Abstract
Engineered cardiac patches (ECPs) hold great promise to repair ischemia-induced damages to the myocardium. Recent studies have provided robust technological advances in obtaining pure cardiac cell populations as well as various novel scaffold materials to generate engineered cardiac tissues that can significantly improve electrical and contractile functions of damaged myocardium. Given the significance in understanding the cellular and extracellular structural as well as compositional details of native human heart wall, in order to fabricate most suitable scaffold material for cardiac patches, herein, we have reviewed the structure of the human pericardium and heart wall as well as the compositional details of cardiac extracellular matrix (ECM). Moreover, several strategies to obtain cardiac-specific scaffold materials have been reviewed, including natural, synthetic and hybrid hydrogels, electrospun fibers, decellularized native tissues or whole organs, and scaffolds derived from engineered cell sheets. This review provides a comprehensive analysis of different scaffold materials for engineering cardiac tissues.
Keywords: Cardiac patch engineering, scaffold materials, cardiac tissue regeneration
1. Clinical Needs for Tissue Engineered Cardiac Patches
Despite the tremendous research and development that has been achieved during the past half century to prevent various cardiovascular complications, heart diseases are still the leading cause of death globally. Nearly half a million people suffer from acute myocardial infarction (MI) and subsequent heart failure worldwide [1, 2]. Among several heart related complications, coronary artery disease (CAD) is the major culprit, responsible for nearly 60 % of cardiac related deaths due to the occlusion of coronary arteries [3]. Current therapeutic interventions include the use of blood thinners, balloon angioplasty/stenting and bypass grafting to restore coronary revascularization. However, these interventions fail to restore ischemia-induced damages to the myocardium. Ischemic damages cause the loss of cardiomyocytes (CMs), which are subsequently replaced by fibrotic scar tissues, leading to arrythmia and subsequent heart failure [4]. Presently, the only treatment option for end-stage heart failure is the whole heart transplantation, which is far-fetched for most of the patients due to the shortage of donors. Therefore, development of efficacious therapies that can regenerate damaged myocardium and prevent end-stage heart failure are critically required. Currently, direct cell delivery and employment of engineered tissue constructs are two potential therapeutic interventions to reduced ischemia-induced myocardium damages.
Cell delivery-based therapies from phase I and phase II clinical studies have demonstrated that contractile cells (i.e. myoblasts, CMs) and non-contractile cells (i.e. smooth muscle cells(SMCs), bone merrow derived human mesenchymal stem cells (BM-hMSCs)) delivered through intramyocardial injection or intravascular infusion can attenuate further damage to the myocardium and reduce the risk of heart failure [5]. However, these cell delivery-based therapies suffer from major drawbacks such as very low cell survival and engraftment rate at the site of injury, which dramatically reduces their therapeutic effectiveness. Data obtained from several animal studies have proven that cells delivered as a part of engineered tissue construct can significantly enhance engraftment rate compared to the traditional intramyocardial injection or intravascular infusion [6]. To date, several strategies have been employed to fabricate engineered cardiac patches (ECPs). For example, Menasche P., et al., published first clinical case report in which, Human embryonic stem cell-derived cardiac progenitor cells were embedded in a fibrin scaffold to develop an ECP for a severe heart failure treatment [7]. After 3 months of implantation in a 68-year-old patient, functional outcomes including LVEF were improved without further complications including arrhythmia, tumor formation or immunosuppression related adversities [7]. Recently, a research group, led by Yoshiki Sawa, has received approval to treat patients with heart failure using hiPSC-CM sheets. Although promising, this approach can only use ~ 100 μm thin cell layers due to lack of in-built microvasculature, which can compromise graft survival after implantation [8]. These studies with promising outcome in human patients have shown the potential of ECPs in the treatment of chronic heart failure. However, several hurdles are still need to be overcome for successful translation of this technology into the clinics worldwide.
Like any other engineered tissue constructs, cardiac tissue engineering also faces two major challenges: (1) selection of an appropriate cell type, and (2) a suitable scaffold to construct the ECP. In this review, we have focused on different scaffold materials that can been employed to fabricate state-of-the-art ECPs. Advantages and limitations of these scaffold materials in promoting CM maturation have also been discussed in detail.
2. Cellular and Extracellular Organization of Heart Wall
The cardiac extracellular matrix (ECM) plays a vital role to maintain the heart functions. It provides physical support to the heart cells and also prevents over-stretching of the heart tissue, while providing freedom of movement for vigorous contractions. In addition to its extraordinary biological and mechanical properties, the cardiac ECM also supports electrical signal propagation, especially after fibrotic scar formation following MI [4]. Therefore, in order to select highly biological, biocompatible and efficient scaffold materials to fabricate ECPs, it is first important to review cellular and extracellular structural details of the native heart.
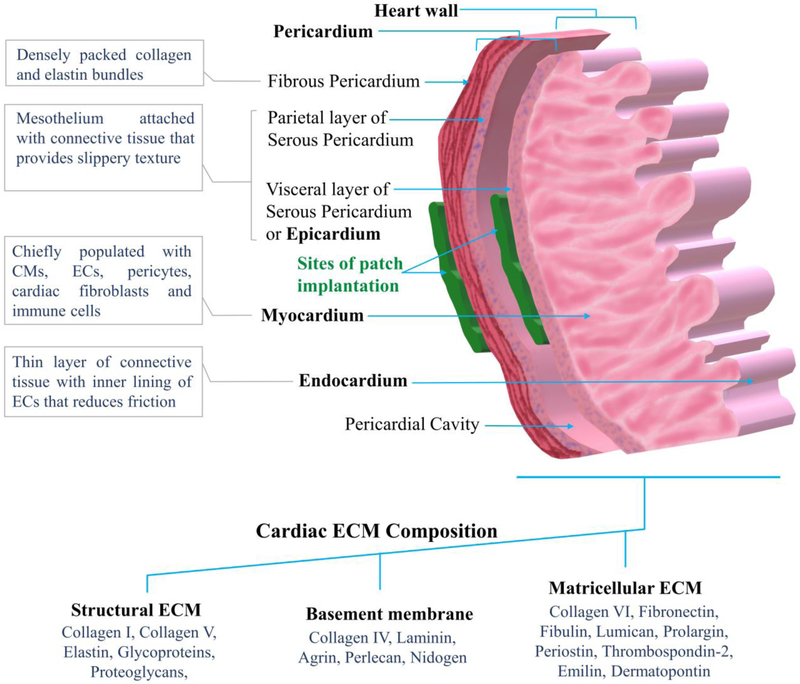
2.1. Pericardium
The heart is surrounded by pericardium, which support it’s vigorous and rapid contraction. The pericardium consists of the outer fibrous pericardium and the inner serous pericardium (Figure 1). The fibrous pericardium is the outer most layer, which is composed of densely packed collagen bundles along with abundant elastin fibers fused in between. The external surface of fibrous pericardium has ECM fibers organized in wave formation, giving rise to its distensibility and some freedom of movement. This layer also contains adipose tissue, nerves, blood vessels and rare population of monocytes and mast cells [9]. The inner serous pericardium forms a double layer. The outer layer of serous pericardium, parietal layer, is fused with the outer fibrous pericardium [10]. The inner visceral layer, also known as epicardium, forms outer heart wall and adheres tightly to the heart surface. Between this double layer is a pericardial cavity, where presents the pericardial fluid, which reduces the friction between the two layers of serous pericardium during the continuous contraction of the heart. The parietal layer contains mesothelial cells, which are microvilli epithelial cells that form and re-absorb pericardial fluid [9, 10]. The pericardium resembles like a bag that prevents over stretching of the heart and attaches it to the mediastinum.
