Graphical abstract
Keywords: COVID-19, SARS-COV-2, Coronavirus, Hyperimmune sera, Animal antibodies, Neutralizing titers
Abstract
Since the very beginning of the COVID-19 pandemic, different treatment strategies have been explored. These mainly involve the development of antimicrobial, antiviral, and/or anti-inflammatory agents as well as vaccine production. However, other potential options should be more avidly investigated since vaccine production on a worldwide level, and the anti-vaccination movement, also known as anti-vax or vaccine hesitancy by many communities, are still real obstacles without a ready solution. This review presents recent findings on the potential therapeutic advantages of heterologous serotherapy for the treatment of COVID-19. We present not only the effective use in animal models of hyperimmune sera against this coronavirus but also strategies, and protocols for the production of anti-SARS-CoV-2 sera. Promising antigens are also indicated such as the receptor-binding domain (RBD) in SARS-CoV-2 S protein, which is already in phase 2/3 clinical trial, and the trimeric protein S, which was shown to be up to 150 times more potent than the serum from convalescent donors. Due to the high death rate, the treatment for those currently infected with coronavirus cannot be ignored. Therefore, the potential use of anti-SARS-CoV-2 hyperimmune sera should be carefully but urgently evaluated in phase 2/3 clinical studies.
1. Introduction
Coronaviruses are spherical, enveloped viruses, composed of a single-stranded RNA molecule (30 kilobases) belonging to the Coronaviridae family [1], [2], [3], [4]. They cause zoonoses, and infection in humans resulting in respiratory clinical manifestations, ranging from mild to severe, which can lead to the patients’ death [5], [6], [7], [8], [9], [10], [11].
The 21st century is characterized by the emergence of three coronavirus zoonoses of great medical importance [12]. The first started in China in 2002 with the Severe Acute Respiratory Syndrome Coronavirus 1 (SARS-CoV-1), the second occurred in Saudi Arabia in 2012 with the Middle East Respiratory Syndrome Coronavirus (MERS-CoV), and the third once again in China with SARS-CoV-2 [5], [13], [14], [15], [16], [17].
SARS-CoV-2 has a wide and rapid spread from person to person, through droplets of saliva, sneezing, coughing, phlegm, handshakes, and contact with contaminated objects and surfaces [18]. In March 2020, the World Health Organization (WHO) already considered the coronavirus disease 2019 (COVID-19) as a pandemic. Up to the end of October 2020, almost 45 million cases of COVID-19, and ca. 1.2 million deaths have been recorded worldwide [19], [20], [21], [22]. Many countries adopted social distancing measures to try to minimize the progress of this pandemic, which also had an important impact on the world economy [23], [24].
The search for the most effective therapy against COVID-19 has driven the research of several different forms of treatment including antivirals [25], [26], [27], a combination of interferons, [28], corticosteroids [29], [30], protease inhibitors [31], [32], convalescent plasma [33], [34], monoclonal, and polyclonal antibodies, and vaccines. However, until now, no therapy has been considered fully efficient and adopted as the official, and unique protocol, despite the drugs remdesivir (USA) and dexamethasone (UK) having been approved for use [5], [7], [13], [35], [36].
Antibody immunotherapy has been studied for many years for the treatment of some highly contagious diseases [37], [38], [39]. For example, the administration of human polyclonal immunoglobulins or hyperimmune animal sera for treatment against viruses [37] such as Hepatitis B and rabies has been applied with high effectiveness. Therapy with human immunoglobulins for the treatment of SARS-CoV-1 has been tested and shown to be successful and more recently, in October 2020, the experimental monoclonal antibody (mAb) cocktail REGN-COV2 from Regeneron Pharmaceuticals was used together with other drugs to treat the president of the United States, Donald Trump infected with the coronavirus [38]. Due to the high cost of these treatments, studies with equine hyperimmune sera in anti-COVID therapies have also begun and should be explored [36], [39].
Vaccines should be preventive against the virus but the treatment for those currently affected cannot be ignored due to the high death toll occurring, among other reasons. Therefore, this review aims to explore studies of the therapeutic potential for the application of heterologous serotherapy as a feasible plan B of reasonable cost to treat COVID-19 patients.
2. COVID-19 and passive immunotherapy
2.1. Physiopathology and risk factors for COVID-19
COVID-19 is an acute respiratory disease caused by SARS-CoV-2 with a complex clinical profile, from asymptomatic or mild symptoms to deadly severe respiratory conditions. The whole complexity and severity of symptoms to the viral infection, and/or patient response, are still being established due to the different pathophysiological responses reported worldwide [40], [41], [42]. Dry cough, fever, and tiredness are the most common and mildest symptoms. Some patients may develop pain (throat, head, and/or body), nasal congestion, heart damage, diarrhea, nausea, vomiting, loss of smell, and taste and, in the most severe cases, difficulty breathing and coagulation problems [1], [41], [43], [44], [45]. Elderly people or those with comorbidities, such as high blood pressure, heart, and lung problems, cancer, and diabetes, are more likely to develop the most severe forms of the disease [40], which triggers the urgent search for antiviral therapies, and vaccines [46].
The disease mechanism is largely unknown although recently a new mechanistic theory has been published. In this, SARS-CoV-2 infection produces an imbalance in the RAS (renin-angiotensin system), resulting in elevation of bradykinin in multiple tissues causing increases in vascular dilation and permeability, leading to hypotension. This process results in the lungs filling with water and hyaluronic acid to form a gel impeding the uptake of oxygen leading to many of the symptoms of COVID-19 [47], [48].
2.2. Vaccine and anti-vaccine movements
Many laboratories around the world have been investing in high-tech research for the development of anti-COVID vaccines [48], [49], [50], [51], [52], [53], [54]. Importantly, the development of a vaccine is a complex process that requires time and high investment. In 2018, a minimum cost of between 2.8 and 3.7 billion dollars was estimated for a vaccine to reach Phase 2a of development [55].
In addition to the high cost of investment in research and development, there are many challenges in this process, which range from large-scale manufacturing to global distribution. Regarding anti-SARS-CoV-2 vaccines currently in the research phase, it is predicted that the production and distribution of these vaccines may suffer economic influences. This is because the demand for this vaccine will be higher than the supply, with the possibility that countries with greater purchasing power acquire most of the vaccines available to the detriment of developing countries [56].
Inequalities among nations are also reflected in the acquisition and distribution of immunobiologicals, especially when this distribution requires storage at controlled temperatures. WHO has already recorded that more than 2.8 million vaccines have been lost due to failure on following cold chain requirements and less than 10% of countries have complied with that Organization's recommendations for effective practices on vaccination schedules [56].
Currently, in many countries, the organization of anti-vaccine movements for COVID-19 are observed, and are gaining increasing popularity around the World [48]. Religious beliefs, social norms, the parents level of education and the socio-demographic context may also influence the decision of whether to vaccinate children [57]. Thus, this can reduce confidence in the effectiveness of this strategy [49], [57], [58]. It is noteworthy that the distance and social isolation also favor the uncontrolled dissemination of false information on social media, which worsens this situation. Since from the very beginning of the process of vaccinating populations, misinformation about vaccines has multiplied, and this may also have an impact on SARS-CoV-2 vaccination coverage [59], [60], [61]. The recent announcement of the vaccine Sputnik-V released in Russia prior to phase 3 trials with an apparent huge political interference increases the fear, especially from those of the anti-vaccine movements. The description of Sputnik-V trials, so far involving only 76 people, reinforces the concern about the safeness of these new vaccines and the urgent need for other low-cost treatment options [62].
2.3. Passive immunotherapy
Passive immunotherapy is one of the alternatives to be further explored for treating coronavirus infections. It has been used in medical practice for some years [37] and stands out as a possibility in the current pandemic scenario [63]. It is a treatment that aims at inducing rapid immunity in a short time and is extremely relevant as a therapy of choice in a pandemic [64].
Over the years, the use of antibodies for antiviral treatments has grown from therapy with hyperimmune sera, evolving into the use of polyclonal and monoclonal antibodies [64]. Passive immunization treatments aim to increase the patient immune response, preventing the disease from progressing to more severe conditions [65]. This strategy has been used recently in European countries affected by COVID-19 as adjunctive therapy to antivirals, and other medications, as it acts directly on viral neutralization, preventing disease progression [64], [66]. The highlights among these biotechnological products are intravenous immunoglobulins, convalescent plasma, monoclonal antibodies, nanobodies, and hyperimmune sera [37], [63].
Intravenous immunoglobulins are obtained from healthy donors, with a high titer of specific antibodies against the target agent. They are used in the treatment, for example, of autoimmune, and coronavirus diseases [67]. Convalescent plasma is collected from individuals cured from the infection of interest. The plasma must be purified, and free from infectious agents, and can be administered to patients with more severe conditions, such as in COVID-19. It is worth mentioning that this therapy has already been used to treat infections such as influenza and cytomegalovirus. However, despite the potential, its main limitation is the number of available donors that have high antibody titers for coronavirus [36], [63], [67], [68], [69], [70], [71].
