Supplemental Digital Content is available in the text.
Keywords: control group, cysteine, neuroimaging, phenotype, white matter
Background and Purpose:
Cysteine altering NOTCH3 variants, which have previously been exclusively associated with the rare hereditary small vessel disease cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, have a population frequency of 1:300 worldwide. Using a large population database, and taking genotype as a starting point, we aimed to determine whether individuals harboring a NOTCH3 cysteine altering variant have a higher load of small vessel disease markers on brain magnetic resonance imaging than controls, as well as a higher risk of stroke and cognitive impairment.
Methods:
A cross-sectional study using integrated clinical, neuroimaging, and whole-exome sequencing data of 92 456 participants from the Geisinger DiscovEHR initiative cohort. The case group consisted of individuals harboring a NOTCH3 cysteine altering variant (n=118). The control group consisted of randomly selected age- and sex-matched individuals who did not have any nonsynonymous variants in NOTCH3 (n=184). Medical records including brain magnetic resonance imagings were evaluated for clinical and neuroimaging findings associated with small vessel disease. Group comparisons were done using Fisher exact test and ordinal logistic regression models. Risk of stroke was assessed using Cox regression.
Results:
Of the 118 cases, 39.0% were men, mean age 58.1±16.9 years; 12.6% had a history of stroke, compared with 4.9% of controls. The risk of stroke was significantly increased after age 65 years (hazard ratio, 6.0 [95% CI, 1.4–26.3]). Dementia, mild cognitive impairment, migraine with aura and depression were equally prevalent in cases and controls. Twenty-nine cases (25%) and 45 controls (24%) had an available brain magnetic resonance imaging. After age 65 years, cases had a higher white matter lesion burden and more lacunes. A severe small vessel disease phenotype compatible with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy was rarely seen.
Conclusions:
Cysteine altering NOTCH3 variants are an important contributor to the risk of stroke, lacunes, and white matter hyperintensities in the elderly population.
Cerebral small vessel disease (SVD) is the cause of about a quarter of ischemic strokes worldwide and is the most common cause of vascular dementia.1 SVD is most commonly sporadic, associated with aging and hypertension, but a minority of SVD is monogenic of which cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is the most common and well studied.2,3
See related article, p 3482
CADASIL is caused by distinctive NOTCH3 missense variants, namely variants leading to a cysteine alteration in one of the 34 epidermal growth factor-like repeat (EGFr) domains of the NOTCH3 protein.4,5 These NOTCH3 missense variants (NOTCH3cys) result in aggregation of the ectodomain of mutant NOTCH3 protein in the tunica media of small arteries,6 which is associated with cerebrovascular dysfunction and cerebral hypoperfusion.7–9 T2-weighted brain MR images consistently show white matter hyperintensities (WMHs) usually by the age of 35 years,10,11 often involving the anterior temporal lobes and external capsules.10,12 In later disease stages, confluent WMHs are superimposed by multiple lacunes and frequently subcortical microbleeds.13 Typically, patients with CADASIL have recurrent ischemic strokes from a mean age of 50 years and vascular cognitive impairment leading to dementia.14,15 Migraine with aura is seen in roughly half of the patients,16,17 and about one-third of patients have mood disorders.18
To date, >280 unique NOTCH3cys variants have been described in CADASIL pedigrees worldwide. Patients with CADASIL with NOTCH3cys variants in epidermal growth factor-like repeat (EGFr) domains 7 to 34 have recently been described to have a later onset of stroke and reduced survival compared with patients with NOTCH3cys variants in EGFr domains 1 to 6.19 Although CADASIL has been assumed to be rare, it was recently shown that NOTCH3cys variants in EGFr domains 7 to 34, identical to those found in CADASIL pedigrees, have an unexpectedly high population frequency (1:300).19,20 A recent study in a population cohort (UK Biobank) revealed that NOTCH3cys variants in EGFr domains 7 to 34 ascertained in the population are associated with a much milder SVD phenotype than CADASIL, and can even be nonpenetrant, with a normal brain magnetic resonance imaging (MRI) up to the eighth decade.21 In UK Biobank, there was no increased risk of stroke associated with these variants compared with controls. However, UK Biobank is known to have a healthy volunteer bias,22 which may give an underestimation of the stroke risk associated with NOTCH3cys variants in EGFr domains 7 to 34 in the population. Therefore, we queried Geisinger DiscovEHR, a biobank with whole-exome sequencing and phenotypic data of 92 456 patient-participants in an integrated health system. We used an inverse approach, identifying all individuals with a NOTCH3cys genotype and subsequently assessing stroke frequency and other clinical and neuroimaging features associated with SVD.