Figure 1.
Cellular and extracellular matrix (ECM) composition of the pericardium and heart wall.
2.2. The heart wall
The heart wall contains three layers: the outer most epicardium, a middle layer called myocardium and innermost endocardium (Figure 1). The epicardium, also known as visceral layer of serous pericardium, is a thin transparent layer consisting of mesothelium and a connective tissue that provides smooth and slippery texture to the heart wall. The myocardium contains chiefly CMs along with a smaller population of non-myocyte resident cells including endothelial cells (ECs), pericytes, fibroblasts, and resident immune cells (mainly macrophage, B-cells and T-cells) [11]. The CMs account for almost 30% of total cells of the human heart [12]. ECs have largest (60%) and fibroblasts have second largest (15%) population in the heart among non-myocyte cells [13]. The myocardium is responsible for the heart’s contraction by propagation of action potential. The innermost endocardium contains a thin layer of connective tissue and an endothelial cell lining that forms a smooth inner layer for the heart chambers, major blood vessels and valves, in order to minimize the surface friction generated by the blood flow [11].
2.3. Native cardiac extracellular matrix
The fibrillar collagen I and collagen V are the main structural components of the heart that forms around 70 % of the adult human cardiac ECM [14]. The basement membranes, which comprise about 20 % of the cardiac ECM, are mainly composed of collagen IV along with smaller amounts of agrin laminin, perlecan and nidogen. The structural ECM that comprises 4% of cardiac ECM, is composed of fibrous glycoproteins and proteoglycans that also maintain the propagation of action potentials generated by pacemaker cells. In addition, lumican, prolargin, periostin, thrombospondin-2, fibronectin, emilin, dermatopontin, fibulin and collagen VI form the matricellular ECM that comprises 3% of the cardiac ECM [15]. In a recent pre-clinical study, human pericardial ECM and porcine cardiac ECM were decellularized and compared for their structural and mechanical properties. The results showed 14 distinct components that are specifically found in human cardiac ECM [16].
Following MI, the myocardial ECM undergoes extensive remodeling to form a rigid scar tissue. The functional mechanism, by which cardiac ECM regulates myocardial regeneration, is largely unknown to date. However, Basset et al., have shown that a component of cardiac ECM, known as agrin, regulates endogenous cardiac regeneration [17]. Agrin, a heparan sulfate proteoglycan, can successfully induce proliferation of human or mice induced pluripotent stem cell (iPSC) derived CMs (iPSC-CMs) through yes-associated protein (Yap) and extracellular signal - regulated kinase (ERK) mediated signaling [17]. These cellular and ECM components of native heart tissue can be mimicked to fabricate highly biological ECPs.
3. Scaffold Materials for Cardiac patch Engineering
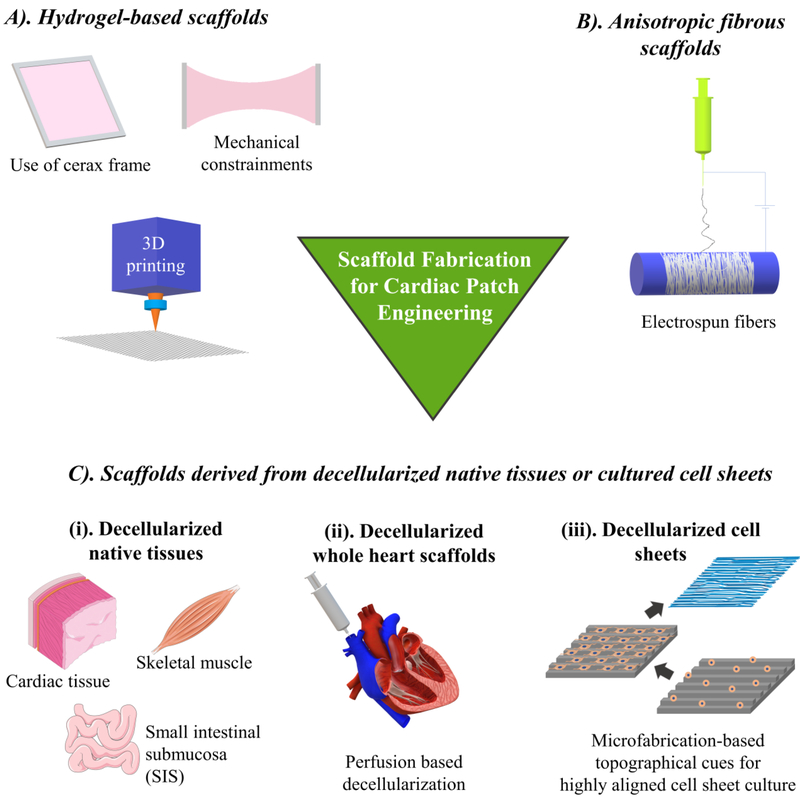
Multiple factors must be considered when selecting materials for cardiac patch fabrication. The heart tissue, by its very nature, is incredibly complex, requiring large amounts of oxygen and nutrients, along with specialized cells (CMs), to ensure the uninterrupted functioning of the heart throughout the lifetime of an individual. To guarantee the proper development of a cardiac patch, it is absolutely essential to meet the following criteria: (1) establishment of an environment that resembles native cardiac ECM, (2) appropriate electromechanical coupling within the cardiac patch, (3) vigorous and stable contractility of the cardiac patch, and (4) inclusion of functional vasculatures [18]. Although not a universal method has been created to engineer a cardiac patch, several approaches have been applied in their fabrication, including application of hydrogels, electrospun fibers, decellularized native tissue or engineered cell layers derived ECM, and the decellularized “whole heart tissue scaffold” (Figure 2). The following sections review the aforementioned scaffold fabrication strategies and discuss their appropriateness for the use in cardiac tissue engineering.
