Abstract
Objectives:
In the literature, inconsistent associations between the primary locations of lung adenocarcinomas (ADCs) with patient prognosis have been reported, due to varying definitions for central and peripheral locations. In this study, we investigated the clinical characteristics and prognoses of ADCs located in the main bronchus.
Methods:
A total of 397,189 lung ADCs registered from 2004-2013 in the National Cancer Database (NCDB) were extracted and divided into main bronchus-located ADCs (2.5%, N=10,111) and non-main bronchus ADCs (97.5%, N=387,078). The ADCs located in the main bronchus and those not in the main bronchus were compared in terms of patient prognosis, lymph node involvement, distant metastases and other clinical features, including rate of curative-intent resection, histologic grade, and stage.
Results:
ADCs located in the main bronchus had significantly worse patient survival than those in the non-main bronchus, both for all patients (HR=1.82, 95% CI 1.78-1.86) and for those undergoing curative-intent resection (HR=2.49, 95% CI 2.23-2.78). Furthermore, ADCs located in the main bronchus had a significantly higher rate of lymph node involvement and distant metastasis than those not in the main bronchus, when stratified by tumor size (trend test, p<e−16). Multivariate analysis of overall survival showed that main bronchus location is a prognostic factor (HR=1.15, 95% CI 1.08-1.23) independent of other clinical factors.
Conclusions:
Main bronchus location is an independent predictor for metastasis and worse outcomes irrespective of stage and treatment. Tumor primary location might be considered in prognostication and treatment planning.
Keywords: Lung adenocarcinoma, tumor location, main bronchus, surgery, patient prognosis, the National Cancer Database
INTRODUCTION
Lung adenocarcinoma (ADC) incidence has been gradually increasing over the past few years, and currently accounts for more than 50% of non-small cell lung cancer (NSCLC) cases1. ADCs generally occur in peripheral lung tissues, but centrally located primary ADCs have not been uncommon in recent years1,2. Tumor location (central vs. peripheral) has been reported to be associated with prognosis of ADC patients. However, the results among different studies are inconsistent or conflicted, mainly due to different definitions of tumor locations, small sample sizes, and non-comprehensive statistical analysis. For example, to define the tumor location, Onn et al used the distance3 ; Ito et al used computed tomography (CT) imaging on the basis of hilar structures4; Ketchedjian et al used whether a tumor can be visualized within the inner third of the lung field or on bronchoscopy5. Furthermore, the main bronchus has not been analyzed as a separate location in terms of the above definitions. Recently, therapeutic interventions by endoscopy or laser therapy are making great advancements in treating tumors in the main bronchus, especially small ones6-8. These findings greatly increase the importance of studying tumors arising from the main bronchus location. In contrast to ADC, previous studies in lung squamous cell carcinoma (SqCC), the second major subtype accounting for 30% of NSCLC cases9, found no significant differences in patient prognosis between central and peripheral locations10,11. Consequently, this study focuses only on lung ADCs.
Anatomically, the main bronchi are the two main air passages that branch from the trachea, about 2 centimeters (cm) in length on the right and 5cm on the left. The main bronchi are closer to vital organs, such as the great vessels and the heart, than the lobular bronchi (secondary) and segmental (tertiary) bronchi. Because the main bronchi are physically close to critical anatomic structures, lung cancer located in the main bronchi could have a high risk of involving surrounding organs and a comparatively lower rate of curative-intent resection. We hypothesized that ADCs located in the main bronchus have worse prognosis and significantly different clinical-pathological characteristics compared with those in other locations, such as the lobes or segmental bronchi.
In this study, we extracted 397,189 cases from the National Cancer Database (NCDB) diagnosed between 2004-2013 in the United States, and compared the ADCs located in the main bronchus and those not in the main bronchus in terms of patient survival outcomes and clinical features, including rate of curative-intent resection, histologic grade, and stage. Furthermore, lymph node involvement and distant metastases were compared between main bronchus and non-main bronchus ADCs, stratified by tumor size. To our best knowledge, there is no similar study on lung ADCs located in the main bronchus.
