Abstract
Colchicine is an anti-inflammatory agent which has been used for decades in the treatment of gout. The drug has a number of dermatological indications like Psoriasis, Sweet's syndrome, aphthosis, Behcet's disease, erythema nodosum, leukocytoclastic vasculitis and is consistently effective in neutrophilic disorders. Thought it is an affordable with minimal side effects, It has remained underutilized. However, it has novel uses and is being considered in COVID-19 due to its action on IL-1β and IL-6. This article presents a concise and up-to-date review focusing on its mechanisms of action and indications.
Keywords: Aphthosis, colchicine, COVID-19, dermatology, familial mediterranean fever, gout, neutrophilic, palmoplantar pustulosis, pustular psoriasis, side effects, uses
Introduction
Colchicine is an alkaloid obtained from the plant Colchicum autumnale, also known as meadow saffron or autumn crocus. The use of Colchicum autumnale was first described for joint pain and swelling in Egyptian literature “Ebers Papyrus” in 1500 BC. Colchicum plants were brought to America by Benjamin Franklin, who suffered from gout himself. The medical compound was first isolated in 1820 by the French chemists PS Pelletier and JB Caventou.
Pharmacokinetics
Colchicine has a good bioavailability after oral administration and is absorbed in the jejunum and ileum. It achieves peak levels in 2 hours, has a half life (t1/2) of 4.4 hours, is deacetylated in liver and excreted in feces (metabolites/active product) The lipophilic nature of colchicine allows ready absorption by multiple cell types and binding to its primary target tubulin; which serves as a drug reservoir. It is metabolized via cytochrome P450 3A4 (CYP3A4). The elimination is via biliary and fecal excretion. The excretion is dependent on multidrug resistance transporter molecule ABCB1 (P-glycoprotein [P-gp]).[1] Around 10–20% is eliminated through the kidneys.
Mechanisms of Action
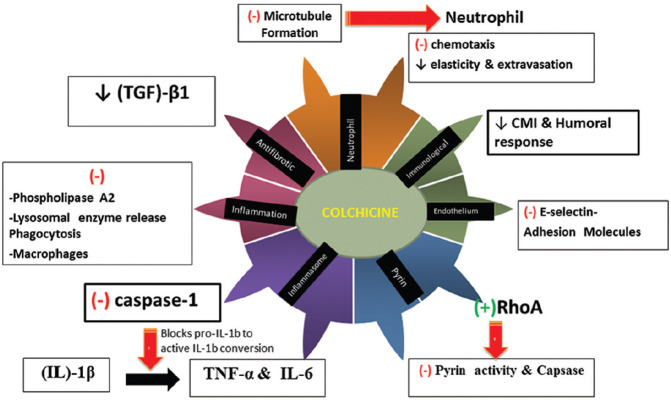
The drug has multiple actions which are detailed below[2,3] and depicted in the Figure 1.
Figure 1.
An overview of mechanisms of action of Colchicine
-
1- Action on microtubules
Colchicine is an anti-mitotic drug that blocks mitotic cells in metaphase. It consists of three hexameric rings termed A, B, and C. Inhibition of polymerization of microtubules in vitro occurs by binding of the tropolone methyl ester; which is structurally similar to ring C of Colchicine.[4] Since microtubules are part of the cytoskeleton in almost every eukaryotic cell, this action is the primary effect of the drug and it acts more on rapidly proliferating cells (e.g., bone marrow, GI tract lining).
-
2- Action on neutrophils
It preferentially concentrates in neutrophils, possibly related to their low expression of ABCB1 (ATP Binding Cassette Subfamily B Member 1), a drug transporter gene. Mutation of this gene leads to colchicine resistance.
Through microtubule depolymerization, colchicine inhibits cell proliferation, chemotaxis, adhesion, mobilization, signal transduction, gene expression, and neutrophil secretion of granule contents. It also inhibits the deformability of neutrophils, which affects the extravasation during inflammation.[5]
Furthermore, Cronstein et al.[6] have shown that colchicine may also alter the distribution of adhesion molecules (E selectin) on the surface of both neutrophils and endothelial cells, leading to a significant inhibition of the interaction between white blood cells (WBC) and endothelial cells interfering with their transmigration.
