Abstract
Objectives
P-wave duration (PWD) is associated with the development of atrial arrhythmias, cardiovascular and all-cause mortality. With this study, we aimed to assess the distribution and determinants of PWD in the general population.
Design
Cross-sectional study using data collected between 2014 and 2016.
Setting
In the population-based cohort CoLaus|PsyCoLaus, Lausanne, Switzerland, we used 12-lead ECGs to measure PWD. Potential demographic, clinical and biological determinants of PWD were collected by questionnaire, anthropometry, blood pressure measurement and biological assays.
Participants
Data from 3459 participants (55% women, 62±10 years, 93% Caucasian) were included. Participants were excluded if they presented with (1) no sinus rhythm or paced rhythm on the study ECG or Wolff-Parkinson-White ECG pattern; (2) missing or non-interpretable ECG; and (3) missing phenotypic data.
Primary outcome measure
Determine (1) the PWD distribution and (2) the demographic, clinical and biological determinants of PWD in a large population-based cohort.
Results
Median and IQR of PWD was 112 (102–120) ms. In the multivariable analyses, PWD was significantly associated with age (p<0.001) and height (p<0.001), with an adjusted regression coefficient (95% CI) of 0.29 ms/years (0.23 to 0.36) and 0.32 ms/cm (0.28 to 0.37), respectively. PWD, given thereafter in ms with adjusted mean±SE, was significantly (p<0.05) associated with (a) gender (woman 110.0±0.4; man 112.1±0.4), (b) body mass index (normal 110.1±0.4; overweight 110.9±0.4; obese 113.0±0.5), (c) abdominal obesity (no 110.5±0.3; yes 111.7±0.4) and (d) hypertension (no 110.4±0.3; yes 111.7±0.4).
Conclusion
PWD is positively associated with age, height, male gender, obesity markers and hypertension. Clinical interpretation of PWD should take these factors into consideration.
Keywords: epidemiology, adult cardiology, cardiac epidemiology, cardiology
Strengths and limitations of this study.
This study evaluated the association between P-wave duration (PWD) and demographic, clinical and biological variables in a general population setting.
A large number of covariates possibly associated with the PWD were analysed.
The study was conducted in a population-based sample allowing for the generalisation of the results to similar Caucasian populations.
Not having a published consensus about the measurement of PWD represents a limit of this study.
Introduction
P-wave duration (PWD) has received increasing attention during the past decades because of its association with the development of atrial arrhythmias (eg, atrial fibrillation and flutter),1–3 as well as cardiovascular (CV) and all-cause mortality.4 A prolonged PWD may reflect the presence of structural cardiac abnormalities (atrial inflammation and fibrosis), which lead to impairment in atrial conduction and interatrial conduction in particular. The latter may promote the development of an interatrial block, reflecting the conduction delay through specific myocardial fibres connecting both atria and known as the Bachmann bundle.4–6 Interatrial block is characterised by a PWD ≥120 ms and a bimodal morphology; it is a distinct entity from left atrial enlargement, although they can have associated electrocardiographic pattern.2 5 Therefore, PWD measured on a baseline ECG, a non-invasive and easily obtained tool, can be considered a marker of structural changes affecting the atrial tissue.7
Many studies associated PWD with ageing7–9 and male gender.7 Moreover, hypertension and obesity cause left ventricular hypertrophy, subsequent ventricular diastolic dysfunction and atrial enlargement, and have been linked to prolonged PWD.4 10 Finally, diabetes is also associated with atrial structural changes, such as fibrosis and dysregulation of connexin protein expression, both affecting PWD.8 Hence, the identification of the demographic, clinical and biological factors associated with a prolonged PWD could be used to detect people at risk of arrhythmia and adverse CV outcomes. Such detection offers the chance to reduce the risk of stroke and heart failure and possibly lower mortality rates.11 Yet, limited information is available on the influence of each determinant on the prolongation of PWD. Although PWD cut-offs of 110–120 ms have been proposed, no standard value of PWD has been defined in an unselected population. We used data from CoLaus|PsyCoLaus cohort to determine (1) the PWD distribution and (2) the demographic, clinical and biological determinants of PWD.
Methods
Study population and design
The CoLaus|PsyCoLaus study is a population-based study investigating the clinical, biological and genetic determinants of CV diseases. The sampling strategy and its aims have been described in detail elsewhere.12 In summary, a non-stratified, representative sample of the population of Lausanne, Switzerland, was recruited between 2003 and 2006, including 6733 participants. The following inclusion criteria were applied: (a) aged between 35 and 75 years; (b) written informed consent; and (c) willingness to take part in the examination and to provide blood samples. Participants were invited to attend the Lausanne University Hospital for data collection at baseline and subsequent follow-ups. Each visit included a health questionnaire, a physical examination and blood tests.