Monoclonal antibodies are antibodies selective for a specific viral antigen and are homogeneous, produced by a single clone of B cell [72]. As the SARS-CoV-2 is an RNA virus, it may mutate and become resistant, which is a great disadvantage to the use of these antibodies. Currently, many studies in this area involve protein S, which is present on SARS-CoV-1 and SARS-CoV-2 and can be used in treatment for both of these. These antibodies can be administered in association with other drugs or as monoclonal antibody cocktails [7], [35], [37], [69], [70], [71], [72], [73].
Nanobodies are active fragments of antibodies (single monomeric variable domains) that, in recent years, have been extensively explored for the treatment of poisoning by venomous animals, bacteria, and parasitic zoonoses. They can recognize antigens and have been studied for therapies against, for example, influenza virus, MERS-CoV, and Congo hemorrhagic fever virus [74]. They are quite thermally stable, easy to reproduce in prokaryotic systems, specific, and can be humanized [73], [74], [75], [76], [77] so have potential as therapeutic tools [78].
Since the 19th century, polyclonal immunoglobulin therapies have been widely used. They are made with pools of human or animal donor serum with high neutralizing titers [79]. Hyperimmune serum contains polyclonal antibodies, which can be used therapeutically to treat viral infections [64]. They are concentrates of heterologous immunoglobulins, formed by intact IgG molecules or digested Fab, and F(ab′)2 fractions [80], [81].
The initial studies on polyclonal immunoglobulin therapies date back to 1891 with Émile Roux, in which hyperimmune horse serum was used successfully to treat tetanus, bubonic, and pneumonic plague [82]. In 1894, hyperimmune sera from animals were used to treat cases of accidents with venomous animals [82], although, it was Dr. Vital Brazil Mineiro da Campanha who first demonstrated the antigenic specificity for treatment with serotherapy [83]. Subsequently, this therapy with hyperimmune serum against specific venoms became established and uses equine plasma as a source of polyclonal antibodies [84]. Some countries already have a high production of hyperimmune serum against the rabies virus, including Brazil with three centers: the Vital Brazil Institute [85], the Butantan Institute [86], and the Ezequiel Dias Foundation [87]. The serum is used prophylactically in cases of human accidents involving animals with or suspected of carrying the rabies virus [88]. Good results of this treatment have resulted in researchers studying this same therapy against influenza [89], and Ebola [90], [91], [92]. Pre-clinical studies with the Ebola virus [90], [91], [92], H5N1 influenza virus [89] and SARS have shown very promising results [89], [90], [91], [92], [93].
3. Perspectives for treating coronaviroses with hyperimmune SERA – Procedures and strategies
3.1. Studies of hyperimmune sera for the treatment of coronaviruses
The literature, since 2005, contains reports of studies using the therapeutic approach with hyperimmune sera in the treatment of coronaviruses (Table 1 ) [39], [69], [84], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106].
Table 1.
Studies presenting a therapeutic approach to hyperimmune sera in the treatment of coronavirus from 2004 to 2020.
| AUTHORS | ARTICLE PROPOSAL |
|---|---|
| PEIRIS et al., 2004 [94] | Review available information on coronavirus, and its therapies. |
| SUBBARAO et al., 2004 [95] | Check of the efficiency of passive antibody immunization in the treatment of SARS-CoV. |
| LU et al., 2005 [93] | Development of an equine hyperimmune serum as a future SARS treatment measure. |
| WANG et al., 2005 [96] | Evaluation of the protective effects of equine serum F (ab′) 2 against SARS-CoV infection. |
| XU et al., 2007 [39] | Check of the safety, immunogenicity, and pharmacokinetics of equine anti-SARS serum - CoV F (ab′) 2 in monkeys. |
| ZHOU et al., 2007 [97] | Evaluation of the preventive, and therapeutic role of anti-SARS-CoV equine serum F (ab′) 2 in elderly mice. |
| LUO et al., 2007 [98] | Establishment of a new animal model for the study of SARS-CoV in Chinese hamster, and evaluation of the therapeutic action of equine anti-SARS serum in this animal model. |
| NEWCOMBE & NEWCOMB, 2007 [99] | Examination of contemporary techniques, and applications of polyclonal antibodies, and evaluation of their future therapeutic potential. |
| ZHAO et al., 2015 [100] | Investigation of the prophylactic, and therapeutic efficacy of antibodies in the hyperimmune serum of dromedary in reducing the viral load of MERS CoV, weight loss, and pulmonary pathology in mice. |
| DIXIT et al., 2016 [84] | Examination of the history of the use of polyclonal antibodies in animals, their developments in safe, and effective products, and the potential application in humans for neglected infectious diseases. |
| DYALL et al., 2017 [69] | Demonstration of existing therapies in the treatment of coronavirus. |
| ZHAO et al., 2017 [101] | Demonstration that antibodies from horses immunized with MERS-Cov virus particles are able to neutralize viral infection in mice. |
| PAN et al., 2020 [102] | Verification of the antigenicity of RBD (receptor binding domain) in mice, production of equine antiserum using RBD as an immunogen, and evaluation of its neutralizing power against SARS-CoV-2. |
| ZYLBERMAN et al., 2020 [103] | Production of the anti-SARS-CoV-2 serum in Argentina, and its process phases, with the start of a phase 2/3 clinical trial scheduled for July 2020. |
| SAPKAL et al., 2020 [104] | Development of equine antisera with high neutralizing activity against SARS-CoV-2. |
| Cunha et al., 2020 [105] | Demonstrated that equine hyperimmune globulin raised against the SARS-CoV-2 spike glycoprotein has extremely high neutralizing titers. |
| Leon et al., 2020 [106] | Development and pre-clinical characterization of two therapeutic equine formulations towards SARS-CoV-2 proteins for the potential treatment of COVID-19. |
The development of an equine serum with fractions of F(ab′)2 for therapeutic use in SARS was reported in 2005 [93]. In this study, two viral strains from humans were isolated, selected for study and used to immunize healthy adult horses. For immunization, they used inactivated viruses with complete and incomplete Freund's adjuvant, according to the established plan. After collection, the plasma was fractionated with ammonium sulfate, dialyzed, digested with pepsin, ultrafiltered, and purified by ion exchange chromatography.
The product of this procedure contained the anti-SARS-CoV-1F(ab′) 2 fractions and was stored at 4 °C. As a result, the horses were able to generate antibodies against the strain by which they were immunized. In addition, these antibodies also neutralized the second strain not used for immunization, and evaluation at the end of the experiments revealed that both strains had some epitopes in common. The peak of IgG production occurred around the seventh week after the first immunization. The recovery rate of plasma F(ab′)2 fractions reached a purity greater than 90%, assessed by SDS-PAGE electrophoresis, concluding that this production process was viable [93].
Some advances have been achieved with the analysis of protective effects of anti-SARS-COV-1F(ab′)2 antibodies, using BALB/c mice as an animal model along with incomplete Freund's adjuvant [96]. A fraction of the animal plasma was collected and the specific anti-SARS antibodies produced by the horses were detected using the Enzyme Linked Immunosorbent Assay (ELISA). The plasma was fractionated with ammonium sulfate, digested with pepsin, followed by thermocoagulation at 57 °C to inactivate the Fc fragments and nonspecific proteins. The product obtained showed 94% purity measured by the folium scan method. In this study, the toxicity of the SARS-COV-1F(ab′)2 antibodies was also assessed by the MTT test (3- (4,5-dimethylthiazol-2yl) -2,5-diphenyl tetrazolium bromide) and the neutralization of SARS-CoV in vitro by F(ab′)2 by the fragments cytopathic effect (CPE), both in Vero E6 cells. The results indicate no toxic effect on Vero E6 cells and that the SARS-COV-1F(ab′)2 antibodies were able to protect the cells from SARS-CoV infection [96].
This anti-SARS-COV-1 serum was able to protect VERO cells in vitro against the viral strain used for immunization as well as another strain, which did not participate in the immunization plan. These data confirmed the information about the similarity between these strains, and how polyclonal antibodies can increase the neutralization capacity [96].
In vivo studies with BALB/c mice reported the analysis of the prophylactic, and therapeutic effects of the anti-SARS-COV-1 serum [96]. In the prophylactic tests, two groups received equine serum: a) serum from horses not immunized against SARS-CoV-1 (control group), and b) serum from horses immunized with purified SARS-CoV-1 particles (BJ-01) and incomplete Freund's adjuvant. In addition, a third group of mice received only the virus and was also used as a second control for the experiment. One day after pre-treatment, three groups, both groups received the SARS-COV-1 virus intranasally since it is a respiratory virus. The SARS-CoV was measured 2 days post-infection by Taqman real-time RT-PCR (Reverse-transcriptase polymerase chain reaction). The dose of 50 µL of anti-SARS-CoV F(ab′)2 fragments was sufficient to neutralize the entire viral load of the animal, a fact that did not occur in the control groups.
