Abstract
Given the COVID-19 pandemic, currently, there are many drugs in clinical trials against this virus. Among the excellent drug targets of SARS-CoV-2 are its proteases (Nsp3 and Nsp5) that plays vital role in polyprotein processing giving rise to functional nonstructural proteins, essential for viral replication and survival. Nsp5 (also known as Mpro) hydrolyzes replicase polyprotein (1ab) at eleven different sites. For targeting Mpro, we have employed drug repurposing approach to identify potential inhibitors of SARS-CoV-2 in a shorter time span. Screening of approved drugs through docking reveals Hyaluronic acid and Acarbose among the top hits which are showing strong interactions with catalytic site residues of Mpro. We have also performed docking of drugs Lopinavir, Ribavirin, and Azithromycin on SARS-CoV-2 Mpro. Further, binding of these compounds (Hyaluronic acid, Acarbose, and Lopinavir) is validated by extensive molecular dynamics simulation of 500 ns where these drugs show stable binding with Mpro. We believe that the high-affinity binding of these compounds will help in designing novel strategies for structure-based drug discovery against SARS-CoV-2.
Communicated by Ramaswamy H. Sarma
Keywords: SARS-CoV-2, COVID-19, Nsp5, Mpro, approved drug, protease
Introduction
Traditional drug development, despite of technical advancement is an expensive, laborious, and time-consuming process. In the pandemic situation like Coronavirus Disease 2019 (COVID-19), where no proper cure is available, drug repurposing or repositioning of an approved drug is a desirable choice. Repurposing is a progressive strategy that helps in identifying a new use for already approved drugs. With undeniable advantages of shorter time requirements and lower costs for development, use of an approved drug against a novel target can fulfill the principle need in this current pandemic (Ashburn & Thor, 2004; Breckenridge & Jacob, 2019; Pushpakom et al., 2019). Therefore, drug repurposing against different targets of newly emerged Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) may deliver faster results.
Our previous report on SARS-CoV-2 has elaborately discussed the identified or probable functions and presence of disorder in its proteome (Giri et al., 2020). Among the known protein targets of SARS-CoV-2, non structural protein 3 and 5 (also called as Mpro or 3CL-protease) proteases are excellent drug targets due to their roles in processing of polyprotein into the constituent nonstructural proteins needed for viral replication and survival (Wu et al., 2020). Mpro cleaves the replicase polyprotein 1ab at eleven different sites and is considered to be the best-characterized enzyme targets for drug development against coronaviruses (Zhang et al., 2020). Recently, one of the crystal structures of SARS-CoV-2 Mpro (PDB ID: 6LU7) in complex with N3 (asynthetic inhibitor) is reported by Zhenming et al. (Anand et al., 2003; Jin et al., 2020).
Previously, we have established several studies to identify some of the existing drugs as inhibitors of other viral proteomes (Kumar et al., 2018, 2019; Kumar, Sharma et al., 2020; Sharma et al., 2020). One such report discussed the inhibitory action of hydroxychloroquine against Zika virus protease (Kumar et al., 2018). Here, in this study, we made an attempt to identify druggable sites in Mpro and also investigated the binding interactions between Mpro and approved drug molecules. We have screened a library of drugs approved by USA, Canada and European authorities, fetched from the Drugbank database, against active-site of SARS-CoV-2 Mpro. As revealed from docking and simulation results, top hit compounds and approved drugs (which are currently in clinical trials) (Cao et al., 2020; ‘COVID-19 ring-based prevention trial with lopinavir/ritonavir’, 2020; ‘Evaluation of the efficacy of the hydroxychloroquine-azithromycin combination in the in the prevention of COVID-19 related SDRA’, 2020; Khalili et al., 2020; ‘Safety and efficacy of hydroxychloroquine associated with azithromycin in SARS-CoV2 virus’, 2020) are showing stable and robust binding with active site residues of enzyme Mpro. High-affinity binding interactions of these compounds could be a basis to design or improve the novel strategies for further clinical trials and structure-based drug discovery against SARS-CoV-2.
Materials and methods
Preparation of protein and ligands
First crystal structure of SARS-CoV-2 main protease (PDB ID: 6LU7) (Jin et al., 2020) in complex with N3 inhibitor is made available quickly for exploring structure-based drug designing opportunities. The structure is prepared and refined using Protein Preparation Wizard of Schrodinger LLC (Madhavi Sastry et al., 2013). The improper bond parameters and sidechain disarrangement in Mpro structure are corrected in Maestro and minimized by OPLS 2005 forcefield. The Mpro structure is used to generate active sites from Sitemap program (Halgren, 2009). A grid is generated at the inhibitor binding site to analyze the interactions of approved drugs with the active site of Mpro. A library of 2454 approved drugs obtained from Drugbank database (Wishart et al., 2018) is prepared in LigPrep module by generating at most 4 stereoisomers and tautomers (total 5025 structure in output) per ligand at pH 7 using Epik program.
Docking studies of mpro with approved drugs
Prepared library of approved drugs is docked against prepared Mpro structure using Glide XP (Extra Precision method) scoring algorithm embedded in Schrodinger. OPLS 2005 forcefield is used for calculating bonded and non-bonded parameters such as van der Waal interactions (Friesner et al., 2006; Halgren et al., 2004). For the detailed protocol of Glide, see our previously reported articles (Kumar et al., 2019; Kumar, Aarthy et al., 2020; Kumar, Saumya et al., 2020; Sharma et al., 2017; Yadav et al., 2020). The interaction of docked compounds with Mpro is analyzed in the 4 Å cutoff of active site. Further, binding energy calculation is done using Prime module (based on Molecular mechanics-Generalized Born Surface Area: MM-GBSA) (Aarthy et al., 2018; Jacobson et al., 2004).
Molecular dynamics (MD) simulations
To study the structural dynamics and binding stability of inhibitors with Mpro at a molecular level, we performed MD simulation in SPC water models using GROMOS54A7 forcefield in Gromacs upto 500 ns (Berendsen et al., 1995). Charge neutralization is done by adding 3 Na+ ions and 0.15 M salt in the cubic simulation box. For energy minimization, 50,000 steps of steepest descent algorithm are executed with Verlet cutoff scheme to calculate the neighboring interactions. Further, equilibration process under NPT and NVT conditions is done for 1 ns. Parrinello-Rahman and V-rescale methods are availed for pressure and temperature coupling, respectively. The inhibitor topologies are generated using PRODRG server. Finally, the production runs for all four systems (Mpro with Hyaluronic acid (HA), Acarbose, Amikacin, and Lopinavir) is performed in periodic boundary conditions for 500 ns each. LINCS algorithm is used for calculating bond parameters (Hess et al., 1997). Particle Mesh Ewald for long-range electrostatics with fourier spacing of 0.16 is used for production MD simulations.
Analysis of MD simulations
The analysis consisting root mean square deviation (RMSD), root mean square fluctuation (RMSF), radius of gyration (Rg) for C-α atoms, are calculated using gmx, rms, rmsf, and gyrate commands, respectively. The number of hydrogen bonds are calculated in VMD using Hydrogen bonds plugin (Humphrey et al., 1996). Further, we have used covar and anaeig commands of gromacs to interpret the cumulative motions occurring in protein by virtue of ligand binding describing the principle component analysis (PCA). We have also measured the solvent accessible surface area (SASA) using sasa command in Gromacs to determine the exposed surface area of protein to the solvents.
Results and discussion
Analysis of active site and other druggable sites of mpro
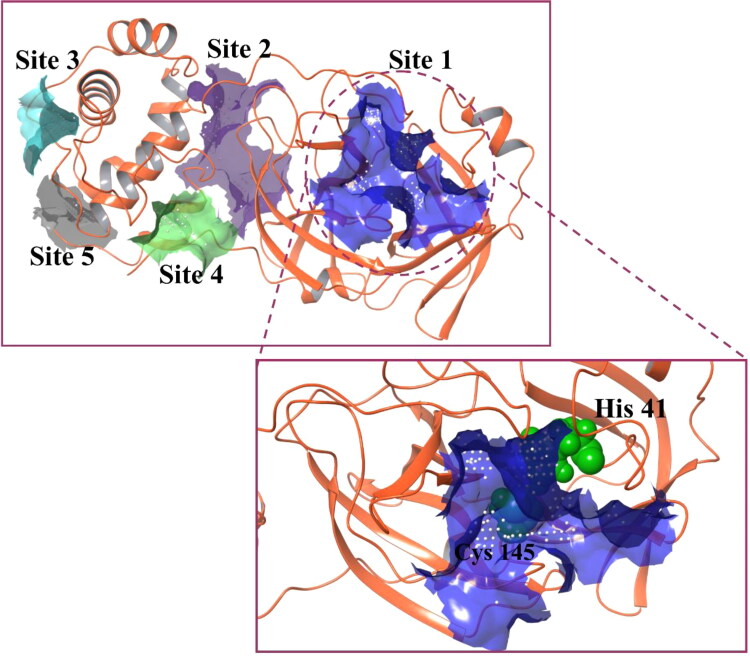
The Mpro crystal structure (PDB ID: 6LU7) is used in this study to screen the approved drug molecules for the purpose of identifying a potential inhibitor candidate. To proceed with the screening process, identification of a druggable site is the primary requirement for a potent outcome. As illustrated in Figure 1, five different druggable sites are predicted in Mpro structure by employing a SiteMap module. Among these five predicted sites, the most appropriate site based on the size and volume of the pocket, hydrophobicity and hydrophilicity of amino acid, site score, and druggability score (Dscore) is selected for docking and simulation analyses (Table 1). Out of all five sites, site 1 obtains the highest site score and Dscore. Importantly, it consists of a catalytic dyad having -His41 and -Cys145, essential for the protease activity of Mpro. Also, a comparison between the predicted site 1 and inhibitor binding site of N3 (co-crystallized N3 inhibitor with Mpro; 6LU7) reveals a similar type of residue distribution in both sites (Table 1). Therefore, we have considered site 1 for further docking studies. A grid was generated at site 1 with the coordinates of −12.49 Å, 15.06 Å and 74.04 Å for X, Y and Z, respectively.
Figure 1.
Predicted druggable sites of Mpro. Five druggable sites are represented with different colors – site 1: blue; site 2: purple; site 3: cyan; site 4: green and site 5: grey. Site 1 shows the highest site score and Dscore, and hence is selected for docking studies.
Table 1.
