Abstract
Background
Many patients with coronavirus disease 2019 (COVID-19) complain from olfactory dysfunction (OD).
Aims/objectives: To evaluate the prevalence, prognosis, and recovery from OD in COVID-19 patients.
Material and methods
In this study, patients with COVID-19 symptoms who were referred to six different tertiary referral centres were recruited after positive results for COVID-19. All patients were assessed for a one-month follow-up after the initial diagnosis of COVID-19.
Results
Three hundred and eleven patients with COVID-19 were recruited in the present study. Two hundred and seven patients (66.6%) had a recent history of OD. One hundred and seventy-eight patients had experienced OD as a primary symptom intercurrent to other COVID-19 symptoms or solely. Sixty-nine patients had OD at the time of presentation to referral centres. Headache and nasal obstruction had significant relationships with recovery from OD in this subgroup, and the platelet count was the most important predictor for the recovery from OD.
One hundred seventy-nine (86.4%) patients were nearly or fully recovered from OD approximately a month after the onset of OD.
Conclusion
Headache, nasal obstruction, and platelet count may have specific roles as prognostic factors in the recovery from OD.
Keywords: Anosmia coronavirus disease 2019 (COVID-19), hyposmia olfactory dysfunction (OD)
Chinese abstract
背景:许多患有冠状毒病2019(COVID-19)的患者抱怨嗅觉功能障碍(OD)。
目的:评价COVID-19患者的OD患病率、预后和恢复。
材料和方法:在这项研究中, 招募了被转诊到六个不同的三级转诊中心的具COVID-19症状后确诊转阳的患者。在初次诊断COVID-19之后, 对所有患者进行了为期一个月的随访评估。
结果:本研究共招募了311名COVID-19患者。有近期OD史的患者共207名(66.6%)。 178名患者经历了OD, 这是与其它COVID-19症状并发或仅发生的主要症状。有69名患者在转诊中心就诊时患有OD。在该亚组中, 头痛和鼻塞与OD的恢复有显著关系, 而血小板计数是OD恢复的最重要的预测指标。OD发作约一个月后, 有179例(86.4%)患者几乎或完全从OD恢复。
结论:头痛、鼻塞和血小板计数可能是OD恢复的预后因素。
Introduction
Coronavirus disease of 2019 (COVID-19) is currently one of the main concerns of every community. Based on previous findings in the literature, that may manifest in fever, cough, sore throat, breathlessness, fatigue, myalgia, arthralgia, diarrhoea, and chest pain [1]. However, in several studies, the latest discoveries have shown decreased smell and taste function as symptoms that are reported by many patients with COVID-19 [1].
Olfactory dysfunction (OD) following upper respiratory tract infections (URTIs) was not a new topic for many Otorhinolaryngologists. Additionally, Coronavirus was initially presented with lower respiratory tract infection. Nevertheless, this presentation and OD by this new virus were notably unknown for many physicians in the early phases of this pandemic wave. However, OD associated with COVID-19 seems to be a unique presentation [2]. There were also few patients with OD in the absence of other symptoms such as fever, cough, or other systemic complaints [3].
Therefore, based on the above-mentioned points, the researchers of the current study aimed to evaluate OD in patients with COVID-19.
Materials and methods
This multicentric cross-sectional study was approved on 18 March 2020 by the local ethics committee with a reference number. All participants with COVID-19 symptoms who were referred to the department of respiratory emergenciesof six different tertiary referral centres of five different cities after evaluation by polymerase chain reaction (PCR) and computed tomography (CT) of the chest were recruited. All aspects of the study were made clear, and all participants agreed and signed the informed consent form after adequate explanations. They were invited to take part in the present study from March 2020 to May 2020. Demographic profile, past medical history (PMH) (including major illnesses or any previous surgery), and history of smoking or addiction were documented. Inclusion criteria for COVID-19 were the presence or a recent history of common symptoms such as fever, cough, dyspnoea, sputum, myalgia, arthralgia, headache, diarrhoea, rhinorrhoea, sore throat, abdominal pain, pharyngeal discomfort, chest pain, decreased smell or taste functions, and polymerase chain reaction (PCR)-positive or COVID-19 imaging findings on computed tomography (CT) of the chest. The date of the presentation of symptoms was asked. OD and all of those related components such as hyposmia, anosmia, parosmia, phantosmia, the date of OD presentation, how OD appeared (suddenly or gradually), OD status (fluctuating or continuously present), and the negative impact of OD on daily activities (mild, moderate, severe, not at all) were assessed by an Otorhinolaryngologist or an infectious disease specialist in all centres. OD patients were followed up later a month after the initial diagnosis of COVID-19, and they were also asked for the recovery from OD (no change, a little, and nearly or fully). Patients who died or left the follow-up were excluded.
Statistical analysis
Statistical analysis was conducted utilising SPSS (version 24; IBM, Armonk, NY). The Chi-square test was utilised to analyse nominal (categorical) data, and independent samples t-test compared the means for groups. The Pearson correlation coefficient was utilised for the evaluation of quantitative data and its statistical relationships. The Mann–Whitney U test (nonparametric) analysed the data were not normally distributed. p-values <.05 was considered statistically significant. Minitab® 19 Statistical Software 19.2020.1 (64 bit) was also utilised for classification and regression tree (CART) modelling. The number of terminal nodes of the model was determined based on an optimal node provided by the program per se. All the quantitative and qualitative variables (i.e. platelet count) were inserted for producing the best model and predicting an excellent CART model. Therefore, the data set was successfully split into increasingly homogenous subgroups. At each stage (node), the Gini algorithm selected an explanatory variable and split value with the best discrimination between two outcome classes. The CART produced an algorithm to predict 30-day outcome of patients who had OD at the time of presentation to referral centres. The minimum number of cases for being split into internal nodes was 10, and the minimum number of cases allowed for the terminal node was 3.