The clinical experience is that colchicine is ineffective in relieving ongoing acute Familial Mediterranean fever (FMF) attacks while being very efficient in preventing the attacks when given chronically. This is because colchicine exerts its prophylactic activity through gene suppression of loci involved in neutrophil migration or other inflammatory processes which requires 12-24 h and a higher dose.[7]
Conversely, in acute gout (local synovitis), the immediate response to colchicine relies on its rapid effect on neutrophil motility and in altering the distribution of adhesion molecules on polymorphonuclear and endothelial cell surfaces.[6] This effect is achieved by lower colchicine concentration and requires a shorter time (30–120 min).[7]
-
3- Anti-inflammatory Action
Apart from its action on neutrophils, there are other important actions of colchicine. At high concentrations, colchicine suppresses phospholipase A2 activation, lysosomal enzyme release, and phagocytosis [Figure 1].[8] It modulates pyrin expression which is one of the mechanisms by which it acts in in FMF[9] . Caspase 1, which is the enzymatic component of the nucleotide-binding oligomerization domain (NOD) like receptor family pyrin domain containing 3 (NLRP3) inflammasome is inhibited by Colchicine. This is turn inhibits the activation pro-interleukin (IL)-1β into IL-1β, and thus consequently decreases the production of cytokines involved in inflammation such as IL-6 and TNF-α. This effect of colchicine may not be its direct effect on the inflammasome and may occur via genetic sequences which are higher up.[10] However it has been found that a blood concentration of colchicine; which is 10–100 times higher the serum concentration; is required to achieve inflammasome inhibition.
The action of the drug in FMF has been further researched and its effect seems to be via the Rat sarcoma homolog gene family, member A (Rho A) protein, a peptide which controls the action of GTPases thereby affecting tubulin dynamics. Pyrin is a pattern recognition receptor (PRR); which is dependent upon modification of Rho A protein and GTPases. Colchicine activates RhoA, which inhibits pyrin activity which is turn inhibits the release of IL-1 & IL-1β thus reducing the inflammatory process.
-
4- Action on macrophages
In macrophages, it activates the nutritional biosensor AMP activated protein kinase, which transduces multiple anti-inflammatory effects of colchicine. It also blunts TNF-α--induced activation of macrophages and reduces TNF-α receptors on the surface of macrophages and endothelial cells.[1,11] Thus TNF-α-induced activation of NF-κB and signal transduction of the TNF-α-NF-κB pathway is inhibited by colchicine. These effects are medicated by its destabilization of the microtubule network.
-
5- Antifibrotic action
Its action in amyloidosis is via a direct suppression of genes, like fibronectin, which inhibits the assembly and deposition of fibers such as amyloid. Also, colchicine has been found to inhibit transforming growth factor (TGF)-β1 activity. This can explain the beneficial effect of colchicine in other conditions of fiber deposition such as in scleroderma and cirrhosis where no direct relationship with the intensity or chronicity of an underlying inflammation exists.
-
6- Immunosuppressive action
This is one of the less studied mechanisms. Colchicine is believed to inhibit both cell-mediated immunity and antibody secretion.[12] It has been shown to inhibit expression of IL-2 receptor on activated T lymphocytes and downregulate surface expression of intercellular adhesion molecule-1 (ICAM-1) and E-selectin on endothelial cells by blocking cell microtubule assembly.[13]
Dosage
While there is variation in the dosage of colchicine in common conditions, 0.5–2 mg daily, in one, two or three divided doses per day, is the routine dose. The total daily dose should be ≤4 mg and in renal patients ≤2 mg. The tablet strength available in India in 0.5 mg, whereas in Western countries, the tablet available is of 0.6 mg.
Renal dysfunction
If the kidney function is deranged, the dose of colchicine should be titrated according to the creatinine clearance:
Creatinine clearance 35–49 mL/min—0.5 mg daily
Creatinine clearance 10–34 mL/min—0.5 mg every 2–3 days
Creatinine clearance <10 mL/min—contraindicated.
Liver dysfunction
Colchicine should be administered cautiously in a patient with liver dysfunction, keeping in mind possible drug interactions (See later).
Non-dermatological Gout
A dose of 0.5 mg once or twice a day, up to a maximum dose of 1 mg per day is employed for the prophylaxis of gout. For the treatment of acute gouty flare; a dose of 1 mg is given, followed by 0.5 mg one hour later.
Familial Mediterranean fever
For adults who are not on any interacting drugs, a dose of 1– 2 mg/day in 1–2 divided doses is given.
If the patient is also taking any of the strong CYP3A4 inhibitors or the P-glycoprotein inhibitors or has taken them within the past 14 days; there is a risk of colchicine toxicity with higher doses of colchicine (Refer to section on Drug Interactions). Therefore, the dose of colchicine is decreased and a maximum dose of 0.5 mg/day in 1–2 divided doses is preferred.
If the patient is taking or has previously taken the moderate CYP3A4 inhibitors within the past 14 days, then a maximum of 1 mg/day in 1–2 divided doses can be given.
Dermatological
Indications
Colchicine is an effective drug for neutrophilic disorders, and we have found it useful in palmoplantar pustulosis, amyloidosis, aphthosis, and vasculitis [Figures 2-4]. A recent study has used it successfully in aphthosis and leucocytoclastic vasculitis[14] while other authors have used it for autoinflammatory diseases,[15] and neutrophilic urticaria.[16] An overview of the indications is given in the table below [Table 1].
Figure 2.