Parameters associated with druggable site of Mpro predicted by SiteMap.
| Druggable sites | Site score | Dscore | Hydro-phobicity | Hydro-philicity | Residue distribution at the druggable site |
|---|---|---|---|---|---|
| N3 binding site (6LU7) | – | – | – | – | 3, 4, 24–27, 41, 49, 140–145, 163–166, 172 |
| Site 1 | 0.99 | 1.036 | 0.699 | 0.876 | 25–27, 41, 44–46, 49, 52, 54, 140–145, 163–168, 172, 187–190, 192 |
| Site 2 | 0.782 | 0.769 | 0.279 | 0.875 | 8, 104, 106–111, 127, 151, 153, 158, 200–203, 240, 246, 249, 292–295 |
| Site 3 | 0.645 | 0.61 | 0.873 | 0.749 | 218–221, 270–271, 274–277, 279 |
| Site 4 | 0.573 | 0.385 | 0.09 | 1.352 | 3–5, 207, 282, 284, 288, 291 |
| Site 5 | 0.541 | 0.444 | 0 | 1.102 | 212–213, 217, 256–257, 304–306 |
Residue distribution in predicted site 1 and experimental N3 binding site is observed to be very similar. Residues of active site in crystal structure and predicted active site, in bold fonts, show similarity with each other.
Molecular docking
2454 approved drug molecules are selected from DrugBank database for molecular docking at predicted site 1 of Mpro of SARS-CoV-2. These selected drugs are first subjected to the ligand preparation module, Ligprep, to generate at most four stereoisomers for each drug molecule (produced 5025 structures). Resulted structures are then subjected further in molecular docking protocol, which generated 9263 binding poses from 5025 input structures based on binding energies. Based on their interactions and docking scores, we have shortlisted top 100 hits which are tabulated in Table 2. Most of these listed compounds are antibiotics and antioxidants or have anti-inflammatory as well as anti-cancer properties (Table 2). In addition to top 100 compounds we have also shortlisted Ribavirin, Lopinavir, and Azithromycin (Table 3) for further analysis as these approved drugs are currently being tested on SARS-CoV-2 infected patients. The investigation of stability and binding interactions of these compounds at Mpro active site 1 will help in designing novel strategies for therapeutics against COVID-19. Detailed description of docking parameters of top hits and selected compounds are given in Tables 2 and 3.
Table 2.
A summary of XP-docking results of top 100 hits and their pharmaceutical usage.
| Sr. No. | Database ID | Compound Name | Uses of drugs | Mol. Weight (Da) | Docking score (kcal/mol) | Glide emodel (kcal/mol) |
|---|---|---|---|---|---|---|
| 1. | DB08818 | Hyaluronic acid | For the treatment of joint disorders and eye-related problems | 776.649 | −13.54 | −83.935 |
| 2. | DB00284 | Acarbose | Anti-diabetic | 645.608 | −12.181 | −62.573 |
| 3. | DB03615 | Ribostamycin | Antibiotic; Anti-HIV | 454.473 | −11.845 | −58.629 |
| 4. | DB00104 | Octreotide | For treatment of acromegaly | 1019.25 | −11.162 | −98.49 |
| 5. | DB01421 | Paromomycin | For the treatment of acute and chronic intestinal amebiasis | 615.629 | −11.159 | −78.346 |
| 6. | DB01698 | Troxerutin | Antioxidant | 610.518 | −11.122 | −95.085 |
| 7. | DB00479 | Amikacin | Antibiotic | 585.602 | −10.966 | −72.087 |
| 8. | DB01172 | Kanamycin | Antibiotic | 484.499 | −10.928 | −58.924 |
| 9. | DB00452 | Neomycin | Antibiotic | 614.644 | −10.071 | −73.753 |
| 10. | DB04846 | Celiprolol | Vasodilator | 379.501 | −9.83 | −63.781 |
| 11. | DB01204 | Mitoxantrone | Anti-neoplastic, for treating multiple sclerosis | 444.481 | −9.732 | −81.091 |
| 12. | DB13074 | Macimorelin | Ghrelin memetic, for treating adult growth hormone deficiency | 474.565 | −9.326 | −92.817 |
| 13. | DB01193 | Acebutolol | For treating hypertension and cardiac arrythmia | 336.426 | −9.184 | −57.125 |
| 14. | DB11190 | Pantethine | For lowering blood cholesterol and triglycerides | 554.721 | −9.076 | −81.72 |
| 15. | DB13270 | Dibekacin | Antibiotic | 451.521 | −9.009 | −59.594 |
| 16. | DB08995 | Diosmin | For treating hemorrhoids, and chronic venous diseases | 608.545 | −8.904 | −78.572 |
| 17. | DB00560 | Tigecycline | Antibiotic | 585.649 | −8.729 | −73.398 |
| 18. | DB00803 | Colistin | Antibiotic | 1155.455 | −8.699 | −105.537 |
| 19. | DB09146 | Iron sucrose | For treating iron deficiency | 866.546 | −8.08 | −41.733 |
| 20. | DB00449 | Dipivefrin | Prodrug of adrenaline, used to control-intra ocular pressure in open eye glaucoma | 351.437 | −8.51 | −59.081 |
| 21. | DB00684 | Tobramycin | Antibiotic | 467.514 | −9.834 | −60.025 |
| 22. | DB08816 | Ticagelor | Platelet aggregation inhibitor | 522.568 | −8.468 | −78.186 |
| 23. | DB00997 | Doxorubicin | Antibiotic | 543.519 | −8.59 | −54.811 |
| 24. | DB00581 | Lactulose | Laxative agent for treating chronic constipation | 342.296 | −8.248 | −48.27 |
| 25. | DB00287 | Travoprost | For treating ocular hypertension | 500.548 | −8.233 | −64.479 |
| 26. | DB00445 | Epirubicin | Anti-tumor effect | 543.519 | −8.219 | −71.773 |
| 27. | DB04465 | Lactose | Used as nutrient and in medical preparations | 342.296 | −8.125 | −45.437 |
| 28. | DB01177 | Idarubicin | Antineoplastic | 497.494 | −8.156 | −62.355 |
| 29. | DB08916 | Afatinib | For treating metastatic non-small cell lung cancer | 485.938 | −8.097 | −86.799 |
| 30. | DB00118 | Ademetionine | Anti-inflammatory, for treating chronic liver diseases | 398.44 | −8.073 | −56.482 |
| 31. | DB00938 | Salmeterol | For treating asthma and chronic obstructive pulmonary disease | 415.566 | −8.069 | −64.203 |
| 32. | DB01082 | Streptomycin | Antibiotic | 581.574 | −10.415 | −62.467 |
| 33. | DB09050 | Ceftolozane | Antibacterial | 666.69 | −9.869 | −94.804 |
| 34. | DB06791 | Lanreotide | For treating acromegaly and carcinoid syndrome | 1096.33 | −9.827 | −86.499 |
| 35. | DB14568 | Ivosidenib | For treating acute myeloid leukemia | 582.97 | −8.657 | −76.875 |
| 36. | DB06663 | Pasireotide | For treating Cushing’s syndrome | 1047.206 | −8.515 | −89.032 |
| 37. | DB00314 | Capreomycin | Antibiotic | 1321.412 | −8.382 | −74.787 |
| 38. | DB06267 | Udenafil | For treating erectile dysfunction | 516.656 | −8.048 | −81.068 |
| 39. | DB08868 | Fingolimod | For treating multiple sclerosis | 307.471 | −8.034 | −63.569 |
| 40. | DB00140 | Riboflavin | Neutraceutical | 376.364 | −8.01 | −64.756 |
| 41. | DB11842 | Angiotensin II | Vasoconstrictor | 1046.179 | −7.913 | −100.789 |
| 42. | DB09026 | Aliskiren | Renin inhibitor, for treating hypertension | 551.758 | −7.927 | −73.187 |
| 43. | DB00798 | Gentamycin | Antibiotic | 477.595 | −7.913 | −49.219 |
| 44. | DB11986 | Entrectinib | For treating ROS-1 positive non-small cell lung cancer and NTRK gene fusion positive solid tumors | 560.65 | −7.855 | −81.876 |
| 45. | DB00481 | Ralofixene | Anti-estrogen, for prevention and treatment of osteoporosis, corticosteroid-induced bone loss, and invasive breast cancer | 473.583 | −7.799 | −71.274 |
| 46. | DB01232 | Saquinavir | HIV protease inhibitor | 670.841 | −7.798 | −77.88 |
| 47. | DB00623 | Fluphenazine | For treating psychosis | 437.522 | −7.793 | −67.712 |
| 48. | DB01076 | Atorvastatin | For treating dyslipidemia and preventing cardiovascular diseases | 558.64 | −7.787 | −78.135 |
