given to each depressive response (possible range 0 to 15). The GDS has good internal consistency and test-retest reliability with older adults (Almeida & Almeida, 1999) and is recommended for use with PD (Meara, Mitchelmore, & Hobson, 1999).
QoL
The Parkinson’s Disease Questionnaire-39 (PDQ-39) assesses life concerns of people with PD across eight domains: mobility, activities of daily living, emotional well-being, stigma, social support, cognition, communications, and bodily discomfort (Jenkinson, Fitzpatrick, & Peto, 1998). Participants reported the frequency of their difficulties in QoL due to PD on a 5-point Likert scale from 0 (Never) to 4 (Always). The PDQ-39 Summary Index (SI) score is the average of eight domain scores (possible range 0 to 100) with a higher score signifying more problematic QoL. The score has adequate reliability and validity (Jenkinson et al., 1997). Previous studies suggest the minimal clinically important difference (MCID) for the PDQ-39 SI as an outcome measure is −4.72 to show improvement and +4.22 to show worsening (Horváth et al., 2017).
Data analysis
Study data were managed using REDCap electronic data capture tools (Harris et al., 2009) hosted at Tufts University. Analyses were performed with IBM SPSS Statistics 25 for Windows. Descriptive statistics and key variable correlation coefficients were calculated separately and together for women and men. Gender differences for each variable were tested, using chi-squares for nominal variables and t-tests for scale variables. Path coefficients for mediation, moderation, and conditional process analyses were estimated with ordinary least squares regression using Hayes’ PROCESS macro for SPSS, version 2.16.1. MCIDs identified for the PDQ-39 were used to interpret the clinical significance of the estimated mediation effects.
We conducted a simple mediation analysis, with facial masking severity as the predictor, experienced stigma (or depression) as the mediator, and QoL as the outcome with the statistical model shown in Fig. 1 (PROCESS Model 4; Hayes, 2013). Path a predicted the mediator from facial masking. Path b predicted QoL from the mediator controlling for facial masking. Path c (total effect of predictor on outcome) predicted QoL from facial masking, and path c’ (direct effect of predictor) predicted QoL from facial masking, controlling for the mediator. The indirect effect of facial masking on QoL through the mediator was estimated as the product of the path a and b coefficients (ab). Because the product of two unstandardized coefficients has an irregular, non-normal sampling distribution, Hayes (2013) recommends that bias-corrected bootstrapped confidence intervals perform better for the interpretation of indirect effects than do inferential tests that are predicated on a normal sampling distribution (e.g. Sobel test). Following his recommendations, we do not report inferential tests of indirect effects. Bias-corrected and accelerated 95% confidence intervals based on 5000 bootstrapped samples are presented for interpretation of the estimated variability of these effects.
Next, we conducted a conditional process (moderated mediation) analysis using the statistical model shown in Fig. 2 (PROCESS Model 7; Hayes, 2013) with gender added to the model as the moderator of the path from facial masking to the mediator. Moderated mediation of this path contributes three pathways from the predictor to the mediator: prediction of the mediator from facial masking (a1), from gender (a2); and from the interaction of facial masking and gender (a3). The coefficients of paths b, c, and c’ remain the same as in the simple mediation model. The Fig. 2 statistical model adds the calculation of indirect effects that are conditional upon gender, and the index of moderated mediation, which describes the magnitude of the difference between the indirect effects for men and women, and its related confidence interval.
Finally, we conducted a moderated parallel multiple mediation analysis using the statistical model shown in Fig. 3 (Process Model 7; Hayes, 2013) with stigma and depression entered as mediators operating in parallel, and gender as the moderator of the path from facial masking to each mediator. This model generates the same a1, a2, a3, and c path coefficients as those calculated separately for the single moderated mediation models of stigma and depression. The Fig. 3 statistical model adds the following controls to the model of moderated mediation (Hayes, 2015). The path c’ coefficient (direct effect on QoL) is controlled by both mediators (stigma and depression). The b coefficients for each mediator are controlled by the QoL effects of the other mediator, in addition to the QoL effects of facial masking (as in the simple mediation model). Conditional indirect effects by gender for each mediator (referred to as specific indirect effects) control for QoL outcomes of the other mediator, and the differences between the indirect effects of women and men are interpreted with this additional control for the other mediator.
Figure 3.
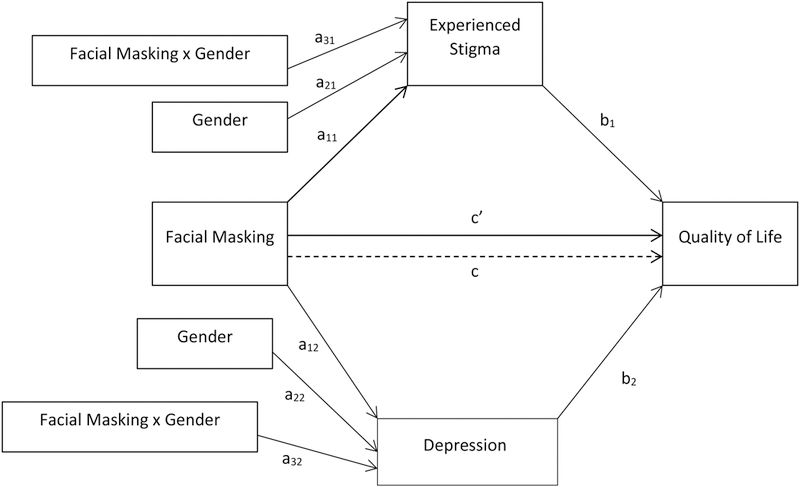
Statistical diagram of the parallel moderated mediation model
Results
Participant Characteristics
Table 1 shows the demographic and disease severity characteristics of the study participants (N = 90). Participants generally had mild to moderate disease severity, as reflected in their Hoehn & Yahr stage and MDS-UPDRS scores (Martinez-Martin et al., 2015; Skorvanek et al., 2017). Participants’ characteristics were similar to those in our paper on stigmatization and QoL outcomes (Ma et al., 2016), in which 73 of the same participants (29 women) were analyzed.
