Abstract
Objective:
Little is known about how menopausal hormone treatment (HT) may influence the development of white matter hyperintensities (WMH) in the brain. This study evaluated the associations of changes in levels of pituitary-ovarian hormones during HT and changes in WMH.
Methods:
Women (n = 78 adherent to treatment) enrolled in the Kronos Early Estrogen Prevention Study (KEEPS) underwent brain MRI, and blood collection prior to and following 48 months of randomization to either 0.45 mg/d oral conjugated equine estrogen (o-CEE) daily, 50 µg/d transdermal 17β estradiol (tE2) or placebo pills and patches. Women in the active treatment groups also received oral 200 mg/d micronized progesterone the first 12 d of the month. Estradiol (E2), estrone (E1), follicle stimulating hormone (FSH), and luteinizing hormone (LH) were measured in serum by high sensitive liquid chromatography/mass spectroscopy at baseline and following 48 months of HT. Longitudinal change in WMH volume was determined from FLAIR MRIs using a semi-automated image segmentation algorithm.
Results:
Serum levels of FSH, LH, E1 or E2 did not associate with WMH volume at baseline. After 48 months of treatment, smaller increases in WMH associated with decreases in FSH from baseline in the tE2 group and increases in E1 in both tE2 and oCEE groups. Changes in LH did not associate with changes in WMH in any group.
Conclusions:
Circulating levels of pituitary-ovarian hormones associate with changes in WMH volume in recently menopausal women using HT. Whether these relationships would be influenced by different doses of tE2 or oCEE remains to be determined.
Keywords: Estrogen, follicle stimulating hormone, luteinizing hormone, hormone therapy, menopause, Pituitary-Ovarian, white matter hyperintensity
Introduction
Sex hormones influence structure and function of the brain through both the organizational effects, those that remain once sex hormones are removed, and the activational effects, those that vary with fluxes in levels of hormones.1–4 Age related declines in learning and memory associate with changes in brain structure which may, in part, be related to decreased levels of circulating estrogens during menopause. Therefore, it is important to understand how different formulations of menopausal hormone treatment (HT) impact brain structure following menopause.
For example, ventricular volumes and volumes of white matter hyperintensities (WMH) on brain MRI increased to a greater extent in women using oral conjugated equine estrogen (oCEE) than in those using placebo.5 While the rates of change in ventricular volumes slowed to placebo rates after the HT was withdrawn, the WMH continued to increase 3 years after HT.6 Additionally, women randomized to transdermal 17β estradiol (tE2) had less accumulation of β- amyloid than placebo, especially those who were positive for APOE ε 4, a risk factor for β- amyloid accumulation.7 CEE consists of a complex of hormones, the most prominent of which is estrone sulfate and with oral administration would be further metabolized in the liver, contrary to the tE2 where, E2 would be absorbed directly into the peripheral circulation before being metabolized in the liver. Thus, the resulting circulating concentrations of E1 and E2 differ between the two treatment groups. Both E1 and E2 decrease secretion of follicle stimulating hormone (FSH) and luteinizing hormone (LH) from the pituitary.8 Gonadotropins may be involved in brain function as demonstrated in observational studies.9 Specifically, rising levels of peripheral LH levels associate with cognitive deficits in older men and women, including patients with Alzheimer’s disease.10–13
The relationship of changes in pituitary-ovarian hormone levels during menopausal HT and the change in WMH is unknown. Therefore, this study evaluated the associations of changes in pituitary-ovarian hormones with changes of brain structure in women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS) at Mayo Clinic.
Methods
The Kronos Early Estrogen Prevention Study (KEEPS; NCT00154180) was a randomized, placebo-controlled, double-blind multicenter clinical trial that evaluated the cardiovascular and cognitive effects of o-CEE, tE2, and placebo in women between 42 and 58 years of age who experienced natural menopause.14 Women were within 5–36 months of their last menstrual period and without cognitive impairment. An ancillary study to evaluate the effects of HT on brain structure by MRI was conducted at Mayo Clinic during the four years of KEEPS.5 Women with contraindications for MRI, or those with neurologic disorders, were excluded. The study was approved by the Mayo Clinic institutional review board (no. 224104) and participants provided written informed consent.
MRI was performed before randomization (baseline) and 48 months after randomization to treatments of 0.45 mg/d o-CEE daily, 50 µg/d tE2 weekly or placebo pills and patches. Women in the active treatment groups also received oral 200 mg/d micronized progesterone for the first 12 d of each month. All participants underwent genotyping for the APOE ε4 allele, presence of which is associated with increased risk of Alzheimer’s disease.
Brain MRI studies were performed on a single 1.5-tesla system, with an 8-channel phased array coil (GE Healthcare A T2-weighted fluid-attenuated inversion recovery (FLAIR) and a T1-weighted 3D high resolution magnetization-prepared rapid acquisition gradient-echo (MPRAGE) sequence were included in the standardized protocol for anatomic segmentation and labeling of WMH. WM was segmented using a semi-automated segmentation algorithm on FLAIR-MRI by a single image analyst, who was blinded to the treatment status.15 The total change in volume was then calculated for the WMH lesion volume during the 48 months of HT.
