Abstract
The aim of the present study was to investigate the association between the severity of constipation and sarcopenia in elderly adults. We conducted a single-center university hospital-based, retrospective cross-sectional study of consecutive outpatients aged ≥65 years from 2017 to 2020. Patients were included in the study if all of the following information were available from medical records: Patient's profile (age, sex, body mass index), laxative/prokinetics use, evaluation of sarcopenia, nutritional status, and questionnaires concerning the severity of constipation [Constipation Scoring System (CSS)], abdominal symptom-related quality of life (QOL) (Izumo scale) and stool shape [Bristol Stool Form Scale (BSFS)]. Multiple regression analysis of risk factors for high CSS score was performed. The results revealed that of the 310 eligible study subjects, [149 men (48.1%) and 161 women (51.9%); mean age, 75.7±6.1 years; mean body mass index, 23.0±3.6 kg/m2], sarcopenia was noted in 83 cases (26.8%). The CSS score was significantly higher in the sarcopenia group than that noted in the non-sarcopenia group (4.9±4.9 vs. 3.6±3.6, P=0.009). The CSS score was significantly associated with the albumin level (r=-0.148), lymphocyte count (r=-0.118), CONUT score (r=0.130), reflux-related QOL score (r=0.155), upper abdominal pain-related QOL score (r=0.171), fullness-related QOL score (r=0.299), constipation-related QOL score (r=0.615), diarrhea-related QOL score (r=0.235) and BSFS score (r=-0.114). In multiple regression analysis, independent predictors for CSS score were sarcopenia [standardized partial regression coefficient (β)=0.107, P=0.032], constipation-related QOL score (β=0.537, P<0.001), laxative/prokinetics use (β=0.211, P<0.001) and BSFS score (β=-0.098, P=0.031) (R2=0.436). In conclusion, sarcopenia, constipation-related QOL score, laxative/prokinetics use and BSFS score are associated with the severity of constipation in elderly adults.
Keywords: constipation, sarcopenia, constipation scoring system, Izumo scale, Bristol Stool Form Scale
Introduction
Constipation is one of the most common gastrointestinal complaints, and its incidence appears to increase with increasing age (1). It was reported that in an internet survey, 28.4% of the respondents considered themselves to be constipated in Japan (2). Since chronic constipation greatly impairs a patient's quality of life (QOL), measures to treat constipation are an important issue in elderly adults (3). In addition, Choung et al reported that neurological and cardiovascular diseases are linked to constipation, and it was suggested that constipation was associated with systemic disease (4). Sarcopenia is an age-related geriatric syndrome that is characterized by the gradual loss of muscle mass, muscle strength, and muscle quality. It was reported that sarcopenia is associated with cardiovascular mortality and all-cause mortality (5). In addition, the prevalence of sarcopenia is higher among subjects with various diseases such as type 2 diabetes, osteoporosis, mild cognitive impairment and Alzheimer disease (6-8). The elderly population has increased rapidly in Japan, and the care of elderly patients has become a serious issue, with sarcopenia attracting much attention as a cause of the heavy burden to families and society (9). Sarcopenia and chronic constipation frequently occur in elderly adults; however, the association between the two diseases has not been clearly established. In the present study, we carried out a single-center university hospital-based retrospective cross-sectional analysis to clarify the association between the severity of constipation and sarcopenia in elderly adults.
Materials and methods
Study design
We conducted a single-center university hospital-based, retrospective cross-sectional study of consecutive outpatients ≥65 years of age who were being treated at the Department of Gastroenterology of Juntendo Tokyo Koto Geriatric Medical Center between April 2017 and March 2020.
Inclusion criteria
Subjects were included if all of the following information was available from their medical records: i) patient profile [age, sex, body mass index (BMI)]; ii) use of laxative/prokinetics; iii) evaluation of sarcopenia; iv) nutritional status [albumin level, cholesterol level, lymphocyte count, CONtrolling NUTritional status (CONUT) score]; v) questionnaires concerning the severity of constipation [Constipation Scoring System (CSS)] vi) questionnaire concerning abdominal symptom-related QOL (Izumo scale) and vii) questionnaire concerning stool shape [Bristol Stool Form Scale (BSFS)].
We included patients for whom we had performed colonoscopy (or barium enema) and chest, abdominal, and pelvic computed tomography within one year. The data on patient profile, medications, questionnaires, and findings concerning sarcopenia were collected at the same time.