Figure 2.
Scaffold fabrication strategies for cardiac patch engineering.
3.1. Hydrogels
Hydrogels are characterized by vast hydrophilic networks of polymer chains, often adopting a three-dimensional (3D) conformation due to the cross-linking within the polymer chain network. As hydrogels are capable of mimicking compositional characteristics of natural ECM to some extent, and can be engineered to control various processes such as cell attachment and molecular responses, therefore they are used extensively in tissue engineering applications [19].
Several materials have been used in the fabrication of hydrogel scaffolds for cardiac patch engineering, including fibrin, collagen, gelatin, chitosan, Matrigel, and their different combinations. Among these, fibrin is the most attractive candidate so far due to its biocompatibility, ease of processing, environmental adaptability and appropriate rate of biodegradation. Polymerized fibrin bears resemblance to natural ECM due to its resulting self-assembled nanometric fibrous structure [20]. Other naturally derived materials, such as collagen, hyaluronic acid, gelatin and chitosan, have been incorporated to form hybrid hydrogel scaffolds to fabricate cardiac patches. Major research groups in the field of cardiac tissue engineering uses the combination of fibrin and Matrigel, which can be polymerized around a cerax frame to form biodegradable hydrogels in desired sizes and shapes [21–23]. Markedly, these hybrid hydrogels can be seeded with CMs to fabricate ECPs with large sizes up to 36 × 36 mm, which is applicable to human hearts [24]. Furthermore, these different sized fibrin-Matrigel based ECPs displayed electrical signal propagation and required freedom of movement during the strong mechanical contraction with twitch force greater than 20 mN [22, 24]. The fibrin-based hybrid scaffolds usually degrade within two weeks of implantation and can be gradually replaced by cardiac cell secreted ECM [25]. Besides Matrigel, fibrin has also been mixed with other proteins to fabricate hybrid hydrogels with desired bio-chemical and bio-mechanical properties. A hybrid fibrin-collagen hydrogel exhibited similar stiffness (0.50 ± 0.22 to 3.73 ± 0.91 kPa) to that of the native myocardium [26]. Moreover, Greater fibrin concentrations within the hydrogel and larger CM cell seeding densities led to more vigorous compaction and beating of the engineered cardiac tissue.
Since CMs containing ECPs are electro-mechanically active, it is important to carefully design hydrogels that have enough tensile strength to support continuous contraction of the patch. One of the strategies to increase hydrogel’s mechanical strength is using interpenetrating network (IPN) hydrogels. In one such study, Zhang et al. [27] developed a hybrid hyaluronic acid-fibrin IPN hydrogel, which had a slower degradation rate than that of the hydrogel purely composed of fibrin. The enhanced fibroblast adhesion and migration was observed by Gsib et al. [28], who also used the mechanically-reinforced IPN hydrogels composed of fibrin, polyethylene oxide, and serum albumin.
Electrical conductivity is another essential property that need to be possessed by the hydrogels to enhance the propagation of electrical signals as well as the synchronicity of beating between the engrafted CMs and the native myocardium. Incorporation of conductive inorganic particles into the hydrogel can make it electrically conductive. In one such study, gold nanoparticles (GNP) were incorporated into chitosan to develop a conductive hydrogel [29]. The electrical conductivity of this hydrogel can be controlled by regulating the amount of GNPs. This GNP containing chitosan scaffold supported the survival, proliferation and migration of BM-hMSCs, providing a promising platform to engineer electrically active cardiac tissues in vitro [29]. In another study, reduced graphene oxide (rGO) was incorporated in a gelatin methacryloyl (GelMA) hydrogel to make it electrically conductive. This rGO containing GelMA significantly improved contraction speed, viability and proliferation of cultured CMs compared to the pure GelMA hydrogel [30].
Besides electrical conductivity, the hydrogels also allows the in-built self-assembled microvascular network formation, which increases the patch survival and engraftment after transplantation. To obtain aligned in-built microvessels in the hydrogel, Schaefer et al., developed a mechanically constrained bilayer fibrin patch containing human blood outgrowth ECs and pericytes in a co-culture. Upon implantation into a nude rat model, this cardiac patch showed rapid engraftment along with the successful inosculation of microvessels with the host vasculature [23]. In a recent study, Garcia et al., developed a device to deliver polyethylene glycol (PEG) hydrogel in a pericardial space using a minimally invasive strategy [31]. This device can create a temporary space between the pericardium and epicardium for the release and gelation of PEG to reduce the risk of embolization, thrombotic occlusion and arrhythmia [31]. Using this approach, hydrogels incorporating various cells, pharmaceutical or therapeutic compounds and nucleic acids can minimal invasively be delivered to the epicardial surface.
Although easy to fabricate and deliver, hydrogels lack biological complexity of the native cardiac ECM, which is composed of myriads of proteins, glycoproteins, proteoglycans, growth factors and other small molecules that are crucial for regulating cell − ECM crosstalk. Also, it is difficult to obtain cardiac specific anisotropic native tissue architecture in hydrogels. Although mechanical stretching can induce cellular alignment to some extent, it is not an efficient method to ensure anisotropy after implantation. The recent emerging 3D bioprinting technology has made it possible to print hydrogels in an anisotropic orientation [32]. However, the printing resolution is far lower than the nano-micron size of native ECM fibers.