The first follow-up visit was conducted between April 2009 and September 2012, and the second follow-up visit between May 2014 and April 2017. Mean follow-up time was 10.7 (range 8.8–13.6) years for the second follow-up. As ECG data were only collected during the second follow-up, only data collected during the second follow-up was considered.
Electrocardiography
Standard 12-lead ECGs were recorded in resting supine position at 10 mm/mV calibration and paper speed of 25 mm/s on a Cardiovit MS-2015 electrocardiograph (Schiller AG, Baar, Switzerland). Digital ECGs were stored in an anonymised database of SEMA data management system (V3.5, Schiller AG). ECG measurements, including PWD values, were automatically determined in ms by Schiller AG algorithms based on all 12-leads and the reconstitution of an average beat.13 A former work demonstrated a good concordance between PWD calculated by Schiller’s algorithm and PWD measured manually.14 Therefore, calculated PWD values were used as references for this study, with two exceptions requiring a manual determination of PWD. The first one included ECGs for which the algorithm was unable to provide a PWD value (eg, artefacted ECGs, unstable baseline or inverted electrodes). The second one pertained to extreme values of automatically calculated PWD (<80 ms (<2 SD) or >150 ms (>2 SD)), as it has been shown that such extreme values are often inaccurate.14 When required, PWD was manually calculated in ms by two investigators (FB and EP). PWD was measured from the beginning of the P-wave defined as the point where the first atrial deflection departs from the isoelectric line and the end of the P-wave defined as the point where the atrial deflection returns to the isoelectric line. The two investigators identified the ECG lead where the measure would be the most accurate and the mean PWD value from the two investigators was used.
Covariates
Lifestyle and sociodemographic data were collected using self-filled questionnaires. Participants were separated into two ethnic groups: Caucasian and other. Smoking status was defined as never, former and current smokers. Alcohol consumption was categorised into non-drinkers, low risk (1–13 units/week), medium risk (14–27 units/week) and high risk (>28 units/week). Personal and family history of CV diseases (myocardial infarction, angina pectoris, percutaneous revascularisation or coronary bypass grafting, stroke or transient ischaemic attack), the use of tricyclic antidepressants were also collected in the questionnaire. Details on anthropometric, blood pressure and biological measures are described in the online supplemental appendix.
bmjopen-2020-038828supp001.pdf (204.8KB, pdf)
Exclusion criteria
Participants were excluded from the analysis if they presented with (1) no sinus rhythm or paced rhythm on the study ECG or Wolff-Parkinson-White ECG pattern (present on the digital or manual analysis); (2) missing or non-interpretable ECG (artefacted, unstable baseline or inverted electrodes); and (3) missing phenotypic data.
Patient and public involvement
No patients or public were involved in this study design, conduct or analysis.
Statistical analyses
Statistical analyses were performed using Stata V.15.1 for Windows (Stata Corporation, College Station, Texas, USA). Results were expressed as number of participants (percentage) for categorical data and mean±SD or percentiles (10th, 25th, 50th, 75th and 90th) for continuous data.
T-test for continuous variables and χ2 for categorical variables were used to compare included and excluded participants as well as participants with a PWD < or ≥120 ms. The bivariate associations between PWD and continuous variables were assessed through simple linear regression. The bivariate associations between PWD and categorical variables were assessed using Kruskal-Wallis test.
Variables significantly associated with PWD in the bivariate analysis were then carried forward for multivariable analysis. The multivariable analysis was conducted using analysis of variance and two models were applied. Model 1 was applied for each individual variable significantly associated with PWD in the bivariate analysis and adjusted for age, height and gender. Model 2 included all variables significantly associated with PWD in Model 1, including age, height and gender. As body mass index (BMI) and abdominal obesity categories were closely related, analyses were conducted separately for each marker (Models 2A and 2B for BMI and waist circumference for abdominal obesity). Results were expressed as multivariable-adjusted mean±SE for categorical values and as adjusted regression coefficient for continuous variables. As a sensitivity analysis, multivariable analysis was repeated after exclusion of all the ECGs that required a manual determination of PWD. A two-tailed p<0.05 was considered statistically significant.
Results
Selection procedure and characteristics of participants
Of the initial 4881 participants at the second follow-up, 3459 (70.9%) were included. The selection procedure is summarised in figure 1. The majority of participants were Caucasian and 55% were women. Mean (±SD) age and BMI were 62±10 years and 26.4±4.6 kg/m2, respectively. Dyslipidaemia was diagnosed in 70% of the participants, 45% had hypertension, 19% smoked and 8.5% were diabetic. Metabolic syndrome was diagnosed in 28% of participants and 9% reported a previous history of CV disease.
Figure 1.