Exclusion criteria
Patients who were unable to walk due to severe osteoarthritis or neuromuscular disease, immobile patients, patients presenting with delirium tremens, and patients with a history of acute cerebrovascular, gastrointestinal, renal, coronary, hepatic, or respiratory events were excluded from this study. We excluded patients found to have the following conditions: History of gastrectomy, inflammatory bowel disease (IBD), malignant disease (gastric, esophageal, colon, lung, pancreatic, liver, bile duct, gallbladder, breast, uterine, ovarian, prostate, and bladder cancer, as well as malignant lymphoma, leukemia, and multiple myeloma), type 1 diabetes mellitus, hypo/hyper-thyroidism, hypo/hyper-parathyroid disorder, or mental illness.
Patients were also excluded if they met any of the following criteria that affect sarcopenia: Severe cardiac, pulmonary, or musculoskeletal disorders; severe neurologic disorders, such as Parkinson's disease or stroke; and patients in Japan's long-term care service.
Definition of sarcopenia
We defined sarcopenia using the diagnostic algorithm recommended by the Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment, which assesses the presence of both low muscle function (low physical performance or low muscle strength) and low muscle mass (10).
In the present study, we considered subjects ≥65 years of age as having sarcopenia if they had a low appendicular skeletal muscle mass with either a low handgrip strength or slow gait speed. The handgrip strength, gait speed, and muscle mass were measured as follows. The handgrip strength was measured using a handgrip dynamometer (Toei Light Co., Ltd., Saitama, Japan). Both hands were tested, and the larger value was noted as the maximum muscle strength. A low grip strength was established according to the sex-specific cut-off for the maximum muscle strength of the subject according to the Asian Working Group for Sarcopenia (AWGS) criteria (<28 kg for men; <18 kg for women). Gait speed was manually assessed using a stopwatch. A slow gait speed was defined as a gait speed of <1.0 m/sec according to the AWGS criteria. Regional fat and lean mass were measured by whole-body dual X-ray absorptiometry (DXA; Prodigy Advance, GE Healthcare). Subjects were positioned for whole-body scans in accordance with the manufacturer's protocol. The whole-body fat mass and lean mass were divided into several regions, such as the arms, legs, and trunk. The appendicular lean mass was estimated as the sum of the lean mass of the two upper limbs and the lean mass of the two lower limbs. The appendicular skeletal muscle mass index (SMI) was calculated as the appendicular lean mass divided by the square of the height (kg/m2). A low appendicular skeletal muscle mass was defined as an SMI of <7.0 kg/m2 in men and <5.4 kg/m2 in women.
Nutritional status
In this study, the CONUT score (between 0 and 12) was used to evaluate the objective nutritional status. This score is composed of the following three parameters: Serum albumin level, total cholesterol level, and total lymphocyte count (11). The serum albumin level indicates the protein reserves, the serum total cholesterol level indicates caloric depletion, and the total lymphocyte count indicates impairment of the immune system due to malnutrition. The CONUT score is based on the following: Albumin (≥3.5 g/dl, 0 points; 3.00-3.49 g/dl, 2 points; 2.50-2.99 g/dl, 4 points; <2.50 g/dl, 6 points), total cholesterol (≥180 mg/dl, 0 points; 140-179 mg/dl, 1 point; 100-139 mg/dl, 2 points; <100 mg/dl, 3 points), and total lymphocyte count (≥1,600/µl, 0 points; 1,200-1,599/µl, 1 point; 800-1,199/µl, 2 points; <800/µl, 3 points). Venous blood samples for serum preparation were obtained in the early morning after 12 h of fasting from all subjects, and the serum concentration of albumin (g/dl), total lymphocyte count, and total cholesterol level (mg/dl) were measured.
Questionnaire concerning the severity of constipation
The CSS questionnaire, which is a self-administered questionnaire concerning the severity of constipation, has been validated for the assessment of constipation in clinical trial settings (12). The CSS comprises 8 items describing the following symptoms of constipation: Frequency of bowel movements, painful evacuation, incomplete evacuation, abdominal pain, length of time per attempt, assistance for evacuation, unsuccessful attempts at evacuation per 24 h, and duration of constipation. The score for each item ranges from 0 to 4 with the exception of ‘assistance for evacuation’, whose score ranges from 0 to 2. Consequently, the overall score for CSS ranges from 0 to 30, with a higher score indicating worse constipation symptoms.
