Abstract
Background
The prognostic impact of comorbidities in patients with sarcomas is not well defined. The aims of this study were to examine the implications of comorbidities and abnormal peripheral blood indices in patients with sarcomas.
Methods
A population-based database was assembled to extract patients with sarcoma in Hong Kong between January 2004 and March 2018. Charlson’s Comorbidity Index (CCI) score and prevalence of comorbidities, neutrophil, lymphocyte and platelet counts at diagnosis were assessed. The prognostic values of CCI, neutrophil-lymphocyte (NLR) and platelet-lymphocyte ratios (PLR) were estimated using Cox proportional hazard models. Restricted cubic spline plots were used to explore the association of baseline NLR and PLR with all-cause and cancer-specific mortality.
Results
Among 3358 eligible patients with sarcomas, 52.2% died after a median 26 months of follow-up. The most common comorbidities were diabetes mellitus (9.8%) and cerebrovascular disease (4.8%). Patients with higher CCI had higher mortality (CCI=3 vs CCI=2; HR 1.49; 95% CI 1.19 to 1.87; p<0.01; CCI ≥7 vs CCI =2; HR 3.20; 95% CI 2.62 to 3.92; p<0.001). Abnormal NLR and PLR levels were associated with higher all-cause mortality (NLR: HR 1.698, p<0.001, 95% CI 1.424 to 2.025; PLR: HR 1.346, p<0.001, 95% CI 1.164 to 1.555) and cancer-related mortality (NLR: HR 1.648, p<0.001, 95% CI 1.341 to 2.024; PLR: HR 1.430, p<0.001, 95% CI 1.205 to 1.697).
Conclusions
This is the largest population-based soft-tissue or bone sarcoma cohort worldwide. Comorbidities have significant negative prognostic impact on the survival of patients with sarcomas. Moreover, NLR and PLR are robust prognostic factors, and abnormal NLR and PLR have negative effects yet non-linear effects on survival.
Keywords: sarcoma, survival, prognosis, comorbidities, peripheral blood indices
Key questions.
What is already known about this subject?
Traditionally, the assessment of tumour-size, involvement of regional lymph nodes and presence or absence of distant metastases are reliable prognosticators. However, the prognostic impact of comorbidities in patients with sarcomas is not well defined.
What does this study add?
From this large population-based cohort, prognostic implications of common medical comorbidities and haematological factors in patients with sarcomas were assessed. It was found that comorbidities, abnormal neutrophil-lymphocyte and platelet-lymphocyte ratios (NLR and PLR) were prognostic factors and had negative effects on survival.
How might this impact on clinical practice?
This study suggests that in the management of patients with sarcoma, high-risk patients on the number of comorbidities present as well as the magnitude of elevation of NLR and/or PLR may potentially be identified.
Introduction
Sarcoma is the term used for cancers of bone and soft-tissue. Most sarcomas arise from mesoderm-derived cells. Sarcomas are heterogeneous, with >50 subtypes recognised in the WHO classification of tumours of soft-tissue and bone.1 In adults, soft-tissue sarcomas (STSs) are about four times more common than sarcomas of bone (13 040 compared with 3450 cases, respectively, in 2018 in the USA).1 Approximately 40% of patients diagnosed with sarcoma will die of the disease, with the majority dying of metastatic disease.2 The aetiology is generally unknown, and the symptoms may initially mimic many benign conditions. Delay of diagnosis has been well described in sarcomas.2 These cancers are uncommon, constituting <1% of cancers occurring in adults and approximately 15% of cancers in children.1 The incidence of sarcomas, however, increase with age.3
Accurate prognostication of patients with cancer has beneficial implications to the individual patient and to entire healthcare systems through appropriate channelling of healthcare resources.3 4 Traditionally, the assessment of tumour-size (T), involvement of regional lymph nodes (N) and presence or absence of distant metastases (M) are reliable prognosticators from a disease biology perspective, and most certainly are the basis of clinical cancer staging. However, given the rarity of sarcomas and the differing biology of various histopathological types, the discerning prognostic power of this approach have been called into question.5 Comorbidity has been proven to be an important prognostic factor for survival in various cancer types, even adjusting for other significant factors such as age, disease stage and treatment.6–10 To our knowledge, findings of prior studies on the impact of comorbidities and its potential impact on survival of sarcoma patients have been inconclusive. On the one hand, two earlier studies which only included adult, high-grade, non-metastatic, primary and adult extremity or trunk STS, respectively, reported no predictive value for comorbidity when compared with patients with and without comorbidities.11 12 On the other hand, a more contemporary study that assessed the prevalence and prognostic impact of comorbidities from a larger population-based series which used a sarcoma-specific registry in Denmark concluded with the contrary.10 In this analysis which assembled information from over 1700 patients spanning a 30-year period from 1979 to 2008 from a single sarcoma institution, the overall prevalence of comorbidity in STS patients were identified at 25%. The prevalence increased with age, and patients with comorbidities had a larger proportion of adverse prognostic factors. Overall, patients with comorbidities had significantly increased overall and disease-specific mortality compared with patients without comorbidities, even on adjustment of important prognostic factors including age. While these data appear compelling, a major limitation to this prior study was the fact that it was from a single institution, and not from a larger population cohort. Moreover, peripheral blood indices of systemic inflammation such as neutrophil-lymphocyte ratio (NLR) and platelets-lymphocyte ratio (PLR) have been shown to be strong prognostic markers in a variety of cancers, including sarcomas.10 13–16
The aims of our study are, through the largest cohort of sarcoma patients ever assembled for population-based study in Asia, (1) to assess the prognostic implications of common medical comorbidities in patients with sarcomas through a large territory-wide, population-based database and (2) to assess the implications of abnormal NLR and PLR on patients’ survival.