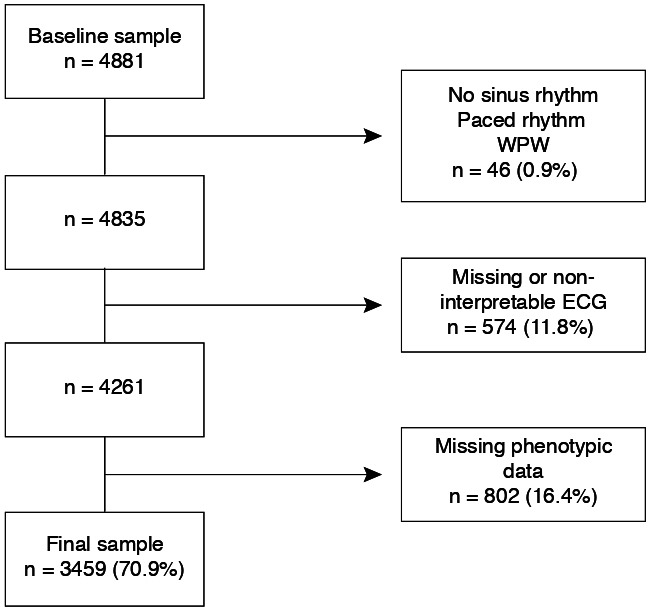
Selection procedure. Percentages were calculated using the baseline sample size as denominator. WPW, Wolff-Parkinson-White.
The characteristics of the excluded population and comparison between participants with PWD <120 ms and ≥120 ms are shown in the online supplemental appendix in online supplemental table 1 and 2, respectively. Significant differences between both populations were observed for age, BMI categories, alcohol use, personal history of CV diseases, hypertension, dyslipidaemia, diabetes and renal failure, as well as for blood levels of troponin and N-terminal pro-B-type natriuretic peptide (NT-pro-BNP).
PWD measure and distribution
A total of 351 ECGs, that is, 10% of the ECGs, needed a manual determination of PWD according to the previously mentioned criteria: 101 (29%) for extreme automatically calculated PWD (> or <2 SD), and 250 (71%) for automatic assessment inability. The PWD distribution in the overall sample is illustrated in figure 2; median (and IQR) of PWD was 112 (102–120) ms. Prevalence of PWD ≥120 ms was 21% (CI: 20%–23%).
Figure 2.
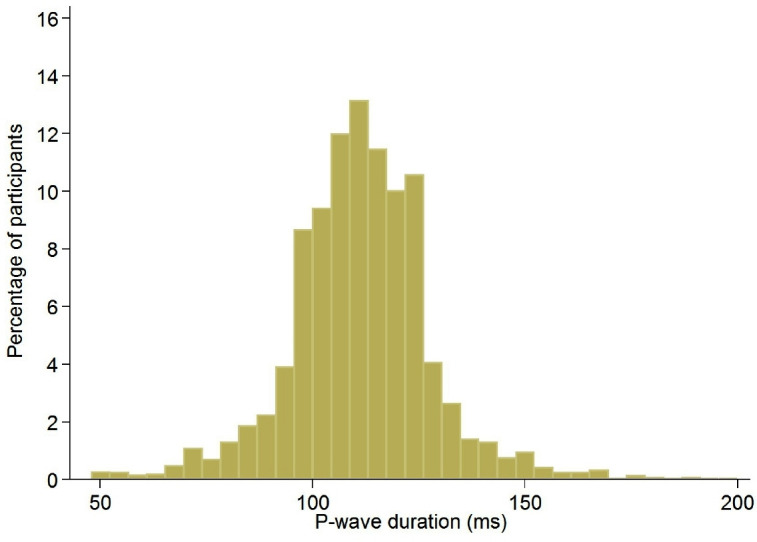
Distribution of P-wave duration in the whole sample, CoLaus|PsyCoLaus study, Lausanne, Switzerland, 2014–2016.
Association of PWD with demographic, clinical and biological markers
The bivariate associations between PWD and the demographic, clinical and biological markers of interest are summarised in table 1 (categorical and continuous variables). Longer PWD were found in elderly; in men; in participants with increased BMI or waist circumference; in former smokers; in participants with increased alcohol consumption, hypertension, dyslipidaemia, diabetes, metabolic syndrome or renal failure; in participants with a personal history of CV disease; in participants with higher levels of troponin or NT-pro-BNP. Figure 3A, B illustrate the linear and positive association of PWD with age and height, respectively. (Online supplemental figure 1 shows mean values of PWD in males and females by age.
Table 1.
