Dear Sirs,
Novel Corona Virus Disease 2019 (COVID-19) poses an unpredictable risk for patients with myasthenia gravis (MG) due to potentially additive effects of bulbar and respiratory muscle weakness and viral lung affection [1]. MG manifestations in conjunction with COVID-19, reported since the outbreak of the pandemic, were highly variable [2] and ranged from isolated ocular symptoms [3] to severe exazerbations with respiratory failure [1, 4, 5] and lethal outcomes of approximately 30% have been reported in a recent series of 15 consecutive patients [6]. Maintenance of standard immunosuppressive treatment is recommended in mild-to-moderate disease [7]; however, information about the management and outcome of patients with severe generalized MG treated with immune-depleting agents such as rituximab is still limited.
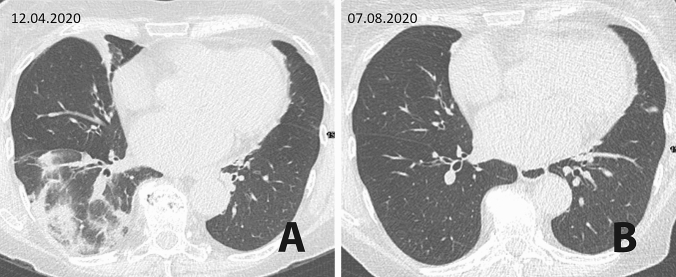
We report the clinical course and 6-month follow-up of a 71-year-old female patient with exacerbation of AChR-antibody positive MG following SARS-CoV-2 infection. MG was diagnosed in November 2018 when the patient presented with dysarthria and swallowing difficulties. Relevant co-morbidities included hypothyreosis and arterial hypertension. A global respiratory insufficiency in January 2019 required mechanical ventilation and intravenous treatment with neostigmine, steroids, and immunoglobulins (IVIg). Oral pyridostigmine and steroids were maintained following extubation. Azathioprine was only given short-term because of liver toxicity. Despite two additional courses of IVIg, disease control was inappropriate and treatment with rituximab was initiated in July 2019; the last dose (1000 mg) was given in January 2020 resulting in significant clinical improvement (MG-ADL score 3) and reduction of steroids to 4 mg per day. In the beginning of March 2020 she developed mild symptoms of an upper respiratory tract infection with fever, cough, and arthralgia. A nasopharyngeal (NP) test was positive for SARS-CoV-2 RNA and she was placed in home quarantine for 2 weeks during which flu-like symptoms regressed. Four weeks after onset of COVID-19, she noted deterioration of her myasthenia with head drop, bulbar symptoms, dyspnea, and mild proximal weakness of lower extremities. On admission, she had no fever, but a nitrite positive urinary tract infection was diagnosed. Treatment with high dose IVIg (0.4 g/kg/day for 5 days) and piperacillin/tazobactam was immediately started. On the second day, the patient developed respiratory insufficiency. A chest CT scan showed signs compatible with COVID-19 pneumonia (Fig. 1a). Another NP test for SARS-CoV-2 RNA (cobas® SARS-CoV-2, F. Hoffmann-La Roche Ltd., Basel, Switzerland) was positive along with fluctuating subfebrile temperature (37.5 °C). Respiratory difficulties were managed with non-invasive positive pressure ventilation, and neostigmine (0.5 mg/ml/h) and steroids 20 mg intravenously. The patient additionally received two doses of 200 ml convalescent plasma (CP) from recovered donors within the first week after admission. Laboratory tests showed pre-existent lymphopenia and CD20 B-cell depletion (< 2/µl) as well as elevations of CRP (max. 16.21 mg/dl), interleukin-6 (max. 284.5 ng/l) and ferritin (max. 1.220µg/l). Serological analysis revealed no specific IgG antibodies against SARS-CoV-2 (Euroimmun, Lübeck, Germany) prior and after CP therapy, while total Ig to SARS-CoV-2 (Wantai, Beijing, SARS-CoV-2 Ab Elisa Kit CE IVD) was slightly increased (index = 3.67; cut-off 1.1) 2 weeks after CP transfusions. The clinical course gradually improved and 5 weeks after myasthenic exacerbation the patient was discharged from hospital in a good condition with only mild exertional dyspnea. However, weekly performed SARS-CoV-2 PCR remained positive until May. IVIg treatment was maintained in a 6-week interval. In July, a negative SARS-CoV-2 test was documented in an out-patient setting. Regarding cardio-pulmonary health, the patient was followed within the prospective observational multicenter COVID study to investigate long-term sequelae in patients hospitalized for SARS-CoV-2 infection (NCT 04416100) that was approved by the local ethics committee (EK 1103/2020). At re-evaluation in August, she still had mild dyspnea (mMRC scale 1), normal total lung capacity (85.7%), mildly reduced diffusion capacity (77%) along with elevated alveolar-arterial gradient (30.4 mmHg) and mildly reduced partial oxygen pressure in arterial blood (69.7 mmHg). Chest CT scan showed considerable improvement of the pneumonia pattern with mild residual parenchymal bands without evidence for pulmonary fibrosis (Fig. 1b). Novel findings included evidence for chronic heart failure with elevated N-terminal-pro-B-type natriuretic peptide (671 ng/l), but preserved ejection fraction.
