ABSTRACT
Background: Cumulative evidence suggests that both traumatic stress and posttraumatic stress disorder (PTSD) are cross-sectionally and prospectively linked to cardiovascular disease (CVD). However, their association with proxy markers of atherosclerosis has hardly been investigated.
Objective: The objective of this general population study was to relate traumatic stress and PTSD to carotid plaque and intima-media thickness (cIMT).
Methods: 3119 adults from the general population were assessed regarding their traditional cardiovascular risk factors, and an ultrasound of the carotid arteries was performed in each participant. Based on a PTSD interview, every participant was assigned to one of three groups: no trauma; trauma, but no PTSD; and trauma with PTSD. The sample was stratified into five age groups.
Results: Trauma exposure was reported by 54.5% of the sample and 2.0% had PTSD. Traumatized participants had increased odds of self-reported CVD events compared to those without trauma exposure, even when accounted for CVD risk factors and other covariates (odds ratio [OR] = 1.51; 95% confidence interval [CI]: 1.03–2.22). This association was driven by those aged 70 years or older. Only in those aged 40 to 49 years, there was an association between cIMT and PTSD. There were no further associations between carotid plaque or cIMT and traumatic stress or PTSD.
Conclusions: Our findings in concert with prior research suggest that the association between traumatic stress, PTSD and atherosclerosis as well as its clinical endpoints is complex and remains inconclusive.
KEYWORDS: Trauma, posttraumatic stress disorder (PTSD), subclinical atherosclerosis, carotid intima-media thickness (cIMT), carotid plaque, cardiovascular disease (CVD)
HIGHLIGHTS:
Although traumatic stress and posttraumatic stress disorder have been linked to cardiovascular disease, they were not related to proxy markers of atherosclerosis in this general population study including 3119 adults.
Short abstract
Antecedentes: La evidencia acumulada sugiere que tanto el estrés traumático como el trastorno de estrés postraumático (TEPT) están vinculados de manera transversal y prospectiva con la enfermedad cardiovascular (ECV). Sin embargo, apenas se ha investigado su asociación con marcadores indirectos de aterosclerosis.
Objetivo: El objetivo de este estudio de población general fue relacionar el estrés traumático y el TEPT con la placa carotídea y el grosor de la íntima-media (GIMc).
Métodos: Se evaluó a 3119 adultos de la población general respecto a factores de riesgo cardiovascular tradicionales y se realizó una ecografía de las arterias carótidas en cada participante. Basado en una entrevista de TEPT, cada participante fue asignado a uno de tres grupos: sin trauma; trauma, pero no TEPT; y trauma con TEPT. La muestra se estratificó en cinco grupos de edad.
Resultados: El 54,5% de la muestra informó exposición al trauma y el 2,0% tenía TEPT. Los participantes traumatizados tenían mayores probabilidades de eventos de ECV auto-reportados en comparación con los sin exposición al trauma, incluso cuando se tienen en cuenta los factores de riesgo de ECV y otras covariables (razón de posibilidades [OR] = 1,51; intervalo de confianza [IC] del 95%: 1,03-2,22). Esta asociación fue mayormente determinada por personas de 70 años o más. Solo en los de 40 a 49 años, hubo una asociación entre cIMT y TEPT. No hubo más asociaciones entre la placa carotídea o el cIMT y el estrés traumático o el TEPT.
Conclusiones: Nuestros hallazgos, en conjunto con investigaciones anteriores, sugieren que la asociación entre el estrés traumático, el TEPT y la aterosclerosis, así como sus criterios de valoración clínicos, es compleja y no es concluyente.
Palabras clave: trauma, trastorno de estrés postraumático (PTSD), enfermedad cardiovascular (ECV), aterosclerosis subclínica, espesor de la íntima-media carotídea (cIMT), placa carotídea
Abstract
背景:累积证据表明, 创伤应激和创伤后应激障碍 (PTSD) 在横断面和前瞻性上均与心血管疾病 (CVD) 相关。然而, 极少研究它们与动脉粥样硬化代用标记物的关系。
目的:这项对一般人群的研究旨在将创伤应激和PTSD与颈动脉斑块和内膜中层厚度 (cIMT) 相关联。
方法:对3119名来自一般人群的成年者的传统心血管风险因素进行了评估, 并对每位参与者进行了颈动脉超声检查。根据PTSD访谈, 每位参与者都被分配至以下三组之一:无创伤组;经历创伤但无PTSD组;和经历创伤并患有PTSD组。样本被分为五个年龄组。
结果:54.5%的样本报告了创伤暴露, 2.0%患有PTSD。即使控制了CVD风险因素和其他协变量, 创伤患者相较于无创伤暴露者的自我报告CVD事件发生几率增加 (优势比[OR] = 1.51; 95%置信区间[CI]:1.03-2.22) 。这个关联是由70岁或以上的人推动的。仅在40至49岁的人群中, cIMT与PTSD之间存在关联。颈动脉斑块或cIMT与创伤应激或PTSD之间没有进一步的关联。
结论:我们的研究结果与先前研究一致表明, 创伤应激, PTSD与动脉粥样硬化之间的关联以及其临床终点尚不明确。
关键词: 创伤, 创伤后应激障碍 (PTSD), 心血管疾病 (CVD), 亚临床动脉粥样硬化, 颈内膜中层厚度 (cIMT), 颈动脉斑块
1. Introduction
Mounting data suggest an association between traumatic stress and clinical endpoints of cardiovascular disease (CVD) including angina pectoris, myocardial infarction (MI), heart failure, and stroke (Hendrickson et al., 2013; Husarewycz, El-Gabalawy, Logsetty, & Sareen, 2014; Song et al. 2019l; Spitzer et al., 2009). Numerous cross-sectional and longitudinal studies report associations between childhood maltreatment including physical and sexual abuse and self-reported CVD, and even suggest a dose–response relation (reviewed by Basu, McLaughlin, Misra, & Koenen, 2017; Su, Jimenez, Roberts, & Loucks, 2015; Suglia et al., 2018). Likewise, traumatic stress during adulthood such as experiencing terrorist attacks (e.g. exposure to the 11 September 2001 World Trade Centre disaster), natural disasters or being involved in war conflicts has cross-sectionally and prospectively been related to CVD (Dyball, Evans, Boos, Stevelink, & Fear, 2019; Hendrickson et al., 2013; Husarewycz et al., 2014; Jordan et al., 2013; Lenane et al., 2019). Again, findings indicate a dose–response relationship between number of traumatic events and CVD endpoints (Husarewycz et al., 2014; Scott et al., 2013; Sledjeski, Speisman, & Dierker, 2008).
