Abstract
Urinary nano-extracellular vesicles (NVs), including exosomes and microvesicles, are considered potential biomarkers for kidney diseases using liquid biopsies. However, clinical application of urinary NVs has not yet been validated. In the present study, the levels of mRNAs in urinary NVs in animal models of kidney disease were assessed. Urine samples were collected from the animal models and urinary NVs were isolated by ultracentrifugation. Gene expression levels of kidney injury markers in urinary NVs and renal tissue were quantified by reverse transcription-quantitative PCR. The mRNA levels of desmin, a podocyte injury marker, in urinary NVs was markedly increased in the puromycin aminonucleoside (PAN) nephritis model, in parallel with enhanced desmin expression in kidney tissues. The expression of regulator of calcineurin 1 and the podocin to nephrin ratio (PNR) were also increased in the PAN nephritis model. Treatment with prednisolone mitigated these changes in gene expression as well as proteinuria. PNR, which is considered a predictive marker of glomerular dysfunction, in urinary NVs was highly correlated with urinary protein excretion (P<0.01). Furthermore, PNR in urinary NVs of Zucker diabetic fatty rats, a diabetic kidney disease model, was correlated with urinary albumin excretion (P<0.01). These results suggest that changes in mRNA levels of urinary NVs reflect the disease status of kidney tissues and their functional alterations. Collectively, mRNA analysis of urinary NVs may be used as a liquid biopsy tool for improved classification and performance of risk prediction to determine the severity of kidney diseases.
Keywords: liquid biopsy, biomarker, nano-extracellular vesicle, exosome, mRNA, renal disease, podocyte injury
Introduction
Chronic kidney disease (CKD), a very common disease, is considered a growing public health issue, with a notable impact on the economy and society. CKD progression without effective treatment may lead to end-stage renal disease (ESRD). The annual cost of treating ESRD is currently >$15 billion and ~$32 billion in Japan and the US, respectively (1,2). Moreover, the number of CKD patients is expected to increase steadily in several countries, and CKD is considered a risk factor for cardiovascular diseases (3). Therefore, new suitable and precision-based diagnostic tools for the prevention and treatment of CKD, including novel drugs, are urgently required. Currently, kidney diseases are primarily diagnosed based on serum creatinine, blood urea nitrogen and urinary albumin levels. However, these biomarkers are insufficient to determine the precise pathological state of a patient with CKD, which can result in heterogeneous outcomes (4,5). Therefore, a novel diagnostic method is required to achieve improved CKD diagnosis and management.
Nano-extracellular vesicles (NVs), including exosomes and microvesicles, are produced by a wide range of cell types and released into various body fluids such as urine, serum and saliva (6). Exosomes are vesicles 30-100 nm in diameter, produced through the fusion between multivesicular bodies and plasma membranes (6). On the contrary, microvesicles are 100-1,000 nm in diameter, which are released from cells through direct shedding of plasma membranes (7). Both types of vesicles contain various components such as cytosol-like proteins and nucleic acids (mRNA, microRNA and DNA) (7). Urine is a very useful source of NVs, as it can be obtained easily and non-invasively, and urinary NVs contain components originating from cells of all regions of the nephron, including glomeruli and renal tubules (8). The components of NVs are considered potential biomarkers for kidney diseases because they may reflect the physiological and pathophysiological states of their cells of origin. Recently, several scientists have reported the practicality of using urinary NVs as a diagnostic tool for kidney disease, prostate cancer and bladder cancer (8,9). However, detailed information on how urinary NVs reflect the physiological and pathological states of the kidney, as well as its functionality, remains elusive. Accordingly, the clinical application of urinary NVs for liquid biopsy has not yet been fully validated.
In the present study, the expression of mRNAs in urinary NVs from rat models of both glomerular nephritis and diabetic kidney disease were assessed to investigate their applicability as a novel diagnostic tool.
Materials and methods
Animals
Male Sprague Dawley (SD), Zucker lean (ZL) and Zucker diabetic fatty (ZDF) rats were purchased from Charles River Laboratories, Inc. Prior to the experimental procedures, animals were acclimatized for at least 5 days and housed under a 12-h light/dark cycle with ad libitum access to water and standard chow, CRF-1 (Oriental Yeast Co., Ltd.). For the puromycin aminonucleoside (PAN)-induced glomerular nephritis model, 73 6-week-old male SD rats received a tail vein injection of 100 mg/kg/5 ml PAN (Sigma-Aldrich; Merck KGaA) in saline buffer. In addition, 24 saline buffer-injected rats were used as the control. Prednisolone was administered as a single oral dose of 10 mg/kg/10 ml prednisolone (Shionogi & Co., Ltd.) 1 h prior to PAN injection using distilled water as the vehicle control. These animals were kept for 1 week and then were euthanized. For the type 2 diabetes model, 12-week-old male ZL (n=6) and ZDF (n=12) rats were used at the beginning of the experiment. These animals were kept for 20 weeks and then were euthanized. All animals were observed at least once daily for monitoring of health. No unforeseen deaths of animals occurred in these studies.
Urine collection and measurements of urinary protein, albumin and creatinine levels
Total urine samples were collected over 24 h from each animal in the metabolic cages at each time-point. Urine samples were used immediately or stored at -20˚C until required. Urinary protein, albumin and creatinine levels were measured using a Rat Urinary Protein assay kit (Chondrex, Inc.), a LBIS™ Rat Albumin ELISA kit (FUJIFILM Wako Pure Chemical Corporation) and a LabAssay™ Creatinine kit (FUJIFILM Wako Pure Chemical Corporation) respectively, according to the manufacturer's protocol.