Table 1.
Participant characteristics
| Total | Men | Women | |
|---|---|---|---|
| Number of participants | 90 | 56 | 34 |
| Age [mean (SD)] | 65.49 (9.72) | 66.34 (8.83) | 64.03 (11.02) |
| Marital status | |||
| Single and never married | 7 | 3 | 4 |
| Married | 65 | 43 | 22 |
| Separate/divorced/widowed | 16 | 8 | 8 |
| Other | 2 | 2 | 0 |
| Education | |||
| High school | 9 | 6 | 3 |
| Some College/Associate Degree | 19 | 8 | 11 |
| Bachelor’s Degree | 22 | 14 | 8 |
| Master’s Degree | 31 | 23 | 8 |
| Doctoral Degree | 9 | 5 | 4 |
| Disease duration [year mean (SD)] | 7.34 (7.06) | 7.85 (7.93) | 6.52 (5.35) |
| Mini-Mental Status Exam [mean (SD)] | 29.20 (1.10) | 29.07 (1.20) | 29.41 (0.89) |
| Hoehn & Yahr stage [mean (SD)] | 2.21 (0.68) | 2.16 (0.65) | 2.29 (0.72) |
| I | 6 | 5 | 1 |
| II | 66 | 40 | 26 |
| III | 11 | 8 | 3 |
| IV | 7 | 3 | 4 |
| MDS-UPDRS Part I [mean (SD)] | 10.50 (5.76) | 9.71 (5.14) | 11.79 (6.53)† |
| MDS-UPDRS Part II [mean (SD)] | 11.12 (7.17) | 10.80 (6.44) | 11.65 (8.30) |
| MDS-UPDRS Part III [mean (SD)] | 33.62 (13.24) | 35.63 (13.30) | 30.32 (12.67)† |
| MDS-UPDRS Part IV [mean (SD)] | 3.37 (4.01) | 2.70 (3.33) | 4.47 (4.78)* |
Note: MDS-UPDRS Movement Disorder Society-Unified Parkinson’s Disease Rating Scale. We tested for gender differences for each variable, chi-squares for nominal variables and t-tests for scale variables. The only gender differences were found in the MDS-UPDRS and the p values are noted.
p < .1,
p < .05.
Description and Correlation of Key Variables
Table 2 shows descriptive results and Pearson correlations for variables included in the mediation models. Women (M = 30.74) reported more problematic quality of life than men (M = 24.30), t(88) = 2.11, p = .04. Women’s PDQ-39 SI score averaged 6.44 point higher than men’s, which is greater than the threshold for MCID for worse QoL (+ 4.22) (Horváth et al., 2017).
Table 2.
Means, Standard Deviations, and Intercorrelations of Model Variables by Gender (N = 90, 34 women)
| Pearson r | |||||
|---|---|---|---|---|---|
| Cronbach’s α | M (SD) | SSCI | GDS | PDQ39 SI |
|
| Facial masking | |||||
| Total | -- | 1.94 (0.90) | 0.56** | 0.37** | 0.49** |
| Women | 2.03 (0.94) | 0.70** | 0.49** | 0.70** | |
| Men | 1.89 (0.89) | 0.45** | 0.26 | 0.30* | |
| Gender difference p-value | .49 | .09 | 0.24 | .01 | |
| SSCI | |||||
| Total | .95 | 1.78 (0.59) | 0.58** | 0.82** | |
| Women | 1.88 (0.67) | 0.66** | 0.87** | ||
| Men | 1.72 (0.53) | 0.49** | 0.75** | ||
| Gender difference p-value | .25 | .26 | .12 | ||
| GDS | |||||
| Total | .79 | 2.43 (2.62) | 0.65** | ||
| Women | 2.88 (3.14) | 0.66** | |||
| Men | 2.16 (2.24) | 0.63** | |||
| Gender difference p-value | .21 | .81 | |||
| PDQ-39 SI | |||||
| Total | .95 | 26.73 (14.27) | |||
| Women | 30.74 (17.06) | ||||
| Men | 24.30 (11.80) | ||||
| Gender difference p-value | .04 | ||||
Notes. SSCI Stigma Scale for Chronic Illness; GDS Geriatric Depression Scale;
PDQ-39 SI Parkinson’s Disease Questionnaire-39 Summary Index. Gender differences are tested by t-test for M (SD) statistics and by Z-test for r’s.
p < .05
p < .01
p < .001
Intercorrelations for the total sample ranged in magnitude from moderate to large degrees of positive association (range = 0.37 – 0.82, ps < .001). Women’s correlation between facial masking and QoL was significantly higher than men’s (Z = 2.47, p < .05), and women’s correlation between facial masking and stigma trended toward being higher for women than for men’s (Z = 1.69, p < .10). No other gender differences approached statistical significance, although correlations were larger in magnitude for women than for men.