Methods
We assembled a retrospective database of all patients with a diagnosis of sarcoma, defined by having coded with the International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) code of 170.x and 171.x whoever attended clinics and hospitals operated by the Hong Kong Hospital Authority (HA) between 1 January 2004 and 31 March 2018. The HA database has been extensively used for conducting high-quality large-scale cohort studies for oncology patients.17 18 The date of diagnosis was defined as the earliest date of which the ICD-9-CM disease diagnostic code was recorded. Allowing for a 1-year window period, patients with index dates before 1 January 2005 were excluded. Primary causes of death were determined by ICD-10 diagnosis code provided by the Births and Deaths General Register in Hong Kong of the Hong Kong Immigration Department. Subjects exited the cohort at the following timepoint: (1) 31 March 2018, being the last date of data capture, (2) death from any cause and (3) censored at the last healthcare service utilisation, whichever came first.
Baseline covariates
The considered baseline covariates were age, sex, clinical characteristics including initial year of sarcoma diagnosis, sarcoma tumour location (bone and articular cartilage, connective and other soft-tissue or both), body mass index (BMI), weight, glycated haemoglobin, systolic and diastolic blood pressure, Charlson Comorbidity Index (CCI), serum lactate dehydrogenase (LDH), absolute neutrophil count, absolute lymphocyte count, platelet count, NLR and PLR, NLR and PLR were generated by the ratios of absolute neutrophil count, absolute platelet count and absolute lymphocyte count, respectively. Based on prior reports, abnormal NLR was defined by NLR ≥2.5.16 Abnormal PLR was defined by PLR ≥182.16 Abnormal serum LDH was defined by (1) for men, <106 U/L or >218 U/L; (2) for women, <103 U/L or >199 U/L.
Outcomes
All-cause mortality and cancer-related mortality were outcomes of interest.
Statistical analysis
Baseline demographic and clinical characteristics for sarcoma patients were presented. Survival rates and 95% CIs using normal distribution at years from one to ten in overall and by demographic and clinical subgroups were determined, while linear trends in survival at 1 year up to 10 years over time from 2005 to 2016 were investigated.
Incidence rates (IR) of each outcome event in overall and subgroup were estimated using the total number of sarcoma patients during the follow-up period divided by person-years at risk. To examine the association between the baseline characteristics and mortality events, Cox proportional hazards regression model assessed the effect of sociodemographic characteristics, sarcoma tumour locations and CCI on each outcome event. HRs and their 95% CI were reported for each variable in the regression model. Log-rank test was used to compare the equality of the survival curves between the subgroups. Exploratory analysis examined the non-linear associations between baseline NLR and PLR and all-cause mortality, fitting a restricted cubic spline function with four knots (5th, 35th, 65th and 95th centiles). Cumulative prevalence of comorbid conditions at diagnosis, 1–5 years after diagnosis were reported.
All statistical analyses were performed using STATA V.13.0 (StataCorp). All significance tests were two tailed and p values<0.05 were taken to indicate statistical significance.
Results
A total of 3358 eligible patients with sarcomas were recruited in the study, including 661 with sarcomas in bone and articular cartilage, 2576 with sarcomas in connective and other soft-tissue (online supplemental figure 1). A total of 121 patients were coded as suffering from sarcomas of both connective and other soft-tissue as well as bone and articular cartilage.
esmoopen-2020-001035supp001.pdf (3.3MB, pdf)
The mean age of patients with bone sarcomas and STSs were 46.8 and 56.5, respectively (online supplemental table 1). A larger proportion adolescent and young adults suffered from ‘bone and articular cartilage’ sarcoma, accounting 26.2% of the total patients with this disease, vs only 16.3% in ‘connective and other soft-tissue’. This is particularly evidence in children, defined as <18 years of age, accounting for 17.5% of bone and cartilage vs 4.7% of connective and other STS). Mean weight and BMI of all patients were 61.1 kg and 24.2 kg/m2, respectively. Mean level of overall serum LDH was 332.6 U/L. Patients with STS had a relatively higher mean CCI than patients in two other groups.