PATIENTS AND METHODS
Database and Study Sample
To test our hypothesis, a large cohort from the NCDB was used. NCDB is maintained jointly by the American Cancer Society and the American College of Surgeons. Information submitted by tumor registries throughout the United States represents an estimated 70% of newly diagnosed cancer cases12. NCDB is a valuable database for cancer research; more than 10,000 papers have been published based on NCDB, including about 1,000 papers on lung cancer. Furthermore, information from such a large database can increase statistical stability and reduce the biases associated with institutional studies.
NCDB data from 2004 to 2013 on lung cancer patients were included in this study, which in total included 1,163,465 NSCLC cases from 1,287 facilities in the United States. The cases were independent and recorded by annual reports from all the CoC-accredited programs. Only patients with a single malignant primary tumor were included in this study, while those who developed a subsequent malignant invasive or in situ primary tumor were excluded from analysis. The ADC locations were determined by the International Classification of Diseases for Oncology (ICD-O)-3 topographic codes of C34.0 (main bronchus location), C34.1 (upper lobe), C34.2 (middle lobe), and C34.3 (lower lobe), and C33.9 (tracheal location). However, there is no data under the category of C33.9 for tracheal tumors. Thus, only C34.0, C34.1, C34.2, and C34.3 categories were included in the analysis, and tracheal tumors were completely excluded. Patients with diagnoses other than ADC were further excluded from the analysis; the remaining 397,189 patients were included in this analysis.
The database provided diagnostic, demographic, and treatment information for each patient, and the clinical stage information was determined using the new 8th edition AJCC/UICC staging system13,14. Patients containing any missing information on resection, histologic grade, or clinical stage were excluded in the following analysis. Patients without survival data were further excluded from survival analysis. Univariate analysis showed that ADCs arising in different lobes had similar prognoses, and thus the primary locations, including upper, middle, and lower lobes, were combined together under non-main bronchus location in the following analyses (Supplemental Figure 1).
Statistical Analyses
Overall survival (OS) was calculated from the date of diagnosis until death or the last follow-up contact date, and presented using Kaplan-Meier (KM) curves. Univariate and multivariate survival analyses were performed with the Cox proportional hazard model. Chi-square analysis was performed to identify whether the main bronchus group and non-main bronchus group had different rates of lymph node involvement and distant metastases stratified by tumor size. The odds ratio of resection was calculated using multivariate logistic regression. All statistical analyses were performed using statistical program R, version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria). R package “survival” (version 2.41-3) was used. All results were considered statistically significant if two-sided p value < 0.05.
RESULTS
Of the 397,189 lung ADC patients from the NCDB database, the main bronchus locations accounted for 2.5% and non-main bronchus locations accounted for the remaining 97.5% (Table 1). Compared with non-main bronchus locations, patients with ADCs located in the main bronchus were more likely to be elderly (age ≥ 65 years), male, and black, less likely to receive surgery, and tended to have more severe histologic grade and more advanced clinical stage once diagnosed (Table 1).
Table 1.
Comparison of demographic and clinical characteristics between main bronchus and non-main bronchus located adenocarcinomas.
| Main bronchus (N=10,111, 2.5 %) |
Non-main bronchus (N=387,078, 97.5 %) |
p value | ||
|---|---|---|---|---|
| Age (years) | <65 | 4,984 (49.3) | 157,754 (40.8) | < 2e−16 |
| ≥65 | 5,127 (50.7) | 229,324 (59.2) | ||
| Gender | Male | 5,077 (50.2) | 185,914 (48) | < 2e−16 |
| Female | 5,034 (49.8) | 201,164 (52) | ||
| Race | White | 8,397 (83) | 324,342 (83.8) | < 2e−16 |
| Black | 1,276 (12.6) | 44,394 (11.5) | ||
| Other | 438 (4.3) | 18,342 (4.7) | ||
| Surgery | Yes | 512 (5.2) | 116,152 (30.6) | < 2e−16 |
| No | 9,392 (94.8) | 262,851 (69.4) | ||
| Unknown* | 207 | 8,075 | ||
| Histologic | Well differentiated | 180 (1.8) | 34,563 (8.9) | < 2e−16 |
| grade | Moderately differentiated | 934 (9.2) | 82,395 (21.3) | |
| Poorly differentiated | 3,200 (31.6) | 101,312 (26.2) | ||
| Undifferentiated | 61 (0.6) | 2,052 (0.5) | ||
| Unknown* | 5,736 (56.7) | 166,756 (43.1) | ||
| Clinical stage | Stage I | 141 (4.3) | 17,165 (16.1) | < 2e−16 |
| Stage II | 200 (6.1) | 8,103 (7.6) | ||
| Stage III | 1,343 (40.7) | 35,060 (32.9) | ||
| Stage IV | 1,616 (49.0) | 46,344 (43.4) | ||
| Unknown* | 6,811 | 280,406 |
Patients containing unknown information were excluded for comparison analysis between two groups.