Stigma as a Mediator
Table 3 and Figure 4 show the results for the simple stigma mediation model. Consistent with correlation results, facial masking was associated with QoL (path c). The unstandardized coefficient suggests that for every one-point difference in facial masking severity between any two participants, there was a 7.70 difference in their QoL scores. Participants with more severe masking had more problematic QoL than participants with less severe masking, and the magnitude of the difference was over the +4.22 MCID threshold for clinically worse PDQ-39 SI scores.
Table 3.
Mediation model of facial masking on quality of life through stigma
| Unstandardized Estimate |
SE | t a | 95% CI | |
|---|---|---|---|---|
| Path a (face → stigma) | 0.37 | 0.06 | 6.41*** | 0.25 – 0.48 |
| Path b (stigma → QoL) | 19.29 | 1.83 | 10.54*** | 15.65 – 22.92 |
| Path c (face → QoL) | 7.70 | 1.47 | 5.24*** | 4.78 – 10.62 |
| Path c’ (face → QoL) | 0.64 | 1.19 | 0.54 | −1.72 – 3.00 |
| Indirect effect (face → stigma → QoL) | 7.06 | 1.54 | - | 4.27 – 10.30 |
p < .001
Bias-corrected bootstrapped confidence intervals are used to interpret results for indirect effects instead of inferential tests (Hayes, 2013).
Figure 4.
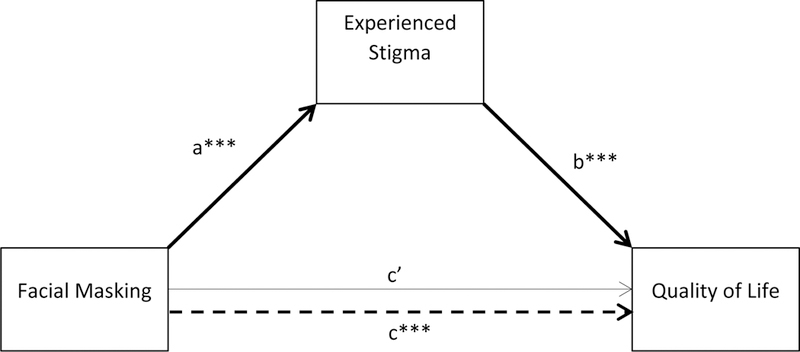
Mediation model of facial masking on quality of life through stigma. Significant pathways are in bold. ***p < .001
The coefficient for the unmoderated regression of stigma on facial masking (path a) suggested that for every one-point difference in facial masking severity between any two participants, there was approximately a 1/3 of a point difference in their stigma scores. The coefficient for QoL regressed on stigma, controlling for facial masking (path b) suggested that for every one-point difference in stigma controlled for facial masking, there was a difference of 19.29 points in the Qol, scores of any two participants, a difference that well exceeds MCID for the PDQ-39 SI. After controlling for experienced stigma, facial masking was not significantly associated with QoL and the coefficient was reduced from path c = 7.70 to path c’ = 0.64, suggesting stigma to be a robust mediator of the effect of masking on QoL.
At the next step, we tested the indirect effect of facial masking on QoL through stigmatization, conditional upon gender (Fig. 2). Table 4 and Figure 5 show that path a3, from facial masking to stigma, demonstrated a significant interaction effect of facial masking and gender on experienced stigma. Women’s facial masking had a larger association with stigmatization than did men’s facial masking.
Table 4.
Moderated mediation model of facial masking on quality of life through stigma by gender
| Unstandardized Estimate |
SE | t a | 95% CI | |
|---|---|---|---|---|
| Path a1 (face → stigma) | 0.27 | 0.07 | 3.68*** | 0.12 – 0.41 |
| Path a2 (gender → stigma) | −0.36 | 0.25 | −1.44 | −0.86 – 0.14 |
| Path a3 (face x gender → stigma) | 0.24 | 0.11 | 2.06* | 0.01 – 0.46 |
| Path b (stigma → QoL) | 19.29 | 1.83 | 10.54*** | 15.65 – 22.92 |
| Path c (face → QoL) | 7.70 | 1.47 | 5.24*** | 4.78 – 10.62 |
| Path c’ (face → QoL) | 0.64 | 1.19 | 0.54 | −1.72 – 3.00 |
| Conditional Indirect Effects | ||||
| Men | 5.16 | 1.74 | - | 2.19 – 9.06 |
| Women | 9.70 | 2.28 | - | 5.12 – 13.95 |
| Index of Moderated Mediation | 4.55 | 2.91 | - | −0.64 – 9.61 |
p < .05,
p < .001
Bias-corrected bootstrapped confidence intervals are used to interpret results for indirect effects instead of inferential tests (Hayes, 2013)
Figure 5.
