Abstract
Air pollution can highly impact the respiratory system in healthy individuals. Studies have indicated that particles with an aerodynamic diameter of ≤2.5 µm (PM2.5) can be considered to be harmful for lung alveoli and bronchial epithelium cells. PM2.5 can be directly inhaled and can deeply penetrate into the lung alveoli, causing lung dysfunction. However, the toxicological mechanism mediated by PM2.5 for respiratory disease has still not been clearly determined. The purpose of the current study was to investigate the effects of PM2.5 on mouse bronchial epithelium cells (MBECs) and explored the possible mechanism mediated by PM2.5 in MBECs. The results of the current study indicated that PM2.5 markedly decreased lung function, including total lung capacity, residual volume, vital capacity and airway resistance in experimental mice. The results demonstrated that PM2.5 markedly induced inflammatory responses, oxidative injury and MBEC apoptosis. PM2.5 increased interleukin (IL)-1β and IL-6 expression, and reactive oxygen species production in MBECs. Furthermore, PM2.5 specifically induced PI3K, AKT and mTOR expression in MBECs. Disruption of PI3K/AKT/mTOR signaling was also indicated to effectively inhibit apoptosis of MBECs. In conclusion, the results of the current study systematically demonstrated the role of apoptosis-mediated MBEC apoptosis in PM2.5-treated mice, and provides a potential strategy for preclinical intervention in patients with PM2.5-induced lung diseases.
Keywords: PM2.5, autophagy, apoptosis, bronchial epithelium cells, PI3K, AKT, mTOR
Introduction
Air pollution can lead to severe respiratory health problems, especially in the elderly population (1-3). Studies have demonstrated that environmental exposure to particulate matter ≤2.5 µm (PM2.5) threatens the human respiratory system, and is currently a worldwide concern (4-6). PM2.5 can be directly inhaled and deeply penetrate into the lung alveoli, which further leads to severe lung dysfunction, including chronic cough, bronchitis, asthma and lung cancer (7-9). DNA damage, cell apoptosis, cell necrosis, autophagy and cell abnormalities have been identified to occur during PM2.5-induced cytotoxicity (10-12). Although a number of reports have attempted to understand PM2.5-induced lung injury, the underlying processes and signaling mechanisms governing the effect of PM2.5 on the lungs have not yet been clearly elucidated (13-16).
A number of signaling pathways have been reported to be associated with PM2.5-induced biological cell processes (17-19). The toxicological effects of PM2.5 can lead to oxidative damage and/or cytokine secretion in human bronchial epithelial cells (20). A previous study indicated that PM2.5 induced apoptosis in L132 cells by changing the transcription rates of p53, β-cell lymphoma-2 and bax genes (9). PM2.5 exposure has also been indicated to induce autophagy via long non-coding RNA loc146880, which was identified to promote the migration and invasion of lung cancer cells (11). In addition, PM2.5 has been demonstrated to induce lung inflammation in mice via the LPS/MyD88 pathway (21). Furthermore, PM2.5-induced oxidative stress increased adhesion molecule expression in human endothelial cells via the ERK/AKT/NF-κB-dependent pathway (22). Furthermore, PM2.5-induced oxidative stress increases intercellular adhesion molecule-1 expression in lung epithelial cells through the interleukin (IL)-6/AKT/STAT3/NF-κB-dependent pathway (23). However, to the best of our knowledge, the toxicological mechanisms of PM2.5-induced cell apoptosis have still not been fully determined.
In the current study, the effects of PM2.5 on inflammation and apoptosis of mouse bronchial epithelium cells (MBECs) was assessed. The possible mechanism mediated by PM2.5 was analyzed in MBECs. The in vivo study was also performed to investigate the role of PM2.5 in experimental mice.
Materials and methods
Animals
A total of 20 eight week old male C57BL/6 (weight, 20-23 g) mice were purchased from Tianjin Medical University. All mice were housed at 23±1˚C, 50±5% humidity with a 12 h light/dark cycle and access to food and water ad libitum. A method of PM2.5 intratracheal instillation was performed on mice, and was performed as previously reported (24). In brief, mice were randomly divided into two groups (n=10 in each group) and lived in PM2.5 and a normal survival (Control) environment. Mice were housed in their respective environments for a total of 21 days. The mice were euthanized using cervical decapitation on day 22.
