Abstract
Purpose:
This is a step-by-step procedural guide to help new and unexperienced surgeons with the implementation of a robotic program for the surgical treatment of morbid obesity.
Methods:
Based on our vast robotic experience, we present our standardized technique and also, with a PubMed research, discuss the different surgical aspects.
Results:
We present our trainings pathway towards the first robotic Roux-en-Y gastric bypass, a step-by-step procedural guide with helpful hints when it comes to troubleshooting and also discuss some surgical aspects.
Conclusion:
The robotic Roux-en-Y gastric bypass is safe and feasible, and might offer some advantages in comparison to the laparoscopic approach.
Keywords: Procedure Guide, Robotic Roux-en-Y Gastric Bypass, Learning Curve, Technical Aspects, Troubleshooting
INTRODUCTION
By 2025, obesity is projected to increase across the European region. If present trends continue, more than half of European countries will have an obesity prevalence of 20% or more.1 Many different surgical approaches are used to treat the severely obese, with proximal Roux-en-Y gastric bypass (RYGB) being the most frequent procedure in Europe,2,3 particularly in the presence of gastroesophageal reflux or type 2 diabetes.2,4 Generally it is performed laparoscopically and the technique is well established.4 Laparoscopic Roux-en-Y gastric bypass (LRYGB) is clearly superior to the open procedure5 with a low complication rate, but is technically demanding with a flat learning curve ranging between 1006 and 500 cases.7 To overcome the technical limits of laparoscopy (particularly the enormous retracting force of the obese abdominal wall and the limited movement capability of its instruments) and to potentially flatten the learning curve,8 the robotic system was introduced into bariatric surgery.
We implemented our robotic program in 2013 and have performed more than 1400 robotic-assisted surgeries ever since, using both the da Vinci SI and XI systems (Intuitive Surgical Sàrl). After gaining experience in hepatobiliary, colorectal, esophageal, and thoracic robotic surgery,10–13 the system was also introduced into our bariatric program in 2016. Since then, more than 200 robotic Roux-en-Y gastric bypass (RRYGB) surgeries and 50 re-do surgeries have been performed in our institution.13 In this article, we present our standardized technique for RRYGB, offer suggestions for successful implementation, highlight potential solutions for common problems, and discuss different technical aspects.
Set up of the Robotic Program
Implementation of robot-assisted surgery requires a multidisciplinary approach, with appropriate training and cooperation of surgical, anesthetic, and technical staff. Besides acquiring the technical skills and becoming accustomed to the complex technique, patient selection and an appropriate frequency of procedures are required to avoid complications.14 The console control must be mastered safely before the first, technically-easy interventions are performed on the patient. In addition to control over the system, the lack of haptic feedback in particular requires habituation and practice. In contrast to laparoscopy, every movement of the instruments must be visually-controlled to avoid injury to surrounding structures. For example, a suture must not be guided outside the visual field to tighten it. Accordingly, laparoscopic "handling" must be retrained.15
We also implemented a certified training for the scrub nurses. In this modular course, the nurses learn the basic principles of the robotic system and troubleshooting in case of a medical or technical emergency.
We began preparing for our first RRYGB with training sessions on the SimNow simulator, followed by a course learning the basic robotic technique on dummies. After overcoming the specific learning curve of the DaVinci robot, we started training on body donors. Nowadays, a new robotic bariatric surgeon can participate in special courses that teach the basics as well as special cases of bariatrics on mini pigs or body donors. After initial training, we recommend that surgeons began with simple surgeries, such as cholecystectomy, hiatoplasty, or even sleeve gastrectomy. After completing a relevant case load, the first few Roux-en-Y gastric bypasses should be performed on female patients with a relative low body mass index (BMI), with the assistant of a proctor.
Preoperative Preparation
The patient is admitted on the day of surgery, and written informed consent is obtained from every patient 24 hours prior to surgery. To reduce the risk of deep vein thrombosis, pneumatic pumps are applied to the patient’s legs. Additionally, a weight-adapted perioperative single shot of antibiotic prophylaxis is administered before the first skin incision. Postoperatively, every patient also receives weight-adapted, low molecular weight heparin.