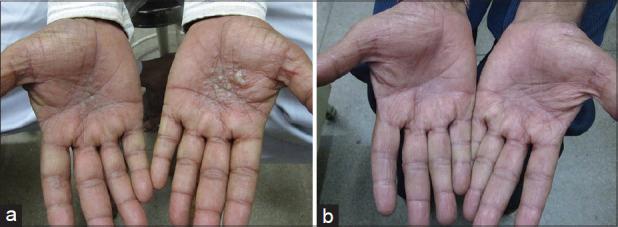
(a) A case of palmoplantar pustulosis. (b) After 10 days of Colchicine, 1 mg twice daily.
Figure 4.
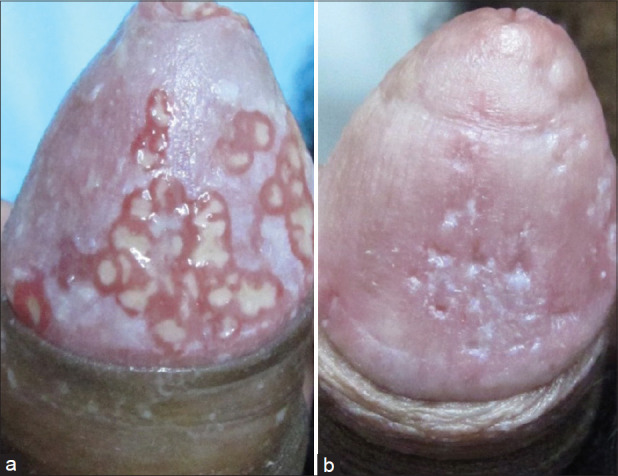
(a) A case of herpetiform penile aphthous ulcers misdiagnosed as herpes genitalis. (b) The same patient after 10 days of Colchicine 1 mg thrice a day.[30]
Table 1.
Uses of colchicine
| Approval status | Indications |
|---|---|
| FDA approved | Acute flares of gout |
| Familial Mediterranean fever | |
| Unapproved[19] | Neutrophilic dermatoses |
| Sweet’s syndrome | |
| Pyoderma gangrenosum | |
| Behcet’s disease | |
| Neutrophilic infiltration | |
| Pustular Psoriasis | |
| Palmoplantar pustulosis | |
| Erythema nodosum | |
| ENL | |
| Recurrent aphthous stomatitis | |
| Vasculitis | |
| Granuloma Faciale | |
| Neutrophilic bullous diseases | |
| Dermatitis herpetiformis | |
| Epidermolysis bullosa acquisita | |
| Linear IgA disease | |
| Subcorneal pustular dermatosis | |
| Autoimmune connective tissue disorders | |
| Dermatomyositis | |
| Scleroderma | |
| Others | |
| Actinic keratosis | |
| Eosinophilic cellulitis | |
| Fibromatosis | |
| Keloids | |
| Lichen planus pigmentosus | |
| Mid dermal elastolysis | |
| Oleoma | |
| Renal amyloidosis secondary to ulcerative colitis | |
| Hidradenitis suppurativa |
An interesting off label use of this drug is as a re-purposed drug for COVID-19 via its action on IL-6 & inflammasomes, which may help in ameliorating the cytokine storm[17] and is being actively researched in COVID-19.[18] Recently the GRECCO-19 trial;[19] an open-label RCT comparing colchicine with control group was carried out among 105 patients. The end point was to analyze difference in peak high-sensitivity troponin levels between the 2 groups. It was found that even though the authors did not see a significant reduction in troponin levels with colchicine, the group who received colchicine had significantly less clinical deterioration which was judged by requirement for mechanical ventilation. The patients treated with colchicine had significantly lower prothrombotic background as suggested by low levels of dimerized plasma fragment D levels.[19]
The use of colchicine in select dermatoses is detailed below:
Palmoplantar pustulosis: Palmoplantar pustulosis is a type of chronic pustular dermatoses, closely related to psoriasis. A study done on 32 patients by Mori et al. administered 1-2 mg of colchicine per day in patients of palmoplantar pustulosis.[20] Partial or complete remission of pustule formation occurred in all but one patient after 2-8 weeks of therapy though we have noted a faster improvement [Figure 2a and b]. The action is via suppression of neutrophil chemotaxis [Figure 1]. In our experience, if the condition is diagnosed correctly, the results are excellent, and some patients are controlled even on low doses (0.5 mg on alternate days).
Pustular psoriasis: Colchicine is believed to be effective in difficult-to-treat or recalcitrant forms of psoriasis like pustular and palmoplantar psoriasis. Zachariae et al.[21] reported complete resolution in 3 of 4 patients with pustular psoriasis. Stefanaki et al.[22] used it to achieve remission of both SCPD and palmoplantar pustular psoriasis in a dapsone-intolerant patient. Taguchi et al. successfully added colchicine to secukinumab and guselkumab, respectively, in two patients with generalized pustular psoriasis, who developed recurrences while on the biologics.[23] While the literature recommends the use of cyclosporine, acitretin or biologicals in pustular psoriasis, colchicine can be used prior to use of other drugs [Figure 3a and b].
