Abstract
Objectives
The HIV epidemic is around 7%–20% among men who have sex with men (MSM) in Southwest China. The low HIV-testing rate highlights the need for tools to identify high-risk MSM in resource-limited regions. Our aim was, therefore, to evaluate the HIV RISK Assessment Tool for HIV prediction and to characterise the primary infection among MSM in Southwest China.
Design
A cross-sectional survey was conducted in Guizhou province between January and December 2018. Participants were recruited from gay communities, among whom the HIV RISK Assessment Tool was evaluated. Logistic regression was used to analyse items associated with HIV and the area under the curve (AUC) of the receiver operating curve was calculated to quantify discrimination performance.
Participants
1330 MSM participants, of which 83 (6.2%) tested as HIV positive.
Results
A higher composite score of the tool (adjusted OR (aOR) 9.33, 95% CI 4.57 to 19.05) was independently associated with HIV infection. Items positively associated with HIV infection included having 2–5 same sex partners (aOR 2.43, 95% CI 1.28 to 4.64), always (aOR 5.93, 95% CI 1.59 to 22.13) or sometimes (aOR 4.25, 95% CI 2.09 to 8.64) having unprotected anal intercourse, taking both insertive and receptive sex roles (aOR 4.95, 95% CI 2.57 to 9.53) or only the receptive sex role (aOR 2.26, 95% CI 1.21 to 4.24). The tool showed an optimal discrimination ability (AUC=0.827), with a specificity of 0.747 and sensitivity of 0.785. Five MSM were identified with primary infection and had similar sexual risk behaviors as HIV-positive participants.
Conclusions
The HIV RISK Assessment Tool showed an overall good performance in predicting HIV risk among MSM in Guizhou province where the prevalence is still severe. This tool also demonstrated a potential to identify primary infection and is worth being promoted in resource-limited regions.
Keywords: epidemiology, HIV & AIDS, public health
Strengths and limitations of this study.
This is the first study to evaluate the HIV infection risk using an HIV RISK Assessment Tool among men who have sex with men (MSM) with a low HIV-testing rate in southwest China.
Primary infection among MSM in southwest China was initially identified using a pooled RNA-testing method.
Sociodemographic variables were adjusted in the analysis of the effectiveness of the HIV RISK Assessment Tool, which thus showed a strong explanatory strength.
The confirmation of the tool in predicting primary infection is restricted, which may be due to the small sample size with limited information and further studies with larger sample sizes are needed.
Introduction
By the end of 2018,1 approximately 860 000 people were living with HIV in China and the concern is increasing regarding the emerging epidemics among men who have sex with men (MSM).2 The overall prevalence of HIV among MSM in China from 2001 to 2018 was estimated to be 5.7%3 and it is predicted that the HIV incidence rate will reach 0.72 per 100 person-years among MSM in the next 20 years in China.4
There are substantial epidemiological variation in HIV between different geographical regions.5 Southwest and northwest China are the most HIV-affected regions across the at-risk groups with substantially higher prevalences in Guizhou, Yunnan and Sichuan provinces.6 It is, therefore, crucial to characterise the HIV epidemic among MSM in southwest China.
HIV testing is the first step in identifying new HIV infections and improving access to prevention and care.7 Although China initiated the ‘Four Free and One Care’ policy in 2003, a significant number of Chinese MSM still do not participate in HIV counselling and testing.8 A meta-analysis revealed that more than half of MSM had never been tested for HIV, and only 38% had been tested in the previous 12 months.9 MSM do not believe themselves to be at risk of HIV and are afraid to be tested positive and also have privacy concerns.10 In fact, systematic discrimination and stigma based on sexual orientation, for instance in healthcare settings, can also deter some MSM from getting an HIV test.11 12 Moreover, MSM with a previous negative result have been shown to be more likely to continue risky behaviors.13
Due to these barriers, other methods to identify individuals at high risk of HIV infection are needed in southwest China aimed at a targeted prevention and control strategy. Several risk models specific to MSM have been developed to quantify this risk including the Denver model,14 the University of North Carolina at Malawi Risk Screening Score model15 and the San Diego Early Test Score model.16 However, these models were mainly developed for Western countries or for Africa.
The HIV RISK Assessment Tool specific to Chinese MSM has, therefore, been developed to identify MSM at different levels of risk of acquiring HIV. This tool can be used by healthcare workers in order to raise awareness among MSM of their risks for contracting HIV. The HIV RISK Assessment Tool helps healthcare workers to adopt individualised prevention interventions for targeted groups, such as pre-exposure prophylaxis (PrEP), early HIV testing and particularly primary infection.
Primary infection, the period following initial HIV infection prior to antibody seroconversion, is when the peak virus concentration is reached in the blood and genital fluids, and has shown a low detection rate.17 18 Transmission is highest during this period19 and therefore, the identification of the primary infection status and its characteristics is also critical for effective intervention strategies to reduce HIV transmission rates. Moreover, the initiation of treatment during the primary infection period is more likely to obtain a functional cure, which is the ideal HIV treatment outcome.
A recent study estimated a risk prediction score for HIV infection among MSM in Beijing20; however it is not clear whether this tool can be used in resource-limited regions. To date, no data have been published on the HIV infection risk and the primary infection among MSM in southwest China.
To fill this gap, we conducted a cross-sectional study to collect behavioural and biological data on MSM in 2018 in Guizhou province located in southwest China. The objective of this study was to validate the HIV RISK Assessment Tool developed by Li et al21 to estimate the HIV infection risk and to analyse the characteristics of MSM participants with primary infection in southwest China.