Questionnaire concerning abdominal symptom-related QOL
The Izumo scale was designed for the quantitative measurement of abdominal symptom-related QOL (13). It is based on a self-reported questionnaire including 15 items in 5 domains: Reflux, Upper abdominal pain, Fullness, Constipation, and Diarrhea. Each item is scored from 0 to 5 on a Likert scale according to the degree of symptoms, as follows: 0=not bothered, 1=not so bothered, 2=slightly bothered, 3=bothered, 4=strongly bothered and 5=intolerably bothered. Each domain comprises three items and each domain has a score from 0 to 15 points to evaluate the severity of symptoms, with higher scores indicating more severe symptoms. Each question is grouped into one of the five domains, in which questions 1, 2 and 3 are grouped into the Reflux domain, questions 4, 5 and 6 into the Upper abdominal pain domain, questions 7, 8 and 9 into the Fullness domain, questions 10, 11 and 12 into the Constipation domain, and questions 13, 14 and 15 into the Diarrhea domain. Domain-specific QOL impairment in the Izumo scale is ranked from 0 (no QOL impairment) to 15. It has good internal consistency and has good correlation with the Gastrointestinal Symptom Rating Scale. In each patient, the sum of the scores obtained for the three questions in each domain (Reflux, Upper abdominal pain, Fullness, Constipation, and Diarrhea) was calculated and defined as the reflux-related QOL score, upper abdominal pain-related QOL score, fullness-related QOL score, constipation-related QOL score, and diarrhea-related QOL score, respectively.
Questionnaire concerning stool shape
Stool consistency and shape were assessed by the Bristol Stool Form Scale (BSFS) (14), which classifies stool into seven categories: 1, nut-like; 2, lumpy sausage; 3, sausage with cracks; 4, smooth snake; 5, soft blobs; 6, fluffy pieces; and 7, watery.
Ethics
This study was conducted in accordance with the tenets of the Declaration of Helsinki. The Juntendo Tokyo Koto Geriatric Medical Center Ethics Committee approved the study and the study protocol (protocol no. 106-12). With regard to informed consent of the participants, the Juntendo Tokyo Koto Geriatric Medical Center Ethics Committee determined that this study was exempt from the need to obtain patient consent. According to the decision of the Juntendo Tokyo Koto Geriatric Medical Center Ethics Committee, we notified the study subjects about our study contents on the homepage of our hospital and guaranteed them the opportunity to refuse participation.
Statistical analyses
Clinical characteristics were compared between the sarcopenia and non-sarcopenia groups and univariate analyses of clinical characteristics were performed using χ2 test and t-test. Correlations between the constipation scoring system (CSS) score and various clinical parameters (age, BMI, albumin level, cholesterol level, lymphocyte count, CONUT score, BSFS score, and reflux-related, upper abdominal pain-related, fullness-related, constipation-related, and diarrhea-related QOL scores) were determined based on Pearson's correlation coefficients. The age, BMI, albumin level, cholesterol level, lymphocyte count, CONUT score, CSS score, BSFS score, and reflux-related, upper abdominal pain-related, fullness-related, constipation-related, diarrhea-related QOL scores are presented as the mean ± standard deviation. Comparison between the CSS score and sarcopenia was analyzed using the box-and-whisker plot method. Multiple regression analysis was performed with CSS score as the dependent variable, and with age, sex, BMI, laxative/prokinetics use, sarcopenia, albumin level, cholesterol level, lymphocyte count, CONUT score, BSFS score, and reflux-related, upper abdominal pain-related, fullness-related, constipation-related, and diarrhea-related QOL scores as independent variables. Multiple regression analysis of the risk factors for high CSS score was performed using a forced entry method and we judged multicollinearity by the variance inflation factor (VIF). All statistical analyses were performed using the SPSS version 19 software program (IBM Corp.). P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical characteristics of the study patients
A flow chart of participants is shown in Fig. 1. The clinical characteristics of the 310 eligible study subjects [149 men (48.1%) and 161 women (51.9%); mean age, 75.7±6.1 years; mean body mass index, 23.0±3.6 kg/m2] are summarized in Table I. The CSS score was significantly higher in the sarcopenia group (n=83) than in the non-sarcopenia group (n=227) (4.9±4.9 vs. 3.6±3.6, P=0.009). In addition, age (P<0.001), male gender (P=0.004), prevalence of laxative/prokinetic use (P=0.009) and CONUT score (P=0.001) were significantly higher, while BMI (P<0.001), albumin level (P=0.003) and cholesterol level (P=0.021) were significantly lower in the sarcopenia group than in the non-sarcopenia group.
