Supplemental Digital Content is available in the text.
Keywords: blood pressure, dental caries, hemorrhage, risk factor, Streptococcus mutans
Background and Purpose:
Cerebral microbleeds (CMB) are associated with stroke and cognitive impairment. We previously reported a high prevalence of CMB in people with Streptococcus mutans expressing Cnm, a collagen-binding protein in the oral cavity. S. mutans is a major pathogen responsible for dental caries. Repeated challenge with S. mutans harboring the cnm gene encoding Cnm induced cerebral bleeding in stroke-prone spontaneously hypertensive rats. The purpose of this longitudinal study is to examine the relationship of cnm-positive S. mutans to the development of CMB.
Methods:
We retrospectively investigated patients with stroke receiving oral microbiological examination and head 3T magnetic resonance imaging evaluations twice in the period 2014 to 2019, allowing >180-day interval. Patients with cnm-positive S. mutans were compared with those without. Quasi-Poisson regression models were used to explore associations between cnm-positive S. mutans and the increase in number of CMB between the 2 magnetic resonance imaging scans.
Results:
A total of 111 patients were identified; 21 (19%) with cnm-positive S. mutans and 90 (81%) without. Clinical history, including blood pressure and the use of antithrombotic agents, were comparable between the 2 groups. New CMB were more commonly observed in patients with cnm-positive S. mutans (52% versus 23%; P=0.008). The incidence of CMB was significantly higher in the group with cnm-positive S. mutans, especially in deep areas, (incidence rate ratios [95% CI], 5.1 [1.9–13.6] for CMB in any brain region; 15.0 [5.4–42.0] for deep CMB), which persisted after adjusting for age, sex, hypertension, and renal impairment (4.7 [1.8–11.9] for CMB in any brain region; 13.9 [4.3–44.5] for deep CMB).
Conclusions:
This study demonstrates that cnm-positive S. mutans is associated with an increased incidence of CMB. Treatment for cnm-positive S. mutans infection may be a novel microbiota-based therapeutic approach for stroke and cognitive impairment.
Small vessel disease (SVD) is a collective term for pathological changes in cerebral small vessels, which contribute to lacunar infarcts, white matter lesions, and cerebral microbleeds (CMB).1 Increasing evidence has identified CMB as an independent risk factor for dementia2 and stroke.3 CMB are associated with an almost 2-fold increased risk of ischemic stroke (IS) and 4-fold risk of intracerebral hemorrhage (ICH).4 On gradient-echo T2*-weighted magnetic resonance imaging (MRI), CMB are described as small, round foci with hypointensities.5–7 The underlying histopathology of CMB is not uniform, representing recent or old hemorrhages, vasculopathies, or hemorrhagic microinfarcts. These heterogeneous pathological substrates likely reflect different causes.8,9
See related article, p 3489
We previously reported a role of systemic inflammation in CMB development.10 Higher circulating levels of high-sensitivity CRP (C-reactive protein), IL (interleukin)-6, and IL-18 are associated with CMB.10 Experimental models for CMB include mice subcutaneously injected with lipopolysaccharide.9,11 CMB are known to be induced by infective endocarditis12 or bacterial sepsis.6
Streptococcus mutans is a Gram-positive bacterium and a major pathogen responsible for dental caries.13 Several cross-sectional studies have shown that oral infection with S. mutans expressing Cnm protein is associated with an increased prevalence of CMB.14,15 Cnm is a cell-surface 120-kDa collagen-binding protein of S. mutans, and its coding gene is cnm.16–19 S. mutans resides on the surface of teeth and frequently induces bacteremia through brushing, flossing, or tooth extraction.20,21 Once in the bloodstream, cnm-positive S. mutans attaches to cerebrovascular basement membranes (BM)17–19 inducing local blood-brain barrier inflammation, resulting in ICH.19 Experimental intravenous administration of cnm-positive S. mutans in stroke-prone spontaneously hypertensive rats and a mouse model of cerebral hemorrhage exacerbates cerebral bleeds.19 Epidemiological studies from many countries have shown ≈20% to 30% of patients with ICH15,22 and 7% to 20% of the general population14,23–25 have cnm-positive S. mutans in their oral cavity. Clarifying the effects of cnm-positive S. mutans on the cerebral vasculature is, therefore, both necessary and urgent.
In this study, we hypothesize that cnm-positive S. mutans contributes to the development of CMB. We investigated the association between cnm-positive S. mutans and incidence of CMB in a longitudinal retrospective study.
Methods
Data Availability Statement
Raw data were generated and preserved at the National Cerebral and Cardiovascular Center. Derived data supporting the findings of this study are available from corresponding authors on request.
Study Design
The current study was approved by the Ethical Committee of the National Cerebral and Cardiovascular Center (M23-073, M25-111 and M27-015) and conducted in accordance with Declaration of Helsinki standards.
Subjects who fully satisfied the following criteria were selected from the database of the National Cerebral and Cardiovascular Center Stroke Registry (https://www.clinicaltrials.gov; Unique identifier: NCT02251665) and included in the analysis: (1) subjects who developed acute IS, transient ischemic attack (TIA), or ICH from February 15, 2014 to April 8, 2018; (2) subjects who signed an informed consent form for the current research, including receiving oral bacterial assessments from February 15, 2014 to April 30, 2018; and (3) subjects receiving 3T-MRI scans for clinical purposes twice, with more than a 180-day interval between examinations, from February 15, 2014 to February 15, 2019. The first MRI scan was used for baseline evaluation and the second for follow-up. If >2× of 3T-MRI scans were performed, the oldest and the latest MRI data were selected. The observational period was defined as the period from baseline to follow-up MRI scans. Subjects with cnm-positive S. mutans (cnm [+] group) were compared to those without (cnm [−] group) unless otherwise noted. The cnm (−) group comprised patients with cnm-negative S. mutans and those without S. mutans.
