Abstract
Long non-coding RNAs (lncRNAs) are involved in carcinogenesis and tumor suppression, and are novel biological tumor regulators. However, the functional roles of lncRNAs and their underlying dysregulation mechanisms in breast cancer are not completely understood. The aim of the present study was to investigate the clinical significance and biological functions of lncRNA TMPO antisense RNA 1 (TMPO-AS1) in breast cancer. TMPO-AS1 levels were measured in human cancer tissues and breast cancer cell lines, and the functional roles of TMPO-AS1 in breast cancer cells were investigated by performing in vitro and in vivo assays. Additionally, luciferase reporter assays were conducted to detect the association between microRNA (miR)-140-5p and TMPO-AS1. TMPO-AS1 expression levels were significantly increased in breast cancer tissues and cell lines compared with adjacent non-cancerous tissues and MCF-10A cells, respectively. In vitro and in vivo studies indicated that TMPO-AS1 knockdown significantly suppressed breast cancer cell viability at 48 and 72 h compared with the small interfering (si)RNA negative control group (NC; siNC). TMPO-AS1 knockdown in vitro inhibited MCF-7 and T47D cell migration and invasion compared with the siNC group. TMPO-AS1 knockdown in metastatic breast cancer cells also decreased metastatic colonization in the mouse lung compared with the short hairpin RNA NC group. Mechanistically, TMPO-AS1 promoted cellular viability and migration as a competing endogenous RNA by sponging miR-140-5p. The results suggested that TMPO-AS1 may serve as a potential therapeutic target in patients with breast cancer.
Keywords: TMPO antisense RNA 1, microRNA-140-5p, viability, metastatic colonization, breast cancer
Introduction
Breast cancer has one of the highest incidence rates among all types of cancer, with patients also displaying high mortality worldwide (1). In the majority of cases, estrogen receptor, progesterone receptor and human EGF-like receptor 2 signaling are important drivers of the development of breast tumors (2-4). Despite advances in the diagnosis and treatment of breast cancer, including radical surgery and adjuvant therapy, end-stage survival rates remain low due to aggressive clinical behavior (5,6). Therefore, identifying novel therapeutic targets for breast cancer is important.
Increasing evidence has indicated the potential role of long non-coding RNAs (lncRNAs) as biomarkers and therapeutic targets for solid tumors (7-10). lncRNAs have been reported to participate in various epigenetic regulatory processes (11,12), serve important roles in numerous biological functions and are also aberrantly expressed in a variety of tumors (13-18). However, the relevance of aberrant lncRNA expression in the biological, prognosis and molecular classification of human breast cancer is not completely understood. The TMPO antisense RNA 1 (TMPO-AS1) gene, located on human chromosome 12, is a recently identified lncRNA consisting of 3,161 nucleotides that has rarely been reported in human diseases (19). Moreover, the functional role and potential regulatory mechanisms underlying TMPO-AS1 in breast cancer are not completely understood. Therefore, the present study aimed to investigate the functions and regulatory molecular mechanisms underlying TMPO-AS1 in breast cancer.
In the present study, the functional roles of lncRNA TMPO-AS1 in human breast cancer were investigated, with a focus on its underlying regulatory mechanisms. Collectively, the present study suggested a novel regulatory mechanism, by which TMPO-AS1 promoted breast cancer progression, providing a new perspective for the study of the molecular mechanisms underlying breast cancer-associated lncRNAs.
Materials and methods
Ethics statement
The present study was approved by the Ethics Committee of the Third Affiliated Hospital of Harbin Medical University (Harbin, China, approval number: 20170529003). All patients provided written in formed consent.
Clinical specimens and cell lines
Breast cancer tissues were collected from patients with breast cancer at The Third Affiliated Hospital of Harbin Medical University. These patients included 40 females; the age range was from 27-64 years, with a mean age of 45.4±5.6 years. At the same time, a total of 15 healthy controls, which included 15 females with an age range between 31-67 years, with a mean age of 48.2±6.9 years serve as the control group. Immediately after resection, the primary and matched adjacent non-cancerous tissues were frozen in liquid nitrogen. Human breast cancer cells and MCF-10A epithelial cells were purchased from ATCC. MCF7, T47D, MDA-MB-231 and SKBR3 cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.), supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin, and 100 mg/ml streptomycin at 37˚C with 5% CO2. BT20 cells were cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin at 37˚C with 5% CO2. MCF-10A cells were cultured in M-171 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with mammary epithelial growth factors (Invitrogen; Thermo Fisher Scientific, Inc.) at 37˚C with 5% CO2.
RNA interference
A specific small interfering (si)RNA targeted against TMPO-AS1 and the corresponding negative control (NC) siRNA were purchased from Shanghai GenePharma Co., Ltd. The following siRNAs were used: siTMPO-AS1 forward, 5'-GAGCCGAACUACGAACCAATT-3' and reverse, 5'-UUGGUUCGUAGUUCGGCUCTT-3'; scrambled siRNA NC forward, 5'-UUCUCCGAACGUGUCACGUTT-3' and reverse, 5'-ACGUGACACGUUCGGAGAATT-3'. miR-140-5p mimic (miR10000431-1-5, 5'-UGAGAACUGAAUUCCAUGGGUU-3'), mimic NC (miR1N0000001-1-5, 5'-UUCUCCGAACGUGUCACGUTT-3'), miR-140-5p inhibitor (miR20000431-1-5, 5'-AACCCAUGGAAUUCAGUUCUCA-3') and inhibitor NC (miR2N0000001-1-5, 5'-UCUACUCUUUCUAGGAGGUUGUGA-3') were obtained from Guangzhou RiboBio Co., Ltd. 1x106 cells were transfected with 50 pg/µl siRNA, miRNA mimic, miRNA inhibitor or corresponding NCs using Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) and harvested 48-72 h after transfection.