Behçet's syndrome: The response rate achieved ranges between 60 and 70% and orogenital and ocular lesions are the most responsive.[24] A dose of 1.5 mg per day was effective in treating erythema nodosum and accompanying arthralgias, as reported by Aktulga et al.[25] To date, few controlled trials of colchicine in the treatment of Behçet's syndrome have been carried out; but a single double-blind study comparing this drug with cyclosporine demonstrated that both medications were equally effective in reducing the symptoms[26] which makes colchicine a cheaper and safer option.
Recurrent aphthous stomatitis: Recurrent aphthous stomatitis affects 20% of the population. Colchicine, 0.5 mg three times a day, effectively induced disease remission in 3 patients.[27] Twenty patients were administered 1.5 mg per day of colchicine by Katz et al., who found reduction of pain by 77% and mean ulcer count by 71%.[28] All patients experienced relapse when the drug was discontinued. A review also recommends colchicine, pentoxifylline, or prednisolone as preferred agents for aphthosis.[29] In our practice, a combination of colchicine and pentoxyphylline is a good option, though in some the results of doxycycline are better and can serve as a good replacement to steroids. A recent case of genital aphthosis misdiagnosed as herpes genitalis had an exquisite response to colchicine making it a useful drug as a therapeutic intervention in such unusual presentations [Figure 4a and b].[30]
Leukocytoclastic vasculitis and urticarial vasculitis: Leukocytoclastic vasculitis involves inflammation of post capillary venules with associated neutrophil infiltration and leukocytoclasis. Colchicine is effective in this disorder.[14,31] Colchicine in a dose of 0.5 mg twice daily was administered to 13 patients by Callen et al.[32] Disease was controlled completely in 9 patients and partial control was observed in 3 patients. The arthralgias also showed response to treatment. A rapid response was noted; usually within the first week of treatment. Drug withdrawal was associated with relapse in many patients but could be rapidly controlled on re-introducing the drug. Analogous to this, urticarial vasculitis can also be successfully treated with colchicine and a retrospective cohort study of 57 patients[33] found colchicine as effective as corticosteroid therapy in hypocomplementaemic urticarial vasculitis.
Bullous diseases: The drug has been tried in various disorders like chronic bullous dermatosis of children (CBDC) where it has been used as an adjuvant drug with prednisone.[34] In Epidermolysis bullosa acquista (EBA), Megahed and Scharffetter-Kochanek[35] have described its use in a dose of 2 mg which let to resolution in 2 weeks, of most mucosal and cutaneous lesions and this has also been reported from India.[36] Aram et al.[37] administered 1.5 mg per day colchicine in a patient of Linear IgA disease; who had not responded to Dapsone. A rapid response was noted within 5 days and complete recovery was seen in 10 weeks. Similarly, Silvers et al. described 3 patients with dermatitis herpetiformis who responded rapidly to colchicine 1.2–1.8 mg/day.[38]
Fibromatosis: Dominguez-Malagon et al.[39] described patients with various types of fibromatosis who were given colchicine with good results. Microscopically, the abnormal long-spacing collagen present in rough endoplasmic reticulum was dissipated after treatment which accounts for its therapeutic results and is accounted by its antifibrotic action [Figure 1]. Another patient with palmar fibromatosis responded well to a twice-weekly dose of 1.2 mg of colchicine.
Sweet's syndrome: This is a typical neutrophilic disorder where beneficial responses to colchicine have been described. Four patients suffering from Sweet's syndrome showed rapid improvement in 2–5 days on a dose of 1.5 mg day; as reported by Suehisa et al.[40]
Amyloidosis: Cutaneous lesions develop in up to 40% of patients with systemic amyloidosis, both primary and secondary. Colchicine not only slows the progression of amyloidosis associated with FMF,[41] but also prevents amyloid deposition. Colchicine is also effective in decreasing the proteinuria seen in renal amyloidosis secondary to ulcerative colitis. Treatment of primary cutaneous amyloidosis has yielded mixed results.
Prevention of coronary artery disease: Addition of colchicine 0.5 mg per day to statin and antiplatelet therapy has a beneficial effect in reducing the risk of cardiovascular events and myocardial infarction;[42] in patients with known stable coronary artery disease. Colchicine not only decreases the levels of inflammatory cytokines, but also prevent cholesterol crystal induced neutrophil egress to the site of atherosclerotic plaque.[43]
Figure 3.