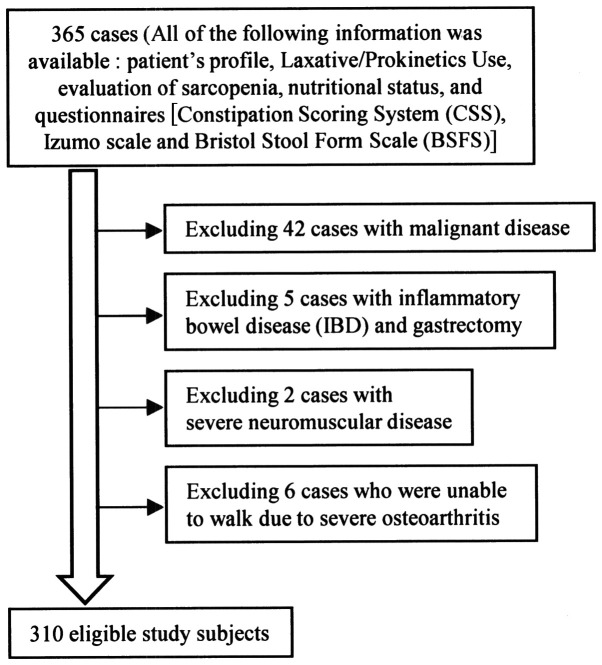
Figure 1.
A flow chart of the participants. Of the 365 study subjects, all of the following information was available from the medical records: Patient's profile, laxative/prokinetics use, evaluation of sarcopenia, nutritional status, and questionnaires [Constipation Scoring System (CSS), Izumo scale and Bristol Stool Form Scale (BSFS)]; 42 cases with malignant disease, 5 cases with inflammatory bowel disease (IBD) and gastrectomy, 2 cases with severe neuromuscular disease and 6 cases who were unable to walk due to severe osteoarthritis were excluded in this study. There were 310 eligible participants.
Table I.
Clinical characteristics of the study patients (n=310).
| Patient profile | Total (n=310) | Sarcopenia (n=83) | Non-sarcopenia (n=227) | P-value |
|---|---|---|---|---|
| Age (years) | 75.7 (±6.1)b | 79.1 (±6.1)b | 74.4 (±5.7)b | <0.001 |
| Sex | ||||
| Male | 149 (48.1)a | 51 (61.4)a | 98 (43.2)a | |
| Female | 161 (51.9)a | 32 (38.6)a | 129 (56.8)a | 0.004 |
| BMI (kg/m2) | 23.0 (±3.6)b | 21.4 (±2.7)b | 23.6 (±3.7)b | <0.001 |
| Internal medicine therapeutic agents | ||||
| Laxative/prokinetics | ||||
| Non-user | 241 (77.7)a | 56 (67.5)a | 185 (81.5)a | |
| User | 69 (22.3)a | 27 (32.5)a | 42 (18.5)a | 0.009 |
| Nutritional status | ||||
| Albumin level (g/dl) | 4.2 (±0.4)b | 4.1 (±0.4)b | 4.2 (±0.3)b | 0.003 |
| Cholesterol level (mg/dl) | 204 (±36)b | 196 (±40)b | 206 (±34)b | 0.021 |
| Lymphocyte count (/µl) | 1,869 (±632)b | 1,768 (±690)b | 1,906 (±607)b | 0.088 |
| CONUT score | 0.9 (±1.2)b | 1.2 (±1.4)b | 0.7 (±1.1)b | 0.001 |
| Severity of constipation | ||||
| CSS score | 4.0 (±4.0)b | 4.9 (±4.9)b | 3.6 (±3.6)b | 0.009 |
| Abdominal symptom-related QOL | ||||
| Reflux-related QOL score | 1.5 (±2.0)b | 1.3 (±1.7)b | 1.5 (±2.1)b | 0.250 |
| Upper abdominal pain-related QOL score | 1.2 (±2.0)b | 0.9 (±2.0)b | 1.3 (±2.1)b | 0.150 |
| Fullness-related QOL score | 1.5 (±2.1)b | 1.3 (±2.0)b | 1.6 (±2.1)b | 0.178 |
| Constipation-related QOL score | 2.3 (±2.7)b | 2.4 (±2.7)b | 2.2 (±2.7)b | 0.520 |
| Diarrhea-related QOL score | 1.6 (±2.4)b | 1.7 (±2.6)b | 1.6 (±2.3)b | 0.814 |
| Stool shape | ||||
| BSFS score | 4.0 (±1.0)b | 4.0 (±1.2)b | 4.0 (±1.0)b | 0.791 |
aNumber (%).
bMean (± standard deviation). BMI, body mass index; CSS, Constipation Scoring System; BSFS, Bristol Stool Form Scale; CONUT, CONtrolling NUTritional status; QOL, quality of life.