3.2. Anisotropic fibrous scaffolds
The native myocardium has highly aligned cellular and ECM organization, which contributes to its strong mechanical properties [33]. Compared with hydrogels, anisotropic fibrous scaffolds can better mimic the nano-micron scale fibrous topography of myocardium. From past few years, advances in electrospinning and microfabrication technology has enabled the fabrication of nano-micron scale fibrous scaffolds that can incorporate CMs to engineer a cardiac patch.
Electrospinning is a highly versatile technique that allows for the design of nanofibers with a variety of different properties that can be tailored to meet the needs of intended applications. In tissue engineering, electrospinning allows for the formation of non-woven fibrous mats that mimic the structure of natural ECM, down to the nanoscale [34]. Several studies have shown promising results from electrospun nanofibrous scaffolds for cardiac patch engineering by using a variety of different materials, such as a poly(l-lactic acid) (PLA)/polyaniline (PANI) blend [35], PLA and chitosan [36], gelatin [37], and silk fibroin/poly(ester-urethane) urea [38]. The performance of these synthetic or biopolymeric materials can be further enhanced by decoration with natural ECM. In one such study, NIH3T3 fibroblasts were cultured on the aligned electrospun poly (L-lactide-cocaprolactone) (PLCL) scaffold and subsequently decellularized to obtain a PLCL scaffold blended with fibroblast-derived ECM (PLCL/FDM) [39]. Compared to the control fibronectin coated PLCL fibers, the PLCL/FDM scaffold promoted the differentiation of H9c2 cardiomyoblast towards CM-like cells and increased the genotypic expression of mature CMs in seeded neonatal rat CMs [39].
The electrical conductivity can be endowed to electrospun fibers simply by including electroconductive inorganic materials in the electrospun fibers or by electrospinning electroconductive polymers. The conductive carbon nanotubes (CNTs) have been incorporated into an aligned poly (glycerol sebacate)/gelatin (PG) electrospun scaffold [40]. The CNTs enhanced not only the electrical conductivity but also the mechanical strength of the scaffold, while maintaining CMs’ survival, alignment, and contractile function. The CMs cultured on CNT incorporated PG scaffold displayed stronger and synchronous beating compared to those cultured on pure PG scaffold [40]. In another study, an electronic, elastic and biodegradable scaffold composed of electrospun albumin and gold electrodes was seeded with CMs to fabricate a cardiac patch. Upon in vivo implantation in rat model, this scaffold successfully degraded within a period of 3 weeks [41]. Arumugam et al. [42] electrospun a conductive scaffold using a piezoelectric polymer β−polyvinylidene fluoride (PVDF). In comparison to a control non-piezoelectric scaffold, the β-PVDF scaffold offered more significant CM differentiation and adhesion in addition to stronger mechanical stability. In another similar approach, microfibrous biodegradable poly(ester carbonate urethane)urea (PECUU) scaffold was enriched with native porcine cardiac ECM and epicardially implanted in rat after two weeks following left coronary ligation [43]. Their results indicated that the incorporation of native ECM in PECUU scaffold mitigated left ventricle wall thinning, scar formation and promoted angiogenesis [43].
Although electrospun fibrous scaffolds can mimic the anisotropy and nano-scale fibrous structure of the native cardiac ECM, similar to hydrogels, they lack the biochemical complexity of the cardiac ECM, which is a blend of various biomacromolecules. To obtain this complex and cardiac specific ECM, decellularization methods have been developed to generate highly biomimetic ECM scaffolds from native tissues or cell layers.
3.3. Scaffolds derived from decellularized native tissues or cultured cell layers
From last four decades, it has been clearly known that native ECM is not an inert scaffold that only provides mechanical support for the cells. Rather, it is involved in essential signaling pathways that regulate key cellular functions [44, 45]. Among myriads of transmembrane proteins, integrin family of proteins are the key mediators that regulate cell-ECM interaction and downstream signaling pathways. Integrins act as interphase that sense the biophysical cues from the extracellular space and transfer this information into cytosol of CMs using adapter protein complexes such as integrin linked kinase (ILK), focal adhesion kinase (FAK), non-catalytic tyrosine kinase adaptor protein (Nck)-2 and particularly interesting new cysteine-histidine rich protein (PINCH) [46]. These protein complexes regulate receptor tyrosine kinase signaling, which is essential for the regulation of CM shape, polarity, adhesion, survival, proliferation, migration and differentiation. Disruption in this signaling can negatively influence functional properties of CMs and result in hypertrophy [47]. Integrin regulated CM adhesion is also influenced by membrane-associated proteins such as the a desintegrin and metalloprotease (ADAMs). ADAMs regulate expression and function of other transmembrane family of proteins especially growth factor receptors, which further regulate the cardiac hypertrophy [48, 49]. To provide this essential cell-ECM interaction in engineered cardiac patches, ECM scaffolds derived from decellularized native tissue have been recognized as ideal candidates, which can be further sterilized and re-seeded with cardiac cells to develop functional cardiac tissues.
Different strategies have been developed for decellularization of tissues including enzymatic, chemical and mechanical degradation, which have been recently reviewed by Taylor et al. [50]. The widely used reagents in most of the cardiac tissue decellularization studies include sodium dodecyl sulfate (SDS) [51], ethanol with supercritical carbon dioxide [52], TritonX [53] and nucleases. Decellularized tissues are commonly characterized based on the residual cellular and nuclear content, ECM composition and its organization, presence of macromolecules and matrix-bound growth factors, mechanical strength as well as maintenance of vascular tree [50].