Results
Demographic characteristics, history, imaging, and PCR for all patients
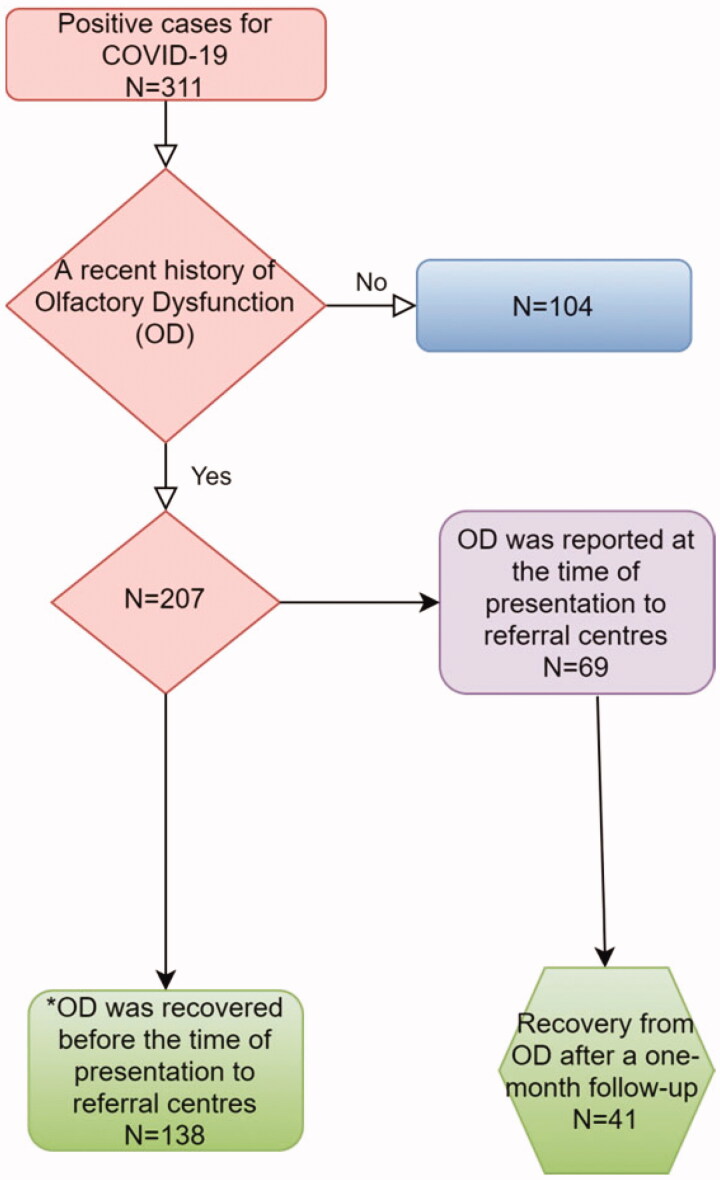
Three hundred and eleven patients who were positive for COVID-19 in PCR or on chest CT scans were recruited in the current study. Amongst all patients, 255(82%) patients were PCR-positive, and 56 (18%) PCR-negative. Two hundred and ninety patients (93.2%) had chest CT findings for COVID-19, and 21 (6.8%) of patients were negative-CT findings (Table 1). Six patients reported OD, but they were PCR-negative in the absence of chest CT findings, so they were excluded.
Table 1.
This table shows number of each group of patient diagnosed with COVID-19 infection.
| Chest CT scan |
Total | |||
|---|---|---|---|---|
| N = 311 | Negative | Positive | ||
| PCR | Negative | 0 | 56 | 56 |
| Positive | 21 | 234 | 255 | |
| Total | 21 | 290 | 311 | |
PCR: Polymerase chain reaction; CT scan: computerized tomography scan.
Out of 311 patients of this study, 223 were male (71.7%) and 88 (28.3%) female patients. The mean and standard deviation (SD) of the age were 47 and 12.42, respectively. Two hundred and seven patients (66.6%) had a recent history of OD. One hundred and seventy-eight patients had experienced OD as a primary symptom intercurrent to other COVID-19 symptoms or solely (p = .000). In 138 of these patients, OD was recovered before the time of presentation to referral centres. However, 69 patients had still OD when they were being visited in these centres (p = .000) (Table 2).
Table 2.
This table presents demographic characteristics, signs, and symptoms for all patients (with or without OD).
| Total (%) | Olfactory dysfunction (OD) |
p-Values | ||
|---|---|---|---|---|
| None n = 104 (%) |
Positive n = 207 (%) |
|||
| Age (Mean ± SD) | 47.00 ± 12.42 | 50.92 ± 14.51 | 45.02 ± 10.71 | *.007 |
| Sex | ||||
| Female | 88 (28.3) | 36 (33.6) | 52 (25.1) | .079 |
| Male | 223 (71.7) | 68 (66.4) | 155 (74.9) | |
| Primary OD | ||||
| None | 133 (42.8) | 104 (100) | 29 (14) | *.000 |
| Positive | 178 (57.2) | 0 (0) | 178 (86) | |
| Current OD | ||||
| None | 242 (77.8) | 104 (100) | 138 (66.7) | *.000 |
| Positive | 69 (22.2) | 0 (0) | 69 (33.3) | |
| Primary Fever | ||||
| None | 122 (39.4) | 42 (40.4) | 80 (38.8) | .792 |
| Positive | 188 (60.6) | 62 (59.6) | 126 (62.2) | |
| Current Fever | ||||
| None | 284 (91.3) | 93 (89.4) | 191 (92.3) | .400 |
| Positive | 27 (8.7) | 11 (10.6) | 16 (7.7) | |
| Primary Chill | ||||
| None | 206 (66.2) | 76 (73.1) | 130 (62.8) | .071 |
| Positive | 105 (33.8) | 28 (26.9) | 77 (37.2) | |
| Current Chill | ||||
| None | 294 (94.5) | 100 (96.2) | 194 (93.7) | .373 |
| Positive | 17 (5.5) | 4 (3.8) | 13 (6.3) | |
| Primary Sweating | ||||
| None | 308 (99.0) | 102 (98.1) | 206 (99.5) | .220 |
| Positive | 3 (1.0) | 2 (1.9) | 1 (0.5) | |
| Current Sweating | ||||
| None | 310 (99.7) | 103 (99.7) | 207 (100) | .158 |
| Positive | 1 (0.3) | 1 (0.3) | 0 (0) | |
| Primary Abdominal Pain | ||||
| None | 304 (97.7) | 102 (98.1) | 202 (97.6) | .782 |
| Positive | 7 (2.3) | 2 (1.9) | 5 (2.4) | |
| Current Abdominal Pain | ||||
| None | 238 (76.5) | 80 (76.9) | 158 (76.3) | .315 |
| Positive | 73 (23.5) | 24 (23.1) | 49 (23.7) | |
| Primary Chest Pain | ||||
| None | 304 (97.7) | 102 (98.1) | 202 (97.6) | .618 |
| Positive | 7 (2.3) | 2 (1.9) | 5 (2.4) | |
| Current Chest Pain | ||||
| None | 307 (97.7) | 101 (97.1) | 206 (99.5) | .076 |
| Positive | 4 (1.3) | 3 (2.9) | 1 (0.5) | |
| Primary Cough | ||||
| None | 230 (74.0) | 80 (76.9) | 150 (72.5) | .398 |
| Positive | 81 (26.0) | 24 (23.1) | 57 (27.5) | |
| Current Cough | ||||
| None | 238 (76.5) | 80 (76.9) | 158 (76.3) | .907 |
| Positive | 73 (23.5) | 24 (23.1) | 49 (23.7) | |
| Primary Sore throat | ||||
| None | 281 (90.4) | 99 (95.2) | 182 (87.9) | *.040 |