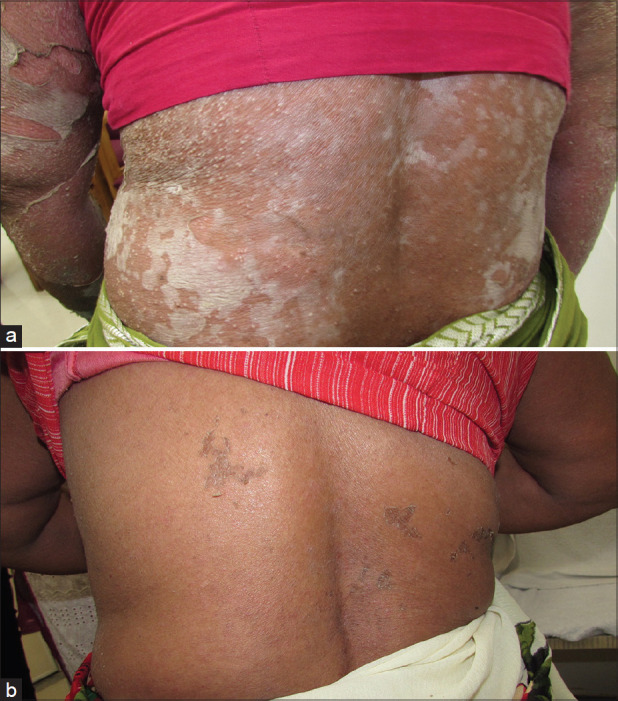
(a) A case of pustular psoriasis who relapsed repeatedly after taking methotrexate and cyclosporine. (b) A complete clearance of lesions after 10 days of Colchicine 1 mg twice daily
Also, a role of Colchicine as a prophylactic agent in patients with high cardiovascular risk has been elicited. A retrospective cohort study done by Solomon et al.[44] found that among 501 patients diagnosed with gout that were treated with colchicine; a protective effect signified by 49% relative risk reduction in the combined outcomes of transient ischemic attack, stroke, and MI was noted; along with 73% relative risk reduction of all-cause mortality.
Further, a substantial amount of evidence points to an increased risk of cardiovascular disease in patients with psoriasis. This link is explained by occurrence of Th1, Th17, and Th22 mediated inflammation in both the diseases.[45,46] Thus colchicine may have a potential role in psoriatic patients with cardiovascular risk factors.
Contraindications
The contraindications to bear in mind when prescribing colchicine are listed in Table 2.[47,48]
Table 2.
Contraindications to the use of Colchicine
| Absolute Contraindications |
| Known hypersensitivity to the drug |
| Concomitant use of a P-glycoprotein (p-gp) inhibitor or CYP3A4 inhibitor in the presence of renal or hepatic impairment |
| Serious GI/cardiac/renal/hepatic impairment. |
| Relative contraindications |
| Pregnancy/Lactation |
| Blood dyscrasias |
| Geriatric patients |
| Children |
Safety Profile
Colchicine is a relatively safe drug in moderate doses. The most common side effect reported with the use of colchicine are gastrointestinal side effects; namely diarrhea (23%), vomiting (17%), and nausea. However, the incidence of these side effects is low in patients receiving low to moderate doses. In patients receiving high doses (2–3 mg/day), the prevalence of gastrointestinal side effects is reported in 80%. An overview of the common and uncommon side effects due to colchicine is listed in Table 3.[47,48]
Table 3.
Overview of side effects with Colchicine
| System involved | Side effects |
|---|---|
| Common side effects | |
| Gastrointestinal | Diarrhoea (23%), nausea, vomiting (17%) |
| Uncommon side effects | |
| Gastrointestinal | Cramps, pain, lactose intolerance |
| Hepatic | Elevated liver transaminases |
| Cutaneous | Maculopapular rash, purpura, alopecia |
| Hematological | Leukopenia, granulocytopenia, thrombocytopenia, pancytopenia, aplastic anemia. |
| Musculoskeletal | myopathy, elevated CPK, myotonia, muscle weakness, muscle pain, rhabdomyolysis |
| Reproductive | oligospermia, azoospermia |
| Genitourinary | Oliguria, renal damage |
*Those underlined are serious side effects
Drug Interactions
Since colchicine is metabolized by CYP3A4, inhibitors of this enzyme result in increase in colchicine levels, predisposing to colchicine toxicity. The P glycoprotein inhibitors also result in increased colchicine levels, owing to competition for tubular secretion in the kidney. Statins should be avoided as acute myopathy has been reported in patients who have been given colchicine along with statins. Colchicine may also decrease the action of vitamin B12, due to reversible malabsorption, thus predisposing to megaloblastic anemia. The salient interactions of colchicine are listed in Table 4.[47,48]
Table 4.
Drug interactions of Colchicine
| Interaction | Mechanism | Drugs |
|---|---|---|
| Drugs that increase the level of Colchicine | Strong CYP3A4 inhibitors | Atazanavir, Nelfinavir, Ritonavir, Sequinavir, Indinavir, Clarithromycin, Telithromycin, Itraconazole, Ketoconazole, Nefazodone |
| Moderate CYP3A4 inhibitors | Amprenavir, Fosamprenavir, Erythromycin, Fluconazole, Aprepitant, Diltiazem, Verapamil. Grapefruit juice. | |
| P Glycoprotein inhibitors | Cyclosporine, Ranolazine, Digoxin grapefruit juice | |
| Food | ||
| Drugs that increase the gastrointestinal side effects | Increased toxicity | NSAIDS, Ethanol |
| Drugs that cause increased bone marrow depression | Increased toxicity | Cyclosporine, Radiation, bone marrow depressants |
| Drugs that lead to increased rhabdomyolysis | Increased toxicity | HMG CoA reductase inhibitors (Statins), Fibrates (Fenofibrate, Gemfibrozil), Digoxin |
| Drug/Lab Test | Increase | Alk phosphatase, AST |
| Decrease | Platelets, WBC, granulocytes | |
| False positive | Urine Hgb | |
| Interference | urinary 17-hydroxycorticosteroids |
Work up and Monitoring
-Baseline investigations
Colchicine may cause aplastic anemia, agranulocytosis, and decreased platelet count in those on long term therapy. Therefore, before starting therapy, a complete blood count, reticulocyte count, liver function tests, and kidney function tests should be done.