Materials and methods
Design and participants
The study was conducted following STROBE (Strengthening the Reporting of Observational studies in Epidemiology) guidelines. A cross-sectional survey was conducted in Guizhou province (including the cities of Anshun, Bijie, Guiyang, Zunyi, Tongren, Qiannan autonomous prefecture) between January and December 2018. A convenience sampling method was used to recruit subjects, and MSM participants were invited by local community-based organisations (CBOs). Each CBO is run by 2–3 full-time staff who are trained and guided by the local Centers for Disease Control and Prevention (CDCs), including Anshun, Bijie, Guiyang, Zunyi, Tongren and Qiannan. CBO staff introduced the study to potential participants when providing voluntary counselling and testing (VCT) services for MSM and doing regularly outreach health education work in the community, such as in gay bars, public bathrooms and parks. Recruited participants were encouraged to also invite their MSM peers to take part.
The inclusion criteria were as follows: (1) aged 18 years or older, (2) biologically male and to have self-reportedly engaged in anal or oral sex with men in the previous 6 months, (3) self-reported as HIV-negative or with an unknown HIV infection status and (4) willing to provide informed consent. Exclusion criteria included MSM without baseline information, including age, marital status, ethnicity and education level, and also whether they had ever received HIV testing, and the test results of HIV RISK Assessment Tool. Each participant was invited to provide a blood sample for HIV testing and received a small amount of money for their participation. The study protocol was reviewed and approved by the Medical Ethics Committee and the review boards of the Guizhou CDC, China (ID: S2017-04).
Sample size calculation
The present cross-sectional study aimed to explore the HIV prevalence and primary infection rate among MSM in Guizhou province. According to previous studies, the prevalence of HIV among MSM in western China is around 7%–20%.22–24 For the logistic regression analysis, the minimum number of cases to be included was calculated as n=10k/p225, where k is the number of covariates (estimated to be 8) and p is the smallest estimation of HIV prevalence in the population (estimated to be 7%). The calculated sample size was approximately 1142. To take a 20% non-response rate into account, the final sample size was increased to 1370.
Patient and public involvement
Study participants consisted of individuals who met the eligibility criteria as outlined above. Participants and other members of public were not involved in the recruitment, design, conduct, reporting or dissemination plans. The results would be disseminated to study participants through staffs at the local CDCs.
Data collection
All eligible participants were interviewed by trained investigators using a structured survey consisting of questions on sociodemographic information, sexual risk behaviors, HIV-related knowledge and risk prediction questions.
Measures
Sociodemographic variables
Sociodemographic variables were collected, including age, ethnicity (Han/Miao minority/others), education level (illiterate/primary/junior high school/senior high school/university or above), marital status (unmarried/married), places frequented to find male partners (internet/bars/clubs/baths/parks/public restrooms) and whether participants had ever had HIV testing (no/yes).
Sexual behaviors
Sexual behaviors were assessed by asking participants to report the number of male and female sexual partners with whom they had had (anal or oral sex), and also to report the frequency of condom use (no/sometimes/always), both with regard to receptive and inserted anal intercourse and vaginal intercourse. Commercial sex was measured by asking participants if they had paid or been paid for sex, which was defined as exchanging sex for money or goods.
Participants were also asked to report whether their sexual partners were HIV positive (no/unknown/yes and received antiretroviral treatment (ART)/yes but not received ART) and whether they were infected with sexual transmitted diseases (STDs), including gonorrhoea, syphilis, chlamydia trachomatis infection, condyloma acuminatum, genital herpes. All the behaviors were assessed in relation to the previous 6 months.
Substance use
Substance use was measured by asking participants if they had used alkyl nitrites (‘poppers’), methamphetamine (‘crystal meth’), sildenafil citrate, 3,4-Methylenedioxymethamphetamine (MDMA), ketamine, cocaine or crack cocaine, or marijuana in the previous 6 months. These variables were dichotomised as yes or no responses.
The HIV RISK Assessment Tool
The HIV RISK Assessment Tool was developed by Li et al through the Delphi method and two rounds of specialist consultation.21 The positivity coefficients through the first and second round of specialist consultation were 100.0% and 94.1%, respectively. The mean of authority coefficients (Cr) was 0.86. Kendall’s W coefficients were 0.55 and 0.46 for the first and second round of specialist consultations, respectively, suggesting that the specialists had similar opinions and that the tool had a high reliability.
Variables included: number of same sex partners (anal or oral sex), HIV-positive same sex partners (no/unknown/yes and received ART/yes but not received ART), unprotected anal intercourse (UAI) with a man (no/sometimes/always), commercial male sex behaviors (no/yes), diagnosis of STDs (no/yes), sex role with a man (only inserted/only receptive/both), recreational drug usage (no/yes) and group sex with men (no/yes). All variables were reported in relation to the past 6 months.
Risk scores for each item were reported as average ORs, which were extracted from studies included in systematic reviews during the tool development process, with the composite risk score for an individual being the summed scored of each item score. The risk scores and descriptions for each item are shown in the online supplemental table 1. We classified those with a composite score of 7.00–12.60 into low HIV risk category, 12.61–14.79 into moderate HIV risk category and 14.80–24.85 into high HIV risk category.
bmjopen-2020-039557supp001.pdf (125.3KB, pdf)
HIV testing
According to China CDC standardised laboratory procedures, ELISA (Wantai Biotech, Beijing, China) was used for screening HIV and western blot (MP Diagnostics, Singapore) was used to confirm the HIV screening results. Specimens with a negative result were tested for primary infection using a qualitative pooled RNA test. Sera from 30 antibody-negative individuals were manually combined using an algorithm adapted from Pilcher et al26 for the Procleix HIV-1 Discriminatory Assay (Gen-Probe, San Diego, California, USA): 200 μL aliquots were combined into 3 pools of 10 specimens. Aliquots of 500 μL from each of three intermediate pools were combined into one master pool. Pool size was selected to have results available within 2 weeks. With an assay sensitivity of 30 copies/mL, 1:30 dilutions associated with 30-sample pools permitted the detection of samples having 900 copies/mL or greater. If HIV-1 RNA is detected in the master pool, the corresponding intermediate pools are detected. Within the HIV RNA-positive intermediate pools, quantitative RNA testing on each individual was then performed using Roche COBAS Amplicor HIV-1 V.1.5.