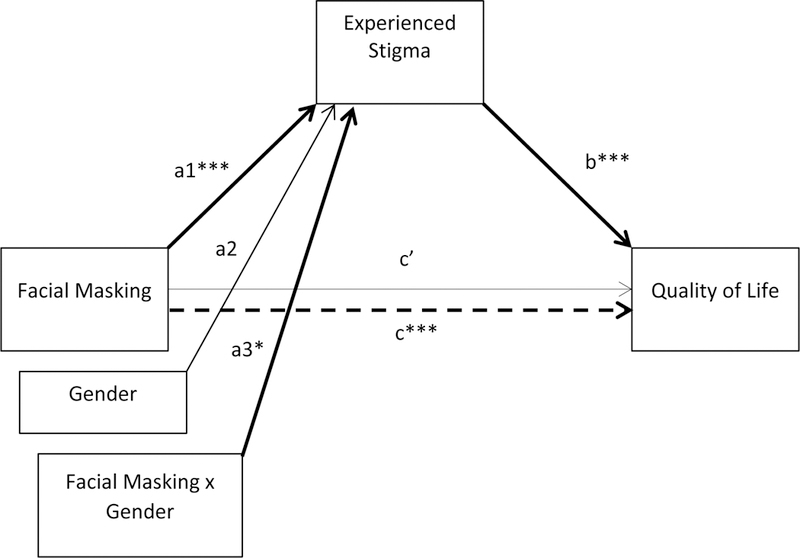
Moderated mediation model of facial masking on quality of life through stigma by gender. Significant pathways are in bold. ***p < .001, *p < .05
The conditional indirect effect of facial masking on QoL for women was 9.70, and was 5.16 for men. These findings show that for both women and men, facial masking exerted an indirect effect on QoL through stigma, and these effects exceeded the MCID for the PDQ-39 SI. Greater facial masking was associated with more self-perceived difficulties in QoL, as mediated through stigma. The index of moderated mediation showed that the difference in this association for women and men was marginally significant (coefficient = 4.55, 90% CI [0.17 – 8.64], p < .10), suggesting a potentially stronger mediational role of stigma for women than for men.
Stigma and Depression as Parallel Mediators
The simple depression mediation model showed that depression also mediated the relationship between facial expression and QoL (see Table 5 and Figure 6). The change in QoL due to facial masking as mediated by depression (2.97 points) did not reach the level for MCID as it did in the stigma model, and there was still a statistically significant and MCID in QoL due to facial masking (4.54 points) even after controlling for the mediating effect of depression. The mediational role of depression appeared to be smaller than stigmatization, but because of some overlap of confidence intervals for indirect effects of both variables, we moved to the next step of testing the gender-moderated depression-mediated model.
Table 5.
Mediation model of facial masking on quality of life through depression
| Unstandardized Estimate |
SE | t a | 95% CI | |
|---|---|---|---|---|
| Path a (face → depression) | 1.06 | 0.29 | 3.69*** | 0.49 – 1.63 |
| Path b (depression → QoL) | 2.97 | 0.45 | 6.68*** | 2.09 – 3.86 |
| Path c (face → QoL) | 7.70 | 1.47 | 5.24*** | 4.78 – 10.62 |
| Path c’ (face → QoL) | 4.54 | 1.29 | 3.52*** | 1.98 – 7.11 |
| Indirect effect (face → depression → QoL) | 3.16 | 1.04 | - | 1.22 – 5.28 |
p < .001
Bias-corrected bootstrapped confidence intervals are used to interpret results for indirect effects instead of inferential tests (Hayes, 2013).
Figure 6.
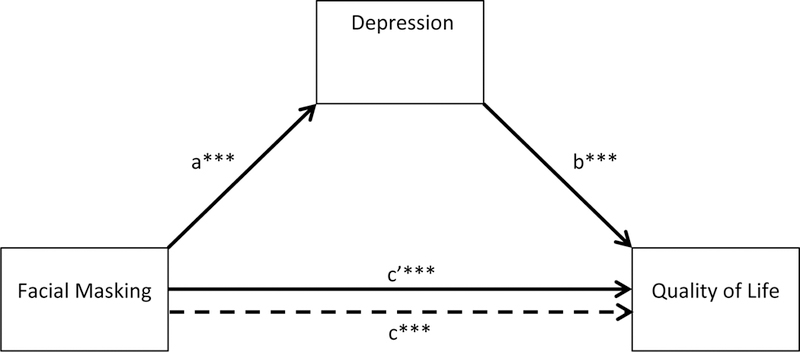
Mediation model of facial masking on quality of life through depression. Significant pathways are in bold. ***p < .001
Table 6 and Figure 7 show a small interaction effect of facial masking and gender on experienced depression that does not reach significance (coefficient = 2.92, 90% CI [−.052 – 5.95], p > .10). An examination of the indirect effect of facial masking on QoL for women (4.84) was at a level of MCID for the PDQ-39 SI, and for men (1.92) did not meet the MCID criterion.
Table 6.
Moderated mediation model of facial masking on quality of life through depression by gender
| Unstandardized Estimate |
SE | t a | 95% CI | |
|---|---|---|---|---|
| Path a1 (face → depression) | 0.65 | 0.37 | 1.75† | −0.09 – 1.38 |
| Path a2 (gender → depression) | −1.36 | 1.27 | −1.07 | −3.87 – 1.16 |
| Path a3 (facexgender → depression | 0.98 | 0.58 | 1.69† | −0.18 – 2.14 |
| Path b (depression → QoL) | 2.97 | 0.45 | 6.68*** | 2.09 – 3.86 |
| Path c (face → QoL) | 7.70 | 1.47 | 5.24*** | 4.78 – 10.62 |
| Path c’ (face → QoL) | 4.54 | 1.29 | 3.52*** | 1.98 – 7.11 |
| Conditional Indirect Effects | ||||
| Men | 1.92 | 1.16 | - | −0.04 – 4.50 |
| Women | 4.84 | 1.52 | - | 1.73 – 7.82 |
| Index of Moderated Mediation | 2.92 | 1.82 | - | −0.65 – 6.43 |
p < .1,
p < .001
Bias-corrected bootstrapped confidence intervals are used to interpret results for indirect effects instead of inferential tests (Hayes, 2013).
Figure 7.