Lung function evaluation
On day 22, all experimental mice were anesthetized using 40 mg/kg of sodium pentobarbital. Animals were intubated with a custom-made laryngoscope blade. Animals were mechanically ventilated with a rodent ventilator. The pulmonary functions, including lung capacity, residual volume, tidal volume and airway resistance were analyzed using the FlexiVent system (SCIREQ) according to manufacturer's protocol.
ELISA
On day 22, blood was collected from each group and the plasma was obtained using centrifugation at 10,000 x g for 10 min at 4˚C. An ELISA kit was to measure the levels of IL-1β (cat. no. MLB00C; Bio-Rad Laboratories, Inc.) and IL-6 (cat. no. M6000B; Bio-Rad Laboratories, Inc.).
Histopathological examination
The lung tissues were fixed with 4% formaldehyde overnight at room temperature and embedded in paraffin, and cut into 4 µm sections. Sections underwent hematoxylin and eosin staining for 30 min at room temperature. Sections were then washed with PBS three times and observed under a light microscope at a magnification of x100 (Olympus Corporation).
Cell culture and treatment
MBECs were purchased from Shanghai Sixin Biotechnology Co., Ltd. MBECs were cultured in endothelial culture medium (cat. no. 1001; ScienCell Research Laboratories, Inc.) containing 10% FBS (cat. no. 0025, ScienCell Research Laboratories, Inc.), 1% endothelial cell growth supplement (cat. no. 1052, ScienCell Research Laboratories, Inc.) and 1% penicillin/streptomycin (Sigma-Aldrich; Merck KGaA) in 5% CO2 at 37˚C. MBECs (1x105/ml) were then seeded onto six-well plates and placed in air containing PM2.5, PM2.5 + PI3K inhibitor (PI3KIR), PI3K inhibitor (PI3KIR; cat. no. 526559; Sigma-Aldrich; Merck KGaA) or 95% air and 5% CO2 at 37˚C for 24 h.
Analysis of reactive oxygen species (ROS) production
A 2',7'-dicholorofluorescein-diacetate (DCFH-DA) probe was used to evaluate the level of ROS production in PM2.5-treated MBECs in six-well plates, as described previously (25,26). DCFH-DA (10 µM) was added into MBECs and cells were cultured for 30 min at 37˚C in the dark. MBECs were then washed three times using ice-cold PBS. ROS production in MBECs was examined at a magnification of x50 using a fluorescence microscope (Olympus Corporation). The fluorescence intensity was calculated using analysis LS 5.0 soft image solution (Olympus Imaging America Inc.).
Western blot analysis
MBECs (5x106) were lysed in RIPA buffer (M-PER reagent for the cells and T-PER reagent for the tissues; Thermo Fisher Scientific, Inc.). Protein concentration was measured using a BCA protein assay kit (Thermo Fisher Scientific, Inc.). Protein (20 µg) was electrophoresed on a 15% SDS-PAGE gel and transferred to a PVDF membrane (EMD Millipore). The membranes were blocked using 5% BSA at 4˚C overnight. The primary rabbit anti-rat antibodies used in the immunoblotting assays were as follows: PI3K (1:1,000; cat. no. ab76315; Abcam), phosphorylated PI3K (1:500; cat. no. ab182651; Abcam), AKT (1:500; cat. no. ab185633; Abcam), phosphorylated AKT (1:1,000; cat. no. ab133458; Abcam) and β-actin (1:2,000; cat. no. ab8226; Abcam). After incubation, the membrane was washed three times in PBST and incubated with HRP-conjugated goat anti-rabbit IgG mAb (1:2,000; cat. no. PV-6001; OriGene Technologies, Inc.) for 1 h at 37˚C. After washing three times with PBST, the membrane was developed using a chemiluminescence assay system (EMD Millipore). Densitometric quantification of the immunoblot data was performed using Quantity-One 1.2 software (Bio-Rad Laboratories, Inc.).