Clinical Characteristics
Clinical information, including incidence of symptomatic stroke and TIA, was collected from medical records. The clinical laboratory results nearest to baseline MRI were used as baseline data. Blood pressure was examined at baseline and follow-up visits. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or history of antihypertensive medication use. Diabetes was considered present through a history of antidiabetic drug or insulin use, a fasting plasma glucose level of ≥126 mg/dL, or glycated hemoglobin A1c level of ≥6.5%. Dyslipidemia was defined as low-density lipoprotein cholesterol level ≥140 mg/dL, high-density lipoprotein cholesterol level ≤40 mg/dL, triglyceride level ≥150 mg/dL, or use of lipid-lowering drugs. Renal impairment was defined as <60 mL/min/1.73 m2 of estimated glomerular filtration rate, according to previous reports.26,27 The presence of atrial fibrillation and current smoking pattern were also noted. Previous IS, TIA and ICH were defined according to the presence of each disease >3 months before the baseline MRI scan, whereas events within 3 months before the baseline MRI were described as recent IS, TIA, or ICH.
Detection of cnm-Positive S. mutans
Dental plaque specimens were collected and inoculated on Mitis-Salivarius medium with bacitracin (Sigma-Aldrich, St. Louis, MO) and 15% sucrose agar plates and anaerobically incubated at 37 °C for 48 hours. S. mutans strains were identified and isolated based on rough morphological features on agar plates, and all strains were cultured in brain heart infusion broth (Becton, Dickinson and Company, Franklin Lakes, NJ) at 37 °C for 24 hours. Bacterial genomic DNA of each strain was extracted, and S. mutans and cnm genes screened using polymerase chain reaction. MKD primer sets for S. mutans and cnm were used to identify cnm-positive and cnm-negative S. mutans.23 Experiments were conducted by researchers blind to clinical information.
MRI Evaluation
Fluid-attenuated inversion recovery and gradient-echo T2*-weighted images were obtained at baseline and follow-up MRI (3T, Magnetom Verio or Spectra; Siemens Medical Solutions, Erlangen, Germany). The presence of CMB on T2*-weighted images was noted according to the Brain Observer MicroBleed Scale.7 CMB were categorized into 3 groups: (1) deep CMB in the deep gray matter in the basal ganglia or thalamus, or white matter in the corpus callosum, internal, external, or extreme capsule, (2) lobar CMB in the cortical gray or subcortical white matter, and (3) subtentorial CMB in the cerebellum or brain stem. CMB in any brain region (any CMB) were also recorded. Newly developed CMB were recorded at follow-up, but not baseline, MRI. All slices were taken parallel to the orbitomeatal line from the base of the skull to the vault. The sequence parameters of T2*-weighted images were as follows: slice thickness, 4.0 mm; interslice gap, 2.0 mm; echo time, 12 ms; repetition time, 550 ms; and flip angle, 20 degrees.
Lacunar infarcts and white matter hyperintensities (WMH) were evaluated by fluid-attenuated inversion recovery images. Lacunar infarcts were defined as supratentorial hypointense lesions of 3 to 15 mm in diameter with a hyperintense rim. Periventricular hyperintensities (PVH) and deep WMH (DWMH) were scored by the Fazekas scale.28 Sequence parameters of fluid-attenuated inversion recovery images were as follows: slice thickness, 5.0 mm; interslice gap, 1.0 mm; echo time, 94 to 114 ms; and repetition time, 12 000 ms.
Severity of SVD
Total severity of SVD was rated as described previously.29 Briefly, 1 point was added if each SVD feature was present: ≥1 of any CMB, ≥1 of lacunar infarcts, irregular PVH extending into deep white matter (Fazekas score 3), and confluent DWMH (Fazekas score 2 or 3). Sum of ratings was used as a total SVD severity (range, 0–4).29
Ratings
SVD markers were independently rated by 2 neurologists. Interrater correlation coefficients were 0.87 for any CMB, 0.94 for deep CMB, 0.94 for lobar CMB, 0.93 for subtentorial CMB, 0.79 for lacunar infarcts, 0.70 for DWMH, and 0.91 for PVH.
Statistical Analyses
Variables were presented as median and interquartile range or numbers and percentages. Mann-Whitney U or Kruskal-Wallis test for continuous data and χ2 or Fisher exacts test for categorical data was used. Quasi-Poisson regression models were applied for associations between cnm-positive S. mutans and number of newly developed CMB during the observational period. The incidence rate ratios (IRR) and their 95% CI were estimated. Based on previous reports,15,27,30 age, sex, hypertension, and renal impairment were set as adjustment factors. We estimated hazard ratios by applying Cox proportional hazard models for associations between cnm-positive S. mutans and symptomatic ICH, IS, and TIA incidence. A P<0.05 (2-tailed) was considered statistically significant. Statistical analysis was conducted using SPSS version 26 (IBM, Armonk, NY) and SAS version 9.4 (SAS Institute, Cary, NC).
Results
Patient Characteristics
From the 3782 patients with acute stroke, 404 patients (11%) received oral bacterial examination (Figure I in the Data Supplement). The clinical profiles of subjects with and without bacterial assessment were similar apart from age, the National Institutes of Health Stroke Scale, and modified Rankin Scale (Table I in the Data Supplement).