Blood collection and blood glucose measurements
Blood samples were collected from the tail vein at each time-point. Blood samples were diluted with distilled water (1:10) and blood glucose was measured using a Glucose Cii Test Wako kit (FUJIFILM Wako Pure Chemical Corporation) according to the manufacturer's protocol.
Isolation of NVs
Urinary NVs were isolated from urine samples using differential centrifugation at 3,000 x g for 10 min at 4˚C. Then, supernatants were centrifuged at 100,000 x g for 1 h at 4˚C and NVs were retrieved from the pellets after gently discarding the supernatants. For nanoparticle tracking analysis (NTA), pellets were suspended in PBS. NTA was performed using the Nanosight LM20 instrument (Nanosight Ltd.) to analyze the distribution of vesicle size and the concentration of particles, as previously reported (10).
mRNA analysis in NVs
For the NVs isolated from the urine of PAN nephritis model rats, total RNA was purified using the RNeasy micro kit (QIAGEN, Inc.) according to the manufacturer's protocol. Briefly, isolated NVs were lysed using lysis buffer containing 1% β-mercaptoethanol, and total RNA was purified using MinElute spin columns with on-column DNase digestion (Qiagen, Inc.). RNA was quantified using a NanoDrop 1000 (Thermo Fisher Scientific, Inc.) and cDNA was synthesized using a SuperScript™ VILO™ cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). Reverse transcription-quantitative (RT-q)PCR was performed using the Biomark HD system (Fluidigm Corporation) with specific TaqMan Gene Expression assays for hypoxanthine phosphoribosyltransferase (Hprt1; Rn01527840_m1), desmin (Rn00574732_m1), aquaporin 1 (Aqp1; Rn00562834_m1), nephrin (Rn00674268_m1), podocin (Rn00709834_m1), and regulator of calcineurin 1 (Rcan1; Rn00596606_m1) (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. For analysis of NVs from ZDF and ZL rats, mRNA was extracted from the isolated NVs using oligo(dT)-immobilized microplates (Hitachi Chemical Diagnostics, Inc.) and quantified by RT-qPCR as previously described (11). All mRNA levels were normalized to the level of Hprt1.
Isolation of glomeruli
Rats were anesthetized using 5% sevoflurane via an inhalation anesthetic system, and the kidneys were immediately excised following euthanasia by exsanguination. After removal of kidney capsules, renal cortexes were minced into very fine fragments. Glomeruli were collected using standard sieving methods as previously described (12). Total RNA was isolated from glomeruli was purified using a RNeasy micro kit, and RT-qPCR was performed using the Biomark HD system for specific TaqMan Gene Expression assays as described above.
Immunohistochemistry
Kidneys were collected from euthanized animals. These samples were immediately fixed in 10% neutralized buffered formalin for 1 week at room temperature. Fixed tissues were then embedded in paraffin for immunohistochemical analysis. Immunostaining of desmin was performed as described previously (13). Briefly, paraffin sections were deparaffinized and incubated overnight at 4˚C with an anti-desmin mouse monoclonal antibody (1:200; cat. no. M0760; Dako; Agilent Technologies, Inc.), and subsequently incubated for 30 min at room temperature with horseradish peroxidase-conjugated anti-mouse IgG goat polyclonal antibody (Nichirei Biosciences Inc.).
Statistical analysis
Data are presented as mean ± standard error of the mean unless otherwise stated. A Student's t-test was used for pairwise comparisons. When comparing >2 groups, statistical differences were evaluated using a one-way ANOVA followed by a Dunnett's multiple comparison test. Repeated measure-based parameters were evaluated using a two-way ANOVA followed by Bonferroni's correction. P<0.05 was considered to indicate a statistically significant difference. For correlation analysis, Pearson correlation coefficients were calculated. All statistical analyses were performed using GraphPad Prism version 7.04 (GraphPad Software, Inc.).