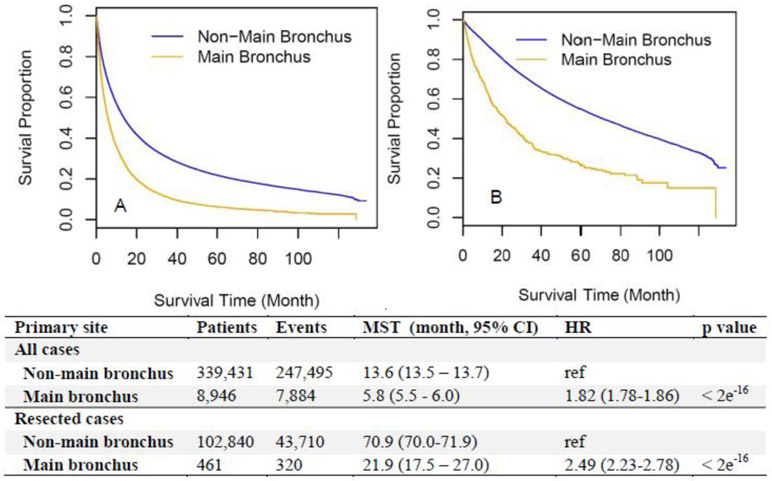
Consistent with the unfavorable clinical features of main bronchus locations shown in Table 1, ADCs arising in the main bronchus had worse survival than those in non-main bronchus locations (upper, middle, and lower lobes) for all patients (median survival time [MST] 5.8 vs. 13.6 months, HR=1.82 [1.78-1.86], Figure 1A). To test if the worse survival of main bronchus ADCs resulted from a lower resection rate (Table 1), survival outcomes of the patients who had undergone curative-intent surgery were compared between main and non-main bronchus locations. However, for the resected patients, main bronchus ADCs still showed significantly worse survival than non-main bronchus located ones (MST 21.9 vs. 70.9 months, HR=2.49 [2.23-2.78], Figure 1B). To rule out the effect of different periods between diagnosis and surgery, survival time calculated from surgery till death or last contact was used and similar results were shown (MST 20.7 vs. 70.1 months, HR=2.50 [2.24-2.80], Supplemental Figure 2).
Figure 1.
Overall survival according to different locations (main bronchus vs. non-main bronchus) among all included patients (A) and a subset who received surgical resection (B). CI, confidence interval; HR, hazard ratio; MST, median survival time.
To further rule out the potential confounder of different clinical stage distributions (Table 1), patients were stratified to early-stage (clinical stage I&II) and advanced-stage (clinical stage III&IV) groups. For the early-stage group, the resection rate was 7.8% (23/295) for main bronchus location and 9.3% (1,986/21,336) for non-main bronchus location, which showed no statistical significance (p=0.43). For the advanced-stage group, the resection rate was 2.9% (67/2,347) for main bronchus location and 4.0% (2,610/65,670) for non-main bronchus location, with a statistically significant difference (p=0.0072, Supplemental Table 1). Interestingly, after adjusting for other clinical variables such as age, gender, race, pathologic grade, and stage, the resection rate was still significantly lower for main bronchus location than for non-main bronchus location (non-main bronchus vs. main bronchus, odds ratio=1.65 [1.29-2.12], Supplemental Table 2).