Methods
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors (lesnik@lumc.nl or rzand@geisinger.edu) upon reasonable request.
Geisinger DiscovEHR Initiative Cohort
As part of the MyCode initiative, individuals agreed to provide blood and DNA samples for research, including genomic analyses as part of the Regeneron-Geisinger DiscovEHR collaboration. MyCode genetic data are linked to data in the Geisinger electronic health records under a protocol approved by the Geisinger Institutional Review Board. Recruitment occurs in primary care and specialty clinics throughout Geisinger Health System without regard to underlying diseases. The mean age of the participants is 57.4±18.1 years (range, 2–89), and 57.9% are women. The majority of participants (97.5%) are White of European descent. The consent rate has been >85%. The details of enrollment, sample collection, and processing have been previously published.23,24
Identification of Cases With a NOTCH3cys Variant and Controls in DiscovEHR
All variants located in exons 2 to 24 of the NOTCH3 gene (ie, the exons encoding the 34 EGFr domains of the NOTCH3 protein) were called through the Genome Analysis Toolkit best practices pipeline and filtered with a genotyping quality of 30, a minimum depth of 10, a minimum allele balance of 20, and a minimum quality by depth of 5.25,26 The cases were defined as those individuals in whom a missense variant was detected, leading to a cysteine amino acid alteration in one of the 34 EGFr domains of the NOTCH3 protein (amino acid position 40—1373; http://www.uniprot.org). The control group consisted of 184 randomly selected individuals without any nonsynonymous variants in NOTCH3 exons 2 to 24, who were age- and sex-matched with the cases. The study was approved by the Geisinger Institutional Review Board, and informed consent was waived. The final data de-identification and electronic health records linkage were managed through the Geisinger Phenomic Analytics and Clinical Data Core, which is independent of the study team.
Assessment of Clinical and Neuroimaging SVD Features in DiscovEHR
Medical records were probed using International Statistical Classification of Diseases and Related Health Problems Tenth Revision (ICD-10 codes) by a team of investigators with expertise in vascular neurology and neuroimaging, blinded for NOTCH3 variant status, age, and sex. ICD-10 codes corresponding with the following diagnoses and cardiovascular risk factors were recorded: stroke, transient ischemic attack (TIA), mild cognitive impairment, dementia, depression, migraine with and without aura, past or current smoking, hypertension, use of statin medications for hyperlipidemia, diabetes type 1 and 2, coronary artery disease, and peripheral vascular disease. Medication list, positive family history of at least one first-degree relative with stroke, and other relevant medical history, such as a diagnosis of multiple sclerosis, were also recorded. To confirm the ICD-10 code for stroke and TIA, complete medical records were reviewed. Stroke was defined as rapidly evolving focal symptoms lasting ≥24 hours with no apparent cause other than of vascular origin. TIA was defined as a transient episode lasting <24 hours of neurological dysfunction caused by focal brain or retinal ischemia, without infarction on brain imaging.
For all cases and controls in whom a brain MRI with at least T1, T2, and T2-weighted fluid-attenuated inversion recovery sequences were available, images were scored by 2 physicians with experience in vascular neurology and neuroimaging (Dr Hack and M.A. Iqbal or Dr Khan), blinded for NOTCH3 variant status, age, sex, and medical history. A trained and board-certified physician (Dr Zand) in vascular neurology and neuroimaging also reviewed the imaging and acted as a tiebreaker. Brain MRIs were scored according to the Standards for Reporting Vascular changes on Neuroimaging Guidelines.27 The following lesions were assessed: the number of lacunes of presumed vascular origin, number of cerebral of microbleeds, the burden of WMH in the periventricular white matter (PVWM) and deep white matter (DWM) according to the simplified Fazekas scale,28 and the presence of WMH in the external capsules and anterior temporal lobes. Global cortical atrophy was assessed using the Pasquier scale.29
Statistical Analysis
Normally distributed continuous variables were summarized as mean±SD and compared between cases and controls using the unpaired 2-sample t test. Statistical comparisons on binary categorical variables between cases and controls were performed using the Fisher exact test. Ordinal logistic regression models were used to compare ordinal categorical variables between cases and controls. Log-rank test was used to compare the time to first stroke between cases and controls. Cox regression was used to correct for sex and cardiovascular risk factors (ie, hypertension, statin use, diabetes type 1 or 2, past or current smoking). The assumption of proportional hazards was assessed by inspecting Schoenfield residuals and log minus log plots. In our Cox regression model, the assumption of proportional hazards was violated because of changes in the hazard ratios around the age of 65 years. Therefore, the survival analysis was divided into 2 time intervals, that is, <65 years and ≥65 years. SPSS 26.0 (Chicago, IL) was used for all statistical analyses.