Not only trauma exposure itself but also posttraumatic stress disorder (PTSD) as its most prototypical psychological sequelae has been linked to CVD (reviewed by Edmondson & von Känel, 2017; Ryder, Azcarate, & Cohen, 2018; Scherrer et al., 2019). While earlier studies in community and clinical samples as well as in war veterans (e.g. O’Toole, Catts, & Trauma, 2008; Seng, Clark, McCarthy, & Ronis, 2006; Spitzer et al., 2009) were limited by their cross-sectional design, more recent prospective cohort studies have estimated the association of PTSD with incident CVD events and/or CVD death with adjusted hazard ratios ranging from 1.5 to 3.3 (e.g. Boscarino, 2008; Kubzansky, Koenen, Spiro, Vokonas, & Sparrow, 2007; Scherrer et al., 2010; Song et al. 2019; Vaccarino et al., 2013). One of the largest prospective cohort studies used Danish national registry data and found that patients diagnosed with PTSD had an increased risk of incident MI by 50% and of stroke by 70% (Gradus et al., 2015). Very similar findings were reported by the Nurses’ Health Study II following 49,978 women for 20 years: Those endorsing four or more PTSD symptoms had a 60% increased risk of incident CVD compared to participants without a history of trauma (Sumner et al., 2015). Additional analysis of the same cohort found the risk of incident CVD to increase by 9% with every additional 5 years of symptomatic PTSD. Interestingly, women whose PTSD resolved did not have an increased risk of CVD (Gilsanz et al., 2017).
There are several major challenges to the field of trauma-PTSD-CVD research. First, apart from few studies (Scherrer et al., 2019; Sumner et al., 2015; Vaccarino et al., 2013) the majority has exclusively focussed on the impact of PTSD on CVD by contrasting PTSD positive subjects with those without trauma exposure. However, there is evidence that multiple traumas may have a cumulative effect on physical health independent of PTSD symptomatology (Scott et al., 2013; Sledjeski et al., 2008; Spitzer et al., 2009). Second, and even more important, assessing CVD outcomes with self-report or unvalidated diagnostic coding may introduce a severe bias. Only very few studies have used outcomes confirmed by medical record review or additional information (Rich-Edwards et al., 2012; Turner, Neylan, Schiller, Li, & Cohen, 2013; Vaccarino et al., 2013). Of note, those participants within a subpopulation of active duty members (n = 23,794) of the prospective Millennium Cohort Study who were screened positive for PTSD symptoms had an increased likelihood of incident self-report but not medical record CVD diagnoses (Crum-Cianflone et al., 2014). The possible bias induced by self-reported diagnoses can be addressed either by using objective measures of cardiovascular health (Turner et al., 2013; Vaccarino et al., 2013) or by investigating proxy markers of (subclinical) atherosclerosis and CVD, respectively. For example, in veterans without coronary artery disease, those with PTSD were more likely to have elevated coronary artery calcium scores indicating coronary atherosclerosis (Ahmadi et al., 2011). Another study found that patients with PTSD due to deportation had higher aortic pulse wave velocity (aPWV) suggestive of higher aortic stiffness than controls without trauma, with the magnitude of aPWV increasing with the severity of PTSD (Walczewska, Rutkowski, Wizner, Cwynar, & Grodzicki, 2011). Likewise, police officers with severe PTSD symptoms showed a nearly twofold reduction in brachial artery flow-mediated dilation as biomarker for subclinical CVD compared to those with low PTSD symptomatology (Violanti et al., 2006); however, carotid intima-media thickness (cIMT) was not related to PTSD symptoms in the same sample (Violanti et al., 2006). By contrast, in 465 middle-aged twins from the Vietnam Era Twin Registry who were free from CVD, both combat exposure and PTSD were associated with cIMT (Goetz et al., 2014). Likewise, among 372 medical students having experienced the long-lasting war in Bosnia–Herzegovina, those with well-defined trauma exposures during the war had a significantly higher cIMT than non-traumatized participants, and trauma was independently associated with higher cIMT after adjustment for CVD risk factors (Vulic et al., 2019). Moreover, and from a health care perspective, objective measures of subclinical CVD may help to identify individuals at elevated risk which might be beneficial in planning and implementing primary and secondary prevention strategies, particularly in groups with high-risk for trauma exposure and subsequent PTSD like police or military personnel. In sum, results on the association between trauma, PTSD and proxy markers of CVD are primarily focussing on highly exposed samples, and remain inconclusive.
In light of these considerations, the objective of our study was to determine whether traumatic stress, the number of traumatic experiences as a proxy for the individual trauma load and/or subsequent PTSD might contribute to CVD in adult community residents by relating them to surrogate markers of atherosclerosis, i.e. cIMT and carotid plaque. These preclinical disease markers are measured non-invasively by ultrasonography and are widely used to assess early stages of atherosclerosis (Tibaut et al., 2019), particularly as cIMT represents a strong predictor of future vascular events (reviewed by Carpenter, Sinclair, & Kunadian, 2016). Considering the wide age range of our study population, and because age is a strong independent risk factor for atherosclerosis and CVD, respectively (Hamczyk, Nevado, Barettino, Fuster, & Andrés, 2020; Paneni, Diaz Cañestro, Libby, Lüscher, & Camici, 2017), this issue was given particular attention by assigning participants to five almost equal age strata (<40 years; 40–49 years; 50–59 years; 60–69 years; and ≥70 years).