E1 (pg/mL), E2 (pg/mL), LH (IU/L), and FSH (IU/L) were measured by high sensitive liquid chromatography/mass spectroscopy from a single fasting serum samples collected at the same time of day at baseline prior to and 48 months after randomization. Hormone levels were measured at the clinical core laboratory at Mayo Clinic, Rochester, MN. Detailed methodology along with the intra-assay and inter-assay coefficients of variability (C.V.’s) for E2, estrone, LH, and FSH, have been reported previously and are included in Table 1.8 In brief, total 17 β-estradiol and estrone were extracted with methylene chloride and after derivatization with dansyl chloride, high-pressure liquid chromatography (HPLC) was used prior to introduction of the derivatized sample extract into the tandem mass spectrometry (LC-MS/MS) (Agilent Technologies, Santa Clara, CA 95051). FSH and LH were measured by respective, specific two-site immunoenzymatic assays performed on a DxI 800 automated immunoassay system (Beckman Instruments, Chaska, MN 55318).
Table 1:
Hormone intra and inter-assay Coefficients of Variability
| Hormone | Intra-assay C.V. | Inter-assay C.V. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17β estradiol (pg/mL) | 0.23 | 0.50 | 0.74 | 35 | 151 | 405 | 0.29 | 0.50 | 0.77 | 32 | 140 | 382 |
| Variation (%) | 11.8 | 7.3 | 6.0 | 1.6 | 1.5 | 1.4 | 10.8 | 8.5 | 6.9 | 5.1 | 4.6 | 4.8 |
| Estrone (pg.mL) | 0.30 | 0.50 | 0.84 | 32 | 142 | 389 | 0.25 | 0.51 | 0.85 | 30 | 131 | 355 |
| Variation (%) | 17.8 | 7.5 | 6.1 | 2.5 | 1.7 | 1.2 | 12.0 | 9.5 | 7.9 | 7.4 | 7.1 | 6.6 |
| Follicle Stimulating Hormone (mIU/mL) | 8.6 | 47.1 | 6.5 | 16.7 | 58.0 | |||||||
| Variation (%) | 32. | 2.8 | 3.6 | 3.2 | 4.7 | |||||||
| Luteinizing Hormone (mIU/mL) | 1.2 | 38.5 | 1.4 | 15.6 | 48.8 | |||||||
| Variation (%) | 4.3 | 4.0 | 9.3 | 6.0 | 6.0 | |||||||
| Testosterone, high sensitivity (ng/dL) | 0.65 | 4.3 | 48 | 118 | 832 | 0.69 | 4.3 | 45 | 117 | 841 | ||
| Variation (%) | 7.4 | 6.1 | 9.0 | 2.3 | 0.9 | 8.9 | 6.9 | 4.0 | 3.6 | 3.5 | ||
Reused with permission from: Kling JM, Dowling NM, Bimonte-Nelson HA, et al. Impact of Menopausal Hormone Formulations on Pituitary-Ovarian Regulatory Feedback. Am J Physiol Regul Integr Comp Physiol. 2019;doi: 10.1152/ajpregu.00234.2019
C.V. = coefficient of variability
Statistical analysis
WMH volumes at baseline, changes in WMH volumes, baseline hormone values, and changes in hormone values were not transformed since they were approximately normally distributed in this particular sample. Analyses including WMH or change in WMH also included an adjustment for the log of total intracranial volume (TIV). We performed sensitivity analyses without adjustment for comparison. The baseline participant characteristics were described using means and standard deviations for continuous variables, or counts and percentages for categorical variables. The oCEE, tE2 and placebo groups were first compared using analysis of variance (ANOVA) or chi-square tests. Where the omnibus ANOVA test was significant, Tukey’s HSD (honestly significant difference) test was used to assess pairwise comparisons of the groups. We reported the comparisons of each treatment group with placebo. Changes in WMH volumes and hormone levels were described and tested in the same way, and then summarized using Pearson partial correlations (adjusted for log TIV) and Pearson correlations (without adjustment for log TIV).
To analyze the effects of APOE ε4 status and smoking on the associations of WMH with hormones, linear regression models were fitted with WMH as a response and one of the hormones, treatment group, smoking status, log (TIV), and APOE ε4 status as explanatory variables. First, full models were fit with a treatment group by APOE ε4 status interaction term in each model. The treatment x APOE ε4 status interactions were not statistically significant, and models without the interaction terms were then fit. Standard diagnostic tests indicated that the assumptions for all of the models in this study were met reasonably well.
Results
One hundred and eighteen women enrolled in KEEPS at Mayo Clinic were invited to participate in the ancillary KEEPS-MRI study. Of these, twelve declined participation and an additional five were excluded because of MRI contraindications or neurologic disorders. There were a total of 101 who underwent MRI at baseline; 78 participants who were compliant to treatment underwent MRI at month 48 and had hormone levels measured at both time points. At baseline, clinical characteristics including hormone levels, cardiovascular risk factors, and global cognitive function were similar across treatment groups (Table 2). There was a higher percentage of APOE ε4 carriers in the tE2 group compared to placebo (46%, p=0.01) (Table 3 and 4). The oCEE group had a higher WMH volume at baseline compared to placebo (p=0.002). Sensitivity analyses did not uncover an effect of baseline WMH differences on any of the associations.
Table 2.