| 49. | DB12615 | Plazomycin | Antibacterial used for treatment of complicated Urinary tract infections. | 592.691 | −9.778 | −72.324 |
| 50. | DB13265 | Hexobendine | Vasodilator | 592.686 | −7.744 | −76.055 |
| 51. | DB01095 | Fluvastatin | Statin used for preventing cardiovascular diseases | 411.466 | −7.721 | −64.343 |
| 52. | DB11827 | Ertugliflozin | For improving glycemic control in type 2 diabetes | 436.89 | −7.706 | −60.905 |
| 53. | DB00211 | Midodrine | Vasoconstrictor, for treating hypotension | 254.282 | −7.689 | −49.714 |
| 54. | DB01598 | Imipenem | Antibacterial | 299.346 | −7.676 | −50.15 |
| 55. | DB00195 | Betaxolol | For treating hypertension | 307.428 | −7.67 | −49.774 |
| 56. | DB00512 | Vancomycin | Antibacterial | 1449.254 | −7.669 | −90.77 |
| 57. | DB08893 | Mirabegron | For treating overactive bladder | 396.506 | −7.544 | −66.775 |
| 58. | DB09335 | Alatrofloxacin | Antibiotic | 558.518 | −7.538 | −83.603 |
| 59. | DB09082 | Vilanterol | For treating COPD and asthma | 486.43 | −7.536 | −75.659 |
| 60. | DB01118 | Amiodarone | Antiarrhythmic | 645.312 | −7.532 | −64.154 |
| 61. | DB09330 | Osimertinib | For treatment of metastatic EGFR-T790M mutation positive non − small cell lung cancer (NSCLC). | 499.619 | −7.503 | −86.439 |
| 62. | DB01203 | Nadolol | Used to lower blood pressure. | 309.401 | −7.5 | −54.254 |
| 63. | DB06717 | Fosaprepitant | Used to prevent nausea associated with chemotherapy treatment. | 614.407 | −7.479 | −80.068 |
| 64. | DB01195 | Flecainide | Anti-arrhythmic agent | 414.343 | −7.452 | −58.535 |
| 65. | DB00955 | Netilmicin | Aminoglycoside antibiotic | 475.587 | −7.442 | −53.689 |
| 66. | DB01297 | Practolol | Treatment of cardiac arrhythmia | 266.336 | −7.426 | −45.129 |
| 67. | DB00176 | Fluvoxamine | Anti-depressant | 318.34 | −7.424 | −50.184 |
| 68. | DB00738 | Pentamidine | Anti-protozoal agent | 340.42 | −7.409 | −54.139 |
| 69. | DB08874 | Fidaxomicin | Anti-biotic used for treatment of diarrhoea | 1058.039 | −7.392 | −67.174 |
| 70. | DB00722 | Lisinopril | Used to treat hypertension, heart failure and myocardial infarction. | 405.488 | −7.378 | −58.31 |
| 71. | DB11712 | Tezacaftor | Used as Cystic Fibrosis membrane conductance regulator | 520.505 | −7.367 | −67.889 |
| 72. | DB01182 | Propafenone | Anti-arrhythmia agent | 341.444 | −7.355 | −61.246 |
| 73. | DB11263 | Polydatin | Possesses anti-inflammatory, immune-regulatory, anti-oxidative and anti-tumor activities. | 390.388 | −7.767 | −58.642 |
| 74. | DB06193 | Pixantone | Used in treatment of relapsed or refractory non-Hogkin’s Lymphoma (NHL) | 325.372 | −7.329 | −62.038 |
| 75. | DB06736 | Aceclofenac | Non-steroildal anti-inflammatory drug. | 354.18 | −7.307 | −47.903 |
| 76. | DB01098 | Rosuvastatin | Lipid lowering drug used to lower risk of cardiovascular disease. | 481.538 | −7.293 | −69.399 |
| 77. | DB11155 | Triclocarban | Anti-bacterial agent effective against Gram positive bacteria. | 315.58 | −7.27 | −46.515 |
| 78. | DB00224 | Indinavir | Antiviral drug used HIV-type I | 613.789 | −7.245 | −87.086 |
| 79. | DB01062 | Oxybutynin | Anti-cholinergic medication | 357.486 | −7.222 | −57.409 |
| 80. | DB06441 | Cangrelor | Reversible P2Y12 inhibitor for patients undergoing percutaneous coronary intervention (PCI) | 776.35 | −7.204 | −99.813 |
| 81. | DB13274 | Micronomicin | Aminoglycoside antibiotic | 463.576 | −7.202 | −52.655 |
| 82. | DB08932 | Macitentan | Used by people with pulmonary arterial hypertension. | 588.273 | −7.201 | −79.928 |
| 83. | DB06608 | Tafenoquine | Used for treatment and prevention of relapse in vivax malaria. | 463.501 | −7.189 | −62.476 |
| 84. | DB00694 | Daunorubicin | Used for treatment of leukemia and other neoplasms. | 527.52 | −8.15 | −55.99 |
| 85. | DB09092 | Xanthinol | Used as a vasodilator | 311.342 | −7.174 | −49.809 |
| 86. | DB00358 | Mefloquine | Anti-malarial drug | 378.312 | −7.168 | −51.161 |
| 87. | DB01624 | Zuclopenthixol | Anti-psychotic agent | 400.965 | −7.145 | −62.147 |
| 88. | DB08860 | Pitavastatin | Lipid lowering drug | 421.461 | −7.129 | −63.977 |
| 89. | DB11732 | Lasmiditan | Used for termination of migranes. | 377.367 | −7.104 | −54.791 |
| 90. | DB00410 | Mupirocin | Broad spectrum antibiotic | 500.622 | −7.099 | −64.487 |
| 91. | DB12783 | Benserazide | Given in combination with Levadopa to minimize side-effects of Levadopa in Parkinson’s disease therapy | 257.246 | −7.094 | −48.452 |
| 92. | DB08882 | Linagliptin | DPP-4 inhibitor used for treatment of type II diabetes. | 472.542 | −7.092 | −63.844 |
| 93. | DB00188 | Bortezomib | Used for treatment of relapsed myeloma and mantle cell lymphoma. | 384.237 | −7.081 | −68.167 |
| 94. | DB01030 | Topotecan | Anti-neoplastic agent used to treat ovarian cancer. | 421.446 | −7.076 | −57.118 |
| 95. | DB01396 | Digitoxin | Used to treat heart failure | 764.939 | −7.066 | −67.588 |
| 96. | DB13532 | Cyclopenthiazide | Diuretic with anti-hypersentitive properties. | 379.87 | −7.062 | −55.037 |
| 97. | DB00675 | Tamoxifen | Used to treat estrogen receptor positive breast cancers. | 371.515 | −7.052 | −59.058 |
| 98. | DB00961 | Mepivacaine | Local anaesthetic | 246.348 | −7.115 | −37.177 |
| 99. | DB06292 | Dapagliflozin | Used for managing diabetes mellitus type 2 | 408.873 | −7.034 | −56.858 |
| 100. | DB00606 | Cyclothiazide | Diuretic used for treatment of edemaassociated with congestive heart failure, hepatic cirrhosis and corticosteroid and estrogen therapy | 389.878 | −7.03 | −57.596 |
Table 3.
A summary of XP-docking results of selected approved drugs in clinical trials for COVID-19.
| Sr. No. | Database ID | Compound name | Uses of drugs | Molecular weight (Da) | Docking score (kcal/mol) | Glide emodel (kcal/mol) |
|---|---|---|---|---|---|---|
| 1. | DB00811 | Ribavirin | Antiviral | 244.205 | −6.813 | −49.566 |
| 2. | DB01601 | Lopinavir | Antiviral | 628.801 | −6.119 | −81.525 |
| 3. | DB00207 | Azithromycin | Antibiotic | 748.985 | −5.636 | −42.186 |
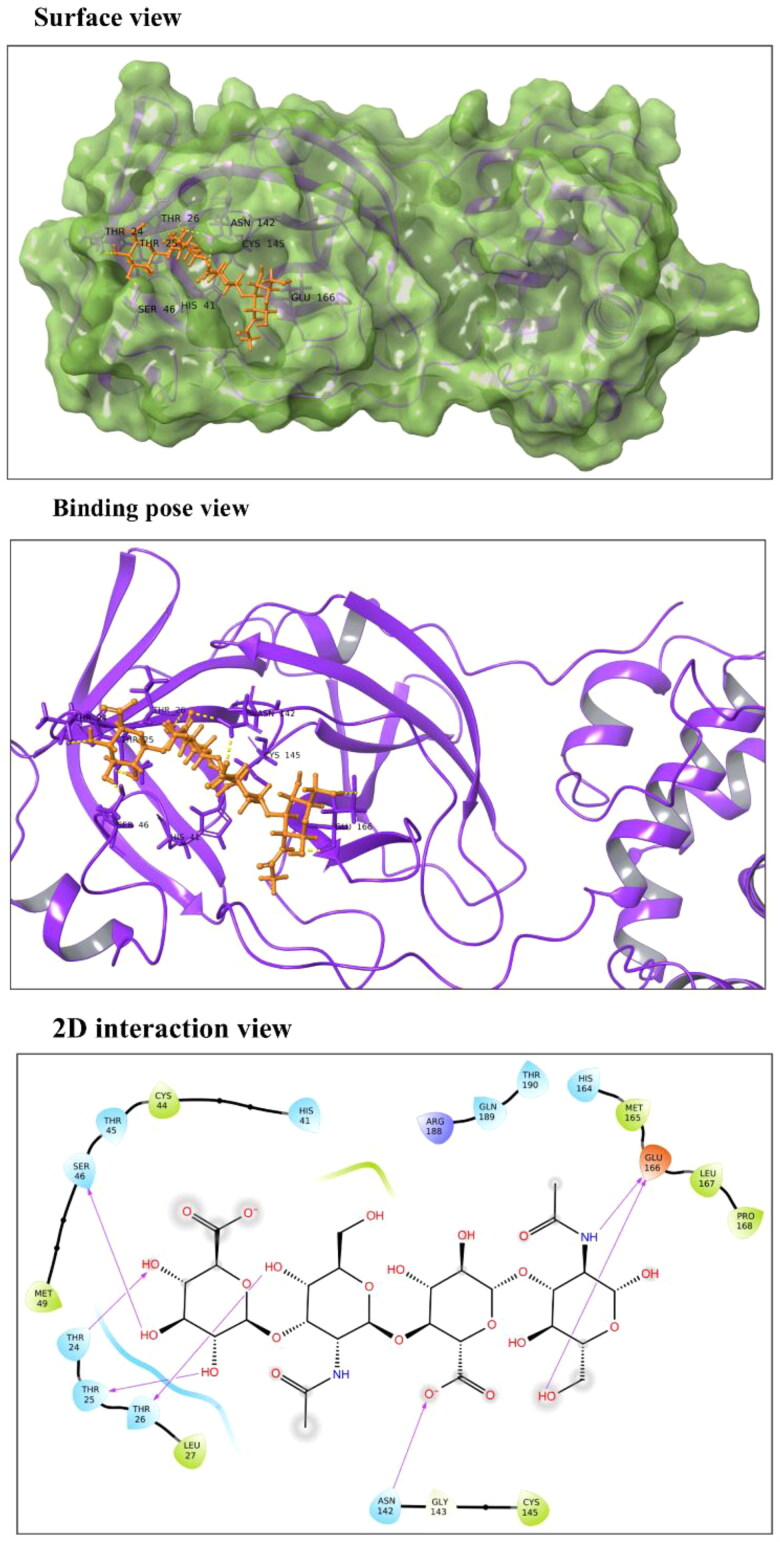
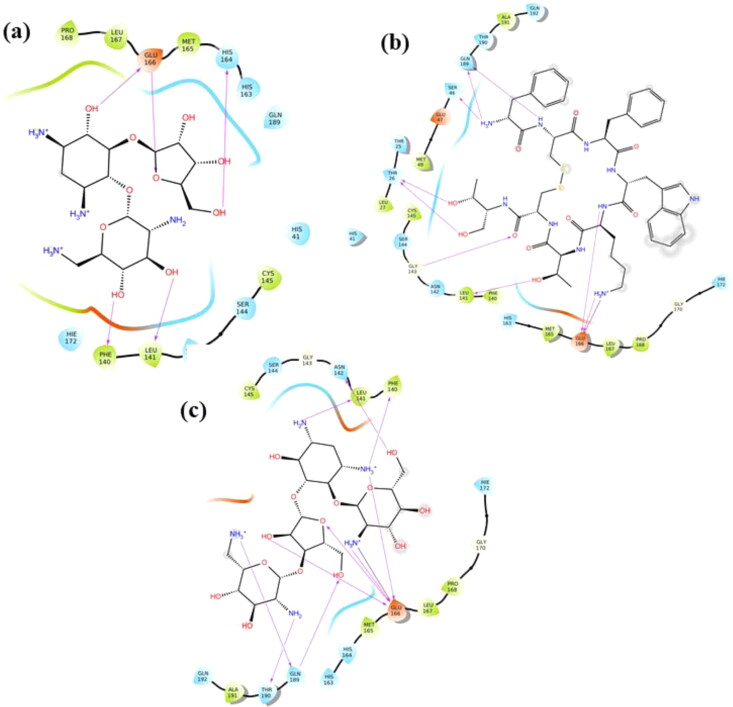
Hyaluronic acid (HA): It is an anionic non-sulfated linear glycosaminoglycan polymer of two saccharide units, D-glucuronic acid and N-acetyl-d-glucosamine. HA is stabilized at the active site 1 of Mpro via hydrogen bonds (H-bonds) with Ser46, Thr24, Thr25, Thr26, Asn142 and Glu166 residues (Figure 2). HA shows docking score and binding energy equivalent to −13.54 kcal/mol and −49.46 kcal/mol, respectively (Table 2). It is majorly found in connective tissues, umbilical cord, vitreous fluid in eyes, and synovial fluid of joints (Fraser et al., 1997). Due to its presence in the extracellular matrix, HA is biocompatible and biodegradable, and therefore, functions as a drug delivery carrier (Widjaja et al., 2014). Using cell culture studies, HA has been demonstrated to work as an antiviral compound against both DNA and RNA viruses such as Coxsackievirus B5, Herpes Simplex Virus type 1 and type 2, Influenza Virus A/H1N1 and Porcine Parvovirus in a nonspecific manner (Cermelli et al., 2011).