To establish stable TMPO-AS1-knockdown MCF-7 cells, 2x106 MCF-7 cells were transfected with 4 mg shTMPO-AS1 (5'-CCGGGAGCCGAACTACGAACCAACTCGAGTTGGTTCGTAGTTCGGCTCTTTTTG-3') or sh-NC (5'-CCGGTTCTCCGAACGTGTCACGTCTCGAGACGTGACACGTTCGGAGAATTTTTG-3') plasmids (Hanheng Biotechnology Co., Ltd.) using HyFect™ DNA Transfection Reagent (Leadgene Biomedical, Inc.). At 24 h post-transfection, stable transfectants were selected using 500 mg/ml puromycin (Sigma-Aldrich; Merck KGaA). The selection medium was replaced every 3 days for 2 weeks, and clones of resistant cells were isolated and allowed to proliferate in medium containing puromycin (500 mg/ml).
RNA isolation, cDNA synthesis and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from transfected cells using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. RNA integrity was evaluated by performing 1.5% agarose gel electrophoresis. Total RNA (1 µg) was reverse transcribed into cDNA using the Superscript III First-Strand Synthesis system (Toyobo Life Science). The following temperature protocol was used for reverse transcription: 37˚C for 15 min, 50˚C for 5 min and 98˚C for 5 min. Subsequently, qPCR was performed using Fast SYBR® Green Master Mix (Thermo Fisher Scientific, Inc.). The following primers were used for qPCR: TMPO-AS1 forward, 5'-GTGCTGCAGGACCGAGG-3' and reverse, 5'-GCTTTGTGTCCGCGAGTTTT-3'; and GAPDH forward, 5'-AACGGATTTGGTCGTATTGG-3' and reverse, 5'-TTGATTTTGGAGGGATCTCG-3'. The specific primer of miR-140-5p was: forward, 5'-GAGTGTCAGTGGTTACCGT-3', and reverse, 5'-GCATGGTCCGAGGTATTC-3'. Primer of U6 was: forward 5'-CTCGCTTCGGCAGCACA-3' and reverse 5'-AACGCTTCACGAATTTGCGT-3' The following thermocycling conditions were used for qPCR: Initial denaturation at 95˚C for 5 min; followed by 40 cycles of denaturation at 95˚C for 15 sec, annealing at 60˚C for 20 sec and elongation at 72˚C for 10 sec; and final extension at 72˚C for 10 min. The relative expression level was determined by the 2-ΔΔCq method (20).
Plasmid construction and transfection
A TMPO-AS1 expression vector was purchased from Baizhi Biomedical Technology (Shanghai) Co., Ltd. to facilitate TMPO-AS1 overexpression in breast cancer cells. The wild-type (WT) and mutant (MUT) TMPO-AS1 sequences were cloned into the pmirGLO vector (Promega Corporation). Cells were seeded (2x105 cells/well) into 6-well plates. At 60-70% confluence, cells were transfected with 4 µg plasmid using Lipofectamine 2000 according to the manufacturer's protocol. At 48 h post-transfection, cells were collected and used for subsequent experiments.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was assessed by performing a CCK-8 assay (Dojindo Molecular Technologies, Inc.) according to the manufacturer's protocol. Cells were seeded (2x103 cells/well) in 96-well plates and cultured for 24 h. Following transfection, cells were cultured for 24, 48 or 72 h. Subsequently, the medium in each well was replaced with 100 µl complete medium containing 10 µl CCK-8 solution, and the plate was incubated for 1 h at 37˚C. Absorbance was measured at wavelengths of 450 nm using a Multiskan Spectrum spectrophotometer (Thermo Fisher Scientific, Inc.).
Colony formation assay
Cells were seeded (1x103 cells/well) into 6-well plates. Following culture for 2 weeks, the colonies were treated with 70% methanol at room temperature for 15 min, followed by staining with 0.1% crystal violet (Sigma-Aldrich; Merck KGaA) at room temperature for 20 min. Following extensive washing with phosphate-buffered saline, the cells were observed under a light microscope (DM1000; Leica Microsystems GmbH). Visible colonies of ≥50 cells were then counted (magnification, x100).
Wound healing and invasion assays
Cell migration was assessed by performing the wound healing assay. At 80-90% confluence, a 10 µl sterile pipette tip was used to create a scratch wound in the cell monolayer. Cells were cultured in media containing 2% FBS for 24 h at room temperature. The wounds were observed using a phase-contrast light microscope (magnification, x100) (DM1000; Leica Microsystems GmbH). The wound was imaged at 0 and 24 h and the percentage of migration was calculated using ImageJ software (version 1.43; National Institutes of Health). To assess cell invasion, an invasion assay was performed using Transwell inserts (pore size, 8 µm). The upper surface of the membrane was coated with Matrigel (BD Biosciences) at 4˚C overnight according to the manufacturer's protocol. Subsequently, cells (2x105) were added to the upper chamber for 24 h at 37˚C. A total of 750 µl DMEM supplemented with 20% fetal bovine serum was added to the lower chamber. Non-invading cells were removed using a cotton swab. Invading cells were fixed with 70% methanol at room temperature for 15 min and stained with leucocrystal violet for 20 min. To assess cell migration, the same procedure was performed without the use of Matrigel. Stained cells were visualized using a light microscope (magnification, x100) (DM1000; Leica Microsystems GmbH).
In vivo lung-colonization assays
BALB/c nude mice (5-6 weeks old, 18-20 g) were purchased from the Laboratory Animal Center of the Harbin Medical University and housed in barrier facilities on a 12-h light/dark cycle under specific pathogen-free conditions. Mice were maintained at 20-25˚C with 40-70% humidity, 12-h light/dark cycles, and free access to food and drinking water. Eating, feeding and operating procedures strictly followed aseptic principles. The mice were treated in accordance with protocols approved by The Third Affiliated Hospital of Harbin Medical University. MCF7 cells (2x106) or PBS were injected into the tail vein of each mouse. At 5 weeks post-injection, the mice were euthanized according to Institutional Animal Care and Use Committee protocols (n=5 per group) (21). The lungs were dissected and stored in liquid nitrogen or fixed in 4% formalin for 30 min at room temperature for further analysis. Next, 5-µm-thick sections were stained with hematoxylin and eosin for 10 min at room temperature and scanned using a Scanscope XT digital slide scanner (Aperio Technologies, Inc.). Digital images of lung sections were used to analyse the metastatic burden. Lung tumour lesions were digitally demarcated, and the number of lesions per section and the individual lesion area were determined using Spectrum software (version 11.2.0.3; Aperio Technologies, Inc.). The metastatic burden was calculated as an average of the total area of tumour lesions divided by the total lung area across four-step sections. The following humane endpoints were used in the present study: i) tumor size exceeded 10% body weight; ii) tumor ulceration; and iii) extreme weight loss. None of the endpoints were observed in the mice during the present study.