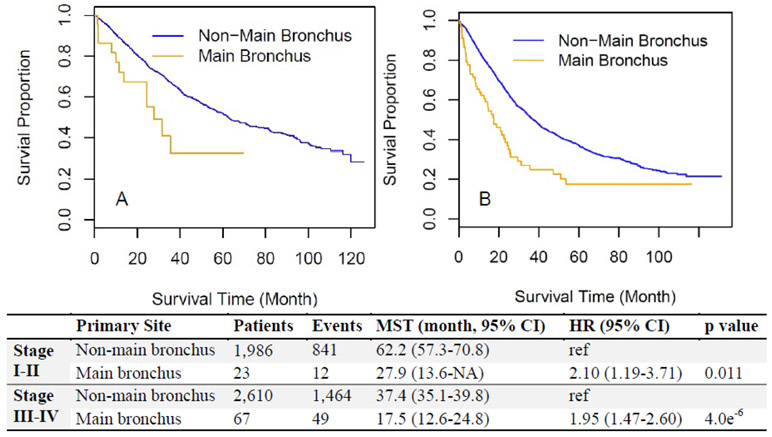
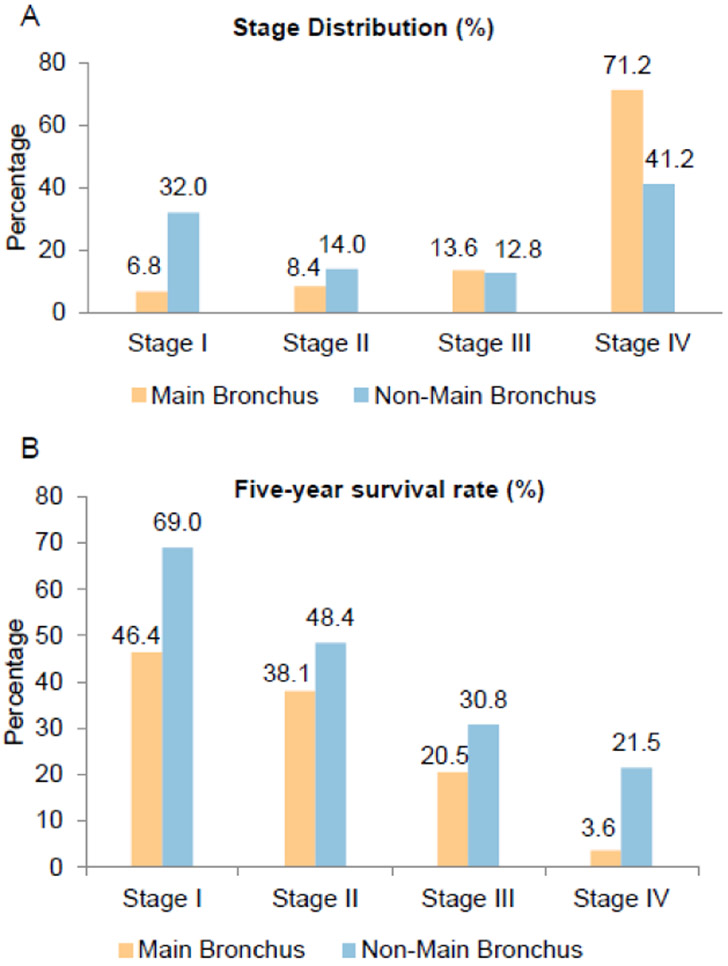
Furthermore, the patients with resected ADC arising from the main bronchus showed worse survival than those from the non-main bronchus location for both the early-stage group (HR=2.10 [1.19-3.71], Figure 2A) and the advanced-stage group (HR=1.95 [1.47-2.60], Figure 2B). This observation remained the same after stratification by all clinical stages; a significantly lower 5-year survival rate than those located in non-main bronchus locations across all stages was observed (Figure 3). A multivariate Cox regression model showed main bronchus location as an independent worse prognostic factor (HR=1.15 [1.08-1.23], Table 2) after adjusting for other confounders, including age, gender, race, histologic grade, surgery, and clinical stage.
Figure 2.
Comparison of overall survival for resected patients between non-main bronchus and main bronchus locations after stratification by different clinical stages. (A) Resected patients in early-stage (stage I-II). (B) Resected patients in advanced-stage (stage III-IV). CI, confidence interval; HR, hazard ratio; MST, median survival time.
Figure 3.
Stage distribution and five-year survival rate according to tumor location (main bronchus vs. non-main bronchus) stratified by clinical stages. (A) Main bronchus location has more advanced stages as compared with non-main bronchus location. (B) Main bronchus location has a worse 5-year survival rate after stratification by clinical stage.
Table 2.