TUNEL assay
TUNEL analysis was conducted using an In Situ Cell Death Detection kit (DeadEnd™ Colorimetric Tunel System; Promega Corporation). For lung tissue, tissue sections (4 µm) were deparaffinized using xylene, rehydrated in graded ethanol, and rehydrated for 3 min. The sections were then incubated with TUNEL (DeadEnd™ Colorimetric Tunel System; Promega Corporation ) for 2 h at 37˚C according to the manufacturer's protocol. For MBECs, cells were treated with 4% paraformaldehyde for 15 min at room temperature. Cells were then washed with PBST three times at room temperature and incubated with TUNEL (DeadEnd™ Colorimetric Tunel System, Promega) for 1 h at 37˚C according to the manufacturer's protocol. Cells were washed with PBS three times at room temperature and then incubated with 5% DAPI (Sigma-Aldrich; Merck KGaA) under Antifade mounting medium (cat. no. P0126; Beyotime Institute of Biotechnology) for 30 min at 37˚C. Finally, images of sections and cells were captured in six fields of view with a ZEISS LSM 510 confocal microscope at 488 nm at a magnification of x100. The apoptosis rate was measured using Developer XD 1.2 software (Definiens AG).
Autophagy assay
MBECs in culture dishes were fixed with 4% paraformaldehyde for 15 min at room temperature, and blocked using 0.5% BSA for 30 min at 37˚C. MBECs were washed with PBST three times at room temperature and incubated with anti-LC3B (1:1,000; cat. no. ab48394; Abcam) for 12 h at 4˚C. Cells were incubated with Alexa Fluor 488-labeled goat anti-rabbit secondary antibodies (1:2,000; Beyotime Institute of Biotechnology) for 12 h at 4˚C. Images were captured using a confocal microscope (ZEISS GmbH; LSM510 META; magnification, x100) and analyzed using AxioVision Rel. 4.6 software (Zeiss GmbH).
Statistical analysis
All data are expressed as means ± SEM and analyzed using SPSS software 17.0 (SPSS Inc.). Data were analyzed using a Student's t-test and multiple groups were analyzed using a one-way ANOVA followed by Tukey's test. P<0.05 was considered to indicate a statistically significant difference.
Results
PM2.5 damages lung function in experimental mice
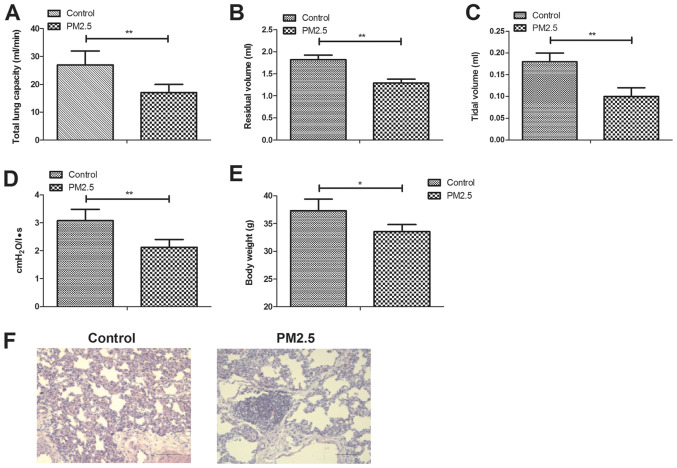
To explore the effects of PM2.5 on lung function, experimental mice were exposed to PM2.5 or clean air as a control. The results indicated that PM2.5 significantly decreased lung function, including total lung capacity, residual volume, vital capacity and airway resistance in experimental mice (Fig. 1A-D). Body weight of mice was significantly decreased by PM2.5 compared with the control (Fig. 1E). H&E staining demonstrated that PM2.5 induced lung injury (Fig. 1F).
Figure 1.
PM2.5 decreased lung function of experimental mice. (A) Total lung capacity, (B) residual volume, (C) vital capacity and (D) airway resistance. (E) Body weight of mice between the PM2.5 and control group. (F) Lung tissue injury of mice between the PM2.5 and control group. *P<0.05 and **P<0.01. Magnification, x40.
PM2.5 increases inflammatory cytokines in an experimental animal model
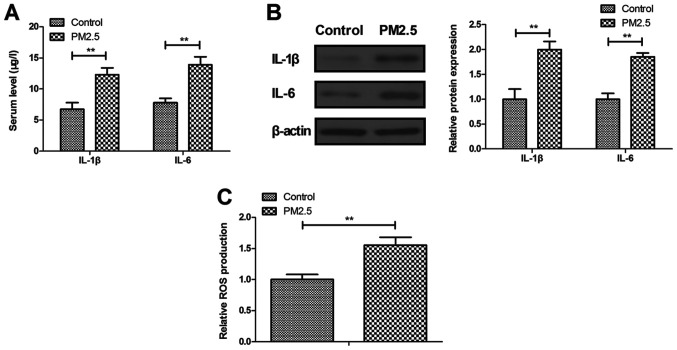
The current study explored the effects of PM2.5 on inflammatory cytokines in an experimental animal model. PM2.5 increased IL-1β and IL-6 expression levels in the serum of the experimental animal model compared with the control (Fig. 2A). IL-1β and IL-6 expression was also markedly increased by PM2.5 in MBECs compared with the control (Fig. 2B). ROS production was indicated to be upregulated in MBECs in PM2.5-treated mice compared with the control (Fig. 2C), which may induce oxidative injury in lung cells.