2. Methods
2.1. Procedure and subjects
Participants were recruited as part of the Study of Health in Pomerania (SHIP), which is an ongoing population-based health examination project in Northeastern Germany. A sample from the adult population (20–79 years) was drawn from population registries considering a German citizenship and residency in West Pomerania. Data collection of the baseline cohort (SHIP-0) was performed between October 1997 and May 2001. SHIP-0 comprised 4310 participants providing informed written consent. The study was approved by the local Ethics Committee. The objectives and design of SHIP are published elsewhere (John et al., 2001; Völzke, 2012). In brief, data were collected in two examination centres specifically established for this study. Participants were offered free transportation to the examination centres and back home, a meal and 15 € as incentives. The data collection comprised four parts: a health- and risk factor-related self-report questionnaire, an oral health examination, a medical examination, and a computer-assisted health-related interview. The latter was conducted by trained and certified interviewers, and there was a continuous quality monitoring (John et al., 2001; Völzke, 2012).
The present study was part of the 5-year follow-up (SHIP-I) performed between December 2002 and December 2006 (Spitzer et al., 2008), i.e. a cross-sectional study nested in a long-term community-based cohort study. For SHIP-I, there were 130 passive non-responders due to migration, and 231 deceased subjects. Of the remaining 3949 eligible persons, 649 were active non-responders. Thus, a total of 3300 participants of the original study were followed up (83.6% response). Of the 3300 participants, 13 (0.4%) did not complete the interview. Further exclusion criteria comprised cognitive impairment as defined by a Mini-Mental State Examination (MMSE) score of 23 or below (N = 116 participants), and missing ultrasound data (N = 52 participants).Thus, 3119 adults living in the community were analysed in the present study.
2.2. Psychological assessment
Cognitive functioning was assessed by means of the MMSE (Folstein, Folstein, & McHugh, 1975) before administering the other measures. MMSE scores of 23 or below were considered to indicate cognitive impairment leading to an exclusion of the participants.
The health-related interview of SHIP-I included the PTSD module of the Structured Clinical Interview for DSM-IV (SCID) (First, Spitzer, Gibbon, & Williams, 1997), frequently administered by traumatic stress professionals (Elhai, Gray, Kashdan, & Franklin, 2005), and the MMSE (Folstein et al., 1975). The PTSD interview begins by directly asking about the exposure to events defined as trauma in DSM-IV (criterion A1). If a participant negated all traumas, the module was terminated. Otherwise the interview was continued to assess the lifetime DSM-IV PTSD symptoms including fear, helplessness, or horror as initial reaction (criterion A2), five re-experiencing symptoms (criterion B), seven avoidance symptoms (criterion C), and five arousal symptoms (criterion D). If participants did not pass the required diagnostic threshold of the respective criterion, the interview was terminated. In case participants met the DSM-IV PTSD criteria A to E (duration of the symptoms for at least 1 month), they were assigned a PTSD diagnosis irrespective of distress and/or impairment (criterion F). Because there is evidence for a graded relationship between the number of lifetime traumas and chronic medical conditions in general and CVD in particular (Husarewycz et al., 2014; Scott et al., 2013; Sledjeski et al., 2008), we considered traumatic stress not only as absent vs. present, but also as dimensional variable reflected by the number of trauma types.
2.3. Medical history, cardiovascular risk factors and ultrasound measurements
Sociodemographic factors, personal medical history and cardiovascular risk factors including elevated lipids, diabetes mellitus, hypertension, and smoking were assessed by a computer-assisted personal interview. Among others, respondents were asked if they had suffered from a physician-diagnosed myocardial infarction, stroke, hypertension, and diabetes mellitus. Dyslipidemia was rated positive if participants reported physician-confirmed elevated lipid levels or a current lipid-lowering medication. Participants also underwent routine medical examination including anthropometric measurements. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in metres. Smoking status was classified as never smoking (= 0), former smoking (= 1), or current smoking (i.e. one or more cigarettes per day; = 2). Physical inactivity was defined as performing sports for less than 1 h per week. Marital status was subdivided into three categories: never married (= 0), married (= 1), and divorced, separated or widowed (= 2). Corresponding to the German school system, educational level was categorized into <10 years (extended elementary school = 0), 10–11 years (junior high school = 1), and >11 years (secondary and high school = 2).
The ultrasound protocol has been defined previously (Luedemann et al., 2002). In brief, ultrasonography was performed on non-fasted participants as part of the medical examination battery. Certified medical assistants examined the extracranial carotid arteries bilaterally with B-Mode ultrasound using a 5 MHz linear array transducer and a high-resolution instrument (Diasonics VST Gateway, Santa Clara, CA USA). The common carotid arteries, the internal carotid arteries as well as the carotid bifurcations on both sides were visualized in multiple longitudinal and transversal planes and evaluated online for the presence of atherosclerotic plaques. Atherosclerotic plaques were defined by the following criteria: focal thickening of the vessel wall relative to adjacent segments (either protrusion into the lumen or localized roughness with increased echogenicity) or a cIMT ≥ 1.3 mm. For the measurement of cIMT, scans through the axis of the distal straight portion (1 cm in length) of both common carotid arteries were digitized and recorded for subsequent offline analysis. Certified readers calculated the mean far wall cIMT by averaging 10 consecutive measurement points (in 1 mm steps) from both sides. The cIMT was defined as the distance between the characteristic echoes from the lumen-intima and media-adventitia interfaces. Intrareader, intrasonographer, interreader, and intersonographer variabilities were assessed semi-annually using scatter plots with regression lines and Bland–Altman plots. All Spearmen correlation coefficients were > .90. In Bland–Altman plots, all mean differences for repeated measurements within the same reader or sonographer were < 1% (95% limits of agreement <10%) and all mean differences for measurements between different readers or sonographers were < 5% (95% limits of agreement <15%).
2.4. Statistical analysis
For between-group comparisons, we applied analyses of variance for continuous variables and χ2-test for categorical variables. The odds of self-reported CVD endpoints in case of positive trauma exposure and/or PTSD were estimated by means of logistic regression analyses. To evaluate the association between traumatic load (assessed by the number of types of traumatic events), PTSD and subclinical atherosclerosis, several regression analyses were performed with cIMT and carotid plaque as dependent variables. Age, sex, educational level, marital status and traditional risk factors (smoking status, BMI, dyslipidemia, hypertension, diabetes, physical inactivity) were included as potential confounders. Because growing older increases the risk of being exposed to traumatic events, we also considered a potential interaction between the traumatic load and age.