Operating room configuration using the da Vinci Xi System
Our operating room configuration for RRYGB using the da Vinci Xi is shown in Figure 1. The surgeon console is positioned so that good communication with the patient-side team can be established, ideally enabling direct eye contact. The anesthesiologist is placed behind the patient’s right shoulder. The assistant sits on the right side of the patient, while the scrub nurse is at the patient’s leg. At least one video monitor showing the endoscopic view is located in direct line of sight for the assistant and the scrub nurse.
Figure 1.
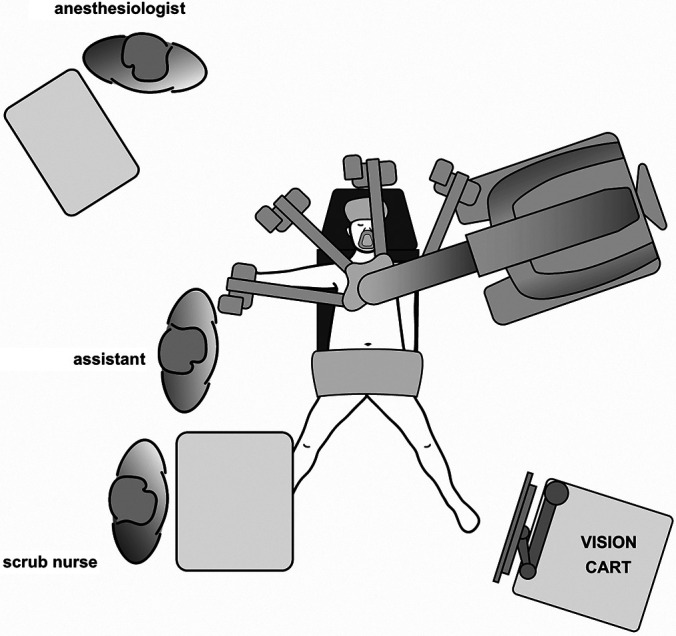
Operation room setup for the DaVinci Xi.
Patient positioning and surgical preparation
Under general anesthesia, the patient is placed in a supine position with the left arm tucked and legs split (“French” position) for access between the legs. A body warmer to prevent patient hypothermia should be applied to the chest. The patient's left arm is positioned alongside the body to allow easier cart docking. Pressure points and bony prominences are padded for protection. The body position is carefully secured with a gel pad or bean bag, and a strap applied across the patient’s thighs to avoid any shifting of the reverse Trendelenburg position. The abdominal area (xiphoid process to supraumbilic) is prepped and draped in the usual sterile fashion. The table is prepared for placement in a reverse Trendelenburg position (∼20°) without tilt.
Port placement and docking
Port placement for the da Vinci Surgical System is based on the concept of the surgical workspace. Slight modifications to the port locations might be necessary due to the patient’s anatomy to ensure enough distance to the target anatomy in shorter patients, and vice versa in taller or extremely obese patients. Extra-long ports are not needed, in our opinion. Their extra length is below the remote center of the trocars, whereas the longer distance in obese patients arises from the increased subcutaneous fat, thus not providing benefit when operating on the upper gastrointestinal tract.
Port placement using the da Vinci XI System [Figure 2]
Figure 2.