Figure 2.
PM2.5 stimulated inflammatory cytokines in an experimental animal model. (A) Serum levels of IL-1β and IL-6. (B) Protein expression of IL-1β and IL-6 in MBECs. (C) ROS production in MBECs. **P<0.01. IL, interleukin; ROS, reactive oxygen species; MBECs, mouse bronchial epithelium cells.
PM2.5 induces apoptosis of lung tissue in experimental mice and MBECs
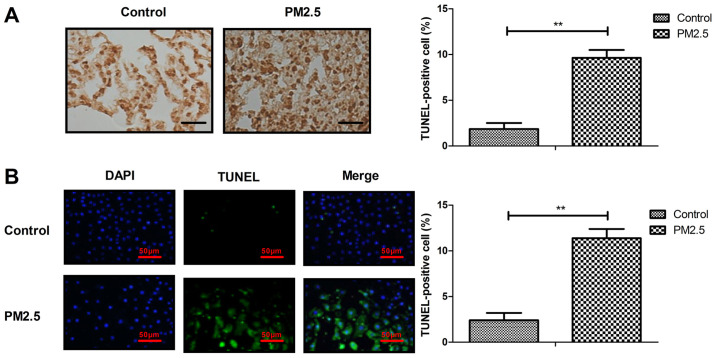
Apoptosis of lung tissue and MBECs was analyzed in vivo and in vitro. As indicated in Fig. 3A, PM2.5 increased lung cell apoptosis in lung tissue compared with the control. The in vitro assay demonstrated that PM2.5 induced the apoptosis of MBECs compared with the control (Fig. 3B). These results indicated that PM2.5 can induce apoptosis of MBECs and in the lung tissue of experimental mice.
Figure 3.
PM2.5 induced apoptosis of lung tissue in experimental mice and MBECs. Effects of PM2.5 on apoptosis of lung cells in (A) lung tissue and (B) MBECs. Magnification, x40; Scale bar, 50 µm. **P<0.01. MBECs, mouse bronchial epithelium cells.
PM2.5 upregulates PI3K, AKT and mTOR expression, and induces autophagy in MBECs
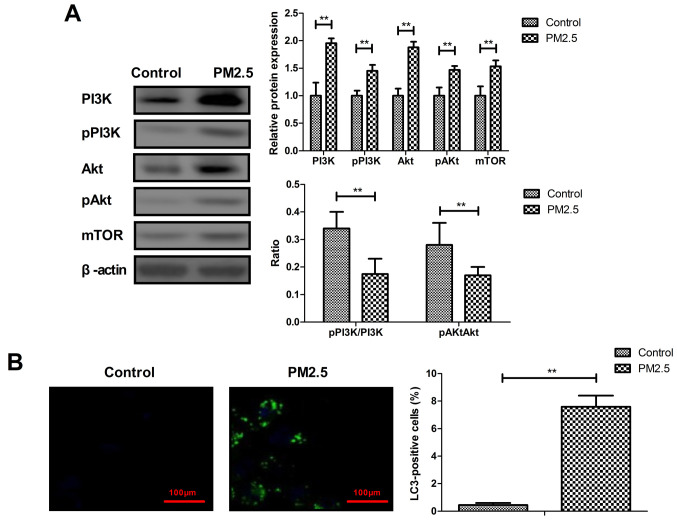
The PI3K, AKT and mTOR expression in MBECs was evaluated in MBECs in vitro. The results demonstrated that PM2.5 increased PI3K, AKT and mTOR expression in MBECs. A statistical difference was identified in the PI3K and AKT phosphorylation level between PM2.5 and the control group (Fig. 4A). PM2.5 was also demonstrated to induced autophagy of MBECs in vitro (Fig. 4B).
Figure 4.