Statistical analysis
All data were analysed using SAS software (V.9.4) and figures were generated using the package ‘pROC’ in R software (V.3.4.3). Univariate and multivariate logistic regressions were used to explore the associations of sociodemographics, history of HIV testing, composite score of HIV Risk Assessment Tool with HIV infection. The association of each item of the HIV RISK Assessment Tool with HIV infection were further assessed by univariate and multivariate logistic regressions adjusted for the sociodemographic variables. The adjusted OR (aOR)of the full model with all items in the tool and adjusted for sociodemographic characters were used as a prediction model to generate a receiver operating characteristic curve (ROC curve) and the area under the curve (AUC) was calculated. aOR and 95% CIs were calculated to evaluate the risk of HIV infection and transmission. A two-sided p value≤0.05 was regarded as statistically significant.
Results
Participant characteristics and HIV prevalence
By the end of 2018, the initial sample consisted of 1365 MSM. Thirty-five participants who had missing sociodemographic data or results of HIV RISK Assessment tool were excluded, leaving a final sample of 1330 MSM, with a mean age of 30.5 years. Of these, the majority were of Han ethnicity (76.2%), were unmarried (71.6%), had at least a senior high school education (81.7%) and sought primary male partners through the internet (73.7%). Only 47.2% had ever received HIV testing (table 1).
Table 1.
Sociodemographic characteristics and their associations with HIV infection among men who have sex with men participants
| Characteristics | N (%)* | HIV positive, N (%)† | Crude OR (95% CI) | P value | Adjusted OR (95% CI)‡ | P value |
| Age, mean±SD (years) | 30.54±8.80 | 30.36±10.28 | ||||
| 18–25 | 435 (32.7) | 34 (7.8) | 0.76 (0.35 to 1.65) | 0.493 | 0.68 (0.22 to 2.14) | 0.512 |
| 26–35 | 575 (43.2) | 33 (5.7) | 0.55 (0.25 to 1.19) | 0.127 | 0.59 (0.21 to 1.67) | 0.322 |
| 36–45 | 230 (17.3) | 7 (3.0) | 0.28 (0.10 to 0.78) | 0.015 | 0.38 (0.13 to 1.13) | 0.081 |
| 46 | 90 (6.8) | 9 (10.0) | 1 | 1 | ||
| Ethnicity | ||||||
| Han | 1011 (76.2) | 67 (6.6) | 2.51 (1.07 to 5.86) | 0.034 | 2.63 (1.09 to 6.31) | 0.031 |
| Miao minority | 101 (7.6) | 10 (9.9) | 3.88 (1.37 to 11.00) | 0.011 | 3.95 (1.32 to 11.82) | 0.014 |
| Other minorities | 218 (16.4) | 6 (2.8) | 1 | 1 | ||
| Marital status | ||||||
| Unmarried | 953 (71.6) | 60 (6.3) | 1.03 (0.63 to 1.70) | 0.895 | 0.92 (0.43 to 1.96) | 0.821 |
| Married | 377 (28.5) | 23 (6.1) | 1 | 1 | ||
| Education | ||||||
| Illiterate/primary | 40 (3.0) | 7 (17.5) | 3.25 (1.36 to 7.79) | 0.008 | 2.80 (0.98 to 7.98) | 0.054 |
| Junior high school | 204 (15.3) | 12 (5.9) | 0.96 (0.49 to 1.86) | 0.897 | 0.64 (0.32 to 1.30) | 0.218 |
| Senior high school | 417 (31.4) | 23 (5.5) | 0.89 (0.53 to 1.51) | 0.677 | 0.66 (0.38 to 1.15) | 0.142 |
| University or above | 669 (50.3) | 41 (6.1) | 1 | 1 | ||
| Internet as major mode of seeking male partner§ | ||||||
| Yes | 969 (73.7) | 67 (6.9) | 1.60 (0.92 to 2.80) | 0.099 | 2.24 (1.18 to 4.25) | 0.013 |
| No | 346 (26.3) | 16 (4.6) | 1 | 1 | ||
| Had received HIV testing | ||||||
| Yes | 629 (47.3) | 27 (4.3) | 0.52 (0.32 to 0.83) | 0.006 | 0.54 (0.32 to 0.89) | 0.016 |
| No | 701 (52.7) | 56 (8.0) | 1 | 1 | ||
| Composite score of HIV RISK Assessment Tool, mean±SD | 12.47±3.16 | 14.64±2.06 | 1.25 (1.17 to 1.35) | <0.001 | ||
| 7.00–12.60 | 665 (50.0) | 11 (1.7) | 1 | 1 | ||
| 12.61–14.79 | 395 (29.7) | 36 (9.1) | 5.96 (3.00 to 11.86) | <0.001 | 5.32 (2.63 to 10.73) | <0.001 |
| 14.80–24.85 | 270 (20.3) | 36 (13.3) | 9.15 (4.58 to 18.26) | <0.001 | 9.33 (4.57 to 19.05) | <0.001 |
*The percentage of the total cohort.
†The percentage of each category which is HIV positive.
‡Adjusted for all variables listed.
§Fifteen had missing values.
Eighty-three (6.2%) participants had been tested as HIV positive. The HIV prevalence was higher among those who had one or more of the following characteristics: (1) were over 46 years old (10.0%); (2) members of the Miao ethnic group (9.9%); (3) sought male partners through the Internet (6.9%); (4) had received only primary education or were illiterate (17.5%) and (5) had never received HIV testing (8.0%) (table 1). The comparison of sociodemographic characteristics among HIV-positive and HIV-negative participants can be seen in online supplemental table 2.