Results
NOTCH3cys Variants in DiscovEHR
There were 131 cases with a NOTCH3cys variant, which corresponds to a frequency of 1:706. In 130 cases, the NOTCH3cys variant was located in one of the EGFr domains 7 to 34; one individual had a NOTCH3cys variant in EGFr domain 5 (Table I in the Data Supplement). There were 25 unique NOTCH3cys variants, of which some were frequent and some only occurred once. The most frequent variant was p.Arg1231Cys (EGFr domain 31) found in 84 individuals. This variant, as well as 9 of the other 24 unique NOTCH3cys variants in DiscovEHR, have been previously reported in CADASIL pedigrees.
Increased Frequency of Stroke but Not of Dementia or Migraine With Aura, in NOTCH3cys Cases
Medical records were available for 118 cases, with a mean age at last visit of 58.1±16.9 years (range, 20.1–93.8 years); 39.0% were men (Table 1). Fifteen cases had a history of stroke, which was significantly more frequent than in controls (12.7% versus 4.9%; P=0.02). In time-to-event analysis, cases had a significantly shorter stroke-free survival than controls (P=0.02; Figure 1). After correction for vascular risk factors and sex, cases had a significantly increased risk of stroke after the age of 65 years (hazard ratio, 6.0 [95% CI, 1.4–26.3]; P=0.02), but before age 65 years, the difference was not statistically significant (hazard ratio, 2.1 [95% CI, 0.7–6.3]; P=0.20). In cases, cardiovascular risk factors and sex were not independently associated with an increased risk of stroke (all P>0.15). Dementia, mild cognitive impairment, migraine with aura and depression were equally prevalent between cases and controls (Table 1). Although there was no significant difference in traditional vascular risk factors between cases and controls, coronary artery disease was significantly more frequent in the control group (26.6% versus 12.7%; P=0.004). A positive family history for stroke or dementia was not more frequent in cases than in controls (Table 1). The case with a NOTCH3cys variant in EGFr domain 5 was 71 years old during her last visit. Her past medical history only reported a possible TIA at an unknown age. There was no neuroimaging available of her.
Table 1.
Small Vessel Disease Features, Vascular Risk Factors, and Family History in Cases With a NOTCH3cys Variant vs Controls
Figure 1.
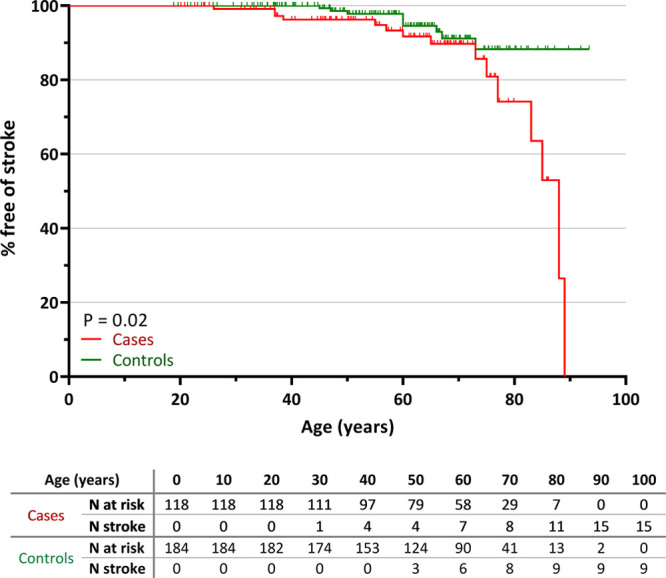
Stroke incidence in cases with a NOTCH3cys variant vs controls. Kaplan-Meier plot showing the proportion free of stroke in cases (n=118) and controls (n=184). Cases had a significantly shorter stroke-free survival compared with controls (P=0.02). The table under the graph shows the number of cases and controls at risk and the number of individuals with a first stroke per 10-y interval.