We identified 111 subjects fulfilling all the criteria and found that cnm-positive S. mutans was present in 21 (19%), and absent in 90 (81%), patients. Among the 90 patients in the cnm (−) group, cnm-negative S. mutans was detected in 69 and no S. mutans in 21. Characteristics of subjects at baseline MRI are described in Table 1. Age, sex, blood pressure, and vascular risk factors were similar between cnm (+) and cnm (−) groups (systolic blood pressure: 126 mm Hg [116–134] versus 130 mm Hg [118–147], P=0.267; diastolic blood pressure: 76 mm Hg [64–85] versus 74 mm Hg [65–84], P=0.946). The 2 groups also exhibited equivalent blood pressure at follow-up evaluation (systolic blood pressure: 125 mm Hg [117–135] versus 122 mm Hg [115–135]; diastolic blood pressure: 75 mm Hg [70–80] versus 70 mm Hg [64–80]). The cnm (+) group showed higher, but not significant, levels of CRP and fibrinogen than the cnm (−) group (CRP: 0.15 mg/dL [0.04–0.79] versus 0.08 mg/dL [0.04–0.23], P=0.250; fibrinogen: 344 mg/dL [274–415] versus 308 mg/dL [273–358], P=0.185).
Table 1.
Clinical Characteristics at the Baseline Evaluation
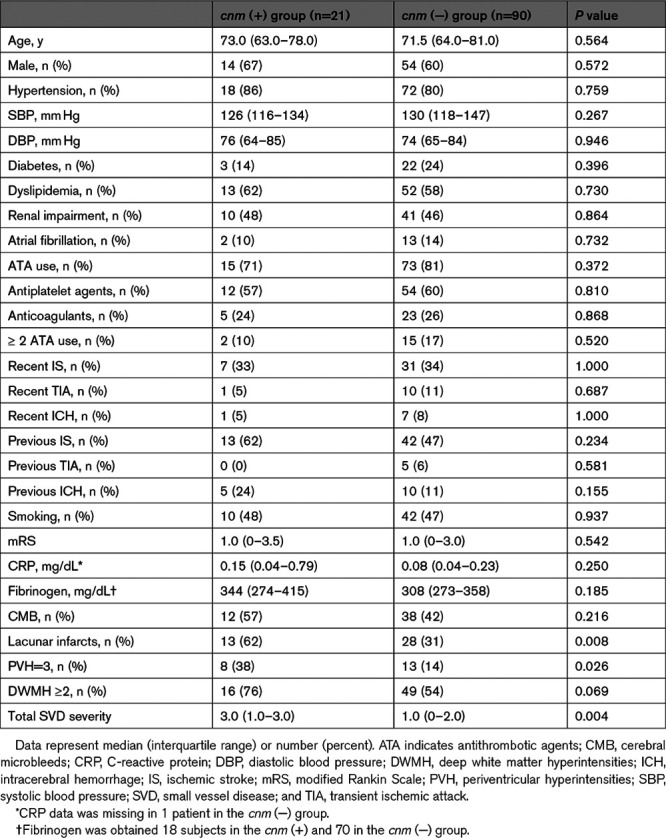
CMB were detected in 12 (57%) of the cnm (+), and 38 (42%) of the cnm (−), group. Lacunar infarcts, PVH, and DWMH were commonly observed in the cnm (+) group (lacunar infarcts: 62% versus 31%, P=0.008; PVH: 38% versus 14%, P=0.026; DWMH: 76% versus 54%, P=0.069). Consequently, total SVD severity was significantly more advanced in the cnm (+) than cnm (−) group (3.0 [1.0–3.0] versus 1.0 [0–2.0], P=0.004).
Cerebral Microbleeds
The numbers of CMB are summarized in Table 2. The cnm (+) group showed a marginally increased number of CMB versus the cnm (−) group at baseline, especially in the deep region, but comparable in lobar and subtentorial regions (any CMB: 2.0 [0–10.5] versus 1.0 [0–5.3], P=0.094; deep CMB: 1.0 [0–7.5] versus 0 [0–2.0], P=0.091) and follow-up (any CMB: 4.0 [0.5–13.5] versus 1.0 [0–6.0], P=0.067; deep CMB: 2.0 [0–10.0] versus 0 [0–2.0], P=0.039).
Table 2.
The Number of CMB at the Baseline and the Follow-Up MRI Scans
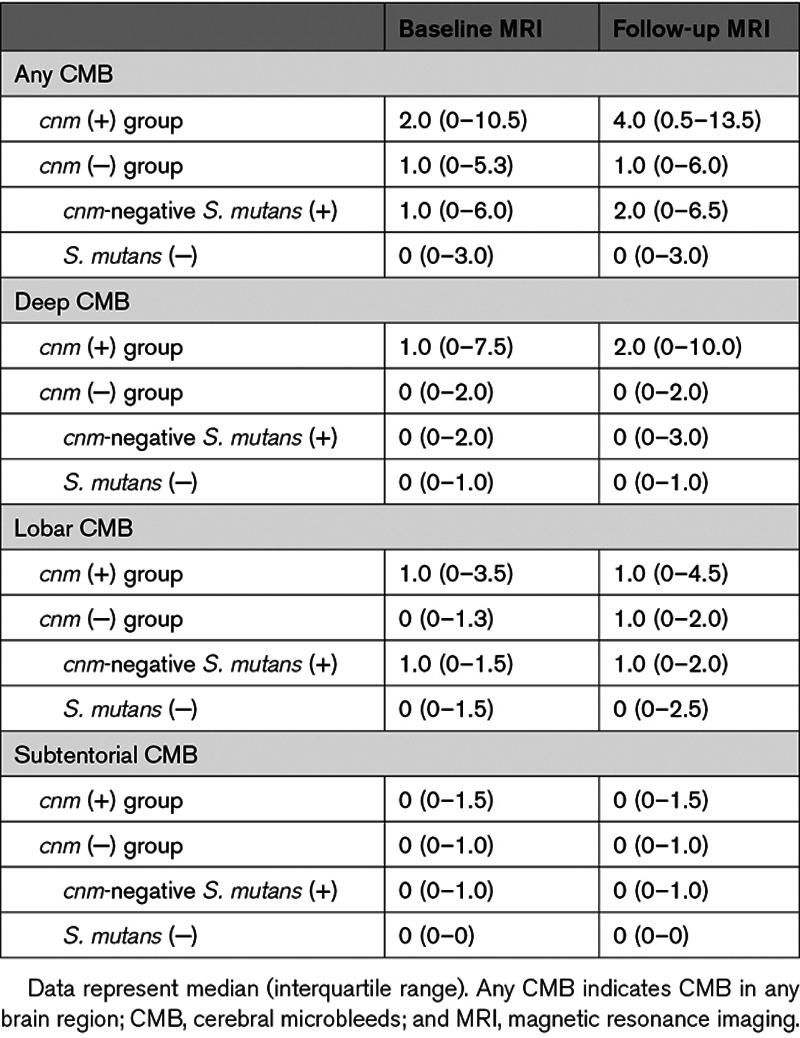
We assessed the development of new CMB from baseline to follow-up MRI. The observational period was similar between the cnm (+) and cnm (−) group (509 [279–584] versus 482 [364–732] days, P=0.405). CMB development was significantly higher in the cnm (+) than cnm (−) group (52% versus 23%, P=0.008; Table 3). In particular, newly developed CMB were more frequent in deep regions (48% versus 9%, P<0.001) in the cnm (+) than cnm (−) group. Mean numbers of new CMB in the cnm (+) and cnm (−) groups were 2.2 versus 0.5 for any CMB, 1.4 versus 0.1 for deep, 0.4 versus 0.4 for lobar, and 0.4 versus 0.1 for subtentorial.