The average composite score of the HIV RISK Assessment Tool was 12.47±3.16 (table 1). Overall, 20.3% and 29.7% of the participants were classified into high and moderate HIV risk category, respectively. Regarding the individual items (sexual behaviours in the last 6 months), 50.8% reported as having had 2–5 male sexual partners, 54.3% were unaware of their partners’ HIV status and 47.4% sometimes had UAI with a man. In the previous 6 months, around 4.5% reported as having ever had commercial sex with a man, 8.1% as having STDs, 2.2% as having ever used recreational drugs and 9.6% having had group sex with men. A total of 46.4% and 16.0% of participants had only inserted or had receptive sex roles, respectively, while 37.6% had taken both sex roles (table 2).
Table 2.
Individual items of HIV RISK Assessment Tool and their respective associations with HIV infection among men who have sex with men participants
| N (%)* | HIV positive, N (%)† |
Crude OR (95% CI) |
P value | Adjusted OR (95% CI)‡ |
P value | Adjusted OR (95% CI)§ |
P value | |
| Behaviours in the prior 6 months | ||||||||
| 1. No. of same sex partners | ||||||||
| 0–1 | 569 (42.8) | 15 (2.6) | 1 | 1 | 1 | |||
| 2–5 | 675 (50.8) | 65 (9.6) | 3.94 (2.22 to 6.98) | <0.001 | 3.90 (2.15 to 7.06) | <0.001 | 2.43 (1.28 to 4.64) | 0.007 |
| 6–9 | 72 (5.4) | 3 (4.2) | 1.61 (0.45 to 5.69) | 0.463 | 1.62 (0.45 to 5.69) | 0.468 | 1.62 (0.39 to 6.72) | 0.507 |
| ≥10 | 14 (1.1) | 0 | NA | NA | NA | |||
| 2. Had had HIV-positive same sex partners | ||||||||
| No | 540 (40.6) | 13 (2.4) | 1 | 1 | 1 | |||
| Unknown | 726 (54.3) | 64 (8.8) | 3.92 (2.14 to 7.19) | <0.001 | 3.56 (1.91 to 6.62) | <0.001 | 1.24 (0.31 to 4.94) | 0.302 |
| Yes and received cART | 42 (3.2) | 3 (7.1) | 3.12 (0.85 to 11.41) | 0.086 | 3.13 (0.83 to 11.72) | 0.091 | 1.44 (0.72 to 2.87) | 0.763 |
| Yes but not received cART | 22 (1.7) | 3 (13.64) | 6.40 (1.68 to 24.35) | 0.007 | 6.60 (1.68 to 25.95) | 0.007 | 1.51 (0.33 to 6.88) | 0.597 |
| 3. Had UAI with a man | ||||||||
| No | 670 (50.4) | 11 (1.6) | 1 | 1 | 1 | |||
| Sometimes | 630 (47.4) | 68 (10.8) | 7.25 (3.80 to 13.84) | <0.001 | 6.66 (3.44 to 12.89) | <0.001 | 4.25 (2.09 to 8.64) | <0.001 |
| Always | 30 (2.3) | 4 (13.3) | 9.22 (2.75 to 30.90) | <0.001 | 8.92 (2.58 to 30.79) | <0.001 | 5.93 (1.59 to 22.13) | 0.008 |
| 4. Had commercial sex with a man | ||||||||
| No | 1270 (95.5) | 81 (6.4) | 1 | 1 | 1 | |||
| Yes | 60 (4.5) | 2 (3.3) | 0.51 (0.12 to 2.11) | 0.35 | 0.50 (0.12 to 2.13) | 0.348 | 0.32 (0.07 to 1.50) | 0.148 |
| 5. Had had an STD | ||||||||
| No | 1222 (91.9) | 77 (6.3) | 1 | 1 | 1 | |||
| Yes | 108 (8.1) | 6 (5.6) | 0.88 (0.37 to 2.06) | 0.759 | 0.82 (0.33 to 2.04) | 0.669 | 0.55 (0.21 to 1.44) | 0.222 |
| 6. Sex role | ||||||||
| Only inserted | 617 (46.4) | 18 (2.9) | 1 | 1 | 1 | |||
| Only receptive | 213 (16.0) | 32 (6.4) | 2.28 (1.26 to 4.10) | 0.006 | 2.33 (1.27 to 4.27) | 0.006 | 2.26 (1.21 to 4.24) | 0.011 |
| Both | 500 (37.6) | 33 (15.5) | 6.10 (3.36 to 11.09) | <0.001 | 6.44 (3.43 to 12.09) | <0.001 | 4.95 (2.57 to 9.53) | <0.001 |
| 7. Use of recreational drugs | ||||||||
| No | 1301 (97.8) | 81 (6.2) | 1 | 1 | 1 | |||
| Yes | 29 (2.2) | 2 (6.9) | 1.12 (0.26 to 4.77) | 0.883 | 1.12 (0.25 to 5.09) | 0.885 | 1.01 (0.20 to 5.15) | 0.988 |
| 8. Had had group sex with men | ||||||||
| No | 1202 (90.4) | 74 (6.2) | 1 | 1 | 1 | |||
| Seldom/always | 128 (9.6) | 9 (7.0) | 1.15 (0.56 to 2.36) | 0.697 | 1.26 (0.59 to 2.69) | 0.554 | 0.98 (0.42 to 2.31) | 0.962 |
*The percentage of the total cohort.
†The percentage of each category which is HIV positive.
‡Adjusted for variables in table 1 except HIV RISK composite score.
§Adjusted for variables listed in table 2.
ART, antiretroviral treatment; STDs, sexually transmitted diseases; UAI, unprotected anal intercourse.