Higher Burden of WMHs and More Lacunes in NOTCH3cys Cases
Brain MRI was available for 29 cases (24.6%) and 44 controls (23.9%). Age at MRI did not differ between cases and controls. Cases had an overall higher WMH burden than controls, but this did not reach statistical significance (Table 2; Figure 2A and 2B). However, Fazekas DWM 3 and Fazekas PVWM ≥1 was significantly more frequent in cases than in controls: 24.1% versus 4.5% (P=0.02) and 82.8% versus 56.8% (P=0.02). Fazekas DWM 0 or PVWM 0 was never seen in cases older than 55 years, and above age 70 years all cases had Fazekas DWM 2 or 3. Conversely, the majority of controls older than 70 years still had Fazekas DWM ≤1 or PVWM ≤1 (Figure 2C and 2D).
Table 2.
Brain MRI Small Vessel Disease Markers in Cases With a NOTCH3cys Variant vs Controls
Figure 2.
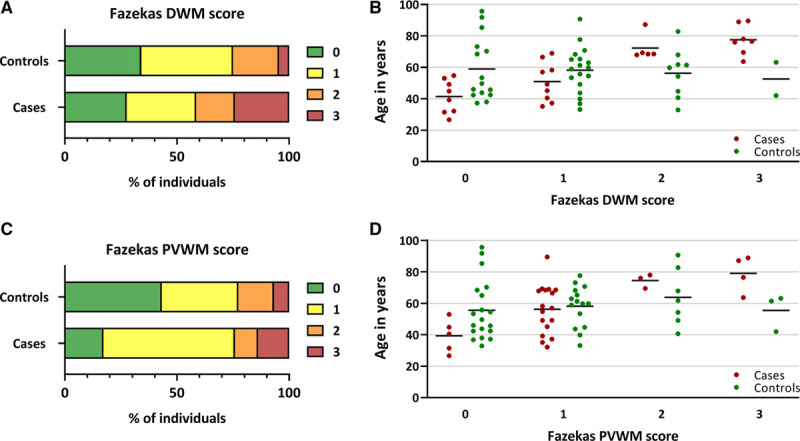
White matter hyperintensity lesion load in cases with a NOTCH3cys variant vs controls. Proportional bar charts showing (A) deep white matter (DWM) Fazekas scores and (B) periventricular white matter (PVWM) Fazekas scores, showing a higher WMH lesion load in cases vs controls. C and D, Scatterplots showing the age distribution per Fazekas score in cases vs controls. The cases with Fazekas DWM 3 did not have an alternative cause for their confluent deep white matter hyperintensities besides the NOTCH3cys variant. Of the 3 controls with Fazekas DWM 3 or PVWM 3, one had been treated with cranial radiotherapy and one had a high vascular risk factor burden. Horizontal black lines represent mean ages.
There was no difference in the presence of WMH in the temporal poles or external capsules between cases and controls with Fazekas DWM ≥2: 16.6% versus 18.2% (P>0.99) and 58.3% versus 54.5% (P>0.99), respectively. Although overall there was no significant difference in the presence of lacunes between cases and controls: 27.6% versus 13.6% (P=0.22; Table 2), after age 65 years lacunes were significantly more prevalent in cases than controls (53.8% versus 7.1%; P=0.01). There were no significant differences in the presence of cerebral microbleeds and global cortical atrophy between cases and controls (Table 2).
Discussion
Using a genotype-first approach, we studied clinical and neuroimaging SVD features in individuals with NOTCH3cys variants in the population, using the exome sequencing data from DiscovEHR. NOTCH3cys variants occurred at a frequency of 1:706 and were almost exclusively located in NOTCH3 EGFr domains 7 to 34. Individuals with a NOTCH3cys variant were at increased risk of stroke, WMHs, and lacunes after age 65 years, but a classical mid-adult onset CADASIL phenotype was not seen. Our findings are in line with accumulating evidence that NOTCH3cys variants do not only cause the rare and severe hereditary SVD CADASIL but are much more commonly associated with a milder SVD phenotype, specifically when these variants are located in EGFr 7 to 34.21,30–32 Given the high population frequency of NOTCH3cys variants (1:300 worldwide), the total number of individuals who are at higher risk of SVD and stroke as a result of a NOTCH3cys variant is significant. On a world population of ≈8 billion, there are an estimated 25 million individuals with a NOTCH3cys variant. Based on our results, we would expect that the majority of the individuals with a NOTCH3cys variant will develop NOTCH3cys-associated SVD after the age of 65 years. NOTCH3cys variants, therefore, are a new genetic risk factor which should be taken into account in SVD risk stratification and prevention.