Results
Characterization of urinary NVs in the PAN nephritis rat model
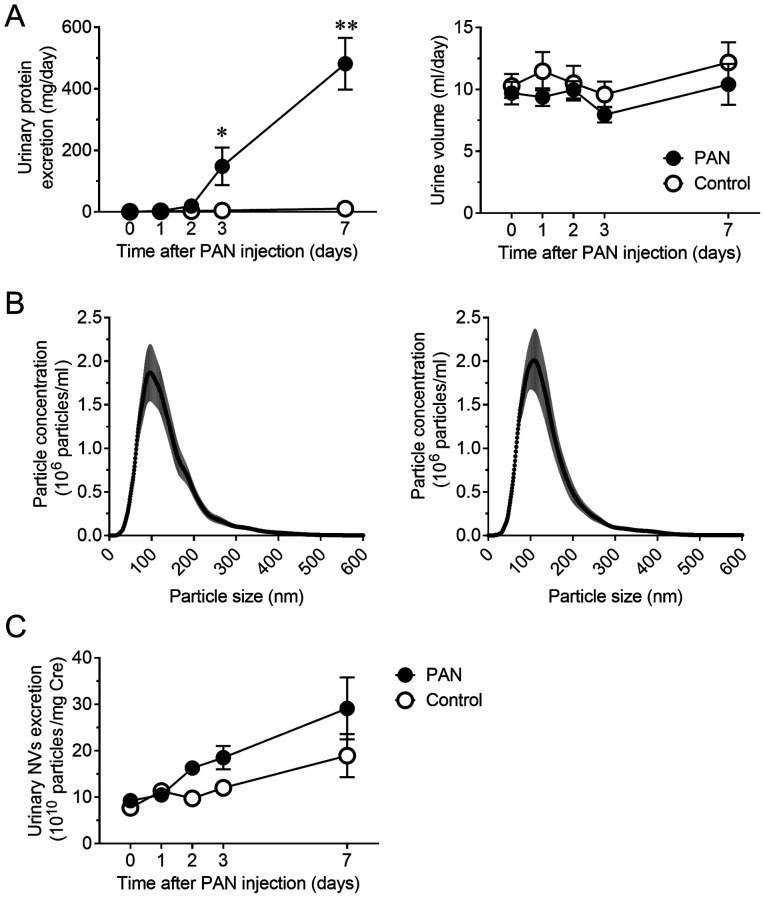
Notable proteinuria developed 3 days after the induction of PAN nephritis in the rat model. Urinary protein excretion significantly increased at days 3 and 7 (P<0.05 and P<0.01, respectively) without a significant change in urine volume (Fig. 1A). The size distribution and concentration of NVs in urine were assessed using NTA to confirm the presence of NVs and compare the vesicle profiles between normal and diseased animals. As previously reported (14,15), most of the obtained vesicles were <200 nm in diameter. The size distribution of NVs was not altered (Fig. 1B), but the concentration of urinary NVs was slightly increased in the PAN nephritis model, even though the difference was not significant (Fig. 1C).
Figure 1.
Disease profile of the PAN nephritis model and excretion of NVs in urine. (A) Time course of urinary protein excretion (left) and urine volume (right) (n=8). (B) Size distribution of NVs in urine from control rats (left) and PAN nephritis model rats (right) on day 7 (n=8). The gray range represents the SEM. (C) Time course of urinary NV excretion (n=8). Data are presented as the mean ± SEM. *P<0.05, **P<0.01 vs. control group. PAN, puromycin aminonucleoside; NVs, nano-extracellular vesicles; SEM, standard error of the mean.
Changes in mRNA levels of urinary NVs and glomeruli in the PAN nephritis rat model
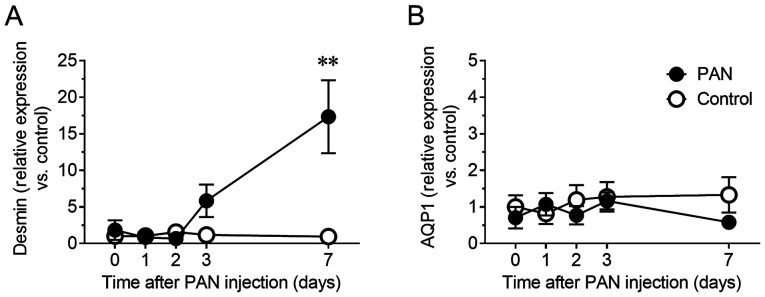
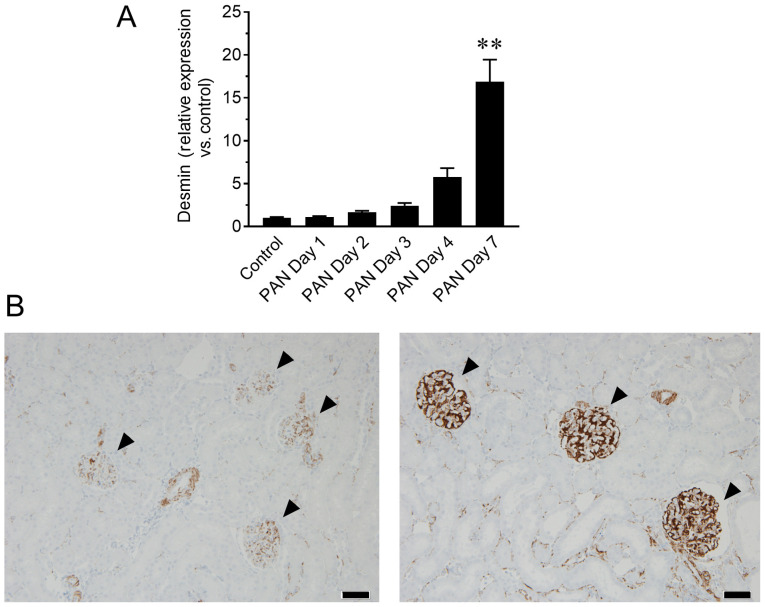
Whether urinary NVs could indicate site-specific damages in glomeruli were next determined. In fact, PAN is an antibiotic well known to cause glomerular specific damage (16). Therefore, the mRNA levels of desmin, a sensitive biomarker for glomerular injury in rodents (17,18), were evaluated in urinary NVs. The relative content of desmin mRNA in urinary NVs of the PAN nephritis model increased 5.8-fold (P=0.44) on day 3 and 17.3-fold (P<0.01) on day 7 (Fig. 2A). To confirm whether this change reflected the status of kidney tissue, mRNA and protein levels of desmin in the glomeruli were assessed by RT-qPCR and immunohistochemistry, respectively. The mRNA levels of desmin in glomeruli showed a similar increasing trend to that of urinary NVs, particularly on day 7 (P<0.01; Fig. 3A), whereas increased desmin immunoreactivity in glomeruli was observed in the PAN nephritis model (Fig. 3B), corroborating the observations from urinary NVs. Meanwhile, the mRNA levels of Aqp1, a proximal tubular marker, in urinary NVs was not altered in the PAN nephritis model (Fig. 2B).