| Positive | 30 (9.6) | 5 (4.8) | 25 (12.1) | |
| Current Sore throat | ||||
| None | 303 (97.4) | 102 (98.1) | 201 (97.1) | .608 |
| Positive | 8 (2.6) | 2 (1.9) | 6 (2.9) | |
| Primary Dyspnoea | ||||
| None | 282 (90.7) | 88 (84.6) | 194 (93.7) | *.009 |
| Positive | 29 (9.3) | 16 (15.4) | 13 (6.3) | |
| Current Dyspnoea | ||||
| None | 238 (76.5) | 80 (76.9) | 158 (76.3) | .907 |
| Positive | 73 (23.5) | 24 (23.1) | 49 (23.7) | |
| Primary Gustatory Dysfunction | ||||
| None | 214 (68.8) | 93 (89.4) | 121 (58.5) | *.000 |
| Positive | 97 (31.2) | 11 (10.6) | 86 (41.5) | |
| Current Gustatory Dysfunction | ||||
| None | 239 (78.4) | 90 (86.5) | 149 (74.1) | *.040 |
| Positive | 66 (21.6) | 14 (13.5) | 52 (25.9) | |
| Primary Nasal Obstruction | ||||
| None | 295 (94.9) | 100 (96.2) | 195 (94.2) | .462 |
| Positive | 16 (5.1) | 4 (3.8) | 12 (5.8) | |
| Current Nasal Obstruction | ||||
| None | 294 (96.4) | 101 (94.1) | 193 (96) | .741 |
| Positive | 11 (3.6) | 3 (2.9) | 8 (4) | |
| Primary Myalgia | ||||
| None | 209 (67.2) | 71 (68.3) | 138 (66.7) | .776 |
| Positive | 102 (32.8) | 33 (31.7) | 69 (33.3) | |
| Current Myalgia | ||||
| None | 279 (89.7) | 88 (84.6) | 191 (92.3) | *.036 |
| Positive | 32 (10.3) | 16 (15.4) | 16 (7.7) | |
| Primary Post Nasal Drip (PND) | ||||
| None | 296 (95.2) | 101 (97.1) | 195 (94.2) | .258 |
| Positive | 15 (4.8) | 3 (2.9) | 12 (5.8) | |
| Current Post Nasal Drip (PND) | ||||
| None | 296 (96.1) | 100 (96.2) | 196 (96.1) | .974 |
| Positive | 12 (3.9) | 4 (3.8) | 8 (3.9) | |
| Primary Fatigue | ||||
| None | 283 (91.0) | 88 (84.6) | 195 (94.2) | *.005 |
| Positive | 28 (9.0) | 16 (15.4) | 12 (5.8) | |
| Current Fatigue | ||||
| None | 300 (96.5) | 100 (96.2) | 200 (96.6) | .834 |
| Positive | 11 (3.5) | 4 (3.8) | 7 (3.4) | |
| Primary Nausea or Vomiting | ||||
| None | 304 (97.7) | 102 (98.1) | 202 (97.6) | .782 |
| Positive | 7 (2.3) | 2 (1.9) | 5 (2.4) | |
| Current Nausea or Vomiting | ||||
| None | 311 (100) | 104 (100) | 207 (100) | … |
| Positive | 0 (0) | 0 (0) | 0 (0) | |
| Primary Loss of Appetite | ||||
| None | 305 (98.1) | 100 (96.2) | 205 (99) | .082 |
| Positive | 6 (1.9) | 4 (3.8) | 2 (1) | |
| Current Loss of Appetite | ||||
| None | 310 (99.7) | 104 (100) | 206 (99.5) | .478 |
| Positive | 1 (0.3) | 0 (0) | 1 (0.5) | |
| Primary Diarrhoea | ||||
| None | 307 (98.7) | 102 (98.1) | 205 (98) | .480 |
| Positive | 4 (1.3) | 2 (1.9) | 2 (1) | |
| Current Diarrhoea | ||||
| None | 309 (99.4) | 104 (100) | 205 (99) | .315 |
| Positive | 2 (0.6) | 0 (0) | 2 (1) | |
| Primary Rhinorrhoea | ||||
| None | 297 (95.9) | 100 (97.1) | 197 (95.2) | .427 |
| Positive | 13 (4.1) | 3 (2.9) | 10 (4.8) | |
| Current Rhinorrhoea | ||||
| None | 303 (97.4) | 103 (99) | 200 (96.6) | .203 |
| Positive | 8 (2.6) | 1 (1) | 7 (3.4) | |
| Primary Sneeze | ||||
| None | 302 (97.2) | 98 (94.2) | 204 (98.6) | .032 |
| Positive | 9 (2.8) | 6 (5.8) | 3 (1.4) | |
| Current Sneeze | ||||
| None | 308 (99) | 104 (100) | 204 (98.6) | .217 |
| Positive | 3 (1) | 0 (0) | 3 (1.4) | |
| Primary Facial Pain | ||||
| None | 305 (98.1) | 104 (100) | 201 (97.1) | .080 |
| Positive | 6 (1.9) | 0 (0) | 6 (2.9) | |
| Current Facial Pain | ||||
| None | 308 (99) | 103 (99) | 205 (99) | .997 |
| Positive | 3 (1) | 1 (1) | 2 (1) | |
| Primary Headache | ||||
| None | 271 (87.1) | 94 (90.4) | 177 (85.5) | .225 |
| Positive | 40 (12.9) | 10 (9.6) | 30 (14.5) | |
| Current Headache | ||||
| None | 289 (93.8) | 98 (94.2) | 191 (93.6) | .835 |
| Positive | 19 (6.2) | 6 (5.8) | 13 (6.4) | |
In this table, symptoms of patients who had experienced OD are presented here as a primary symptom or at the time of presentation as 'current symptom'. The Asterisks in the table denote statistically significant p-values.