Characteristics of the participants at baseline
| oCEE (n = 23) |
tE2 (n = 24) |
Placebo (n = 31) |
P-value | Effect size η2 | |
|---|---|---|---|---|---|
| Age at baseline, year | 53 (2) | 53 (2) | 53 (2) | 0.51 | 0.02 |
| Education | 0.83 | ||||
| High school or less | 2 (9%) | 1 (5%) | 2 (6%) | ||
| Some college / College graduate | 16 (73%) | 15 (68%) | 19 (61%) | ||
| Some graduate / Graduate | 4 (18%) | 6 (27%) | 10 (32%) | ||
| Smoking status | 0.26 | ||||
| Nonsmoker | 16 (73%) | 13 (57%) | 24 (77%) | ||
| Smoker (past or current) | 6 (27%) | 10 (43%) | 7 (23%) | ||
| Time past menopause, months | 21 (11) | 19 (8) | 16 (9) | 0.19 | 0.04 |
| Treatment onset past baseline MRI, d | 14 (30) | 21 (31) | 30 (68) | 0.47 | 0.02 |
| APOE ε4 carrier | 2 (9%) | 11 (46%) | 7 (23%) | 0.01 | |
| Migraines | 2 (9%) | 0 (0%) | 4 (13%) | 0.24 | |
| Global cognitive function (z-scores) | −0.09 (0.79) | 0.03 (0.75) | 0.21 (0.65) | 0.33 | 0.03 |
| Mean systolic blood pressure, mm Hg | 125 (11) | 120 (17) | 121 (11) | 0.36 | 0.03 |
| Mean diastolic blood pressure, mm Hg | 78 (6) | 74 (9) | 75 (7) | 0.24 | 0.04 |
| Waist circumference, cm | 83 (21) | 82 (12) | 85 (11) | 0.79 | 0.01 |
| Body mass index, kg/m2 | 29 (4) | 26 (4) | 27 (4) | 0.06 | 0.07 |
| Coronary arterial calcification present (Agatston score) | 4 (17%) | 2 (8%) | 3 (10%) | 0.61 | |
| Carotid intima-media thickness (mm) | 0.73 (0.11) | 0.71 (0.09) | 0.71 (0.08) | 0.72 | 0.01 |
| Low-density lipoprotein, mg/dL | 123 (24) | 122 (33) | 115 (28) | 0.53 | 0.02 |
| High-density lipoprotein, mg/dL | 69 (10) | 72 (10) | 72 (13) | 0.74 | 0.01 |
| Triglycerides, mg/dL | 84 (49) | 88 (37) | 78 (44) | 0.68 | 0.01 |
| White matter hyperintensity volume, cm3 | 2.77 (1.31)a | 2.49 (1.60)b | 1.52 (0.71) | 0.002* | 0.05* |
| FSH (IU/L) | 96 (32) | 98 (44) | 78 (34) | 0.08 | 0.06 |
| LH (IU/L) | 43 (14) | 43 (18) | 36 (15) | 0.12 | 0.002 |
| E1 (pg/mL) | 31 (15) | 30 (10) | 32 (21) | 0.93 | 0.01 |
| E2 (pg/mL) | 10 (12) | 13 (15) | 17 (34) | 0.58 | 0.05 |
models adjusted for log-transformed TIV
Abbreviation; oCEE = oral conjugated equine estrogens. FSH = follicle stimulating hormone, LH = luteinizing hormone, E1 = estrone, E2 = estradiol
Data are n (%) or mean (SD)
Pairwise comparison to placebo < 0.01
Pairwise comparison to placebo < 0.05
Table 3.
Linear regression analysis between total change in WMH volume (cm3) and hormones, model was fit with interaction between treatment group and APOE status.
| FSH | LH | E1 | E2 | |||||
|---|---|---|---|---|---|---|---|---|
| Est (95% CI) | P | Est (95% CI) | P | Est (95% CI) | P | Est (95% CI) | P | |
| Intercept | −8.0 (−20.8, 4.82) | 0.22 | −9.08 (−22.6, 4.43) | 0.19 | −9.75 (−22.4, 2.93) | 0.13 | −9.20 (−22.3, 3.85) | 0.16 |
| Hormone | 0.006 (0.002, 0.01) | 0.007 | 0.006 (−0.005, 0.02) | 0.27 | −0.005 (−0.008, −0.001) | 0.008 | −0.004 (−0.008, −0.000) | 0.05 |
| o-CEE | 0.41 (0.05, 0.78) | 0.03 | 0.24 (−0.12, 0.59) | 0.19 | 0.53 (0.12, 0.93) | 0.01 | 0.28 (−0.07, 0.63) | 0.12 |
| tE2 | 0.26 (−0.16, 0.68) | 0.21 | 0.11 (−0.31, 0.53) | 0.61 | 0.16 (−0.24, 0.56) | 0.42 | 0.20 (−0.23, 0.62) | 0.36 |
| APOE | −0.27 (−0.74, 0.20) | 0.25 | −0.20 (−0.69, 0.29) | 0.42 | −0.22 (−0.69, 0.25) | 0.35 | −0.23 (−0.71, 0.26) | 0.35 |
| Smoker | 0.21 (−0.07, 0.50) | 0.14 | 0.25 (−0.05, 0.54) | 0.10 | 0.23 (−0.06, 0.51) | 0.12 | 0.24 (−0.05, 0.54) | 0.10 |
| TIV | 1.12 (−0.65, 2.88) | 0.21 | 1.28 (−0.59, 3.14) | 0.18 | 1.36 (−0.38, 3.11) | 0.12 | 1.29 (−0.51, 3.08) | 0.16 |
| o-CEE x APOE | 0.87 (−0.06, 1.80) | 0.07 | 0.81 (−0.17, 1.78) | 0.10 | 0.87 (−0.06, 1.80) | 0.07 | 0.82 (−0.13, 1.78) | 0.09 |
| tE2 x APOE | 0.75 (0.07, 1.43) | 0.03 | 0.62 (−0.08, 1.33) | 0.08 | 0.62 (−0.05, 1.30) | 0.07 | 0.68 (−0.01, 1.38) | 0.05 |
Abbreviations; oCEE = oral conjugated equine estrogens, tE2 = transdermal estradiol, FSH = follicle stimulating hormone, LH = luteinizing hormone, E1 = estrone, E2 = estradiol, TIV = total intracranial volume
Table 4.