Multivariate Cox regression of primary location on overall survival, adjusted by age, sex, race, histologic grade, surgery, and clinical stage. Univariate analysis result for each included clinical features is shown as “unadjusted HR”.
| Unadjusted HR (95% CI) |
p value | Adjusted HR* (95% CI) |
p value | ||
|---|---|---|---|---|---|
| Primary Location | Main Bronchus vs. Non-Main Bronchus | 1.29 (1.21-1.37) | 5e−15 | 1.15 (1.08-1.23) | 7.74e−6 |
| Age | ≥65 y vs. <65 y | 1.19 (1.16-1.22) | < 2e−16 | 1.34 (1.31-1.37) | < 2e−16 |
| Gender | Female vs. Male | 0.77 (0.76-0.79) | < 2e−16 | 0.80 (0.78-0.81) | < 2e−16 |
| Race | Black vs. White | 0.95 (0.92-0.98) | 0.0024 | 0.94 (0.92-0.98) | 0.0018 |
| Other vs. White | 0.78 (0.73-0.82) | < 2e−16 | 0.73 (0.69-0.77) | 0.0003 | |
| Histologic | Moderate vs. Well | 1.24 (1.20-1.29) | < 2e−16 | 1.19 (1.14-1.24) | < 2e−16 |
| Grade† | Poor vs Well | 1.58 (1.53-1.64) | < 2e−16 | 1.45 (1.39-1.50) | < 2e−16 |
| Undifferentiated vs. Well | 1.49 (1.33-1.66) | 1.36e−16 | 1.38 (1.24-1.54) | 7.35e−9 | |
| Surgery | Yes vs. No | 0.34 (0.33-0.36) | < 2e−16 | 0.43 (0.41-0.46) | < 2e−16 |
| Clinical | II vs. I | 1.41 (1.34-1.48) | < 2e−16 | 1.40 (1.34-1.48) | < 2e−16 |
| Stage | III vs. I | 1.67 (1.61-1.73) | < 2e−16 | 1.61 (1.55-1.67) | < 2e−16 |
| IV vs. I | 3.14 (3.03-3.25) | < 2e−16 | 2.92 (2.81-3.03) | < 2e−16 |
CI, confidence interval; HR, hazard ratio.
Adjusted for all other clinical variables in the table.
Defined by extent of differentiation, which describes the tumor's resemblance to normal tissue.
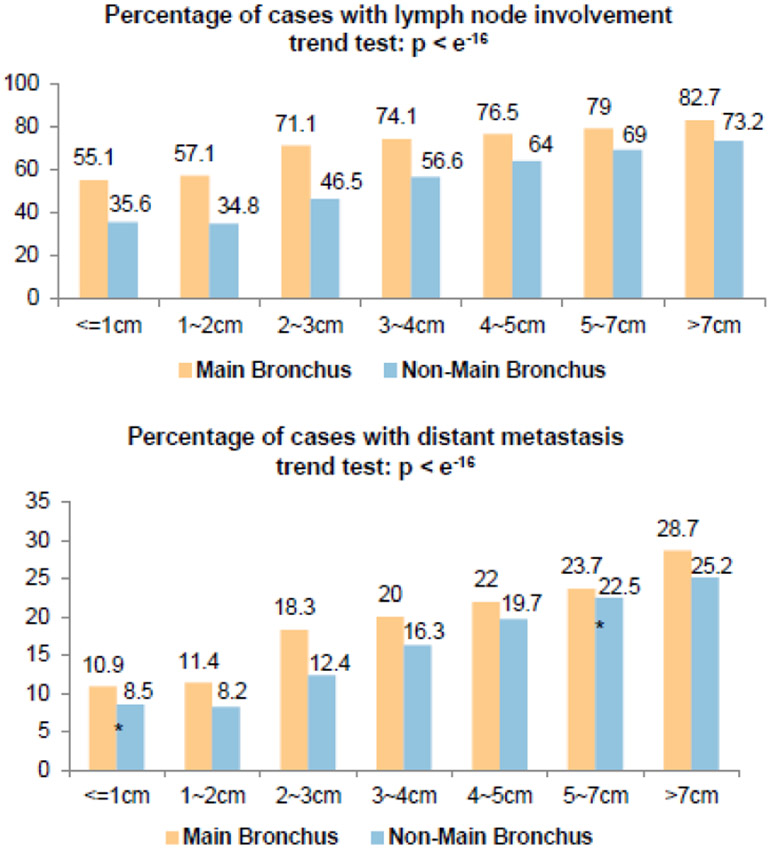
To test the hypothesis that main bronchus ADCs have different clinical characteristics than non-main bronchus ones, even when the tumor sizes are similar, patients were stratified by tumor size (Figure 4, Supplemental Table 3). In almost every patient subgroup with similar tumor size, the ADCs located in the main bronchus showed a significantly higher percentage of lymph node involvement (Figure 4A) and distant metastasis (Figure 4B). Consistent with previous reports15, in both main bronchus and non-main bronchus locations, tumor size was positively correlated with probability of both lymph node involvement and distant metastasis (p value < e−16, trend test, Figure 4).