Although SVD is associated with mild cognitive impairment and vascular dementia,1,33 we did not find an increased frequency of mild cognitive impairment and dementia in the individuals with a NOTCH3cys variant. However, the frequency of cognitive impairment may be an underestimation as this was evaluated using only ICD-10 codes, and the majority of patients did not have a formal neuropsychological evaluation. The role of NOTCH3cys variants in mild cognitive impairment and vascular dementia in the elderly population needs to be addressed in large prospective studies. Only 4.2% of individuals with a NOTCH3cys variant had migraine with aura, whereas this has been reported in 45% to 70% of patients with CADASIL.16,17 A possible explanation for this discrepancy could be that NOTCH3cys variants most commonly found in CADASIL, that is, those located in EGFr domains 1 to 6, predispose to a higher risk for migraine with aura than variants in EGFr domains 7 to 34. This hypothesis is also supported by the low prevalence of migraine with aura observed in Asian CADASIL cohorts, in whom NOTCH3cys variants in EGFr domains 7 to 34 are much more common.34,35
The individuals with a NOTCH3cys variant in DiscovEHR did not have an increased frequency of WMH in the anterior temporal poles or external capsules compared with controls, although the presence of WMH in these areas is suggestive of CADASIL,10,12 which is explained by the relatively mild phenotype and low WMH burden of individuals with a NOTCH3cys variant in DiscovEHR.
In line with previous population studies, the vast majority of NOTCH3cys variants in DiscovEHR are located in one of NOTCH3 EGFr domains 7 to 34, with the p.Arg1231Cys variant occurring most frequently.19–21 As such, it is becoming increasingly clear that there is an extreme variability in disease severity associated with NOTCH3cys EGFr 7 to 34 variants, implying a role for strong disease modifiers. Variants located in EGFr domains 1 to 6, on the other hand, seem to almost always lead to a severe mid-adult onset CADASIL phenotype, as they are frequent in CADASIL pedigrees and rare in the population. It was not possible to investigate the effect of specific variants on SVD phenotype in DiscovEHR because of the frequency and distribution of NOTCH3cys variants in this population.
Studies in CADASIL cohorts have shown that hypertension and smoking are disease modifiers,18,36,37 but in this population study, we found no additional effect of cardiovascular risk factors on stroke risk in individuals with a NOTCH3cys variant. This may be because of a sample size limitation, with insufficient power to detect small effects. Future studies with larger population cohorts are required to investigate the effect of cardiovascular risk factors on the NOTCH3cys-associated disease spectrum.
Our study has several limitations. Brain MRIs were of individuals who had an indication for neuroimaging, leading to a selection bias in both cases and controls. Furthermore, the prevalence of clinical symptoms was likely underestimated because of the retrospective nature of the study and the use of ICD-10 codes. However, complete medical records were reviewed to confirm the ICD-10 diagnosis for stroke and TIA. Stratification of stroke subtypes could not be reliably performed, as brain MRIs during the acute phase were generally not available. Finally, the fact that controls had a 2× higher frequency of coronary artery disease was unexpected, especially as vascular risk factor burden was equal between cases and controls. A protective effect of NOTCH3cys variants on coronary artery disease has never been reported in CADASIL and is unlikely from a pathomechanistic perspective.38 A sampling bias cannot be ruled out, but the fact that controls had a higher frequency of coronary artery disease suggests that the effect of NOTCH3cys variants on stroke risk may be underestimated.
In conclusion, this study shows that highly distinctive NOTCH3cys variants, which have a frequency of 1:300 worldwide, are an important contributor to the risk of stroke, lacunes, and WMHs in the elderly population.
Acknowledgments
Dr Hack contributed to acquisition of data, analyzing and interpreting the data, drafting the manuscript and in statistical analysis. Dr Rutten designed and conceptualized the study, interpreted the data, drafted the manuscript. She also performed critical revision of the manuscript for important intellectual content and supervision. T. N. Person contributed to acquisition of data and critical revision of the manuscript for important intellectual content. Dr Li interpreted the data and contributed to critical revision of the manuscript for important intellectual content. Dr Khan contributed to acquisition of data and critical revision of the manuscript for important intellectual content. Dr Griessenauer interpreted the data and performed critical revision of the manuscript for important intellectual content. Regeneron Genetics Center performed acquisition of data and critical revision of the manuscript for important intellectual content. Dr Abedi interpreted the data and performed critical revision of the manuscript for important intellectual content. Dr Lesnik Oberstein designed and conceptualized the study, interpreted the data, drafted the manuscript, and performed critical revision of the manuscript for important intellectual content and supervision. Dr Zand performed acquisition of data, interpretation of data, critical revision of the manuscript for important intellectual content, and supervision. The authors extend their thanks to Muhammad Ahmad Iqbal and Joseph Hornak for assisting with clinical charts review. We thank Amy Kolinovsky for assisting as the Geisinger’s certified honest broker for this study.