3.3.1. Decellularized native tissues
A traditional tissue engineering approach has been implemented to fabricate ECPs by seeding cells in a native ECM scaffold decellularized from tissues such as heart, skeletal muscle and small intestinal submucosa (SIS) [54, 55]. Among these studies murine heart tissues have been used in several studies to fabricate ECPs [56, 57]. In one such study, human iPSC (hiPSC) derived CMs (hiPSC-CMs) and fibroblasts were seeded in ECM scaffolds decellularized from rat hearts [56]. This native cardiac ECM significantly improved hiPSC-CMs’ maturation by increasing their electrophysiological activity compared to hiPSC-CM aggregates. Upon implantation on the infarcted area, the cardiac patch reduced infarct size and improved the percentage of ejection fraction and fractional shortening. Additionally, the hiPSC-CMs responded well to drugs that influence CMs’ activity [56], enabling this platform suitable for the pharmaceutical drug screening. Besides murine ECM scaffolds, ECM decellularized from porcine hearts and SIS are also being studied [55, 58]. The porcine myocardial scaffold seeded with both bone marrow mononuclear cells [59] and adipose derived stem cells [60] exhibited angiogenic potential at the implantation site by increasing the vasculature formation. Cardiac tissues from human donors have also been decellularized and recellularized to develop ECPs. In one of the studies, a 0.3 mm-thick cardiac tissue was isolated from patients with end-stage-non-ischemic dilated cardiomyopathy and was decellularized to obtain a human cardiac ECM [61]. Murine ESCs, iPSCs and mesenchymal stromal cells were grown on this cardiac ECM demonstrated that the cardiac specific ECM significantly enhanced the differentiation of ESCs and iPSCs (but not mesenchymal stromal cells) toward CMs phenotype compared to those grown on Matrigel and Geltrex [61]. In another study, human ESCs were modified to express transcription activator like effector nucleases to efficiently generate CM like cells [62]. When these cells were reseeded into the decellularized human ventricles, they showed robust electrophysiological and contractile function [62]. In a more recent study, donated human heart tissues were decellularized using SDS and Triton-X and recellularized with non-transgenic hiPSC-CMs [63]. After 120 days of culture, these cardiac tissue constructs presented typical sarcomeric structure, electrical conduction and contractile force generation. In addition, the whole heart scaffolds were also partially recellularized by iPSC-CMs. Along with electrical propagation, these engineered hearts performed metabolic function as well as left ventricular pressure development [63]. These studies have significantly advanced the technology that can rapidly generate human cardiac grafts for next generation therapeutic intervention as well as disease modelling.
3.3.2. Decellularized whole heart scaffolds
From the past few years, efforts have been made to engineer an entire heart by repopulating cardiac cells into the decellularized whole heart. Perfusion based decellularization of the whole heart was first demonstrated by Ott et al. in 2008, which can efficiently maintain the native tissue structure, vasculature and ECM organization [64]. Yasui et al., repopulated the decellularized female Wistar rat heart with neonatal rat cardiac cells (mixed population of CMs, ECs and SMCs) to observe excitation − propagation during CM beating [65]. Although the repopulated heart showed stable excitation − propagation, the direction of propagation was disorganized, indicating the symptom of arrythmia. Additionally, the repopulated CMs showed reduced expression of a gap junction protein, connexin43, which resulted in this arrhythmogenic propensity [65]. Robertson et al., for the first time, showed that it was possible to recellularize the whole decellularized rat heart including arterial and venous beds as well as cavities, through inferior vena cava and brachiocephalic artery [66]. In a more recent study, “humanized rat hearts” were created by seeding human ESC-derived cardiac progenitor cells (CPCs) into the decellularized rat hearts [67]. The bovine fibroblast growth factor (bFGF) was immobilized in the decellularized rat hearts, which enhanced cellular migration, proliferation and differentiation. In addition, the bFGF improved the differentiation of CPCs into CMs, ECs and SMCs. These humanized hearts displayed aligned myofilaments along with the synchronous contraction after 12 days of perfusion [67]. Although these studies offer an efficient template to engineer contracting human hears, several translational challenges need to be addressed, including obtaining heart scaffold materials with minimum batch-to batch variations, large scale cardiac cell population, improvisation in cell repopulation and enhancing functional properties without severe arrhythmogenic propensity. Currently, the more viable option to repair MI induced damaged myocardium is the implantation of ECP derived from decellularized native tissues or cell layers.
3.3.3. Decellularized cell sheets
Although decellularized native tissue provides highly bio-mimetic scaffolds with structural and compositional details closer to native ECM, several roadblocks remain in this approach, including scarcity of donors, huge batch-to batch variations, pathogen transfer, and undesirable immune responses toward allogenic or xenogeneic ECM [68]. The application of decellularized specie-specific and tissue-specific cell layers, grown from pathogen-free cells, can potentially avoid these issues while serving as a more bio-mimetic microenvironment. Cardiac fibroblasts (CF) are one of the suitable cell sources for this approach. Schmuck et al., characterized the compositions of a decellularized rat CF sheet, which contained 82% fibronectin, 13% collagen type I, 3.4% collagen type III, 0.2 % collagen type V and 1.3% elastin, along with 18 non -structural bioactive molecules [69]. Due to the high fibronectin content, these scaffolds are easy to attach to native epicardium without glue or sutures. ECM derived from mouse fibroblasts (NIH3T3) cell sheet was analyzed for its potential to promote the differentiation of H9c2 cardiomyoblasts into CMs [70]. The H9c2 cells cultured on this ECM for 7 days under differentiation medium showed significantly higher expression of CM specific markers such as alpha actinin, cardiac troponin, myosin light chain 2 (Myl2) and troponin T (Tnnt), compared to gelatin and fibronectin hydrogels. Moreover, cross-linking of this fibroblast derived ECM with genipin increased its stiffness by 100 times (up to 8.5 kPa), which displayed a significant impact on myoblast differentiation into CMs compared to non-crosslinked ECM [70]. In order to understand the influence of ECM remodeling during heart failure and its crosstalk with cardiac cells, Pagano et al., obtained decellularized ECM from CFs isolated from right atrial appendage of healthy or diseased human hearts [71]. Human resident cardiac progenitor cells cultured on normal cardiac ECM secreted significantly higher levels of osteopontin, fibroblast growth factor (FGF)-6, FGF7, 3’-nucleotidase (NT-3), insulin like growth factor binding protein- 4 (IGFBP4) and tissue inhibitor of metalloproteinases (TIMP)-2, compared to the pathological ECM, indicating that cells cultured on normal ECM had an overall reduced anti-remodeling paracrine secretion profile [71]. These findings provide novel insights into cellular responses toward pathological remodeling of the human cardiac ECM. Besides structural and functional maturation of cardiac cells in an engineered patch, it is also crucial to develop in-built vasculature that can support graft survival after implantation. Several pre-vascularization strategies have been developed to date to form functional channels within 3D tissues, which can get anastomosed with host after implantation [72, 73]. Our group has used decellularized human dermal fibroblast (hDF) derived ECM to form highly dense and well-oriented microvascular network by co-culturing of hMSCs and ECs [74–76]. To obtained highly aligned ECM scaffolds, cells were cultured on PDMS substrates with nano-micron scale aligned grooves that allowed fabrication of highly aligned cell sheets [77, 78]. In a most recent study, we have developed an anisotropic, completely biological, and prevascularized cardiac patch with microvascular features representative of native myocardium by co-culturing hMSC and ECs on a highly aligned hDF-ECM [79].