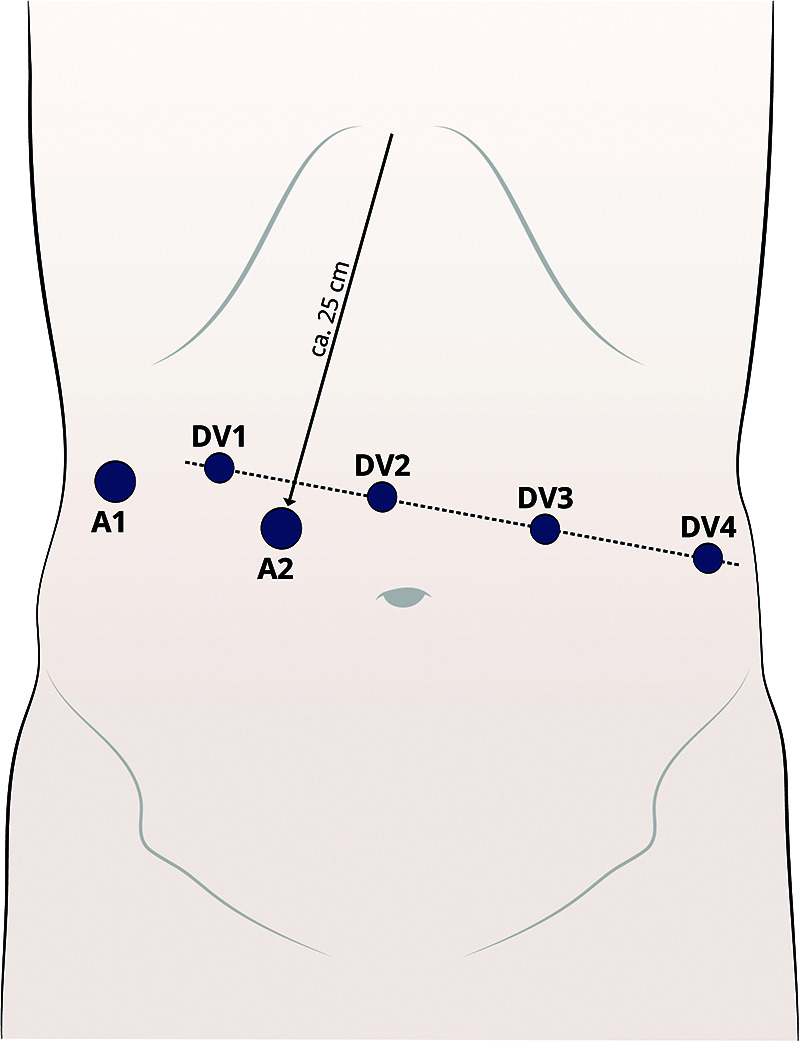
Trocar placement.
The pneumoperitoneum is established [15 mmHg] using a 12 mm Fios First Entry trocar (Applied Medical, Rancho Santa Margarita, CA, USA) about 25 cm below the xyphoid (Assistant trocar 2) [A2] on the right paramedian. This is inserted while pulling the skin towards the sealing to increase the intra-abdominal space. The port positions are marked only on the abdominal wall over the pneumoperitoneum for consistency of port positions. Another 12 mm or 5 mm (depending on the retractor) Fios trocar (Assistant trocar 1) [A1] is inserted at the right lateral flank. This is mainly used for liver retraction. From A1, a line is drawn across the upper abdomen at a 90° angle to the xyphoid-A2 line. All da Vinci (DV) ports are placed along this line. Port 1 (8 mm) [DV1] is placed 8 – 10 cm lateral to port A1. Port 2 (8 mm) [DV2] is placed 8 – 10 cm lateral to port 1. This will be the camera port. Port 3 (8 mm) [DV3] and port 4 (8 mm) are placed [DV4] 8 – 10 cm lateral to each other on the patient’s left side.
Patient cart docking using the da Vinci XI System
Before docking the patient cart, the patient table is prepared. The operating table is lowered to enable clearance for the da Vinci arms, ensuring that there are no obstructions overhead or in front of the patient’s cart. In particular, the patient’s face is secured so that there is no injury while docking. The patient cart is rolled up from the left side of the patient, and the target laser is aligned with the camera port (DV2). Normally, no repositioning of the patient and/or operating table is necessary. If there has to be a change in positioning, the integrated table motion feature should be used to ensure collision-free movement. This might be especially useful in revisional bariatric surgery; however, in our opinion it does not offer any apparent benefit in a standard robotic gastric bypass. Once docked, targeting is performed on the stomach.
PROCEDURE STEPS FOR RRYGB
The following step-by-step procedure guide represents our current technique.
General tips to avoid intraoperative complications
Proper traction and countertraction should be used to create an adequate exposure. A standardized assistant’s role might also be helpful. It is important to develop and stay in the correct tissue plane and be aware of the variations in vasculature and anatomy. Use of bariatric length (45 cm) laparoscopic instrumentation avoids interference for the assistant.