-Follow up investigations-
Complete blood count picture with reticulocyte count should be done every 3 months.
Use in Special Populations
Pregnancy: Colchicine is a category C drug. It is better to avoid its use in pregnancy and should only be prescribed, if the benefits outweigh the fetal risk.
Lactation: Around 10% of the drug is excreted in the breast milk. Therefore, it is advisable to avoid breastfeeding with concomitant intake of the drug.
Hepatic dysfunction: Colchicine should be used with caution in patients with known liver dysfunction or who present with elevation of transaminases while on the medication.
Renal dysfunction: The dose of colchicine should be titrated in patients with lower creatinine clearance. If the creatinine clearance is less than 10 mL/min, then colchicine is contraindicated.
Conclusion
Colchicine has myriad uses in dermatology and a favorable safety profile. Its predominant action on neutrophils makes it a very cheap and effective option for dermatoses mediated by neutrophils. Also, its action in various other disorders is closely linked to its action on pyrins, inflammasomes, and cytokines. What makes it more unique is its cost and easy availability and the drug needs to explored more often than is listed in literature. The lack of consistent response may be related to variations in its blood levels that are dependent on the genotyping variations in level of ABCB1 (P-glycoprotein [P-gp]). It is now being re purposed as a drug to treat the cytokine storm in COVID-19.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Terkeltaub RA. Colchicine update: 2008. Semin Arthritis Rheum. 2009;38:411–9. doi: 10.1016/j.semarthrit.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Slobodnick A, Shah B, Pillinger MH, Krasnokutsky S. Colchicine: Old and new. Am J Med. 2015;128:461–70. doi: 10.1016/j.amjmed.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Viollet B, Terkeltaub R, Liu-Bryan R. AMP-activated protein kinase suppresses urate crystal-induced inflammation and transduces colchicine effects in macrophages. Ann Rheum Dis. 2016;75:286–94. doi: 10.1136/annrheumdis-2014-206074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreu JM, Timasheff SN. Interaction of tubulin with single ring analogue of colchicine. Biochemistry. 1982;21:534–43. doi: 10.1021/bi00532a019. [DOI] [PubMed] [Google Scholar]
- 5.Paschke S, Weidner AF, Paust T, Marti O, Beil M, Ben-Chetrit E. Technical advance: Inhibition of neutrophil chemotaxis by colchicine is modulated through viscoelastic properties of subcellular compartments. J Leukoc Biol. 2013;94:1091–6. doi: 10.1189/jlb.1012510. [DOI] [PubMed] [Google Scholar]
- 6.Cronstein BN, Molad Y, Reibman J, Balakhane E, Levin RI, Weissmann G. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J Clin Invest. 1995;96:994–1002. doi: 10.1172/JCI118147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Chetrit E, Bergmann S, Sood R. Mechanism of the anti-inflammatory effect of colchicine in rheumatic diseases: A possible new outlook through microarray analysis. Rheumatology (Oxford) 2006;45:274–82. doi: 10.1093/rheumatology/kei140. [DOI] [PubMed] [Google Scholar]
- 8.Paya M, Terencio MC, Ferrandiz ML, Alcaraz MJ. Involvement of secretory phospholipase A2 activity in the zymosan air pouch model of inflammation. Br J Pharmacol. 1996;117:1773–9. doi: 10.1111/j.1476-5381.1996.tb15353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mansfield E, Chae JJ, Komarow HD, Brotz TM, Frucht DM, Aksentijevich I, et al. The familial Mediterranean fever protein, pyrin, associates with microtubules and colocalizes with actin filaments. Blood. 2001;98:851–9. doi: 10.1182/blood.v98.3.851. [DOI] [PubMed] [Google Scholar]
- 10.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 11.Dalbeth N, Lauterio TJ, Wolfe HR. Mechanism of action of colchicine in the treatment of gout. Clin Ther. 2014;36:1465–79. doi: 10.1016/j.clinthera.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Yang LP. Oral colchicine (Colcrys): In the treatment and prophylaxis of gout. Drugs. 2010;70:1603–13. doi: 10.2165/11205470-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Perico N, Ostermann D, Bontempeill M, Morigi M, Amuchastegui CS, Zoja C, et al. Colchicine interferes with L-selectin and leukocyte function-associated antigen-1 expression on human T lymphocytes and inhibits T cell activation. J Am Soc Nephrol. 1996;7:594–601. doi: 10.1681/ASN.V74594. [DOI] [PubMed] [Google Scholar]