In vivo tumor growth model
BALB/c nude mice (5-6 weeks old, 18-20 g) were purchased from the Laboratory Animal Center of the Harbin Medical University and housed in barrier facilities on a 12-h light/dark cycle under specific pathogen-free conditions. Breast cancer cells were stably transfected with sh-TMPO-AS1 or sh-NC, washed with PBS and resuspended in DMEM (1x108 cells/ml). Subsequently, 100 µl cell suspension was subcutaneously injected into the posterior side of BALB/c mice (n=5 per group). Tumor size and volume were recorded every 5 days. At 30 days post-injection, the mice were euthanized, and the tumors separated for measurement and weighing. The following humane endpoints were used in the present study: i) tumor size exceeded 10% body weight; ii) tumor ulceration; and iii) extreme weight loss. None of the endpoints were observed in the mice during the present study.
Luciferase reporter assay
Luciferase reporter gene assay was implemented using the Dual-Luciferase Reporter Assay System (Promega Corporation) according to the manufacturer's instructions. Cells were seeded into 24-well plates and transfected with a wild-type (WT)-TMPO-AS1 luciferase reporter gene vector, a mutant (Mut)-TMPO-AS1 vector containing a 7-bp mutation on the predicted miR-140-5p binding site within TMPO-AS1 (Hanheng Biotechnology Co., Ltd.), along with the aforementioned miR-150-5p mimic or inhibitor using Lipofectamine 3000 (Life Technologies; Thermo Fisher Scientific, Inc.). Following 48 h, cells were lysed using passive lysis buffer (Promega Corporation) and the luciferase activity was detected. Luciferase activity was normalized against Renilla. All experiments were performed at least three times.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software (version 6.0; GraphPad Software, Inc.). Data are presented as the mean ± SEM. Comparisons between two groups were analyzed using the paired Student's t-test. Comparisons among multiple groups were analyzed using one-way or repeated measures ANOVA followed by Bonferroni's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
TMPO-AS1 expression is significantly increased in breast cancer tissues and cell lines
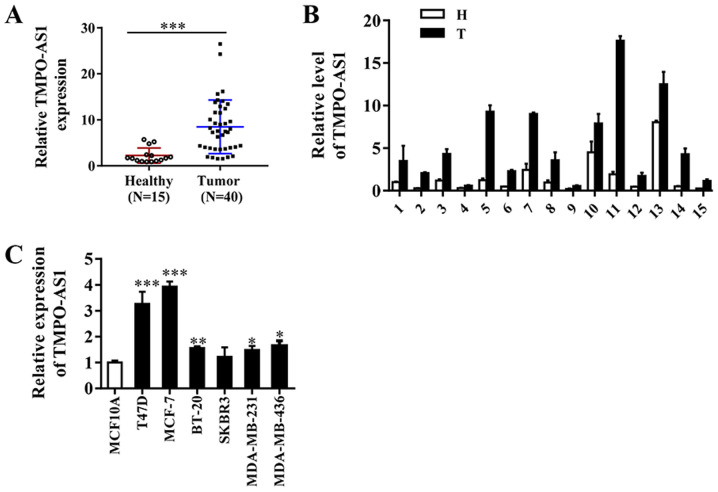
TMPO-AS1 expression was assessed in 40 breast cancer and 15 adjacent non-cancerous tissue samples. The results indicated that TMPO-AS1 expression was markedly upregulated in breast cancer tissues compared with adjacent non-cancerous tissues (Fig. 1A and B). RT-qPCR was performed to further assess differences in TMPO-AS1 expression in breast cancer and immortalized breast epithelial cells. Compared with MCF-10A cells, the expression levels of TMPO-AS1 were significantly increased in breast cancer cell lines, except for the SKBR3 cell line (Fig. 1C). The results suggested that increased expression of TMPO-AS1 may be associated with breast cancer progression.
Figure 1.
TMPO-AS1 is highly expressed in breast cancer tissues and cell lines. (A) Relative expression of TMPO-AS1 in breast cancer (n=40) and adjacent non-cancerous (n=15) tissues. ***P<0.001 vs. indicated group. (B) Relative expression levels of TMPO-AS1 in 15 paired breast cancer and adjacent non-cancerous tissues. (C) Relative TMPO-AS1 expression levels in breast cancer and breast epithelial cell lines. Data are presented as the mean ± SD of three independent experiments. *P<0.05, **P<0.01 and ***P<0.001 vs. MCF-10A group. TMPO-AS1, TMPO antisense RNA 1; N, normal; T, tumor.