3. Results
The study population comprised 1619 women (51.9%) and 1500 men (48.1%) with a mean age of 53.6 years (SD = 15.0; range: 25–86 years). Of the 3119 community residents included in the present study, 1699 subjects (54.5%) had been exposed to at least one traumatic event with a mean of 1.60 (SD = 0.96; range: 1–7). Sixty-one participants met criteria for PTSD (2.0% of the total study population, and 3.6% of those with trauma exposure). Participants were assigned to one of the following groups: no trauma exposure (no trauma; N = 1420), trauma, but no PTSD (trauma; N = 1638), and trauma with subsequent PTSD (PTSD; N = 61). As illustrated by Table 1, there were significant differences between the three subsamples with regard to relevant sociodemographic characteristics and CVD risk factors (apart from physical inactivity).
Table 1.
Sociodemographic and clinical characteristics of the study population.
| No trauma (N = 1420) |
Trauma w/o PTSD (N = 1638) |
Trauma with PTSD (N = 61) |
χ2/F | P ≤ | |
|---|---|---|---|---|---|
| Women, % | 53.7 | 49.8 | 67.2 | 10.657 | .005 |
| Age, years | 49.5 ± 13.1 | 57.1 ± 15.6 | 54.7 ± 16.5 | 105.518 | .001 |
| Marital status, % | 67.939 | .001 | |||
| Never married | 17.7 | 12.8 | 11.5 | ||
| Married | 69.9 | 65.9 | 49.2 | ||
| Separated, divorced, widowed | 12.5 | 21.3 | 39.9 | ||
| School education, % | 73.834 | .001 | |||
| <10 years | 30.8 | 44.0 | 50.8 | ||
| 10 − 11 years | 54.5 | 40.7 | 45.9 | ||
| >11 years | 14.7 | 15.3 | 3.3 | ||
| Smoking, % | 17.873 | .001 | |||
| never | 41.9 | 41.9 | 26.2 | ||
| former | 33.3 | 37.7 | 39.3 | ||
| current | 24.8 | 20.5 | 34.4 | ||
| BMI, kg/m2 | 27.5 ± 4.8 | 28.1 ± 4.9 | 28.7 ± 5.5 | 6.593 | .001 |
| Dyslipidemia, % | 20.1 | 30.8 | 29.5 | 46.298 | .001 |
| Diabetes, % | 6.6 | 13.4 | 18.0 | 41.137 | .001 |
| Hypertension, % | 44.3 | 54.1 | 63.9 | 34.143 | .001 |
| Physical inactivity, % | 63.5 | 64.8 | 75.4 | 3.838 | .147 |
| Traumatic events, N | - | 1.59 ± .95 | 2.03 ± 1.21 | 12.688 | .001 |
| MI/stroke, % | 3.2 | 9.1 | 8.2 | 45.084 | .001 |
| Plaques, % | 49.9 | 64.9 | 67.2 | 72.111 | .001 |
| cIMT, mm | .71 ± .13 | .76 ± 0.17 | .74 ± .15 | 45.100 | .001 |
BMI = body mass index; MI/stroke = self-reported physician-diagnosed myocardial infarction or stroke; PTSD = posttraumatic stress disorder; cIMT = intima-media thickness
Self-reported MI and/or stroke were significantly more frequent in participants with trauma exposure compared to those without traumatic experiences (9.1% vs. 3.2%; χ2 = 44.978, p < .001; OR = 3.05; 95% CI: 2.17–4.28). This association remained significant when accounting for non-modifiable (i.e. age, sex) and traditional risk factors (i.e. smoking status, BMI, dyslipidemia, hypertension, diabetes, physical inactivity) as well as sociodemographic variables (OR = 1.57; 95% CI: 1.07–2.30). Among participants with trauma exposure, there was no significant difference in self-reported MI and/or stroke between those with PTSD compared to those without PTSD (8.2% vs. 9.1%; see also Table 1). The age-dependency of self-reported MI and/or stroke in relation to the trauma categories (i.e. no trauma exposure; trauma, but no PTSD; trauma with PTSD) is depicted in Figure 1.
Figure 1.
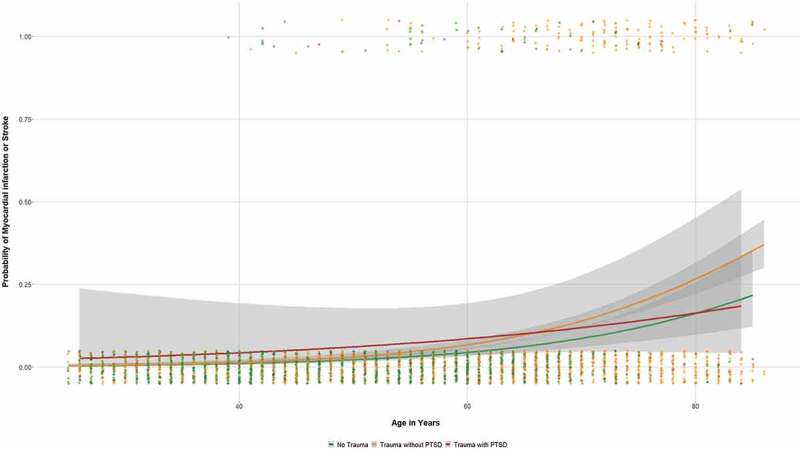
Probability of self-reported physician-diagnosed myocardial infarction or stroke as a function of age in relation to trauma categories (grey area = 95% CI).
As can be seen, the association between CVD events and trauma exposure was exclusively driven by the older participants. Thus, a more detailed look subdividing the study population into different age strata revealed that MI and/or stroke was significantly more frequent only in those with trauma exposure but without PTSD aged 70 years or older with an unadjusted OR = 2.47 (95% CI: 1.20–5.11) that was no longer significant when adjusting for the above-mentioned confounders (OR = 1.92; 95% CI: .89–4.16). Among the other age strata, those with traumatic experiences did not report CVD events more often than those without trauma exposure (cf. Table S1 in the Supplement).
Non-traumatized respondents had significantly less carotid plaques as determined by ultrasound than those with trauma exposure (49.9% vs. 65.0%; χ2 = 71.981, p < .001; OR = 1.86; 95% CI: 1.61–2.15; cf. Table 1). However, the odds of carotid plaques became insignificant when accounted for age as strongest non-modifiable risk factor (OR = 1.02; 95 CI: 0.84–1.24). This finding is also illustrated in Figure 2. Correspondingly, there were no differences in carotid plaque frequencies between non-traumatized and traumatized participants in the different age strata (cf. Table S1 in the Supplement).