- 14.Anzengruber F, Graf V, Hafner J, Meienberger N, Guenova E, Dummer R. Efficacy and safety of colchicine in inflammatory skin diseases: A retrospective, monocentric study in a large tertiary center. J Dermatolog Treat. 2019;20:1–6. doi: 10.1080/09546634.2019.1690621. [DOI] [PubMed] [Google Scholar]
- 15.Tripathi SV, Leslie KS. Autoinflammatory diseases in dermatology.CAPS, TRAPS, HIDS, FMF, Blau, CANDLE. Dermatol Clin. 2013;31:387–404. doi: 10.1016/j.det.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Belani H, Gensler L, Bajpai U, Meinhardt E, Graf J, Pincus L, et al. Neutrophilic urticaria with systemic inflammation: A case series. JAMA Dermatol. 2013;149:453–8. doi: 10.1001/jamadermatol.2013.2705. [DOI] [PubMed] [Google Scholar]
- 17.Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol. 2020;39:2085–94. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalili N, Kashefizadeh A, Nafar M, Poorrezagholi F, Firouzan A, Samadian F, et al. Adding colchicine to the antiretroviral medication-lopinavir/ritonavir (Kaletra) in hospitalized patients with non-severe Covid-19 pneumonia: A structured summary of a study protocol for a randomrized controlled trial. Trials. 2020;21:489. doi: 10.1186/s13063-020-04455-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deftereos SG, Giannopoulos G, Vrachatis DA, Siasos GD, Giotaki SG, Gargalianos P, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: The GRECCO-19 randomized clinical trial. JAMA Netw Open. 2020;3:e2013136. doi: 10.1001/jamanetworkopen.2020.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori S, Hino K, Izumi H. Clinical manifestations and treatment of pustulosis palmaris et plantaris. J Dermatol. 1976;86:671. [PubMed] [Google Scholar]
- 21.Zachariae H, Kragballe K, Herlin T. Colchicine in generalized pustular psoriasis: Clinical response and antibody-dependent cytotoxicity by monocytes and neutrophils. Arch Dermatol Res. 1982;274:327–33. doi: 10.1007/BF00403737. [DOI] [PubMed] [Google Scholar]
- 22.Stefanaki C, Kontochristopoulos G, Kedikoglou S, Hatziolou E, Zakopoulou N. Subcorneal pustular dermatosis associated with palmo-plantar pustular psoriasis: Response to colchicine therapy. J Dermatol. 2004;31:946–8. doi: 10.1111/j.1346-8138.2004.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 23.Taguchi R, Takamura S, Teraki Y. Combination therapy with biologic and colchicine for generalized pustular psoriasis. Int J Dermatol. 2020 doi: 10.1111/ijd.14959. doi: 101111/ijd 14959 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Mizushima Y, Matsumura N, Mori M, Shimizu T, Fukushima B, Mimura Y, et al. Colchicine in Behçet's disease. Lancet. 1977;2:1037. doi: 10.1016/s0140-6736(77)92945-2. [DOI] [PubMed] [Google Scholar]
- 25.Aktulga E, Altaç M, Müftüoglu A, Ozyazgan Y, Pazarli H, Tüzün Y, et al. A double blind study of colchicine in Behçet's disease. Haematologica. 1980;65:399–402. [PubMed] [Google Scholar]
- 26.Masuda K, Nakajima A, Urayama A, Nakae K, Kogure M, Inaba G. Double-masked trial of cyclosporin versus colchicine and long-term open study of cyclosporin in Behçet's disease. Lancet. 1989;1:1093–6. doi: 10.1016/s0140-6736(89)92381-7. [DOI] [PubMed] [Google Scholar]
- 27.Ruah CB, Stram JR, Chasin WD. Treatment of severe recurrent aphthous stomatitis with colchicine. Arch Otolaryngol Head Neck Surg. 1988;114:671–5. doi: 10.1001/archotol.1988.01860180085037. [DOI] [PubMed] [Google Scholar]
- 28.Katz J, Langevitz P, Shemer J, Barak S, Livneh A. Prevention of recurrent aphthous stomatitis with colchicine: An open trial. J Am Acad Dermatol. 1994;31:459–61. doi: 10.1016/s0190-9622(94)70211-x. [DOI] [PubMed] [Google Scholar]
- 29.Altenburg A, El-Haj N, Micheli C, Puttkammer M, Abdel-Naser MB, Zouboulis CC. The treatment of chronic recurrent oral aphthous ulcers. Dtsch Arztebl Int. 2014;111:665–73. doi: 10.3238/arztebl.2014.0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saxena S, Tandon S, Sardana K, Bajaj S. Herpetiform aphthous genital ulcers misdiagnosed as herpes genitalis in a young male and their effective response to colchicine therapy. Int J STD AIDS. 2019;30:1340–3. doi: 10.1177/0956462419870672. [DOI] [PubMed] [Google Scholar]