The total follow-up time was 12 099 person-years with a median 26 months of follow-up. The estimated IR of all-cause mortality was 14.50/100 person-years (95% CI 13.83 to 15.19) (online supplemental table 2). Male patients had higher incidence of all-cause mortality (15.72/100 person-years, 95% CI 14.74 to 17.74) than female (13.23/100 person-years, 95% CI 12.32 to 14.19). Patients over 80 years had highest IR (53.37/100 person-years, 95% CI 47.58 to 59.66) among all age groups. In terms of causes of death (online supplemental table 3), 71.8% of all patients died with ‘neoplasm’ as the primary cause (70.1% in bone and articular cartilage; 71.9% in connective and other soft-tissue group). Specifically, 22% died of ‘sarcoma’ as the primary cause of death. Other neoplastic causes of death included lung cancer (5.76%) and liver cancer (1.31%). Interestingly, nasopharyngeal cancer, a disease that is endemic in the Southern Chinese population, accounted for 1.77% of causes of death within our sarcoma population cohort. The most common non-neoplastic cause of death was ‘diseases of the respiratory system’, with pneumonia-related mortality account for 8.15% of all deaths within this cohort. Other non-neoplastic causes of death included diseases of the ‘circulatory system’ and ‘digestive system’ reported at 2.96% and 1.20%, respectively.
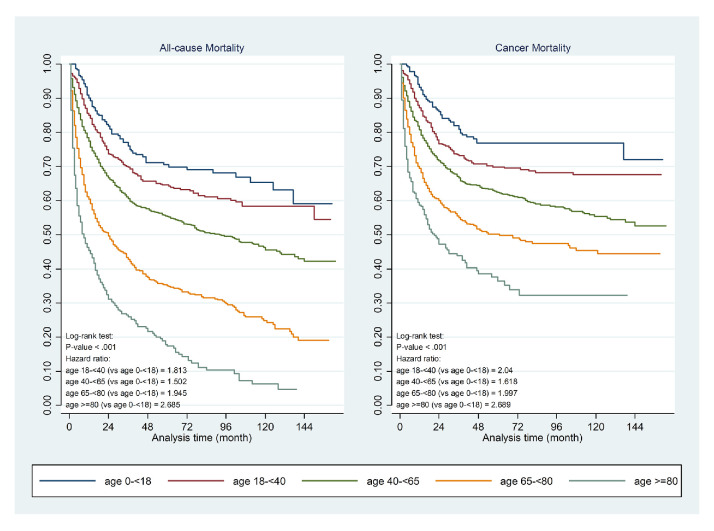
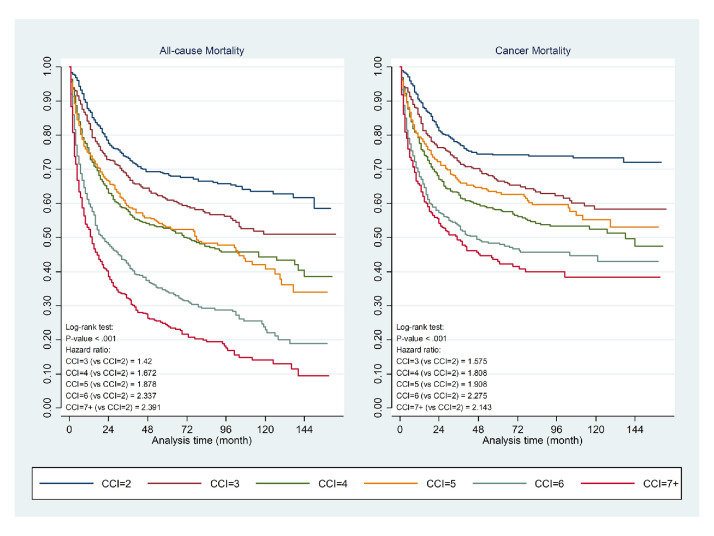
HRs were estimated for factors that might influence all-cause mortality and cancer-related mortality of patients as reported in table 1. Specifically, Age 18–40 (HR 1.813, 95% CI 1.293 to 2.542; HR 2.040, 95% CI 1.366 to 3.048), 65–80 (HR 1.945, 95% CI 1.132 to 3.343; HR 1.997, 95% CI 1.075 to 3.711) and over 80 years old (HR 2.685, 95% CI 1.526–4.725; HR=2.689, 95% CI 1.403 to 5.154) were shown as risk factors of both all-cause mortality and cancer-related mortality when compared with patients<18 years of age. Higher risk of mortality was also found to be associated with increased CCI. CCI ≥7 led to 2.391 times risks of all-cause mortality (p=0.001). Patients with abnormal NLR, PLR and serum LDH were under the risk of all-cause mortality by 1.698 (p<0.001), 1.346 (p<0.001) and 1.355 (p<0.001) times, respectively.
Table 1.