Linear regression analysis between total change in WMH volume (cm3) and hormones, model was fit without interaction between treatment group and APOE status.
| FSH | LH | E1 | E2 | |||||
|---|---|---|---|---|---|---|---|---|
| Est (95% CI) | P | Est (95% CI) | P | Est (95% CI) | P | Est (95% CI) | P | |
| Intercept | −5.22 (−18.2, 7.72) | 0.42 | −6.66 (−20.2, 6.91) | 0.33 | −7.03 (−19.7, 5.66) | 0.27 | −6.50 (−19.6, 6.60) | 0.33 |
| Hormone | 0.005 (0.001, 0.01) | 0.02 | 0.005 (−0.006, 0.02) | 0.37 | −0.004 (−0.008, −0.001) | 0.01 | −0.004 (−0.008, 0.001) | 0.09 |
| o-CEE | 0.55 (0.19, 0.90) | 0.003 | 0.38 (0.04, 0.71) | 0.03 | 0.66 (0.26, 1.06) | 0.001 | 0.42 (0.09, 0.76) | 0.01 |
| tE2 | 0.48 (0.11, 0.85) | 0.01 | 0.31 (−0.04, 0.65) | 0.08 | 0.36 (0.03, 0.69) | 0.03 | 0.40 (0.04, 0.77) | 0.03 |
| APOE | 0.17 (−0.14, 0.48) | 0.27 | 0.18 (−0.14, 0.51) | 0.27 | 0.17 (−0.13, 0.48) | 0.26 | 0.18 (−0.13, 0.50) | 0.25 |
| Smoker | 0.17 (−0.11, 0.46) | 0.23 | 0.21 (−0.08, 0.50) | 0.15 | 0.20 (−0.08, 0.48) | 0.17 | 0.21 (−0.08, 0.49) | 0.16 |
| TIV | 0.73 (−1.06, 2.51) | 0.42 | 0.93 (−0.94, 2.80) | 0.32 | 0.98 (−0.77, 2.72) | 0.27 | 0.90 (−0.90, 2.70) | 0.32 |
Abbreviations; oCEE = oral conjugated equine estrogens, tE2 = transdermal estradiol, FSH = follicle stimulating hormone, LH = luteinizing hormone, E1 = estrone, E2 = estradiol, TIV = total intracranial volume
At baseline, there were no statistically significant associations between FSH (p=0.81), LH (0.51), E1 (p=0.93) or E2 (p=0.79) levels and WMH volume. Significant changes in hormone levels were observed in the oCEE and tE2 groups over the 48 months of the study, but not in the placebo group (Table 5, Figure 1); statistically significant changes in total WMH volume were observed in all groups. During the four years of HT, a greater decrease in FSH in the tE2 group associated with a smaller increase in WMH volume (Table 6, Figure 2). In the tE2 and oCEE groups, a greater increase in E1 associated with a smaller increase in WMH volume (Table 6, Figure 2). These relationships remained the same when outliers in the tE2 group were excluded. There were no significant associations between changes in LH or E2 and changes in WMH. Testosterone and androstenedione were also measured in KEEPS. Values of these hormones did not associate with WHM volume and are not further described.
Table 5.
Summary of total change in WMH volume and hormones levels adjusted for TIV
| Total change | oCEE | tE2 | Placebo | Overall | Pairwise P-value |
|
|---|---|---|---|---|---|---|
| (n = 23) | (n = 24) | (n = 31) | P-value | oCEE / Placebo | tE2 / Placebo | |
| WMH (cm3) | 0.54 (0.60) | 0.52 (0.77) | 0.18 (0.28) | 0.06* | 0.12* | 0.11* |
| FSH (IU/L) | −23 (21) | −33 (38) | 7 (26) | <0.001 | 0.001 | <0.001 |
| LH (IU/L) | −6 (14) | −13 (16) | −3 (10) | 0.04 | 0.81 | 0.04 |
| E1 (pg/mL) | 65 (60) | 19 (22) | −4 (22) | <0.001 | <0.001 | 0.07 |
| E2 (pg/mL) | 5 (15) | 29 (39) | −10 (34) | <0.001 | 0.22 | <0.001 |
Data are mean (SD)
Abbreviations; oCEE = oral conjugated equine estrogens, tE2 = transdermal estradiol, FSH = follicle stimulating hormone, LH = luteinizing hormone, E1 = estrone, E2 = estradiol, TIV = total intracranial volume, WMH = white matter hyperintensity
models adjusted for log-transformed TIV
FIG. 1.