Table 3.
The Frequency of New CMB Development
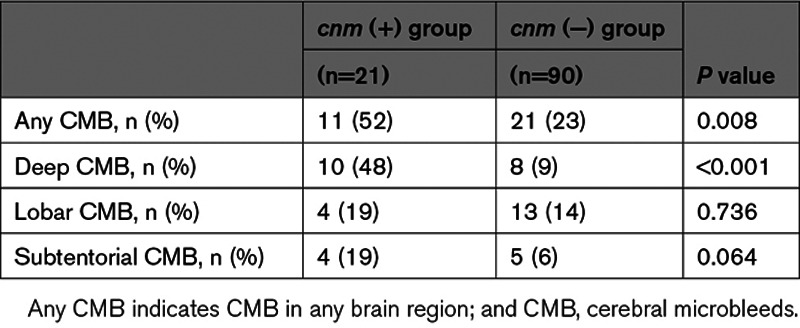
We estimated the IRR considering newly developed CMB and observational period. IRR for CMB in deep and subtentorial, but not lobar, regions were significantly higher using unadjusted analysis (any CMB: IRR, 5.1 [95% CI, 1.9–13.6], P=0.001; deep CMB: IRR, 15.0 [95% CI, 5.4–42.0], P<0.001; subtentorial CMB: IRR, 6.4 [95% CI, 1.3–30.9], P=0.020; lobar CMB: IRR, 1.3 [95% CI 0.2–7.7], P=0.808). Statistical significance for any and deep CMB was confirmed after adjusting for age, sex, hypertension, and renal impairment (any CMB: IRR, 4.7 [95% CI, 1.8–11.9], P=0.001; deep CMB: IRR, 13.9 [95% CI, 4.3–44.5], P<0.001; Table 4). Representative images showing the increase in deep CMB are illustrated in Figure II in the Data Supplement.
Table 4.
IRRs of Newly Developed CMB
Progression of Other SVD Markers
We next evaluated progression of SVD features other than CMB. Frequency of lacunar infarcts (cnm [+] versus cnm [−]: 67% versus 36%), PVH (38% versus 17%), and DWMH (76% versus 57%) on follow-up MRI was subtly increased from baseline. The change in frequency of each SVD feature other than CMB during the observation period was equivalent between cnm (+) and cnm (−) groups (lacunar infarcts: 5% versus 4%, P=1.000; PVH: 0% versus 2%, P=1.000; DWMH: 0% versus 2%, P=1.000).
Stroke and TIA
Symptomatic stroke and TIA frequency during the observation period was investigated. ICH, IS, and TIA incidence was similar in cnm (+) and cnm (−) groups (ICH: 2 [10%] versus 3 [3%], hazard ratios, 5.3 [95% CI, 0.7–38.8]; IS: 6 [29%] versus 23 [26%], hazard ratios, 1.4 [95% CI, 0.6–3.4]; TIA, 1 [5%] versus 3 [3%], hazard ratios, 1.7 [95% CI, 0.2–16.8]).
Comparison Between the 3 Groups
S. mutans, whether cnm positive or not, may contribute to mycotic aneurysms and cerebral hemorrhage.31 We, therefore, compared the 3 groups: (1) subjects with cnm-positive S. mutans, (2) those with cnm-negative S. mutans, and (3) those without S. mutans. Background profiles were similar among the 3 groups, except for some imaging markers of SVD, such as lacunar infarcts, WMH, and total SVD severity (Table II in the Data Supplement). Development of CMB was most prominent in cnm-positive S. mutans subjects (Table III in the Data Supplement). No significant difference was observed between subjects with cnm-negative S. mutans and those without S. mutans.
Discussion
We found harboring cnm-positive S. mutans was closely related to an increased incidence of CMB, especially in the deep area, together with a high prevalence of lacunar infarcts and WMH.