Figure 2.
Molecular interaction of Hyaluronic Acid (HA) at Mpro active site 1. In surface and binding pose views, ligand is represented with orange color and interacting residues are labeled. In 2D interaction view, arrows correspond to H-bonds formed between HA and Mpro.
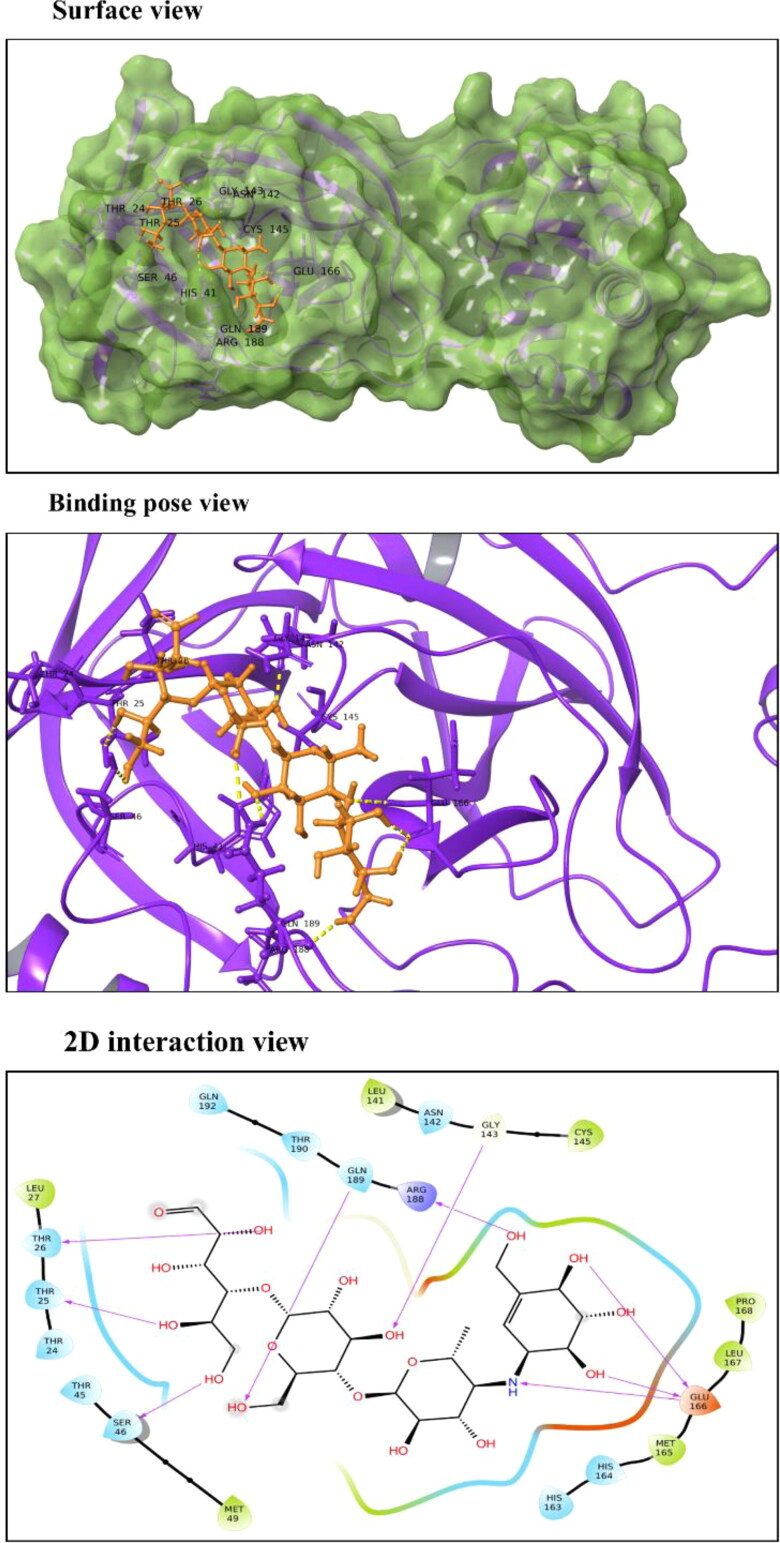
Acarbose: Also known as acarbosa and acarbosum, it is an approved oral drug prescribed to Diabetes Mellitus type II patients. Acarbose is observed to interact with Thr25, Thr26, Ser46, Gly143, Glu166 and Gln189 residues of Mpro (Figure 3). These residues are a part of domains I and II which constitute β-barrels enclosing the active site. It binds to Mpro with a significant docking score of −12.181 kcal/mol and binding energy of −45.54 kcal/mol. It reduces the amount of glucose in blood by delaying the absorption of carbohydrates. Acarbose is a complex oligosaccharide that works by inhibiting the α-amylase and glycosidase enzymes of pancreatic and intestinal cells, thereby preventing the hydrolysis of poly-carbohydrates to glucose (DiNicolantonio et al., 2015).
Figure 3.
Molecular interaction of Acarbose with Mpro active site 1. In surface and binding pose views, ligand is represented with orange color and interacting residues are labeled. In 2D interaction view, arrows correspond to H-bonds formed between Acarbose and Mpro.
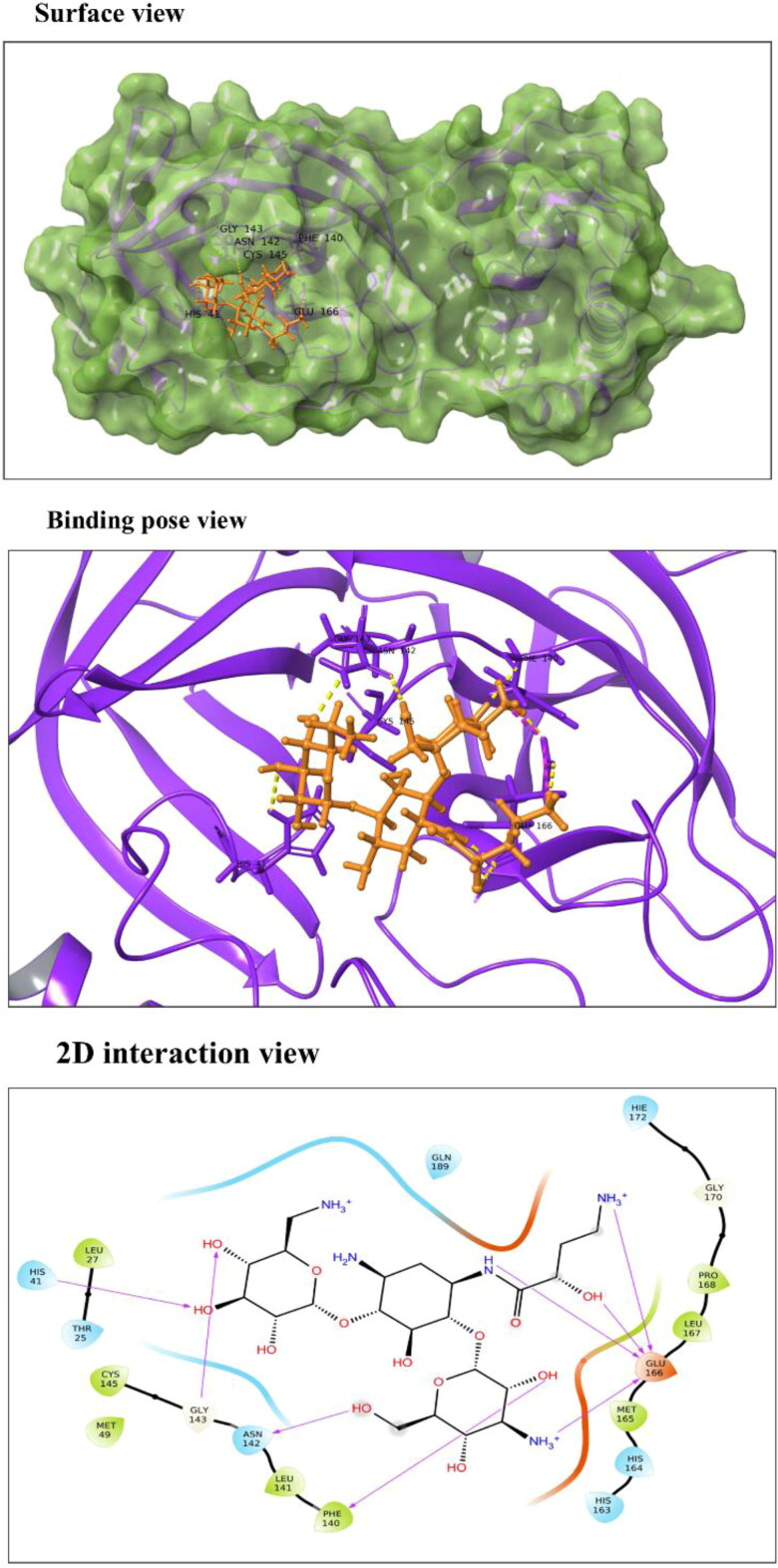
Amikacin: According to our results, docking score of drug Amikacin is −10.966 kcal/mol, and binding energy of −52.25 kcal/mol. Drug-interaction view exposes the interaction of Amikacin with His41 residue of Mpro which is one of the two residues of catalytic dyad conserved in catalytics site of coronaviruses (Figure 4). It also interacts with Phe140, Asn142, Gly143 and Glu166 residues present in domain II. It is a well-known broad-spectrum anti-bacterial drug prescribed for the treatment of life-threatening gram-negative bacterial infections (Ramirez & Tolmasky, 2017; Tamma et al., 2012). Recently in 2018, Amikacin liposome inhalation suspension (ALIS, Arikayce®) is approved for treating patients suffering from mycobacterium avium complex lung diseases therapy (Shirley, 2019).