Factors associated with all-cause mortality and cancer-related mortality of patients with sarcoma
| All-cause mortality | Cancer-related mortality | |||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Male | 1.123 | (0.983 to 1.282) | 0.087 | 1.008 | (0.863 to 1.177) | 0.921 |
| Age group (<18, children and adolescent) | ||||||
| 18 to <40, young adults | 1.813 | (1.293 to 2.542) | 0.001* | 2.040 | (1.366 to 3.048) | <0.001* |
| 40 to <65, adults | 1.502 | (0.927 to 2.435) | 0.098 | 1.618 | (0.936 to 2.798) | 0.085 |
| 65 to <80, young elderly | 1.945 | (1.132 to 3.343) | 0.016* | 1.997 | (1.075 to 3.711) | 0.029* |
| ≥80, elderly | 2.685 | (1.526 to 4.725) | 0.001* | 2.689 | (1.403 to 5.154) | 0.003* |
| Sarcoma tumour site (both) | ||||||
| Bone and articular cartilage | 0.972 | (0.698 to 1.355) | 0.869 | 0.915 | (0.625 to 1.339) | 0.648 |
| Connective and other soft-tissue | 1.093 | (0.801 to 1.493) | 0.575 | 1.083 | (0.759 to 1.544) | 0.660 |
| Charlson Comorbidity Index (2) | ||||||
| 3 | 1.420 | (0.955 to 2.112) | 0.083 | 1.575 | (1.017 to 2.442) | 0.042* |
| 4 | 1.672 | (1.058 to 2.642) | 0.028* | 1.808 | (1.091 to 2.997) | 0.022* |
| 5 | 1.878 | (1.169 to 3.017) | 0.009* | 1.908 | (1.125 to 3.236) | 0.016* |
| 6 | 2.337 | (1.418 to 3.853) | 0.001* | 2.275 | (1.298 to 3.987) | 0.004* |
| ≥7 | 2.391 | (1.441 to 3.969) | 0.001* | 2.143 | (1.210 to 3.799) | 0.009* |
| Abnormal NLR* | 1.698 | (1.424 to 2.025) | <0.001* | 1.648 | (1.341 to 2.025) | <0.001* |
| Abnormal PLR* | 1.346 | (1.164 to 1.555) | <0.001* | 1.430 | (1.205 to 1.697) | <0.001* |
| Abnormal serum LDH* | 1.355 | (1.182 to 1.554) | <0.001* | 1.302 | (1.108 to 1.529) | 0.001* |
| Chemotherapy ever used (not ever used) | ||||||
| Anthracyclines not ever used | 1.511 | (1.270 to 1.797) | <0.001* | 1.567 | (1.282 to 1.917) | <0.001* |
| Anthracyclines-based chemotherapy as first line | 1.511 | (1.249 to 1.828) | <0.001* | 1.560 | (1.255 to 1.941) | <0.001* |
| Non-anthracyclines as first line but anthracyclines ever used | 1.547 | (1.101 to 2.174) | 0.012* | 1.799 | (1.238 to 2.614) | 0.002* |
*Abnormal NLR: NLR ≥2.5; abnormal PLR: PLR ≥182; abnormal Serum LDH: <106 or >218 (for men); <103 or >199 (for women).
LDH, lactate dehydrogenase; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio.
The trends of overall survival at 1–10-years by year of sarcoma diagnosis is displayed in online supplemental figure 2. The data of survival rates through 10 years after diagnosis of overall and by subgroups are shown in table 2.
Table 2.
Survival rates at 1 year, 2 years, 3 years, 5 years and 10 years after a diagnosis of sarcoma overall and by subgroups
| Subgroups | Survival rate % (95% CI) | ||||
| 1 year | 2 years | 3 years | 5 years | 10 years | |
| Overall | 72.3 (70.7 to 73.9) | 61.3 (59.6 to 63.0) | 55.5 (53.7 to 57.2) | 49.4 (47.5 to 51.2) | 40.4 (38.3 to 42.6) |
| Sex | |||||
| Male | 71.5 (69.3 to 73.6) | 59.6 (57.2 to 61.9) | 53.3 (50.8 to 55.8) | 47.7 (45.2 to 50.2) | 37.1 (34.1 to 40.1) |
| Female | 73.2 (70.9 to 75.4) | 63.2 (60.7 to 65.6) | 57.9 (55.3 to 60.4) | 51.2 (48.5 to 53.9) | 44.1 (41.1 to 47.1) |
| Age group | |||||
| <18, children and adolescent | 90.1 (85.6 to 93.2) | 81.9 (76.3 to 86.2) | 76.0 (69.9 to 81.1) | 70.5 (63.9 to 76.2) | 65.3 (57.4 to 72.1) |
| 18 to <40, young adults | 85.0 (81.6 to 87.9) | 73.8 (69.6 to 77.4) | 69.8 (65.4 to 73.7) | 64.5 (59.9 to 68.8) | 58.4 (53.2 to 63.3) |
| 40 to <65, adults | 77.4 (75.1 to 79.6) | 66.7 (64.1 to 69.2) | 60.6 (57.9 to 63.3) | 55.3 (52.5 to 58.1) | 45.6 (42.1 to 48.9) |
| 65 to <80, young elderly | 60.5 (56.9 to 63.9) | 49.7 (46.0 to 53.3) | 42.7 (39.1 to 46.4) | 35.1 (31.4 to 38.8) | 24.9 (20.9 to 29.1) |
| ≥80, elderly | 45.4 (40.0 to 50.7) | 31.1 (26.1 to 36.2) | 26.0 (21.3 to 31.0) | 17.3 (13.0 to 22.2) | 6.3 (3.0 to 11.2) |