Suggested da Vinci Instruments
Arm 1: Fenestrated bipolar forceps. Arm 2: endoscope 30° down (left hand). Arm 3: Ultracision (Harmonic ACE Curved Shears) (right hand) (to be temporarily exchanged with large needle driver). Arm 4: tip-up fenestrated grasper (right hand).
We suggest that adhesiolysis be performed using robotic or laparoscopic instrumentation through the da Vinci and/or laparoscopic assistant ports, as needed.
1. Inspection
After insertion of the remaining da Vinci instruments under sight and adequate trocar and arm depth, the liver paddle is first inserted via the A1 and the liver is retracted towards the right-upper quadrant. The abdominal space is carefully inspected. If unsure whether the jejunal loop is long enough, the transverse colon is turned over and a length of 100 cm is approximated on the jejunum from the ligament of Treitz in the aboral direction, to define the site where the jejunal loop will be anastomosed to the stomach for the gastrojejunostomy. The greater omentum is divided above the colon transversum. Be aware of different vasculature and use a straight dissection line. If the greater omentum is dissected in a diagonal manner, partial omentum necrosis can occur, leading to a higher spike in C-reactive protein (CRP) values.
2. Preparation of the stomach pouch
To preserve the left gastric artery, a retrogastric tunnel is formed starting 6 cm below the gastroesophageal junction from the lesser curvature. To ensure the correct size of the pouch, reducing the risk of stenosis or dumping syndrome, an 18-mm bougie is inserted orally by the anesthesiologist after removing the gastric tube. Using a linear stapler (Echelon Flex, 45 mm, Ethicon, Johnson & Johnson, Cincinnati, OH, USA), the stomach pouch is formed with the bougie as calibration. We prefer a narrow, but long pouch, which provides the surgeon with many different options in case of surgical problems and/or during revisional bariatric surgery. This should only be done after the surgeon has ensured that the gastric tube has been removed. At the lowest point, far from the lesser curvature, the pouch is opened using Ultracision.
3. Fashioning the gastrojejunostomy
Starting from the ligament of Treitz, 100 cm of jejunum is measured aborally using the fenestrated grasper as a surrogate for a ruler, opened antimesenterically, and a linear stapler (Echelon Flex, 45 mm, gold cartridge, Ethicon, Johnson & Johnson, Cincinnati, OH, USA) is inserted in the proximal direction by the bedside assistant through the A2. Under controlled closing of the stapler, the jejunum can be mobilized antecolically to the stomach pouch. The other end of the stapler is inserted into the stomach pouch, and thus the side-to-side gastrojejunostomy is fashioned after a good positioning of both ends to create only a small enterostomy. After the stapler is fired, it is fundamental that the branches are not opened completely in an uncontrolled manner, otherwise the gastrojejunostomy will tear open. The stapler should only be gently removed after it is opened slightly. For completing this anastomosis, the Harmonic ACE Curved Shears on the third arm are temporarily exchanged with the large needle driver. To close the enterotomy a 15 cm unidirectional 2–0 Stratafix (Ethicon, Johnson & Johnson, Cincinnati, OH, USA) suture is used for a continuous seromuscular suture. Arm 4 is used for optimizing the position of the small bowel loop whilst Arms 1 and 3 are dynamically used for suturing. Starting from each side of the enterostomy, two sutures are needed to complete the anastomosis. The sutures are finished off in the middle with the last stiches being guided in opposite directions. This ensures an anchoring in the tissue. If the suture gets too short at the end of the continuous suture and/or bleeding through the branch channel occurs, clips are applied to increase the anchoring capabilities. A short cutting of the sutures avoids local adhesions.
4. Fashioning the jejunojejunostomy
Proximal to the anastomosis, the jejunum is separated with the same linear stapler. 1,5m distal from the gastrojejunostomy, again using the fenestrated grasper as surrogate for a ruler, the jejunum is opened antimesenterically, and a side-to-side jejunojejunostomy is created using the 45-mm linear stapler. As before the stapler should be opened carefully to avoid stress on the anastomosis. The enterotomy is closed using a single suture with Stratafix 3–0 (Ethicon, Johnson & Johnson, Cincinnati, OH, USA) in a seromuscular running technique.