The strong linkage of cnm-positive S. mutans and deep CMB aligns with previous cross-sectional studies.14,15 Estimated IRR for deep CMB was high in comparison with other known risk factors in previous reports.30,32,33 Deep CMB were considered as biomarkers for hypertensive arteriopathy,3,34 but their pathogenesis cannot be fully explained by hypertension, as they are occasionally found in subjects without high blood pressure.14,32
An important hallmark of S. mutans expressing Cnm protein13 is its binding activity to components of vascular BM, such as collagen-IV17 and laminin.18 Collagen-binding activity is positively correlated with cnm mRNA expression in S. mutans.23 Conversely, neither cnm-negative S. mutans nor cnm knockout strains of S. mutans can attach to soft tissues such as vessel walls.17 Aging and hypertension induce endothelial injury and BM thickening, resulting in collagen-IV and laminin exposure in cerebral small arteries.35,36 Once cnm-positive S. mutans adheres to BM, infiltration of neutrophils may activate local inflammation, increasing blood-brain barrier permeability, and production of enzymes, such as matrix metalloproteinase-9,19 inducing ICH or CMB (Figure). Endothelial injury related to aging and hypertension is prominent in the deep cerebral vessels,37 likely contributing to an increase in the deep CMB rather than lobar CMB, by cnm-positive S. mutans.
Figure.
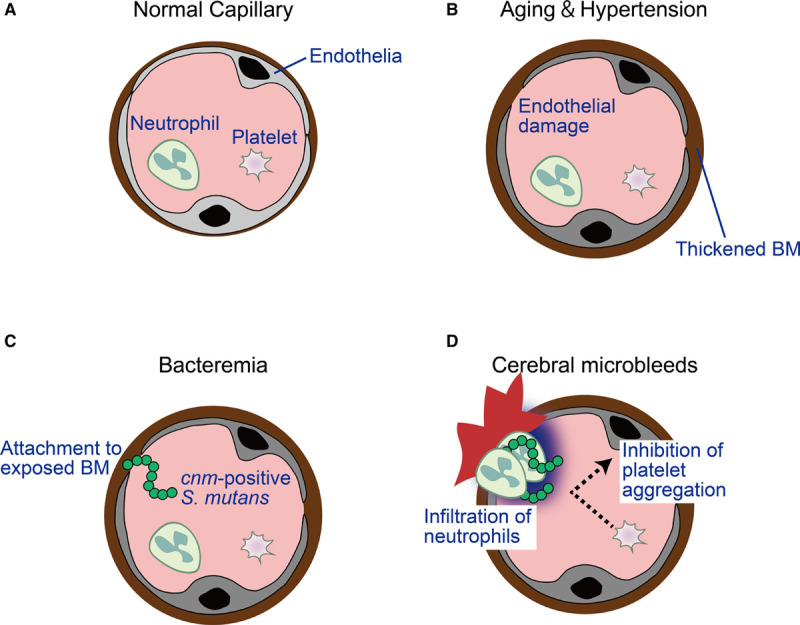
Hypothetical model of the mechanism contributing to develop cerebral microbleeds (CMB) by the infection of cnm-positive Streptococcus mutans (S. mutans). A, Normal vessel. Cerebral bleeding may occur at the level of arterioles and capillaries. B, Aging and hypertension results in endothelial damage and thickened basement membranes (BM). C, Bacteremia of S. mutans are induced by brushing, flossing or tooth extraction. Unlike cnm-negative S. mutans, cnm-positive S. mutans can attach to the BM. D, Once cnm-positive S. mutans binds to the vessel wall, infiltration of neutrophils results in local inflammation. The negative charges on the surface of cnm-positive S. mutans inhibit aggregation of platelets, which also possess negative charges on the surfaces. CMB are eventually induced.
Furthermore, unlike cnm-negative S. mutans, the cnm-positive S. mutans can suppress collagen-induced platelet aggregation.19 All S. mutans, whether cnm-positive or not, have negative zeta potential values, an indicator of cell-surface charge, although cnm-positive S. mutans possesses lower zeta potential values.19 Since platelets also possess negative potentials, cnm-positive S. mutans may inhibit platelet adhesion and aggregation, accelerating thus cerebral bleeding. Zeta potential values differ among strains of cnm-positive S. mutans and lower zeta potential values significantly correlate with decreased collagen-induced platelet aggregation.19
We previously reported cnm-positive S. mutans is significantly associated with severe dental caries.23 S. mutans expressing collagen-binding protein can strongly bind to the type-I collagen-composed dentin tooth layer, accelerating development of carious lesions.17 The increased predisposition of cnm-positive S. mutans to invade dental caries provides opportunities for S. mutans to enter the bloodstream and cerebral circulation. Poor oral health could facilitate dental bacteremia and cerebrovascular health.17,38 The collagen-binding activity of cnm-positive S. mutans to type-I collagen in teeth and type-IV collagen in cerebrovascular BM facilitates CMB.
S. mutans, including cnm-positive, are commonly transmitted by vertical infection, colonizing mouths of infants at around 2 years.23,39 Mothers and caretakers of children are the major sources of S. mutans,40 which generally remain after colonization16 but are not easily implanted again in adulthood.41,42 Therefore, preventing vertical cnm-positive S. mutans infection could represent a major preventative factor in SVD and CMB.
Here, overall prevalence of CMB was 45%, higher than previous IS cohorts,9,43 and equivalent to IS and ICH mixed stroke cohorts.43 The current study included about 20% of subjects with a history of ICH. Additionally, high magnetic field strength of MRI may have affected CMB frequency. Only patients receiving 3T-MRI scans were included, which is suitable for CMB detection and superior to 1.5T-MRI.5
Although CMB may predict future ICH,3,4,44 the incidence of symptomatic ICH in the cnm (+) group was similar to the cnm (−) group. Circulating inflammatory marker level was increased, but nonsignificantly, in the cnm (+) group, which may be a consequence of a small sample size. Thus, to definitively establish an association between cnm-positive S. mutans, symptomatic ICH, and inflammatory marker levels, a large-scale prospective investigation is warranted. This study leads to new hypotheses and provides useful data to guide power calculations and effect sizes for future larger-scale investigations.