PM2.5 increased PI3K, AKT and mTOR expression and induced autophagy in MBECs. (A) PI3K, AKT and mTOR expression and phosphorylation in MBECs. (B) Autophagy of MBECs between PM2.5 and the control group. **P<0.01. MBECs, mouse bronchial epithelium cells.
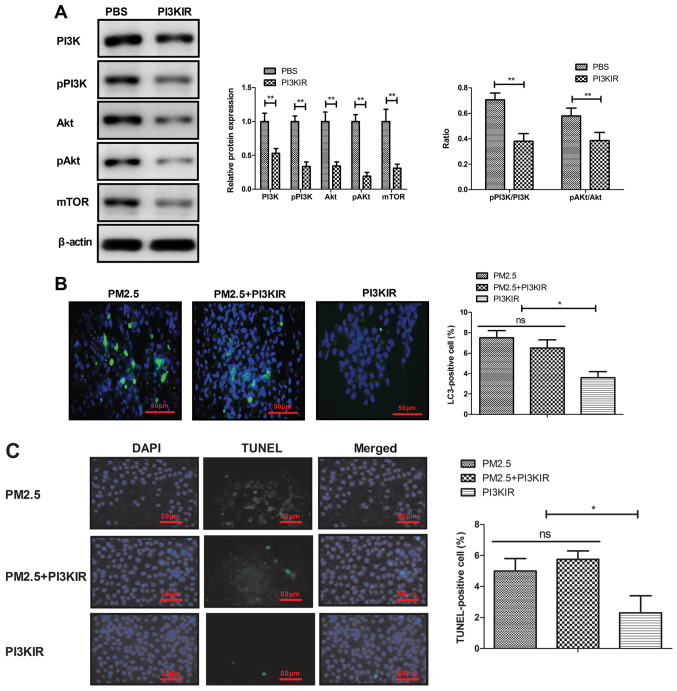
PM2.5 induces apoptosis of MBECs via the PI3K/AKT/mTOR signaling pathway
The current study also explored the potential mechanism mediated by PM2.5 in MBECs. The results demonstrated that PI3KIR significantly decreased PI3K, AKT and mTOR expression in MBECs (Fig. 5A). PI3K inhibitor also blocked PM2.5-induced autophagy and apoptosis of MBECs (Fig. 5B-C). These results indicated that disruption of PI3K/AKT/mTOR signaling can effectively block autophagy and apoptosis of MBECs induced by PM2.5.
Figure 5.
PM2.5 induced apoptosis of MBECs via PI3K/AKT/mTOR signal pathway (A) Effects of PI3K inhibitor (PI3KIR) on PI3K, AKT and mTOR expression in MBECs. (B-C) Effects of PI3K inhibitor (PI3KIR) on (B) PM2.5-induced autophagy and (C) apoptosis of MBECs. *P<0.05; **P<0.01. MBECs, mouse bronchial epithelium cells; p, phosphorylated.
Discussion
PM2.5 has been demonstrated to rapidly induce inflammatory responses, oxidative injury and cell apoptosis or death in human bronchial epithelium cells (21,26,27). A previous study indicated that PM2.5-induced oxidative stress increased adhesion molecule expression in human endothelial cells via the ERK/AKT/NF-κB pathway (22). In the current study, the effects of PM2.5 on lung function in experimental mice and on the apoptosis of MBECs was explored in vitro and in vivo. The results indicated that PM2.5 decreased the overall lung function of experimental mice and specifically induced autophagy of MBECs to increase apoptosis. Disruption of PI3K/AKT/mTOR pathway effectively reduced autophagy level and apoptosis of MBECs. These results indicated that the mechanism of autophagy-mediated MBECs apoptosis induced by PM2.5 is mediated by the PI3K/AKT/mTOR pathway, and provided a potential strategy for the treatment of lung diseases induced by PM2.5.
Inflammatory cytokine levels serve a crucial role in the progression of lung disease (28). PM2.5 has been indicated to lead to increasing IgE, intracellular adhesion molecule-1, vascular cell adhesion molecule-1, EOS, interferon-γ, IL-4, IL-5, IL-33 and thymic stromal lymphopoietin in a rat with PM2.5-induced allergic rhinitis (29). In addition, PM2.5 was indicated to induce ROS formation and NADPH oxidase expression in 16HBE cells and bone marrow stromal cells, which suggested that PM2.5 increased inflammatory activation mediated by ROS induction in the respiratory tract (30). Furthermore, PM2.5 promoted pro-inflammatory cytokine IL-6 and IL-1β signaling activation (31). The current study demonstrated that PM2.5 increased IL-1β and IL-6 expression in the serum of an experimental animal model and upregulated IL-1β and IL-6 expression in MBECs. PM2.5 has been revealed to induce respiratory damage, and a mechanistic basis for preventing outcomes in polluted environments has been identified previously (32). The results of the current study demonstrated that ROS production in MBECs were upregulated in MBECs in PM2.5-treated mice, which further resulted in oxidative injury of experimental mice.