5. Drainage and closure
To test the gastrojejunostomy a methylene blue test is conducted. The systolic blood pressure is then raised above 130 mmHg to identify any signs of bleeding. Any bleeding is treated with either the bipolar forceps, with a clip application, or a suture. After repositioning the greater omentum, a drainage is placed through the A1. All trocars are retrieved under view. The pneumoperitoneum is released though the A2 and the patient cart can be undocked.
Postoperative care
Postoperative care in our department is standardized. Depending on the BMI and comorbidities, the patient is either brought to the recovery room, the intermediate care, or intensive care units. We want the patient to mobilize themselves on the day of surgery. Bloodwork is regularly done on the first and second postoperative day. The drainage tube is normally taken out on the second to third postoperative day. In our institution, the patients are discharged if CRP values fall, a daily fluid intake of 1000 – 1500 ml is achieved, the drainage is drawn, the wound healing is inconspicuous, the pain levels are low under medication, and the patient is fully mobilized. We regularly see the patients in our outpatient center at both four weeks and 12 months after surgery.
Alterations in the technique
Robotic stapling
If the surgeon prefers using a robotic-assisted stapling device, small changes have to be made concerning the trocar placement. To ensure a good angle when creating the stomach pouch, the trocars in the DV1 and DV3 position have to be switched to 12 mm da Vinci trocars. Potential benefits are the direct control of the stapler by the console surgeon and mechanical bending of the device. However, due to the lack of haptic feedback, there is a potential risk of tissue damage of the intestine when inserted into the jejunum while being grasped by two robotic arms. In this case, the bedside assistant should hold the jejunal loop to feel the tension of the tissue. It also requires another instrument change, increasing the overall operation time. Due to its positioning, the stapler has a closer distance towards the field of operation, potentially reducing the movement range. In our opinion, assistant-driven stapling offers more flexibility and a better distance towards the anastomoses, especially when fashioning the jejunojejunostomy, but needs a much more experienced bedside assistant.
Anastomosis techniques
There are several anastomotic techniques available for the RRYGB. They include linear stapler anastomosis, circular stapler anastomosis, and complete “hand”-sewn suture. There are studies comparing different anastomotic techniques within LRYGB. The linear anastomosis may be associated with a reduced risk of anastomosis stricture and wound infection, as well as with a shorter operative time. No difference was found with regard to leakage rates.16 Hand-sewn and circular stapler anastomoses are techniques with similar safety and effectiveness, with the hand-sewn anastomosis having a lower rate of bleeding complications and surgical wound infection, although requiring greater experience in laparoscopic hand suturing.17 Whether those statements are also valid for RRYGB remains to be shown. We have kept to the well-established technique using the linear stapler to reduce the patient’s risk in the implementation phase.
Suture
A continuous seromuscular running suture with resorbable braided suture material can also be used for the anastomoses. In this case, the bedside assistants job is to guide and tense the suture throughout. However, we prefer the fixed-barbed sutures because they enable the robotic surgeon to perform a simple continuous suture without the need for recurrent retraction.18
Instruments used
Besides our preferred method using the Ultracision Harmonic ACE Curved Shears, there are also other options available for the energy dissection, such as the vessel sealer (Intuitive Surgical Sàrl), scissors, and hook. We do not normally use scissors with bipolar or the hook with monopolar energy, because in our opinion they do not deliver the required hemostatic capability like the Ultracision, thus regularly leading to the use of extra laparoscopic clips. The vessel sealer is very effective in its hemostatic capabilities and is also bendable. The joint is quite far away from the tip of the instrument, making it a bit harder to get used to. In our opinion, it is not suitable for the enterotomy, meaning that either the assistant has to perform enterotomy or another instrument has to be used, increasing the time and costs of the procedure. This is why we use the Ultracision, even though it is not bendable and is significantly more expensive compared to the hook and/or scissor.
Troubleshooting
With the implementation of a new robotic procedure, problems associated with it naturally arise. In Table 1, we outline typical problems occurring during a RRYGB and our potential solutions.
Table 1.