4. Conclusion Remarks and Future Outlook
In the past decade, several strategies have been applied to promote CMs maturation in the cardiac patch including provision of electrical [80, 81] and mechanical stimulus [82]. Whereas, in recent studies, ECM has emerged as an important cell signaling regulator that can promote proliferation [17] as well as electrophysiological and structural maturation of CMs [83]. Meanwhile, several other recent studies have shown that ECPs with mixed population of cells (CMs, SMCs and ECs) enhanced outcomes, especially longer cell survival after transplantation, compared to ECPs with only CMs [24, 84, 85].
Despite improvising advances in tissue biofabrication and massive production of CMs, significant hurdles remain in ECP development, such as the electrical function and contractile force generation to the adult heart level. Presently, researchers in the field are aiming to develop ECPs that can generate a contractile force in the range of 2–4 mN/mm2, along with an electrical conductive velocity higher than 25 cm/s [2]. Besides attaining these functional properties, it is critical to achieve a functional integration between native myocardium and implanted ECP. Recently, two studies have demonstrated the functional electrical synchronization between intracardially injected human embryonic stem cells (hESC) derived CMs (hESC-CMs) and the native heart in guinea pigs [86] and pigtail macaques [87]. In these studies, hESC-CM were genetically modified to express fluorescent calcium indicator, in order to visualize calcium ion flux generated by hESC-CMs exclusively after engraftment. Their results indicated that calcium transients generated by engrafted cells were synchronized with electrocardiogram (ECG) of the hosts’ hearts, which gave researchers a hope that CMs engrafted in a ECP could not only survive, but also get electrically synchronized with native heart. In another study, Jackman et al., implanted fluorescent calcium indicator-expressing neonatal rat ventricular cells containing ECP on the epicardial surface of adult rat ventricle to study electrical synchronization between the patch and native heart [21]. However, their results ECP failed to couple with the native heart, possibly due to the scar tissue formation, which acted as an insulating barrier between the patch and heart [21]. To date, obtaining functional integration between ECPs and the host remains a critical hurdle. It is possible that appropriate cardiac specific ECM can lead to achieve this synchronization by restoring the complex crosstalk between the ECM, CMs as well as non-CM resident cells. The other critical issue is the survival of the implanted ECP. Recently, Yoshiki Sawa’s group, has received approval to treat patients with heart failure using hiPSC-CM sheets [8]. However, these cell layers lack in-built vasculatures. Therefore, they could only use ~ 100 μm thin cell layers for implantation to reduce the risk of graft survival.
To date, several prevascularization strategies have been developed to generate tissue-specific highly organized microvascular networks in tissue scaffolds to ensure graft survival [88]. Some of these studies have incorporated ECs and/or pericytes into ECPs, in order to promote in-built vasculature that can promote anastomosis with host upon implantation [89, 90]. Recently, we have developed a completely biological ECP patch with microvascular size features as well as interpapillary distance representative of native myocardium [79]. We anticipate that the tissue prevascularization and its successful anastomosis with host vessel upon implantation will significantly increase the graft survival and promote its functional integration with native myocardium. After achieving beneficial effects in small and large animal models, slowly but steadily, cardiac-specific ECM scaffold materials are impending their way toward human clinical trials [7, 91]. These next generation engineered cardiac tissues will undoubtably make their place in clinics in the near future.
Acknowledgements
This study was supported by the National Institutes of Health (1R15CA202656 and 1R15HL145654–01) and the National Science Foundation (1703570) to FZ. It was also supported by NIH 1U01HL134764–01 to TJK.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest Statement
The authors have declared that no conflict of interest exists.
References:
- 1.Benjamin EJ, et al. , Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation, 2018. 137(12): p. e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, et al. , Can We Engineer a Human Cardiac Patch for Therapy? Circ Res, 2018. 123(2): p. 244–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin Emelia J, et al. , Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation, 2019. 139(10): p. e56–e528. [DOI] [PubMed] [Google Scholar]
- 4.Yue L, Xie J, and Nattel S, Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res, 2011. 89(4): p. 744–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tompkins BA, et al. , What Is the Future of Cell-Based Therapy for Acute Myocardial Infarction. Circulation research, 2017. 120(2): p. 252–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackman CP, et al. , Human Cardiac Tissue Engineering: From Pluripotent Stem Cells to Heart Repair. Current opinion in chemical engineering, 2015. 7: p. 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menasche P, et al. , Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J, 2015. 36(30): p. 2011–7. [DOI] [PubMed] [Google Scholar]
- 8.Cyranoski D, Reprogrammed’ stem cells approved to mend human hearts for the first time. . Nature, 2018. 557: p. 619–620 [DOI] [PubMed] [Google Scholar]
- 9.Ishihara T, et al. , Histologic and ultrastructural features of normal human parietal pericardium. Am J Cardiol, 1980. 46(5): p. 744–53. [DOI] [PubMed] [Google Scholar]
- 10.Braga-Vilela AS, et al. , Extracellular matrix of porcine pericardium: biochemistry and collagen architecture. J Membr Biol, 2008. 221(1): p. 15–25. [DOI] [PubMed] [Google Scholar]