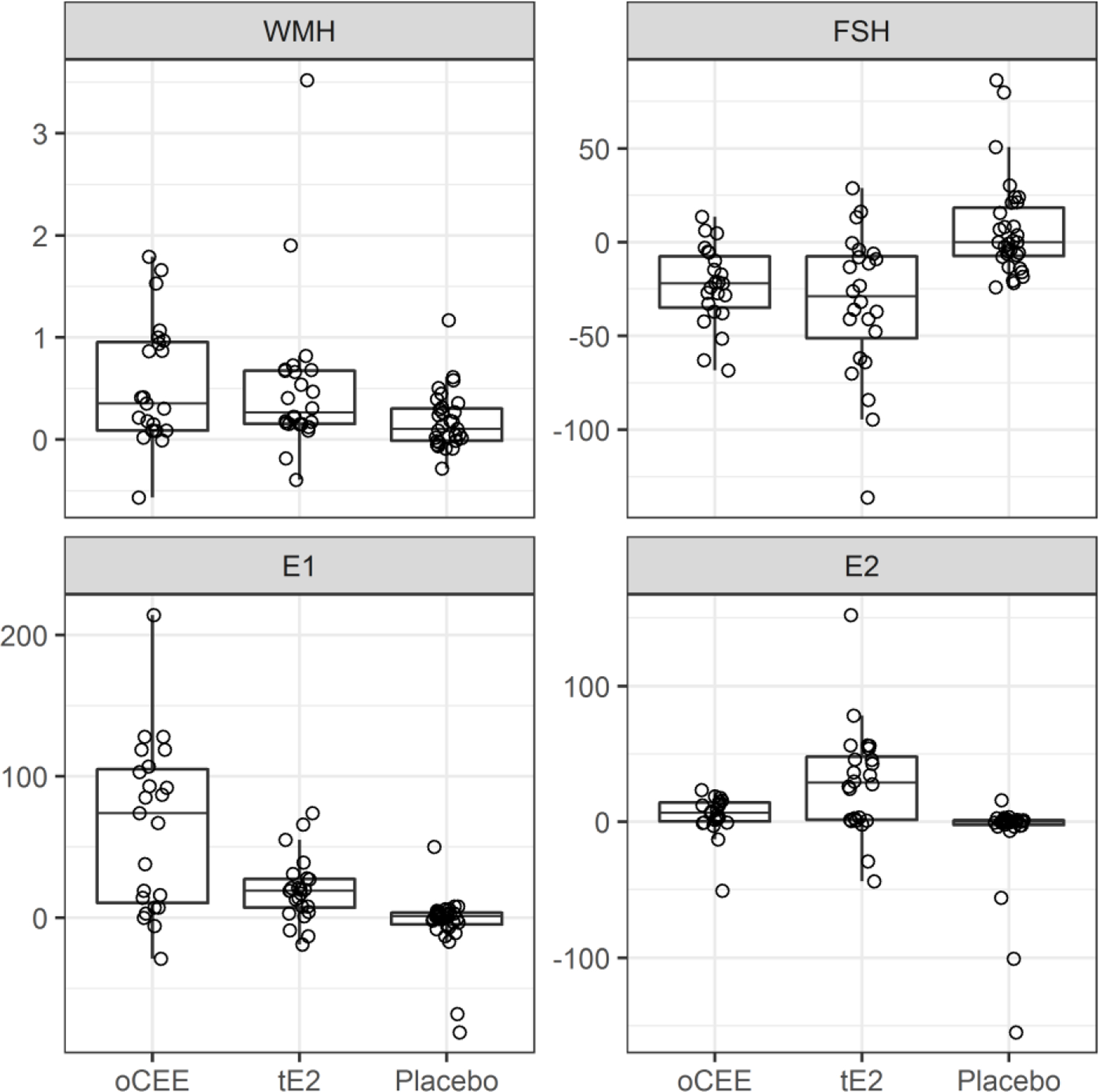
Boxplots of total change in WMH volume and total changes in hormones levels from baseline to month 48. The horizontal lines of the box from bottom to top show the 25th, 50th, and 75th percentiles of the data. The height of the box, 75th to 25th percentile, is the interquartile range (IQR). Whiskers extend 1.5*IQR from the box. Points farther out are considered outliers and drawn individually. E1, estrone; E2, estradiol, FSH, follicle-stimulating hormone; oCEE, oral conjugated equine estrogen; tE2, transdermal estradiol; WMH, white matter hyperintensity.
Table 6.
Pearson’s partial correlations (p-values) between total change in WMH volume and total changes in hormones levels from baseline to month 48 for each treatment group. Models adjusted for the TIV.
| oCEE (n = 23) |
tE2 (n = 24) |
Placebo (n = 31) |
|
|---|---|---|---|
| FSH | 0.24 (0.28) | 0.46 (0.03) | −0.18 (0.34) |
| LH | 0.11 (0.62) | 0.10 (0.64) | −0.09 (0.64) |
| E1 | −0.43 (0.04) | −0.41 (0.054) | 0.21 (0.27) |
| E2 | −0.18 (0.43) | −0.38 (0.08) | 0.14 (0.46) |
Abbreviations; oCEE = oral conjugated equine estrogens, tE2 = transdermal estradiol, FSH = follicle stimulating hormone, LH = luteinizing hormone, E1 = estrone, E2 = estradiol, TIV = total intracranial volume
FIG. 2.