Troubleshooting of Common Problems During Robotic Roux-en-Y Gastric Bypass
| Problem | Solution |
|---|---|
| Ultracision is too short | Insert the trocar deeper into the skin, but make sure to reposition the trocar afterwards to reduce friction and increase precision, which will minimize tissue trauma |
| Jejunojejunostomy directly in front of the camera | Insert the two left DV trocars deeper into the skin. But note the length of the Ultracision |
| Increased risks of tissue damage by the first arm when hepatomegaly is present | Use a liver retractor and place the DV1 trocar more caudally |
| Tissue damage when measuring the length of the limps | Only grasp the small intestine with atraumatic instruments (bipolar forceps, tip-up fenestrated grasper) to minimize friction. Never grasp tissue with two robotic instruments close to each other. Always spread out the point of grasping and only do so under visual control |
| Dissection of stomach wall when inserting stapler and/or insufficient anastomosis | Ensure the full thickness of the gastrotomy. Evaluate the possibility of using a running suture. If unsure, refashion the anastomosis |
| Tissue damage when using the robotic stapler | Always have ALL of your instruments in your field of view, especially when inserting the robotic stapler |
DV, da Vinci.
DISCUSSION
The role of the robotic system in bariatric surgery is still unclear. It offers the advantage of a separation between the restoring forces of the abdominal wall, especially in patients with a high BMI, and the surgeon, making it more comfortable. As an international comparison, patients in Germany have the highest BMI values at the time of surgery (49.1 kg/m2, compared to 34.2 kg/m2 in South Korea, for example).3
Use of the robotic technique has hardly played a role so far in Germany, as the higher costs led several centers to promptly discontinue their robotic programs after their initial experience. The costs for the use of robotic systems can be divided into three groups: the initial purchase, maintenance, and disposable parts. This means that in Germany, there are currently about 2000€extra costs per case.20 Due to the tightly calculated fees and the lack of additional fees for the use of the robot, robot-assisted bariatric interventions cannot cover these costs. By reducing the operation times after the learning curve and the "idle time" of the robot room, the costs per case can be reduced by 25% to 1500€. In their work, Hagen et al. even describe lower total costs for RRYGB procedures due to lower complication rates.21 Due to higher margins, the costs in the USA play an unsignificant role in the evaluation of the benefits of the surgical robot. In general, a robot program should be managed on an interdisciplinary basis to increase utilization and thus minimize the costs per intervention.14
To be able to perform bariatric surgery independently, the American Society for Metabolic and Bariatric Surgery requires participation in 100 bariatric procedures. Similarly, the DGAV also recommends 100 interventions in accordance with the recommendations of Schauer et al.6 to obtain recognition as a surgeon for bariatric surgery. In 2005, the only randomized controlled trial on the learning curve of RRYGB was conducted. Here, shorter surgery times were observed in the robotic group.8 Li and Bindal also described in meta-analyses a shortened learning curve when using the robot.22,23 However, according to a Canadian study, a plateau of the learning curve and thus stable surgery times are only achieved after more than 600 interventions.23 Thus, the benefit of robot-assisted bariatric surgery seems to depend on the surgeon's current level of experience. Within the training phase and beyond, as our own experience shows, RRYGB can be superior compared to the LRYGB but surgeons who have performed more than 500 LRYGB probably won’t benefit when using the robotic system.19 Since last year, several papers based on the data provided by the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) were published.20–23 Raul et al.21 described a better postoperative outcome in the robotic-assisted group compared to the laparoscopic approach their propensity score-matched comparative analysis. The robotic approach might also improve the outcome in revisional bariatric surgery.20
Additionally, technical possibilities combined with robotic surgery allow faster; therefore, safer surgical education, reducing patient morbidity and mortality. The rapid development in this field of surgery is certainly not yet complete, as highlighted by the planned market launch of the Da Vinci Single Port System or the introduction of new robotic platforms. These developments will fuel competition and should reduce the purchasing and running costs of a robotic system, thus hopefully making it more suitable for small output centers in the future.
Contributor Information
Jan-Niclas Kersebaum, University Medical Center Schleswig-Holstein, Campus Kiel, Department of General, Visceral-, Thoracic-, Transplantation-, and Pediatric Surgery, Arnold-Heller-Str. 3, 24105 Kiel, Germany.