Sources of Funding
This study is supported by the Regeneron Genetics Center, the Netherlands Organisation for Health Research and Development (ZonMW 91717325) and the Dutch Alzheimer Foundation.
Disclosures
Dr Hack is funded by the Netherlands Organisation for Health Research and Development (ZonMW 91717325) and received support from the Dutch Alzheimer Foundation. Dr Rutten is funded by the Netherlands Brain Foundation (HA2016-02-03) and received institutional support from Leiden University Medical Center. Dr Abedi has financial research support from the Defense Threat Reduction Agency (DTRA) grant No. HDTRA1-18-1-0008 sub-awarded to Geisinger and funds from the National Institutes of Health (NIH) grant No. R56HL116832 sub-awarded to Geisinger. Dr Lesnik Oberstein has financial research support from the Netherlands Organisation for Health Research and Development (ZonMW 91717325) and the Netherlands Brain Foundation (HA2016-02-03) and receives institutional support from Leiden University Medical Center. Dr Zand has financial research support from Bucknell University Initiative Program, Roche—Genethech Biothenology Company, the Geisinger Health Plan Quality fund, and receives institutional support from Geisinger Health System. The other authors report no conflicts.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CADASIL
- cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy
- DWM
- deep white matter
- EGFr
- epidermal growth factor-like repeat
- ICD-10
- International Statistical Classification of Diseases and Related Health Problems Tenth Revision
- MRI
- magnetic resonance imaging
- NOTCH3 cys variants
- cysteine altering missense NOTCH3 variants
- PVWM
- periventricular white matter
- SVD
- cerebral small vessel disease
- TIA
- transient ischemic attack
- WMH
- white matter hyperintensity
Drs Oberstein and Zand Shared last authorship.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.120.030343.
For Sources of Funding and Disclosures, see page 3568.
Contributor Information
Remco J. Hack, Email: r.j.hack@lumc.nl.
Julie W. Rutten, Email: j.w.rutten@lumc.nl.
Thomas N. Person, Email: tnperson@geisinger.edu.
Jiang Li, Email: jli@geisinger.edu.
Ayesha Khan, Email: akhan2@geisinger.edu.
Christoph J. Griessenauer, Email: christoph.griessenauer@gmail.com.
Vida Abedi, Email: vabedi@geisinger.edu.
References
- 1.Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18:684–696. doi: 10.1016/S1474-4422(19)30079-1 [DOI] [PubMed] [Google Scholar]
- 2.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009;8:643–653. doi: 10.1016/S1474-4422(09)70127-9 [DOI] [PubMed] [Google Scholar]
- 3.Cannistraro RJ, Badi M, Eidelman BH, Dickson DW, Middlebrooks EH, Meschia JF. CNS small vessel disease: a clinical review. Neurology. 2019;92:1146–1156. doi: 10.1212/WNL.0000000000007654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joutel A, Vahedi K, Corpechot C, Troesch A, Chabriat H, Vayssière C, Cruaud C, Maciazek J, Weissenbach J, Bousser MG, et al. Strong clustering and stereotyped nature of Notch3 mutations in CADASIL patients. Lancet. 1997;350:1511–1515. doi: 10.1016/S0140-6736(97)08083-5 [DOI] [PubMed] [Google Scholar]
- 5.Rutten JW, Haan J, Terwindt GM, van Duinen SG, Boon EM, Lesnik Oberstein SA. Interpretation of NOTCH3 mutations in the diagnosis of CADASIL. Expert Rev Mol Diagn. 2014;14:593–603. doi: 10.1586/14737159.2014.922880 [DOI] [PubMed] [Google Scholar]
- 6.Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, Piga N, Chapon F, Godfrain C, Tournier-Lasserve E. The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest. 2000;105:597–605. doi: 10.1172/JCI8047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuominen S, Miao Q, Kurki T, Tuisku S, Pöyhönen M, Kalimo H, Viitanen M, Sipilä HT, Bergman J, Rinne JO. Positron emission tomography examination of cerebral blood flow and glucose metabolism in young CADASIL patients. Stroke. 2004;35:1063–1067. doi: 10.1161/01.STR.0000124124.69842.2d [DOI] [PubMed] [Google Scholar]