A previous study indicated that PM2.5 activated a number of apoptosis pathways in human epithelial lung cells (L132) in culture (9). PM2.5 also induced autophagy-mediated cell death via NOS2 signaling in human bronchial epithelium cells (33). The direct toxic effect of PM2.5 on human umbilical vein endothelial cells provides a novel insight into the mechanism of cardiovascular diseases caused by PM2.5 exposure (34). The results of the present study revealed that PM2.5 upregulated PI3K, AKT and mTOR expression and induced autophagy in MBECs. To the best of our knowledge, the results of the current study first reported that PM2.5 induced the apoptosis and autophagy of MBECs via the PI3K/AKT/mTOR signaling pathway.
In conclusion, the current study indicated that PM2.5 led to lung injury, increased tissue inflammatory factors, increased ROS production, and induced apoptosis and autophagy in MBECs. The results indicated that PM2.5 induced apoptosis and autophagy via the PI3K/AKT/mTOR signaling pathway, which may provide a potential target in alleviating lung injury in PM2.5-induced lung diseases.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YZ performed the experiments and analyzed the data. XH designed the current study and wrote the manuscript. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
This retrospective study was approved by the Ethics Committee of The Second Hospital of Tianjin Medical University (Tianjin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lee D, Rushworth A, Sahu SK. A Bayesian localized conditional autoregressive model for estimating the health effects of air pollution. Biometrics. 2014;70:419–429. doi: 10.1111/biom.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischer NL, Merialdi M, van Donkelaar A, Vadillo-Ortega F, Martin RV, Betran AP, Souza JP. Outdoor air pollution, preterm birth, and low birth weight: Analysis of the world health organization global survey on maternal and perinatal health. Environ Health Perspect. 2014;122:425–430. doi: 10.1289/ehp.1306837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong GW. Air pollution and health. Lancet Respir Med. 2014;2:8–9. doi: 10.1016/S2213-2600(13)70284-4. [DOI] [PubMed] [Google Scholar]
- 4.Choi JH, Kim JS, Kim YC, Kim YS, Chung NH, Cho MH. Comparative study of PM2.5- and PM10-induced oxidative stress in rat lung epithelial cells. J Vet Sci. 2004;5:11–18. [PubMed] [Google Scholar]
- 5.Nam HY, Choi BH, Lee JY, Lee SG, Kim YH, Lee KH, Yoon HK, Song JS, Kim HJ, Lim Y. The role of nitric oxide in the particulate matter (PM2.5)-induced NFkappaB activation in lung epithelial cells. Toxicol Lett. 2004;148:95–102. doi: 10.1016/j.toxlet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Harder SD, Soukup JM, Ghio AJ, Devlin RB, Becker S. Inhalation of PM2.5 does not modulate host defense or immune parameters in blood or lung of normal human subjects. Environ Health Perspect. 2001;109 (Suppl 4):599–604. doi: 10.1289/ehp.01109s4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng F, Guo X, Liu H, Fang X, Yang M, Chen W. Effects of dust storm PM2.5 on cell proliferation and cell cycle in human lung fibroblasts. Toxicol In Vitro. 2007;21:632–638. doi: 10.1016/j.tiv.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Dagher Z, Garcon G, Billet S, Verdin A, Ledoux F, Courcot D, Aboukais A, Shirali P. Role of nuclear factor-kappa B activation in the adverse effects induced by air pollution particulate matter (PM2.5) in human epithelial lung cells (L132) in culture. J Appl Toxicol. 2007;27:284–290. doi: 10.1002/jat.1211. [DOI] [PubMed] [Google Scholar]