Figure 2.
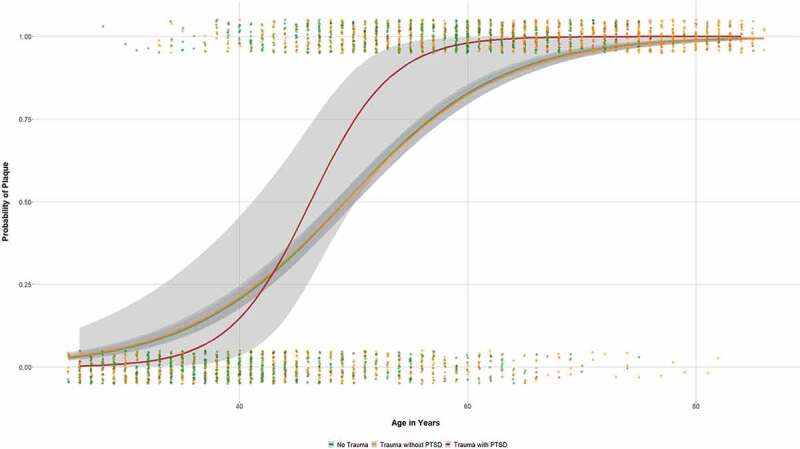
Probability of plaque occurrence as a function of age in relation to trauma categories (grey area = 95% CI).
Similarly, analysing the entire study population, cIMT was higher in those with traumatic experiences compared to participants without trauma exposure (.76 ± .17 vs. .71 ± .13; F = 89.120, p < .001), but there was no difference between traumatized respondents with and without PTSD (cf. Table 1). Again, introducing age as covariate rendered trauma insignificant (F = 2.74, p = .098; cf. Figure 3).
Figure 3.
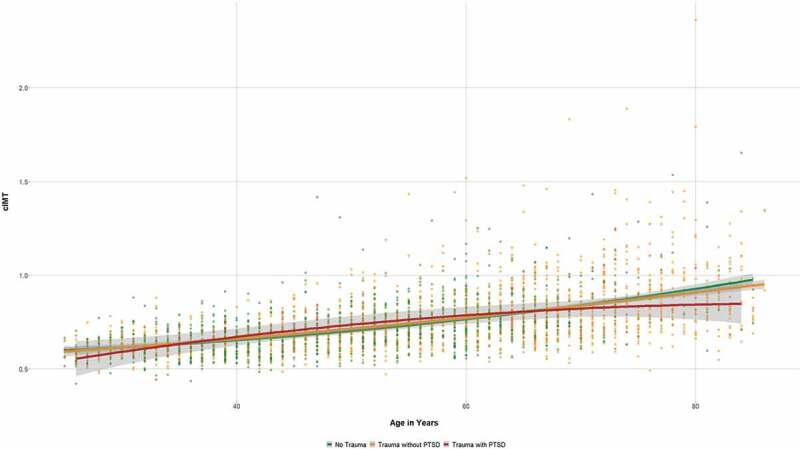
Carotid IMT (in mm) as a function of age in relation to trauma categories (grey area = 95% CI).
Interestingly, there was a significant difference in cIMT between non-traumatized participants, traumatized participants without PTSD and participants with PTSD in one age strata: Among respondents aged between 40 and 49 years, cIMT was significantly higher in those with PTSD compared to those without PTSD independent of their trauma exposure (.76 ± .20 vs. .68 ± .09; F = 8.003, p = .005; cf. Table S1 in the Supplement). However, graphical inspection as well as application of the Tukey criterion (1.5 interquartile ranges) revealed one outlier among those 11 participants with PTSD in this age stratum (cf. Figure 3). Exclusion of this outlier rendered differences in cIMT between traumatized participants without PTSD (.68 ± .09) and those with PTSD (N = 10; .70 ± .08) insignificant (F = .326, p = .569).
Re-analyses of the data after exclusion of participants with a self-reported history of MI and/or stroke (N = 199) basically revealed the same findings: Considering the entire study population, both frequency of carotid plaques and cIMT were significantly higher in those with trauma exposure than in non-traumatized individuals, while PTSD positive respondents and those with trauma exposure but no PTSD did not differ with regard to these variables. When introducing age as a covariate, the above-mentioned findings became insignificant (data not presented).
As depicted in Table 2, trauma load as assessed by number of trauma types contributed to cIMT and plaque occurrence independent of trauma and PTSD status, respectively, in unadjusted analyses. However, when accounting for age, other sociodemographic covariates and traditional risk factors, findings became insignificant.
Table 2.
Relation between traumatic stress, traumatic load (assessed by number of traumatic events), PTSD status and surrogate markers of CVD (i.e. mean cIMT and plaque occurrence).
| Mean cIMT |
Plaque |
|||
|---|---|---|---|---|
| |
Unad. |
Adjusteda |
Unad. |
Adjusteda |
| B (SE) | B (SE) | OR (95% CI) | OR (95% CI) | |
| Group | ||||
| Traumab PTSD Trauma loadc |
.053 ±.01***.032 ±.02.038 ±.00*** | −.004 ±.01 -.013 ±.02 -.006 ±.01 |
1.85 (1.60–2.13)*** 2.06 (1.19–3.54)** 1.54 (1.43–1.67)*** |
.95 (.68–1.33) 1.49 (.64–3.56) 1.31 (.75–2.19) |
| Trauma load*aged | .000 ±.00* | .000 ±.00 | 1.01 (1.01–1.01)*** | .99 (.99–1.00) |
aAll regression analyses were adjusted for age, sex, educational level, marital status, smoking, BMI, dyslipidemia, hypertension, diabetes, physical inactivity as well as the interaction between age and traumatic load
bDenotes trauma exposure in general, i.e. irrespective of PTSD status
cNumber of types of traumatic experiences
dinteractions between the number of types of traumatic events and age
* p <.05** p <.01*** p <.001
4. Discussion
Our study adds to and extends prior research on the association between traumatic stress, PTSD and atherosclerosis or CVD, respectively, in several ways. First, we found that traumatized respondents had a 50% increased odd of self-reported MI and/or stroke compared to non-traumatized participants, even when accounted for age, sex, and traditional risk factors. However, a more detailed look revealed that the link between self-reported CVD events and trauma exposure was carried by those aged 70 years or older, but was no longer significant adjusted for major risk factors. One might conclude that it is insufficient to consider age as an important covariate in epidemiological studies using populations with a wide age range (Dong et al., 2004; Goodwin & Stein, 2004; Romans, Belaise, Martin, Morris, & Raffi, 2002). Rather, including non-linear age associations and stratifying larger samples into different age groups as done in our study might account better for the strong age dependency of atherosclerosis and CVD, respectively (Hamczyk et al., 2020; Paneni et al., 2017).