Baseline characteristics of the study population and bivariate associations between P-wave duration and different demographic, clinical and biological markers, CoLaus|PsyCoLaus study, Lausanne, Switzerland, 2014–2016
| N | P-wave duration (ms) | P value | |||||
| 10% | 25% | 50% | 75% | 90% | |||
| All | 3459 (100%) | 94 | 102 | 112 | 120 | 128 | |
| Age group (years) | |||||||
| 40–49 | 441 (12.7) | 88 | 98 | 108 | 116 | 122 | <0.001 |
| 50–59 | 1189 (34.4) | 94 | 102 | 110 | 118 | 124 | |
| 60–69 | 975 (28.2) | 96 | 104 | 112 | 120 | 128 | |
| 70–79 | 704 (20.4) | 96 | 104 | 114 | 124 | 132 | |
| 80+ | 150 (4.3) | 101 | 108 | 118 | 126 | 134 | |
| Gender | <0.001 | ||||||
| Men | 1543 (44.6) | 98 | 106 | 114 | 122 | 132 | |
| Women | 1916 (55.4) | 90 | 100 | 110 | 118 | 124 | |
| Race/ethnicity | 0.155 | ||||||
| Other | 257 (7.4) | 92 | 102 | 110 | 118 | 126 | |
| Caucasian | 3202 (92.6) | 94 | 102 | 112 | 120 | 128 | |
| BMI categories | <0.001 | ||||||
| Normal | 1405 (40.6) | 92 | 102 | 110 | 118 | 126 | |
| Overweight | 1415 (40.9) | 94 | 104 | 112 | 120 | 128 | |
| Obese | 639 (18.5) | 94 | 104 | 114 | 122 | 130 | |
| Abdominal obesity | <0.001 | ||||||
| No | 2160 (62.5) | 94 | 102 | 110 | 118 | 126 | |
| Yes | 1298 (37.5) | 92 | 104 | 112 | 122 | 130 | |
| Smoking | 0.004 | ||||||
| Never | 1455 (42.1) | 92 | 102 | 110 | 120 | 128 | |
| Former | 1349 (39.0) | 94 | 104 | 112 | 120 | 130 | |
| Current | 655 (18.9) | 94 | 102 | 110 | 118 | 126 | |
| Alcohol use | <0.001 | ||||||
| Non-drinker | 908 (26.3) | 90 | 100 | 110 | 120 | 128 | |
| Low risk | 2076 (60.0) | 94 | 102 | 112 | 120 | 128 | |
| Medium risk | 386 (11.2) | 98 | 104 | 114 | 122 | 130 | |
| High risk | 89 (2.6) | 98 | 106 | 116 | 122 | 130 | |
| Personal history of CVD | 0.003 | ||||||
| No | 3150 (91.1) | 94 | 102 | 112 | 120 | 128 | |
| Yes | 309 (8.9) | 94 | 104 | 112 | 124 | 132 | |
| Family history for CVD | 0.119 | ||||||
| No | 2115 (61.1) | 94 | 104 | 112 | 120 | 128 | |
| Yes | 1344 (38.9) | 92 | 102 | 110 | 120 | 128 | |
| Hypertension | <0.001 | ||||||
| No | 1915 (55.4) | 92 | 102 | 110 | 118 | 124 | |
| Yes | 1544 (44.6) | 96 | 106 | 114 | 122 | 130 | |
| Dyslipidaemia | 0.004 | ||||||
| No | 1015 (29.3) | 92 | 102 | 110 | 120 | 126 | |
| Yes | 2444 (70.7) | 94 | 104 | 112 | 120 | 128 | |
| Diabetes | 0.012 | ||||||
| No | 3160 (91.5) | 94 | 102 | 112 | 120 | 128 | |
| Yes | 294 (8.5) | 94 | 104 | 112 | 122 | 132 | |
| Metabolic syndrome | <0.001 | ||||||
| No | 2503 (72.4) | 94 | 102 | 110 | 120 | 126 | |
| Yes | 955 (27.6) | 94 | 104 | 112 | 122 | 132 | |
| Renal failure | 0.006 | ||||||
| No | 3086 (89.2) | 94 | 102 | 112 | 120 | 128 | |
| Yes | 373 (10.8) | 94 | 104 | 112 | 122 | 130 | |
| Antidepressant medications | 0.860 | ||||||
| No | 3424 (99.0) | 94 | 102 | 112 | 120 | 128 | |
| Yes | 35 (1.0) | 92 | 100 | 112 | 120 | 126 | |
| Troponin ≥14 ng/L | <0.001 | ||||||
| No | 2311 (91.1) | 94 | 104 | 112 | 120 | 130 | |
| Yes | 226 (8.9) | 94 | 106 | 118 | 126 | 136 | |
| CRP ≥5 mg/L | 0.354 | ||||||
| No | 3174 (91.8) | 94 | 102 | 112 | 120 | 128 | |
| Yes | 285 (8.2) | 96 | 104 | 112 | 120 | 126 | |
| NT-pro-BNP ≥125 ng/L | <0.001 | ||||||
| No | 1450 (67.4) | 92 | 102 | 110 | 118 | 126 | |
| Yes | 702 (32.6) | 92 | 104 | 112 | 122 | 132 | |
Please refer to the methods and online supplemental appendix for the definition of each characteristic. Results are expressed as deciles or quartiles. Between-group comparisons performed using non-parametric Kruskal-Wallis test.