Figure 2.
Changes in podocyte and tubular injury marker expression in urinary NVs. (A) Time course of relative mRNA levels of desmin, a podocyte injury marker, in urinary NVs. (B) Time course of relative mRNA levels of Aqp1, a tubular marker, in urinary NVs. n=4-8. Data are presented as the mean ± standard error of the mean. **P<0.01 vs. control group. NVs, nano-extracellular vesicles; Aqp1, aquaporin 1.
Figure 3.
Changes in desmin expression in glomeruli. (A) Changes in relative desmin gene expression in isolated glomeruli at different time-points. n=6-10. Data are presented as the mean ± standard error of the mean. **P<0.01 vs. control group. (B) Representative images of immunohistochemistry of desmin in the kidney of control (left) and PAN nephritis model (right) rats at day 7. Arrowheads, glomeruli. Scale bar, 50 µm. PAN, puromycin aminonucleoside.
Analysis of urinary NV mRNAs as pharmacological biomarkers
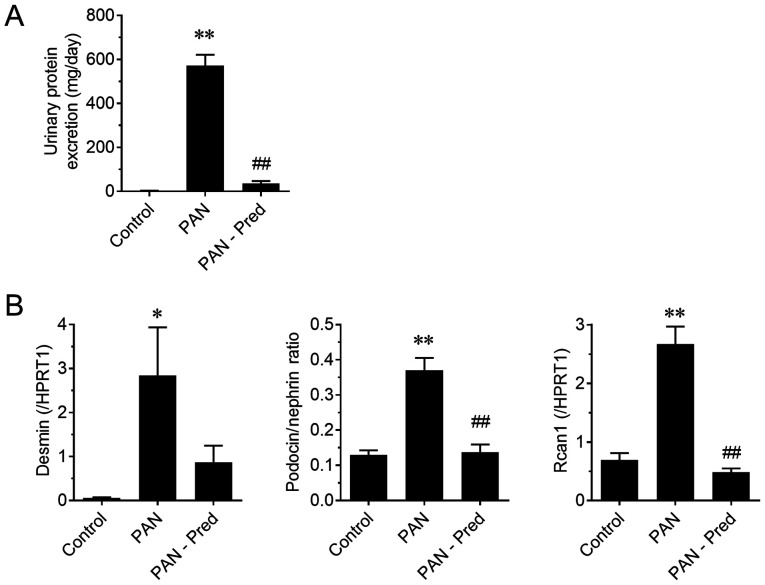
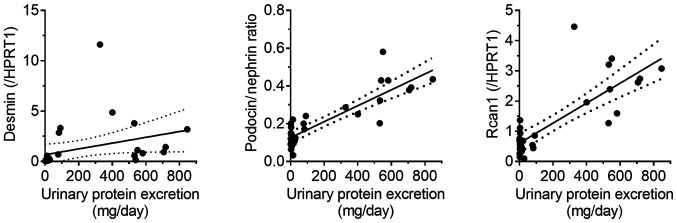
To evaluate the potential of mRNAs in urinary NVs as pharmacological biomarkers, the effect of prednisolone on the PAN nephritis model was assessed. The induced urinary protein excretion of the PAN nephritis model was significantly mitigated by treatment with prednisolone (P<0.01; Fig. 4A), confirming previously reported observations (19). Fig. 4B shows the effect of prednisolone on the mRNA levels of urinary NVs. The expression of two podocyte injury markers, the mRNA levels of desmin and the podocin to nephrin ratio (PNR), the latter being a potential podocyte loss prediction marker reported by Fukuda et al (20), significantly increased in the PAN nephritis model (P<0.01), whereas a decreasing tendency and a significant decrease in the prednisolone-treated group was observed (P=0.10 and P<0.01, respectively). The mRNA levels of Rcan1, which is upregulated during active calcineurin signaling and is therefore considered a target of immunosuppressants for nephritis treatment (21), also increased in urinary NVs of the PAN nephritis model (P<0.01), and it was reversed by prednisolone treatment (P<0.01). Additionally, the correlations between mRNA levels and disease severity were assessed (Fig. 5). A positive correlation was observed between PNR and urinary protein excretion, as well as between Rcan1 levels and urinary protein excretion (r2=0.75 and 0.64, respectively; P<0.01 in both cases). A similar but weaker correlation was observed between desmin mRNA levels and urinary protein excretion (r2=0.12; P=0.07).
Figure 4.
Effect of Pred on proteinuria and mRNA levels of urinary NVs in the PAN nephritis model. (A) Effect of Pred on urinary protein excretion and (B) mRNAs levels of genes associated with podocyte injury in urinary NVs. Data are presented as the mean ± standard error of the mean. n=10. *P<0.05, **P<0.01 vs. control group; ##P<0.01 vs. the PAN group. Pred, prednisolone; NVs, nano-extracellular vesicles; HPRT1, hypoxanthine phosphoribosyltransferase.