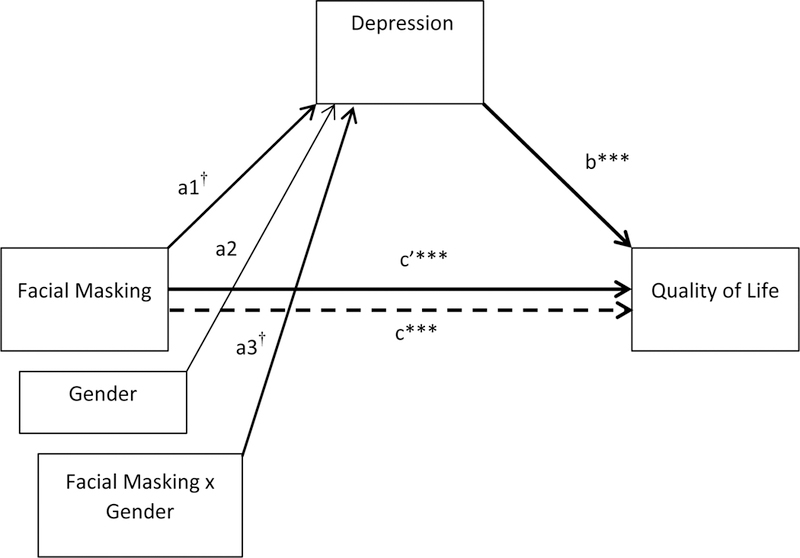
Moderated mediation model of facial masking on quality of life through depression by gender. Significant pathways are in bold. †p < .1, *p < .05
Table 7 and Figure 8 show the mediation results when depression and stigma were simultaneously entered into a parallel moderated mediator model. For both women (coefficient = 7.90) and men (coefficient = 4.20), facial masking showed an indirect effect on QoL through stigma (controlling for depression). This was also true for both women (coefficient = 2.35) and men (coefficient = 0.93) when depression was the mediator (controlling for stigma). Women’s facial masking indirect effect on the PDQ-39 SI via stigma (7.90) surpassed the MCID (+4.22) criterion, while men’s indirect effect via stigma (4.20) approximated the MCID criterion. Neither women’s nor men’s facial masking indirect effects on the PDQ-39 SI via depression met the MCID criterion. As measured by the index of moderated mediation, the indirect effect was marginally stronger in women than in men for both stigma and depression mediation (stigma: coefficient = 3.70, 90% CI [.29 – 7.44], p < .10; depression: coefficient = 1.42, 90% CI [0.07 – 3.32], p < .10).
Table 7.
Parallel Moderated Mediation model of facial masking on quality of life through stigma and depression by gender
| Unstandardized Estimate |
SE | ta | 95% CI | |
|---|---|---|---|---|
| Path a11 (face → stigma) | 0.27 | 0.07 | 3.68*** | 0.12 – 0.41 |
| Path a21 (gender → stigma) | −0.36 | 0.25 | −1.44 | −0.86 – 0.14 |
| Path a31 (facexgender → stigma) | 0.24 | 0.11 | 2.06* | 0.01 – 0.46 |
| Path a12 (face → depression) | 0.65 | 0.37 | 1.75† | −0.10 – 1.38 |
| Path a22 (gender → depression) | −1.36 | 1.27 | −1.07 | −3.87 – 1.16 |
| Path a32 (facexgender → depression) | 0.98 | 0.58 | 1.69† | −0.18 – 2.14 |
| Path b1 (stigma → QoL) | 15.71 | 1.96 | 8.02*** | 11.82 – 19.60 |
| Path b2 (depression → QoL) | 1.45 | 0.39 | 3.73*** | 0.68 – 2.22 |
| Path c (face → QoL) | 7.70 | 1.47 | 5.24*** | 4.78 – 10.62 |
| Path c’ (face → QoL) | 0.41 | 1.11 | 0.37 | −1.79 – 2.62 |
| Conditional Indirect Effects | ||||
| Stigma | ||||
| Men | 4.20 | 1.44 | - | 1.80 – 7.40 |
| Women | 7.90 | 2.02 | - | 4.12 – 11.85 |
| Depression | ||||
| Men | 0.93 | 0.57 | - | 0.05 – 2.36 |
| Women | 2.35 | 0.89 | - | 0.80 – 4.29 |
| Indices of Moderated Mediation | ||||
| Stigma | 3.70 | 2.16 | - | −0.43 – 8.07 |
| Depression | 1.42 | 0.97 | - | −0.20 – 3.62 |
p < .1,
p < .05,
p < .001
Bias-corrected bootstrapped confidence intervals are used to interpret results for indirect effects instead of inferential tests (Hayes, 2013).
Figure 8.