Independent association of sociodemographic characteristics and HIV RISK Assessment Score with HIV infection
In the multivariate logistic regression, Han ethnicity (aOR 2.63, 95% CI 1.09 to 6.31) and Miao minority ethnic group (aOR 3.95, 95% CI 1.32 to 11.82) had a higher HIV infection rate compared with other minorities. Seeking partners through Internet (aOR 2.24, 95% CI 1.18 to 4.25) (vs other places) was positively associated with HIV infection, whereas having received HIV testing (aOR 0.54, 95% CI 0.32 to 0.89) was negatively associated with HIV infection. Notably, the composite score of HIV RISK Assessment Tool was independently associated with HIV infection. Compared with those who had a score of 7.00–12.60, participants who had a composite score of 12.61–14.79 (aOR 5.32, 95% CI 2.63 to 10.73) and 14.80–24.85 (aOR 9.33, 95% CI 4.57 to 19.05) were significantly associated with an increasing HIV infection risk, respectively, (p<0.001 in trend χ2 test) (table 1).
HIV RISK Assessment Tool and its prediction effect
In the multivariate logistic regression model, adjusted for sociodemographic variables listed in table 1, those with 2–5 same sex partners, not knowing HIV status of partners, with HIV positive same sex partners without clinical treatment, always or sometimes having UAI or having both sex roles or only a receptive sex role were significantly associated with increased HIV infection risk, and with a higher aOR than those in the original tool. In the fully adjusted model adjusting for each individual item and sociodemographic variables, participants who had 2–5 same sex partners (aOR 2.43, 95% CI 1.28 to 4.64) were more likely to have HIV infection compared with those who only had 0–1 same sex partner. Those who always had UAI (aOR 5.93, 95% CI 1.59 to 22.13) or sometimes had UAI (aOR 4.25, 95% CI 2.09 to 8.64) (vs having no UAI) had a higher HIV infection risk. In addition, MSM who had both sex roles (aOR 4.95, 95% CI 2.57 to 9.53) or only a receptive sex role (aOR 2.26, 95% CI 1.21 to 4.24) compared with those only having an inserted sex role were positively associated with HIV infection (table 2).
The evaluation of the optimal probability of the fully adjusted model of multivariate logistic regression was based on 83 seroconversions out of 1330 MSM participants. The AUC for ROC was 0.827, illustrating the precise prediction of the HIV RISK Assessment Tool. Using 0.916 as a cut-off of probability, the optimum sensitivity was 78.5% and specificity was 74.7%. The Youden index was 0.532 calculated from the formula=sensitivity+specificity−1 (figure 1).
Figure 1.
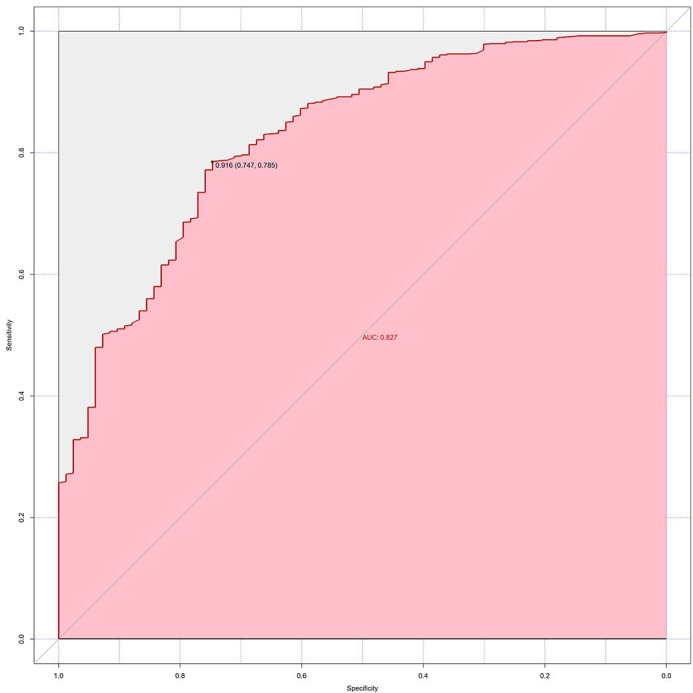
Receiver operating characteristic curve for the individual items of HIV RISK Assessment Tool model.
Primary HIV infection
As shown in table 3, five participants were identified as having primary infection. Four out of five HIV RISK Assessment composite scores were above the average with a mean score of (13.22±3.04). A total of 2/270 and 2/395 were in the high and moderate score category of HIV RISK Assessment composite score. All were of Han ethnicity and their HIV-1 subtype was CRF07_BC without antidrug resistance. A total of 60% had never received HIV testing, while two had a CD4+ T cell count of over 400 cells/µL. Regarding sexual behaviors, 80% had 2–5 same sex partners, or sometimes had UAI with a man, and 60% confirmed that their partners were HIV negative. None had commercial sex or had group sex with men or had STDs. A total of 80% had taken receptive or inserted sex roles, while one had both sex roles.
Table 3.
Characteristics of the five men who have sex with men with primary HIV infection
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
| Age (years) | 35 | 23 | 48 | 21 | 46 |
| Ethnicity | Han | Han | Han | Han | Han |
| Marital status | Ever married | Never married | Ever married | Never married | Ever married |
| Education level | University or above | University or above | Junior high school | University or above | University or above |
| Internet as major mode of male partner identification | Yes | Yes | Yes | Yes | Yes |
| Had received HIV testing | Yes | No | Yes | Yes | No |
| CD4+ T cell count before ART (cells/μL) | 405 | 384 | 309 | 322 | 478 |
| HIV viral loads | >10 000 000 | >10 000 000 | >10 000 000 | 1 400 331 | 5 056 987 |
| HIV subtype | CRF07_BC | CRF07_BC | CRF07_BC | CRF07_BC | CRF07_BC |
| Antiretroviral drug resistance | No | No | No | No | No |
| HIV RISK Assessment Tool | |||||
| 1. No. of same sex partners | 2–5 | 2–5 | 2–5 | 2–5 | 0–1 |
| I2. Had had HIV-positive same sex partners | No | Unknown | No | No | Unknown |
| 3. Had had UAI with a man | Sometimes | Sometimes | Sometimes | Sometimes | Never |
| 4. Had had commercial sex with a man | No | No | No | No | No |
| 5. Had had an STD | No | No | No | No | No |
| 6. Sex role | Receptive | Receptive | Inserted | Both | Inserted |
| 7. Had used recreational drugs | No | No | No | No | No |
| 8. Had had group sex with men | No | No | No | No | No |
| Composite score of HIV RISK Assessment Tool | 15.75 | 14.11 | 13.46 | 14.80 | 8.00 |
| The category of composite score | High | Moderate | Moderate | High | Low |
ART, antiretroviral therapy; UAI, unprotected anal intercourse.