Figure 4.
Percentage of patients with lymph node involvement and distant metastasis stratified by tumor size and location. Within each subgroup with similar tumor size, except for the ones indicated by *, percentages of both lymph node involvement and distant metastasis are significantly different between main bronchus and lobes; detailed data are listed in Supplementary Table 1.
DISCUSSION
This study investigated the prognostic significance of tumor location, defined as main bronchus or non-main bronchus, for lung ADC. Our results show that main bronchus location is an independent predictor for prognosis, in addition to age, sex, race, surgery, histologic grade, and clinical stage. The ADCs located in the main bronchus show a higher percentage of lymph node involvement and distant metastasis after stratification for tumor size.
Primary tumor location is one of the important factors for determining the optimal treatment and prognosis for patients with a malignant tumor. Lung cancer can be divided into central and peripheral tumors by its location. Several definitions have been used for central tumors according to the CT findings3,4 and/or bronchoscope findings5. However, the international classification of diseases for oncology (ICD-O) has been internationally recognized as the definitive classification of neoplasms, since it was published in 1976, and it consists of two coding systems-the topographical code and the morphological code16. In the current study, we adopted the ICD-O topographical code from NCDB for distinguishing main bronchus location versus non-main bronchus location. As far as we know, our analysis is the first to address adenocarcinoma arising in the main bronchus location. In this study, we found that main bronchus ADCs had a significantly lower resection rate than that of ADCs in other locations after adjusting for clinical variables (Supplemental Table 1, 2) , which likely reflects particular anatomic considerations and a frequent need for technically challenging procedures such as total pneumonectomy or bronchial sleeve resection5,8,17. By contrast, more peripheral ADCs may be adequately treated with lobectomy and, in some cases, possibly sub-lobar resections. Even when considering only those cases that underwent curative resection, main bronchus ADCs had significantly worse outcomes than ADCs in other locations. Similar findings were also observed in advanced stage, non-surgical cases.
The present study adds considerably to earlier work suggesting a prognostic effect of lung tumor location. In an analysis of almost 34,000 patients identified in the National Health Insurance Research Database (NHIRD) in Taiwan between 2002 and 2008, Wang et al18 showed that tumor location is associated with patient survival. However, that earlier report had only 10% the number of patients as the current study, did not include information on histologic subtype, and reported association between tumor location and outcome only in univariate analysis. By contrast, the current study includes multivariate survival analysis, as well as analyses stratified by clinical stage and surgical treatment. These considerations are critical, as the potentially confounding effects of clinical stage and treatment would otherwise hinder the demonstration of an association between tumor main bronchus location and worse survival. Although the percentage of missing information for clinical stage is high in the NCDB database since this data was retrospectively collected, the sample size for analysis after excluding patients with missing clinical stage information is still the largest in this study. Additionally, Wang et al18 found that patients with lower-lobe cancers had lower survival rates than those of the upper-/middle-lobes, while the difference between the upper-, middle-, and lower-lobes is negligible compared to the difference between the three lobes and the main bronchus (Supplemental Figure 1). As a result, we combined the upper-, middle-, and lower-lobes into a single non-main bronchus group.