| Sarcoma tumour site | |||||
| Bone and articular cartilage | 73.7 (70.1 to 77.0) | 65.0 (61.0 to 68.6) | 59.6 (55.5 to 63.4) | 52.6 (48.3 to 56.6) | 44.5 (39.7 to 49.2) |
| Connective and other soft-tissue | 71.7 (69.8 to 73.4) | 60.5 (58.5 to 62.4) | 54.9 (52.8 to 56.9) | 49.3 (47.2 to 51.4) | 39.9 (37.4 to 42.4) |
| Both | 78.8 (70.3 to 85.2) | 58.8 (49.3 to 67.1) | 46.5 (37.0 to 55.4) | 34.8 (25.8 to 43.9) | 28.6 (19.5 to 38.5) |
| NLR | |||||
| <2.5 | 87.9 (85.7 to 89.8) | 78.7 (76.0 to 81.1) | 72.5 (69.5 to 75.3) | 66.4 (63.2 to 69.4) | 56.5 (52.4 to 60.4) |
| ≥2.5 | 61.1 (58.8 to 63.3) | 48.3 (45.9 to 50.6) | 42.0 (39.7 to 44.4) | 35.5 (33.2 to 37.8) | 27.1 (24.5 to 29.7) |
| PLR | |||||
| <182 | 81.9 (79.9 to 83.8) | 71.5 (69.1 to 73.7) | 65.1 (62.6 to 67.5) | 58.4 (55.7 to 61.0) | 48.2 (44.9 to 51.5) |
| ≥182 | 57.5 (54.8 to 60.1) | 44.7 (42.0 to 47.4) | 38.7 (36.0 to 41.4) | 32.6 (30.0 to 35.3) | 25.2 (22.4 to 28.1) |
| Serum LDH, U/L | |||||
| 106–218 (for men); 103–199 (for women) |
67.5 (63.7 to 70.9) | 53.1 (49.2 to 56.9) | 44.8 (40.9 to 48.7) | 39.6 (35.7 to 43.6) | 31.7 (27.4 to 36.0) |
| <106 or >218 (for men); <103 or >199 (for women) |
55.2 (51.7 to 58.6) | 43.8 (40.3 to 47.2) | 37.2 (33.7 to 40.6) | 30.5 (27.2 to 33.9) | 24.3 (20.8 to 27.9) |
LDH, lactate dehydrogenase; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio.
In the study population, age was proven to be an important prognosticator for mortality. Comparing to children (<18 years), the HR for all causes of death were significantly higher in adolescent and young adults, defined as 18 to <40 years (HR 1.81; 95% CI 1.29 to 2.54; p=0.001), and in the elderly (65 to <80; HR 1.94; 95% CI 1.13 to 3.34; p=0.016; ≥80; HR 2.69; 95% CI 1.57 to 4.73; p=0.001). A similar pattern was also seen in cancer-related mortality. Figure 1 depicts the Kaplan-Meier curves of all-cause and cancer-specific mortality in relation to age of presentation.
Figure 1.
Kaplan-Meier curves of all-cause and cancer-specific mortality in relation to age of sarcoma presentation.
Comorbidities and its impact on prognosis
21.3% and 33.7% of patients had at least one or more associated comorbidities defined within the CCI in the ‘bone and articular cartilage’ and ‘connective and other soft-tissue’ at time of sarcoma diagnosis, respectively. The mean CCI of the entire population cohort was 4.6 (Bone and articular cartilage group 4.0; connective and soft-tissue group 4.8). Discounting the comorbidity of ‘musculoskeletal and chronic orthopaedic disorders’ the most common comorbidity present at sarcoma diagnosis was diabetes mellitus, reported at 9.8% in the entire cohort (7.0% in bone and articular cartilage group and 10.7% in connective and soft-tissue group). Moreover, prevalence of diabetes mellitus further increased to 12.5% at 5 years after diagnosis, occurring in one-eighth of the entire study population (online supplemental table 4). Other common comorbidities included cerebrovascular disease (4.8%), other chronic ischaemic heart disease (3.8%), chronic lung disease (2.9%), congestive heart failure (2.6%), liver disease (2.4%) and peptic ulcer disease (2.4%). Within the study cohort, increasing number of comorbidities, as defined by a higher CCI score, was also associated with an incremental high risk for all-cause as well as cancer specific mortality. This remained true after correction for age and presence of history of any solid tumours as factors that may influence the CCI score. Figure 2 indicates the impact of CCI in relation to all-cause and cancer-specific mortality.
Figure 2.
Kaplan-Meier curve of Charlson Comorbidity Index (CCI) in relation to all-cause and cancer-specific mortality.