Discussion
Our study was conducted in Guizhou province where little research has been conducted although the HIV prevalence is severe. The HIV prevalence was 6.2% in our sample, which is in line with a meta-analysis showing that the overall HIV prevalence among MSM is 5.0% in mainland China and 6.3% in western China.27 The Miao minority had a significantly greater odds of HIV infection, and it has been reported that ethnic affiliation could be a powerful predictor of HIV infection, since it reflects exposure to specific cultural standards, social policies and social structures.28 Those who had had HIV testing had a lower HIV infection rate, which may be attributed to their awareness of the HIV risk and their likelihood of regulating related behaviors. It has been previously reported that internet-based sex-seeking behavior promotes HIV infection risk.29
A notable finding was that the HIV RISK Assessment Tool performed well in predicting HIV infection. This tool was previously developed for identifying high-risk behaviors associated with HIV infection,30 and the formal external validation was evaluated by Luo et al.20 Our data showed that its composite score was independently associated with HIV infection, with higher composite scores relating to a higher HIV infection risk. Its individual items including having 2–5 male partners, having HIV-positive partners without treatment, always or sometimes having UAI, taking both sex roles or only a receptive role should be given a higher weight in the scoring system. In addition, the aOR after adjusting for sociodemographic characters in our study could be used as a reference when calculating composite risk score in southwest China. The accuracy of the HIV RISK Assessment Tool with an ROC–AUC of 0.827 also confirmed its external validity. It is thus worth of promoting among MSM where frequent VCT is uncommon.13 31 Self-perceived HIV infection risk is an important precursor of health-seeking behaviors.32 However, it has been reported that there is a large discordance between self-perceived and actual HIV infection risk.33 This gap thus needs to be filled by an objective risk assessment in order to help HIV-vulnerable populations understand their risk and better proactively seek HIV prevention services.34
Our study confirmed the implications of the HIV RISK Assessment Tool in clinical usage. First, a definitive HIV test confirmation delays the final determination of the serostatus, and the potential patients would thus not be linked to care before diagnosis. Using this score evaluation might help to identify high-risk MSM whose contact information could be collected before the infection status has been confirmed.35 Second, this tool could be a possible substitute to evaluate the HIV infection risk for people who are reluctant to get HIV testing in clinical settings. Those who reached a high composite score in screening should be further encouraged to receive HIV testing. Moreover, since this tool was developed by critical evaluation, overcoming the geographical restrictions of study populations, it could be used more broadly with different MSM populations.31 The tool could also be used for repeated assessments to track changes in HIV infection risk among MSM, providing feedback to health workers or counsellors.
The identification of high-risk MSM is beneficial in terms of the effective and efficient strategic allocation of budgets. PrEP is a promising strategy that has been found to be effective in HIV control36 but with a slow uptake37 since PrEP is expensive and carries potential risks related to side effects, renal and bone toxicity and HIV drug resistance. There is consensus that it should be targeted towards those at highest HIV infection risk in order to maximise its clinical, public health and cost benefits.38 The use of HIV RISK Assessment tool to identify those MSM who are at higher risk of HIV seroconversion could improve targeted prevention interventions,39 where current PrEP access is limited, as many providers and clinics offering PrEP likely have a limited capacity.
Our results also indicated that primary infection may be prevalent among those young, highly educated adults who seek partners through the Internet. The results are in accordance with other studies, manifesting that young MSM in high school and college are at high risk for HIV infection with poor HIV knowledge awareness and high-risk sexual behaviors in China,40–42 especially in Guizhou and Yunnan provinces.43 Participants of primary infection had the similar sexual behaviors and high composite scores as high-risk MSM. Participants with primary infection also had a higher composite score compared with HIV-negative MSM. These results suggest the potential usage of the HIV RISK Assessment Tool in identifying those with primary infection, and individuals with a high composite score but negative HIV test results should also be concerned about primary infection, and the subsequent pooled RNA testing is recommended. What is more, this method avoids RNA testing on each individual, which is quite cost-effectiveness and is well suitable in resource-limited regions. The correct and direct identification of individuals with primary infection would provide the opportunity to start immediate treatment and to provide PrEP to MSM in established partnerships with index patients in an attempt to block transmission.44
However, this study has several limitations that need to be considered. First, the developers of the tool did not assess the internal reliability measures such as Cronbach’s alpha, but measured the Kendall’s W coefficient and the mean authority coefficients instead. Despite this, the tool has still been demonstrated to be reliable. Second, the small sample size of the study restricted the confirmation and usefulness of the tool in screening primary infection, and further studies with large sample sizes are needed. Moreover, we recruited participants mainly through VCT and doing outreach health education work. This may involve sampling bias as the social network collected was not random and may be limited to a specific group or geographical area. In addition, some of our data were self-reported, which might lead to under-reporting due to the social desirability. Self-reporting is also a subject to recall bias.
Conclusions
Our study showed that MSM in Guizhou province continue to engage in risky behaviours, and a high prevalence of HIV infection was found. We confirm the validity of the HIV RISK Assessment Tool in revealing the extent of the HIV epidemic, and its application should be considered among those reluctant to receive HIV testing and in resource-limited regions. Finally, our results highlight the need to notice primary infection among young MSM with risk behaviours, as well as those with high composite scores who should be targeted for VCT promotion and intervention.