- 8.Moreton FC, Cullen B, Delles C, Santosh C, Gonzalez RL, Dani K, Muir KW. Vasoreactivity in CADASIL: comparison to structural MRI and neuropsychology. J Cereb Blood Flow Metab. 2018;38:1085–1095. doi: 10.1177/0271678X17710375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin X, Zhou Y, Yan S, Lou M. Effects of cerebral blood flow and white matter integrity on cognition in CADASIL patients. Front Psychiatry. 2018;9:741 doi: 10.3389/fpsyt.2018.00741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singhal S, Rich P, Markus HS. The spatial distribution of MR imaging abnormalities in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy and their relationship to age and clinical features. AJNR Am J Neuroradiol. 2005;26:2481–2487. [PMC free article] [PubMed] [Google Scholar]
- 11.Samões R, Alves JE, Taipa R, Silva J, Melo Pires M, Pereira-Monteiro JM. CADASIL: MRI may be normal in the fourth decade of life - a case report. Cephalalgia. 2016;36:1082–1085. doi: 10.1177/0333102415618613 [DOI] [PubMed] [Google Scholar]
- 12.O’Sullivan M, Jarosz JM, Martin RJ, Deasy N, Powell JF, Markus HS. MRI hyperintensities of the temporal lobe and external capsule in patients with CADASIL. Neurology. 2001;56:628–634. doi: 10.1212/wnl.56.5.628 [DOI] [PubMed] [Google Scholar]
- 13.van den Boom R, Lesnik Oberstein SA, Ferrari MD, Haan J, van Buchem MA. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: MR imaging findings at different ages–3rd-6th decades. Radiology. 2003;229:683–690. doi: 10.1148/radiol.2293021354 [DOI] [PubMed] [Google Scholar]
- 14.Opherk C, Peters N, Herzog J, Luedtke R, Dichgans M. Long-term prognosis and causes of death in CADASIL: a retrospective study in 411 patients. Brain. 2004;127pt 112533–2539. doi: 10.1093/brain/awh282 [DOI] [PubMed] [Google Scholar]
- 15.Buffon F, Porcher R, Hernandez K, Kurtz A, Pointeau S, Vahedi K, Bousser MG, Chabriat H. Cognitive profile in CADASIL. J Neurol Neurosurg Psychiatry. 2006;77:175–180. doi: 10.1136/jnnp.2005.068726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guey S, Mawet J, Hervé D, Duering M, Godin O, Jouvent E, Opherk C, Alili N, Dichgans M, Chabriat H. Prevalence and characteristics of migraine in CADASIL. Cephalalgia. 2016;36:1038–1047. doi: 10.1177/0333102415620909 [DOI] [PubMed] [Google Scholar]
- 17.Tan RY, Markus HS. CADASIL: migraine, encephalopathy, stroke and their inter-relationships. PLoS One. 2016;11:e0157613 doi: 10.1371/journal.pone.0157613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adib-Samii P, Brice G, Martin RJ, Markus HS. Clinical spectrum of CADASIL and the effect of cardiovascular risk factors on phenotype: study in 200 consecutively recruited individuals. Stroke. 2010;41:630–634. doi: 10.1161/STROKEAHA.109.568402 [DOI] [PubMed] [Google Scholar]
- 19.Rutten JW, Van Eijsden BJ, Duering M, Jouvent E, Opherk C, Pantoni L, Federico A, Dichgans M, Markus HS, Chabriat H, et al. The effect of NOTCH3 pathogenic variant position on CADASIL disease severity: NOTCH3 EGFr 1-6 pathogenic variant are associated with a more severe phenotype and lower survival compared with EGFr 7-34 pathogenic variant. Genet Med. 2019;21:676–682. doi: 10.1038/s41436-018-0088-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutten JW, Dauwerse HG, Gravesteijn G, van Belzen MJ, van der Grond J, Polke JM, Bernal-Quiros M, Lesnik Oberstein SA. Archetypal NOTCH3 mutations frequent in public exome: implications for CADASIL. Ann Clin Transl Neurol. 2016;3:844–853. doi: 10.1002/acn3.344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutten JW, Hack RJ, Duering M, Gravesteijn G, Dauwerse JG, Overzier M, van den Akker EB, Slagboom E, Holstege H, Nho K, et al. Broad phenotype of cysteine-altering NOTCH3 variants in UK Biobank: CADASIL to nonpenetrance. Neurology. 2020;95:e1835–e1843. doi: 10.1212/WNL.0000000000010525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, Collins R, Allen NE. Comparison of sociodemographic and health-related characteristics of UK biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026–1034. doi: 10.1093/aje/kwx246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams MS, Buchanan AH, Davis FD, Faucett WA, Hallquist MLG, Leader JB, Martin CL, McCormick CZ, Meyer MN, Murray MF, et al. Patient-centered precision health in a learning health care system: geisinger’s genomic medicine experience. Health Aff (Millwood). 2018;37:757–764. doi: 10.1377/hlthaff.2017.1557 [DOI] [PubMed] [Google Scholar]