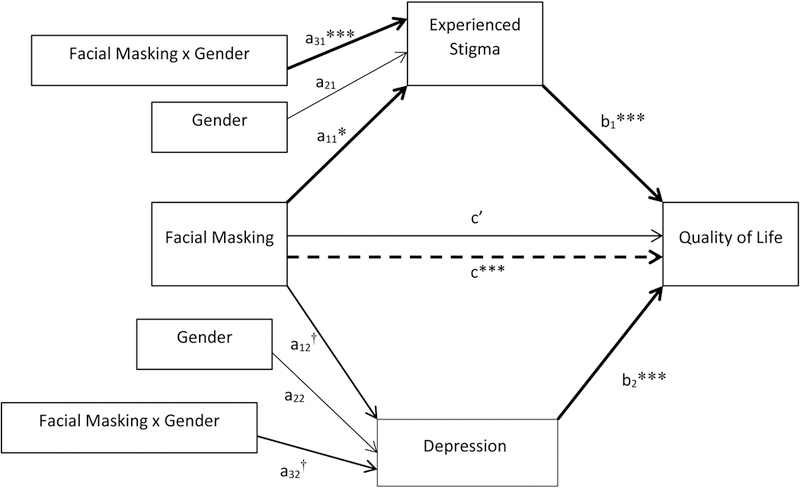
Parallel Moderated Mediation model of facial masking on quality of life through stigma and depression by gender. Significant pathways are in bold. †p < .1, *p < .05, ***p < .001
Discussion
In this study, we examined the associations among facial masking, experienced stigma, depression, and QoL for women and men with PD. Our hypotheses were generally supported. First, people with PD who self-rated experiencing more difficulty in facial expression tended to feel more stigmatized and this relationship contributed to their compromised QoL at clinically meaningful levels. Second, a one-point increment of facial masking was associated with larger increase of experienced stigma in women than in men. The stronger relationship between facial masking and experienced stigma in turn contributed to marginally more compromised QoL in women than in men.
The relation between facial masking and experienced stigma in PD is consistent with qualitative research that explored the experience of having facial masking (Nijhof, 1995; Abudi et al., 1997; Chiong-Rivero et al., 2011) as well as controlled experiments that presented samples of videotaped people with different degrees of facial masking to health care practitioners and older adult observers (Hemmesch et al., 2009, Tickle-Degnen et al., 2011; Hemmesch, 2014). The current study also supports the qualitative research on patients’ experiences of the impact of facial masking on health status that implies the connection between facial masking and subjective well-being (Chiong-Rivero et al., 2011).
The present study, although cross-sectional in nature, provides further quantitative evidence for the association between facial masking and social outcomes like stigma from the patient’s perspective (Gunnery et al., 2016). In addition, previous studies found that negative bias of practitioners and older adult observers toward higher masking was stronger when judging women than men. Our research provides corroborating evidence showing a moderating effect of gender on the relation between self-rated facial masking and experienced stigma. Overall, the results suggest that social norms that expect women to be more expressive than men appear to not only affect the first impressions of people with PD, but also become incorporated in one’s self-perceptions.
We also found a strong relationship between experienced stigma and QoL (r = 0.82), which replicates our previous findings in the subset of baseline data (r = 0.83) (Ma et al., 2016). We extend the previous findings by identifying that self-reported facial masking was a source of experienced stigma. In addition, the strong correlations between stigma and QoL for both women (r = 0.87) and men (r = 0.75), as well as the significant coefficients after controlling for facial masking (path b in Table 3, path b in Table 4) suggest a notable role of experienced stigma in QoL across gender.
Exploratory analysis of the role of depression in the relationship between facial masking and QoL showed that depression also mediates the relationship between facial masking and QoL, but to a lesser extent than experienced stigma and at a level that is not clinically meaningful. The indirect path from self-reported facial masking to quality of life through stigma was still present when controlling for the effects of depression. These findings are not surprising given the overlap in the constructs of the depression, stigma, and QoL, but also provide some discriminant validity in showing that two different measures of self-reported negative experience in PD do not mediate the relationship between facial masking and QoL at the same statistical or clinically meaningful level. Though our inclusion criteria did not exclude people based on depression, overall the sample was largely non-depressed (M = 2.1 on a 15-point scale), the role of depression in the relationships between facial masking, stigma, and QoL warrants further investigation in a sample that has more variation in depression.
Our findings highlight the important role of facial masking in QoL in people with PD. Individuals may become aware of their facial masking when they note their impaired ability to express themselves during interaction with others. As social interaction is a quick exchange of verbal and nonverbal information, an inappropriate facial expression or lack of facial expression may induce unexpected, negative reactions from others. The failure to meet the social norms in communication implies social deviance and incompetence, which may in turn lead the individuals to devalue themselves and experience psychological distress. Such stigmatized feelings may further contribute to compromised social, psychological, and physical well-being.
In this study, severity of facial masking was self-reported by people with PD, which may be different from clinician-judged scores. Although it could be argued that clinicians are trained raters, clinicians only observe the individual with PD for a short period, while the individual with PD’s personal experience of facial masking is integrated into their everyday life (Lyons & Tickle-Degnen, 2005). Self-ratings reflect patient perspectives and lived experiences, which are important to understand for clinical outcomes, as is recognized in the recent call to develop patient-centered outcome research (Frank, Basch, & Selby, 2014). The present study suggests the importance of understanding how people with PD perceive and interpret their facial masking. Interventions that attend to stigma issues related to facial masking may be helpful to enhance QoL in people with PD. Because women appear to be more susceptible to experience stigma related to facial masking, special attention should be paid to this population. Strategies at both personal and societal levels may be needed to reduce stigma attached to facial masking. For example, a personal-level strategy is to acknowledge the presence of the stigmatizing condition accompanied by willingness to discuss it (Hebl & Kleck, 2000). At the societal level, the media may serve to normalize stigmatizing conditions by revealing and discrediting negative stereotypes associated with stigma (Hebl, Tickle, & Heatherton, 2000).
Interventions to decrease facial masking may also be helpful in decreasing stigma and consequently increasing quality of life. Previous research has found the Lee Silverman Voice Treatment has shown some promise in decreasing facial masking (Dumer et al., 2014). Interventions that train people with PD in how to better control their face, and also educational interventions that help the family and close friends of people with PD to better understand how PD affects a person’s ability to express their emotions accurately may also be helpful in breaking this path from facial masking to QoL through stigma.