- 9.Dagher Z, Garcon G, Billet S, Gosset P, Ledoux F, Courcot D, Aboukais A, Shirali P. Activation of different pathways of apoptosis by air pollution particulate matter (PM2.5) in human epithelial lung cells (L132) in culture. Toxicology. 2006;225:12–24. doi: 10.1016/j.tox.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 10.Gu LZ, Sun H, Chen JH. Histone deacetylases 3 deletion restrains PM2.5-induced mice lung injury by regulating NF-κB and TGF-β/Smad2/3 signaling pathways. Biomed Pharmacother. 2017;85:756–762. doi: 10.1016/j.biopha.2016.11.094. [DOI] [PubMed] [Google Scholar]
- 11.Deng X, Feng N, Zheng M, Ye X, Lin H, Yu X, Gan Z, Fang Z, Zhang H, Gao M, et al. PM2.5 exposure-induced autophagy is mediated by lncRNA loc146880 which also promotes the migration and invasion of lung cancer cells. Biochim Biophys Acta Gen Subj. 2017;1861:112–125. doi: 10.1016/j.bbagen.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Badyda AJ, Grellier J, Dabrowiecki P. Ambient PM2.5 exposure and mortality due to lung cancer and cardiopulmonary diseases in polish cities. Adv Exp Med Biol. 2017;944:9–17. doi: 10.1007/5584_2016_55. [DOI] [PubMed] [Google Scholar]
- 13.Zwozdziak A, Sowka I, Willak-Janc E, Zwozdziak J, Kwiecinska K, Balinska-Miskiewicz W. Influence of PM1 and PM2.5 on lung function parameters in healthy schoolchildren-a panel study. Environ Sci Pollut Res Int. 2016;23:23892–23901. doi: 10.1007/s11356-016-7605-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferraz ER, Rainho CR, Fernandes AS, Felzenszwalb I. Differential toxicity of an organic PM2.5 extract to human lung cells cultured in three dimensions (3D) and monolayers. J Toxicol Environ Health A. 2016;79:221–231. doi: 10.1080/15287394.2016.1143902. [DOI] [PubMed] [Google Scholar]
- 15.Abbas I, Verdin A, Escande F, Saint-Georges F, Cazier F, Mulliez P, Courcot D, Shirali P, Gosset P, Garçon G. In vitro short-term exposure to air pollution PM2.5-0.3 induced cell cycle alterations and genetic instability in a human lung cell coculture model. Environ Res. 2016;147:146–158. doi: 10.1016/j.envres.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 16.Fu J, Jiang D, Lin G, Liu K, Wang Q. An ecological analysis of PM2.5 concentrations and lung cancer mortality rates in China. BMJ Open. 2015;5(e009452) doi: 10.1136/bmjopen-2015-009452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rumelhard M, Ramgolam K, Hamel R, Marano F, Baeza-Squiban A. Expression and role of EGFR ligands induced in airway cells by PM2.5 and its components. Eur Respir J. 2007;30:1064–1073. doi: 10.1183/09031936.00085907. [DOI] [PubMed] [Google Scholar]
- 18.Billet S, Garcon G, Dagher Z, Verdin A, Ledoux F, Cazier F, Courcot D, Aboukais A, Shirali P. Ambient particulate matter (PM2.5): Physicochemical characterization and metabolic activation of the organic fraction in human lung epithelial cells (A549) Environ Res. 2007;105:212–223. doi: 10.1016/j.envres.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Meng Z, Zhang Q. Damage effects of dust storm PM2.5 on DNA in alveolar macrophages and lung cells of rats. Food Chem Toxicol. 2007;45:1368–1374. doi: 10.1016/j.fct.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Dergham M, Lepers C, Verdin A, Cazier F, Billet S, Courcot D, Shirali P, Garçon G. Temporal-spatial variations of the physicochemical characteristics of air pollution Particulate Matter (PM2.5-0.3) and toxicological effects in human bronchial epithelial cells (BEAS-2B) Environ Res. 2015;137:256–267. doi: 10.1016/j.envres.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 21.He M, Ichinose T, Yoshida S, Ito T, He C, Yoshida Y, Arashidani K, Takano H, Sun G, Shibamoto T : PM2.5-induced lung inflammation in mice: Differences of inflammatory response in macrophages and type II alveolar cells. J Appl Toxicol. 2017;37:1203–1218. doi: 10.1002/jat.3482. [DOI] [PubMed] [Google Scholar]