Peripheral blood indices and its impact on prognosis
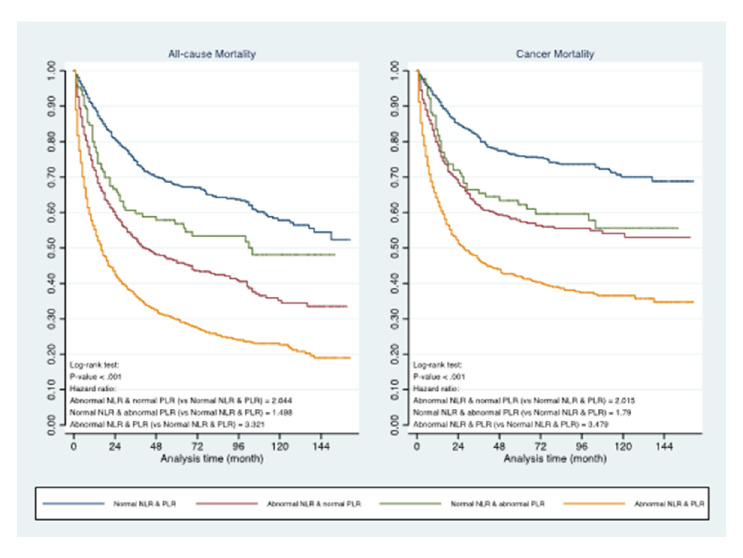
Within the 3358 patients who satisfied inclusion criteria for analysis, 3020 patients had neutrophils, lymphocytes and platelet results available for NLR and PLR calculation. The earliest haematology result after the date of index diagnosis of sarcoma were used for analysis. In total, 1989 (65.9%) of patients had abnormal NLR, defined as NLR >/=2.5. 1438 (47.6%) patients had abnormal PLR, as defined by PLR >/=192. The HR for all-cause mortality in patients with abnormal NLR and abnormal PLR vs normal NLR and PLR were 1.698 (95% CI 1.42 to 2.025) and 1.346 (95% CI 1.164 to 1.555), respectively. Similar results are also seen in cancer-specific mortality (Abnormal NLR: HR 1.648 (95% CI 1.341 to 2.204) and Abnormal PLR: 1.430 (95% CI 1.205 to 1.697)). Patients with both abnormal NLR and PLR had the worst prognoses (figure 3). Moreover, the relationship between NLR and PLR with mortality was found to be non-linear. Restricted cubic spline plots of four knots which provided the best fit were chosen to test this hypothesis (online supplemental figure 3). There is evidence that the magnitude of detriment of an increased NLR and PLR plateaus off at higher values.
Figure 3.
Prognostic impact of abnormal neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR) and presence of both abnormal NLR and PLR in all-cause and cancer-specific mortality.
Discussion
In this report, we have assembled the largest population-based soft-tissue or bone sarcoma database worldwide. We have shown that comorbidities are prevalent in patients with sarcomas at the time of diagnosis in the Asian population, with over 20% in bone and articular cartilage and over 30% of connective and other STS patients having at least one documented medical comorbidity at time of diagnosis. Moreover, the presence of and an increasing number of concomitant comorbidities are associated with worse prognoses.
Interestingly, we have shown that diabetes mellitus as being the most common comorbidity identified in our cohort (9.8%), followed by cerebrovascular disease (4.8%) and other chronic ischaemic heart disease (3.8%). While there are not many datasets available internationally for comparison, when reviewing data available from existing literature, the higher prevalence of diabetes mellitus over other comorbidities appear to be unique to our population. Specifically, in a report that used the Dutch Pathology Registry linked to the PHARMO database investigating 553 patients with an STS diagnoses between years 2000 and 2007,19 the OR for patients with STS versus a cancer-free control cohort of diabetes mellitus was 1.8 (95% CI 1.2 to 2.6), and was comparable to other comorbidities including cardiovascular disease (OR 2.0; 95% CI 1.7 to 2.6) and respiratory disease (OR 2.2; 95% CI 1.6 to 3.0).19 The reason for a higher prevalence of diabetes mellitus in our population is not well understood, and is likely multifactorial. Population studies, however, have shown that even in ethnically identical Chinese populations in Hong Kong, Taiwan and Mainland China, there were significant higher prevalence rates of diabetes mellitus reported in 1995–2003 in the Hong Kong (OR 1.5; 95% CI 1.4 to 1.7) and Taiwanese population (OR 2.0; 95% CI 1.8 to 2.2), as compared with the Mainland, even on adjustment for age and diagnostic criteria.20 Acknowledging the presence of comorbidities, and its implications on prognoses is of importance to the specific patient in question as well as the entire population. Similar to our findings, a prior study on data obtained from the Danish Sarcoma Registry of over 2167 sarcoma patients nationwide diagnosed between 2000 and 2013 have indicated the presence of comorbidities at diagnosis had significant negative impact on patients’ mortality in patients who only had localised disease on presentation, but not in patients with metastatic disease.21 There is thus a need for us to identify comorbidities early in a patients’ sarcoma disease journey.
Putting our findings and data available in the literature into perspective, it appears that the implications of early identification of comorbidities in sarcoma patients, as well as early diagnosis of sarcomas in patients with established comorbidities can both have potential positive impacts on patients’ survival. Sarcomas are rare malignancies and are often diagnosed at later stages of disease.1 With over 20% of sarcomas patients having established comorbidities at presentation, there may be a role to reinforce awareness and improve on the index of suspicion of potential diagnoses of sarcomas among primary care physicians who are already treating these patients for their comorbidities. As an example, the endocrinologist caring for a patient’s diabetes mellitus may be the first ‘port of call’ for a patient complaining of a rapidly enlarging lump. The endocrinologist thus needs to recognise the potential malignant implications of this and be able to able to arrange for early investigations and referrals to sarcoma specialists to ensure timely and appropriate treatment. Further prospective studies may also be conducted to delineate specific at-risk populations, such as those with an established diagnosis of diabetes mellitus. On the other hand, patients who already have an established diagnosis of sarcoma in conjunction with one or more of the comorbidities have been shown to have a worse survival outcome in our series. Aggressive management of comorbidities may thus be beneficial to survival, especially in patients with localised disease on presentation.