Subsequently, another test was carried out to verify the therapeutic properties of equine serum [96]. It was similar to the first test, but in this case, the virus was first inoculated, and the serum was given the next day. As in the previous test, the mice that received the anti-SARS-COV-1 serum were protected from viral infection. In conclusion, the equine anti-SARS-COV-1 serum has been shown to be effective in both preventive and therapeutic studies [96]. It has also been shown that the passive transfer of antibodies from the hyperimmune serum is able to protect mice from SARS-CoV infections, and has a neutralizing activity that can be used in humans. This corroborates the findings of all authors already mentioned previously [96].
Sapkal and collaborators [104] also used the human SARS-CoV-2 virus, inactivated with gamma rays, to immunize horses between 4 and 10 years old. The plasma, which showed a high level of neutralization against SARS-CoV-2, was collected, digested with pepsin and fractionated with caprylic acid. Purity was assessed by SDS-PAGE and antibody titers by ELISA. The product was shown to be pure and effective, capable of recognizing antigen at the maximum dilution of 1: 81,920 of serum. It is a process that can be prepared on a large scale at a lower cost and is promising for testing in humans.
Hamsters are another animal model used to verify the therapeutic effects of the equine anti-SARS serum and represented an important step aiming at carrying out preclinical studies [98]. In a first test, viruses were administered intranasally and analyzed for any response to viral infection. After euthanasia, the lungs of the animals were removed to determine viral titers, by analyzing the CPE, the location where the virus was housed, and the pathology presented by immunohistochemistry [98].
The viruses were monitored until the ninth day of infection, and the animals were responsive to viral infection, with edema, inflammation, and necrosis. A second test aimed to verify the prophylactic effects of anti-SARS-COV-1 equine hyperimmune serum against the virus in this animal model. For this, two groups were tested, the first received the plasma with the antibody of the horse immunized with the virus, and the other was the negative control group, receiving plasma from horses that had not been immunized [98], similarly to previous experiments [96].
In both groups, the serum was inoculated the day before the virus. In the group that received anti-SARS serum, the concentration of 10 mg kg−1 of antibody was able to completely neutralize the inoculated virus on the third day after infection, and without pathological pulmonary changes. The concentration of 5 mg kg−1 neutralized 50% of the viral load, but the animals showed some pulmonary pathology. This highlights the fact that viral neutralization is concentration dependent. As expected, the control group did not present any type of viral protection since they did not receive any specific antibody against SARS-CoV [98].
A third test evaluated the therapeutic effects of the serum. It was similar to the second test, but the serum was inoculated in the animals one day after the viral infection. The concentration of 50 mg kg−1 of serum reduced 4log10 of viral load, with the same results on days 3, and 4 after infection. Increasing the concentration to 100 mg × kg−1 did not yield better results. The study demonstrated that the animal model was susceptible to SARS-CoV, and the severity of pathological manifestations was dependent on the intensity of the viral load, as occurs in humans. The data demonstrated that the Chinese hamster is an efficient animal model for studies with SARS-CoV [98].
The effects of equine serum against a corona virus were also analyzed for MERS-CoV [101]. The horses were immunized with viral particles, resulting from infection of Sf9 cells with recombinant baculovirus that co-expressed genes of proteins S, M, and E, according to a pre-established immunization plan. Two weeks after immunizations, plasmas were collected and analyzed by ELISA to verify the ability of the horse to produce antibodies against the virus. Plasma fractions were collected and purified immunoaffinity chromatography (proteins A, and G), digestion with pepsin, generating a product with 91% purity, evaluated by SDS-PAGE electrophoresis [101].
BALB/c mice were used as an animal model and, in the first study, they were inoculated with strains of MERS-CoV to verify the manifestation of symptoms. Interestingly, the anti-MERS serum showed preventive and therapeutic effects against the MERS-CoV virus. Furthermore, in all three studies, outlined above the efficiency demonstrated for the production of F (ab′) 2 fragments presented a purity rate greater than 90% [93], [96], [101].
In another study by Zhou et al. [97], the preventive and therapeutic role of equine anti-SARS plasma was assessed in elderly mice as an animal model [97]. This study is important because it can simulate the preclinical study for the elderly, whose age is one of the risk factors for this viral infection. Four groups were formed, and the mice received the inoculation of: a) group 1: the virus only (positive control); b) group 2: the plasma one day before, or c) group 3: one day after viral inoculation; and d) group 4: the equine plasma not immunized for SARS (negative control). Mice from the positive and negative control groups (groups 1, and 4) were susceptible to viral infection. The protective and therapeutic behavior of plasma in elderly mice was similar to that observed in previous studies [96], [98], pointing to the safety of the plasma use for the treatment of coronaviruses in the elderly.
The possibility of reinfection was also assessed in BALB/c mice that had primary SARS-CoV infections [95]. The ability of the equine anti-SARS plasma to show a prophylactic effect against the virus infection in mice was also evaluated. In the first experiment, the recovered mice reinfected by the virus showed no evidence of increased viral replication. In a second experiment, two groups of animals were evaluated: a) pre-treated with anti-SARS hyperimmune serum, and b) pre-treated with serum from animals that did not have anti-SARS antibodies. The mice that received serum with anti-SARS antibodies were protected, and the viral replication in the respiratory tract was controlled, a fact that corroborates the therapeutic efficacy of the hyperimmune serum in the treatment of coronavirus infections [95].
In addition, the use of anti-MERS serum from camels in Egypt, and Saudi Arabia against infections of this virus was evaluated in mice [100]. The hyperimmune serum of these animals was able to reduce the infectious process in the mice, demonstrating that there is viral neutralization. The researchers believe that this test can, and should be extrapolated to humans. Since Saudi Arabian camels have high levels of antibodies, without the need for additional exogenous immunization, this therapy can be implemented immediately, and on a large scale [100].
3.2. Therapy with polyclonal and monoclonal antibodies
Therapy with polyclonal antibodies is old, and very well established for the treatment of accidents with venomous animals, and digoxin poisoning [99]. Although this therapy presents risks of hypersensitivity reactions in patients, the production process has been modernized over the years. The animals used as bioreactors are controlled, monitored, kept isolated, and constantly evaluated. Immunoglobulins can be digested, promoting the removal of the Fc fraction, which together with other non-specific proteins are removed from the product. These steps increase the degree of purity whereas the adverse effects are decreased. Importantly, antibodies from these animals are often the only option for emergency treatment [99].
The advantages and disadvantages between polyclonal and monoclonal antibodies are discussed in the literature, including aspects such as specificity and financial issues. The overall conclusion is that the use of polyclonal antibodies is the best therapy in these cases. Monoclonal antibodies target a specific epitope when fighting infection, while polyclonal antibodies have the capacity to neutralize multiple epitopes. The production of monoclonal antibodies is more expensive, and a cocktail of these antibodies is often necessary to achieve the best treatment. In addition, most infected people do not have access to a high value-added medicine such as monoclonals. Another disadvantage of therapy with monoclonal antibodies, compared to polyclonal antibodies is an antigenic escape, since the virus has a great possibility of mutation. These facts make the massive production of monoclonals unfeasible and/or disadvantageous in cases of epidemics and pandemic diseases such as SARS-CoV [37], [89], [95], [96], [99].
Previously mentioned studies [96], [101] concluded that the use of homologous antibodies should be a favored option against therapy with heterologous antibodies, mainly due to the reduced possibility of occurrence of adverse reactions. However, homologous therapy has many disadvantages, including the fact that plasma from convalescent donors presents the risk of viral transmission, such as hepatitis and HIV, and the difficulty in finding enough donors to collect the material from in this therapy, especially in the case of a pandemic. The passive transfer of antibodies from the hyperimmune serum can protect mice from SARS-CoV infections and has a potential neutralizing profile that can be used in humans. This corroborates other authors mentioned previously [94], [96], [98].
3.3. Safety in the use of anti-SARS-Cov sera
The safety and efficacy of administering anti-SARS equine F (ab′) 2 serum in monkeys were also evaluated, which is another major step in a preclinical study [39]. After the production of equine serum, the authors divided the monkeys into two large groups a) 9 monkeys for testing in pharmacokinetic studies, and b) 18 animals, in safety tests. In the pharmacokinetic tests, the half-life of fragments F (ab′) 2 varied from 41 to 46 h [39]. The total clearance was not influenced by the difference in the dose administered, and multiple injections did not affect the concentration × time curve, whereas the pharmacokinetic parameters (mean residence time, volume of distribution, and elimination rate) were constant. In the safety tests, tolerance, and immune responses were similar between the study and the control groups. However, some animals that received the serum showed erythema at the injection site and an enlarged spleen. Importantly, three weeks later, the two groups returned to normal condition and the adverse reactions disappeared. This shows that there was an immune response and that the model animal soon recovered. No tested animal evolved to death by administering the serum. These two results indicated that it is safe to use the equine anti-SARS serum with F (ab́) 2 fragments for the treatment of coronaviruses, as part of a combination therapy with other drugs [39].