There are some limitations to this study. First, it involved Japanese subjects only, making predictions for other countries uncertain and demonstrating the need for multinational validation studies. Second, this was a retrospective study, posing potential risk of selection bias. Only 11% of the total stroke patients had oral bacterial evaluation due to age and factors, leading to difficulty providing informed consent, such as impaired consciousness, cognitive impairments, and advanced frailty. This resulted in the lower age and scores of National Institutes of Health Stroke Scale and modified Rankin Scale in patients receiving bacterial assessments (Table I in the Data Supplement). Finally, all patients had a history of stroke and the effect of cnm-positive S. mutans on CMB development should be examined in a population-based cohort in any future study.
In conclusion, cnm-positive S. mutans was associated with increased CMB incidence. Though the results should be verified by large-scale prospective studies, a close association between cnm-positive S. mutans and CMB development suggests treatments targeting cnm-positive S. mutans may act as novel therapeutic approaches for dementia and stroke.
Acknowledgments
We indebted to Yuko Kiyama and Natsuki Hanada for technical assistance and Dr Ahmad Khundakar for editorial assistance and helpful comments.
Sources of Funding
This study was funded by Grant-in-Aid for Japan Society for the Promotion of Science Fellows to Dr Saito (19J00106), Grant-in-Aid for Challenging Exploratory Research to Dr Ihara (16K14573, 19K22610), Mitsui Sumitomo Insurance Welfare Foundation to Dr Ihara., SENSHIN Medical Research Foundation to Dr Ihara, Invitational Fellowships for Research in Japan to Dr Friedland, and the Jewish Heritage Fund for Excellence to Dr Friedland.
Disclosures
Dr Yoshimoto reports other support from Takeda Pharmaceutical Company Limited during the conduct of the study. Dr Nakahara reports grants from Boehringer Ingelheim, grants and personal fees from Daiichi Sankyo, grants and personal fees from Eisai, grants and personal fees from Otsuka, grants from Pfizer, and grants and personal fees from Sanofi outside the submitted work. Dr Koga reports honoraria from Otsuka, Takeda, Bayer, Pfizer, Bristol-Myers Squibb, Daiichi Sankyo, Ono, Mitsubishi Tanabe Pharma Corporation, and Boehringer Ingelheim. Dr Toyoda reports lecture honoraria from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, and Daiichi Sankyo. Dr Ihara reports research support not attributed in the artic from Shimadzu Corporation and Otsuka Pharmaceutical. The other authors report no conflicts.
Supplemental Materials
Tables I–III
Figures I–II
Supplementary Material
Nonstandard Abbreviations and Acronyms
- any CMB
- cerebral microbleeds in any brain region
- BM
- basement membranes
- CRP
- C-reactive protein
- DWMH
- deep white matter hyperintensities
- ICH
- intracerebral hemorrhage
- IL
- interleukin
- IRR
- incidence rate ratios
- IS
- ischemic stroke
- MRI
- magnetic resonance imaging
- PVH
- periventricular hyperintensities
- SVD
- small vessel disease
- TIA
- transient ischemic attack
- WMH
- white matter hyperintensities
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.120.029607.
For Sources of Funding and Disclosures, see page 3638.
Contributor Information
Satoshi Hosoki, Email: saitou.satoshi.43m@kyoto-u.jp.
Shuichi Tonomura, Email: tonomura.shuichi58@ncvc.go.jp.
Hiroyuki Ishiyama, Email: pockn.4.hy@ncvc.go.jp.
Takeshi Yoshimoto, Email: yoshimototakeshi1982@gmail.com.
Shuhei Ikeda, Email: ikeda.shuhei83@ncvc.go.jp.
Hajime Ikenouchi, Email: ikehajime@ncvc.go.jp.
Yumi Yamamoto, Email: yumi.yamamoto@ncvc.go.jp.
Yorito Hattori, Email: yoh2019@ncvc.go.jp.
Kaori Miwa, Email: miwa@osaka-njm.net.
Robert P. Friedland, Email: robert.friedland@louisville.edu.
Roxana O. Carare, Email: R.O.Carare@soton.ac.uk.
Jin Nakahara, Email: nakahara@jnn.sakura.ne.jp.
Norihiro Suzuki, Email: nrsuzuki@a2.keio.jp.
Masatoshi Koga, Email: koga@ncvc.go.jp.
Kazunori Toyoda, Email: toyoda@ncvc.go.jp.
Ryota Nomura, Email: rnomura@dent.osaka-u.ac.jp.
Kazuhiko Nakano, Email: nakano@dent.osaka-u.ac.jp.
Misa Takegami, Email: takegami@ncvc.go.jp.