Furthermore, we have confirmed the prognostic importance of NLR and PLR in our large population cohort of patients with sarcomas. Aside from commonly used clinical and/or histopathological factors including tumour grade, size and site, a variety of host–response factors such as performance status and systemic inflammatory response can affect clinical outcomes. We have confirmed that NLR and PLR are indeed robust prognostic markers which are readily available in most patients at diagnosis. As the haematological indices needed to generate NLR and PLR values were obtained at time of index presentation with a sarcoma diagnosis, it is unlikely that these results, and their prognostic implications, are associated with surgery, chemotherapy or radiotherapy use. While prior studies have shown that high NLR and/or PLR are associated with worse overall survival in sarcoma patients,13 16 22 23 to the best of our knowledge, our report is the first to elucidate the fact that the relationship of NLR and PLR with overall survival is non-linear and plateaus off at higher NLR and PLR values. This has important implications on modelling and on establishment of nomograms. Our cohort is also the largest ever assembled histopathological and haematological factors in reported literature.
As data used for analysis for this study were obtained from a territory-wide clinical database, one limitation to our study is the integrity of our data in terms of diagnosis is dependent on accurate coding of diagnoses by clinicians. A total of 121 within the 3358 patients who met eligibility criteria (3.6%) were concurrently coded for ICD-9-CM 170.x as well as ICD-9-CM 171.x, indicating the presence of both bone & articular cartilage as well as connective and STS. While it is possible that this specific combination coding is indicative of possible metastatic disease on presentation, we cannot rule out the fact that this was entered erroneously. Interestingly, these patients did indeed have worse survival outcomes when compared with the two larger cohorts. However, given the small numbers, we do not think that this will have any significant effect on the conclusions drawn from this study. Another limitation is the lack of specific histology documented in these patients within this database as at the time of data capture for this study, the Hong Kong HA anatomical pathology database were not fully linked with the epidemiological, haematological and biochemical modules. We fully acknowledge the heterogeneity in natural history and treatment responses in between different types of sarcomas, our study’s intention was to provide a macroscopic landscape analysis on bone- and STSs in general. The large size of our assembled cohort, in conjunction with the robustness of haematological, biochemical and survival data obtained through the Hong Kong HA clinical database and the Hong Kong Immigration Department’s Register of Births and Deaths, respectively, have allowed us to contribute to existing literature.
Conclusions
This study revealed clinical and haematological factors that have prognostic implications in patients with sarcomas. Comorbidities are important factors that affect survival of patients with sarcomas. Specifically, an increase in number of comorbidities are associated with a worse prognosis. Diabetes mellitus, present in 9.8% of sarcoma patients at diagnosis, appeared to be the most common comorbidity in our Hong Kong population. Moreover, haematological factors including elevated NLR and elevated PLR are confirmed to be robust negative prognostic markers within our large assembled cohort. Interestingly, the magnitude of prognostic implication is non-linear, and the incremental risk of death associated with of elevated NLR and PLR diminishes and plateaus at higher values. Given the rarity of sarcomas, our findings suggest that there may be a role for early identification of patients with sarcomas in patients who are already being seen for comorbidities in primary care. In particular, high-risk patients based on the number of comorbidities present as well as the magnitude of elevation of NLR and/or PLR, may potentially be identified. With aggressive management of such comorbidities, there may potentially be a role for improvement in patients’ survival.
Acknowledgments
Authors would like to thank Chu Wa Ho for statistical assistance, and acknowledge the Central Panel on Administrative Assessment of External Data Requests, Hong Kong Hospital Authority Head Office, for the provision of Hospital Authority data.
Footnotes
Twitter: @herbloong, @CarlosWongHKU
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: HH-fL reports receipt of research funding of from MSD and Mundipharma for projects outside of this submitted work. HH-fL has also received personal fees from Novartis, Pfizer, Eisai and Boehringer-Ingelheim for advisory activities outside the scope of this submitted work. CK-HW reports receipt of research funding from the EuroQoL Group Research Foundation, the Hong Kong Research Grants Council, and the Hong Kong Health and Medical Research Fund. No other disclosures were reported.
Patient consent for publication: Not required.