Figure 4.
Molecular interaction of Amikacin with Mpro active site 1. In surface and binding pose views, ligand is represented with orange color and interacting residues are labeled. In 2D interaction view, arrows corresponds to H-bonds formed between Amikacin and Mpro.
Ribostamycin: Docking score of Ribostamycin on SARS-CoV-2 protease is estimated to be −11.845 kcal/mol with a glide binding energy of −36.45 kcal/mol (Table 2). The Mpro-Ribostamycin complex is stabilized by five H-bonds formed between Phe140, Leu141, His164 and Glu166 residues of protease and the compound (Figure 5(a)). It is an aminoglycosidic anti-bacterial compound but is demonstrated to have an antiviral activity against HIV-1 as well (Ennifar et al., 2006). Our previous in-silico study has also reported nsp2 cysteine protease of chikungunya virus as one of the targets of Ribostamycin (Kumar et al., 2019).
Figure 5.
2D Molecular interaction views of (a) Ribostamycin, (b) Octreotide and (c) Paromomycin with Mpro. Arrows correspond to H-bonds formed by the drug molecule with residues of Mpro.
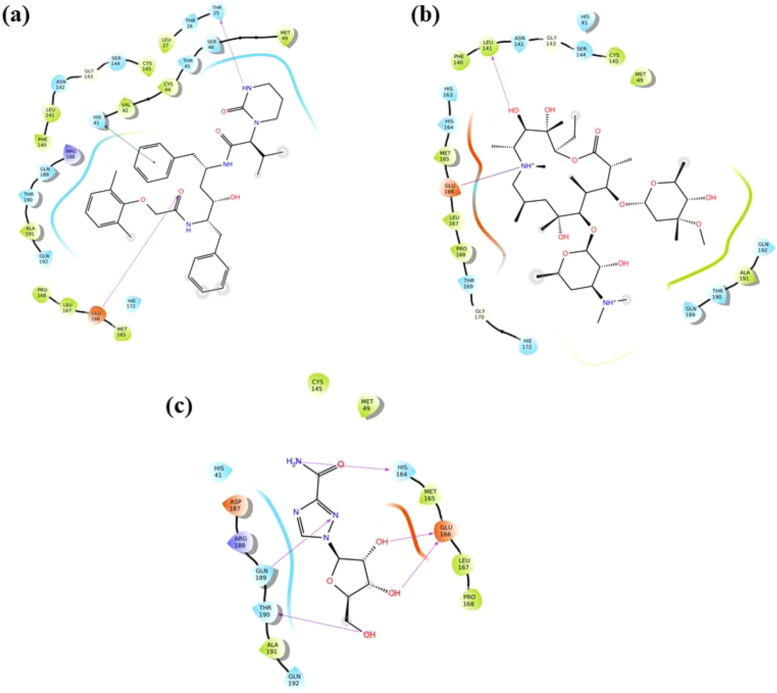
Octreotide: The long-acting octapeptide has a cyclic structure mimicking to that of natural hormone somatostatin. Due to similar pharmacologic properties to endogenous hormone, it is a potent inhibitor of growth hormone, luteinizing hormone, insulin and glucagon (Battershill & Clissold, 1989; McKeage et al., 2003). Our docking analysis has given it a very significant binding score of −11.162 kcal/mol. The drug form H-bonds with Thr26, Ser46, Gly143, Leu141, Glu166 and Gln189 residues and a salt bridge interaction with Glu166 of protease with a binding energy of −66.41 kcal/mol (Figure 5(b)). It is recommended for the treatment of acromegaly and thyrotrophinomas (Battershill & Clissold, 1989; McKeage et al., 2003). As an analogue of somatostatin, Octreotide has been reported to bind with somatostatin receptor and exert its influence on downstream signaling (Hofland & Lamberts, 1996). In a direct way, this drug has not been reported against any of the viral infection, as per our knowledge. However, several octapeptide substrates have been functionally characterized against Nsp3 helicase protein of all serotypes of Dengue virus (Li et al., 2005).
Paromomycin: It is prescribed as a first line drug for the treatment of intestinal amebiasis and visceral leishmaniasis (Davidson et al., 2009; Shalev-Benami et al., 2017). In our docking study, it is observed to interact via H-bonds with Phe140, Leu141, Asn142, Glu166, Gln189 and Thr190 residues of Mpro and shows a powerful docking score of −11.159 kcal/mol (Figure 5(c)). Furthermore, the stability of complex is increased by a salt bridge interaction between NH3+ and Glu166 residue (Details of binding parameters are given in Table 2).
Lopinavir: According to our docking results (docking score of −6.119 kcal/mol), Lopinavir-protease complex is stabilized by two H-bonds formed by Thr25, and Glu166 amino acids of protease with the drug (Table 3). In conjunction with H-bonds, a Pi-Pi stacking interaction with His41 further increases the stability of complex upto −46.98 kcal/mol of binding energy (Figure 6(a)). It is an FDA-approved peptidomimetic compound containing a hydroxyethylene scaffold that mimics the peptide bond. Co-formulated capsules with Ritonavir, marketed as Kaletra® are widely used for the treatment of HIV (Chandwani & Shuter, 2008). As an anti-retroviral drug, Lopinavir in combination with Ritonavir is demonstrated to suppress viral replication.
Figure 6.
2D Molecular interaction views of (a) Lopinavir, (b) Azithromycin and (c) Ribavirin with Mpro. Arrows, blue-red straight line and green straight line corresponds to H-bonds, salt bridge and pi-pi interactions formed between the drug molecules with residues of Mpro.
Azithromycin: It shows a docking score of −5.636 kcal/mol and is observed to form a single H-bond with Leu141 in addition with a salt bridge interaction between NH+ and Glu166 residue (Figure 6(b)). The Mpro-Azithromycin complex exhibits a binding energy equivalent to −46.25 kcal/mol. Approved by FDA in 1991, it is a broad-spectrum antibiotic drug administered orally during respiratory, enteric, and genitourinary infections (Peters et al., 1992). As a macrolide having a 15-membered ring, it shows bacteriostatic activity against Gram-negative as well as Gram-positive bacteria. Although considered safe for use during pregnancy, it occasionally causes diarrhea in breast-feeding infants (McMullan & Mostaghim, 2015; Peters et al., 1992). As mentioned above, presently, Azithromycin is in clinical trials along with Hydroxychloroquine for the treatment of COVID-19 infection (‘Evaluation of the efficacy of the hydroxychloroquine-azithromycin combination in the in the prevention of COVID-19 related SDRA’, 2020; ‘Safety and efficacy of hydroxychloroquine associated with azithromycin in SARS-CoV2 virus’, 2020). However, FDA has approved it for emergency use against SARS-CoV-2.
Ribavirin: In our study, docking analysis of Ribavirin revealed a docking score equivalent to −6.813 kcal/mol (Table 3). The drug establishes H-bonds with His164, Glu166, Gln189 and Thr190 residues of SARS-CoV-2 main protease (binding energy −35.63 kcal/mol) (Figure 6(c)). It is a broad-spectrum FDA-approved antiviral drug used against a number of DNA and RNA viruses including respiratory syncytial virus, parainfluenza virus and hepatitis C virus (Krilov, 2001; Reddy et al., 2009). It is a synthetic guanosine analogue which interfers with viral RNA synthesis. Administered orally, Ribavirin is prescribed in combination with pegylated interferon-α2 for treating hepatitis C virus infection (Loustaud-Ratti et al., 2016; Reddy et al., 2009). On the basis of its in-vitro efficacy against SARS-CoV and MERS-CoV, it is being clinically tested on COVID-19 patients (Khalili et al., 2020).
The catalytic site of Mpro and nearby residues in contact such as Phe140, Leu141, His164, Glu166, Glu189 are observed to be important for binding of these drugs. Therefore out of 100 shortlisted drugs, we have chosen HA, Acarbose, and Amikacin on the basis of their high docking scores and molecular interactions with catalytic site and nearby residues of Mpro. Additionally, binding stability of the drug Lopinavir is also investigated through molecular simulation.
Molecular dynamics simulation
The stability of protein-ligand complexes with respect to protein conformation is determined by MD simulations. In general, a small deviation in protein conformation during the course of simulation indicates a stable protein structure. We analyzed the atomic distance and fluctuation in protein upon binding of drug molecule at the active site 1 of Mpro. Acarbose, HA, Amikacin and Lopinavir complexes with Mpro are monitored for 500 ns using our in-house high-performance cluster facility.