Correlations between the CSS score and various clinical parameters
The results of Pearson's correlation coefficients are shown in Table II. The CSS score was significantly and positively correlated with the CONUT score (r=0.130, P=0.011), reflux-related QOL score (r=0.155, P=0.003), upper abdominal pain-related QOL score (r=0.171, P=0.001), fullness-related QOL score (r=0.299, P<0.001), constipation-related QOL score (r=0.615, P<0.001) and diarrhea-related QOL score (r=0.235, P<0.001), while it was significantly and negatively correlated with the albumin level (r=-0.148, P=0.004), lymphocyte count (r=-0.118, P=0.019) and BSFS score (r=-0.114, P=0.023).
Table II.
Correlations between the constipation scoring system (CSS) score and various clinical parameters.
| Clinical parameters | r | P-value |
|---|---|---|
| Age (years) | 0.083 | 0.07 |
| BMI (kg/m2) | -0.077 | 0.09 |
| Albumin level (g/dl) | -0.148 | <0.01 |
| Cholesterol level (mg/dl) | -0.086 | 0.07 |
| Lymphocyte count (/µl) | -0.118 | <0.05 |
| CONUT score | 0.130 | <0.05 |
| Reflux-related QOL score | 0.155 | <0.01 |
| Upper abdominal pain-related QOL score | 0.171 | <0.01 |
| Fullness-related QOL score | 0.299 | <0.001 |
| Constipation-related QOL score | 0.615 | <0.001 |
| Diarrhea-related QOL score | 0.235 | <0.001 |
| BSFS score | -0.114 | <0.05 |
r, Spearman's correlation coefficient; BMI, body mass index; CONUT, CONtrolling NUTritional status; BSFS, Bristol Stool Form Scale.
Association between the severity of constipation and sarcopenia
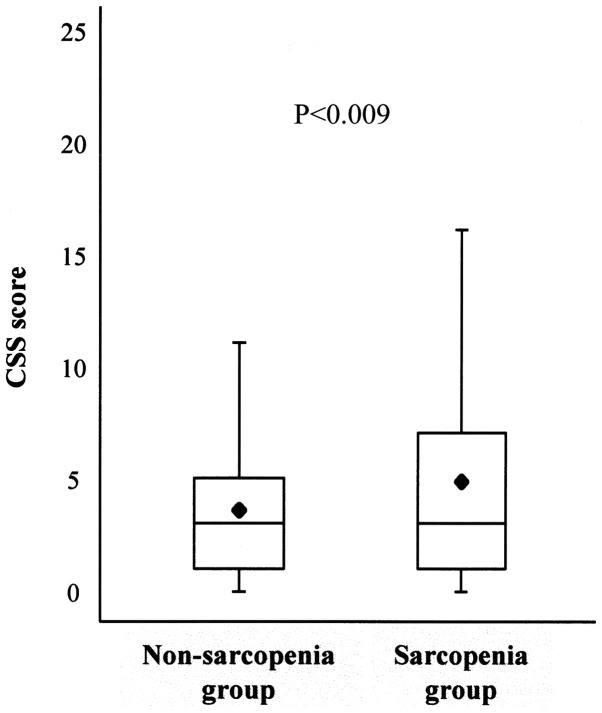
The association between the CSS score and sarcopenia was analyzed using the box-and-whisker plot method. The lower hinge, median, and upper hinge of the box corresponded to the 25, 50, and 75th percentiles, respectively. The mean of the data set is indicated by the rhombus. The CSS score was significantly higher in subjects with sarcopenia than in subjects without sarcopenia (4.9±4.9 vs. 3.6±3.6, P<0.001) (Table I, Fig. 2).
Figure 2.
Association between the CSS score and sarcopenia. Box and whisker plots of the Constipation Scoring System (CSS) score in the non-sarcopenia and sarcopenia groups show the minimum and maximum values. The lower hinge, median, and upper hinge of the box correspond to the 25, 50, and 75th percentiles, respectively. The mean in each group is indicated by the rhombus.
Results of the multiple regression analysis
The multiple regression analysis results are shown in Table III. In the multiple regression analysis, independent predictors for CSS score were sarcopenia (standardized partial regression coefficient [β]=0.107, P=0.032), constipation-related QOL score (β=0.537, P<0.001), laxative/prokinetics use (β=0.211, P<0.001) and BSFS score (β=-0.098, P=0.031) (R2=0.436).
Table III.