Ethics approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethics approval of this study was granted by Joint Chinese University of Hong Kong – New Territories East Cluster Clinical Research Ethics Committee (Ref No. CRE-2016.104).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data may be obtained from a third party and are not publicly available. The data underlying the results presented in the study are available from Administrative Assessment of External Data Requests, Hong Kong Hospital Authority Head Office (contact via hacpaaedr@ha.org.hk). Restrictions apply to the availability of these data, which were used under licence for this study. Analysis codes are available on request from CKHW, yet data sharing is prohibited by the Hospital Authority.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Brouns F, Stas M, De Wever I. Delay in diagnosis of soft tissue sarcomas. Eur J Surg Oncol 2003;29:440–5. 10.1016/S0748-7983(03)00006-4 [DOI] [PubMed] [Google Scholar]
- 3.Ferrari A, Sultan I, Huang TT, et al. Soft tissue sarcoma across the age spectrum: a population-based study from the surveillance epidemiology and end results database. Pediatr Blood Cancer 2011;57:943–9. 10.1002/pbc.23252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hui D, Paiva CE, Del Fabbro EG, et al. Prognostication in advanced cancer: update and directions for future research. Support Care Cancer 2019;27:1973–84. 10.1007/s00520-019-04727-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loong HH, Wong K-H, Tse T. Controversies and consensus of neoadjuvant chemotherapy in soft-tissue sarcomas. ESMO Open 2018;3:e000293. 10.1136/esmoopen-2017-000293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bøje CR, Dalton SO, Grønborg TK, et al. The impact of comorbidity on outcome in 12 623 Danish head and neck cancer patients: a population based study from the DAHANCA database. Acta Oncol 2013;52:285–93. 10.3109/0284186X.2012.742964 [DOI] [PubMed] [Google Scholar]
- 7.Lund L, Jacobsen J, Nørgaard M, et al. The prognostic impact of comorbidities on renal cancer, 1995 to 2006: a Danish population based study. J Urol 2009;182:35–40. discussion 40. 10.1016/j.juro.2009.02.136 [DOI] [PubMed] [Google Scholar]
- 8.Piccirillo JF, Costas I. The impact of comorbidity on outcomes. ORL J Otorhinolaryngol Relat Spec 2004;66:180–5. 10.1159/000079875 [DOI] [PubMed] [Google Scholar]
- 9.Read WL, Tierney RM, Page NC, et al. Differential prognostic impact of comorbidity. J Clin Oncol 2004;22:3099–103. 10.1200/JCO.2004.08.040 [DOI] [PubMed] [Google Scholar]
- 10.Maretty-Nielsen K, Aggerholm-Pedersen N, Safwat A, et al. Prevalence and prognostic impact of comorbidity in soft tissue sarcoma: a population-based cohort study. Acta Oncol 2014;53:1188–96. 10.3109/0284186X.2014.888494 [DOI] [PubMed] [Google Scholar]
- 11.Nakamura T, Grimer R, Gaston C, et al. The value of C-reactive protein and comorbidity in predicting survival of patients with high grade soft tissue sarcoma. Eur J Cancer 2013;49:377–85. 10.1016/j.ejca.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 12.Gadgeel SM, Harlan LC, Zeruto CA, et al. Patterns of care in a population-based sample of soft tissue sarcoma patients in the United States. Cancer 2009;115:2744–54. 10.1002/cncr.24307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szkandera J, Absenger G, Liegl-Atzwanger B, et al. Elevated preoperative neutrophil/lymphocyte ratio is associated with poor prognosis in soft-tissue sarcoma patients. Br J Cancer 2013;108:1677–83. 10.1038/bjc.2013.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. 10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 15.Templeton AJ, Rodríguez-Lescure Á, Ruíz A, et al. Prognostic role for the derived neutrophil-to-lymphocyte ratio in early breast cancer: a GEICAM/9906 substudy. Clin Transl Oncol 2018;20:1548–56. 10.1007/s12094-018-1885-5 [DOI] [PubMed] [Google Scholar]
- 16.Chan ATC, Hui EP, Ngan RKC, et al. Analysis of plasma Epstein-Barr virus DNA in nasopharyngeal cancer after chemoradiation to identify high-risk patients for adjuvant chemotherapy: a randomized controlled trial. J Clin Oncol 2018:3091–100. 10.1200/JCO.2018.77.7847 [DOI] [PubMed] [Google Scholar]
- 17.Tsoi KKF, Ho JMW, Chan FCH, et al. Long-Term use of low-dose aspirin for cancer prevention: a 10-year population cohort study in Hong Kong. Int J Cancer 2019;145:267–73. 10.1002/ijc.32083 [DOI] [PubMed] [Google Scholar]
- 18.Lam TH, Wong KH, Chan KK, et al. Recommendations on prevention and screening for breast cancer in Hong Kong. Hong Kong Med J 2018;24:298–306. 10.12809/hkmj177037 [DOI] [PubMed] [Google Scholar]
- 19.van Herk-Sukel MPP, Shantakumar S, Overbeek LIH, et al. Occurrence of comorbidities before and after soft tissue sarcoma diagnosis. Sarcoma 2012;2012:1–7. 10.1155/2012/402109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong KC, Wang Z. Prevalence of type 2 diabetes mellitus of Chinese populations in mainland China, Hong Kong, and Taiwan. Diabetes Res Clin Pract 2006;73:126–34. 10.1016/j.diabres.2006.01.007 [DOI] [PubMed] [Google Scholar]
- 21.Raedkjaer M, Maretty-Kongstad K, Baad-Hansen T, et al. The impact of comorbidity on mortality in Danish sarcoma patients from 2000-2013: a nationwide population-based multicentre study. PLoS One 2018;13:e0198933. 10.1371/journal.pone.0198933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Idowu OK, Ding Q, Taktak AFG, et al. Clinical implication of pretreatment neutrophil to lymphocyte ratio in soft tissue sarcoma. Biomarkers 2012;17:539–44. 10.3109/1354750X.2012.699554 [DOI] [PubMed] [Google Scholar]
- 23.Que Y, Qiu H, Li Y, et al. Preoperative platelet-lymphocyte ratio is superior to neutrophil-lymphocyte ratio as a prognostic factor for soft-tissue sarcoma. BMC Cancer 2015;15:648. 10.1186/s12885-015-1654-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-001035supp001.pdf (3.3MB, pdf)