OD was reported by patients as hyposmia (54.3%), anosmia (27.3%), parosmia (13.1%), and phantosmia (10.2%) solely or in a combination of these symptoms (for all variables p < .05).
Signs, symptoms, and underlying conditions
Two hundred and seven patients (66.6%) had experienced OD. In these patients, OD was started before (4.2%), simultaneously (32.1%), or after (63.7%) the presentation of other COVID-19 symptoms. In the latter cases, OD was started mainly after three days following other COVID-19 symptoms (Figure 1). These patients reported OD as a primary symptom (86%) or at the time of presentation to referral centres (33.3%). Other primary symptoms such as fever (62.2%), gustatory dysfunction (41.5%), chill (37.2%), myalgia (33.3%), cough (27.5%), headache (14.5%), and sore throat (12.1%), were, respectively, reported by patients (Table 2). The distribution of sore throat and gustatory dysfunction as primary symptoms was significantly higher in OD patients (p < .05). In OD patients, amongst those who had gustatory dysfunction (47.5%), that was reported as hypogeusia (68%) and dysgeusia (18.5%) (for all variables p < .05). Amongst all patients, 10.6% and 13.5% had only gustatory dysfunction in the absence of OD as a primary symptom or at the time of presentation to centres, respectively (p < .05). The results also revealed that the distribution of dyspnoea, sneezing, and fatigue as primary symptoms was more noted in patients without OD (p < .05) (Table 2). The distribution of myalgia as a symptom at the time of presentation was significantly higher in non-OD patients (p < .05).
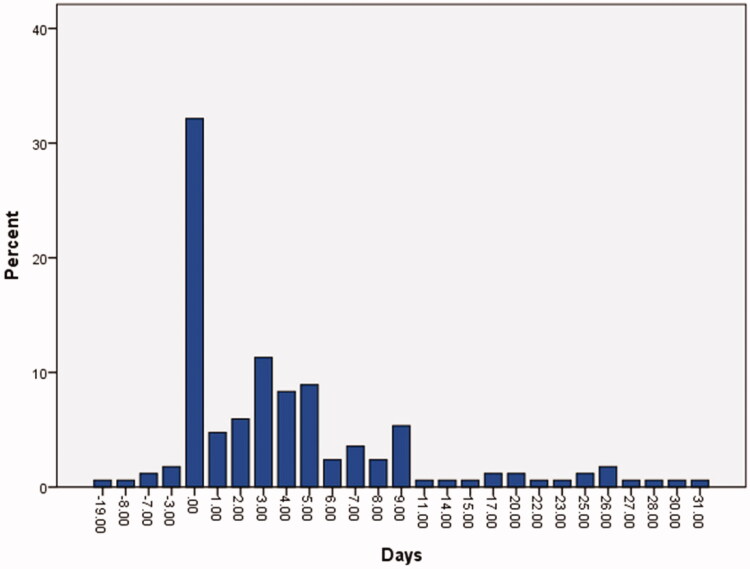
Figure 1.
This graph represents the differences between the number of days for the presentation of OD and COVID-19 symptoms in all patients who had a recent history of OD (207 cases). OD was reported by these patients before (4.2%), intercurrent to (32.1%), or a few days after (63.7%) other COVID-19 symptoms.
A history of underlying conditions was significantly higher in non-OD patients. Hypertension and history of COVID-19 in family members were significantly higher in non-OD and OD patients, respectively (p < .05). A history of cancer, asthma, liver, kidney, pulmonary, and cardiovascular diseases was more prominent in non-OD patients (p < .05). Diabetes was the most common (21.1%) underlying condition, and liver diseases and cancers were less common in all patients (p < .05). The distribution of other underlying conditions was not significantly different (p > .05) (Table 3).
Table 3.
This table shows underlying conditions for all patients (with or without OD).
| Olfactory dysfunction (OD) |
||||
|---|---|---|---|---|
| Total (%) | None n = 104 (%) |
Positive n = 207 (%) |
p-Values | |
| Past Medical History (Hx) | ||||
| None | 167 (53.6) | 41 (39.4) | 126 (37.6) | *.000 |
| Positive | 144 (46.3) | 63 (60.6) | 81 (39.1) | |
| Diabetes | ||||
| None | 245 (78.8) | 80 (78.9) | 165 (78.7) | .571 |
| Positive | 66 (21.2) | 24 (23) | 42 (21.3) | |
| Hyperlipidemia | ||||
| None | 306 (98.4) | 101 (97) | 205 (99) | .204 |
| Positive | 5 (1.6) | 3 (3) | 2 (1) | |
| Hypertension | ||||
| None | 258 (83.0) | 78 (75) | 180 (87) | *.008 |
| Positive | 53 (17.0) | 26 (25) | 27 (13.0) | |
| Liver Diseases | ||||
| None | 309 (99.4) | 102 (98) | 207 (100.0) | *.045 |
| Positive | 2 (0.6) | 2 (2) | 0 (0.0) | |
| Kidney Diseases | ||||
| None | 302 (97.1) | 96 (92.3) | 206 (99.5) | *.000 |
| Positive | 9 (2.9) | 8 (7.7) | 1 (0.5) | |
| Hyperthyroidism | ||||
| None | 300 (96.8) | 100 (96.2) | 200 (97.1) | .660 |
| Positive | 10 (3.2) | 4 (3.8) | 6 (2.9) | |
| Hypothyroidism | ||||
| None | 307 (98.7) | 102 (98.1) | 205 (99) | .480 |
| Positive | 4 (1.3) | 2 (1.9) | 2 (1) | |
| Pulmonary Disease | ||||
| None | 302 (97.1) | 96 (92.3) | 206 (99.5) | *.000 |
| Positive | 9 (2.9) | 8 (7.7) | 1 (0.5) | |
| Cardiovascular Diseases | ||||
| None | 288 (92.6) | 88 (84.6) | 200 (96.6) | *.000 |
| Positive | 23 (7.4) | 16 (15.4) | 7 (3.4) | |
| Cancer | ||||
| None | 308 (99.0) | 101 (97.1) | 207 (100.0) | *.014 |
| Positive | 3 (1.0) | 3 (2.9) | 0 (0.0) | |
| Asthma | ||||
| None | 293 (94.2) | 93 (89.4) | 200 (96.6) | *.010 |
| Positive | 18 (5.8) | 11 (10.6) | 7 (3.4) | |
| Family history of COVID-19 | ||||
| None | 218 (70.1) | 89 (85.6) | 129 (63.3) | *.000 |
| Positive | 91 (29.9) | 15 (14.4) | 76 (36.7) | |
A history of underlying conditions was significantly higher in non-OD patients (p < .05). Hypertension and history of COVID-19 in family members were significantly higher in non-OD and OD patients, respectively. A history of cancer, asthma, liver, kidney, pulmonary, and cardiovascular diseases was more prominent in Non-OD patients (p < .05). The Asterisks in the table denote statistically significant p-values.