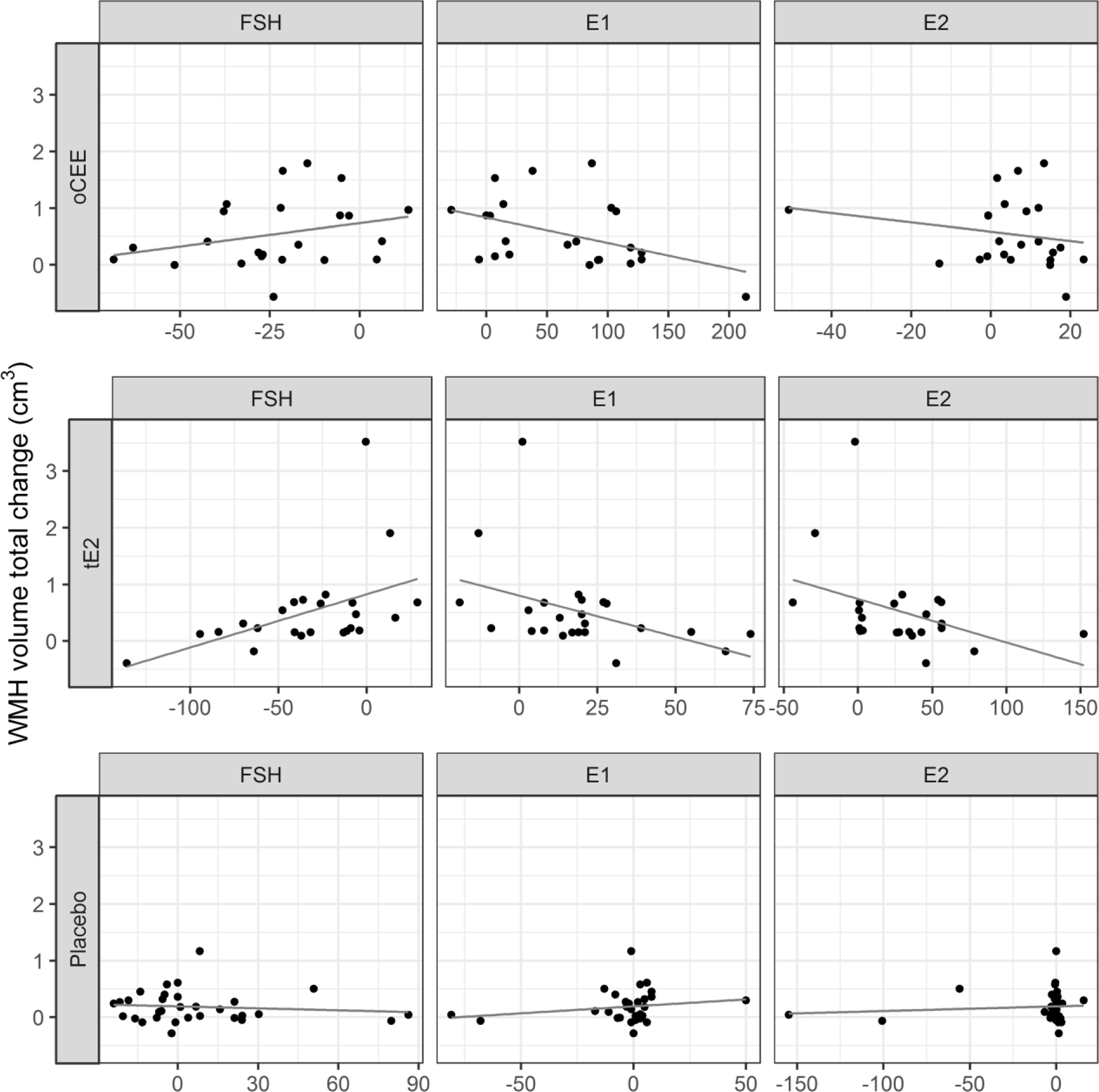
Scatterplots between total change in WMH volume and total changes in hormones levels by treatment group. The line represents the predicted linear relationship between the change in hormone and change in WMH. Each point represents a participant. E1, estrone; E2, estradiol, FSH, follicle-stimulating hormone; oCEE, oral conjugated equine estrogen; tE2, transdermal estradiol; WMH, white matter hyperintensity
Neither APOE ε4 status nor smoking modified the association between changes in WMH and changes in any of the hormone levels.
Discussion
In recently menopausal women participating in KEEPS who were randomized to one of two common formulations of menopausal HT, greater decreases in FSH associated with smaller increases in WMH volume over time only in the tE2 group. In other words, the type of HT appears to influence circulating hormones differently8 and a relationship was found between their changing levels and specific brain structure changes over time. These results may be related to the stronger negative feedback mechanisms for E2 and FSH with tE2 compared to those involved with E1. Furthermore, conversion of estrone sulfate to E2 and feedback regulation of FSH may be less robust with oCEE compared to tE2. 8,16 These different relationships among the hormones and WMH may help explain why not all HT formulations have the same effect on neuropsychologically implicated menopausal symptoms such as mood and anxiety.17 In addition, not all HT formulations may influence hypertension and small vessel ischemic disease, as these conditions associate with the presence of WMH.18 Previously reported in KEEPS, WMH volume increased for all groups in KEEPS over time, although the rate of increase in WMH volume was only found to be statistically significantly greater in the oCEE group compared to placebo.6 In the present study, greater increases in E1 associated with smaller increases in WMH volume in both the tE2 and oCEE groups suggesting an inhibitory effect of E1 on mechanisms associated with development of WMH.