This paper presents preliminary results from the baseline data from the 3-year SocM-PD project (Tickle-Degnen et al., 2014). Some limitations of this study should be noted. Unequal sample sizes of women and men may have diminished our statistical power to detect gender differences, as we found marginally significant difference between women and men in the indirect effects of facial masking on QoL through stigma. Moreover, although our conditional process model aims to shed light on the mechanism linking facial masking and QoL, the cross-sectional nature of this study limits the causal interpretation.
This study provides quantitative evidence for the indirect effect of self-rated facial masking on QoL through experienced stigma in PD. People with PD, especially women, associate facial masking with experiencing stigma, which in turn contributes to decreased QoL. The contribution of stigma is above and beyond that of depression which is another measure of negative experience. The results highlight the importance of understanding how women and men with PD perceive and interpret their facial masking. Interventions to enhance QoL may attend to stigma issues related to facial masking. Approaches at both personal and societal levels to reduce stigma may need to be developed and tested.
Highlights:
People with Parkinson’s can experience a decrease in facial expressivity.
Those who experience less facial expressivity report lower quality of life (QoL).
Stigma mediates the relationship between facial expressivity and QoL.
The mediating effect of stigma is especially strong for women.
Interventions to enhance QoL may attend to stigma issues related to facial masking.
Acknowledgments
Research reported in this publication was supported by the United States National Institutes of Health: specifically, the National Institute of Nursing Research, Award Number R01NR013522, the National Institute on Aging Award Number F32AG046034, and the National Center for Advancing Translational Sciences, Award Number UL1TR002544. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The first author did this research at Tufts University under the Fulbright Senior Research Grants.
Footnotes
Disclosures: The authors report no disclosures.
References
- Abudi S, Bar-Tal Y, Ziv L, & Fish M (1997). Parkinson’s disease symptoms -- patients’ perceptions. Journal of Advanced Nursing, 25, 54–59. doi: 10.1046/j.1365-2648.1997.1997025054.x [DOI] [PubMed] [Google Scholar]
- Almeida OP, & Almeida SA (1999). Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD‐10 and DSM‐IV. International Journal of Geriatric Psychiatry, 14, 858–865. doi: [DOI] [PubMed] [Google Scholar]
- Bologna M, Fabbrini G, Marsili L, Defazio G, Thompson PD, & Berardelli A (2013). Facial bradykinesia. Journal of Neurology, Neurosurgery, and Psychiatry, 84, 681–685. doi: 10.1136/jnnp-2012-303993 [DOI] [PubMed] [Google Scholar]
- Briton NJ, & Hall JA (1995). Beliefs about female and male nonverbal communication. Sex Roles, 32, 79–90. doi: 10.1007/BF01544758 [DOI] [Google Scholar]
- Caap-Ahlgren M, & Lannerheim L (2002). Older Swedish women’s experiences of living with symptoms related to Parkinson’s disease. Journal of Advanced Nursing, 39, 87–95. doi: 10.1046/j.1365-2648.2002.02245.x [DOI] [PubMed] [Google Scholar]
- Chiong-Rivero H, Ryan GW, Flippen C, Bordelon Y, Szumski NR, Zesiewicz TA, Vassar S, Weidmer B, Garcia RE, Bradley M, & Vickrey BG (2011). Patients’ and caregivers’ experiences of the impact of Parkinson’s disease on health status. Patient Related Outcome Measures, 2011(2), 57–70. doi: 10.2147/PROM.S15986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumer AI, Oster H, McCabe D, Rabin LA, Spielman JL, Ramig LO, & Borod JC (2014). Effects of the Lee Silverman Voice Treatment (LSVT® LOUD) on hypomimia in Parkinson’s disease. Journal of the International Neuropsychological Society, 20, 302–312. doi: 10.1017/S1355617714000046 [DOI] [PubMed] [Google Scholar]
- Earnshaw VA, & Quinn DM (2012). The impact of stigma in healthcare on people living with chronic illnesses. Journal of Health Psychology, 17, 157–168. doi: 10.1177/1359105311414952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P (1989). The argument and evidence about universals in facial expressions of emotion In Wagner H & Manstead A (Eds.), Handbook of social psychophysiology (pp. 143–164). New York: Wiley. [Google Scholar]
- Frank L, Basch E, & Selby JV (2014). The PCORI perspective on patient-centered outcomes research. JAMA, 312, 1513–1514.32. doi: 10.1001/jama.2014.11100 [DOI] [PubMed] [Google Scholar]
- Girard JM, Cohn JF, Mahoor MH, Mavadati SM, Hammal Z, & Rosenwald DP (2014). Nonverbal social withdrawal in depression: Evidence from manual and automatic analyses. Image and Vision Computing, 32, 641–647. doi: 10.1016/j.imavis.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez‐Martin P, ... & Dubois B (2008). Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Movement Disorders, 23, 2129–2170. doi: 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- Gunnery S, Habermann B, Saint-Hilaire M, Thomas C, & Tickle-Degnen L (2016). The relationship between the experience of hypomimia and social wellbeing in people with Parkinson’s disease and their care partners. Journal of Parkinson’s disease, 6, 625–630. doi: 10.3233/JPD-160782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42, 377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach: Guilford Press. [Google Scholar]