Thorben Möller, University Medical Center Schleswig-Holstein, Campus Kiel, Department of General, Visceral-, Thoracic-, Transplantation-, and Pediatric Surgery, Arnold-Heller-Str. 3, 24105 Kiel, Germany.
Witigo von Schönfels, University Medical Center Schleswig-Holstein, Campus Kiel, Department of General, Visceral-, Thoracic-, Transplantation-, and Pediatric Surgery, Arnold-Heller-Str. 3, 24105 Kiel, Germany.
Terbish Taivankhuu, University Medical Center Schleswig-Holstein, Campus Kiel, Department of General, Visceral-, Thoracic-, Transplantation-, and Pediatric Surgery, Arnold-Heller-Str. 3, 24105 Kiel, Germany.
Thomas Becker, University Medical Center Schleswig-Holstein, Campus Kiel, Department of General, Visceral-, Thoracic-, Transplantation-, and Pediatric Surgery, Arnold-Heller-Str. 3, 24105 Kiel, Germany.
Jan-Hendrik Egberts, University Medical Center Schleswig-Holstein, Campus Kiel, Department of General, Visceral-, Thoracic-, Transplantation-, and Pediatric Surgery, Arnold-Heller-Str. 3, 24105 Kiel, Germany.
Jan Henrik Beckmann, University Medical Center Schleswig-Holstein, Campus Kiel, Department of General, Visceral-, Thoracic-, Transplantation-, and Pediatric Surgery, Arnold-Heller-Str. 3, 24105 Kiel, Germany; Department of General, Visceral-, Thoracic-, Transplantation-, and Pediatric Surgery, University Medical Center Schleswig-Holstein, Campus Kiel (Drs Kersebaum, Möller, von Schönfels, Taivankhuu, Becker, Egberts and Beckmann)..
References:
- 1.Pineda E, Sanchez-Romero LM, Brown M, et al. Forecasting future trends in obesity across Europe: the value of improving surveillance. Obes Facts. 2018;11:360–371. [DOI] [PMC free article] [PubMed]
- 2.Angrisani L, Santonicola A, Iovino P, et al. IFSO Worldwide Survey 2016: Primary, Endoluminal, and Revisional Procedures. Obes Surg. 2018;28(12):3783–3794. [DOI] [PubMed] [Google Scholar]
- 3.Welbourn R, Hollyman M, Kinsman R, et al. Bariatric Surgery Worldwide: Baseline Demographic Description and One-Year Outcomes from the Fourth IFSO Global Registry Report 2018. Obes Surg. 2019;29(3):782–795. [DOI] [PubMed] [Google Scholar]
- 4.Wittgrove AC, Clark GW, Tremblay LJ. Laparoscopic Gastric Bypass, Roux-en-Y: Preliminary Report of Five Cases. Obes Surg Incl Laparosc Allied Care. 1994;4(4):353–357. [DOI] [PubMed] [Google Scholar]
- 5.Banka G, Woodard G, Hernandez-Boussard T, Morton JM. Laparoscopic vs open gastric bypass surgery: Differences in patient demographics, safety, and outcomes. Arch Surg. 2012;147(6):550–556. [DOI] [PubMed] [Google Scholar]
- 6.Schauer P, Ikramuddin S, Hamad G, Gourash W. The learning curve for laparoscopic Roux-en-Y gastric bypass is 100 cases. Surg Endosc. 2003;17(2):212–215. [DOI] [PubMed] [Google Scholar]
- 7.Doumouras AG, Saleh F, Anvari S, Gmora S, Anvari M, Hong D. Mastery in Bariatric Surgery. Ann. Surg. 2018;267(3):489–494. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez BR, Mohr CJ, Morton JM, Safadi BY, Alami RS, Curet MJ. Comparison of totally robotic laparoscopic Roux-en-Y gastric bypass and traditional laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis 2005; vol. 1(6):549–554. [DOI] [PubMed] [Google Scholar]
- 9.Egberts JH, Stein H, Aselmann H, Jan-Hendrik A, Becker T. Fully robotic da Vinci Ivor-Lewis esophagectomy in four-arm technique-problems and solutions. Dis Esophagus. 2017;30(1):1–9. [DOI] [PubMed] [Google Scholar]