Second, to the best of our knowledge, this is the first general population study on the relationship between traumatic experiences, PTSD and proxy markers of atherosclerosis, i.e. cIMT and carotid plaque. Prior studies have exclusively focussed on specific populations like police officers (Violanti et al., 2006), students surviving the Bosnian war (Vulic et al., 2019) or Vietnam veterans (Goetz et al., 2014). While cIMT was related to both combat exposure and PTSD in middle-aged twin war veterans (Goetz et al., 2014), and to traumatic experiences in first-year medical students who were preschool children during the Bosnian war (Vulic et al., 2019), it was not associated with PTSD symptoms in police officers (Violanti et al., 2006). In line, neither cIMT nor carotid plaque was associated with trauma or PTSD in our study. Thus, we are in need of future research to resolve these conflicting results.
Third, in contrast to earlier studies focussing on either traumatic events (e.g. Husarewycz et al., 2014) or PTSD (e.g. Gradus et al., 2015), thus unable to disentangle their differential impact on CVD, our investigation is among the few studies that simultaneously assess trauma exposure without PTSD and PTSD (Scherrer et al., 2019; Sumner et al., 2015; Vaccarino et al., 2013). Our findings indicate that neither trauma exposure without PTSD nor PTSD were linked to surrogate markers of atherosclerosis and CVD, respectively, in a cross-sectional design. This is in line with a recently published prospective study indicating that PTSD is not an independent risk factor for incident CVD (Scherrer et al., 2019). In contrast, a longitudinal study in women reported that both trauma exposure in general and elevated PTSD symptoms increase the risk of CVD (Sumner et al., 2015).
It has to be kept in mind that cIMT and carotid plaque represent surrogates of CVD reflecting arterial structure, but not function (Vlachopoulos et al., 2015). Considering our and others’ null results (Violanti et al., 2006), it may be more useful to assess arterial function by means of endothelial dysfunction or peripheral arterial stiffness as proxy markers of CVD. Consistently, aPWV was higher and FMD was lower in those with PTSD compared to controls (Violanti et al., 2006; Walczewska et al., 2011). So far, only one study (Violanti et al., 2006) has simultaneously examined surrogate markers of both arterial structure (cIMT) and function (FMD): While FMD was significantly lower in police officers with more PTSD symptoms, cIMT was not associated with symptom severity (Violanti et al., 2006).
Although our study holds some major strengths including the population-based design, the controlling for potential confounders, the exclusion of individuals with cognitive impairment, the assessment of traumatic load and PTSD with a psychometrically sound interview (Elhai et al., 2005) as well as the adherence to a standardized ultrasound protocol to assess widely accepted markers of atherosclerosis, some methodological limitations merit discussion. First, because our investigation was cross-sectional, the reported associations do not allow any causal inferences. Second, the lifetime prevalence of trauma exposure in our study (54.5%) may appear high for a general population sample while the lifetime prevalence of PTSD (2.0% of the total sample) was low. However, the World Mental Health (WMH) surveys indicate very similar findings for both experience of traumatic events and PTSD in other central European countries (Benjet et al., 2016; Scott et al., 2013). Nevertheless, small numbers of participants with PTSD may have compromised statistical power, especially regarding the age-stratified analyses. Thus, we may have failed to find smaller associations between PTSD and measures of atherosclerosis. Moreover, inter-rater reliability for the diagnostic interviewers was not assessed. Finally, because the PTSD interview was terminated in case the diagnostic threshold was missed, we were not able to consider a continuous PTSD symptom measure which might have increased power and would have allowed analysing associations of subthreshold PTSD conditions with cardiovascular health. Finally, the present study was part of the 5-year follow-up with a response rate of 83.6%, which might have introduced selection bias.
In closing, our null findings on the relationship between traumatic stress, PTSD and proxy markers of atherosclerosis in our general population sample contrast the large body of research indicating positive cross-sectional and longitudinal associations. These discrepancies suggest that potential associations as well as their underlying mechanisms are complex, remain inconclusive and warrant further research.
Supplementary Material
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here
Data availability statement
For data protection issues, data from ‘Study of Health in Pomerania’ (SHIP) are not open for public use, but are available upon request (https://www2.medizin.uni-greifswald.de/cm/fv/ship/daten-beantragen/).