Assessment of root mean square deviation (RMSD), root mean square fluctuation (RMSF) and radius of gyration (Rg)
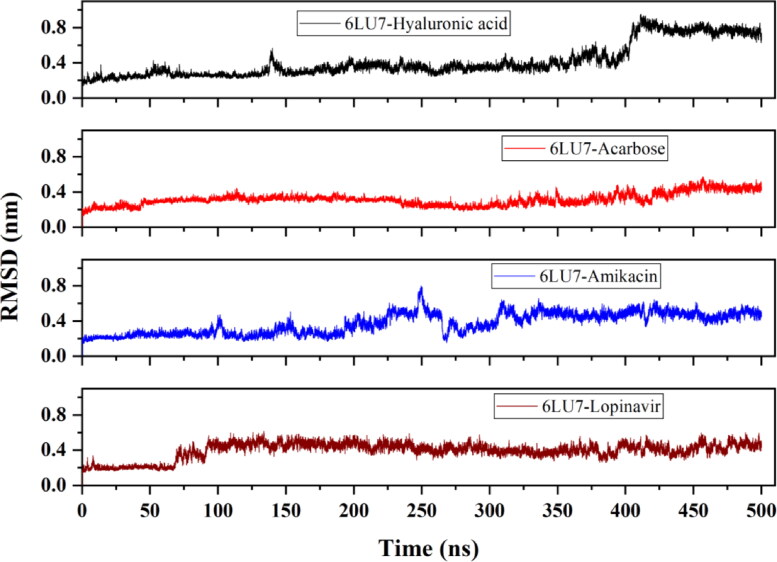
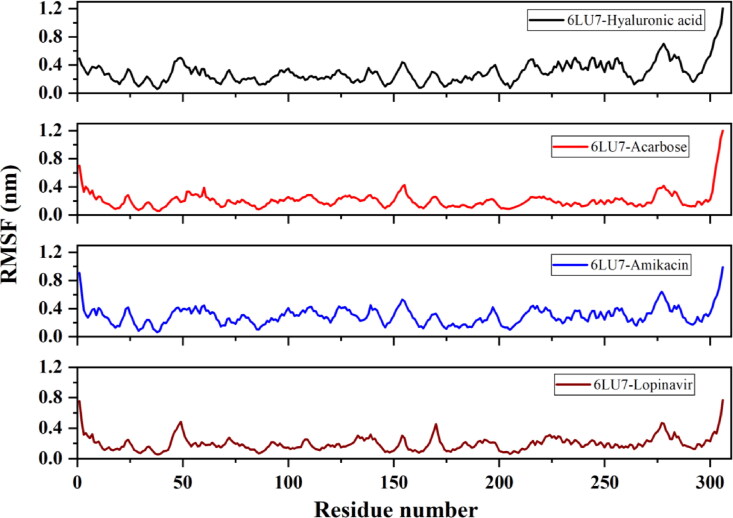
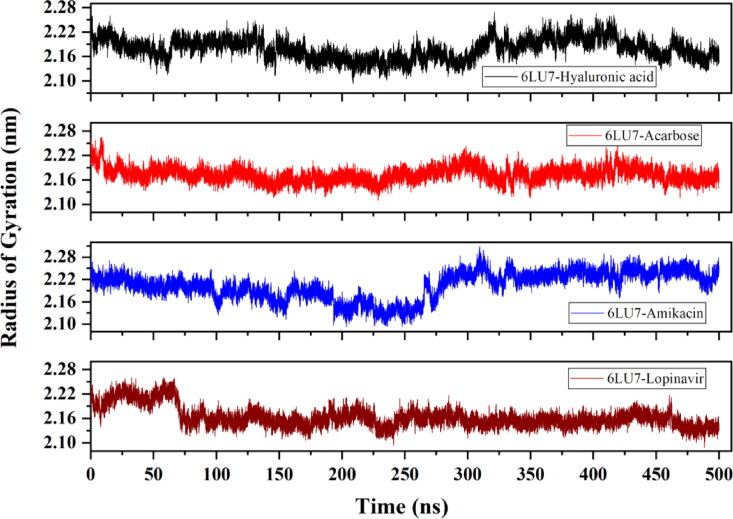
The average RMSD of 6LU7-HA, 6LU7-Acarbose, 6LU7-Amikacin and 6LU7-Lopinavir complex is observed to be 0.42 nm, 0.31 nm, 0.37 nm and ∼0.4 nm, respectively. 6LU7-HA complex attains a stable conformation till 15 ns, after which deviation starts increasing and the complex become unstable near 400 ns. For 6LU7-Acarbose, RMSD keeps on fluctuating between 0.2 nm and 0.3 nm during the entire simulation period. Nonetheless, these RMSD values are still much lower than that of other two compounds 6LU7-HA and 6LU7-Amikacin (Figure 7). The RMSF plot identifies flexible regions in protein-ligand complexes. Residues with low RMSF values indicate its contribution to the structured regions, while higher residual fluctuation indicates the presence of unstructured regions such as loops and turns in protein. The binding of an inhibitor or druggable compound through one or more stable bonds, however, reduces the fluctuation in residues. As illustrated in Figure 8, Lopinavir bound protease complex is observed to have higher fluctuating (0.4–0.6 nm) peaks showing less stable binding. Contrary to this, 6LU7-Acarbose, 6LU7-HA and 6LU7-Amikacin complexes are showing less fluctuations (between 0.1 to 0.3 nm). Similarly, Rg for 6LU7-HA and 6LU7-Acarbose are observed to be 2.18 nm and 2.17 nm, respectively. For 6LU7-Amikacin, and 6LU7-Lopinavir complexes, respective mean Rg values are calculated to be 2.2 nm and 2.16 nm. Graphs in Figure 9 illustrate the Rg for complex of Mpro (6LU7) with HA, Amikacin, Acarbose, and Lopinavir. The time-dependent trace of Rg is stable for HA, and Acarbose throughout the simulation. In case of Amikacin, the complex is showing higher fluctuation between 2.15 nm to 2.25 nm beyond 275 ns. Although, 6LU7-Lopinavir, at initial stage of simulation acquires a high Rg upto 2.25 nm, however, beyond 60 ns itdecreases and becomes stable till 425 ns.
Figure 7.
Evaluation of root mean square deviation (RMSD) from molecular dynamic trajectory of Mpro (6LU7) in complex with HA, Acarbose, Amikacin and Lopinavir.
Figure 8.
Evaluation of root mean square fluctuation (RMSF) of Mpro (6LU7) residues upon binding with HA, Acarbose, Amikacin and Lopinavir.
Figure 9.
Evaluation of protein structure compactness through radius of gyration (Rg) for HA, Acarbose, Amikacin and Lopinavir bound Mpro (6LU7).
Assessment of principle component analysis (PCA) and solvent solvent accessible surface area (SASA)
Binding of ligand induces local as well as global fluctutaions in protein structure that are hard to distinguish. In order to identify the simultaneously occurring conformational changes, PCA is performed for C-α atoms of protease. It is a multivariate statistical technique which builds a co-varinace or correlation matrix for methodically calculating eigenvectors and eigenvalues. PCA generates eigenvectors which represents the reduced collective atomic motions in protein structure (David & Jacobs, 2014).
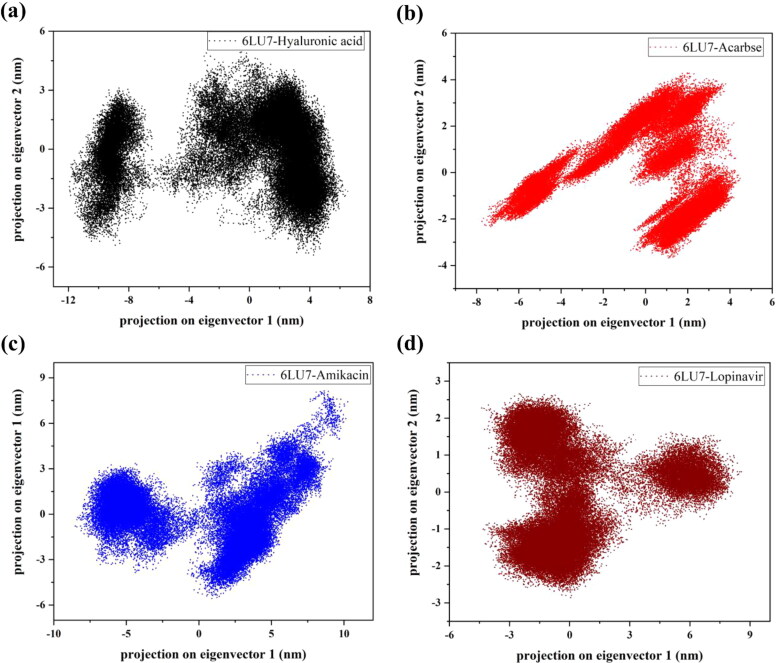
The effect of ligand stabilization on protein structure is shown in Figure 10 where PC1 and PC2 majorly contributes to global conformations of 6LU7-ligand complexes. As observed in the analysis, dense clusters are formed in all drug bound forms of protease but Amikacin and HA have higher range from −10 to 10 nm and −12 to 6 nm, respectively. However, Lopinavir has shown stable clusters with a range of −5 to 5 nm and similarly, the second top hit drug, Acarbose is observed to form clusters of stable positions (−7 to 2 nm). This indicates that binding of Acarbose has reduced the global conformational changes occurring in Mpro while HA has slightly higher values, may be due to little fluctuation in the last 100 ns period of simulation.
Figure 10.
Depiction of principal component analysis (PCA). Projection on eigenvector 1 against eigenvector 2 for HA, Acarbose, Amikacin and Lopinavir bound Mpro (6LU7).
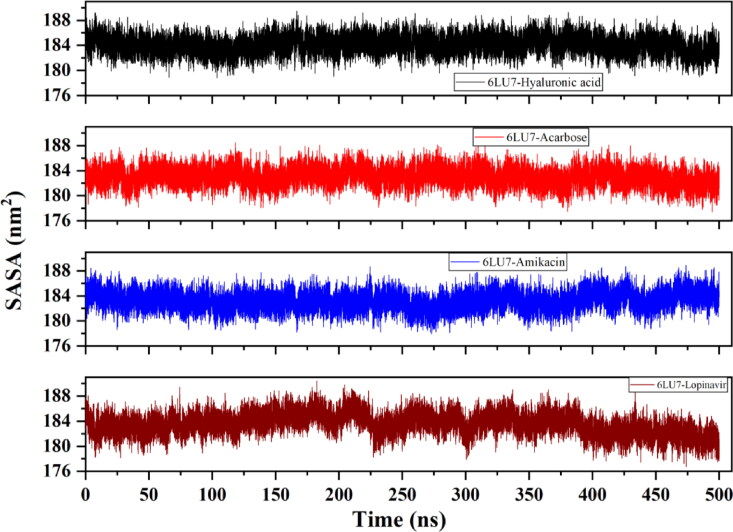
Hydrophobic force deriving protein folding results in formation of hydrophobic cores with hydrophilic residues assembling near the surface of a protein structure. Binding of ligand can significantly affect the SASA of a protein, in which overall differences related to ligand-induced stability in complex are frequently estimated. Therefore, we also investigated the SASA parameter in this study. Figure 11 depicts the SASA values of C-α atoms of Mpro complexes upon binding with screened drugs. In correlation with RMSD, RMSF and Rg analysis, 6LU7-Lopinavir has a gradually increasing surface area (188 nm2) till 200 ns illustrating higher protein surface exposure. The complex is then observed to be fluctuating a little which again decreases after 400 ns to 178 nm2. However, Acarbose and HA has a stable trend of SASA values than Amikacin bound Mpro with an average value of 184 nm2 but Amikacin experiences higher fluctuations after 250 ns. This also confirms that the top screened drugs Acarbose and HA have gained firm binding with Mpro.
Figure 11.
Evaluation of solvent accessible surface area (SASA) of Mpro (6LU7) upon binding with HA, Acarbose, Amikacin and Lopinavir. Acarbose and HA bound Mpro have attained more stable states during simulation.
Numerous attempts of drug repurposing have been made against SARS-CoV-2 Mpro enzyme. In one of the reports, Sang et al. explored inhibitory effect of approved anti-HIV drugs, which have shown stable interactions with residues Met49, Leu141, Cys145, Met165, Pro168 and Glu189 of Mpro (Sang et al., 2020). In another study, synergistic effect of Lopinavir, Oseltamivir and Ritonavir are investigated. These drugs are stabilized by interaction with Lue287, Tyr101, Asp33, His41, Asn142 and Glu166 residues of Mpro (6LU7) (Muralidharan et al., 2020). Khan et al. showed stable binding of two compounds Paritaprevir and Raltegravir with SARS-CoV-2 Mpro (shows multiple interactions with Thr24, Ser46, Leu50, Asn142, Cys145 and Pro168) (Khan et al., 2020). Another approach of using combination of drugs is frequently observed to be highly effective. Lopinavir-Ritonavir complex shows a stable binding affinity of −10.6 kcal/mol with SARS-CoV-2 Mpro. The combination drug is found to interact with one of the catalytic dyad residue His41, and further His164, Glu166, Arg188 and Gln192 of Mpro active site (Kumar, Singh et al., 2020).