The interrelationships of pituitary and ovarian hormones are expected to differ based on whether HT is used, and if used, the formulation and route of administration. The current study found no association between changes in FSH and WMH volume in the placebo or oCEE group. The findings in the tE2 group support the concept that the changes in WMH may be related to pituitary-ovarian hormones. The results also are consistent with previous KEEPS MRI findings that indicate tE2 has a greater effect than oCEE on preserving the dorsolateral prefrontal cortex compared to placebo.6 Although the trends are the same, the range of values for E1 in the tE2 group and E2 in the oCEE are narrow, thus precluding evaluations of dose effects. Estrogen receptors have greater affinity for E2 than E1 and E1 may be converted to E2 all of which may affect an individual response to the hormone. Thus, evaluating other doses or ranges of HT treatments should be explored.
Previous studies show that differences in levels of FSH and LH in postmenopausal women associate with various clinical outcomes. For example, for postmenopausal women not using HT, low FSH levels associated with an increased risk of prediabetes and diabetes,19 as well as increased cardiovascular risk,20 although the associations were partially explained by obesity or adiposity. Higher levels of peripheral LH correlated with cognitive deficits in aging women.9 These studies examined individual levels of gonadotropin hormone, when indeed, it is more likely that pituitary ovarian hormone interactions may influence clinical outcomes. An interaction of the gonadotropin and ovarian hormones likely exerts effects through feedback loops, which may differ by tissue and anatomical location, in part, related to type and number of estrogen receptors.
In KEEPS, women randomized to transdermal E2 and who were APOE ε4 carriers had less accumulation of β-amyloid (a putative biomarker of Alzheimer disease pathology) than those on placebo, and o-CEE.6,7 Although the mechanism of amyloid-beta protein formation are complex and likely explained by multiple factors, results of the current study related to FSH levels and WMH volumes are intriguing and may provide future area for study as a possible or partial explanation for amyloid-beta deposition. Previous studies of postmenopausal women not on HT found that elevated gonadotropin levels were significantly higher in women with Alzheimer’s disease compared to cognitively normal controls.21 Whether or not treatment with HT, through its influence on gonadotropins, may decrease formation of amyloid-beta plaques remains to be determined.
Limitations
Women in KEEPS experienced natural menopause, therefore, the relationships noted in the present study may not apply to women who underwent oophorectomy or hysterectomy after or before the natural age of menopause. Other hormones, such as progesterone, testosterone, thyroid hormones, sex hormone binding globulin, and corticosteroids, were not included in this analysis. These hormones may affect the interpretability of the observed associations and menopausal state of the gonadotropic-pituitary axis. Furthermore, serum hormone levels reflect the total hormone level and not the free or bioavailable state since sex hormone binding globulin was not included in the analysis. Finally, our findings are based on observation of associations; thus, the results are hypothesis generating and may set the stage for future studies which could lead to enhanced understanding of hormonal interactions in women, with and without exogenous hormone treatment, as they age.
Conclusion
Changes in circulating levels of pituitary-ovarian hormones during menopausal HT associate with changes in WMH volume in recently menopausal women. The relationships seen may help explain why different HT formulations lead to different structural brain changes in menopausal women and differentially affect mood, anxiety, risk for hypertension, and small vessel ischemic disease. Whether these relationships would be influenced by different doses of either tE2 or oCEE remains to be determined and evaluating other doses of HT should be explored. Additionally, long term follow up in a larger cohort of women measuring all pituitary ovarian hormones would further clarify these relationships.
Supplementary Material
Table 4a.
P-values comparing models with and without interaction between treatment group and APOE status (tables 2 and 3)
| Hormone | P-value |
|---|---|
| FSH | 0.054 |
| LH | 0.12 |
| E1 | 0.09 |
| E2 | 0.09 |
Abbreviations; FSH = follicle stimulating hormone, LH = luteinizing hormone, E1 = estrone, E2 = estradiol
Acknowledgments:
All serum assays were performed at the Immunochemical Core Lab (ICL) at Mayo Clinic Rochester. We gratefully acknowledge the dedicated efforts of all the investigators and staff at the KEEPS clinical centers, the KEEPS Data Coordinating Center at KLRI, and the NIH Institutes supporting ancillary studies. Above all, we recognize and thank the KEEPS participants for their dedication and commitment to the KEEPS research program.
Sources of funding: Supported in part by a Mayo Clinic Mentored Research Award. Grant NIA RF1 AG057547 to KK, and VMM; P50 AG44170 to VMM. KEEPS was funded by grants from the Aurora Foundation to the Kronos Longevity Research Institute, from the National Institutes of Health (NIH) HL90639 to VMM, Mayo Clinic CTSA 1 UL1 RR024150, from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. The manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official view of NCATS or NIH. Study medications were supplied in part by Bayer Health Care and by Abbott Pharmaceuticals. Funding for FSH:LH assays was made available through support offered by the Department of Obstetrics, Gynecology & Reproductive Sciences at Yale and the Benneck-Polan Family Foundation.