- 31.Plotnick S, Hupert AS, Kantor G. Colchicine and leukocytoclastic vasculitis. Arthritis Rheum. 1989;32:1489–90. doi: 10.1002/anr.1780321125. [DOI] [PubMed] [Google Scholar]
- 32.Callen JP. Colchicine is effective in controlling chronic cutaneous leukocytoclastic vasculitis. J Am Acad Dermatol. 1985;13:193–200. doi: 10.1016/s0190-9622(85)70158-2. [DOI] [PubMed] [Google Scholar]
- 33.Jachiet M, Flageul B, Deroux A, Le Quellec A, Maurier F, Cordoliani F, et al. The clinical spectrum and therapeutic management of hypocomplementemic urticarial vasculitis: Data from a French nationwide study of fifty-seven patients. Arthritis Rheumatol. 2015;67:527–34. doi: 10.1002/art.38956. [DOI] [PubMed] [Google Scholar]
- 34.Zeharia A, Hodak E, Mukamel M, Danziger Y, Mimouni M. Successful treatment of chronic bullous dermatosis of childhood with colchicine. J Am Acad Dermatol. 1994;30:660–1. doi: 10.1016/s0190-9622(09)80120-5. [DOI] [PubMed] [Google Scholar]
- 35.Megahed M, Scharffetter-Kochanek K. Epidermolysis bullosa acquisita: Successful treatment with colchicine. Arch Dermatol Res. 1994;286:35–46. doi: 10.1007/BF00375841. [DOI] [PubMed] [Google Scholar]
- 36.Agrawal P, George R, Thomas M, Has C, Pas H, Schmidt E, Leverkus M. A childhood subepidermal autoimmune bullous disease resembling mechanobullous epidermolysis bullosa acquisita. Br J Dermatol. 2015;173:871–4. doi: 10.1111/bjd.13833. [DOI] [PubMed] [Google Scholar]
- 37.Aram H. Linear IgA bullous dermatosis: Successful treatment with colchicine. Arch Dermatol. 1984;120:960–1. [PubMed] [Google Scholar]
- 38.Silvers DN, Juhlin EA, Berczeller PH, McSorley J. Treatment of dermatitis herpetiformis with colchicine. Arch Dermatol. 1980;116:1373–84. [PubMed] [Google Scholar]
- 39.Dominguez-Malagon HR, Alfeiran-Ruiz A, Chavarria-Xircotencatl P, Duran-Hernandez MS. Clinical and cellular effects of colchicine in fibromatosis. Cancer. 1992;69:2478–83. doi: 10.1002/1097-0142(19920515)69:10<2478::aid-cncr2820691016>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 40.Suehisa S, Tagami H, Inoue F, Matsumoto K, Yoshikuni K. Colchicine in the treatment of acute febrile neutrophilic dermatosis (Sweet's syndrome) Br J Dermatol. 1983;108:99–101. doi: 10.1111/j.1365-2133.1983.tb04584.x. [DOI] [PubMed] [Google Scholar]
- 41.Zemer D, Pras M, Sohar E, Modan M, Cabili S, Gafni J. Colchicine in the prevention and treatment of the amyloidosis of familial Mediterranean fever. N Engl J Med. 1986;314:1001–5. doi: 10.1056/NEJM198604173141601. [DOI] [PubMed] [Google Scholar]
- 42.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404–10. doi: 10.1016/j.jacc.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 43.Nidorf SM, Eikelboom JW, Thompson PL. Colchicine for secondary prevention of cardiovascular disease. Curr Atheroscler Rep. 2014;16:391. doi: 10.1007/s11883-013-0391-z. [DOI] [PubMed] [Google Scholar]
- 44.Solomon DH, Liu CC, Kuo IH, Zak A, Kim SC. Effects of colchicine on risk of cardiovascular events and mortality among patients with gout: A cohort study using electronic medical records linked with Medicare claims. Ann Rheum Dis. 2016;75:1674–9. doi: 10.1136/annrheumdis-2015-207984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jindal S, Jindal N. Psoriasis and cardiovascular diseases: A literature review to determine the causal relationship. Cureus. 2018;10:e2195. doi: 10.7759/cureus.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prodanovich S, Kirsner RS, Kravetz JD, Ma F, Martinez L, Federman DG. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700–3. doi: 10.1001/archdermatol.2009.94. [DOI] [PubMed] [Google Scholar]
- 47.Robinson KP, Chan JJ. Colchicine in dermatology: A review. Australas J Dermatol. 2018;59:278–85. doi: 10.1111/ajd.12795. [DOI] [PubMed] [Google Scholar]
- 48.Choubey V, Mittal S, Narang I, Sardana K. Miscellaneous agents. In: Sardana K, editor. Systemic Drugs in Dermatology. New Delhi: Jaypee Publishers; 2016. pp. 681–83. [Google Scholar]