The safety and efficacy in the use of polyclonal antibodies for the treatment of neglected tropical diseases are also described in the literature [84]. This is a therapy applied since 1890 against various diseases. Over the years, production processes have been modernized, which has made safer products. The production process follows the same standards as the studies cited above [93], [96] with some adaptations, but they present the common strategy of digesting the antibody and purification, which reduces the possibility of hypersensitivity reactions types 1, and 3. The World Health Organization (WHO) guide provides more than one production route, which always maintains quality during manufacturing processes. This ensures that each producer seeks profitable large-scale production, but with a primary focus on patient safety [81].
In humans, when hyperimmune sera are administered, it takes three weeks for the body to be fully cleared. This is confirmed by clinical trials and post-marketing surveillance [84]. This human study for already commercialized sera, combined with the preclinical studies for SARS and MERS described above increases confidence so that the anti-COVID serum can be evaluated in clinical trials [38], [94], [95], [97], [99], [100], [101].
The hyperimmune sera have been used to treat infections such as tetanus, diphtheria, rabies, accidents with venomous animals, SARS-CoV-1, MERS-CoV, Ebola and avian influenza virus safely and with effective results [104]. Initially, the hyperimmune sera produced generated products that contained fractions of other animal proteins that were not IgG, favoring the manifestations of adverse reactions, compromising the application of the serum safely. Over the years, technical-scientific developments made it possible for productive technologies to incorporate immunoglobulin digestion processes and new purification techniques [84]. As the technology of production evolves, these adverse events, which are no more than 5%, have decreased [84]. Removal of FC fragments reduces the possibility of side effects [104] and as a result, adverse events are rare, as demonstrated, for example, with the administration of anti-rabies serum where 1/45000 treated patients have anaphylactic reactions and less than 0.5% late reactions [84].
3.4. Studies involving SARS-COV-2
Studies have also been undertaken in mice on the antigenicity of the Receptor Binding Domain (RBD) of SARS-CoV-2 [102]. RBD is the protein in the SARS-CoV virus S protein receptor binding domain and binds to the angiotensin-converting enzyme 2 (ACE 2) of the target cell, allowing viral coupling [2], [107]. Equine serum F (ab′) 2 was produced by the use of RBD as an immunogen, and its neutralizing power against SARS-CoV-2 was verified.
RBDs were produced by plasmids, and, together with Freund's adjuvant, were used to immunize mice. Three immunizations were performed within two weeks, and 10 days after each immunization, serum was collected from the animals. The serum was tested by ELISA to monitor the antibody production of the mice. The result of this test confirmed that the RBD generated an immune response so that it was selected as an immunogen for assay with horses. Subsequently, PAN and collaborators [102] used RBD, and complete Freund's adjuvant in the first immunization, and RBD, and incomplete Freund's adjuvant in subsequent immunizations. After seven days of each immunization, serum was collected from the horses, and the titers were evaluated by ELISA.
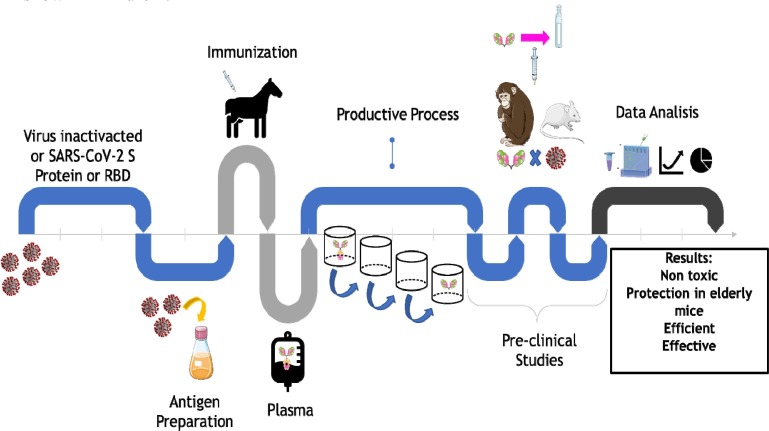
The horses produced antibodies that were incubated and digested by pepsin, fractionation by ammonium sulfate, and submitted to chromatographic purification, to obtain purified F (ab′)2 fragments. These were tested against SARS viruses and produced a neutralizing effect. The authors observed that the horse's plasma was 20 to 100 times more potent than the plasma of convalescent patients with SARS-CoV-2. This advantage, combined with the polyvalent property of several RBD epitopes, make this therapy more promising than the use of monoclonal antibodies and convalescent plasma [37], [89], [90], [91], [96], [99], [101]. The processes involved in studies regarding the evaluation of hyperimmune sera and animal models are shown in Fig. 1 .
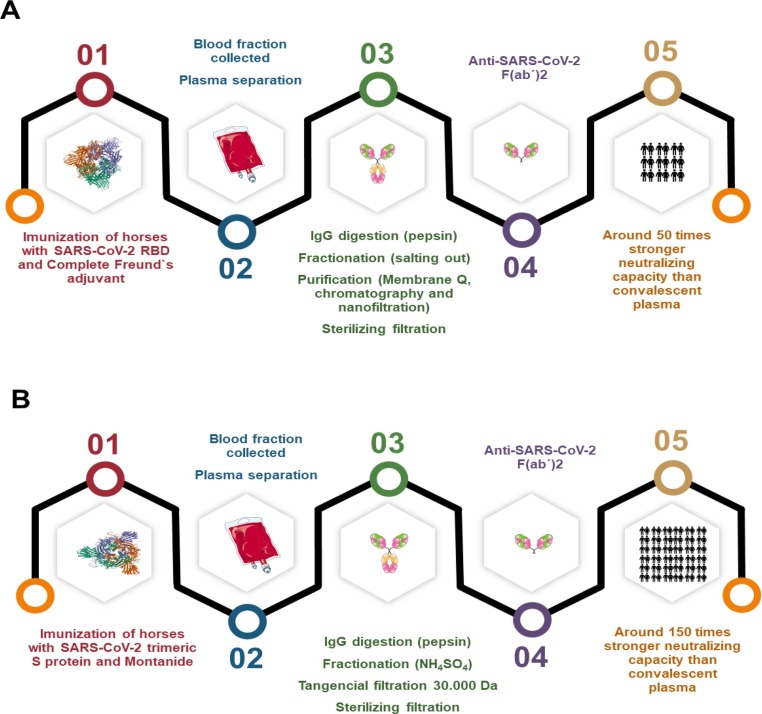
Fig. 1.
Representation of protocols for the production of hyperimmune serum for testing in animal models.
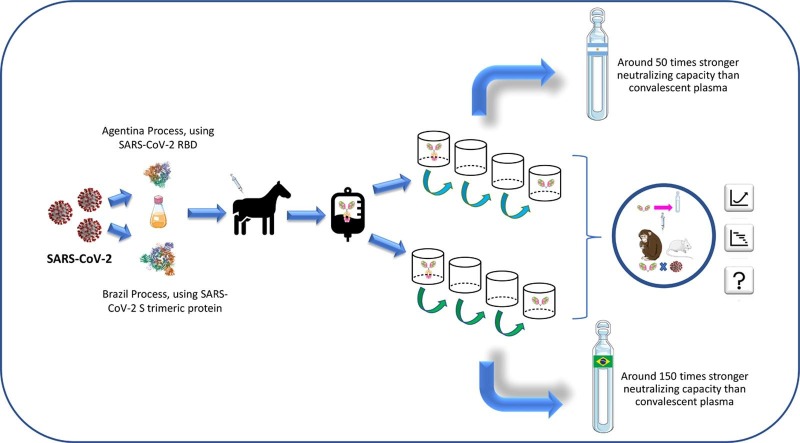
Recently, Zylberman et al. [103] in Argentina produced an anti-SARS-CoV-2 serum and reported the start of phase 2/3 clinical studies in July 2020 [103]. The authors used the same principles of Pan and collaborators [102] to produce RBD as the immunogen. However, they used 10 times less concentration of RBD during the immunization process of the horses without a reduction in the antibody titers generated. This is advantageous in a large-scale production process since the animal goes through several immunization processes. The less antigen received to maintain adequate antibody production, the less adverse effects of immunization can be seen, which is important for animal welfare [102], [103].
The researchers drew up an immunization plan and observed that the antibody titers produced were higher after the second immunization. They reached a plateau after the third immunization, which determined the animals bleeding period at 34 days. The antibody levels were monitored by ELISA and the fraction of plasma underwent a process of digestion with pepsin, saline fractionation by salting out, purification with chromatography with Q membrane, nanofiltration, and sterilizing filtration. The product serum had ca. 50 times stronger neutralizing capacity of SARS-COV-2 than that of convalescent plasma [103]. These F(ab′) 2 antibodies are known to be safe as they are devoid of the Fc domain and avoid triggering of antibody dependent cytokine storms [103].
Recently, Cunha et al. [105] in a study involving two different Brazilian research institutions with expertise on hyperimmune serum production, the Instituto Vital Brazil (IVB) and the Federal University of Rio de Janeiro (UFRJ) have produced an anti-SARS-COV-2 equine serum, following the same technological platform for the production of anti-rabies serum at IVB. Horses were immunized with the trimeric spike glycoprotein of SARS-COV-2 and evaluated for antibody production [105].