Figure 5.
Correlation between gene expression and urinary protein excretion. Solid line, regression line; dotted lines, 95% confidence interval of the slope of the regression line. Rcan1, regulator of calcineurin 1; HPRT1, hypoxanthine phosphoribosyltransferase.
PNR in ZDF the kidney disease model
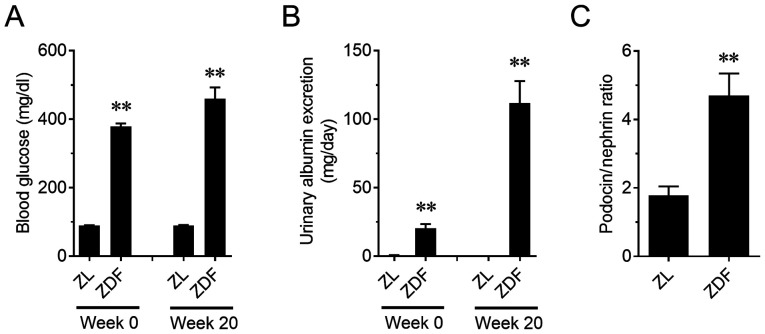
The relationship between kidney functionality and PNR in another type of kidney disease model was assessed. ZDF rats, a type 2 diabetes model, showed a significant increase in blood glucose on weeks 0 and 20 (P<0.01; Fig. 6A). A progressive increase in urinary albumin excretion was also observed in ZDF rats (P<0.01; Fig. 6B). In addition, PNR in ZDF rats on week 20 increased compared to that in the ZL rats (P<0.01; Fig. 6C). Furthermore, PNR was positively correlated with urinary albumin excretion, similar to the PAN nephritis model (r2=0.66; P<0.01; Fig. 7).
Figure 6.
Changes in blood glucose, urinary albumin excretion and in the podocin to nephrin ratio in urinary NVs of ZDF rats. (A) Changes in blood glucose levels and (B) urinary albumin excretion. n=6-12. (C) Changes in the podocin to nephrin ratio in urinary NVs. n=5-8. Data are presented as the mean ± standard error of the mean. **P<0.01 vs. ZL group. NVs, nano-extracellular vesicles; ZDF, Zucker diabetic fatty; ZL, Zucker lean.
Figure 7.
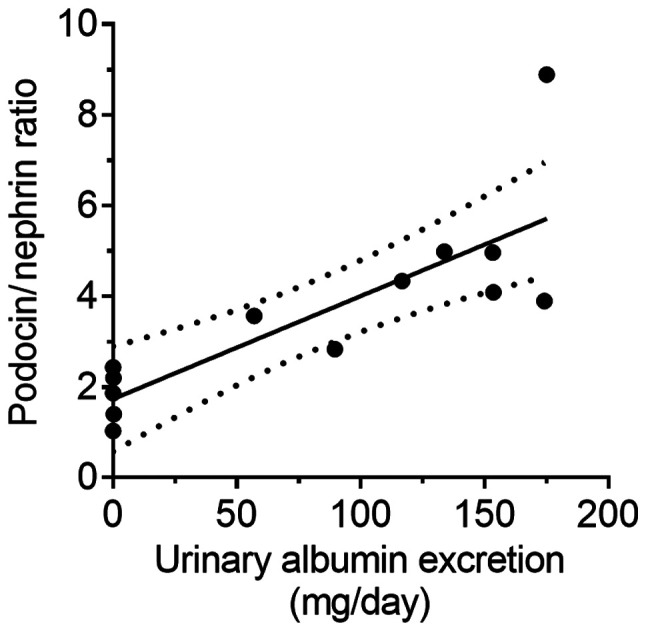
Correlation between podocin to nephrin ratio and urinary albumin excretion in Zucker diabetic fatty rats. Solid line, regression line; dotted lines, 95% confidence interval of the slope of the regression line.
Discussion
Liquid biopsy of blood using NVs has been actively investigated for oncology, in particular for breast cancer (22), lung cancer (23) and pancreatic (24) cancer. However, there is relatively little knowledge regarding the feasibility of liquid biopsy using NVs as biomarkers for renal disease. Various reports on the validity of urinary NVs for risk prediction of renal disease have highlighted urinary exosomal microRNAs as potential biomarkers for lupus nephritis (25), renal fibrosis (26,27) and early renal injury in essential hypertension (28). Moreover, it has been reported that urinary exosomal Wilms tumor 1 mRNA, which codes for a podocyte-derived transduction factor, is a candidate biomarker for diabetic nephropathy (29). Another group also suggested the potential of using exosomal mRNA levels of C-C motif chemokine ligand 2 as a diagnostic tool for IgA nephropathy (30). However, it is still unclear whether changes in the mRNA levels of kidney injury markers in urinary NVs are linked to the actual status of renal disease. In the present study, the mRNA levels of desmin and PNR as podocyte injury markers, as well as the mRNA levels of Rcan1 as a pathogenic marker, using urinary NVs were assessed, and their applicability as biomarkers for renal dysfunction and injury were demonstrated.