Association between the constipation scoring system (CSS) score and other variables in the multiple regression analysis.
| Variables | B | SE | 95% CI of B | β | t | VIF | P-value |
|---|---|---|---|---|---|---|---|
| Age | -0.059 | 0.031 | 0.120, 0.002 | -0.091 | -1.905 | -0.081 | 0.058 |
| Sex | 0.477 | 0.372 | -0.256, 1.210 | 0.060 | 1.280 | 0.055 | 0.201 |
| BMI | -0.033 | 0.052 | -0.136, 0.070 | -0.029 | -0.625 | -0.027 | 0.533 |
| Laxative/prokinetics use | 2.027 | 0.432 | 1.176, 2.878 | 0.211 | 4.688 | 0.200 | <0.001 |
| Sarcopenia | 0.966 | 0.447 | 0.086, 1.845 | 0.107 | 2.160 | 0.092 | 0.032 |
| Albumin level | -1.113 | 0.569 | -2.233, 0.008 | -0.098 | -1.954 | -0.083 | 0.052 |
| Cholesterol level | -0.006 | 0.006 | -0.017, 0.006 | -0.051 | -0.971 | -0.041 | 0.332 |
| Lymphocyte count | 0.000 | 0.000 | -0.001, 0.000 | -0.051 | -0.917 | -0.039 | 0.360 |
| CONUT score | -0.166 | 0.227 | -0.612, 0.279 | -0.049 | -0.735 | -0.031 | 0.463 |
| Reflux-related QOL score | -0.078 | 0.101 | -0.278, 0.121 | -0.038 | -0.771 | -0.033 | 0.442 |
| Upper abdominal pain-related QOL score | -0.048 | 0.104 | -0.253, 0.157 | -0.025 | -0.463 | -0.020 | 0.644 |
| Fullness-related QOL score | 0.109 | 0.116 | -0.120, 0.337 | 0.056 | 0.937 | 0.040 | 0.350 |
| Constipation-related QOL score | 0.803 | 0.078 | 0.650, 0.957 | 0.537 | 10.313 | 0.441 | <0.001 |
| Diarrhea-related QOL score | 0.079 | 0.090 | -0.097, 0.256 | 0.047 | 0.884 | 0.038 | 0.378 |
| BSFS score | -0.382 | 0.176 | -0.728, -0.035 | -0.098 | -2.169 | -0.093 | 0.031 |
Multiple regression analysis was performed with the constipation scoring system (CSS) score as the dependent variable, and with age, sex, BMI, laxative/prokinetics use, sarcopenia, albumin level, cholesterol level, lymphocyte count, CONUT score, reflux-related QOL score, upper abdominal pain-related QOL score, fullness-related QOL score, constipation-related QOL score, diarrhea-related QOL score, and BSFS score as independent variables. B, partial regression coefficient; SE, standard error; CI, confidence interval; β, standardized partial regression coefficient; t, t-ratio; VIF, variance inflation factor; CONUT, CONtrolling NUTritional status; BSFS, Bristol Stool Form Scale; BMI, body mass index; QOL, quality of life.
Discussion
To the best of our knowledge, no previous studies have investigated the association between the severity of constipation and sarcopenia in elderly adults. The present study demonstrated that independent predictors for the Constipation Scoring System (CSS) score were sarcopenia, constipation-related quality of life (QOL) score, laxative/prokinetics use and Bristol Stool Form Scale (BSFS) score in multiple regression analysis.
In this study, we demonstrated that there was a positive association between the severity of constipation and sarcopenia in elderly adults. Functional constipation can be classified into normal transit constipation, slow transit constipation, and functional defecation disorder (15). The process of defecation is carried out in the following order. First, the anorectal angle assumes a linear shape by the musculus puborectalis relaxing at the same time to increase abdominal pressure. Furthermore, defecation is finally performed by relaxation of the musculus sphincter ani externus (16). Therefore, in the defecation process, pelvic floor dysfunction and/or a decrease in abdominal pressure may cause functional defecation disorder. Kepenekci et al (17) demonstrated that age was the major factor associated with the development of pelvic floor dysfunction. Dimpfl et al (18) reported that aging and vaginal childbirth lead to histomorphological changes in the pelvic floor muscle that are consistent with changes of myogenic origin. Muscle weakness by sarcopenia may cause pelvic floor dysfunction and/or a decrease in abdominal pressure. As a result, painful evacuation effort and feeling incomplete evacuation are caused, and the severity of constipation might increase. In addition, due to sarcopenia, the decrease of the muscular strength necessary for the movement to the restroom might decrease stool frequency.