- 24.Carey DJ, Fetterolf SN, Davis FD, Faucett WA, Kirchner HL, Mirshahi U, Murray MF, Smelser DT, Gerhard GS, Ledbetter DH. The Geisinger MyCode community health initiative: an electronic health record-linked biobank for precision medicine research. Genet Med. 2016;18:906–913. doi: 10.1038/gim.2015.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broad Institute. GATK. 2018. https://software.broadinstitute.org/gatk/download/. Accessed January 31, 2018
- 26.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, et al. The genome analysis toolkit: a mapreduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, et al. ; STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351 [DOI] [PubMed] [Google Scholar]
- 29.Pasquier F, Leys D, Weerts JG, Mounier-Vehier F, Barkhof F, Scheltens P. Inter- and intraobserver reproducibility of cerebral atrophy assessment on MRI scans with hemispheric infarcts. Eur Neurol. 1996;36:268–272. doi: 10.1159/000117270 [DOI] [PubMed] [Google Scholar]
- 30.Masoli JAH, Pilling LC, Kuchel GA, Melzer D. Clinical outcomes of CADASIL-associated NOTCH3 mutations in 451,424 European ancestry community volunteers. Transl Stroke Res. 2019;10:339–341. doi: 10.1007/s12975-018-0671-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee YC, Chung CP, Chang MH, Wang SJ, Liao YC. NOTCH3 cysteine-altering variant is an important rsisk factor for stroke in the Taiwanese population. Neurology. 2020;94:e87–e96. doi: 10.1212/WNL.0000000000008700 [DOI] [PubMed] [Google Scholar]
- 32.Rutten JW, van den Akker EB, Lesnik Oberstein SAJ. Commentary to: Masoli Clinical outcomes of CADASIL-Associated NOTCH3 mutations in 451,424 European ancestry community volunteers. (Translational Stroke Research Oct 2018). Transl Stroke Res. 2019;10:458–459. doi: 10.1007/s12975-018-0681-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jokinen H, Koikkalainen J, Laakso HM, Melkas S, Nieminen T, Brander A, Korvenoja A, Rueckert D, Barkhof F, Scheltens P, et al. Global burden of small vessel disease-related brain changes on MRI predicts cognitive and functional decline. Stroke. 2020;51:170–178. doi: 10.1161/STROKEAHA.119.026170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi JC, Song SK, Lee JS, Kang SY, Kang JH. Headache among CADASIL patients with R544C mutation: prevalence, characteristics, and associations. Cephalalgia. 2014;34:22–28. doi: 10.1177/0333102413497598 [DOI] [PubMed] [Google Scholar]
- 35.Chen S, Ni W, Yin XZ, Liu HQ, Lu C, Zheng QJ, Zhao GX, Xu YF, Wu L, Zhang L, et al. Clinical features and mutation spectrum in Chinese patients with CADASIL: a multicenter retrospective study. CNS Neurosci Ther. 2017;23:707–716. doi: 10.1111/cns.12719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chabriat H, Hervé D, Duering M, Godin O, Jouvent E, Opherk C, Alili N, Reyes S, Jabouley A, Zieren N, et al. Predictors of clinical worsening in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: prospective cohort study. Stroke. 2016;47:4–11. doi: 10.1161/STROKEAHA.115.010696 [DOI] [PubMed] [Google Scholar]
- 37.Ling Y, De Guio F, Duering M, Jouvent E, Hervé D, Godin O, Dichgans M, Chabriat H. Predictors and clinical impact of incident lacunes in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke. 2017;48:283–289. doi: 10.1161/STROKEAHA.116.015750 [DOI] [PubMed] [Google Scholar]
- 38.Lesnik Oberstein SA, Jukema JW, Van Duinen SG, Macfarlane PW, van Houwelingen HC, Breuning MH, Ferrari MD, Haan J. Myocardial infarction in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Medicine (Baltimore). 2003;82:251–256. doi: 10.1097/01.md.0000085054.63483.40 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors (lesnik@lumc.nl or rzand@geisinger.edu) upon reasonable request.