A first experimental batch of serum was produced for the concept tests, in order to prove that the production process does not inactivate the purified antibodies (Fig. 2 ).
Fig. 2.
Schematic representation of the hyperimmune serum production processes. A – Production processes by Zylberman et al. [103]. B – Production processes by Cunha et al. [105]. Protein images were obtained from the Protein Data Bank (A: <https://www.rcsb.org/3d-view/6XM4/1>). (B: <https://www.rcsb.org/3d-view/6ZOZ/1>).
During this production process, the plasma was digested with pepsin, fractionated with ammonium sulfate, and ultrafiltered, resulting in a concentrate of F (ab′) 2 antibodies. The neutralization titer exceeded that of the convalescent plasma from three Brazilian donors by up to 150 times, demonstrating the efficiency of the process, and enhancing the prospect of the next stages of pre-clinical, and clinical studies [103]. Fig. 2 illustrates the production processes of anti-COVID-19 hyperimmune serum by Zylberman et al. [103] (Fig. 2 A) and Cunha et al. [105] (Fig. 2B).
The data obtained indicate that the production method of the hyperimmune serum produced by Cunha et al. [105] generates a serum with greater immunogenic potency compared to the method used by Zylberman et al. [103].
4. Conclusions
The potential for spreading SARS-CoV-2 with many cases evolving to death in a short period of time, coupled with the financial and logistical inequalities for the acquisition of the vaccine among nations, as well as the increasing anti-vaccine movements, make the use of hyperimmune serum a highly promising therapy against COVID-19. In addition, this strategy is strongly feasible as a plan B, especially when plan A (vaccines) are not available in the frontline and/or are not totally accepted by the population, and/or people are already infected by the virus.
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
Acknowledgments
The authors would like to thank the National Council for Scientific, and Technological Development (CNPq), Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro (FAPERJ - E-26/202.447/2019), Instituto Vital Brazil, and Universidade Federal Fluminense for the support provided for the development of this research.
References
- 1.Yuan X.i., Yang C., He Q., Chen J., Yu D., Li J., Zhai S., Qin Z., Du K.e., Chu Z., Qin P. Current and perspective diagnostic techniques for COVID-19. ACS Infect. Dis. 2020;6(8):1998–2016. doi: 10.1021/acsinfecdis.0c00365. [DOI] [PubMed] [Google Scholar]
- 2.Ahidjo B.A., Loe M.W.C., Ng Y.L., Mok C.K., Chu J.J.H. Current Perspective of antiviral strategies against COVID-19. ACS Infect. Dis. 2020;6(7):1624–1634. doi: 10.1021/acsinfecdis.0c00236. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Q.A., Kato-Weinstein J., Li Y., Deng Y.i., Granet R., Garner L., Liu C., Polshakov D., Gessner C., Watkins S. Potential therapeutic agents and associated bioassay data for COVID-19 and related human coronavirus infections. ACS Pharmacol. Transl. Sci. 2020;3(5):813–834. doi: 10.1021/acsptsci.0c00074.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: A review. Clinical Immunol. 2020;215:108427. doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zumla A., Chan J.F.W., Azhar E.I., Hui D.S.C., Yuen K.-Y. Coronaviruses — drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15(5):327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.AminJafari A., Ghasemi S. The possible of immunotherapy for COVID-19: A systematic review. Int. Immunopharmacol. 2020;83:106455. doi: 10.1016/j.intimp.2020.106455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felsenstein S., Herbert J.A., McNamara P.S., Hedrich C.M. COVID-19: Immunology and treatment options. Clinical Immunol. 2020;215:108448. doi: 10.1016/j.clim.2020.108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Y. Kasmi, K. Khataby, A. Souiri, M.M. Ennaji, Chapter 7: Coronaviridae: 100 years of emergence, and re-emergence, in: M.M. Ennaji (Ed.), Emerging, and Reermeging Viral Pathogens, Volume 1: Fundamental, and Basic Virology Aspects of Human, Animal, and Plant Pathogens, 2020, pp. 127–149. https://reader.elsevier.com/reader/sd/pii/B9780128194003000077?token=F0BA95214E32950AC1A799527F7D66F8067B7729B1DF8908366E9F0A1A4DFF49C41FECEE558821C77DD18DEFF1184116.
- 9.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., Pan P., Wang W., Hu D., Liu X., Zhang Q., Wu J. Coronavirus infections and immune responses. J. Med. Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.L. Yang, Y. Zhang, Y. Wei, Y. Li, X. Wang, Y. Liu, D. Tian, X. Jia, R. Gong, W. Liu, IgY antibodies against Ebola virus possess post-exposure protection, and excellent thermostability. bioRxiv, 2020. Preprint at https://www.researchgate.net/publication/341570625_IgY_antibodies_against_Ebola_virus_possess_post-exposure_protection_, and_ excellent_thermostability. doi: https://doi.org/10.1101/2020.05.21.108159. [DOI] [PMC free article] [PubMed]
- 11.Zhang J.-Z., Wang X.-M., Xing X., Xu Z., Zhang C., Song J.-W., Fan X., Xia P., Fu J.-L., Wang S.-Y., Xu R.-N., Dai X.-P., Shi L.-S., Huang L., Jiang T.-J., Shi M., Zhang Y., Zumla A., Maeurer M., Bai F., Wang F.-S. Single-cell l, andscape of immunological responses in patients with COVID-19. Nat. Immunol. 2020;21:1107–1118. doi: 10.1038/s41590-020-0762-x. [DOI] [PubMed] [Google Scholar]
- 12.Falcinelli S.D., Chertow D.S., Kindrachuk J. (2016) Integration of global analyses of host molecular responses with clinical data to evaluate pathogenesis, and advance therapies for emerging, and re-emerging viral infections. ACS Infect. Dis. 2016;2(11):787–799. doi: 10.1021/acsinfecdis.6b00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behzadi M.A., Leyva-Grado V. Overview of current therapeutics, and novel c, andidates against influenza, respiratory syncytial virus, and Middle East respiratory syndrome coronavirus infections. Front. Microbiol. 2019;10:1327. doi: 10.3389/fmicb.2019.01327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song Z., Xu Y., Bao L., Zhang L., Yu P., Qu Y., Zhu H., Zhao W., Han Y., Qin C. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11(1):59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J., Shi P.-Y., Li H., Zhou J. Broad spectrum antiviral agent niclosamide, and its therapeutic potential. ACS Infect. Dis. 2020;6(5):909–915. doi: 10.1021/acsinfecdis.0c00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva S.J.R., Silva C.T.A., Guarines K.M., Mendes R.P.G., Pardee K., Kohl A., Pena L. Clinical, and laboratory diagnosis of SARS-CoV-2, the virus causing COVID-19. ACS Infect. Dis. 2020;2020 doi: 10.1021/acsinfecdis.0c00274. [DOI] [PubMed] [Google Scholar]
- 17.Gupta T., Gupta S.K. Potential adjuvants for the development of a SARS-CoV-2 vaccine based on experimental results from similar coronaviruses. Int. Immunopharmacol. 2020;86:106717. doi: 10.1016/j.intimp.2020.106717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schrank C.L., Minbiole K.P., Wuest W.M. Are quaternary ammonium compounds, the workhorse disinfectants, effective against Severe Acute Respiratory Syndrome-Coronavirus-2? ACS Infect. Dis. 2020;6(7):1553–1557. doi: 10.1021/acsinfecdis.0c00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization (WHO), Q, and A: How is COVID-19 transmitted? 2020, https://www.who.int/news-room/q-a-detail/q-a-how-is-covid-19-transmitted.
- 20.WHO, WHO Coronavirus Disease (COVID-19) Dashboard, 2020. https://covid19.who.int/.