BMI, body mass index; CRP, C reactive protein; CVD, cardiovascular disease; NT-pro-BNP, N-terminal pro-B-type natriuretic peptide.
Figure 3.
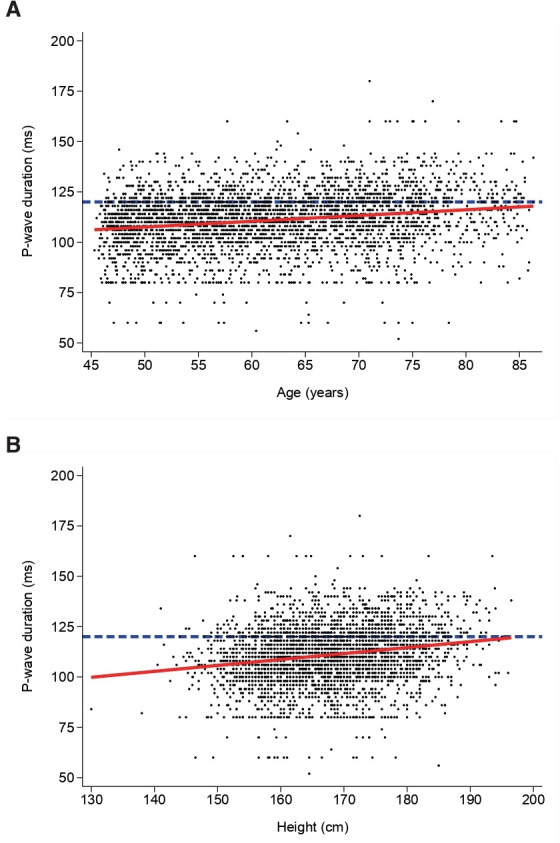
(A) Association between P-wave duration and age. (B) Association between P-wave duration and height. The dashed blue horizontal line represents the sample mean; the red line represents the linear regression.
bmjopen-2020-038828supp002.pdf (237KB, pdf)
The multivariable associations of PWD with the demographic, clinical and biological markers are summarised in table 2. After adjusting for age, height and gender in the Model 1, only BMI categories, abdominal obesity and hypertension remained significantly associated with PWD. Even after inclusion of all variables in the Model 2, age, height, gender, obesity markers (BMI or abdominal obesity) and hypertension were significantly associated with PWD. These statistically significant associations were confirmed for both models even after exclusion of manually analysed ECGs. Results are presented in online supplemental table 3 of the online supplemental appendix.
Table 2.
Multivariable associations between P-wave duration and different demographic, clinical and biological markers, CoLaus|PsyCoLaus study, Lausanne, Switzerland, 2014–2016
| Characteristics | Model 1 | P value | Model 2A | P value | Model 2B | P value |
| Age (continuous) | – | 0.32 (0.28 to 0.37) | <0.001 | 0.31 (0.26 to 0.36) | <0.001 | |
| Height (continuous) | – | 0.29 (0.23 to 0.36) | <0.001 | 0.27 (0.21 to 0.34) | <0.001 | |
| Gender | <0.001 | <0.001 | ||||
| Woman | – | 110.0±0.4 | 109.8±0.4 | |||
| Man | – | 112.1±0.4 | 112.4±0.4 | |||
| BMI categories | <0.001 | <0.001 | ||||
| Normal | 109.9±0.4 | 110.1±0.4 | ||||
| Overweight | 110.9±0.3 | 110.9±0.4 | ||||
| Obese | 113.2±0.5 | 113.0±0.5 | ||||
| Abdominal obesity | <0.001 | 0.016 | ||||
| No | 110.4±0.3 | 110.5±0.3 | ||||
| Yes | 112.0±0.4 | 111.7±0.4 | ||||
| Smoking | 0.628 | |||||
| Never | 111.0±0.3 | – | – | |||
| Former | 111.1±0.4 | – | – | |||
| Current | 110.5±0.5 | – | – | |||
| Alcohol use | 0.859 | |||||
| Non-drinker | 110.7±0.4 | – | – | |||
| Low risk | 110.9±0.3 | – | – | |||
| Medium risk | 111.4±0.7 | – | – | |||
| High risk | 111.6±1.4 | – | – | |||
| Personal history of CVD | 0.925 | |||||
| No | 110.9±0.2 | – | – | |||
| Yes | 111.0±0.8 | – | – | |||
| Hypertension | <0.001 | 0.010 | 0.002 | |||
| No | 110.1±0.3 | 110.4±0.3 | 110.3±0.3 | |||
| Yes | 112.0±0.3 | 111.7±0.4 | 111.8±0.4 | |||
| Dyslipidaemia | 0.318 | |||||
| No | 110.6±0.4 | – | – | |||
| Yes | 111.1±0.3 | – | – | |||
| Diabetes | 0.548 | |||||
| No | 111.0±0.2 | – | – | |||
| Yes | 110.5±0.8 | – | – | |||
| Metabolic syndrome | 0.113 | |||||
| No | 110.7±0.3 | – | – | |||
| Yes | 111.5±0.4 | – | – | |||
| Renal failure | 0.866 | |||||
| No | 111.0±0.2 | – | – | |||
| Yes | 110.8±0.7 | – | – | |||
| Troponin ≥14 ng/L | 0.920 | |||||
| No | 112.4±0.3 | – | – | |||
| Yes | 112.5±1.0 | – | – | |||
| NT-pro-BNP ≥125 ng/L | 0.310 | |||||
| No | 110.5±0.4 | – | – | |||
| Yes | 111.2±0.5 | – | – |
– not included in the model. Please refer to the methods and online supplemental appendix for the definition of each characteristic. Results are expressed as adjusted coefficient (95% CI) for continuous variables and as mean±SE for categorical variables. Model 1: adjusted for age (continuous), height (continuous) and gender. Full model (2A and 2B): including all variables indicated.