- 11.Gray GA, et al. , Resident cells of the myocardium: more than spectators in cardiac injury, repair and regeneration. Current Opinion in Physiology, 2018. 1: p. 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, et al. , Necrotic Myocardial Cells Release Damage‐Associated Molecular Patterns That Provoke Fibroblast Activation In Vitro and Trigger Myocardial Inflammation and Fibrosis In Vivo. 2015. 4(6): p. e001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinto Alexander R, et al. , Revisiting Cardiac Cellular Composition. Circulation Research, 2016. 118(3): p. 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson TD, et al. , Quantification of decellularized human myocardial matrix: A comparison of six patients. Proteomics Clin Appl, 2016. 10(1): p. 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bejleri D and Davis ME, Decellularized Extracellular Matrix Materials for Cardiac Repair and Regeneration. 2019. 8(5): p. 1801217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perea-Gil I, et al. , Head-to-head comparison of two engineered cardiac grafts for myocardial repair: From scaffold characterization to pre-clinical testing. Sci Rep, 2018. 8(1): p. 6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassat E, et al. , The extracellular matrix protein agrin promotes heart regeneration in mice. Nature, 2017. 547: p. 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vunjak-Novakovic G, et al. , Challenges in Cardiac Tissue Engineering. Tissue Engineering Part B: Reviews, 2010. 16(2): p. 169–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naahidi S, et al. , Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnology Advances, 2017. 35(5): p. 530–544. [DOI] [PubMed] [Google Scholar]
- 20.Roura S, Gálvez-Montón C, and Bayes-Genis A, Fibrin, the preferred scaffold for cell transplantation after myocardial infarction? An old molecule with a new life. Journal of Tissue Engineering and Regenerative Medicine, 2017. 11(8): p. 2304–2313. [DOI] [PubMed] [Google Scholar]
- 21.Jackman CP, et al. , Engineered cardiac tissue patch maintains structural and electrical properties after epicardial implantation. Biomaterials, 2018. 159: p. 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang D, et al. , Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials, 2013. 34(23): p. 5813–5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaefer JA, et al. , A cardiac patch from aligned microvessel and cardiomyocyte patches. 2018. 12(2): p. 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shadrin IY, et al. , Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nature communications, 2017. 8(1): p. 1825–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wendel JS, et al. , Functional consequences of a tissue-engineered myocardial patch for cardiac repair in a rat infarct model. Tissue Eng Part A, 2014. 20(7–8): p. 1325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaiser NJ, et al. , Optimizing Blended Collagen-Fibrin Hydrogels for Cardiac Tissue Engineering with Human iPSC-derived Cardiomyocytes. ACS Biomaterials Science & Engineering, 2019. 5(2): p. 887–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, et al. , Hyaluronic acid-fibrin interpenetrating double network hydrogel prepared in situ by orthogonal disulfide cross-linking reaction for biomedical applications. Acta Biomaterialia, 2016. 38: p. 23–32. [DOI] [PubMed] [Google Scholar]
- 28.Gsib O, et al. , Evaluation of Fibrin-Based Interpenetrating Polymer Networks as Potential Biomaterials for Tissue Engineering. Nanomaterials, 2017. 7(12): p. 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baei P, et al. , Electrically conductive gold nanoparticle-chitosan thermosensitive hydrogels for cardiac tissue engineering. Materials Science and Engineering: C, 2016. 63: p. 131–141. [DOI] [PubMed] [Google Scholar]
- 30.Shin SR, et al. , Reduced Graphene Oxide-GelMA Hybrid Hydrogels as Scaffolds for Cardiac Tissue Engineering. Small, 2016. 12(27): p. 3677–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia JR, et al. , A Minimally Invasive, Translational Method to Deliver Hydrogels to the Heart Through the Pericardial Space. JACC: Basic to Translational Science, 2017. 2(5): p. 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao L, et al. , Myocardial Tissue Engineering With Cells Derived From Human-Induced Pluripotent Stem Cells and a Native-Like, High-Resolution, 3-Dimensionally Printed Scaffold. Circ Res, 2017. 120(8): p. 1318–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaneko N, et al. , Three-dimensional reconstruction of the human capillary network and the intramyocardial micronecrosis. Am J Physiol Heart Circ Physiol, 2011. 300(3): p. H754–61. [DOI] [PubMed] [Google Scholar]
- 34.Sill TJ and von Recum HA, Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials, 2008. 29(13): p. 1989–2006. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, et al. , Electrospun conductive nanofibrous scaffolds for engineering cardiac tissue and 3D bioactuators. Acta Biomaterialia, 2017. 59: p. 68–81. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Wang S, and Zhang R, Composite poly(lactic acid)/chitosan nanofibrous scaffolds for cardiac tissue engineering. International Journal of Biological Macromolecules, 2017. 103: p. 1130–1137. [DOI] [PubMed] [Google Scholar]
- 37.Elamparithi A, et al. , Gelatin electrospun nanofibrous matrices for cardiac tissue engineering applications. International Journal of Polymeric Materials and Polymeric Biomaterials, 2017. 66(1): p. 20–27. [Google Scholar]
- 38.Du J, et al. , Potential applications of three-dimensional structure of silk fibroin/poly(ester-urethane) urea nanofibrous scaffold in heart valve tissue engineering. Applied Surface Science, 2018. 447: p. 269–278. [Google Scholar]
- 39.Suhaeri M, et al. , Novel Platform of Cardiomyocyte Culture and Coculture via Fibroblast-Derived Matrix-Coupled Aligned Electrospun Nanofiber. ACS Applied Materials & Interfaces, 2017. 9(1): p. 224–235. [DOI] [PubMed] [Google Scholar]
- 40.Kharaziha M, et al. , Tough and flexible CNT-polymeric hybrid scaffolds for engineering cardiac constructs. Biomaterials, 2014. 35(26): p. 7346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feiner R, et al. , Multifunctional degradable electronic scaffolds for cardiac tissue engineering. Journal of Controlled Release, 2018. 281: p. 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arumugam R, et al. , β-PVDF based electrospun nanofibers – A promising material for developing cardiac patches. Medical Hypotheses, 2019. 122: p. 31–34. [DOI] [PubMed] [Google Scholar]
- 43.D’Amore A, et al. , Bi-layered polyurethane – Extracellular matrix cardiac patch improves ischemic ventricular wall remodeling in a rat model. Biomaterials, 2016. 107: p. 1–14. [DOI] [PubMed] [Google Scholar]
- 44.Meier L and Hay ED, Stimulation of corneal differentiation by interaction between cell surface and extracellular matrix. I. Morphometric analysis of transfilter “induction”. J Cell Biol, 1975. 66(2): p. 275–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holmes JW, Borg TK, and Covell JW, Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng, 2005. 7: p. 223–53. [DOI] [PubMed] [Google Scholar]