- 21.Xiong T.Y., Redwood S., Prendergast B., Chen M. Coronaviruses, and the cardiovascular system: acute, and long-term implications. Eur. Heart J. 2020;41:1798–1800. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu H., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course, and risk factors for mortality of adults inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prekumar L., Segovia-Chumbez B., Jadi R., Martinez D.R., Raut R., Markmann A., Cornaby, Bartelt L., Weiss S., Park Y., Edwards C.E., Weimer E., Scherer E.M., Rouphael N., Edupuganti S., Weiskopf D., Tse L.V., Hou Y.J., Margolis D., Sette A., Collins M.H., Schmitz J., Baric R.S., de Silva A.M. The receptor binding domain of the viral spike protein is an immunodominant, and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 2020;5(48) doi: 10.1126/sciimmunol.abc8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saul S., Einav S. Old drugs for a new virus: repurposed approaches for combating COVID-19. ACS Infect. Dis. 2020 doi: 10.1021/acsinfecdis.0c00343. [DOI] [PubMed] [Google Scholar]
- 25.Choy K.T., Wong A.Y.L., Kaewpreedee P., Sia S.F., Chen D., Hui K.P.Y., Chu D.K.W., Chan M.C.W., Cheung P.P.-H., Huang X., Peiris M., Yen H.-L. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir Res. 2020;178:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shannon A., Le N.T.T., Selisko B., Eydoux C., Alvarez K., Guillemot J.C., Decroly E., Peersen O., Ferron F., Canard B. Remdesivir, and SARS-CoV-2: Structural requirements at both nsp12 RdRp, and nsp14 Exonuclease active-sites. Antivir Res. 2020;178:104793. doi: 10.1016/j.antiviral.2020.104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pizzorno A., Padey B., Dubois J., Julien T., Traversier A., Dulière V., Brun P., Lina B., Rosa-Calatrava M., Terrier O. In vitro evaluation of antiviral activity of single, and combined repurposable drugs against SARS-CoV-2. Antivir. Res. 2020:104878. doi: 10.1016/j.antiviral.2020.104878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sallard E., Lescure F.X., Yazdanpanah Y., Mentre F., Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antivir Res. 2020;178:104791. doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H., Chen C., Hu F., Wang J., Zhao Q., Gale R.P., Liang Y. Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review, and meta-analysis. Leukemia. 2020:1–9. doi: 10.1038/s41375-020-0848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zha L., Li S., Pan L., Tefsen B., Li Y., French N., Chen L., Yang G., Villanueva E.V. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19) Med. J. Australia. 2020;212(9):416–420. doi: 10.5694/mja2.50577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan S.A., Zia K., Ashraf S., Uddin R., Ul-Haq Z. Identification of chymotrypsin-like protease inhibitors of SARS-CoV-2 via integrated computational approach. J. Biomol. Struct. Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1751298. [DOI] [PubMed] [Google Scholar]
- 32.Jiménez-Alberto A., Ribas-Aparicio R.M., Aparicio-Ozores G., Castelán-Vega J.A. Virtual screening of approved drugs as potential SARS-CoV-2 main protease inhibitors. Comput. Biol. Chem. 2020:107325. doi: 10.1016/j.compbiolchem.2020.107325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chai K.L., Valk S.J., Piechotta V., Kimber C., Monsef I., Doree C., Wood E.M., Lamikanra A.A., Roberts D.J., McQuilten Z., So-Osman C., Estcourt L.J., Skoetz N. Convalescent plasma or hyperimmune immunoglobulin for people with COVID‐19: a living systematic review. Cochrane Database Syst. Rev. 2020;(10) doi: 10.1002/14651858.CD013600.pub3. [DOI] [PubMed] [Google Scholar]
- 34.Farrugia A., MacPherson J., Busch M.P. Convalescent plasma - this is no time for competition. Transfusion. 2020;60(7):1644–1646. doi: 10.1111/trf.15922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabaan A. Middle East respiratory syndrome coronavirus: five years later. Expert Rev Respir Med. 2017;11(11):901–912. doi: 10.1080/17476348.2017.1367288. [DOI] [PubMed] [Google Scholar]
- 36.De Alwis R., Chen S., Gan E., Ooi E.O. Impact of immune enhancement on Covid-19 polyclonal hyperimmune globulin therapy, and vaccine development. EBioMedicine. 2020;55:102768. doi: 10.1016/j.ebiom.2020.102768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berry J., Gaudet R. Antibodies in infectious diseases: polyclonals, monoclonals, and niche biotechnology. New Biotechnol. 2011;28(5):489–501. doi: 10.1016/j.nbt.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeFrancesco L. COVID-19 antibodies on trial. Nat. Biotechnol. 2020;38(11):1242–1252. doi: 10.1038/s41587-020-0732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y., Jia Z., Zhou L., Wang L., Li J., Liang Y., Zhao T.T., Ni B., Wu Y. Evaluation of the safety, immunogenicity, and pharmacokinetics of equine anti-Sars–Cov F(Ab′)2 in macaque. Int. Immunopharmacol. 2007;7:1834–1840. doi: 10.1016/j.intimp.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO, Q, and A on coronaviruses (COVID-19), 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-,and-answers-hub/q-a-detail/q-a-coronaviruses.
- 41.Kuri-Cervantes L., Pampena M.B., Meng W., Rosenfeld A.M., Ittner C.A.G., Weisman A.R., Agyekum R.S., Mathew D., Baxter A.E., Vella L.A., Kuthuru O., Apostolidis S.A., Bershaw L., Dougherty J., Greenplate A.R., Pattekar A., Kim J., Han N., Gouma S., Weirick M.E., Arevalo C.P., Bolton M.J., Goodwin C.E., Anderson E.M., Hensley S.E., Jones T.K., Mangalmurti N.S., Prak E.T.L., Wherry E.J., Meyer N.J., Betts M.R. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 2020;5(4.9):eabd7114. doi: 10.1126/sciimmunol.abd7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.di Mauro G., Scavone C., Concetta R., Francesco R., Annalisa C. SARS-Cov-2 infection: Response of human immune system, and possible implications for the rapid test, and treatment. Int. Immunopharmacol. 2020;84:106519. doi: 10.1016/j.intimp.2020.106519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin X., Lian J.-S., Hu J.-H., Gao J., Zheng L., Zhang Y.-M., Hao S.-R., Jia H.-Y., Cai H., Zhang X.-L., Yug G.D., Xu K.-J., Wang X.-Y., Gu J.-Q., Zhang S.-Y., Ye C.-Y., Jin C.-L., Lu Y.-F., Yu X., Yu X.-P., Huang J.-R., Xu K.-L., Ni Q., Yu C.-B., Zhu B., Li Y.-T., Liu J., Zhao H., Zhang X., Yu L., Guo Y.-Z., Su J.-W., Tao J.-J., Lang G.-J., Wu X.-X., Wu W.-R., Qv T.-T., Xiang D.-R., Yi P., Shi D., Chen Y., Ren Y., Qiu Y.-Q., Li L.-J., Sheng J., Yang Y. Epidemiological, clinical, and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menni M., Valdes A.M., Freidin M.B., Sudre C.H., Nguyen L.H., Drew D.A., Ganesh S., Varsavsky T., Cardoso M.J., Moustafa J.S.E.-S., Visconti A., Hysi P., Bowyer R.C.E., Mangino M., Falchi M., Wolf J., Ourselin S., Chan A.T., Steves C.J., Spector T.D. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat. Med. 2020;26:1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothan H.A., Byrareddy S.N. The epidemiology, and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freitas T.F., Durie I.A., Murray J., Longo J.E., Miller H.C., Crich D., Hogan R.J., Tripp R.A., Pegan S.D. Characterization, and noncovalent inhibition of the deubiquitinase, and de ISGylase activity of SARS-CoV-2 papain-like protease. ACS Infect. Dis. 2020;6(8):2099–2109. doi: 10.1021/acsinfecdis.0c00168. [DOI] [PubMed] [Google Scholar]
- 47.Roche J.A., Roche R. A hypothesized role for dysregulated bradykinin signaling in COVID-19 respiratory complications. FASEB J. 2020;34(6):7265–7269. doi: 10.1096/fj.202000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michael R. Garvin, Christiane Alvarez, J. Izaak Miller, Erica T. Prates, Angelica M. Walker, B. Kirtley Amos, Alan E. Mast, Amy Justice, Bruce Aronow, Daniel Jacobson. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm, eLife Computational and Systems Biology Medicine, July 7 2020. [DOI] [PMC free article] [PubMed]
- 49.Boodoosingh R., Olayemi L.O., Sam F.A. COVID-19 vaccines: Getting Anti-vaxxers involved in the discussion. World Dev. 2020:105177. doi: 10.1016/j.worlddev.2020.105177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Queiroz N.M.G.P., Marinho F.V., Chagas M.A., Leite L.C.C., Homan E.J., de Magalhães M.T.Q., Oliveira S.C. Vaccines for COVID-19: perspectives from nucleic acid vaccines to BCG as delivery vector system. Microbes Infect. 2020 doi: 10.1016/j.micinf.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Putter J.S., Seghatchian J. An update on COVID-19 infection control measures, plasma-based therapeutics, corticosteroid pharmacotherapy and vaccine research. Transfus Apher Sci. 2020;4:102934. doi: 10.1016/j.transci.2020.102934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ita K. Coronavirus Disease (COVID-19): Current Status and Prospects for Drug and Vaccine Development. Arch. Med. Res. 2020:31154–31161. doi: 10.1016/j.arcmed.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sultana J., Mazzaglia G., Luxi N., Cancellieri A., Capuano A., Ferrajolo C., de Waure C., Ferlazzo G., Trifirò G. Potential effects of vaccinations on the prevention of COVID-19: rationale, clinical evidence, risks and public health considerations. Expert Rev Vaccines. 2020;17 doi: 10.1080/14760584.2020.1825951. [DOI] [PubMed] [Google Scholar]