NVs were isolated by ultracentrifugation. Although it was reported that the amount and profile of the obtained NVs should depend on the isolation method (31), the size of the particles isolated in the present study was similar to that described in previous reports (14,15), indicating successful NV purification. No changes were detected in urinary NV excretion or particle size profiles in the PAN nephritis model. On the contrary, increased levels of desmin mRNA in urinary NVs reflected similar changes in glomeruli. However, there was no variation in the mRNA levels of Aqp1, known as a tubular marker, suggesting that tubules were not directly injured in the PAN nephritis model. Aqp1 expression has been reported to be decreased in an ischemia/reperfusion-induced acute kidney injury model in rats and during kidney transplantation in humans (32), consistent with the podocyte-specific toxicity of PAN (33). Moreover, Spanu et al (34) reported that the mRNA levels of cystatin C in urinary NVs was correlated with renal cortical expression and urinary cystatin C protein levels (34). Taking the results of the present study and those of previous reports together, it is hypothesized that changes in mRNA levels of urinary NVs reflect alterations in gene expression in the component cells of the kidney organ, and thus the analysis of urinary NVs can provide information on the pathological state of renal tissues in a non-invasive manner.
The applicability of urinary NVs as pharmacological biomarkers was also examined. The PNR increased in the PAN nephritis model, and was significantly decreased upon treatment with prednisolone. PNR in the urinary sediment was previously reported as a promising biomarker of podocyte stress in glomeruli (20,35), since shifts in PNR are thought to be due to alterations occurring in podocytes. The results of the present study are consistent with these reports, implying that the pharmacological effects of a drug for podocyte protection can be detected by analyzing isolated urinary NVs.
In addition, the mRNA levels of Rcan1 in urinary NVs were increased in the PAN nephritis model, whereas such increases were compensated by prednisolone treatment. The expression of Rcan1 has been reported to be induced through the activation of calcineurin/nuclear factor of activated T cells signaling, and the activation of this cascade can cause several kidney diseases, such as minimal change disease (36) and glomerulosclerosis (37). Calcineurin is known as a target of cyclosporine and tacrolimus, two common therapeutic agents for glomerular nephritis (21). Moreover, Ding et al (38) reported that calcineurin activity was upregulated by PAN treatment of podocytes in vitro, and it was suggested that calcineurin inhibitors protect against podocyte injury in a PAN-induced nephritis model (39). In addition, it has been reported that prednisolone and other glucocorticosteroids decrease the activity of calcineurin (40,41). Therefore, it is concluded that the levels of urinary NV Rcan1 can be used as a biomarker to monitor kidney injury in a non-invasive manner.
Moreover, experiments using ZDF rats, a type 2 diabetes mellitus model, were performed to investigate the applicability of urinary NV mRNA analysis in another type of kidney disease. Similar to the PAN nephritis model, ZDF rats display albuminuria with glomerular and podocyte injuries (42). As observed in the PAN nephritis model, an increased PNR in urinary NVs was observed, and a correlation between PNR and urinary albumin excretion was also observed in ZDF rats. These results suggest that PNR in urinary NVs can be considered a useful biomarker for renal dysfunction linked to glomerular injuries, and demonstrates the versatility of urinary NVs as diagnostic tools in various types of kidney diseases.
In conclusion, the present study showed that changes in mRNA levels of urinary NVs may serve as reliable predictors of physiological and pathological alterations in the kidney. Based on these results, it is suggested that this method should be validated further and potentially used as a liquid biopsy tool for kidney disease.
Acknowledgements
The authors are grateful to Professor Akiyoshi Fukamizu (University of Tsukuba) for meaningful discussions. We would also like to thank Dr K Kikkawa, D A Umeda, Dr N Shirata, Mr. T Iguchi (Mitsubishi Tanabe Pharma Corporation) and Dr M Obana (Osaka university) for their support.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
KF and KA designed the study and performed the experiments, analyzed data as well as drafted and finalized the manuscript. TM performed experiments and was involved in data analysis, interpretation of the results and manuscript preparation. MT and TK performed the experiments. HM was involved in the design of the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal experiments were performed in accordance with the institutional guidelines and approved in advance by the Committee for Animal Experiments of Mitsubishi Tanabe Pharma Corporation (approval nos. AJ12-0869, AJ14-0783 and AJ14-0827).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wang V, Vilme H, Maciejewski ML, Boulware LE. The economic burden of chronic kidney disease and end-stage renal disease. Semin Nephrol. 2016;36:319–330. doi: 10.1016/j.semnephrol.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Takemoto Y, Naganuma T. Economic issues of chronic kidney disease and end-stage renal disease. Contrib Nephrol. 2019;198:87–93. doi: 10.1159/000496533. [DOI] [PubMed] [Google Scholar]