- 22.Rui W, Guan L, Zhang F, Zhang W, Ding W : PM2.5-induced oxidative stress increases adhesion molecules expression in human endothelial cells through the ERK/AKT/NF-κB-dependent pathway. J Appl Toxicol. 2016;36:48–59. doi: 10.1002/jat.3143. [DOI] [PubMed] [Google Scholar]
- 23.Liu CW, Lee TL, Chen YC, Liang CJ, Wang SH, Lue JH, Tsai JS, Lee SW, Chen SH, Yang YF, et al. PM2.5-induced oxidative stress increases intercellular adhesion molecule-1 expression in lung epithelial cells through the IL-6/AKT/STAT3/NF-κB-dependent pathway. Part Fibre Toxicol. 2018;15(4) doi: 10.1186/s12989-018-0240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Li Y, Shi Z, Wu J, Yang X, Feng L, Ren L, Duan J, Sun Z. Metabolic impact induced by total, water soluble and insoluble components of PM2.5 acute exposure in mice. Chemosphere. 2018;207:337–346. doi: 10.1016/j.chemosphere.2018.05.098. [DOI] [PubMed] [Google Scholar]
- 25.Ye JZ, Su YB, Lin XM, Lai SS, Li WX, Ali F, Zheng J, Peng B. Alanine enhances aminoglycosides-induced ROS production as revealed by proteomic analysis. Front Microbiol. 2018;9(29) doi: 10.3389/fmicb.2018.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang D, Ma M, Zhou W, Yang B, Xiao C. Inhibition of miR-32 activity promoted EMT induced by PM2.5 exposure through the modulation of the Smad1-mediated signaling pathways in lung cancer cells. Chemosphere. 2017;184:289–298. doi: 10.1016/j.chemosphere.2017.05.152. [DOI] [PubMed] [Google Scholar]
- 27.Zhao H, Yang B, Xu J, Chen DM, Xiao CL : PM2.5-induced alterations of cell cycle associated gene expression in lung cancer cells and rat lung tissues. Environ Toxicol Pharmacol. 2017;52:77–82. doi: 10.1016/j.etap.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Gauger PC, Vincent AL, Loving CL, Henningson JN, Lager KM, Janke BH, Kehrli ME Jr, Roth JA. Kinetics of lung lesion development and pro-inflammatory cytokine response in pigs with vaccine-associated enhanced respiratory disease induced by challenge with pandemic (2009) A/H1N1 influenza virus. Vet Pathol. 2012;49:900–912. doi: 10.1177/0300985812439724. [DOI] [PubMed] [Google Scholar]
- 29.Wang YL, Gao W, Li Y, Wang YF. Concentration-dependent effects of PM2.5 mass on expressions of adhesion molecules and inflammatory cytokines in nasal mucosa of rats with allergic rhinitis. Eur Arch Otorhinolaryngol. 2017;274:3221–3229. doi: 10.1007/s00405-017-4606-8. [DOI] [PubMed] [Google Scholar]
- 30.Jin X, Su R, Li R, Cheng L, Li Z. Crucial role of pro-inflammatory cytokines from respiratory tract upon PM2.5 exposure in causing the BMSCs differentiation in cells and animals. Oncotarget. 2018;9:1745–1759. doi: 10.18632/oncotarget.23158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roper C, Chubb LG, Cambal L, Tunno B, Clougherty JE, Fattman C, Mischler SE. Association of IL-6 with PM2.5 components: Importance of characterizing filter-based PM2.5 following extraction. Water Air Soil Pollut. 2017;228(pii: 43) doi: 10.1007/s11270-016-3219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin X, Xue B, Zhou Q, Su R, Li Z. Mitochondrial damage mediated by ROS incurs bronchial epithelial cell apoptosis upon ambient PM2.5 exposure. J Toxicol Sci. 2018;43:101–111. doi: 10.2131/jts.43.101. [DOI] [PubMed] [Google Scholar]
- 33.Zhu XM, Wang Q, Xing WW, Long MH, Fu WL, Xia WR, Jin C, Guo N, Xu DQ, Xu DG : PM2.5 induces autophagy-mediated cell death via NOS2 signaling in human bronchial epithelium cells. Int J Biol Sci. 2018;14:557–564. doi: 10.7150/ijbs.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Z, Shao T, Qin M, Miao X, Chang Y, Sheng W, Wu F, Yu Y. The effects of autophagy on vascular endothelial cells induced by airborne PM2.5. J Environ Sci (China) 2018;66:182–187. doi: 10.1016/j.jes.2017.05.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.