- 54.Tregoning J.S., Brown E.S., Cheeseman H.M., Flight K.E., Higham S.L., Lemm N.M., Pierce B.F., Stirling D.C., Wang Z., Pollock K.M. Vaccines for COVID-19. Clin. Exp. Immunol. 2020;15 doi: 10.1111/cei.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gouglas Dimitrios, Le Tung Thanh, Henderson Klara, Kaloudis Aristidis, Danielsen Trygve, Hammersland Nicholas Caspersen, Robinson James M., Heaton Penny M., Røttingen John-Arne. Estimating the cost of vaccine development against epidemic infectious diseases: a cost minimisation study. The Lancet. 2018;6(12):e1386–e1396. doi: 10.1016/S2214-109X(18)30346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shin M.D., Shukla S., Chung Y.H., Beiss V., Chan S.K., Ortega-Rivera O.A., Wirth D.M., Chen A., Sack M., Pokorski N.F., Steinmetz N.F. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020;15:646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 57.Dubé E., Vivion M., Macdonald N. Vaccine hesitancy, vaccine refusal and the anti-vaccine movement: influence, impact and implications. Expert Rev. Vaccines. 2015;14(11):99–117. doi: 10.1586/14760584.2015.964212. [DOI] [PubMed] [Google Scholar]
- 58.Edelstein M., Müller M., Ladhani S., Yarwood J., Salathé M., Ramsay M. Keep calm and carry on vaccinating: Is anti-vaccination sentiment contributing to declining vaccine coverage in England? Vaccine. 2020;38(33):5297–5304. doi: 10.1016/j.vaccine.2020.05.082. [DOI] [PubMed] [Google Scholar]
- 59.Khan Y.H., Mallhi T.H., Alotaibi N.H., Alzarea A.I., Alanazi A.S., Tanveer N., Hashmi F.K. Threat of COVID-19 Vaccine hesitancy in pakistan: the need for measures to neutralize misleading narratives. Am. J. Trop. Med. Hygiene. 2020;103(2):603–604. doi: 10.4269/ajtmh.20-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puri N., Coomes E.A., Haghbayan H., Gunaratne K. Social media and vaccine hesitancy: new updates for the era of COVID-19 and globalized infectious diseases. Human Vaccines Immunotherapeutics. 2020:1–8. doi: 10.1080/21645515.2020.1780846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hotez P.J. COVID19 meets the Antivaccine Movement. Microbes Infect. 2020;22(4):162–164. doi: 10.1016/j.micinf.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.T.K. Burki, The Russian vaccine for COVID-19. The Lancet Respiratory Medicine. https://doi.org/10.1016/S2213-2600(20)30402-1. [DOI] [PMC free article] [PubMed]
- 63.Bonam S.R., Kaveri S.V., Sakuntabhai A., Gilardin L., Bayry J. Adjunct immunotherapies for the management of severely ill Covid-19 patients. Cell Reports Medicine. 2020;1:100016. doi: 10.1016/j.xcrm.2020.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lotfi M., Hamblin M.R., Rezaei N. COVID-19: Transmission, prevention, and potential therapeutic opportunities. Clin. Chim. Acta. 2020;508:254–266. doi: 10.1016/j.cca.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gasparyan A., Misra D., Yessirkepov M., Zimba O. Perspectives of immune therapy in coronavirus disease 2019. J. Korean Med. Sci. 2020;35(18):e176. doi: 10.3346/jkms.2020.35.e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kazatchkine M.D., Goldman M., Vincent J.-L. Antibody-based therapies for Covid-19: Can Europe move faster? PLoS Med. 2020;17:E1003127. doi: 10.1371/journal.pmed.1003127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nguyen A.A., Habiballah S.B., Platt C.D., Geha R.S., Chou J.S., Mcdonald D.R. Immunoglobulins in the treatment of Covid-19 infection: Proceed with caution! Clin Immunol. 2020;126:108459. doi: 10.1016/j.clim.2020.108459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker L.M., Burton D.R. Passive immunotherapy of viral infections:'super-antibodies' enter the fray. Nat. Rev. Immunol. 2018;18(5):297. doi: 10.1038/nri.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dyall J., Gross R., Kindrachuk J., Johnson R., Olinger G., Hensley L., Frieman M., Jahrling P. Middle East respiratory syndrome, and severe acute respiratory syndrome: current therapeutic options, and potential targets for novel therapies. Drugs. 2017;77(18):1935–1966. doi: 10.1007/s40265-017-0830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saghazadeh A., Rezaei N. Towards treatment planning of Covid-19: Rationale, and hypothesis for the use of multiple immunosuppressive agents: anti-antibodies, immunoglobulins, and corticosteroids. Int. Immunopharmacol. 2020;84:1065601. doi: 10.1016/j.intimp.2020.106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goodarzi P., Mahdavi F., Mirzaei R., Hasanv H., Sholeh M., Zamani F., Sohrabi M., Tabibzadeh A., Jeda A.S., Niya M.H.K., Keyvani H., Karampoor S. Coronavirus disease 2019 (COVID-19): Immunological approaches, and emerging pharmacologic treatments. Int. Immunopharmacol. 2020;88 doi: 10.1016/j.intimp.2020.106885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castelli M.S., McGonigle P., Hornby P.J. The pharmacology, and therapeutic applications of monoclonal antibodies. Pharmacol. Res. Perspect. 2019;7(6):e00535. doi: 10.1002/prp2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han H.J., Liu J.W., Yu H., Yu X.J. Neutralizing monoclonal antibodies as promising therapeutics against middle east respiratory syndrome coronavirus infection. Viruses. 2018;10(12):680. doi: 10.3390/v10120680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lafaye P., Li T. Use of camel single-domain antibodies for the diagnosis, and treatment of zoonotic diseases. Comp immunol microb. 2018;60:17–22. doi: 10.1016/j.cimid.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alirahimi E., Kazemi-Lomedasht F., Shahbazzadeh D., Habibi-Anbouhi M., Hosseininejad M., Sotoudeh N., Ghaderi H., Muyldermans S., Behdani M. (2018) Nanobodies as novel therapeutic agents in envenomation. Bioch Biophys Acta, Gen Subj. 1862;12:2955–2965. doi: 10.1016/j.bbagen.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 76.Luiz M., Pereira S., Prado N., Gonçalvez N., Kayano A., Moreira-Dill L., Sobrinho J., Zanchi F., Fuly A., Fernandes C., Zuliani J., Soares A., Stabeli R., Fernandes C. Camelid single-domain antibodies (VHHs) against crotoxin: A basis for developing modular building blocks for the enhancement of treatment or diagnosis of crotalic envenoming. Toxins. 2018;10(4):142. doi: 10.3390/toxins10040142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.He L., Tai W., Li J., Chen Y., Gao Y., Li J., Chen Y., Gao Y., Li J., Sun S., Zhou Y., Du L., Zhao G. Enhanced ability of oligomeric nanobodies targeting MERS coronavirus receptor-binding domain. Viruses. 2019;11(2):166. doi: 10.3390/v11020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Vlieger D., Ballegeer M., Rossey I., Schepens B., Saelens X. Single-domain antibodies, and their formatting to combat viral infections. Antibodies. 2019;8(1):1. doi: 10.3390/antib8010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pelletier J.P.R., Mukhtar F. Immunologic Concepts in Transfusion Medicine. 2020. Passive monoclonal, and polyclonal antibody therapies; pp. 251–348. [DOI] [Google Scholar]
- 80.Nascimento A., Pinto I.F., Chu V., Aires-Barros M.R., Conde J.P., Azevedo A.M. Studies on the purification of antibody fragments. Sep Purific Technol. 2018;195:388–397. doi: 10.1016/j.seppur.2017.12.033. [DOI] [Google Scholar]
- 81.WHO, Guidelines for the production, control, and regulation of snake antivenom immunoglobulins, 2017. http://www.who.int/bloodproducts/snake_antivenoms/snakeantivenomguide. [DOI] [PubMed]
- 82.Gutiérrez J.M., León G., Angulo Y. Antivenoms for snakebite envenomings. Inflamm. Allergy Drug Targets. 2011;10(5):369–380. doi: 10.2174/187152811797200669. [DOI] [PubMed] [Google Scholar]
- 83.Squaiella-Baptistão C.C., Sant'Anna O.A., Marcelino J.R., Tambourgi D.V. The history of antivenoms development: Beyond Calmette, and Vital Brazil. Toxicon. 2018;150:86–95. doi: 10.1016/j.toxicon.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 84.Dixit R., Herz J., Dalton R., Booy R. Benefits of using heterologous polyclonal antibodies, and potential applications to new, and undertreated infectious pathogens. Vaccine. 2016;34(9):1152–1161. doi: 10.1016/j.vaccine.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Instituto Vital Brazil, Soros, 2020. http://www.vitalbrazil.rj.gov.br/soros_produzidos.htm.
- 86.Instituto Butantan, Soros e vacinas, 2020. http://www.butantan.gov.br/soros-e-vacinas/soros.
- 87.Fundação Ezequiel Dias, Produtos, 2020. http://www.funed.mg.gov.br/produtos-2/.
- 88.E. SM, L.A. El-Tantawy, W.R. Abdel Azis, I. HM, O. AA Al-Shamandy, Evaluation of anti-rabies hyperimmune serum prepared using different adjuvants. JAVS. 5 (2020)17–21. doi: https://doi.org/10.21608/JAVS.2020.21969.1003.