BMI, body mass index; CVD, cardiovascular disease; NT-pro-BNP, N-terminal pro-B-type natriuretic peptide.
Discussion
To our knowledge, this is the first study assessing the PWD and its demographic, clinical and biological determinants in the Swiss population. On a study sample of 3459 participants, we found that age, height, gender, obesity markers and hypertension were associated with PWD. Moreover, we attested that 21% of the study population had a PWD ≥120 ms, possibly reflecting an interatrial block, which is in line with other population-based studies.1 2
PWD and age
We found that ageing was associated with prolonged PWD, a finding also reported by others in several studies.4 7 9 15 Electrophysiologic studies and electroanatomic mapping of the atria demonstrated abnormal conduction in older subjects,4 which appeared to be related to atrial structural changes and to interstitial fibrosis in particular.16 Collagenous septa separate small groups of muscular fibres, causing electrical uncoupling.16 In addition, ageing is also associated with atrial dilatation, which contributes to prolonged PWD.17
PWD and height
Height has been associated with electrocardiographic modifications, particularly with the prolongation of PR interval and QRS duration.18 Still, little is known regarding the association between height and PWD. In our study, we observed that tall individuals had longer PWD. This finding fits with the literature showing that height is a strong determinant of left atrial size, and that left atrial enlargement is associated with mechanical stress responsible for slower atrial conduction.18
PWD and gender
Men had longer PWD than women, a finding also reported elsewhere.4 7 9 15 The multivariable models we used may not account for all the anthropometric differences between men and women.9 For instance, the heart size has been shown to be greater in men than in women.17 Other factors may play a role, such as the effect of sex hormones but there is currently a paucity of data regarding the possible effect of sex hormones on PWD.17
PWD and obesity markers
BMI categories or abdominal obesity were positively associated with PWD, a finding also reported elsewhere.4 7 19 Interestingly, obesity has well known effects on the heart, an entity known as metabolic cardiomyopathy. Obesity is responsible for structural and functional changes in cardiomyocytes independently of coronary artery disease or hypertension.19 Regarding the metabolic cardiomyopathy, an important pathophysiological mechanism is the systemic pro-inflammatory status induced by obesity. This eventually induces low-grade inflammation in the heart with dysfunction of subcellular components (mitochondrial dysfunction, oxidative stress and impaired calcium handling), inflammatory cell infiltration and neurohumoral activation.20 Advanced stages are characterised by apoptosis, adipocytosis, fibrosis and atrial remodelling.20 A second important pathophysiological mechanism is the fatty acid accumulation in the cardiomyocytes. The normal heart reaches a balance between free fatty acid (FFA) uptake and oxidation. The increased level of circulating FFA observed in obesity eventually results in accumulation of lipid droplets within the cardiomyocytes, impacting cardiac function and promoting apoptosis also known as lipotoxic cardiomyopathy.20 21 Studies have shown an association between cardiomyocyte fat content and ECG changes, including longer PWD.19 22
PWD and hypertension
Hypertension was positively correlated with PWD after multivariable adjustments in both models, an association also reported by others.7 11 Left ventricular hypertrophy and diastolic dysfunction caused by high blood pressure are responsible for increased atrial strain with subsequent dilatation and fibrosis.11 23 These changes have repercussions on electrical conduction and, therefore, PWD.23
Study strengths and limitations
Our study was conducted in a population-based sample allowing for the generalisation of the results to similar Caucasian populations. The large sample size of our study population, together with the full set of collected data, allowed us to explore associations between PWD and a number of relevant variables. All the significant associations found in our study can be easily related with underlying (patho)physiological mechanisms.