Fig. 1.
a Chest CT scan showing ground glass opacities and consolidations in the right lower lobe, compatible with COVID-19 pneumonia. b Follow-up after four months shows mild residual parenchymal bands
This patient experienced concurrent myasthenic crisis and COVID-19-related pneumonia with a delay of 4 weeks after initial symptoms of SARS-CoV-2 infection associated with systemic features of hyperinflammation supporting aberrant immune stimulation as potential mechanism of both complications [1, 8]. Considering pre-treatment with rituximab as high-risk for severe COVID-19 disease [7], convalescent plasma [9] was added to the treatment with IVIg and steroids resulting in clinical improvement. The absence of specific IgG antibodies in the serum of the patient after CP transfusion and persistence of viral RNA over several months, however, reflect the need for controlled prospective studies to evaluate efficacy of CP treatment [10]. Fatal outcomes of COVID-19 in patients treated with rituximab in combination with methotrexate and steroids for inflammatory rheumatic disease were reported [11], while patients with pure X-linked agammaglobulinemia with compromised immunity recovered [12]. In this line, we hypothesize that in the absence of a specific humoral IgG response recovery from COVID-19 in our patient was mediated by a specific T-cell response [13]. Immunomodulatory effects of IVIg may have ameliorated virus-induced hyperinflammation and contributed to the favourable outcome; however, a direct anti-viral effect is unlikely as currently available IVIg lots produced from pre-pandemic plasma do not contain cross-reacting neutralizing antibodies against SARS-CoV-2 [14].
In conclusion, this case with generalized MG illustrates a benign course of COVID-19 infection with only mild cardio-respiratory sequelae at follow-up indicating that in CD20-depleted patients the cytotoxic immune response against SARS-CoV-2 by cytotoxic T cells can eventually substitute the lack of specific antibodies.
Acknowledgements
We thank the patient for her permission to report her disease course and Prof. M.B. Fischer for intellectual contribution.
Author contributions
JVW, JL-R, and RB conducted most of the data collection and wrote the manuscript. MK, SW, JVW, and WNL were responsible for primary patient care and follow-up. BP, RH, and RB were involved in intensive medical care. GW performed CT-scans and designed the figure. SS and JL-R re-evaluated the cardio-pulmonary status at follow-up. FD and MR analysed antibodies against SARS-CoV-2. All co-authors contributed to the editing and the discussion of the manuscript and approved the final version.
Funding
None.
Compliance with ethical standards
Conflicts of interest
The authors declare no financial or other conflicts of interest.
Ethical approval
The patient gave her written informed consent to report her case and was enrolled in the observational multicenter CovILD follow-up study for hospitalized patients with SARS-Cov-2 infection (NCT 04416100) approved by the local ethics committee of the Medical University of Innsbruck (EK 1103/2020).
References
- 1.Delly F, Syed MJ, Lisak RP, Zutshi D. Myasthenic crisis in COVID-19. J Neurol Sci. 2020;414:116888. doi: 10.1016/j.jns.2020.116888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anand P, Slama MCC, Kaku M, et al. COVID-19 in patients with myasthenia gravis. Muscle Nerve. 2020;62(2):254–258. doi: 10.1002/mus.26918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sriwastava S, Tandon M, Kataria S, Daimee M, Sultan S. New onset of ocular myasthenia gravis in a patient with COVID-19: a novel case report and literature review. J Neurol. 2020;12:1–7. doi: 10.1007/s00415-020-10263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salik I, Rodhouse HB, Barst S. Myasthenic crisis in the setting of coronavirus disease 2019 (COVID-19) J Clin Anesth. 2020;67:110001. doi: 10.1016/j.jclinane.2020.110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hübers A, Lascano AM, Lalive PH. Management of patients with generalised myasthenia gravis and COVID-19: four case reports. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323565. [DOI] [PubMed] [Google Scholar]
- 6.Camelo-Filho AE, Silva AMS, Estephan EP, et al. Myasthenia gravis and COVID-19: clinical characteristics and outcomes. Front Neurol. 2020 doi: 10.3389/fneur.2020.01053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.International MG/COVID-19 Working Group. Jacob S, Muppidi S, Guidon A, Guptill J, Hehir M, Howard JF, Illa I, Mantegazza R, Murai H, Utsugisawa K, Vissing J, Wiendl H, Nowak RJ. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J Neurol Sci. 2020;412:116803. doi: 10.1016/j.jns.2020.116803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGonagle D, Sharif K, O'Regan A, Bridgewood C. The role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bloch EM. Convalescent plasma to treat COVID-19. Blood. 2020;136(6):654–655. doi: 10.1182/blood.2020007714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulze-Koops H, Krueger K, Vallbracht I, Hasseli R, Skapenko Increased risk for severe COVID-19 in patients with inflammatory rheumatic diseases treated with rituximab. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218148. [DOI] [PubMed] [Google Scholar]
- 12.Soresina A, Moratto D, Chiarini M, et al. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr Allergy Immunol. 2020 doi: 10.1111/pai.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiskopf D, Schmitz KM, Raadsen MP, et al. Phenotype and kinetics of SARS-CoV-2-specific T-cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol. 2020 doi: 10.1126/sciimmunol.abd2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwaiger J, Karbiener M, Aberham C, Farcet MR, Kreil TR. No SARS-CoV-2 neutralization by intravenous immunoglobulins produced from plasma collected before the 2020 pandemic. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa593. [DOI] [PMC free article] [PubMed] [Google Scholar]