- 89.Racine T., Denizot M., Pannetir D., Nguyen L., Pasquier A., Raoul H., Saluzzo J.-F., Kobinger G., Veas F., Herbreteau C. In vitro characterization, and in vivo effectiveness of ebola virus specific equine polyclonal F(ab′)2. J. Infect. Dis. 2019;220(1):41–45. doi: 10.1093/infdis/jiz068. [DOI] [PubMed] [Google Scholar]
- 90.Zheng X., Wong G., Zhao Y., Wang H., He S., Bi Y., Chen W., Jin H., Gai W., Chu D., Cao Z., Wang C., Fan Q., Chi H., Gao Y., Wang T., Feng N., Yan F., Huang G., Zheng Y., Li N., Li Y., Qian J., Zou Y., Kobinger G., Gao G.F., Qiu X., Yang S., Xia X. Treatment with hyperimmune equine immunoglobulin or immunoglobulin fragments completely protects rodents from Ebola virus infection. Sci. Rep. 2016;6(1):1–11. doi: 10.1038/srep24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang H., Wong G., Zhu W., He S., Zhao Y., Yan F., Niaz M., Yuhai R., Zirui B., Kending Z., Hongli C., Zengguo J., Xuexing C., Weiwei G., Bai J., Chen W., Zou Y., Gao Y., Gao G., Yang S., Xia X., Qiu X. Equine-origin immunoglobulin fragments protect nonhuman primates from Ebola virus disease. J. Virol. 2019;93(5):e01548–e01618. doi: 10.1128/JVI.01548-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mirza K.A., Mehmood M., Anwar H., Noreen S. Raising of polyclonal hyperimmune sera in broilers against avian influenza virus subtypes H5N1, H7N3, H9N2, and Newcastle disease virus for diagnostics, and therapeutics. IJVSAH. 2018;3(5):95–99. [Google Scholar]
- 93.Lu J.-H., Guo Z.-M., Han W.-Y., Wang G.-L., Zhang D.-M., Wang Y.-F., Sun S.-Y., Yang Q.-H., Zheng H.-Y., Wong B.-L., Zhong N.-S. Preparation, and development of equine hyperimmune globulin F (ab′) 2 against severe acute respiratory syndrome coronavirus 1. Acta Pharmacol. Sin. 2005;26(12):1479–1484. doi: 10.1111/j.1745-7254.2005.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peiris J., Guan Y., Yuen K. Severe acute respiratory syndrome. Nat. Med. 2004;10(12):S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Subbarao K., Mcauliffe J., Vogel L., Fahle G., Fisher S., Tatti K., Packard M., Shieh W.-Ju., Zaki S., Murphy B. Prior infection, and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J. Virol. 2004;78(7):3572–3577. doi: 10.1128/jvi.78.7.3572-3577.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang X., Ni B., Du X., Zhao G., Gao W., Shi X., Zhang S., Zhang L., Wang D., Luo D., Xing L., Jiang H., Li W., Jiang M., Mao L., He Y., Xiao Y., Wu Y. Protection of mammalian cells from severe acute respiratory syndrome coronavirus infection by equine neutralizing antibody. Antivir Ther. 2005;10(5):681–690. PMID: 16152762. [PubMed] [Google Scholar]
- 97.Zhou L., Ni B., Luo D., Zhao G., Jia Z., Zhang L., Lin Z., Wang L., Zhang S., Xing L., Li J., Liang Y., Shi X., Zhao T.T., Zhou L., Wu Y., Wang X. Inhibition of infection caused by severe acute respiratory syndrome-associated coronavirus by equine neutralizing antibody in aged mice. Int. Immunopharmacol. 2007;7(3):392–400. doi: 10.1016/j.intimp.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luo D., Ni B., Zhao G., Jia Z., Zhou L., Pacal M., Zhang S., Xing L., Lin Z., Wang L., Li J., Liang Y., Shi X., Zhao T., Zou L., Wu Y., Wang X. Protection from infection with severe acute respiratory syndrome coronavirus in a Chinese hamster model by equine neutralizing F(ab′)2. Viral Immunol. 2007;20(3):495–502. doi: 10.1089/vim.2007.0038. [DOI] [PubMed] [Google Scholar]
- 99.Newcombe C., Newcombe A. Antibody production: polyclonal-derived biotherapeutics. J. Chromatogr. B. 2007;848(1):2–7. doi: 10.1016/j.jchromb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 100.Zhao J., Perera R., Kayali G., Meyerholz D., Perlman S., Peiris M. Passive immunotherapy with dromedary immune serum in an experimental animal model for Middle East respiratory syndrome coronavirus infection. J. Virol. 2015;89(11):6117–6120. doi: 10.1128/JVI.00446-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao Y., Wang C., Qiu B., Li C., Wang H., Jin H., Gai W., Zheng X., Wang T., Sun W., Yan F., Gao Y., Wang Q., Yan J., Chen L., Perlman S., Zhong N., Zhao J., Xia X. Passive immunotherapy for Middle East Respiratory Syndrome coronavirus Infection with equine immunoglobulin or immunoglobulin fragments in a mouse model. Antivir Res. 2017;137:125–130. doi: 10.1016/j.antiviral.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pan X., Zhou P., Fan T., Wu Y., Zhang J., Shi X., Shang W., Fang L., Jiang X., Shi J., Sun Y., Zhao S., Cong R., Chen Z., Xiao G. Immunoglobulin Fragment F (ab')2 Against Rbd Potently Neutralizes Sars-Cov-2 in vitro. Antivir Res. 2020:104868. doi: 10.1016/j.antiviral.2020.104868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zylberman V., Sanguineti S., Pontoriero A., Higa S., Cerutti M., Seijo S., Pardo R., Muñoz L., Intrieri M., Alzogaray V., Avaro M., Benedetti E., Berguer P., Bocanera L., Bukata L., Bustelo M., Campos A., Colonna M., Correa E., Cragnaz L., Dattero M., Dellafiori M., Foscaldi S., González J., Guerra L., Klinke S., Labanda M., Lauché C., López J., Martínez A., Otero L., Peyric E., Ponziani P., Ramondino R., Rinaldi J., Rodríguez S., Russo J., Russo M., Saavedra S., Seigelchifer M., Sosa S., Vilariño C., Biscayart P., Corley E., Spatz L., Baumeister E., Goldbaum F. Development of a hyperimmune equine serum therapy for COVID-19 in Argentina. Medicina. 2020;80:1–6. PMID: 32658841. [PubMed] [Google Scholar]
- 104.G. Sapkal, A. Yadav, G.R. Deshpande, P.D. Yadav, M.K. Deshpande, M. Phagiwala, R. Jain, A. Shete, N. Gupta, S. Ponnuru, K. Palakurthi, V. Paradkar, P. Abraham, Development of equine antisera with high neutralizing activity against SARS-CoV-2, 2020. Preprint at: https://www.researchsquare.com/article/rs-83582/v1. doi: https://doi.org/10.21203/rs.3.rs-83582/v1.
- 105.L.E.R. Cunha, A.A. Stolet, M.A. Strauch, V.A.R. Pereira, C.H. Dumard, P.N.C. Souza, J. G. Fonseca, F.E. Pontes, L.G.R. Meirelles, J.W.M. Albuquerque, C.Q. Sacramento, N. Fintelman-Rodrigues, T.M. Lima, R.G.F. Alvim, R.B. Zingali, G.A.P. Oliveira, T.M.L. Souza, A. Tanuri, A.M.O. Gomes, A.C. Oliveira, H.L.M. Guedes, L.R. Castilho, J.L. Silva, Equine hyperimmune globulin raised against the SARS-CoV-2 spike glycoprotein has extremely high neutralizing titers, 2020. Preprint at https://www.biorxiv.org/content/10.1101/2020.08.17.254375v1.full.pdf+html. doi: https://doi.org/10.1101/2020.08.17.254375.
- 106.G. León, M. Herrera, M. Vargas, M. Arguedas, A. Sánchez, A. Segura, A. Gómez, G. Solano, E. Corrales-Aguilar, K. Risner, A. Narayanan, C. Bailey, M. Villalta, A. Hernández, A. Sánchez, D. Cordero, D. Solano, G. Durán, E. Segura, M. Cerdas, D. Umaña, E. Moscoso, R. Estrada, J. Gutiérrez, M. Méndez, A.C. Castillo, L. Sánchez, J.M. Gutiérrez, C. Díaz, A. Alape, Development and pre-clinical characterization of two therapeutic equine formulations towards SARS-CoV-2 proteins for the potential treatment of COVID-19, 2020. Preprint at https://www.biorxiv.org/content/10.1101/2020.10.17.343863v1, doi: https://doi.org/10.1101/2020.10.17.343863.
- 107.Lukassen S., Chua R.L., Trefzer T., Kahn N.C., Schneider M.A., Muley T., Winter H., Meister M., Veith C., Boots A.W., Hennig B.P., Kreuter M., Conrad C., Eils R. SARS-CoV-2 receptor ACE2, and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020;39:e105114. doi: 10.15252/embj.20105114. [DOI] [PMC free article] [PubMed] [Google Scholar]