- 10.Aselmann H, Egberts JH, Beckmann JH, et al. Roboterassistierte pyloruserhaltende Pankreaskopfresektion. Der Chir. 2017;88(5):411–421. [DOI] [PubMed] [Google Scholar]
- 11.Aselmann H, Möller T, Kersebaum J-N, et al. Roboterassistierte Leberresektion. Der Chir. 2017;88(6):476–483. [DOI] [PubMed] [Google Scholar]
- 12.Aselmann H, Kersebaum J-N, Bernsmeier A, et al. Robotic-assisted total mesorectal excision (TME) for rectal cancer results in a significantly higher quality of TME specimen compared to the laparoscopic approach—report of a single-center experience. Int J Colorectal Dis. 2018;33(11):1575–1581. [DOI] [PubMed] [Google Scholar]
- 13.Beckmann JH, Bernsmeier A, Kersebaum J-N, et al. The Impact of Robotics in Learning Roux-en-Y Gastric Bypass: a Retrospective Analysis of 214 Laparoscopic and Robotic Procedures. Obes Surg. March 2020:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egberts JH, Beham A, Ghadimi M. Aufbau eines Roboterprogramms, Zentralblatt für Chir - Zeitschrift für Allg Visz Thorax- und Gefäßchirurgie. 2016;141(02):143–144. [DOI] [PubMed] [Google Scholar]
- 15.Lux MM, Marshall M, Erturk E, Joseph J V. Ergonomic evaluation and guidelines for use of the daVinci robot system. J Endourol. 2010;24(3):371–375. [DOI] [PubMed] [Google Scholar]
- 16.Giordano S, Salminen P, Biancari F, Victorzon M. Linear stapler technique may be safer than circular in gastrojejunal anastomosis for laparoscopic Roux-en-Y gastric bypass: A meta-analysis of comparative studies. Obesity Surgery. 2011;21(12): 1958–1964. [DOI] [PubMed] [Google Scholar]
- 17.Abellán I, López V, Lujan J, et al. Stapling Versus Hand Suture for Gastroenteric Anastomosis in Roux-en-Y Gastric Bypass: a Randomized Clinical Trial. Obes Surg. 2015;25(10):1796–1801. [DOI] [PubMed] [Google Scholar]
- 18.Beckmann JH, Kersebaum J-N, von Schönfels W, et al. Use of barbed sutures in robotic bariatric bypass surgery: a single-center case series. BMC Surg. 2019;19(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beckmann JH, Becker T, Schafmayer C. Roboter-assistierte bariatrische Chirurgie in Deutschland. CHAZ. 2017;20(6):294–298. [Google Scholar]
- 20.Nasser H, Munie S, Kindel TL, Gould JC, Higgins RM. Comparative analysis of robotic versus laparoscopic revisional bariatric surgery: perioperative outcomes from the MBSAQIP database. In: Surgery for Obesity and Related Diseases. vol. 16.Elsevier Inc.;2020:397–405. [DOI] [PubMed] [Google Scholar]
- 21.Sebastian R, Howell MH, Chang KH, et al. Robot-assisted versus laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a propensity score-matched comparative analysis using the 2015–2016 MBSAQIP database. Surg Endosc. 2019;33(5):1600–1612. [DOI] [PubMed] [Google Scholar]
- 22.Papasavas P, Seip RL, Stone A, Staff I, McLaughlin T, Tishler D. Robot-assisted sleeve gastrectomy and Roux-en-y gastric bypass: results from the metabolic and bariatric surgery accreditation and quality improvement program data registry. Surg Obes Relat Dis. 2019;15(8):1281–1290. [DOI] [PubMed] [Google Scholar]
- 23.Pastrana M, Stoltzfus J, Claros L, El Chaar M. Outcomes of robotic bariatric surgery in super-obese patients: first report based on MBSAQIP database. Surg Obes Relat Dis. 2020;16(1):71–79. [DOI] [PubMed] [Google Scholar]