Our study has also some limitations. First, there is no published consensus about the measurement of PWD. Most studies used automated measurements provided by different softwares. Each software has its own specific algorithm susceptible to give slightly different values.24 Second, we used the SEMA software to automatically calculate PWD values. A number of studies have included additional markers of atrial electromechanical function (ie, P-wave indices, such as P-wave terminal force), which were not part of the results provided by the software we used. Third, a significant number of ECG (10%) needed a manual determination of PWD; this might have biased the results. However, even after exclusion of the manually analysed ECGs, associations remained unchanged.
Finally, another limitation of our study is our inability to support causal association between PWD and structural myocardial abnormalities (atrial enlargement, fibro-fatty infiltration) due to the lack of anatomohistological and echocardiographic information, which was not part of our study design.
Future directions
Considering the metabolic syndrome’s burden, incidence of CV events and death will continue to be high. PWD, as an intermediate phenotype reflecting subclinical, structural and functional changes in the atria, can be a useful marker to both assess and monitor the risk of developing atrial fibrillation and worse CV outcomes.1–3 11 Based on our results, it would be interesting to know the evolution of the participants according to their PWD. Data from echocardiography or other imaging techniques, as well as post-mortem materials, could be useful to prove the fatty or fibrotic infiltration of the atria or left atrial enlargement. Moreover, future work could determine if preventive interventions (eg, lifestyle and dietary intervention) based on the PWD have positive effects on clinical outcomes. The ongoing follow-up of the CoLaus|PsyCoLaus cohort would provide some information in the near future.
Conclusion
In a cross-sectional study conducted on a large sample of a population-based cohort, PWD was associated with age, height, male gender, obesity markers and hypertension. Most of these associations could possibly relate to both structural and functional changes affecting the atrial tissue.
Supplementary Material
Footnotes
Contributors: FB conducted the literature search, interpreted the results and wrote the manuscript. PM-V performed the statistical analyses and contributed to write a part of the manuscript. DG interpreted the results and thoroughly revised the manuscript for important intellectual content. FB and EP calculated manually, when required, the PWD. PV and GW participated to conceiving the study. All authors have read and approved this version of the manuscript.
Funding: This work was and is supported by research grants from GlaxoSmithKline, the Faculty of Biology and Medicine of Lausanne, and the Swiss National Science Foundation [33CSCO-122661, 33CS30-139468 and 33CS30-148401). The funding sources had no involvement in the study design, data collection, analysis and interpretation, writing of the report or decision to submit the article for publication.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The Ethics Committee of Canton Vaud (www.cer-vd.ch) approved the CoLaus|PsyCoLaus study (reference 16/03); the approval was renewed for the first (reference 33/09) and the second (reference 26/14) follow-ups. The full decisions can be obtained from the authors on request. The study was performed in agreement with the Helsinki Declaration and in accordance with the applicable Swiss legislation. All participants gave their signed informed consent before entering the study.
Provenance and peer review: Not commissioned; externally peer-reviewed.
Data availability statement: Data may be obtained from a third party and are not publicly available. Due to the sensitivity of the data and the lack of consent for online posting, individual data cannot be made accessible. Only metadata will be made available in digital repositories. Metadata requests can also be performed via the study website www.colaus-psycolaus.ch.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Hari KJ, Nguyen TP, Soliman EZ. Relationship between P-wave duration and the risk of atrial fibrillation. Expert Rev Cardiovasc Ther 2018;16:837–43. 10.1080/14779072.2018.1533814 [DOI] [PubMed] [Google Scholar]
- 2.Skov MW, Ghouse J, Kühl JT, et al. Risk prediction of atrial fibrillation based on electrocardiographic interatrial block. J Am Heart Assoc 2018;7:JAHA.117.008247. 10.1161/JAHA.117.008247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eranti A, Carlson J, Kenttä T, et al. Orthogonal P-wave morphology, conventional P-wave indices, and the risk of atrial fibrillation in the general population using data from the Finnish hospital discharge register. Europace 2020;22:1173–81. 10.1093/europace/euaa118 [DOI] [PubMed] [Google Scholar]