Out of 207 patients, OD was started in 164 (79.2%) abruptly and in 43 (20.8%) gradually (p = .032). In 59% of patients, a fluctuating OD, and in 41% a continuous (or constant) OD was observed (p = .000). The negative impact of OD on daily activities for all patients was asked by doctors – an Otorhinolaryngologist or an infectious disease specialist in each centre, responses were in 59 (28.6%), 34 (16.5%), 24 (11.7%), and 89 (43.2%) patients, mild, moderate, severe, and 'not at all', respectively (p = .000).
Sixty-nine patients had OD at the time of presentation to referral centres (p = .000). These patients were evaluated prospectively as a distinct subgroup. Therefore, they were requested to complete their one-month follow-up. In this group, there were no significant sex differences (50.7% of females versus 49.3% males). The mean (SD) of age in these patients was 41 (11). Out of 69 patients in this subgroup, 41 (59.5%) were PCR-positive for COVID-19. Twenty-eight (40.5%) patients were PCR-negative, but they had chest CT findings for COVID-19 (p = .000). Sixty- three patients (91.3%) had positive chest CT findings (at least a ground-glass pattern) for COVID-19, and 6 (8.7%) patients were negative-CT findings but positive for PCR. OD was reported by these patients as hyposmia (84%), anosmia (19.1%), parosmia (19.1%), and phantosmia (16.6%) solely or in a combination of these symptoms (for all variables p < .05). It was observed that 17.4% and 4.3% of patients had a history of diabetes and hypertension, respectively. Therefore, diabetes was the most common underlying condition in these patients. The frequent symptoms that were started in this group as 'primary symptoms' were OD (58%), taste dysfunction (44.9%), cough (36.2%), headache (29%), sore throat (17.4%), nasal obstruction (15.9), dyspnoea (13%), fatigue (11.6%), nasal discharge (5.8%), and nausea or vomiting (2.9%).
OD was presented with other COVID-19 symptoms in 40.6% of patients concurrently, and before the onset of other symptoms in 5.7% (Figure 2). The mean (SD) of the presentation of OD was 2.57 (5.78) days following the onset of other symptoms. Laboratory tests were also analysed separately in this group (Table 4).
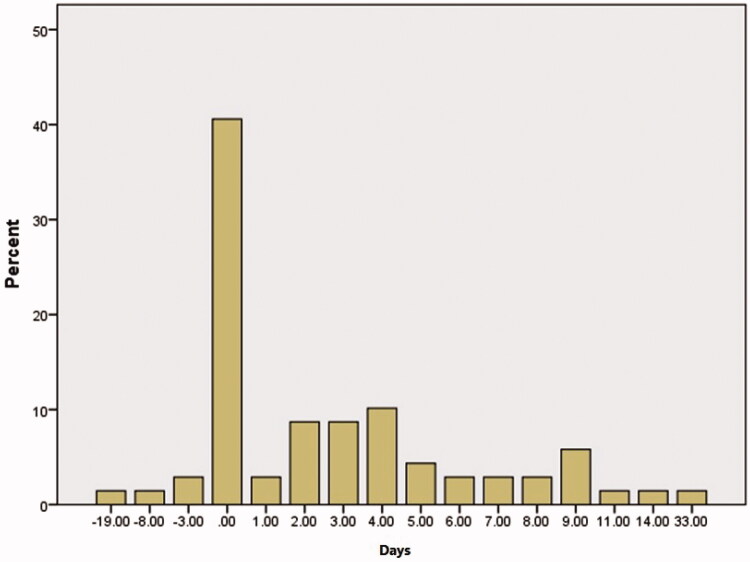
Figure 2.
This graph represents the differences between the number of days for the presentation of COVID-19 symptoms and OD in patients who had OD at the time of presentation to referral centres (69 cases).
Table 4.
This table shows laboratory testing data for patients with OD at the time of presentation.