TMPO-AS1 knockdown inhibits breast cancer cell viability in vitro and in vivo
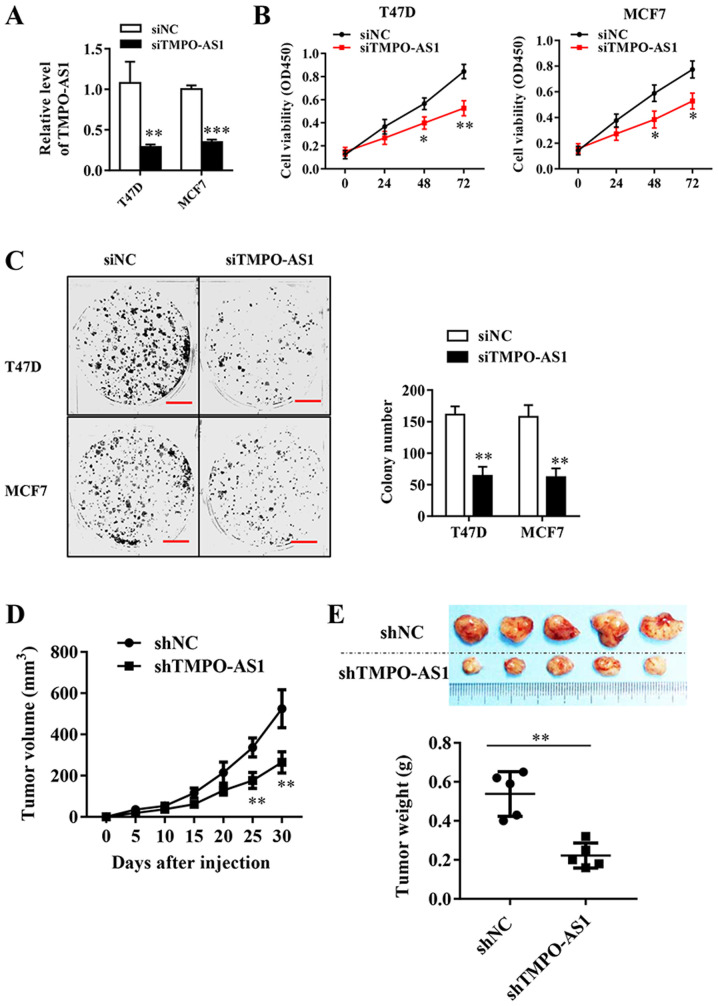
To directly evaluate the role of TMPO-AS1 in breast cancer, T47D and MCF7 cells were transfected with TMPO-AS1 siRNA, which significantly inhibited the expression of endogenous TMPO-AS1 compared with the siNC group (Fig. 2A). The effects of TMPO-AS1 knockdown on breast cancer cell viability were analyzed by performing a CCK-8 assay. The results suggested that TMPO-AS1 knockdown significantly inhibited breast cancer cell viability at 48 and 72 h compared with the siNC group (Fig. 2B). Similarly, TMPO-AS1 knockdown significantly reduced the colony forming abilities of MCF-7 and T47D cells compared with siNC (Fig. 2C). To determine the effects of TMPO-AS1 on tumor growth in vivo, tumor xenograft experiments were performed using nude mice and established stable TMPO-AS1-knockdown MCF cell lines (Fig. 2D). The tumor volumes were recorded every 5 days, and the results indicated that TMPO-AS1 knockdown significantly inhibited tumor growth in vivo from 25 days post-injection compared with the shNC group (Fig. 2E). At the end of the experiment, the weight of each tumor was measured, which indicated that TMPO-AS1 knockdown significantly reduced tumor size compared with the shNC group (Fig. 2F). In summary, TMPO-AS1 knockdown significantly inhibited cellular viability both in vivo and in vitro.
Figure 2.
TMPO-AS1 knockdown inhibits breast cancer cell viability and migration. (A) Transfection efficiency of siTMPO-AS1. (B) Effects of TMPO-AS1 knockdown on T47D and MCF7 cell viability were detected by performing the Cell Counting Kit-8 assay. (C) The effects of TMPO-AS1 knockdown on T27D and MCF7 cell viability were detected by performing colony formation assays. Scale bar, 50 µm. (D) Transfection efficiency of shTMPO-AS1 and shNC. (E) Tumor volumes were measured every day for 5 days to examine the effects of TMPO-AS1 knockdown on tumor growth. Tumor weights were recorded at the end of the experimental period. Data are presented as the mean ± SD of three independent experiments. *P<0.05, **P<0.01 and ***P<0.001 vs. siNC or shNC group. TMPO-AS1, TMPO antisense RNA 1; si, small interfering RNA; sh, short hairpin RNA; NC, negative control; OD, optical density.
TMPO-AS knockdown inhibits breast cancer cell migration and invasion in vitro and in vivo
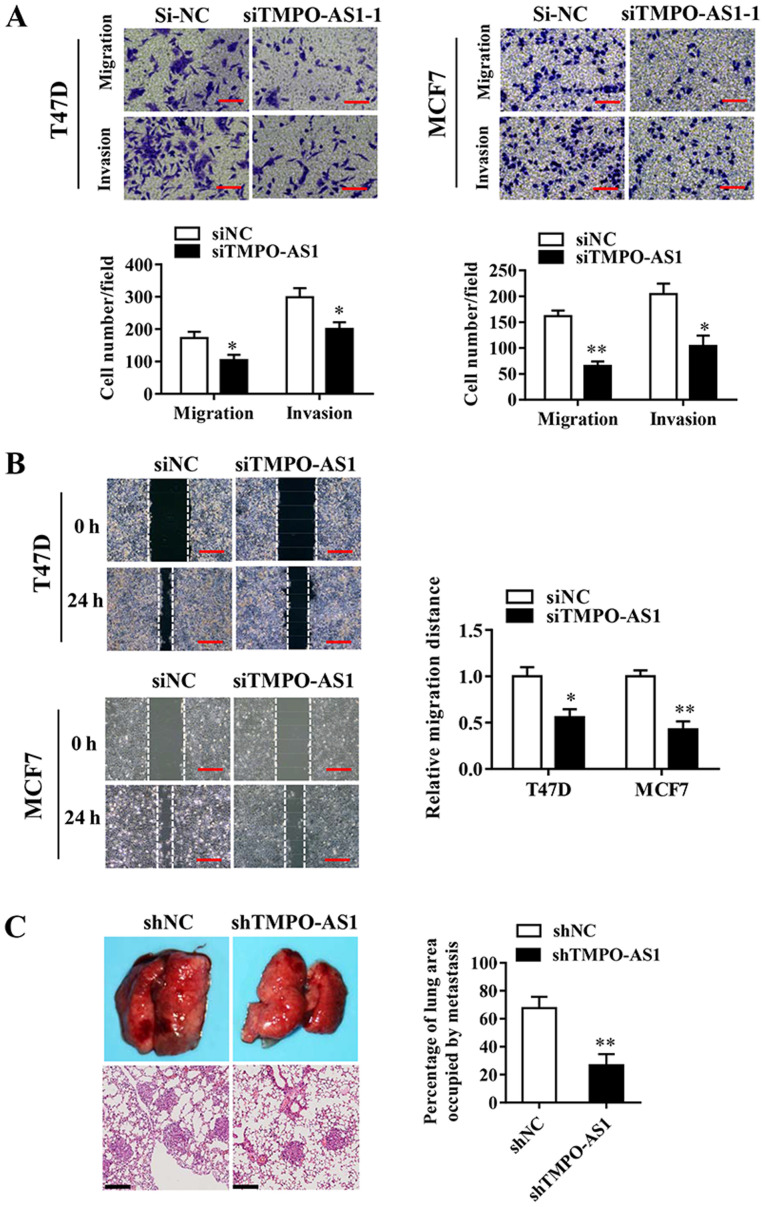
The effects of TMPO-AS1 knockdown on the invasive abilities of T47D and MCF7 cells were examined, and the association between TMPO-AS1 and breast cancer progression was investigated. Cell migration/invasion assays were performed using 24-well Transwells, coated without (migration) or with (invasion) Matrigel. The Transwell invasion assay results indicated that TMPO-AS1 knockdown significantly inhibited T47D and MCF7 breast cancer cell invasion and migration compared with the siNC group (Fig. 3A and B). In addition, the effects of TMPO-AS1 on lung metastasis were observed by injecting TMPO-AS1-knockdown breast cancer cells into nude mice via the tail vein. At 5 weeks post-injection, TMPO-AS1 knockdown significantly reduced the number of pulmonary nodules compared with the shNC group (Fig. 3C). The results indicated that TMPO-AS1 knockdown impaired breast cancer cell invasion both in vitro and in vivo.