Our simulation analysis of 6LU7-HA, 6LU7-Acarbose, 6LU7-Amikacin and 6LU7-Lopinavir complex reveals stable binding of Acarbose, and HA at Mpro active site. Interaction of Lopinavir stabilized by multiple non-covalent bonds with catalytic as well as surrounding residues (such as Asn142 and Glu166) of Mpro is in line with the previously reported literature (Kumar, Singh et al., 2020; Muralidharan et al., 2020). Antiviral effects of HA against both DNA and RNA viruses are already well described (Cermelli et al., 2011). Although Amikacin and Acarbose are not much known to exert antiviral effects, however, they have shown significant docking and simulation results. Therefore, these drugs may have inhibitory potential and are need to be tested experimentally for their efficacy against SARS-CoV-2.
A study by Victorovich et al., has observed that among more than 250 nucleotide mutations (known yet), there are more number of synonymous Cytosine to Uracil transitions in ORF 1a and 1b which have caused mutational U-pressure in RNA plus strand of SARS-CoV-2 and that can not be repaired by proofread machinery of coronaviruses (Victorovich et al., 2020).
Conclusion
The current scenario of the COVID-19 pandemic urgently needs drugs to treat patients. However, at the same time, we need to understand the mechanisms or the interactions of those drugs on the molecular targets on SARS-CoV-2. In this study, we have screened the approved drugs against the Mpro of SARS-CoV-2 to investigate their binding efficacies and molecular interactions. We found that HA, Acarbose, Ribostamycin, Paromomycin, and Amikacin have good binding efficacy with the target. The interaction with the catalytic dyad (-His41, -Cys145) at the active site, seen in Amikacin, and Lopinavir may be responsible for inhibiting Mpro. Additionally, HA and Acarbose have also given the stable binding in 500 ns MD simulation time as observed from atomic distance, fluctuations and PCA. Further, the inhibition of Mpro may lead to the inhibition of SARS-CoV-2 replication and propagation. As elucidated, these drugs may act as potential inhibitors against COVID-19 by targeting Mpro of SARS-CoV-2. However, in-vitro validation of these identified drugs is essential to test the inhibition potency.
Authors contribution
RG, NG and GN: Conception and design. RG: review and writing of the manuscript, and study supervision. PK, TB, AK, BRG, SK: acquisition andinterpretation of data, writing of the manuscript.
Acknowledgements
All the authors would like to thank IIT Mandi for providing HPC facilities. RG would like to thank the Department of Biotechnology, Govt of India (BT/11/IYBA/2018/06). TB is grateful to the Department of Science and Technology for INSPIRE fellowship. PK, SK, AK, and BRG are supported by MHRD fellowship, Govt. of India.
Glossary
Abbreviations
- COVID-19
Coronavirus Disease 2019
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- Mpro
Non Structural Protein 5
- XP
Extra Precision
- MD
Molecular Dynamics
- RMSD
Root Mean Square Deviation
- RMSF
Root Mean Square Fluctuation
- Rg
Radius of Gyration
- PCA
Principle Component Analysis
- SASA
Solvent Accessible Surface Area
- Dscore
Druggability Score
- HA
Hyaluronic Acid
Funding Statement
This work was supported by Department of Biotechnology, Ministry of Science and Technology.
Disclosure statement
All authors declare that there is no financial or any other type of competing interest.
References
- Aarthy, M., Kumar, D., Giri, R., & Singh, S. K. (2018). E7 oncoprotein of human papillomavirus: Structural dynamics and inhibitor screening study. Gene, 658, 159–177. 10.1016/j.gene.2018.03.026 [DOI] [PubMed] [Google Scholar]
- Anand, K., Ziebuhr, J., Wadhwani, P., Mesters, J. R., & Hilgenfeld, R. (2003). Coronavirus main proteinase (3CLpro) structure: Basis for design of anti-SARS drugs. Science, 300(5626), 1763–1767. 10.1126/science.1085658 [DOI] [PubMed] [Google Scholar]
- Ashburn, T. T., & Thor, K. B. (2004). Drug repositioning: Identifying and developing new uses for existing drugs. Nature Reviews. Drug Discovery, 3(8), 673–683. 10.1038/nrd1468 [DOI] [PubMed] [Google Scholar]
- Battershill, P. E., & Clissold, S. P. (1989). Octreotide. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in conditions associated with excessive peptide secretion. Drugs, 38(5), 658–702. . 10.2165/00003495-198938050-00002 [DOI] [PubMed] [Google Scholar]
- Berendsen, H. J. C., van der Spoel, D., & van Drunen, R. (1995). GROMACS: A message-passing parallel molecular dynamics implementation. Computer Physics Communications, 91(1-3), 43–56. 10.1016/0010-4655(95)00042-E [DOI] [Google Scholar]
- Breckenridge, A., & Jacob, R. (2019). Overcoming the legal and regulatory barriers to drug repurposing. Nature Reviews. Drug Discovery, 18(1), 1–2. 10.1038/nrd.2018.92 [DOI] [PubMed] [Google Scholar]
- Cao, B., Wang, Y., Wen, D., Liu, W., Wang, J., Fan, G., Ruan, L., Song, B., Cai, Y., Wei, M., Li, X., Xia, J., Chen, N., Xiang, J., Yu, T., Bai, T., Xie, X., Zhang, L., Li, C. … Wang, C. (2020). A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. The New England Journal of Medicine, 382(19), 1787–1799. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermelli, C., Cuoghi, A., Scuri, M., Bettua, C., Neglia, R. G., Ardizzoni, A., Blasi, E., Iannitti, T., & Palmieri, B. (2011). In vitro evaluation of antiviral and virucidal activity of a high molecular weight hyaluronic acid. Virology Journal, 8(1), 141. 10.1186/1743-422X-8-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandwani, A., & Shuter, J. (2008). Lopinavir/ritonavir in the treatment of HIV-1 infection: A review. Therapeutics and Clinical Risk Management, 4(5), 1023–1033. 10.2147/TCRM.S3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2020). COVID-19 ring-based prevention trial with lopinavir/ritonavir – Full text view – ClinicalTrials.gov. Retrieved April 29, 2020, from https://clinicaltrials.gov/ct2/show/NCT04321174
- David, C. C., & Jacobs, D. J. (2014). Principal component analysis: A method for determining the essential dynamics of proteins BT. In Livesay D. R. (Ed.), Protein dynamics: Methods and protocols (pp. 193–226). Totowa, NJ: Humana Press. 10.1007/978-1-62703-658-0_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, R. N., den Boer, M., & Ritmeijer, K. (2009). Paromomycin. Transactions of the Royal Society of Tropical Medicine and Hygiene, 103(7), 653–660. 10.1016/j.trstmh.2008.09.008 [DOI] [PubMed] [Google Scholar]
- DiNicolantonio, J. J., Bhutani, J., & O’Keefe, J. H. (2015). Acarbose: Safe and effective for lowering postprandial hyperglycaemia and improving cardiovascular outcomes. Open Heart, 2(1), e000327. 10.1136/openhrt-2015-000327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennifar, E., Paillart, J.-C., Bodlenner, A., Walter, P., Weibel, J.-M., Aubertin, A.-M., Pale, P., Dumas, P., & Marquet, R. (2006). Targeting the dimerization initiation site of HIV-1 RNA with aminoglycosides: From crystal to cell. Nucleic Acids Research, 34(8), 2328–2339. 10.1093/nar/gkl317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser, J. R. E., Laurent, T. C., & Laurent, U. B. G. (1997). Hyaluronan: Its nature, distribution, functions and turnover. Journal of Internal Medicine, 242(1), 27–33. 10.1046/j.1365-2796.1997.00170.x [DOI] [PubMed] [Google Scholar]
- Friesner, R. A., Murphy, R. B., Repasky, M. P., Frye, L. L., Greenwood, J. R., Halgren, T. A., Sanschagrin, P. C., Mainz, D. T., Friesner, R. A., Murphy, R. B., Repasky, M. P., Frye, L. L., Greenwood, J. R., Halgren, T. A., Sanschagrin, P. C., & Mainz, D. T. (2006). Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. Journal of Medicinal Chemistry, 49(21), 6177–6196. 10.1021/jm051256o [DOI] [PubMed] [Google Scholar]
- Giri, R., Bhardwaj, T., Shegane, M., Gehi, B. R., Kumar, P., Gadhave, K., Oldfield, C. J., & Uversky, V. N. (2020). When darkness becomes a ray of light in the dark times: Understanding the COVID-19 via the comparative analysis of the dark proteomes of SARS-CoV-2, human SARS and bat SARS-like coronaviruses. BioRxiv, 2020.03.13.990598. 10.1101/2020.03.13.990598. [DOI]
- Halgren, T. A. (2009). Identifying and characterizing binding sites and assessing druggability. Journal of Chemical Information and Modeling, 49(2), 377–389. 10.1021/ci800324m [DOI] [PubMed] [Google Scholar]
- Halgren, T. A., Murphy, R. B., Friesner, R. A., Beard, H. S., Frye, L. L., Pollard, W. T., & Banks, J. L. (2004). Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. Journal of Medicinal Chemistry, 47(7), 1750–1759. 10.1021/jm030644s [DOI] [PubMed] [Google Scholar]
- Hess, B., Bekker, H., Berendsen, H. J. C., & Fraaije, J. G. E. M. (1997). LINCS: A linear constraint solver for molecular simulations. Journal of Computational Chemistry, 18(12), 1463–1472. [DOI] [Google Scholar]
- Hofland, L. J., & Lamberts, S. W. J. (1996). Somatostatin receptors and disease: Role of receptor subtypes. Baillière’s Clinical Endocrinology and Metabolism, 10(1), 163–176. 10.1016/S0950-351X(96)80362-4 [DOI] [PubMed] [Google Scholar]
- Humphrey, W., Dalke, A., & Schulten, K. (1996). VMD: Visual molecular dynamics. Journal of Molecular Graphics, 14(1), 33–38. 10.1016/0263-7855(96)00018-5 [DOI] [PubMed] [Google Scholar]
- Jacobson, M. P., Pincus, D. L., Rapp, C. S., Day, T. J. F., Honig, B., Shaw, D. E., & Friesner, R. A. (2004). A hierarchical approach to all-atom protein loop prediction. Proteins: Structure, Function, and Bioinformatics, 55(2), 351–367. 10.1002/prot.10613 [DOI] [PubMed] [Google Scholar]