| Minimum | Maximum | Mean | Standard deviation | |
|---|---|---|---|---|
| White blood cells | 1100 | 13900 | 6597.73 | 2408.849 |
| Neutrophils % | 52.00 | 88.00 | 69.6957 | 10.96073 |
| Lymphocytes % | 8.40 | 41.00 | 20.9821 | 8.78328 |
| Platelets | 102000 | 410000 | 205772.73 | 75238.802 |
| Haemoglobin concentration (Hb) | 9.5 | 16.7 | 13.632 | 1.6973 |
| C-reactive protein (CRP) | 2 | 150 | 53.49 | 48.189 |
| Lactate dehydrogenase (LDH) | 272 | 750 | 474.71 | 148.721 |
| Erythrocyte sedimentation rate (ESR) | 3 | 100 | 46.29 | 25.671 |
| Lymphocyte count | 176.00 | 2457.00 | 1216.6107 | 491.13153 |
| Neutrophils count | 814.00 | 11537.00 | 4384.3217 | 2412.83608 |
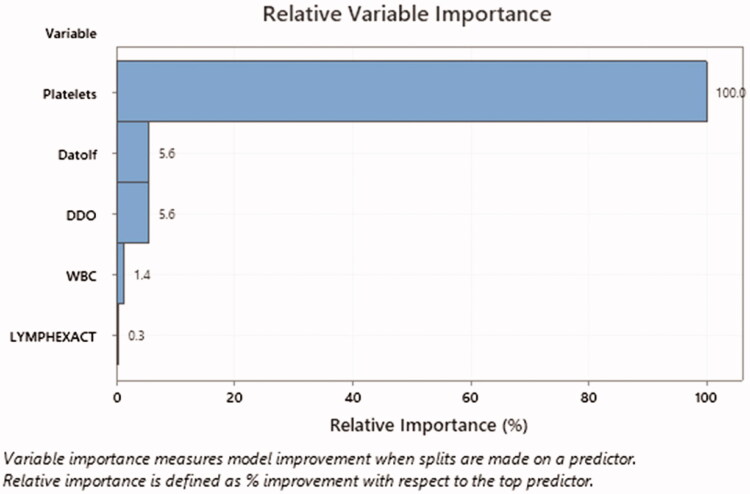
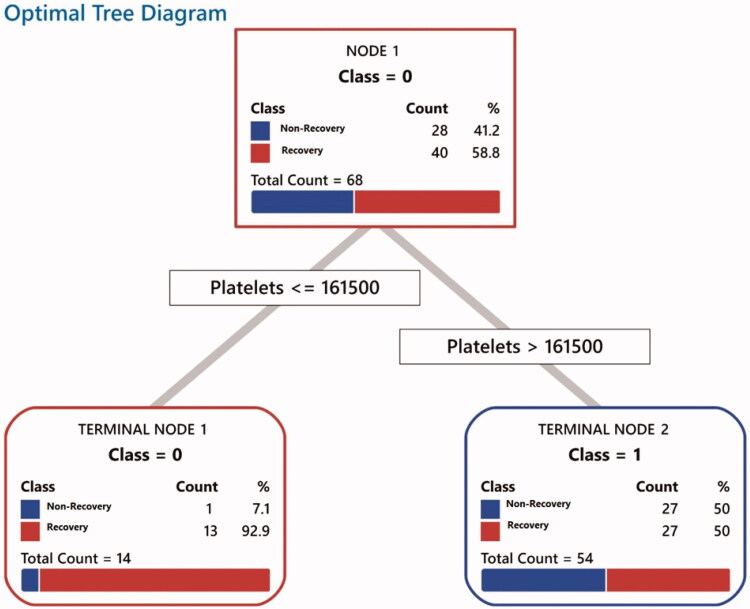
The frequent symptoms were reported by patients at the time of presentation to referral centres in this group as 'current symptoms' were gustatory dysfunction (69.6%), cough (47.8%), dyspnoea (30.4%), headache (10.1%), nasal discharge (8.7%), fever (7.2%), nasal obstruction (7.2%), fatigue (5.8%), sore throat (4.3%), and nausea or vomiting (0%). In these patients, recovery from OD had a significant relationship with the number of days that OD was started (p < .05). Patients who were referred on the 14th day after the onset of other COVID-19 symptoms had a higher rate of recovery from OD as to the patients with the same symptoms presented on the 11th day. In patients who did not have a headache or had nasal obstruction as primary symptoms, a remarkable recovery from OD was observed (p = .002 and p = .000, respectively). There was no significant relationship between nasal obstruction and the number of days for OD presentation. There were no significant relationships between recovery and underlying medical conditions, or other primary symptoms in this group. Platelet count ≤161.500 per microliter of blood had an important relationship with recovery as the most important predictor for recovery even more prominent than the number of days for OD presentation, or other lab tests in these patients (Figures 3 and 4).
Figure 3.
This horizontal bar graph represents the most important predictor for recovery from OD. The most important predictor for recovery is platelet count according to these results.
Figure 4.
This algorithm outlines platelet count and patient classification. Patients who have platelet count ≤161,500 (class 0) have a higher rate of recovery from OD. These horizontal bar graphs in both classes are divided into two different colours. The red colour represents patients who were recovered from OD following a one-month follow-up.
The associations between OD and risk factors
In the evaluation of associations between OD and other variables, past medical history (PMH), sore throat, dyspnoea, fatigue, gustatory dysfunction, sneezing, Angiotensin II receptor blockers (ARBs), and a family medical history of COVID-19 had significant associations with OD (p < .05) (Table 5).
Table 5.
Association between OD and risk factors for all patients are presented in this table.
| Odds ratio (OR) | 95% confidence interval (CI) for OR |
p-Values | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Sex | 1.578 | 0.946 | 2.632 | .079 |
| Past medical history (PMH) | 0.418 | 0.258 | 0.678 | *.000 |
| Fever | 1.067 | 0.659 | 1.727 | .792 |
| Sore throat | 2.720 | 1.010 | 7.326 | *.040 |
| Dyspnoea | 0.369 | 0.170 | 0.799 | *.009 |
| Myalgia | 1.076 | 0.650 | 1.781 | .776 |
| Fatigue | 0.338 | 0.154 | 0.746 | *.005 |
| Nasal Obstruction | 1.538 | 0.484 | 4.893 | .462 |
| Gustatory Dysfunction | 6.009 | 3.034 | 11.901 | *.000 |
| Post-Nasal Drip (PND) | 2.072 | 0.572 | 7.510 | .258 |
| Chest Pain | 0.500 | 0.031 | 8.075 | .618 |
| Headache | 1.593 | 0.746 | 3.400 | .225 |
| Rhinorrhoea | 1.692 | 0.455 | 6.286 | .427 |
| Sneezing | 0.240 | 0.059 | 0.981 | *.032 |
| Alcohol-based hand sanitizers | 0.488 | 0.141 | 1.688 | .727 |
| Angiotensin II receptor blockers (ARBs) | 0.296 | 0.111 | 0.778 | *.010 |
| Angiotensin converting enzyme inhibitors (ACEinh) | 1.547 | 0.546 | 4.380 | .480 |
| Beta blockers | 0.609 | 0.233 | 1.593 | .308 |
| Hx of smoking | 0.616 | 0.269 | 1.410 | .248 |
| Opioid addiction | 0.330 | 0.054 | 2.007 | .207 |
| A family medical history of COVID-19 | 3.496 | 1.888 | 6.473 | *.000 |
| Chest CT | 1.824 | 0.682 | 4.875 | .225 |
| PCR | 0.756 | 0.394 | 1.453 | .400 |
The asterisks in the table denote statistically significant p-values.
Recovery
Out of 207 patients with a history of OD, 138 cases were nearly or fully recovered (66.6%) when they were being visited in referral centres. In these patients, OD was reported as a primary symptom. Sixty- nine patients still had OD when they presented to referral centres. Therefore, they were assessed for a 1-month follow-up. Forty-one patients (59.4%) were nearly or fully recovered. In total, 179 patients (86.4%) were recovered from OD approximately a month after the onset of OD. The answers in four (2%) and twenty-four patients (11.6%) were respectively ‘a little' or ‘no change' (p = .000) (Figure 5).