Figure 3.
TMPO-AS1 knockdown inhibits breast cancer cell migration and invasion in vitro and in vivo. (A) The Transwell migration and invasion assay resulted indicated that TMPO-AS1 knockdown inhibited T47D and MCF7 cell migration and invasion. Scale bar, 20 µm. (B) The wound healing assay results suggested that TMPO-AS1 knockdown inhibited T47D and MCF7 cell migration. Scale bar, 40 µm. (C) Lung colonization of TMPO-AS1-knockdown and control MCF-7 cells was assessed using an in vivo lung metastasis model (n=5 per group). Representative lung images (upper) and hematoxylin and eosin-stained sections (lower; scale bar, 100 µm). The percentage of the lung area occupied by tumors was quantified. Data are presented as the mean ± SD of three independent experiments. *P<0.05 and **P<0.01 vs. siNC or shNC group. TMPO-AS1, TMPO antisense RNA 1; si, small interfering RNA; NC, negative control; sh, short hairpin RNA; ns, not significant.
TMPO-AS1 is a molecular sponge for miR-140-5p
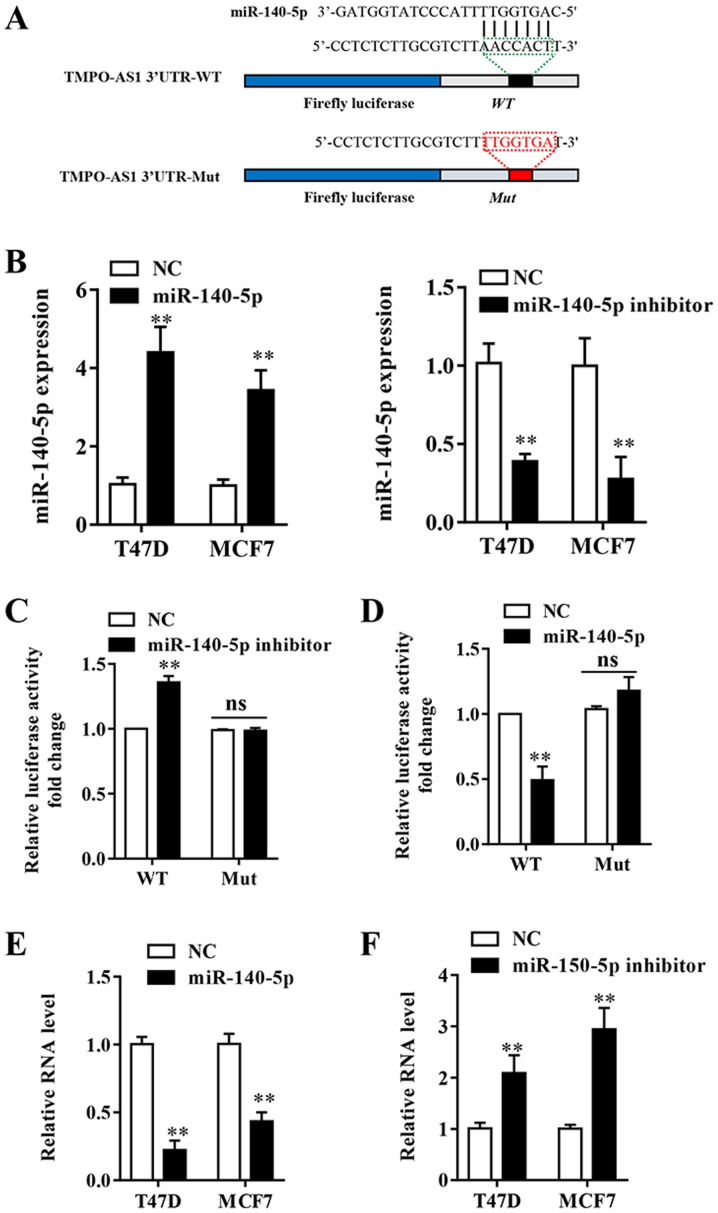
lncRNAs can exert their regulatory functions as competitive endogenous RNAs (ceRNAs) (22). To further determine the potential molecular mechanisms underlying lncRNAs in breast cancer, the StarBase v2.0 (http://starbase.sysu.edu.cn/) and miRcode (version 11; http://mircode.org/) online tools were used to predict the potential binding partners of TMPO-AS1. miR-140-5p was predicted to be a potential target for TMPO-AS1 (Fig. 4A). RT-qPCR was performed to assess the transfection efficiency of miR-140-5p mimic and miR-140-5p inhibitor. The results indicated that miR-140-5p expression was significantly decreased in the miR-140-5p inhibitor group and significantly increased in the miR-140-5p mimic group compared with the NC group (Fig. 4B). A Dual-Luciferase reporter assay was conducted and the results indicated that miR-140-5p mimic significantly inhibited the luciferase activity of TPSO-AS1-WT compared with the NC group, whereas miR-140-5p inhibitor significantly increased the luciferase activity of TPSO-AS1-WT compared with the NC group in MCF-7 cells (Fig. 4C and D). Following mutation of the predicted binding sites within TMPO-AS1, the effects of miR-140-5p mimic and miR-140-5p inhibitor on luciferase activity were abolished (Fig. 4C and D). The results indicated that TMPO-AS1 directly bound to miR-140-5p. Furthermore, miR-140-5p inhibitor significantly upregulated TMPO-AS1 expression compared with the NC group, whereas miR-140-5p mimic significantly decreased the expression of TMPO-AS1 compared with the NC group in both cell lines (Fig. 4E and F). The results suggested that miR-140-5p directly and negatively regulated the expression of lncRNA TMPO-AS1.