- Jin, Z., Du, X., Xu, Y., Deng, Y., Liu, M., Zhao, Y., Zhang, B., Li, X., Zhang, L., Peng, C., Duan, Y., Yu, J., Wang, L., Yang, K., Liu, F., Jiang, R., Yang, X., You, T., Liu, X. … Yang, H. (2020). Structure of Mpro from COVID-19 virus and discovery of its inhibitors. Nature, 582(7811), 289–293. 10.1038/s41586-020-2223-y [DOI] [PubMed] [Google Scholar]
- Khalili, J. S., Zhu, H., Mak, N. S. A., Yan, Y., & Zhu, Y. (2020). Novel coronavirus treatment with ribavirin: Groundwork for an evaluation concerning COVID-19. Journal of Medical Virology, 92(7), 740–746. 10.1002/jmv.25798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, R. J., Jha, R. K., Amera, G. M., Jain, M., Singh, E., Pathak, A., Singh, R. P., Muthukumaran, J., & Singh, A. K. (2020). Targeting SARS-CoV-2: A systematic drug repurposing approach to identify promising inhibitors against 3C-like proteinase and 2′-O-ribose methyltransferase. Journal of Biomolecular Structure and Dynamics, 1–14. 10.1080/07391102.2020.1753577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krilov, L. R. (2001). Respiratory syncytial virus: Update on infection, treatment, and prevention. Current Infectious Disease Reports, 3(3), 242–246. 10.1007/s11908-001-0026-3 [DOI] [PubMed] [Google Scholar]
- Kumar, A., Liang, B., Aarthy, M., Singh, S. K., Garg, N., Mysorekar, I. U., & Giri, R. (2018). Hydroxychloroquine inhibits Zika virus NS2B-NS3 protease. ACS Omega., 3(12), 18132–18141. 10.1021/acsomega.8b01002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, D., Aarthy, M., Kumar, P., Singh, S. K., Uversky, V. N., & Giri, R. (2020). Targeting the NTPase site of Zika virus NS3 helicase for inhibitor discovery. Journal of Biomolecular Structure & Dynamics, 38(16), 4811–4827. 10.1080/07391102.2019.1689851 [DOI] [PubMed] [Google Scholar]
- Kumar, D., Sharma, N., Aarthy, M., Singh, S. K., & Giri, R. (2020). Mechanistic insights into Zika virus NS3 helicase inhibition by epigallocatechin-3-gallate. ACS Omega, 5(19), 11217–11226. 10.1021/acsomega.0c01353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, P., Kumar, D., & Giri, R. (2019). Targeting the nsp2 cysteine protease of chikungunya virus using FDA approved library and selected cysteine protease inhibitors. Pathogens, 8(3), 128. 10.3390/pathogens8030128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, P., Saumya, K. U., & Giri, R. (2020). Identification of peptidomimetic compounds as potential inhibitors against MurA enzyme of Mycobacterium tuberculosis. Journal of Biomolecular Structure & Dynamics, 38(17), 4917–4997. 10.1080/07391102.2019.1696231 [DOI] [PubMed] [Google Scholar]
- Kumar, Y., Singh, H., & Patel, C. N. (2020). In silico prediction of potential inhibitors for the Main protease of SARS-CoV-2 using molecular docking and dynamics simulation based drug-repurposing. Journal of Infection and Public Health, 13(9), 1210–1223. 10.1016/j.jiph.2020.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Lim, S. P., Beer, D., Patel, V., Wen, D., Tumanut, C., Tully, D. C., Williams, J. A., Jiricek, J., Priestle, J. P., Harris, J. L., & Vasudevan, S. G. (2005). Functional profiling of recombinant NS3 proteases from all four serotypes of dengue virus using tetrapeptide and octapeptide substrate libraries. The Journal of Biological Chemistry, 280(31), 28766–28774. 10.1074/jbc.M500588200 [DOI] [PubMed] [Google Scholar]
- Loustaud-Ratti, V., Debette-Gratien, M., Jacques, J., Alain, S., Marquet, P., Sautereau, D., Rousseau, A., & Carrier, P. (2016). Ribavirin: Past, present and future. World Journal of Hepatology, 8(2), 123–130. 10.4254/wjh.v8.i2.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavi Sastry, G., Adzhigirey, M., Day, T., Annabhimoju, R., & Sherman, W. (2013). Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. Journal of Computer-Aided Molecular Design, 27(3), 221–234. 10.1007/s10822-013-9644-8 [DOI] [PubMed] [Google Scholar]
- McKeage, K., Cheer, S., & Wagstaff, A. J. (2003). Octreotide long-acting release (LAR): A review of its use in the management of acromegaly. Drugs, 63(22), 2473–2499. 10.2165/00003495-200363220-00014 [DOI] [PubMed] [Google Scholar]
- McMullan, B. J., & Mostaghim, M. (2015). Prescribing azithromycin. Australian Prescriber, 38(3), 87–90. 10.18773/austprescr.2015.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan, N., Sakthivel, R., Velmurugan, D., & Gromiha, M. M. (2020). Computational studies of drug repurposing and synergism of lopinavir, oseltamivir and ritonavir binding with SARS-CoV-2 protease against COVID-19. Journal of Biomolecular Structure and Dynamics, 1–6. 10.1080/07391102.2020.1752802. [DOI] [PubMed] [Google Scholar]
- Peters, D. H., Friedel, H. A., & McTavish, D. (1992). Azithromycin. Drugs, 44(5), 750–799. 10.2165/00003495-199244050-00007 [DOI] [PubMed] [Google Scholar]
- Pushpakom, S., Iorio, F., Eyers, P. A., Escott, K. J., Hopper, S., Wells, A., Doig, A., Guilliams, T., Latimer, J., McNamee, C., Norris, A., Sanseau, P., Cavalla, D., & Pirmohamed, M. (2019). Drug repurposing: Progress, challenges and recommendations. Nature Reviews. Drug Discovery, 18(1), 41–58. 10.1038/nrd.2018.168 [DOI] [PubMed] [Google Scholar]
- Ramirez, M. S., & Tolmasky, M. E. (2017). Amikacin: Uses, resistance, and prospects for inhibition. Molecules, 22(12), 2267. 10.3390/molecules22122267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, K. R., Nelson, D. R., & Zeuzem, S. (2009). Ribavirin: Current role in the optimal clinical management of chronic hepatitis C. Journal of Hepatology, 50(2), 402–411. 10.1016/j.jhep.2008.11.006 [DOI] [PubMed] [Google Scholar]
- (2020). Safety and efficacy of hydroxychloroquine associated with azithromycin in SARS-CoV2 virus (Coalition Covid-19 Brasil II) – Full text view – ClinicalTrials.gov. Retrieved April 29, 2020, from https://clinicaltrials.gov/ct2/show/NCT04321278
- Sang, P., Tian, S.-H., Meng, Z.-H., & Yang, L.-Q. (2020). Anti-HIV drug repurposing against SARS-CoV-2. RSC Advances, 10(27), 15775–15783. 10.1039/D0RA01899F [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev-Benami, M., Zhang, Y., Rozenberg, H., Nobe, Y., Taoka, M., Matzov, D., Zimmerman, E., Bashan, A., Isobe, T., Jaffe, C. L., Yonath, A., & Skiniotis, G. (2017). Atomic resolution snapshot of Leishmania ribosome inhibition by the aminoglycoside paromomycin. Nature Communications, 8(1), 1589. 10.1038/s41467-017-01664-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, N., Kumar, P., & Giri, R. (2020). Polysaccharides like pentagalloylglucose, parishin A and stevioside inhibits the viral entry by binding the Zika virus envelope protein. Journal of Biomolecular Structure and Dynamics, 1–13. 10.1080/07391102.2020.1797538 [DOI] [PubMed] [Google Scholar]
- Sharma, N., Murali, A., Singh, S. K., & Giri, R. (2017). Epigallocatechin gallate, an active green tea compound inhibits the Zika virus entry into host cells via binding the envelope protein. International Journal of Biological Macromolecules, 104(Pt A), 1046–1054. 10.1016/j.ijbiomac.2017.06.105 [DOI] [PubMed] [Google Scholar]
- Shirley, M. (2019). Amikacin liposome inhalation suspension: A review in Mycobacterium avium complex lung disease. Drugs, 79(5), 555–562. 10.1007/s40265-019-01095-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamma, P. D., Cosgrove, S. E., & Maragakis, L. L. (2012). Combination therapy for treatment of infections with gram-negative bacteria. Clinical Microbiology Reviews, 25(3), 450–470. 10.1128/CMR.05041-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. National Library of Medicine. ( 2020). Evaluation of the efficacy of the hydroxychloroquine-azithromycin combination in the in the prevention of COVID-19 related SDRA – Full Text View – ClinicalTrials.gov. Retrieved April 29, 2020, from https://clinicaltrials.gov/ct2/show/NCT04347512
- Victorovich, K. V., Rajanish, G., Aleksandrovna, K. T., Krishna, K. S., Nicolaevich, S. A., & Vitoldovich, P. V. (2020). Translation-associated mutational U-pressure in the first ORF of SARS-CoV-2 and other coronaviruses. BioRxiv, 2020.05.05.078238. 10.1101/2020.05.05.078238. [DOI] [PMC free article] [PubMed]
- Widjaja, L. K., Bora, M., Chan, P. N. P. H., Lipik, V., Wong, T. T. L., & Venkatraman, S. S. (2014). Hyaluronic acid-based nanocomposite hydrogels for ocular drug delivery applications. Journal of Biomedical Materials Research Part A, 102(9), 3056–3065. 10.1002/jbm.a.34976 [DOI] [PubMed] [Google Scholar]
- Wishart, D. S., Feunang, Y. D., Guo, A. C., Lo, E. J., Marcu, A., Grant, J. R., Sajed, T., Johnson, D., Li, C., Sayeeda, Z., Assempour, N., Iynkkaran, I., Liu, Y., Maciejewski, A., Gale, N., Wilson, A., Chin, L., Cummings, R., Le, D., … Wilson, M. (2018). DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Research, 46(D1), D1074–D1082. 10.1093/nar/gkx1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C., Liu, Y., Yang, Y., Zhang, P., Zhong, W., Wang, Y., Wang, Q., Xu, Y., Li, M., Li, X., Zheng, M., Chen, L., & Li, H. (2020). Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharmaceutica Sinica. B, 10(5), 766–788. 10.1016/j.apsb.2020.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav, R., Selvaraj, C., Aarthy, M., Kumar, P., Kumar, A., Singh, S. K., & Giri, R. (2020). Investigating into the molecular interactions of flavonoids targeting NS2B-NS3 protease from Zika virus through in-silico approaches. Journal of Biomolecular Structure and Dynamics, 1–13. 10.1080/07391102.2019.1709546. [DOI] [PubMed] [Google Scholar]
- Zhang, L., Lin, D., Sun, X., Curth, U., Drosten, C., Sauerhering, L., Becker, S., Rox, K., & Hilgenfeld, R. (2020). Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science, 368(6489), 409–412. 10.1126/science.abb3405 [DOI] [PMC free article] [PubMed] [Google Scholar]