Figure 5.
This chart represents the recovery in all patients who had COVID-19 and olfactory dysfunction (OD). Out of 207 patients with a recent history of OD, 138 cases reported a nearly or fully recovery (66.6%) before the time of presentation to referral centres. In these patients, OD was reported as a primary symptom. Sixty-nine patients still had OD when they presented to referral centres. Therefore, they were assessed for a 1-month follow-up prospectively. In this subgroup, 41 patients (59.4%) were nearly or fully recovered after 1 month. In total, 179 patients (86.4%) were recovered from OD approximately a month after the onset of OD. *These patients reported OD as a primary symptom (a recent history of OD) along with other COVID-19 symptoms. Although OD was recovered when they presented to the referral centre, they still had other COVID-19 symptoms and a positive test for COVID-19.
Discussion
The olfactory dysfunction (OD) was initially a unique presentation and an extraordinary characteristic of COVID-19. In the common causes of OD – sinonasal disease and postinfectious – patients who present with OD, they often have or experienced sinonasal symptoms [4]. Although OD may present as a transient symptom due to an acute infection, postinfectious OD caused by upper respiratory tract infections (URTIs) may continue while other symptoms have resolved [5]. Postviral OD (PVOD) is most commonly caused by a wide variety of viruses including, parainfluenza type 2, influenza, human rhinoviruses, Epstein Barr virus, human immunodeficiency virus (HIV), and coronavirus [5].
Based on recent findings, many patients around the world had OD during the COVID‑19 pandemic [6]. The angiotensin-converting enzyme 2 (ACE2) receptors are expressed in the nervous system. Therefore, this target will help the coronavirus to move within the olfactory and nervous system to stimulate neuronal damages [7]. PVOD also may occur due to damage to the olfactory epithelium, nerve, and central pathways in the brain [8]. In the current study, in patients who had OD at the time of presentation to referral centres, the headache was reported as a primary symptom in 29% of patients (p = .000). The presence of headache may worsen and increase the risk of OD (p = .000; OR 4.531, 95% CI 2.266–9.075). Therefore, the presence of this symptom may confirm damages to the olfactory epithelium, nerve, and central pathways in the brain that may occur during the COVID-19.
In the current study, 71.7% of patients were male, and the mean (SD) of the age was 47 (12.40). The proportion of male cases with COVID-19 was around 50% as reported by Juanjuan Zhang et al. [9], although this proportion was slightly higher in the first period of this epidemic. The researchers of the current study found that 57.2% of patients had OD at the time of presentation initially with or without other COVID-19 symptoms. Although it was not significant (p < .05), OD was higher in men (77.5%).
In a study by Yi-Min Wan et al. [10] OD (anosmia and hyposmia) was observed in 60–70% of patients with SARS-CoV-2 compared with other coronaviruses. Patients with 30–40 and 50–60 years of age were also more prone to PVOD as reported by J Tian and colleagues [11]. In the current study in a subgroup of patients – 69 cases – who had OD at the time of presentation, there were no significant sex differences (50.7% of females versus 49.3% males). Therefore, OD might be observed commonly in younger patients with no differences between females and males.
In a study by Tian et al. [11] the most common nasal symptoms at the time of URTIs were obstruction, rhinorrhoea, and sneezing intercurrent to other symptoms such as sore throat, sputum, and cough. The current study revealed that OD was presented with other COVID-19 symptoms in 32.1% of patients concurrently, and before the onset of other symptoms in 4.2%. The distribution of sore throat and gustatory dysfunction in cases with OD was also significantly higher. The distribution of dyspnoea, sneezing, and fatigue as primary symptoms was significantly more prominent in patients without OD. Therefore, based on the findings of the current study, patients who experience OD might have a mild form of COVID-19.
In some cases, OD might be the only presentation in the absence of other nasal symptoms. OD was presented at a median time of 3 days following other COVID-19 symptoms as reported by Marlene M. Speth et al. [12]. They discovered that 34.9% of OD patients did not experience nasal symptoms. Yi-Min Wan et al. [10] also reported a pure form of OD without nose block or other signs of respiratory infections. In a study by Jerome R. Lechien et al. [13] on 417 mild to moderate COVID-19 patients, researchers assumed that 85.6% of patients had OD related to COVID-19, and OD was also manifested in 11.8%, 65.4%, and 22.8% of patients, before, after, and at the same time of the presentation of other symptoms, respectively.
In the current study, fever (60.6%), OD (57.2%), chill (33.8%), myalgia (32.8%), gustatory dysfunction (31.2%), and cough (26.%) were consequently the most common symptoms similar to other findings [2, 14]. Although fever may present with other symptoms, screening COVID-19 by a temporal thermometer was not appropriate enough to control the initial wave of transmission at early phases of this pandemic [15], particularly, those who had only mild symptoms such as OD and sore throat in the absence of other symptoms.
In previous studies [4, 10, 15], the authors reported that OD frequently co-occurred with gustatory dysfunction. In the current study, gustatory dysfunction was reported in 41.5% of patients as a primary symptom along with OD. Among those who had gustatory dysfunction (47.5%), that was reported as hypogeusia (68%) and dysgeusia (18.5%) (for all variables p < .05).
Smoking may upregulate ACE2 receptors and these receptors may act as targets for Coronavirus as reported by Samuel James Brake et al. [16]. The researchers of the current study also concluded that smoking could not significantly decrease the risk of OD (OR = 0.616, 95% CI: 0.269–1.410, and p = .248).
In the current study, 179 patients (86.4%) were recovered from OD approximately a month after the onset of OD. In a subgroup of patients who had OD at the time of presentation to referral centres, 58.8% were nearly or fully recovered. In a study by Chiesa-Estomba et al. [17], 367 (49%) out of 751 patients were completely recovered from OD after a mean follow-up of 47 ± 7 days. Victor Gorzkowski et al. [18] also reported in a study on 229 patients with COVID‐19, 78.4% of patients were recovered within the fourth and the fifteenth day after olfactory loss onset with a complete olfactory recovery in 51.43% of patients. Luigi Angelo Vaira et al. [19] assumed that initial objective olfactory and gustatory assessment do not show significant prognostic value in predicting the severity of the COVID-19 course. Nevertheless, the persistence of OD at 20 days, correlated with a more severe course.