Figure 4.
TMPO-AS1 is a molecular sponge for miR-140-5p. (A) miR-140-5p binding sites in TMPO-AS1 were predicted via bioinformatics analysis. (B) Transfection efficiency of miR-140-5p mimic and miR-140-5p inhibitor. (C-D) Luciferase reporter assays were performed using MCF7 cells co-transfected with TMPO-AS1-WT or TMPO-AS1-Mut reporter plasmids and (C) miR-140-5p inhibitor or (D) miR-140-5p mimic. Relative TMPO-AS1 expression levels in (E) miR-140-5p mimic- and (F) miR-140-5p inhibitor-transfected T47D and MCF7 cells. Data are presented as the mean ± SD of three independent experiments. **P<0.01 vs. corresponding NC group. TMPO-AS1, TMPO antisense RNA 1; miR, microRNA; WT, wild-type; Mut, mutant; NC, negative control; 3'UTR, 3'-untranslated regions.
TMPO-AS1 promotes breast cancer progression by competitively binding to miR-140-5p
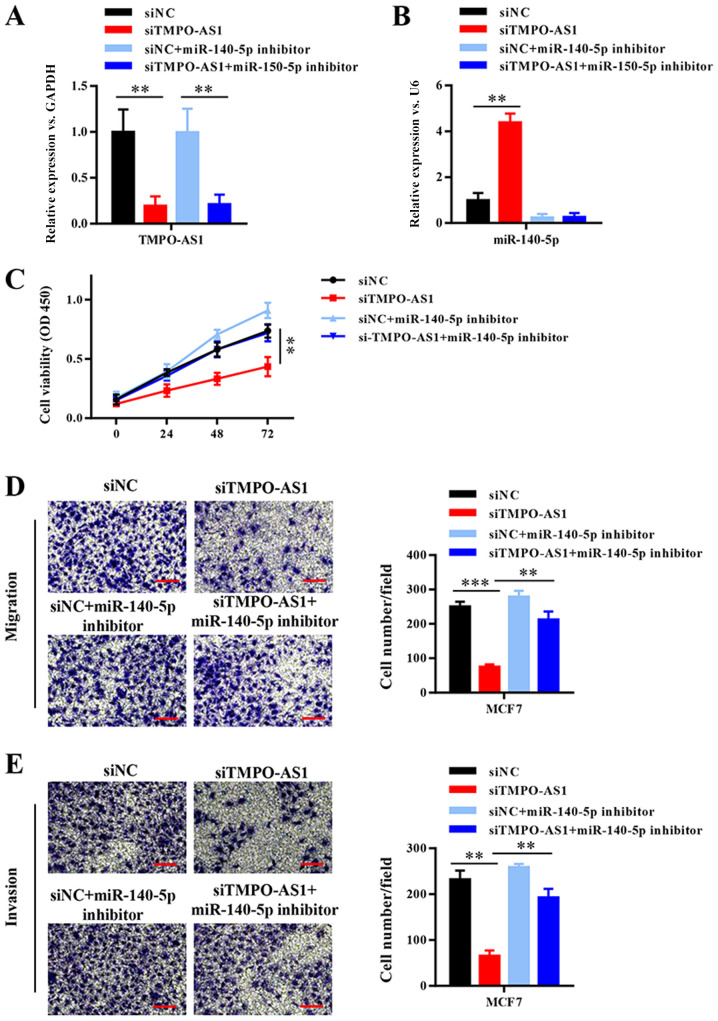
To further investigate whether TMPO-AS1-mediated promotion of breast cancer cell malignancy was dependent on negative regulation by miR-140-5p, TMPO-AS1 was knocked down in MCF-7 cells, which were then transfected with miR-140-5p inhibitor (Fig. 5A and B). Compared with the siNC group, TMPO-AS1 knockdown significantly inhibited cell viability, migration and invasion, whereas miR-140-5p inhibition reversed TMPO-AS1 knockdown-mediated effects (Fig. 5C-E). The results suggested that TMPO-AS1 promoted breast cancer progression by competitively binding to miR-140-5p.
Figure 5.
TMPO-AS1 promotes breast cancer progression by competitively binding miR-140-5p. (A and B) TMPO-AS1 and miR-140-5p expression levels in MCF7 cells. (C) The Cell Counting Kit-8 assay results indicated that TMPO-AS1 knockdown inhibited MCF7 cell viability, which was reversed by miR-140-5p inhibition. The Transwell (D) migration and (E) invasion assay results suggested that TMPO-AS1 knockdown inhibited MCF7 cell migration and invasion, which was reversed by miR-140-5p inhibitor. Scale bar, 20 µm. Data are presented as the mean ± SD of three independent experiments. **P<0.01 and ***P<0.001. TMPO antisense RNA 1; miR, microRNA; si, small interfering RNA; NC, negative control; OD, optical density.