References
- Ahmadi, N., Hajsadeghi, F., Mirshkarlo, H. B., Budoff, M., Yehuda, R., & Ebrahimi, R. (2011). Post-traumatic stress disorder, coronary atherosclerosis, and mortality. The American Journal of Cardiology, 108(1), 29–10. [DOI] [PubMed] [Google Scholar]
- Basu, A., McLaughlin, K. A., Misra, S., & Koenen, K. C. (2017). Childhood maltreatment and health impact: The examples of cardiovascular disease and type 2 diabetes mellitus in adults. The Clinical Psychologist, 24, 125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjet, C., Bromet, E., Karam, E. G., Kessler, R. C., McLaughlin, K. A., Ruscio, A. M., … Koenen, K. C. (2016). The epidemiology of traumatic event exposure worldwide: Results from the world mental health survey consortium. Psychological Medicine, 46(2), 327–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscarino, J. A. (2008). A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: Implications for surveillance and prevention. Psychosomatic Medicine, 70(6), 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter, M., Sinclair, H., & Kunadian, V. (2016). Carotid intima media thickness and its utility as a predictor of cardiovascular disease: A review of evidence. Cardiology in Review, 24(2), 70–75. [DOI] [PubMed] [Google Scholar]
- Crum-Cianflone, N. F., Bagnell, M. E., Schaller, E., Boyko, E. J., Smith, B., Maynard, C., … Smith, T. C. (2014). Impact of combat deployment and posttraumatic stress disorder on newly reported coronary heart disease among US active duty and reserve forces. Circulation, 129(18), 1813–1820. [DOI] [PubMed] [Google Scholar]
- Dong, M., Giles, W. H., Felitti, V. J., Dube, S. R., Williams, J. E., Chapman, D. P., & Anda, R. F. (2004). Insights into causal pathways for ischemic heart disease: Adverse childhood experiences study. Circulation, 110(13), 1761–1766. [DOI] [PubMed] [Google Scholar]
- Dyball, D., Evans, S., Boos, C. J., Stevelink, S. A. M., & Fear, N. T. (2019). The association between PTSD and cardiovascular disease and its risk factors in male veterans of the Iraq/Afghanistan conflicts: A systematic review. International Review of Psychiatry, 70(1), 34–48. [DOI] [PubMed] [Google Scholar]
- Edmondson, D., & von Känel, R. (2017). Post-traumatic stress disorder and cardiovascular disease. The Lancet Psychiatry, 4(4), 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhai, J. D., Gray, M. J., Kashdan, T. B., & Franklin, C. L. (2005). Which instruments are most commonly used to assess traumatic event exposure and posttraumatic effects?: A survey of traumatic stress professionals. Journal of Traumatic Stress, 18(5), 541–545. [DOI] [PubMed] [Google Scholar]
- First, M. B., Spitzer, R. L., Gibbon, M., & Williams, J. B. (1997). Structured clinical interview for DSM-IV axis i disorders. Washington, D.C.: American Psychiatric Press. [Google Scholar]
- Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Gilsanz, P., Winning, A., Koenen, K. C., Roberts, A. L., Sumner, J. A., Chen, Q., Glymour, M. M., Rimm, E. B., & Kubzansky, L. D. (2017). Posttraumatic stress disorder symptom duration and remission in relation to cardiovascular disease risk among a large cohort of women. Psychological Medicine, 47(8), 1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz, M., Shah, A., Goldberg, J., Cheema, F., Shallenberger, L., Murrah, N. V., … Vaccarino, V. (2014, November). Posttraumatic stress disorder, combat exposure, and carotid intima-media thickness in male twins. American Journal of Epidemiology, 180(10), 989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin, R. D., & Stein, M. B. (2004). Association between childhood trauma and physical disorders among adults in the USA. Psychological Medicine, 34(3), 509–520. [DOI] [PubMed] [Google Scholar]
- Gradus, J. L., Farkas, D. K., Svensson, E., Ehrenstein, V., Lash, T. L., Milstein, A., … Sørensen, H. T. (2015). Associations between stress disorders and cardiovascular disease events in the Danish population. BMJ Open, 5(12), e009334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamczyk, M. R., Nevado, R. M., Barettino, A., Fuster, V., & Andrés, V. (2020). Biological versus chronological aging: JACC Focus Seminar. Journal of the American College of Cardiology, 75(8), 919–930. [DOI] [PubMed] [Google Scholar]
- Hendrickson, C. M., Neylan, T. C., Na, B., Regan, M., Zhang, Q., & Cohen, B. E. (2013). Lifetime trauma exposure and prospective cardiovascular events and all-cause mortality: Findings from the heart and soul study. Psychosomatic Medicine, 75(9), 849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husarewycz, M. N., El-Gabalawy, R., Logsetty, S., & Sareen, J. (2014). The association between number and type of traumatic life experiences and physical conditions in a nationally representative sample. General Hospital Psychiatry, 36(1), 26–32. [DOI] [PubMed] [Google Scholar]
- John, U., Greiner, B., Hensel, E., Ludemann, J., Piek, M., Sauer, S., … Kessler, C. (2001). Study of health in pomerania (SHIP): A health examination survey in an east German region: Objectives and design. Sozial- und Prventivmedizin SPM, 46(3), 186–194. [DOI] [PubMed] [Google Scholar]
- Jordan, H. T., Stellman, S. D., Morabia, A., Miller-Archie, S. A., Alper, H., Laskaris, Z., … Cone, J. E. (2013). Cardiovascular disease hospitalizations in relation to exposure to the September 11, 2001 world trade center disaster and posttraumatic stress disorder. Journal of the American Heart Association, 2(5), e000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky, L. D., Koenen, K. C., Spiro, A., 3rd, Vokonas, P. S., & Sparrow, D. (2007). Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the normative aging study. Archives of General Psychiatry, 64(1), 109–116. [DOI] [PubMed] [Google Scholar]
- Lenane, Z., Peacock, E., Joyce, C., Frohlich, E. D., Re, R. N., Muntner, P., & Krousel-Wood, M. (2019). Association of post-traumatic stress disorder symptoms following hurricane katrina with incident cardiovascular disease events among older adults with hypertension. The American Journal of Geriatric Psychiatry, 27(3), 310–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedemann, J., Schminke, U., Berger, K., Piek, M., Willich, S. N., Doring, A., … Kessler, C. (2002). Association between behavior-dependent cardiovascular risk factors and asymptomatic carotid atherosclerosis in a general population. Stroke, 33(12), 2929–2935. [DOI] [PubMed] [Google Scholar]
- O’Toole, B. I., Catts, S. V., & Trauma, P. T. S. D. (2008). physical health: An epidemiological study of Australian Vietnam veterans. Journal of Psychosomatic Research, 64(1), 33–40. [DOI] [PubMed] [Google Scholar]