In the current study, platelet count was the most important predictor for recovery from OD even more prominent than the number of days to presentation to referral centres. As mentioned above, some coronavirus particles may influence and infect the endothelial cells by ACE2 receptors. This endothelium, especially in the small vessels, with slower blood flow, may lead to thrombosis into the microcirculation of the olfactory system. This mechanism may lead to thrombus formation as reported by Xin Zhou et al. [20]. Consequently, patients who have lower platelet count may have a lower degree of thrombosis into the vessels of the olfactory system. Therefore, antiplatelet medications as a prophylactic agent for OD due to COVID-19 might be helpful for its inhibitory consequences on platelet activation in those who have a higher count of platelets.
Conclusions
The OD as a novel characteristic of COVID-19 may manifest in a mild symptom even in the absence of other symptoms. Headache, nasal obstruction, and platelet count may have specific roles as prognostic factors in the recovery from OD. This study also indicates that a mild form of COVID-19 may manifest in OD in younger patients.
Methodological considerations/limitations
First, missing data and some errors throughout data collection and follow-up in this multicentric cross-sectional study by different practitioners from other cities were unavoidable. Although a questioner form was designed to collect data by practitioners from different centres in the same method. Second, because of unknown nature of this virus (COVID-19) especially in the first wave of this pandemic, and also decreasing the exposure to this new virus for the healthcare system, the researchers of the current study could not evaluate patients with unnecessary tests that are designed for evaluation of olfactory dysfunction. Third, the researchers of this study had a plan to take biopsies from the olfactory bulb to confirm the involvement of this component of the olfactory system by Coronavirus. Nevertheless, only two patients died with OD at the presentation to referral centres, and their family did not permit for obtaining a biopsy. Fourth, this study was conducted in referral centres. Therefore in 138 patients, OD was recovered at the time of presentation to these referral centres, but they still had other COVID-19 symptoms and positive test results. To assess the progress and recovery from OD, 69 patients who had OD at the time of presentation to referral centres underwent a close follow-up for one month with the main centre in the capital.
Acknowledgement
All researchers of the current study would like to express deep gratitude to Otorhinolaryngology Research Center of Tehran University of Medical Sciences for supporting this research work.
Funding Statement
Otorhinolaryngology Research Center, Tehran University of Medical Sciences; Grant no. 99-1-112-47182.
Ethical approval
All procedures were performed in this study were based on the ethical standards and with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from individual participant included in the study. The local research ethics committee approved the present study with a reference number: IR.TUMS.VCR.1398.1079
Disclosure statement
No potential conflict of interest was reported by the authors. The authors certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on them or on any organization with which they are associated.
References
- 1.Levinson R, Elbaz M, Ben-Ami R, et al. Anosmia and dysgeusia in patients with mild SARS-CoV-2 infection. medRxiv; 2020. DOI: 10.1101/2020.04.11.20055483. [DOI] [PubMed] [Google Scholar]
- 2.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngol. 2020;277(8):2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beltrán-Corbellini Á, Chico-García JL, Martínez-Poles J, et al. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case–control study. Eur J Neurol. 2020;27(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miwa T, Furukawa M, Tsukatani T, et al. Impact of olfactory impairment on quality of life and disability. Arch Otolaryngol Head Neck Surg. 2001;127(5):497–503 [DOI] [PubMed] [Google Scholar]
- 5.Sugiura T. An epidemiological study of postviral olfactory disorder. Acta Oto-Laryngol. 1998;118(544):191–196. [DOI] [PubMed] [Google Scholar]
- 6.Whitcroft KL, Hummel T.. Olfactory dysfunction in COVID-19: diagnosis and management. JAMA. 2020;323(24):2512–2514. [DOI] [PubMed] [Google Scholar]
- 7.Baig AM, Khaleeq A, Ali U, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. [DOI] [PubMed] [Google Scholar]
- 8.Jafek BW, Murrow B, Michaels R, et al. Biopsies of human olfactory epithelium. Chem Senses. 2002;27(7):623–628. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Litvinova M, Wang W, et al. Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outside Hubei province, China: a descriptive and modelling study. Lancet Infect Dis. 2020;20(7):793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan Y-M, Deng X, Tan E-K.. Olfactory dysfunction and COVID-19. Lancet Psychiat0. 2020;7(8):663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian J, Wei YX, Li L, et al. [Analysis of clinical characteristics of 141 patients with postviral olfactory dysfunction]. Lin Chuang er bi Yan Hou Tou Jing Wai ke za Zhi = J Clin Otorhinolaryngol Head Neck Surg. 2017;31(10):749–752. [DOI] [PubMed] [Google Scholar]
- 12.Speth MM, Singer-Cornelius T, Oberle M, et al. Olfactory dysfunction and sinonasal symptomatology in COVID-19: prevalence. Otolaryngol Head Neck Surg. 2020;163(1):114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lechien JR, Chiesa-Estomba CM, Fakhry N, et al. In reference to Anosmia and Ageusia: common findings in COVID-19 patients. Laryngoscope. 2020;130(9):E506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singhal T. A review of Coronavirus Disease-2019 (COVID-19). Indian J Pediatr. 2020;87(4):281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin-Sanz E, Riestra J, Yebra L, et al. Prospective study in 355 patients with suspected COVID-19 infection. Value of cough, subjective hyposmia, and hypogeusia. The Laryngoscope. 2020. DOI: 10.1002/lary.28999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brake SJ, Barnsley K, Lu W, et al. Smoking upregulates angiotensin-converting enzyme-2 receptor: a potential adhesion site for novel Coronavirus SARS-CoV-2 (Covid-19). JCM. 2020;9(3):841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiesa-Estomba CM, Lechien JR, Radulesco T, et al. Patterns of smell recovery in 751 patients affected by the COVID-19 outbreak. Eur J Neurol. 2020;27:2318–2321. DOI: 10.1111/ene.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorzkowski V, Bevilacqua S, Charmillon A, et al. Evolution of olfactory disorders in COVID-19 patients. The Laryngoscope. DOI: 10.1002/lary.28957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaira LA, Hopkins C, Petrocelli M, et al. Do olfactory and gustatory psychophysical scores have prognostic value in COVID-19 patients? A prospective study of 106 patients. J Otolaryngol - Head Neck Surg. 2020;49(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X, Li Y, Yang Q.. Antiplatelet therapy after percutaneous coronary intervention in patients with COVID-19: implications from clinical features to pathologic findings. Circulation. 2020;141(22):1736–1738. [DOI] [PubMed] [Google Scholar]