Discussion
Despite the variety of available treatments, the mortality rate of breast cancer remains one of the highest among all cancer types worldwide (23), and the incidence of breast cancer has increased rapidly (24). At present, the efficacy and prognosis of patients with breast cancer are largely dependent on clinical and pathological parameters (25). However, the underlying mechanisms regulating the development of breast cancer are not completely understood. Therefore, the identification of novel molecular markers associated with breast cancer malignancy is important. In the present study, TMPO-AS1 was significantly upregulated in breast cancer tissues and cell lines compared with adjacent non-cancerous tissues and MCF-10A cells, respectively.
lncRNAs are RNA molecules >200 nucleotides in length that do not encode protein (26). In previous years, lncRNAs have been reported to be involved in a number of biological and pathological processes, and numerous lncRNAs have been associated with the development and progression of malignancies, including breast cancer (27). For instance, HOX transcriptional antisense RNA is involved in breast cancer metastasis by reprogramming the chromatin state (28), and kinase-activated long intergenic non-coding RNAs promote tumor growth by activating the hypoxia-inducible factor 1 signaling pathway (29). In the present study, the results indicated that TMPO-AS1 was functionally associated with the tumorigenicity and metastasis of breast cancer. Previous studies have suggested that highly expressed lncRNAs may serve oncogenic roles in tumorigenesis (30-32). The RT-qPCR results in the present study indicated that TMPO-AS1 expression was significantly higher in breast cancer tissues compared with adjacent non-cancerous tissues. Compared with MCF-10A immortalized mammary epithelial cells, the expression of TMPO-AS1 was significantly increased in the breast cancer cell lines, except for the SKBR3 cell line. In addition, compared with the siNC and shNC groups, TMPO-AS1 knockdown inhibited breast cancer cell viability and invasion in vivo and in vitro. The results suggested that TMPO-AS1 may serve a tumorigenic role in breast cancer. Besides, use of 2% FBS in the wound healing assay was a limitation of the present study.
Although a large number of lncRNAs are reportedly involved in human diseases (33), the underlying regulatory mechanisms are not completely understood. miRs are ~22 nucleotides in length and serve an essential role in regulating target gene expression by base-pairing with complementary sites in the 3'-untranslated (3'UTRs), 5'UTRs, and coding regions of target mRNAs (34-36). Based on the previously proposed ceRNA hypothesis, multiple lncRNA transcripts have been confirmed to possess binding sites for endogenous miRNAs, the binding of which allows for the regulation of both lncRNA and miRNA activity (37-39). In the present study, bioinformatics analysis indicated that TMPO-AS1 contained miR-140-5p binding sites, which was further verified by performing a dual luciferase reporter assay. The CCK-8 and Transwell assays were also performed to further determine whether TMPO-AS1 promoted cell viability, migration and invasiveness by inhibiting miR-140-5p. The results suggested that miR-140-5p knockdown reversed TMPO-AS1 knockdown-mediated effects on cell viability, migration and invasion. In summary, the results suggested that TMPO-AS1 may serve as an oncogene that promotes breast cancer progression by negatively regulating the tumor suppressor miR-140-5p. Therefore, the TMPO-AS1/miR-140-5p complex may serve as a novel target for the treatment of breast cancer.
In conclusion, the results of the present study indicated that lncRNA TMPO-AS1 expression was increased in breast cancer. The function of TMPO-AS1 in breast cancer cells also suggested that it displayed carcinogenic characteristics during the development of breast cancer. In addition, the results suggested that TMPO-AS1 promoted breast cancer cell viability and migration by sponging miR-140-5p. The results may facilitate the detection of lncRNAs to guide the development of improved diagnostic and therapeutic strategies for breast cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
DZ and QZ conceived and designed the study, drafted and revised the manuscript and supervised the project. DZ, QZ and WL performed the experiments. DZ, QZ, XZ, YH, HY, YY and GZ analyzed and interpreted the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by The Animal Care and Use Committee of the Third Affiliated Hospital of Harbin Medical University (Harbin, China; approval number: 20170529003).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66:31–42. doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- 2.Graveel CR, Calderone HM, Westerhuis JJ, Winn ME, Sempere LF. Critical analysis of the potential for microRNA biomarkers in breast cancer management. Breast Cancer (Dove Med Press) 2015;7:59–79. doi: 10.2147/BCTT.S43799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonarakis ES. AR signaling in human malignancies: Prostate cancer and beyond. Cancers (Basel) 2018;10(22) doi: 10.3390/cancers10010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahim B, O'Regan R. AR signaling in breast cancer. Cancers (Basel) 2017;9(21) doi: 10.3390/cancers9030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anastasiadi Z, Lianos GD, Ignatiadou E, Harissis HV, Mitsis M. Breast cancer in young women: An overview. Updates Surg. 2017;69:313–317. doi: 10.1007/s13304-017-0424-1. [DOI] [PubMed] [Google Scholar]
- 6.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends-an update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 7.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santosh B, Varshney A, Yadava PK. Non-coding RNAs: Biological functions and applications. Cell Biochem Funct. 2015;33:14–22. doi: 10.1002/cbf.3079. [DOI] [PubMed] [Google Scholar]