- 3.Glassock RJ, Warnock DG, Delanaye P. The global burden of chronic kidney disease: Estimates, variability and pitfalls. Nat Rev Nephrol. 2017;13:104–114. doi: 10.1038/nrneph.2016.163. [DOI] [PubMed] [Google Scholar]
- 4.Levin A, Stevens PE. Early detection of CKD: The benefits, limitations and effects on prognosis. Nat Rev Nephrol. 2011;7:446–457. doi: 10.1038/nrneph.2011.86. [DOI] [PubMed] [Google Scholar]
- 5.Rysz J, Gluba-Brzózka A, Franczyk B, Jabłonowski Z, Ciałkowska-Rysz A. Novel biomarkers in the diagnosis of chronic kidney disease and the prediction of its outcome. Int J Mol Sci. 2017;18(1702) doi: 10.3390/ijms18081702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun F, Muller RU. Urinary extracellular vesicles as a source of biomarkers reflecting renal cellular biology in human disease. Methods Cell Biol. 2019;154:43–65. doi: 10.1016/bs.mcb.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 9.De Palma G, Di Lorenzo VF, Krol S, Paradiso AV. Urinary exosomal shuttle RNA: Promising cancer diagnosis biomarkers of lower urinary tract. Int J Biol Markers. 2019;34:101–107. doi: 10.1177/1724600819827023. [DOI] [PubMed] [Google Scholar]
- 10.Gardiner C, Ferreira YJ, Dragovic RA, Redman CW, Sargent IL. doi: 10.3402/jev.v2i0.19671. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J Extracell Vesicles 2: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakami T, Oakes M, Ogura M, Tovar V, Yamamoto C, Mitsuhashi M. Development of glomerulus-, tubule-, and collecting duct-specific mRNA assay in human urinary exosomes and microvesicles. PLoS One. 2014;9(e109074) doi: 10.1371/journal.pone.0109074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li B, Yao J, Morioka T, Oite T. Nitric oxide increases albumin permeability of isolated rat glomeruli via a phosphorylation-dependent mechanism. J Am Soc Nephrol. 2001;12:2616–2624. doi: 10.1681/ASN.V12122616. [DOI] [PubMed] [Google Scholar]
- 13.Kakimoto T, Okada K, Hirohashi Y, Relator R, Kawai M, Iguchi T, Fujitaka K, Nishio M, Kato T, Fukunari A, Utsumi H. Automated image analysis of a glomerular injury marker desmin in spontaneously diabetic Torii rats treated with losartan. J Endocrinol. 2014;222:43–51. doi: 10.1530/JOE-14-0164. [DOI] [PubMed] [Google Scholar]
- 14.Baranyai T, Herczeg K, Onodi Z, Voszka I, Modos K, Marton N, Nagy G, Mager I, Wood MJ, El Andaloussi S, et al. Isolation of exosomes from blood plasma: Qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS One. 2015;10(e0145686) doi: 10.1371/journal.pone.0145686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu M, Ouyang Y, Wang Z, Zhang R, Huang PH, Chen C, Li H, Li P, Quinn D, Dao M, et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci USA. 2017;114:10584–10589. doi: 10.1073/pnas.1709210114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ. Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol. 2009;296:F213–F229. doi: 10.1152/ajprenal.90421.2008. [DOI] [PubMed] [Google Scholar]
- 17.Floege J, Alpers CE, Sage EH, Pritzl P, Gordon K, Johnson RJ, Couser WG. Markers of complement-dependent and complement-independent glomerular visceral epithelial cell injury in vivo. Expression of antiadhesive proteins and cytoskeletal changes. Lab Invest. 1992;67:486–497. [PubMed] [Google Scholar]
- 18.Hoshi S, Shu Y, Yoshida F, Inagaki T, Sonoda J, Watanabe T, Nomoto K, Nagata M. Podocyte injury promotes progressive nephropathy in zucker diabetic fatty rats. Lab Invest. 2002;82:25–35. doi: 10.1038/labinvest.3780392. [DOI] [PubMed] [Google Scholar]
- 19.Yatsu T, Aoki M, Tanaka A. Effect of zelandopam, a dopamine D1-like receptor agonist, in puromycin aminonucleoside nephrosis rats. Eur J Pharmacol. 2005;510:121–126. doi: 10.1016/j.ejphar.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda A, Wickman LT, Venkatareddy MP, Sato Y, Chowdhury MA, Wang SQ, Shedden KA, Dysko RC, Wiggins JE, Wiggins RC. Angiotensin II-dependent persistent podocyte loss from destabilized glomeruli causes progression of end stage kidney disease. Kidney Int. 2012;81:40–55. doi: 10.1038/ki.2011.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spurney RF. Non-immunologic actions of calcineurin inhibitors in proteinuric kidney diseases. Front Endocrinol (Lausanne) 2014;5(181) doi: 10.3389/fendo.2014.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alimirzaie S, Bagherzadeh M, Akbari MR. Liquid biopsy in breast cancer: A comprehensive review. Clin Genet. 2019;95:643–660. doi: 10.1111/cge.13514. [DOI] [PubMed] [Google Scholar]