References
- 1.Ihara M, Yamamoto Y. Emerging evidence for pathogenesis of sporadic cerebral small vessel disease. Stroke. 2016;47:554–560. doi: 10.1161/STROKEAHA.115.009627 [DOI] [PubMed] [Google Scholar]
- 2.Akoudad S, Wolters FJ, Viswanathan A, de Bruijn RF, van der Lugt A, Hofman A, Koudstaal PJ, Ikram MA, Vernooij MW. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol. 2016;73:934–943. doi: 10.1001/jamaneurol.2016.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasi M, Cordonnier C. Clinical relevance of cerebral small vessel diseases. Stroke. 2020;51:47–53. doi: 10.1161/STROKEAHA.119.024148 [DOI] [PubMed] [Google Scholar]
- 4.Debette S, Schilling S, Duperron MG, Larsson SC, Markus HS. Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta-analysis. JAMA Neurol. 2019;76:81–94. doi: 10.1001/jamaneurol.2018.3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nandigam RN, Viswanathan A, Delgado P, Skehan ME, Smith EE, Rosand J, Greenberg SM, Dickerson BC. MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR Am J Neuroradiol. 2009;30:338–343. doi: 10.3174/ajnr.A1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corrêa DG, Cruz Júnior LC, Bahia PR, Gasparetto EL. Intracerebral microbleeds in sepsis: susceptibility-weighted MR imaging findings. Arq Neuropsiquiatr. 2012;70:903–904. doi: 10.1590/s0004-282x2012001100017 [DOI] [PubMed] [Google Scholar]
- 7.Cordonnier C, Potter GM, Jackson CA, Doubal F, Keir S, Sudlow CL, Wardlaw JM, Al-Shahi Salman R. improving interrater agreement about brain microbleeds: development of the brain observer microbleed scale (BOMBS). Stroke. 2009;40:94–99. doi: 10.1161/STROKEAHA.108.526996 [DOI] [PubMed] [Google Scholar]
- 8.van Veluw SJ, Biessels GJ, Klijn CJ, Rozemuller AJ. Heterogeneous histopathology of cortical microbleeds in cerebral amyloid angiopathy. Neurology. 2016;86:867–871. doi: 10.1212/WNL.0000000000002419 [DOI] [PubMed] [Google Scholar]
- 9.Pétrault M, Casolla B, Ouk T, Cordonnier C, Bérézowski V. Cerebral microbleeds: beyond the macroscope. Int J Stroke. 2019;14:468–475. doi: 10.1177/1747493019830594 [DOI] [PubMed] [Google Scholar]
- 10.Miwa K, Tanaka M, Okazaki S, Furukado S, Sakaguchi M, Kitagawa K. Relations of blood inflammatory marker levels with cerebral microbleeds. Stroke. 2011;42:3202–3206. doi: 10.1161/STROKEAHA.111.621193 [DOI] [PubMed] [Google Scholar]
- 11.Sumbria RK, Grigoryan MM, Vasilevko V, Krasieva TB, Scadeng M, Dvornikova AK, Paganini-Hill A, Kim R, Cribbs DH, Fisher MJ. A murine model of inflammation-induced cerebral microbleeds. J Neuroinflammation. 2016;13:218 doi: 10.1186/s12974-016-0693-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okazaki S, Sakaguchi M, Hyun B, Nagano K, Tagaya M, Sakata Y, Sakaguchi T, Kitagawa K. Cerebral microbleeds predict impending intracranial hemorrhage in infective endocarditis. Cerebrovasc Dis. 2011;32:483–488. doi: 10.1159/000331475 [DOI] [PubMed] [Google Scholar]
- 13.Ito S, Misaki T, Naka S, Wato K, Nagasawa Y, Nomura R, Otsugu M, Matsumoto-Nakano M, Nakano K, Kumagai H, et al. Specific strains of Streptococcus mutans, a pathogen of dental caries, in the tonsils, are associated with IgA nephropathy. Sci Rep. 2019;9:20130 doi: 10.1038/s41598-019-56679-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe I, Kuriyama N, Miyatani F, Nomura R, Naka S, Nakano K, Ihara M, Iwai K, Matsui D, Ozaki E, et al. Oral Cnm -positive Streptococcus mutans expressing collagen binding activity is a risk factor for cerebral microbleeds and cognitive impairment. Sci Rep. 2016;6:38561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tonomura S, Ihara M, Kawano T, Tanaka T, Okuno Y, Saito S, Friedland RP, Kuriyama N, Nomura R, Watanabe Y, et al. Intracerebral hemorrhage and deep microbleeds associated with cnm-positive Streptococcus mutans; a hospital cohort study. Sci Rep. 2016;6:20074 doi: 10.1038/srep20074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nomura R, Ogaya Y, Nakano K. Contribution of the collagen-binding proteins of Streptococcus mutans to bacterial colonization of inflamed dental pulp. PLoS One. 2016;11:e0159613 doi: 10.1371/journal.pone.0159613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nomura R, Naka S, Nemoto H, Otsugu M, Nakamura S, Ooshima T, Nakano K. Potential high virulence for infective endocarditis in Streptococcus mutans strains with collagen-binding proteins but lacking PA expression. Arch Oral Biol. 2013;58:1627–1634. doi: 10.1016/j.archoralbio.2013.06.008 [DOI] [PubMed] [Google Scholar]
- 18.Sato Y, Okamoto K, Kagami A, Yamamoto Y, Igarashi T, Kizaki H. Streptococcus mutans strains harboring collagen-binding adhesin. J Dent Res. 2004;83:534–539. doi: 10.1177/154405910408300705 [DOI] [PubMed] [Google Scholar]
- 19.Nakano K, Hokamura K, Taniguchi N, Wada K, Kudo C, Nomura R, Kojima A, Naka S, Muranaka Y, Thura M, et al. The collagen-binding protein of Streptococcus mutans is involved in haemorrhagic stroke. Nat Commun. 2011;2:485 doi: 10.1038/ncomms1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. Bacteremia associated with toothbrushing and dental extraction. Circulation. 2008;117:3118–3125. doi: 10.1161/CIRCULATIONAHA.107.758524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandes CP, Oliveira FA, Silva PG, Alves AP, Mota MR, Montenegro RC, Burbano RM, Seabra AD, Lobo Filho JG, Lima DL, et al. Molecular analysis of oral bacteria in dental biofilm and atherosclerotic plaques of patients with vascular disease. Int J Cardiol. 2014;174:710–712. doi: 10.1016/j.ijcard.2014.04.201 [DOI] [PubMed] [Google Scholar]