In addition to different histology subtypes, lower curative-intent resection and higher clinical stages, our results showed that ADCs located in the main bronchus had a significantly higher lymph node involvement and distant metastatic rate than those located in the non-main bronchus locations, even for small size (≤2 cm) ADCs (Figure 4). These results were consistent with those of Sun et al and Decaluwe et al19,20. In comparison with Sun’s study, we confirmed that ADCs located in the main bronchus showed correlation with both lymph node involvement and distant metastasis, supporting our hypothesis that the worse outcomes of main bronchus tumors may reflect early lymphatic and hematogenous spread. Further histologic characterization may also be relevant to our observed survival differences. Moon et al’s study21 of 308 Korean patients with pulmonary ADCs showed that central ADCs rather than peripheral ones had different proportions of ADC subtypes (e.g., acinar, papillary, lepidic, etc.). That study did not show a significant difference in survival between central and peripheral cases, which may be due to the relatively small sample size.
In summary, we found that tumor primary location has an important prognostic impact for lung ADCs, with main bronchus location associated with worse outcomes, more lymph node involvement and more distant metastasis, irrespective of clinical stage and curative-intent surgery. Further investigation into underlying mechanisms, including association with tobacco use, gene profiling, and other molecular factors, is warranted. The prognostic impact of main bronchus location may be relevant to the design of clinical trials, as well as the planning and discussion of standard clinical care.
Supplementary Material
HIGHLIGHTS.
Main bronchus adenocarcinomas have worse prognosis than the non-main bronchus ones.
Main bronchus adenocarcinomas have a higher rate of lymph node involvement.
Main bronchus adenocarcinomas have a higher rate of distant metastasis.
Main bronchus location is an independent predictor of worse outcomes.
ACKNOWLEDGEMENTS
We thank the NCDB project for collecting this invaluable information and making it publically available. The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytical or statistical methodology employed, or the conclusions drawn from these data by the investigator. This work was supported by the National Institutes of Health [5R01CA152301, P50CA70907, 5P30CA142543, 1R01GM115473, K24CA201543 and 1R01CA172211], the Cancer Prevention and Research Institute of Texas [RP120732], and the Fundamental Research Funds for the Central Universities, China (3332015060), the Key Project of International Cooperation of Science and Technology innovation between Governments, the National Key Research and Development Plan of China (No.2016YEE0103400), and the National Natural Science Foundation of China (No.81572288)
Footnotes
DISCLOSURE
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura H, Saji H. Worldwide trend of increasing primary adenocarcinoma of the lung. Surg Today. 2014;44(6):1004–1012. [DOI] [PubMed] [Google Scholar]
- 3.Onn A, Choe DH, Herbst RS, Correa AM, Munden RF, Truong MT, Vaporciyan AA, Isobe T, Gilcrease MZ, Marom EM. Tumor cavitation in stage i non-small cell lung cancer: Epidermal growth factor receptor expression and prediction of poor outcome. Radiology. 2005;237(1):342–347. [DOI] [PubMed] [Google Scholar]
- 4.Ito M, Yamashita Y, Miyata Y, Ohara M, Tsutani Y, Ikeda T, Misumi K, Harada H, Omori K. Prognostic impact of the primary tumor location based on the hilar structures in non-small cell lung cancer with mediastinal lymph node metastasis. Lung Cancer. 2012;76(1):93–97. [DOI] [PubMed] [Google Scholar]
- 5.Ketchedjian A, Daly BD, Fernando HC, Florin L, Hunter CJ, Morelli DM, Shemin RJ. Location as an important predictor of lymph node involvement for pulmonary adenocarcinoma. J Thorac Cardiovasc Surg. 2006;132(3):544–548. [DOI] [PubMed] [Google Scholar]
- 6.Chhajed PN, Somandin S, Baty F, Mehta AJ, Azzola A, Leuppi J, Tamm M, Brutsche MH. Therapeutic bronchoscopy for malignant airway stenoses: Choice of modality and survival. J Cancer Res Ther. 2010;6(2):204–209. [DOI] [PubMed] [Google Scholar]