On the other hand, several studies reported a neurologic change in the intestinal tract in patients with constipation. Hanani et al (19) suggested that there is an increase in the number of abnormally appearing myenteric ganglia in the human colon with age, which may contribute to disturbed colonic motility in the aging population. Bassotti et al (20) reported that patients with chronic constipation have several abnormalities reconductable to alterations in the enteric nervous system, abnormalities mainly characterized by a constant decrease in enteric glial cells and interstitial cells of Cajal. Kwon and Yoon (21) suggested that there are many neurologic insults on sarcopenia at various levels from the brain to the neuromuscular junctions (NMJs) to generate a volitional task. Regarding the neurologic change in the intestinal tract, the histopathological association between chronic constipation and sarcopenia is unclear at present; however, a common neuropathy may underlie both constipation and sarcopenia.
In addition, we found that the severity of constipation was positively related to the constipation-related QOL score and laxative/prokinetics use, and negatively related to stool shape (BSFS score) in this study. Wald et al (3) suggested that chronic constipation greatly impairs a patient's QOL. There were few reports of the association between use of laxative/prokinetics and severity of constipation in Japan. By the recent internet survey on the actual situation of constipation in the Japanese population, 77.1% of patients with functional constipation used laxatives (22). In our study, it was shown that laxative/prokinetics users may have severe constipation. In a previous study, Tanabe et al (23) reported that QOL was significantly impaired in the constipated group, and that the BSFS score in the constipation group was significantly lower than that in the control group, indicating that the stool was harder in the constipation group than in the control group.
There were several limitations to this study. First, the single-center university hospital-based retrospective cross-sectional design prevented us from establishing causal associations between the severity of constipation and sarcopenia. Second, the sample size was limited and we did not investigate the life background of the subjects such as history of smoking/alcohol use, exercise habits, meal contents, occupation/career, education level, marital status, and medication use except laxative/prokinetics. Thus, the findings in this study should be considered preliminary owing to the relatively small sample size. Therefore, it is possible that the present data are not generalizable to all community-dwelling elderly individuals.
In conclusion, we found that there was a positive association between the severity of constipation and sarcopenia in elderly adults in a single-center university hospital-based, retrospective cross-sectional study. It may be important in the future to consider the therapy for constipation in elderly adults with a comprehensive view that considers the prevention of sarcopenia as well as meals, daily life guidance and medical therapy. The findings in this study should be considered preliminary owing to the relatively small sample size. Further studies are required to understand these associations including biological mechanisms.
Acknowledgements
The authors thank all of the participants for cooperating with the study.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
DAs and AN conceived and designed the study. DAs analyzed the data. DAs, TT, YI, DAb, YS, KeM, HU, KoM, HK, YA, TO, MH and AN performed the data collection. All authors contributed to the writing of the manuscript and reviewed and approved the final manuscript.
Ethics approval and consent to participate
The present study was conducted in accordance with the tenets of the Declaration of Helsinki. The Juntendo Tokyo Koto Geriatric Medical Center Ethics Committee approved the study and the study protocol. According to the decision of the Juntendo Tokyo Koto Geriatric Medical Center Ethics Committee, we notified the study subjects concerning the contents of our study on the homepage of our hospital and guaranteed them the opportunity to refuse participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
References
- 1.Higgins PD, Johanson JF. Epidemiology of constipation in North America: A systematic review. Am J Gastroenterol. 2004;99:750–759. doi: 10.1111/j.1572-0241.2004.04114.x. [DOI] [PubMed] [Google Scholar]
- 2.Tamura A, Tomita T, Oshima T, Toyoshima F, Yamasaki T, Okugawa T, Kondo T, Kono T, Tozawa K, Ikehara H, et al. Prevalence and Self-recognition of chronic constipation: Results of an internet survey. J Neurogastroenterol Motil. 2016;22:677–685. doi: 10.5056/jnm15187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wald A, Scarpignato C, Kamm MA, Mueller-Lissner S, Helfrich I, Schuijt C, Bubeck J, Limoni C, Petrini O. The burden of constipation on quality of life: Results of a multinational survey. Aliment Pharmacol Ther. 2007;26:227–236. doi: 10.1111/j.1365-2036.2007.03376.x. [DOI] [PubMed] [Google Scholar]