- Hayes AF (2015). An index and test of linear moderated mediation, Multivariate Behavioral Research, 50, 1–22. doi: 10.1080/00273171.2014.962683 [DOI] [PubMed] [Google Scholar]
- Hebl MR, & Kleck RE (2000). The social consequences of physical disability In Heatherton TF, Kleck RE, Hebl MR & Hull JG (Eds.), The Social Psychology of Stigma (pp. 419–439). New York: Guilford. [Google Scholar]
- Hebl MR, Tickle J, & Heatherton TF (2000). Awkward moments in interactions between nonstigmatized and stigmatized individuals In Heatherton TF, Kleck RE, Hebl MR & Hull JG (Eds.), The Social Psychology of Stigma (pp. 275–306). New York: Guilford. [Google Scholar]
- Hemmesch AR, Tickle-Degnen L, & Zebrowitz LA (2009). The influence of facial masking and sex on older adults’ impressions of individuals with Parkinson’s disease. Psychology and Aging, 24, 542–549. doi: 10.1037/a0016105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmesch AR (2014). The detrimental effects of atypical nonverbal behavior on older adults’ first impressions of individuals with Parkinson’s disease. Psychology and Aging, 29, 521–527. doi: 10.1037/a0036637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth K, Aschermann Z, Kovács M, Makkos A, Harmat M, Janszky J, ... & Kovács N (2017). Changes in Quality of Life in Parkinson’s Disease: How Large Must They Be to Be Relevant? Neuroepidemiology, 48, 1–8. doi: 10.1159/000455863 [DOI] [PubMed] [Google Scholar]
- Jenkinson C, Fitzpatrick R, & Peto V (1998). The Parkinson’s disease questionnaire: User manual for the PDQ-39, PDQ-8 and PDQ summary index. Oxford, UK: University of Oxford. [Google Scholar]
- Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, & Hyman N (1997). The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age and Ageing, 26, 353–357. doi: 10.1093/ageing/26.5.353 [DOI] [PubMed] [Google Scholar]
- Lyons KD, & Tickle-Degnen L (2005). Reliability and validity of a videotape method to describe expressive behavior in persons with Parkinson’s disease. American Journal of Occupational Therapy, 59, 41–49. doi: 10.5014/ajot.59.1.41 [DOI] [PubMed] [Google Scholar]
- Ma HI, Saint-Hilaire M, Thomas CA, & Tickle-Degnen L (2016). Stigma as a key determinant of health-related quality of life in Parkinson’s disease. Quality of Life Research, 25, 3037–3045. doi: 10.1007/s11136-016-1329-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Martín P, Rodríguez-Blázquez C, Alvarez M, Arakaki T, Arillo VC, Chaná P, ... & Serrano-Duenas M (2015). Parkinson’s disease severity levels and MDS-Unified Parkinson’s Disease Rating Scale. Parkinsonism & Related Disorders, 21, 50–54. doi: 10.1016/j.parkreldis.2014.10.026 [DOI] [PubMed] [Google Scholar]
- Meara J, Mitchelmore E, & Hobson P (1999). Use of the GDS-15 geriatric depression scale as a screening instrument for depressive symptomatology in patients with Parkinson’s disease and their carers in the community. Age and Ageing, 28, 35–38. doi: 10.1093/ageing/28.1.35 [DOI] [PubMed] [Google Scholar]
- Nijhof G (1995). Parkinson’s disease as a problem of shame in public appearance. Sociology of Health & Illness, 17, 193–205. doi: 10.1111/1467-9566.ep10933386 [DOI] [Google Scholar]
- Peto V, Jenkinson C, Fitzpatrick R, & Greenhall R (1995). The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Quality of Life Research, 4, 241–248. doi: 10.1007/BF02260863 [DOI] [PubMed] [Google Scholar]
- Rao D, Choi SW, Victorson D, Bode R, Peterman A, Heinemann A, & Cella D (2009). Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI). Quality of Life Research, 18, 585–595. doi: 10.1007/s11136-009-9475-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass SE, Hecht MA, & Ploutz-Snyder R (1998). Interpersonal sensitivity: Expressivity or perceptivity? Journal of Personality and Social Psychology, 74, 238–249. doi: 10.1037/0022-3514.74.1.238 [DOI] [PubMed] [Google Scholar]
- Skorvanek M, Martinez‐Martin P, Kovacs N, Rodriguez‐Violante M, Corvol JC, Taba P, ... & Alvarez‐Sanchez M (2017). Differences in MDS‐UPDRS Scores Based on Hoehn and Yahr Stage and Disease Duration. Movement Disorders Clinical Practice, 4, 536–544. doi: 10.1002/mdc3.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevelink SAM, Wu IC, Voorend CGN, & van Brakel WH (2012). The psychometric assessment of internalized stigma instruments: a systematic review. Stigma Research and Action, 2, 100–118. doi: 10.5463/SRA.v1i1.11 [DOI] [Google Scholar]
- Tickle-Degnen L, Saint-Hilaire M, Thomas CA, Habermann B, Martinez LSS, Terrin N, ... & Naumova EN (2014). Emergence and evolution of social self-management of Parkinson’s disease: Study protocol for a 3-year prospective cohort study. BMC neurology, 14(1), 95. doi: 10.1186/1471-2377-14-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickle-Degnen L, Zebrowitz LA, & Ma HI (2011). Culture, gender and health care stigma: Practitioners’ response to facial masking experienced by people with Parkinson’s disease. Social Science & Medicine, 73, 95–102. doi: 10.1016/j.socscimed.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]