Role of the Sponsors: The sponsors had no input into the design or conduct of the study or the writing, review or approval of this manuscript.
Footnotes
ClinicalTrials.gov number is NCT00154180.
IRB numbers for KEEPS institutions: The Mayo Clinic KEEPS IRB: 2241-04
Financial disclosures/conflicts of interest: Kejal Kantarci serves on the data safety monitoring board for Takeda Global Research and Development Center, Inc.; receives research support from Avid Radiopharmaceuticals and Eli Lilly, and receives funding from NIH and Alzheimer’s Drug Discovery Foundation. Other authors have nothing to disclosure.
References
- 1.Sherwin BB Estrogen and Cognitive Functioning in Women. Endocrine Reviews. 2003. 24(2): p. 133–151. [DOI] [PubMed] [Google Scholar]
- 2.Luine VN. Estradiol and cognitive function: Past, present and future. Hormones and Behavior. 2014;66:602–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn. Mem.2009;16248–266 [DOI] [PubMed] [Google Scholar]
- 4.Koebele & Bimonte-Nelson HA. Trajectories and phenotypes with estrogen exposures across the lifespan: What does Goldilocks have to do with it? Horm Behav. 2015. August;74:86–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantarci K, Tosakulwon N, Lesnick TG, et al. Effects of hormone therapy on brain structure: A randomized trial. Neurology. 2016;87:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kantarci K, Tosakulwong N, Lesnick TG, et al. Brain structure and cognition 3years after the end of an early menopausal hormone therapy trial. Neurology. 2018;90(16):e1404–e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantarci K, Lowe VJ, Lesnick TG, et al. Early Postmenopausal Transdermal 17β-Estradiol Therapy and Amyloid-β Deposition. Journal of Alzheimer’s disease. 2016;53:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kling JM, Dowling NM, Bimonte-Nelson HA, et al. Impact of Menopausal Hormone Formulations on Pituitary-Ovarian Regulatory Feedback. Am J Physiol Regul Integr Comp Physiol. 2019; 10.1152/ajpregu.00234.2019 [DOI] [PMC free article] [PubMed]
- 9.Bhatta S, Blair JA, Casadesus G. Luteinizing Hormone Involvement in Aging Female Cognition: Not All Is Estrogen Loss. Front Endocrinol Lausanne 9:544, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyde Z, Flicker L, Almeida OP, McCaul KA, et al. Higher luteinizing hormone is associated with poor memory recall: the health in men study. J Alzheimers Dis. (2010) 19:943–51. 10.3233/JAD-2010-1342 [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues MA, Verdile G, Foster JK, et al. Gonadotropins and cognition in older women. J Alzheimers Dis. (2008) 13:267–74. 10.3233/JAD-2008-13304 [DOI] [PubMed] [Google Scholar]
- 12.Short RA, Bowen RL, O’Brien PC, Graff-Radford NR. Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin Proc. (2001) 76:906–9. 10.4065/76.9.906 [DOI] [PubMed] [Google Scholar]
- 13.Verdile G, Yeap BB, Clarnette RM, et al. Luteinizing hormone levels are positively correlated with plasma amyloid-beta protein levels in elderly men. J Alzheimers Dis. (2008) 14:201–8. 10.3233/JAD-2008-14208 [DOI] [PubMed] [Google Scholar]
- 14.Harman SM, Brinton EG, Cedars M, et al. KEEPS: the Kronos Early Estrogen Prevention Study. Climacteric. 2005;8:3–12. [DOI] [PubMed] [Google Scholar]
- 15.Raz L, Jayachandran M, Tosakulwong N, et al. Thrombogenic microvesicles and white matter hyperintensities in postmenopausal women. Neurology. 2013;80:911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connell MB. Pharmacokinetic and pharmacologic variation between different estrogen products. J Clin Pharmacol September;35(9 Suppl):18S–24S, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Gleason CE, Dowling NM, Wharton W et al. Effects of Hormone Therapy on Cognition and Mood in Recently Postmenopausal Women: Findings from the Randomized, Controlled KEEPS-Cognitive and Affective Study. PLOS Medicine. 2015. 12(6):e1001833. 10.1371/journal.pmed.1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes JN 1, Harvey RE, Zuk SM, et al. Aortic hemodynamics and white matter hyperintensities in normotensive postmenopausal women. J Neurol. 2017. May;264(5):938–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang N, Kuang L, Han B, et al. Follicle-Stimulating hormone associates with prediabetes and diabetes in postmenopausal women. Acta Diabetol. 2016;53(2):227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang N, Shao H, Chen Y, et al. Follicle-Stimulating Hormone, Its Association with Cardiometabolic Risk Factors, and 10-Year Risk of Cardiovascular Disease in Postmenopausal Women. J Am Heart Assoc. 2017;6(9):e005918, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Short RA, Bowen RL, O’Brien PC, Graff-Radford NR. Elevated gonadotropin levels in patients with Alzheimers disease. Mayo Clin Proc. 2001;76(9):906–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