Supplementary Material
Acknowledgments
We would like to thank all participants for their participation in the study. The authors wish to thank all study participants and investigators for taking part in the study. We also thank Adrian Wallwork for revising the paper.
Footnotes
MZ and JH contributed equally.
Contributors: MZ, NH and YZ conceived, designed and led the study. MZ, ZY, XZ, YY, XF, LF and YZ investigated, conducted the study and collected data. JH, YD and NH conducted the formal analysis and JH wrote the original draft. JH, YD and NH revised and edited the manuscript. All authors supervised the study and approved of the final version submitted.
Funding: This study was supported by Guizhou Provincial Bureau of Science & Technology (grant no: 2017-2886) and Shanghai Municipal Health Bureau (grant no: GWTD2015S05) and Natural Science Foundation of China (grant no: 81361120385).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. Data are available upon request.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.UNAIDS China’s epidemic and response. Available: http://www.unaids.org.cn/page139?_l=en [Accessed 24 Sep 2020].
- 2.Yin Y, Liu Y, Zhu J, et al. . The prevalence, temporal trends, and geographical distribution of HIV-1 subtypes among men who have sex with men in China: a systematic review and meta-analysis. Epidemiol Infect 2019;147:e83. 10.1017/S0950268818003400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong M-J, Peng B, Liu Z-F, et al. . The prevalence of HIV among MSM in China: a large-scale systematic analysis. BMC Infect Dis 2019;19:1000. 10.1186/s12879-019-4559-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang KR, Peng LP, Gu J, et al. . [Impact of the 90-90-90 goal and pre-exposure prophylaxis on HIV transmission and elimination in men who have sex with men in China: A mathematical modeling study]. Zhonghua Liu Xing Bing Xue Za Zhi 2018;39:1507–14. 10.3760/cma.j.issn.0254-6450.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Guo W, D GL L. Prevalence of AIDS-related sexual behaviors and HIV infection status in young men who have sex with men in China: a meta-analysis. Zhonghua Liu Xing Bing Xue Za Zhi 2014;5:542–7. [DOI] [PubMed] [Google Scholar]
- 6.Qian S, Wei G, Wang L, et al. . Spatial analysis of AIDS aggregation epidemic in China based on geographic information system. Chin J Health Statistics 2014;6:1064–6. [Google Scholar]
- 7.Garnett GP, Hallett TB, Takaruza A, et al. . Providing a conceptual framework for HIV prevention cascades and assessing feasibility of empirical measurement with data from East Zimbabwe: a case study. Lancet HIV 2016;3:e297–306. 10.1016/S2352-3018(16)30039-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma Q, Xia S, Pan X, et al. . Rapid HIV antibody testing among men who have sex with men who visited a gay bathhouse in Hangzhou, China: a cross-sectional study. BMJ Open 2015;5:e8661. 10.1136/bmjopen-2015-008661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou H, Hu N, Xin Q, et al. . Hiv testing among men who have sex with men in China: a systematic review and meta-analysis. AIDS Behav 2012;16:1717–28. 10.1007/s10461-012-0225-y [DOI] [PubMed] [Google Scholar]
- 10.Xu W, Zheng Y, Kaufman MR. Predictors of recent HIV testing among Chinese men who have sex with men: a barrier perspective. AIDS Patient Care STDS 2018;32:408–17. 10.1089/apc.2018.0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Logie CH, Lacombe-Duncan A, Brien N, et al. . Barriers and facilitators to HIV testing among young men who have sex with men and transgender women in Kingston, Jamaica: a qualitative study. J Int AIDS Soc 2017;20:21385. 10.7448/IAS.20.1.21385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phanuphak N, Anand T, Jantarapakde J, et al. . What would you choose: online or Offline or mixed services? feasibility of online HIV counselling and testing among Thai men who have sex with men and transgender women and factors associated with service uptake. J Int AIDS Soc 2018;21:e25118. 10.1002/jia2.25118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin L, Zhao Y, Peratikos MB, et al. . Risk prediction score for HIV infection: development and internal validation with cross-sectional data from men who have sex with men in China. AIDS Behav 2018;22:2267–76. 10.1007/s10461-018-2129-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haukoos JS, Lyons MS, Lindsell CJ, et al. . Derivation and validation of the Denver human immunodeficiency virus (HIV) risk score for targeted HIV screening. Am J Epidemiol 2012;175:838–46. 10.1093/aje/kwr389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wahome E, Fegan G, Okuku HS, et al. . Evaluation of an empiric risk screening score to identify acute and early HIV-1 infection among MSM in coastal Kenya. AIDS 2013;27:2163–6. 10.1097/QAD.0b013e3283629095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoenigl M, Weibel N, Mehta SR, et al. . Development and validation of the San Diego early test score to predict acute and early HIV infection risk in men who have sex with men. Clin Infect Dis 2015;61:468–75. 10.1093/cid/civ335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pilcher CD, Joaki G, Hoffman IF, et al. . Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS 2007;21:1723–30. 10.1097/QAD.0b013e3281532c82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manak MM, Eller LA, Malia J, et al. . Identification of acute HIV-1 infection by hologic aptima HIV-1 RNA qualitative assay. J Clin Microbiol 2017;55:2064–73. 10.1128/JCM.00431-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bassett IV, Chetty S, Giddy J, et al. . Screening for acute HIV infection in South Africa: finding acute and chronic disease. HIV Med 2011;12:46–53. 10.1111/j.1468-1293.2010.00850.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo Q, Huang X, Li L, et al. . External validation of a prediction tool to estimate the risk of human immunodeficiency virus infection amongst men who have sex with men. Medicine 2019;98:e16375. 10.1097/MD.0000000000016375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Jiang Z, Song W, et al. . Development of HIV infection risk assessment tool for mell who have sex with men based on Delphi method. Zhonghua Liu Xing Bing Xue Za Zhi 2017;10:1426–30. [DOI] [PubMed] [Google Scholar]