- 4.Choung RS, Rey E, Richard Locke G III, Schleck CD, Baum C, Zinsmeister AR, Talley NJ. Chronic constipation and Co-morbidities: A prospective population-based nested Case-control study. United European Gastroenterol J. 2016;4:142–151. doi: 10.1177/2050640614558476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkins JL, Whincup PH, Morris RW, Lennon LT, Papacosta O, Wannamethee SG. Sarcopenic obesity and risk of cardiovascular disease and mortality: A population-based cohort study of older men. J Am Geriatr Soc. 2014;62:253–260. doi: 10.1111/jgs.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anbalagan VP, Venkataraman V, Pradeepa R, Deepa M, Anjana RM, Mohan V. The prevalence of presarcopenia in Asian Indian individuals with and without type 2 diabetes. Diabetes Technol Ther. 2013;15:768–775. doi: 10.1089/dia.2013.0068. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimura N, Muraki S, Oka H, Iidaka T, Kodama R, Kawaguchi H, Nakamura K, Tanaka S, Akune T. Is osteoporosis a predictor for future sarcopenia or vice versa? Four-year observations between the second and third ROAD study surveys. Osteoporos Int. 2017;28:189–199. doi: 10.1007/s00198-016-3823-0. [DOI] [PubMed] [Google Scholar]
- 8.Sugimoto T, Ono R, Murata S, Saji N, Matsui Y, Niida S, Toba K, Sakurai T. Prevalence and associated factors of sarcopenia in elderly subjects with amnestic mild cognitive impairment or Alzheimer disease. Curr Alzheimer Res. 2016;13:718–726. doi: 10.2174/1567205013666160211124828. [DOI] [PubMed] [Google Scholar]
- 9.Woo J, Leung J, Morley JE. Defining sarcopenia in terms of incident adverse outcomes. J Am Med Dir Assoc. 2015;16:247–252. doi: 10.1016/j.jamda.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21:300–307. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, Rodríguez F, Fernández G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20:38–45. [PubMed] [Google Scholar]
- 12.Agachan F, Chen T, Pfeifer J, Reissman P, Wexner SD. A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon Rectum. 1996;39:681–685. doi: 10.1007/BF02056950. [DOI] [PubMed] [Google Scholar]
- 13.Furuta K, Ishihara S, Sato S, Miyake T, Ishimura N, Koshino K, Tobita H, Moriyama I, Amano Y, Adachi K, et al. Development and verification of the Izumo Scale, new questionnaire for quality of life assessment of patients with gastrointestinal symptoms. Nihon Shokakibyo Gakkai Zasshi. 2009;106:1478–1487. (In Japanese) [PubMed] [Google Scholar]
- 14.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–944. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 15.Locke GR III, Pemberton JH, Phillips SF. AGA technical review on constipation. American Gastroenterological Association. Gastroenterology. 2000;119:1766–1778. doi: 10.1053/gast.2000.20392. [DOI] [PubMed] [Google Scholar]
- 16.Bouras EP, Tangalos EG. Chronic constipation in the elderly. Gastroenterol Clin North Am. 2009;38:463–480. doi: 10.1016/j.gtc.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Kepenekci I, Keskinkilic B, Akinsu F, Cakir P, Elhan AH, Erkek AB, Kuzu MA. Prevalence of pelvic floor disorders in the female population and the impact of age, mode of delivery, and parity. Dis Colon Rectum. 2011;54:85–94. doi: 10.1007/DCR.0b013e3181fd2356. [DOI] [PubMed] [Google Scholar]
- 18.Dimpfl T, Jaeger C, Mueller-Felber W, Anthuber C, Hirsch A, Brandmaier R, Schuessler B. Myogenic changes of the levator ani muscle in premenopausal women: The impact of vaginal delivery and age. Neurourol Urodyn. 1998;17:197–205. doi: 10.1002/(sici)1520-6777(1998)17:3<197::aid-nau4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Hanani M, Fellig Y, Udassin R, Freund HR. Age-related changes in the morphology of the myenteric plexus of the human colon. Auton Neurosci. 2004;113:71–78. doi: 10.1016/j.autneu.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Bassotti G, Villanacci V, Creţoiu D, Creţoiu SM, Becheanu G. Cellular and molecular basis of chronic constipation: Taking the functional/idiopathic label out. World J Gastroenterol. 2013;19:4099–4105. doi: 10.3748/wjg.v19.i26.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon YN, Yoon SS. Sarcopenia: Neurological point of view. J Bone Metab. 2017;24:83–89. doi: 10.11005/jbm.2017.24.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamura Y, Yamamoto S, Funaki Y, Ohashi W, Yamamoto K, Ozeki T, Yamaguchi Y, Tamura Y, Izawa S, Hijikata Y, et al. Internet survey on the actual situation of constipation in the Japanese population under 70 years old: Focus on functional constipation and Constipation-predominant irritable bowel syndrome. J Gastroenterol. 2020;55:27–38. doi: 10.1007/s00535-019-01611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanabe A, Adachi K, Yamaguchi Y, Izawa S, Yamamoto S, Hijikata Y, Ebi M, Funaki Y, Ogasawara N, Goto C, et al. Gut Environment and dietary habits in healthy japanese adults and their association with bowel movement. Digestion. 2019;21:1–11. doi: 10.1159/000501961. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.