- 23.Cui S, Cheng Z, Qin W, Jiang L. Exosomes as a liquid biopsy for lung cancer. Lung Cancer. 2018;116:46–54. doi: 10.1016/j.lungcan.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Nuzhat Z, Kinhal V, Sharma S, Rice GE, Joshi V, Salomon C. Tumour-derived exosomes as a signature of pancreatic cancer-liquid biopsies as indicators of tumour progression. Oncotarget. 2017;8:17279–17291. doi: 10.18632/oncotarget.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez-Hernandez J, Forner MJ, Pinto C, Chaves FJ, Cortes R, Redon J. Increased urinary exosomal MicroRNAs in patients with systemic lupus erythematosus. PLoS One. 2015;10(e0138618) doi: 10.1371/journal.pone.0138618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lv LL, Cao YH, Ni HF, Xu M, Liu D, Liu H, Chen PS, Liu BC. MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am J Physiol Renal Physiol. 2013;305:F1220–F1227. doi: 10.1152/ajprenal.00148.2013. [DOI] [PubMed] [Google Scholar]
- 27.Chun-Yan L, Zi-Yi Z, Tian-Lin Y, Yi-Li W, Bao L, Jiao L, Wei-Jun D. Liquid biopsy biomarkers of renal interstitial fibrosis based on urinary exosome. Exp Mol Pathol. 2018;105:223–228. doi: 10.1016/j.yexmp.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Hernandez J, Olivares D, Forner MJ, Ortega A, Solaz E, Martinez F, Chaves FJ, Redon J, Cortes R. Urinary exosome miR-146a is a potential marker of albuminuria in essential hypertension. J Transl Med. 2018;16(228) doi: 10.1186/s12967-018-1604-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abe H, Sakurai A, Ono H, Hayashi S, Yoshimoto S, Ochi A, Ueda S, Nishimura K, Shibata E, Tamaki M, et al. Urinary exosomal mRNA of WT1 as diagnostic and prognostic biomarker for diabetic nephropathy. J Med Invest. 2018;65:208–215. doi: 10.2152/jmi.65.208. [DOI] [PubMed] [Google Scholar]
- 30.Feng Y, Lv LL, Wu WJ, Li ZL, Chen J, Ni HF, Zhou LT, Tang TT, Wang FM, Wang B, et al. Urinary exosomes and exosomal CCL2 mRNA as biomarkers of active histologic injury in IgA nephropathy. Am J Pathol. 2018;188:2542–2552. doi: 10.1016/j.ajpath.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 31.He L, Zhu D, Wang J, Wu X. A highly efficient method for isolating urinary exosomes. Int J Mol Med. 2019;43:83–90. doi: 10.3892/ijmm.2018.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonoda H, Yokota-Ikeda N, Oshikawa S, Kanno Y, Yoshinaga K, Uchida K, Ueda Y, Kimiya K, Uezono S, Ueda A, et al. Decreased abundance of urinary exosomal aquaporin-1 in renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2009;297:F1006–F1016. doi: 10.1152/ajprenal.00200.2009. [DOI] [PubMed] [Google Scholar]
- 33.Xia L, Zhou M, Kalhorn TF, Ho HT, Wang J. Podocyte-specific expression of organic cation transporter PMAT: Implication in puromycin aminonucleoside nephrotoxicity. Am J Physiol Renal Physiol. 2009;296:F1307–F1313. doi: 10.1152/ajprenal.00046.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spanu S, van Roeyen CR, Denecke B, Floege J, Muhlfeld AS. Urinary exosomes: A novel means to non-invasively assess changes in renal gene and protein expression. PLoS One. 2014;9(e109631) doi: 10.1371/journal.pone.0109631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukuda A, Wickman LT, Venkatareddy MP, Wang SQ, Chowdhury MA, Wiggins JE, Shedden KA, Wiggins RC. Urine podocin: Nephrin mRNA ratio (PNR) as a podocyte stress biomarker. Nephrol Dial Transplant. 2012;27:4079–4087. doi: 10.1093/ndt/gfs313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang K, Sun W, Zhang L, Xu X, Wang J, Hong Y. miR-499 ameliorates podocyte injury by targeting calcineurin in minimal change disease. Am J Nephrol. 2018;47:94–102. doi: 10.1159/000486967. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Jarad G, Tripathi P, Pan M, Cunningham J, Martin DR, Liapis H, Miner JH, Chen F. Activation of NFAT signaling in podocytes causes glomerulosclerosis. J Am Soc Nephrol. 2010;21:1657–1666. doi: 10.1681/ASN.2009121253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding F, Li X, Li B, Guo J, Zhang Y, Ding J. Calpain-mediated cleavage of calcineurin in puromycin aminonucleoside-induced podocyte injury. PLoS One. 2016;11(e0155504) doi: 10.1371/journal.pone.0155504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen X, Jiang H, Ying M, Xie Z, Li X, Wang H, Zhao J, Lin C, Wang Y, Feng S, et al. Calcineurin inhibitors cyclosporin A and tacrolimus protect against podocyte injury induced by puromycin aminonucleoside in rodent models. Sci Rep. 2016;6(32087) doi: 10.1038/srep32087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sipka S, Szücs K, Szántó S, Kovács I, Lakos G, Antal-Szalmás P, Szegedi G, Gergely P. Inhibition of calcineurin activity and protection against cyclosporine A induced cytotoxicity by prednisolone sodium succinate in human peripheral mononuclear cells. Immunopharmacology. 2000;48:87–92. doi: 10.1016/s0162-3109(00)00180-6. [DOI] [PubMed] [Google Scholar]
- 41.Sipka S, Szucs K, Szántó S, Kovács I, Lakos G, Kiss E, Antal-Szalmás P, Szegedi G, Gergely P. Glucocorticosteroid dependent decrease in the activity of calcineurin in the peripheral blood mononuclear cells of patients with systemic lupus erythematosus. Ann Rheum Dis. 2001;60:380–384. doi: 10.1136/ard.60.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Funk J, Ott V, Herrmann A, Rapp W, Raab S, Riboulet W, Vandjour A, Hainaut E, Benardeau A, Singer T, Jacobsen B. Semiautomated quantitative image analysis of glomerular immunohistochemistry markers desmin, vimentin, podocin, synaptopodin and WT-1 in acute and chronic rat kidney disease models. Histochem Cell Biol. 2016;145:315–326. doi: 10.1007/s00418-015-1391-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.