- 9. doi: 10.1038/nature05874. ENCODE Project Consortium; Birney E, Stamatoyannopoulos JA, Dutta A, Guigó R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, et al: Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447: 799-816, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ, Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al. A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666–681. doi: 10.1016/j.ccr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Han P, Chang CP. Long non-coding RNA and chromatin remodeling. RNA Biol. 2015;12:1094–1098. doi: 10.1080/15476286.2015.1063770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Zhu G, Ma Y, Qu H. lncRNA CCAT1 contributes to the growth and invasion of gastric cancer via targeting miR-219-1. J Cell Biochem. 2019;120:19457–19468. doi: 10.1002/jcb.29239. [DOI] [PubMed] [Google Scholar]
- 14.Han M, Wang Y, Gu Y, Ge X, Seng J, Guo G, Zhang X, Zhao Y, Dou D. lncRNA GHET1 knockdown suppresses breast cancer activity in vitro and in vivo. Am J Transl Res. 2019;11:31–44. [PMC free article] [PubMed] [Google Scholar]
- 15.He P, Zhang Z, Huang G, Wang H, Xu D, Liao W, Kang Y. miR-141 modulates osteoblastic cell proliferation by regulating the target gene of lncRNA H19 and lncRNA H19-derived miR-675. Am J Transl Res. 2016;8:1780–1788. [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Q, Tang Y, Tang C, Cong H, Wang X, Shen X, Ju S. Diminished LINC00173 expression induced miR-182-5p accumulation promotes cell proliferation, migration and apoptosis inhibition via AGER/NF-kappaB pathway in non-small-cell lung cancer. Am J Transl Res. 2019;11:4248–4262. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Li S, Choi YL, Lee J, Gong Z, Liu X, Pei Y, Jiang A, Ye M, Mao M, et al. Systematic identification of cancer-related long noncoding RNAs and aberrant alternative splicing of quintuple-negative lung adenocarcinoma through RNA-Seq. Lung Cancer. 2017;109:21–27. doi: 10.1016/j.lungcan.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Yin YZ, Zheng WH, Zhang X, Chen YH, Tuo YH. LINC00346 promotes hepatocellular carcinoma progression via activating the JAK-STAT3 signaling pathway. J Cell Biochem. 2019;121:735–742. doi: 10.1002/jcb.29319. [DOI] [PubMed] [Google Scholar]
- 19.Mitobe Y, Ikeda K, Sato W, Kodama Y, Naito M, Gotoh N, Miyata K, Kataoka K, Sasaki H, Horie-Inoue K, Inoue S. Proliferation-associated long noncoding RNA, TMPO-AS1, is a potential therapeutic target for triple-negative breast cancer. Cancer Sci. 2020;111:2440–2450. doi: 10.1111/cas.14498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Ngan E, Stoletov K, Smith HW, Common J, Muller WJ, Lewis JD, Siegel PM. LPP is a Src substrate required for invadopodia formation and efficient breast cancer lung metastasis. Nat Commun. 2017;8(15059) doi: 10.1038/ncomms15059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sumazin P, Yang X, Chiu HS, Chung WJ, Iyer A, Llobet-Navas D, Rajbhandari P, Bansal M, Guarnieri P, Silva J, Califano A. An extensive microRNA-mediated network of RNA-RNA interactions regulates established oncogenic pathways in glioblastoma. Cell. 2011;147:370–381. doi: 10.1016/j.cell.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Zhou J, Wang Z, Wang P, Li S. Upregulation of SOX2 activated LncRNA PVT1 expression promotes breast cancer cell growth and invasion. Biochem Biophys Res Commun. 2017;493:429–436. doi: 10.1016/j.bbrc.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Patani N, Martin LA, Dowsett M. Biomarkers for the clinical management of breast cancer: International perspective. Int J Cancer. 2013;133:1–13. doi: 10.1002/ijc.27997. [DOI] [PubMed] [Google Scholar]
- 26.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitobe Y, Takayama KI, Horie-Inoue K, Inoue S. Prostate cancer-associated lncRNAs. Cancer Lett. 2018;418:159–166. doi: 10.1016/j.canlet.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin A, Li C, Xing Z, Hu Q, Liang K, Han L, Wang C, Hawke DH, Wang S, Zhang Y, et al. The LINK-A lncRNA activates normoxic HIF1α signalling in triple-negative breast cancer. Nat Cell Biol. 2016;18:213–224. doi: 10.1038/ncb3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong HT, Liu Q, Zhao T, Yao F, Xu Y, Chen B, Wu Y, Zheng X, Jin F, Li J, Xing P. Long non-coding RNA LOXL1-AS1 drives breast cancer invasion and metastasis by antagonizing miR-708-5p expression and activity. Mol Ther Nucleic Acids. 2020;19:696–705. doi: 10.1016/j.omtn.2019.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiu B, Chi Y, Liu L, Chi W, Zhang Q, Chen J, Guo R, Si J, Li L, Xue J, et al. LINC02273 drives breast cancer metastasis by epigenetically increasing AGR2 transcription. Mol Cancer. 2019;18(187) doi: 10.1186/s12943-019-1115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lv L, He L, Chen S, Yu Y, Che G, Tao X, Wang S, Jian Z, Zhang X. Long non-coding RNA LINC00114 facilitates colorectal cancer development through EZH2/DNMT1-induced miR-133b suppression. Front Oncol. 2019;9(1383) doi: 10.3389/fonc.2019.01383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abe M, Bonini NM. MicroRNAs and neurodegeneration: Role and impact. Trends Cell Biol. 2013;23:30–36. doi: 10.1016/j.tcb.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Da Sacco L, Masotti A. Recent insights and novel bioinformatics tools to understand the role of microRNAs binding to 5' untranslated region. Int J Mol Sci. 2012;14:480–495. doi: 10.3390/ijms14010480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brummer A, Hausser J. MicroRNA binding sites in the coding region of mRNAs: Extending the repertoire of post-transcriptional gene regulation. Bioessays. 2015;36:617–626. doi: 10.1002/bies.201300104. [DOI] [PubMed] [Google Scholar]
- 37.Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, Bozzoni I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karreth FA, Tay Y, Perna D, Ala U, Tan SM Rust AG, DeNicola G, Webster KA, Weiss D, Perez-Mancera PA, et al. In vivo identification of tumor-suppressive PTEN ceRNAs in an oncogenic BRAF-induced mouse model of melanoma. Cell. 2011;147:382–395. doi: 10.1016/j.cell.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.