- 4.Magnani JW, Gorodeski EZ, Johnson VM, et al. P wave duration is associated with cardiovascular and all-cause mortality outcomes: the National health and nutrition examination survey. Heart Rhythm 2011;8:93–100. 10.1016/j.hrthm.2010.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayés de Luna A, Platonov P, Cosio FG, et al. Interatrial blocks. A separate entity from left atrial enlargement: a consensus report. J Electrocardiol 2012;45:445–51. 10.1016/j.jelectrocard.2012.06.029 [DOI] [PubMed] [Google Scholar]
- 6.Huo Y, Mitrofanova L, Orshanskaya V, et al. P-wave characteristics and histological atrial abnormality. J Electrocardiol 2014;47:275–80. 10.1016/j.jelectrocard.2014.01.011 [DOI] [PubMed] [Google Scholar]
- 7.Lehtonen AO, Langén VL, Puukka PJ, et al. Incidence rates, correlates, and prognosis of electrocardiographic P-wave abnormalities - a nationwide population-based study. J Electrocardiol 2017;50:925–32. 10.1016/j.jelectrocard.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 8.Magnani JW, Williamson MA, Ellinor PT, et al. P wave indices: current status and future directions in epidemiology, clinical, and research applications. Circ Arrhythm Electrophysiol 2009;2:72–9. 10.1161/CIRCEP.108.806828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Havmoller R, Carlson J, Holmqvist F, et al. Age-Related changes in P wave morphology in healthy subjects. BMC Cardiovasc Disord 2007;7:22. 10.1186/1471-2261-7-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehtonen AO, Puukka P, Varis J, et al. Prevalence and prognosis of ECG abnormalities in normotensive and hypertensive individuals. J Hypertens 2016;34:959–66. 10.1097/HJH.0000000000000882 [DOI] [PubMed] [Google Scholar]
- 11.Alonso A, Soliman EZ, Chen LY, et al. Association of blood pressure and aortic distensibility with P wave indices and PR interval: the multi-ethnic study of atherosclerosis (MESA). J Electrocardiol 2013;46:359:e1–6. 10.1016/j.jelectrocard.2013.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Firmann M, Mayor V, Vidal PM, et al. The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc Disord 2008;8:6. 10.1186/1471-2261-8-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kligfield P, Badilini F, Denjoy I, et al. Comparison of automated interval measurements by widely used algorithms in digital electrocardiographs. Am Heart J 2018;200:1–10. 10.1016/j.ahj.2018.02.014 [DOI] [PubMed] [Google Scholar]
- 14.Haefliger D, Pruvot E, Monney P, et al. Validation de la mesure de la durée de l’onde P dans une étude de population, 2014. [Google Scholar]
- 15.van der Ende MY, Siland JE, Snieder H, et al. Population-Based values and abnormalities of the electrocardiogram in the general Dutch population: the lifelines cohort study. Clin Cardiol 2017;40:865–72. 10.1002/clc.22737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spach MS, Dolber PC. Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle. Evidence for electrical uncoupling of side-to-side fiber connections with increasing age. Circ Res 1986;58:356–71. 10.1161/01.RES.58.3.356 [DOI] [PubMed] [Google Scholar]
- 17.Liu X-K, Jahangir A, Terzic A, et al. Age- and sex-related atrial electrophysiologic and structural changes. Am J Cardiol 2004;94:373–5. 10.1016/j.amjcard.2004.04.040 [DOI] [PubMed] [Google Scholar]
- 18.Kofler T, Thériault S, Bossard M, et al. Relationships of measured and genetically determined height with the cardiac conduction system in healthy adults. Circ Arrhythm Electrophysiol 2017;10. 10.1161/CIRCEP.116.004735 [DOI] [PubMed] [Google Scholar]
- 19.Babcock MJ, Soliman EZ, Ding J, et al. Pericardial fat and atrial conduction abnormalities in the multiethnic study of atherosclerosis (MESA). Obesity 2011;19:179–84. 10.1038/oby.2010.121 [DOI] [PubMed] [Google Scholar]
- 20.Nishida K, Otsu K. Inflammation and metabolic cardiomyopathy. Cardiovasc Res 2017;113:389–98. 10.1093/cvr/cvx012 [DOI] [PubMed] [Google Scholar]
- 21.Alí A, Boutjdir M, Aromolaran AS. Cardiolipotoxicity, inflammation, and arrhythmias: role for interleukin-6 molecular mechanisms. Front Physiol 2018;9:1866. 10.3389/fphys.2018.01866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman DJ, Wang N, Meigs JB, et al. Pericardial fat is associated with atrial conduction: the Framingham heart study. J Am Heart Assoc 2014;3:e000477. 10.1161/JAHA.113.000477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okutucu S, Aytemir K, Oto A. P-wave dispersion: what we know till now? JRSM Cardiovasc Dis 2016;5:2048004016639443 10.1177/2048004016639443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schläpfer J, Wellens HJ. Computer-Interpreted electrocardiograms: benefits and limitations. J Am Coll Cardiol 2017;70:1183–92. 10.1016/j.jacc.2017.07.723 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-038828supp001.pdf (204.8KB, pdf)
bmjopen-2020-038828supp002.pdf (237KB, pdf)