- 7.Petrella F, Borri A, Casiraghi M, Cavaliere S, Donghi S, Galetta D, Gasparri R, Guarize J, Pardolesi A, Solli P, Tessitore A, Venturino M, Veronesi G, Spaggiari L. Operative rigid bronchoscopy: Indications, basic techniques and results. Multimed Man Cardiothorac Surg. 2014;2014. [DOI] [PubMed] [Google Scholar]
- 8.Shadmehr MB, Farzanegan R, Graili P, Javaherzadeh M, Arab M, Pejhan S, Karam MB, Abbasidezfouli A. Primary major airway tumors; management and results. Eur J Cardiothorac Surg. 2011;39(5):749–754. [DOI] [PubMed] [Google Scholar]
- 9.Bubendorf L, Lantuejoul S, de Langen AJ, Thunnissen E. Nonsmall cell lung carcinoma: Diagnostic difficulties in small biopsies and cytological specimens: Number 2 in the series "pathology for the clinician" edited by peter dorfmuller and alberto cavazza. Eur Respir Rev. 2017;26(144). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomashefski JF Jr., Connors AF Jr., Rosenthal ES, Hsiue IL. Peripheral vs central squamous cell carcinoma of the lung. A comparison of clinical features, histopathology, and survival. Archives of pathology & laboratory medicine. 1990;114(5):468–474. [PubMed] [Google Scholar]
- 11.Kinoshita T, Ohtsuka T, Hato T, Goto T, Kamiyama I, Tajima A, Emoto K, Hayashi Y, Kohno M. Prognostic factors based on clinicopathological data among the patients with resected peripheral squamous cell carcinomas of the lung. J Thorac Oncol. 2014;9(12):1779–1787. [DOI] [PubMed] [Google Scholar]
- 12.Raval MV, Bilimoria KY, Stewart AK, Bentrem DJ, Ko CY. Using the ncdb for cancer care improvement: An introduction to available quality assessment tools. J Surg Oncol. 2009;99(8):488–490. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Wang S, Zhou Y, Lai S, Xiao G, Gazdar A, Xie Y. Evaluation of the 7th and 8th editions of the ajcc/uicc tnm staging systems for lung cancer in a large north american cohort. Oncotarget. 2017;Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V, International Association for the Study of Lung Cancer S, Prognostic Factors Committee AB, Participating I, International Association for the Study of Lung Cancer S, Prognostic Factors Committee Advisory B, Participating I. The iaslc lung cancer staging project: Proposals for revision of the tnm stage groupings in the forthcoming (eighth) edition of the tnm classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. [DOI] [PubMed] [Google Scholar]
- 15.Yang F, Chen H, Xiang J, Zhang Y, Zhou J, Hu H, Zhang J, Luo X. Relationship between tumor size and disease stage in non-small cell lung cancer. BMC Cancer. 2010;10:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Percy C, Holten Vv, Muir CS, Organization WH. International classification of diseases for oncology. 1990. [Google Scholar]
- 17.Bagan P, Berna P, Pereira JC, Le Pimpec Barthes F, Foucault C, Dujon A, Riquet M. Sleeve lobectomy versus pneumonectomy: Tumor characteristics and comparative analysis of feasibility and results. Ann Thorac Surg. 2005;80(6):2046–2050. [DOI] [PubMed] [Google Scholar]
- 18.Wang BY, Huang JY, Cheng CY, Lin CH, Ko J, Liaw YP. Lung cancer and prognosis in taiwan: A population-based cancer registry. J Thorac Oncol. 2013;8(9):1128–1135. [DOI] [PubMed] [Google Scholar]
- 19.Decaluwe H, Stanzi A, Dooms C, Fieuws S, Coosemans W, Depypere L, Deroose CM, Dewever W, Nafteux P, Peeters S, Van Veer H, Verbeken E, Van Raemdonck D, Moons J, De Leyn P, Leuven Lung Cancer G. Central tumour location should be considered when comparing n1 upstaging between thoracoscopic and open surgery for clinical stage i non-small-cell lung cancer. Eur J Cardiothorac Surg. 2016;50(1):110–117. [DOI] [PubMed] [Google Scholar]
- 20.Sun W, Yang X, Liu Y, Yuan Y, Lin D. Primary tumor location is a useful predictor for lymph node metastasis and prognosis in lung adenocarcinoma. Clinical lung cancer. 2017;18(1):e49–e55. [DOI] [PubMed] [Google Scholar]
- 21.Moon Y, Lee KY, Sung SW, Park JK. Differing histopathology and prognosis in pulmonary adenocarcinoma at central and peripheral locations. Journal of thoracic disease. 2016;8(1):169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.