- 22.Guanghua L, Yi C, Shuai T, et al. . HIV, syphilis and behavioral risk factors among men who have sex with men in a drug-using area of southwestern China: results of 3 cross-sectional surveys from 2013 to 2015. Medicine 2018;97:e404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng X, Zhong X, Peng B, et al. . Prevalence and associated risk characteristics of HIV infection based on anal sexual role among men who have sex with men: a multi-city cross-sectional study in Western China. Int J Infect Dis 2016;49:111–8. 10.1016/j.ijid.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Jia M, Chen M, et al. . Prevalence and the associated risk factors of HIV, STIs and HBV among men who have sex with men in Kunming, China. Int J STD AIDS 2017;28:1115–23. 10.1177/0956462416688818 [DOI] [PubMed] [Google Scholar]
- 25.Peduzzi P, Concato J, Kemper E, et al. . A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373–9. 10.1016/S0895-4356(96)00236-3 [DOI] [PubMed] [Google Scholar]
- 26.Pilcher CD, McPherson JT, Leone PA, et al. . Real-time, universal screening for acute HIV infection in a routine HIV counseling and testing population. JAMA 2002;288:216–21. 10.1001/jama.288.2.216 [DOI] [PubMed] [Google Scholar]
- 27.Cao Y, Meng XY, Weng H, et al. . [Prevalence of AIDS-related sexual behaviors and HIV infection status in young men who have sex with men in China: a Meta-analysis]. Zhonghua Liu Xing Bing Xue Za Zhi 2016;37:1021–7. 10.3760/cma.j.issn.0254-6450.2016.07.023 [DOI] [PubMed] [Google Scholar]
- 28.Pan SW, Li D, Carpiano RM, et al. . Ethnicity and HIV epidemiology research in China. Lancet 2016;388:1052–3. 10.1016/S0140-6736(16)31541-0 [DOI] [PubMed] [Google Scholar]
- 29.Pan S, Xu J-J, Han X-X, et al. . Internet-based sex-seeking behavior promotes HIV infection risk: a 6-year serial cross-sectional survey to MSM in Shenyang, China. Biomed Res Int 2016;2016:2860346 10.1155/2016/2860346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li LL, He N. [Research progress and enlightenment of HIV infection risk assessment model in men who have sex with men]. Zhonghua Yu Fang Yi Xue Za Zhi 2018;52:862–8. 10.3760/cma.j.issn.0253-9624.2018.08.016 [DOI] [PubMed] [Google Scholar]
- 31.Tang S, Tang W, Meyers K, et al. . HIV epidemiology and responses among men who have sex with men and transgender individuals in China: a scoping review. BMC Infect Dis 2016;16:588. 10.1186/s12879-016-1904-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clifton S, Nardone A, Field N, et al. . HIV testing, risk perception, and behaviour in the British population. AIDS 2016;30:943–52. 10.1097/QAD.0000000000001006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiwattanacheewin K, Sindhu S, Teitelman A, et al. . Predictors of intention to use HIV testing service among sexually experienced youth in Thailand. AIDS Educ Prev 2015;27:139–52. 10.1521/aeap.2015.27.2.139 [DOI] [PubMed] [Google Scholar]
- 34.Seekaew P, Pengnonyang S, Jantarapakde J, et al. . Discordance between self-perceived and actual risk of HIV infection among men who have sex with men and transgender women in Thailand: a cross-sectional assessment. J Int AIDS Soc 2019;22:e25430. 10.1002/jia2.25430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin L, Zhao Y, Peratikos MB, et al. . Risk prediction score for HIV infection: development and internal validation with cross-sectional data from men who have sex with men in China. AIDS Behav 2018;22:2267–76. 10.1007/s10461-018-2129-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilton J, Kain T, Fowler S, et al. . Use of an HIV-risk screening tool to identify optimal candidates for PrEP scale-up among men who have sex with men in Toronto, Canada: disconnect between objective and subjective HIV risk. J Int AIDS Soc 2016;19:20777. 10.7448/IAS.19.1.20777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirby T, Thornber-Dunwell M. Uptake of PrEP for HIV slow among MSM. Lancet 2014;383:399–400. 10.1016/S0140-6736(14)60137-9 [DOI] [PubMed] [Google Scholar]
- 38.Gomez GB, Borquez A, Case KK, et al. . The cost and impact of scaling up pre-exposure prophylaxis for HIV prevention: a systematic review of cost-effectiveness modelling studies. PLoS Med 2013;10:e1001401. 10.1371/journal.pmed.1001401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molina J-M, Capitant C, Spire B, et al. . On-Demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015;373:2237–46. 10.1056/NEJMoa1506273 [DOI] [PubMed] [Google Scholar]
- 40.Yang Z, Huang Z, Dong Z, et al. . Prevalence of high-risky behaviors in transmission of HIV among high school and college student MSM in China: a meta-analysis. BMC Public Health 2015;15:1272. 10.1186/s12889-015-2614-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen M, Liao Y, Liu J, et al. . Comparison of sexual knowledge, attitude, and behavior between female Chinese college students from urban areas and rural areas: a hidden challenge for HIV/AIDS control in China. Biomed Res Int 2016;2016:8175921 10.1155/2016/8175921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Xu J, Reilly KH, et al. . Prevalence of HIV and syphilis infection among high school and college student MSM in China: a systematic review and meta-analysis. PLoS One 2013;8:e69137. 10.1371/journal.pone.0069137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Tang W, Li Y, et al. . The HIV/AIDS epidemic among young people in China between 2005 and 2012: results of a spatial temporal analysis. HIV Med 2017;18:141–50. 10.1111/hiv.12408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dijkstra M, de Bree GJ, Stolte IG, et al. . Development and validation of a risk score to assist screening for acute HIV-1 infection among men who have sex with men. BMC Infect Dis 2017;17:425. 10.1186/s12879-017-2508-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-039557supp001.pdf (125.3KB, pdf)