- 22.Inenaga C, Hokamura K, Nakano K, Nomura R, Naka S, Ohashi T, Ooshima T, Kuriyama N, Hamasaki T, Wada K, et al. A potential new risk factor for stroke: streptococcus mutans with collagen-binding protein. World Neurosurg. 2018;113:e77–e81. doi: 10.1016/j.wneu.2018.01.158 [DOI] [PubMed] [Google Scholar]
- 23.Nomura R, Nakano K, Taniguchi N, Lapirattanakul J, Nemoto H, Grönroos L, Alaluusua S, Ooshima T. Molecular and clinical analyses of the gene encoding the collagen-binding adhesin of Streptococcus mutans. J Med Microbiol. 2009;58pt 4469–475. doi: 10.1099/jmm.0.007559-0 [DOI] [PubMed] [Google Scholar]
- 24.Momeni SS, Ghazal T, Grenett H, Whiddon J, Moser SA, Childers NK. Streptococcus mutans serotypes and collagen-binding proteins Cnm/Cbm in children with caries analysed by PCR. Mol Oral Microbiol. 2019;34:64–73. doi: 10.1111/omi.12254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamba GS, Dufour D, Nainar SMH, Cioffi I, Lévesque CM, Gong SG. Association of Streptococcus mutans collagen binding genes with severe childhood caries. Clin Oral Investig. 2020;24:3467–3475. doi: 10.1007/s00784-020-03217-4 [DOI] [PubMed] [Google Scholar]
- 26.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A; Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 27.Kim SH, Shin DW, Yun JM, Lee JE, Lim JS, Cho BL, Kwon HM, Park JH. Kidney dysfunction and cerebral microbleeds in neurologically healthy adults. PLoS One. 2017;12:e0172210 doi: 10.1371/journal.pone.0172210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351 [DOI] [PubMed] [Google Scholar]
- 29.Hatate J, Miwa K, Matsumoto M, Sasaki T, Yagita Y, Sakaguchi M, Kitagawa K, Mochizuki H. Association between cerebral small vessel diseases and mild parkinsonian signs in the elderly with vascular risk factors. Parkinsonism Relat Disord. 2016;26:29–34. doi: 10.1016/j.parkreldis.2016.02.011 [DOI] [PubMed] [Google Scholar]
- 30.Poels MM, Ikram MA, van der Lugt A, Hofman A, Krestin GP, Breteler MM, Vernooij MW. Incidence of cerebral microbleeds in the general population: the Rotterdam Scan Study. Stroke. 2011;42:656–661. doi: 10.1161/STROKEAHA.110.607184 [DOI] [PubMed] [Google Scholar]
- 31.Alawieh A, Chaudry MI, Turner RD, Turk AS, Spiotta AM. Infectious intracranial aneurysms: a systematic review of epidemiology, management, and outcomes. J Neurointerv Surg. 2018;10:713–721. [DOI] [PubMed] [Google Scholar]
- 32.Liu W, Liu R, Sun W, Peng Q, Zhang W, Xu E, Cheng Y, Ding M, Li Y, Hong Z, et al. ; CASISP Study Group. Different impacts of blood pressure variability on the progression of cerebral microbleeds and white matter lesions. Stroke. 2012;43:2916–2922. doi: 10.1161/STROKEAHA.112.658369 [DOI] [PubMed] [Google Scholar]
- 33.Akoudad S, Aarts N, Noordam R, Ikram MA, Tiemeier H, Hofman A, Stricker BH, Vernooij MW, Visser LE. Antidepressant use is associated with an increased risk of developing microbleeds. Stroke. 2016;47:251–254. doi: 10.1161/STROKEAHA.115.011574 [DOI] [PubMed] [Google Scholar]
- 34.Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, Krestin GP, Breteler MM. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70:1208–1214. doi: 10.1212/01.wnl.0000307750.41970.d9 [DOI] [PubMed] [Google Scholar]
- 35.Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease–systematic review and meta-analysis. Neurobiol Aging. 2009;30:337–352. doi: 10.1016/j.neurobiolaging.2007.07.015 [DOI] [PubMed] [Google Scholar]
- 36.Burns EM, Kruckeberg TW, Gaetano PK. Changes with age in cerebral capillary morphology. Neurobiol Aging. 1981;2:283–291. doi: 10.1016/0197-4580(81)90037-3 [DOI] [PubMed] [Google Scholar]
- 37.Lammie GA. Pathology of small vessel stroke. Br Med Bull. 2000;56:296–306. doi: 10.1258/0007142001903229 [DOI] [PubMed] [Google Scholar]
- 38.Meurman JH, Hämäläinen P. Oral health and morbidity–implications of oral infections on the elderly. Gerodontology. 2006;23:3–16. doi: 10.1111/j.1741-2358.2006.00102.x [DOI] [PubMed] [Google Scholar]
- 39.Caufield PW, Cutter GR, Dasanayake AP. Initial acquisition of mutans streptococci by infants: evidence for a discrete window of infectivity. J Dent Res. 1993;72:37–45. doi: 10.1177/00220345930720010501 [DOI] [PubMed] [Google Scholar]
- 40.Lapirattanakul J, Nakano K. Mother-to-child transmission of mutans streptococci. Future Microbiol. 2014;9:807–823. doi: 10.2217/fmb.14.37 [DOI] [PubMed] [Google Scholar]
- 41.Krasse B, Edwardsson S, Svensson I, Trell L. Implantation of caries-inducing streptococci in the human oral cavity. Arch Oral Biol. 1967;12:231–236. doi: 10.1016/0003-9969(67)90042-8 [DOI] [PubMed] [Google Scholar]
- 42.Berkowitz RJ, Jordan HV, White G. The early establishment of Streptococcus mutans in the mouths of infants. Arch Oral Biol. 1975;20:171–174. doi: 10.1016/0003-9969(75)90005-9 [DOI] [PubMed] [Google Scholar]
- 43.Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain. 2007;130pt 81988–2003. doi: 10.1093/brain/awl387 [DOI] [PubMed] [Google Scholar]
- 44.Wilson D, Charidimou A, Ambler G, Fox ZV, Gregoire S, Rayson P, Imaizumi T, Fluri F, Naka H, Horstmann S, et al. Recurrent stroke risk and cerebral microbleed burden in ischemic stroke and TIA: A meta-analysis. Neurology. 2016;87:1501–1510. doi: 10.1212/WNL.0000000000003183 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data were generated and preserved at the National Cerebral and Cardiovascular Center. Derived data supporting the findings of this study are available from corresponding authors on request.