- Paneni, F., Diaz Cañestro, C., Libby, P., Lüscher, T. F., & Camici, G. G. (2017). The aging cardiovascular system: Understanding it at the cellular and clinical levels. Journal of the American College of Cardiology, 69(15), 1952–1967. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards, J. W., Mason, S., Rexrode, K., Spiegelman, D., Hibert, E., Kawachi, I., … Wright, R. J. (2012). Physical and sexual abuse in childhood as predictors of early-onset cardiovascular events in women. Circulation, 126(8), 920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romans, S., Belaise, C., Martin, J., Morris, E., & Raffi, A. (2002). Childhood abuse and later medical disorders in women. An epidemiological study. Psychotherapy and Psychosomatics, 71(3), 141–150. [DOI] [PubMed] [Google Scholar]
- Ryder, A. L., Azcarate, P. M., & Cohen, B. E. (2018). PTSD and physical health. Current Psychiatry Reports, 20(12), 116. [DOI] [PubMed] [Google Scholar]
- Scherrer, J. F., Chrusciel, T., Zeringue, A., Garfield, L. D., Hauptman, P. J., Lustman, P. J., … True, W. R. (2010). Anxiety disorders increase risk for incident myocardial infarction in depressed and non-depressed veterans administration patients. American Heart Journal, 159(5), 772–779. [DOI] [PubMed] [Google Scholar]
- Scherrer, J. F., Salas, J., Cohen, B. E., Schnurr, P. P., Schneider, F. D., Chard, K. M., … Lustman, P. J. (2019). Comorbid conditions explain the association between posttraumatic stress disorder and incident cardiovascular disease. Journal of the American Heart Association, 8(4), e011133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, K. M., Koenen, K. C., Aguilar-Gaxiola, S., Alonso, J., Angermeyer, M. C., Benjet, C., … Kessler, R. C. (2013). Associations between lifetime traumatic events and subsequent chronic physical conditions: A cross-national, cross-sectional study. PLoS One, 8(11), e80573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seng, J. S., Clark, M. K., McCarthy, A. M., & Ronis, D. L. (2006). PTSD and physical comorbidity among women receiving Medicaid: Results from service-use data. Journal of Traumatic Stress, 19(1), 45–56. [DOI] [PubMed] [Google Scholar]
- Sledjeski, E. M., Speisman, B., & Dierker, L. C. (2008). Does number of lifetime traumas explain the relationship between PTSD and chronic medical conditions? Answers from the national comorbidity survey-replication (NCS-R). Journal of Behavioral Medicine, 31(4), 341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, H., Fang, F., Arnberg, F. K., Mataix-Cols, D., Fernández de la Cruz, L., Almqvist, C., Fall, K., Lichtenstein, P., Thorgeirsson, G., & Valdimarsdóttir, U. A. (2019). Stress related disorders and risk of cardiovascular disease: Population based, sibling controlled cohort study. BMJ (Clinical ResearchEd.), 365, l1255. [DOI] [PMC free article] [PubMed]
- Spitzer, C., Barnow, S., Volzke, H., John, U., Freyberger, H. J., & Grabe, H. J. (2008). Trauma and posttraumatic stress disorder in the elderly: Findings from a German community study. The Journal of Clinical Psychiatry, 69(5), 693–700. [DOI] [PubMed] [Google Scholar]
- Spitzer, C., Barnow, S., Volzke, H., John, U., Freyberger, H. J., & Grabe, H. J. (2009). Trauma, posttraumatic stress disorder, and physical illness: Findings from the general population. Psychosomatic Medicine, 71(9), 1012–1017. [DOI] [PubMed] [Google Scholar]
- Su, S., Jimenez, M. P., Roberts, C. T. F., & Loucks, E. B. (2015). The role of adverse childhood experiences in cardiovascular disease risk: A review with emphasis on plausible mechanisms. Current Cardiology Reports, 17(10), 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia, S. F., Koenen, K. C., Boynton-Jarrett, R., Chan, P. S., Clark, C. J., Danese, A., … Zachariah, J. P. (2018). Childhood and adolescent adversity and cardiometabolic outcomes: A scientific statement from the American heart association. Circulation, 137(5), 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner, J. A., Kubzansky, L. D., Elkind, M. S. V., Roberts, A. L., Agnew-Blais, J., Chen, Q., & Koenen, K. C. (2015). Trauma exposure and posttraumatic stress disorder symptoms predict onset of cardiovascular events in women. Circulation, 132(4), 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibaut, M., Caprnda, M., Kubatka, P., Sinkovič, A., Valentova, V., Filipova, S., … Petrovic, D. (2019). Markers of atherosclerosis: Part 2 - genetic and imaging markers. Heart, Lung and Circulation, 28(5), 678–689. [DOI] [PubMed] [Google Scholar]
- Turner, J. H., Neylan, T. C., Schiller, N. B., Li, Y., & Cohen, B. E. (2013). Objective evidence of myocardial ischemia in patients with posttraumatic stress disorder. Biological Psychiatry, 74(11), 861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino, V., Goldberg, J., Rooks, C., Shah, A. J., Veledar, E., Faber, T. L., … Bremner, J. D. (2013). Post-traumatic stress disorder and incidence of coronary heart disease: A twin study. Journal of the American College of Cardiology, 62(11), 970–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violanti, J. M., Andrew, M. E., Burchfiel, C. M., Dorn, J., Hartley, T., & Miller, D. B. (2006). Posttraumatic stress symptoms and subclinical cardiovascular disease in police officers. International Journal of Stress Management, 13(4), 541–554. [Google Scholar]
- Vlachopoulos, C., Xaplanteris, P., Aboyans, V., Brodmann, M., Cífková, R., Cosentino, F., … Townsend, R. R. (2015). The role of vascular biomarkers for primary and secondary prevention. A position paper from the European society of cardiology working group on peripheral circulation: Endorsed by the association for research into arterial structure and physiology (ARTERY) society. Atherosclerosis, 241(2), 507–532. [DOI] [PubMed] [Google Scholar]
- Völzke, H. (2012). Study of health in pomerania (SHIP). Concept, design and selected results. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz, 55(6–7), 790–794. [DOI] [PubMed] [Google Scholar]
- Vulic, D., Secerov Zecevic, D., Burgic, M., Vujkovic, Z., Ristic, S., Marinkovic, J., … Wong, N. D. (2019). Post-trauma cardiovascular risk factors and subclinical atherosclerosis in young adults following the war in Bosnia and Herzegovina. European Journal of Psychotraumatology, 10(1), 1601988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczewska, J., Rutkowski, K., Wizner, B., Cwynar, M., & Grodzicki, T. (2011). Stiffness of large arteries and cardiovascular risk in patients with post-traumatic stress disorder. European Journal of Psychotraumatology, 32(6), 730–736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For data protection issues, data from ‘Study of Health in Pomerania’ (SHIP) are not open for public use, but are available upon request (https://www2.medizin.uni-greifswald.de/cm/fv/ship/daten-beantragen/).