- 46.Tirziu D, Giordano FJ, and Simons M, Cell communications in the heart. Circulation, 2010. 122(9): p. 928–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lal H, et al. , Integrins and proximal signaling mechanisms in cardiovascular disease. Front Biosci (Landmark Ed), 2009. 14: p. 2307–34. [DOI] [PubMed] [Google Scholar]
- 48.Asakura M, et al. , Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med, 2002. 8(1): p. 35–40. [DOI] [PubMed] [Google Scholar]
- 49.Tirziu D, Giordano FJ, and Simons M, Cell communications in the heart. Circulation, 2010. 122(9): p. 928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor DA, et al. , Decellularized matrices in regenerative medicine. Acta Biomater, 2018. 74: p. 74–89. [DOI] [PubMed] [Google Scholar]
- 51.Momtahan N, et al. , Automation of Pressure Control Improves Whole Porcine Heart Decellularization. Tissue Eng Part C Methods, 2015. 21(11): p. 1148–61. [DOI] [PubMed] [Google Scholar]
- 52.Seo Y, Jung Y, and Kim SH, Decellularized heart ECM hydrogel using supercritical carbon dioxide for improved angiogenesis. Acta Biomater, 2018. 67: p. 270–281. [DOI] [PubMed] [Google Scholar]
- 53.Merna N, et al. , Optical imaging predicts mechanical properties during decellularization of cardiac tissue. Tissue Eng Part C Methods, 2013. 19(10): p. 802–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong X, et al. , Skeletal Extracellular Matrix Supports Cardiac Differentiation of Embryonic Stem Cells: a Potential Scaffold for Engineered Cardiac Tissue. Cellular Physiology and Biochemistry, 2018. 45(1): p. 319–331. [DOI] [PubMed] [Google Scholar]
- 55.Mosala Nezhad Z, et al. , Small intestinal submucosa extracellular matrix (CorMatrix®) in cardiovascular surgery: a systematic review. Interactive cardiovascular and thoracic surgery, 2016. 22(6): p. 839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Q, et al. , Functional engineered human cardiac patches prepared from nature’s platform improve heart function after acute myocardial infarction. Biomaterials, 2016. 105: p. 52–65. [DOI] [PubMed] [Google Scholar]
- 57.Hong X, et al. , Skeletal Extracellular Matrix Supports Cardiac Differentiation of Embryonic Stem Cells: a Potential Scaffold for Engineered Cardiac Tissue. Cell Physiol Biochem, 2018. 45(1): p. 319–331. [DOI] [PubMed] [Google Scholar]
- 58.Methe K, et al. , An alternative approach to decellularize whole porcine heart. BioResearch open access, 2014. 3(6): p. 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang B, et al. , Fabrication of cardiac patch with decellularized porcine myocardial scaffold and bone marrow mononuclear cells. Journal of biomedical materials research. Part A, 2010. 94(4): p. 1100–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shah M, et al. , A Thin Layer of Decellularized Porcine Myocardium for Cell Delivery. Scientific reports, 2018. 8(1): p. 16206–16206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oberwallner B, et al. , Human cardiac extracellular matrix supports myocardial lineage commitment of pluripotent stem cells. Eur J Cardiothorac Surg, 2015. 47(3): p. 416–25; discussion 425. [DOI] [PubMed] [Google Scholar]
- 62.Garreta E, et al. , Myocardial commitment from human pluripotent stem cells: Rapid production of human heart grafts. Biomaterials, 2016. 98: p. 64–78. [DOI] [PubMed] [Google Scholar]
- 63.Guyette JP, et al. , Bioengineering Human Myocardium on Native Extracellular Matrix. Circ Res, 2016. 118(1): p. 56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ott HC, et al. , Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med, 2008. 14(2): p. 213–21. [DOI] [PubMed] [Google Scholar]
- 65.Yasui H, et al. , Excitation propagation in three-dimensional engineered hearts using decellularized extracellular matrix. Biomaterials, 2014. 35(27): p. 7839–7850. [DOI] [PubMed] [Google Scholar]
- 66.Robertson MJ, et al. , Optimizing recellularization of whole decellularized heart extracellular matrix. PLoS One, 2014. 9(2): p. e90406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rajabi S, et al. , Human embryonic stem cell-derived cardiovascular progenitor cells efficiently colonize in bFGF-tethered natural matrix to construct contracting humanized rat hearts. Biomaterials, 2018. 154: p. 99–112. [DOI] [PubMed] [Google Scholar]
- 68.Lu H, et al. , Autologous extracellular matrix scaffolds for tissue engineering. Biomaterials, 2011. 32(10): p. 2489–99. [DOI] [PubMed] [Google Scholar]
- 69.Schmuck EG, et al. , Cardiac fibroblast-derived 3D extracellular matrix seeded with mesenchymal stem cells as a novel device to transfer cells to the ischemic myocardium. Cardiovascular engineering and technology, 2014. 5(1): p. 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suhaeri M, et al. , Cardiomyoblast (h9c2) differentiation on tunable extracellular matrix microenvironment. Tissue engineering. Part A, 2015. 21(11–12): p. 1940–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pagano F, et al. , Normal versus Pathological Cardiac Fibroblast-Derived Extracellular Matrix Differentially Modulates Cardiosphere-Derived Cell Paracrine Properties and Commitment. Stem cells international, 2017. 2017: p. 7396462–7396462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharma D, Chica J, and Zhao F. Mesenchymal stem cells for pre-vascularization of engineered tissues. 2018. [Google Scholar]
- 73.Riemenschneider SB, et al. , Inosculation and perfusion of pre-vascularized tissue patches containing aligned human microvessels after myocardial infarction. Biomaterials, 2016. 97: p. 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang L, et al. , Prevascularization of natural nanofibrous extracellular matrix for engineering completely biological three-dimensional prevascularized tissues for diverse applications. J Tissue Eng Regen Med, 2018. 12(3): p. e1325–e1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen L, et al. , Pre-vascularization Enhances Therapeutic Effects of Human Mesenchymal Stem Cell Sheets in Full Thickness Skin Wound Repair. Theranostics, 2017. 7(1): p. 117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen L, et al. , Protocols for Full Thickness Skin Wound Repair Using Prevascularized Human Mesenchymal Stem Cell Sheet. Methods Mol Biol, 2019. 1879: p. 187–200. [DOI] [PubMed] [Google Scholar]
- 77.Sharma D, et al. , Polydopamine and collagen coated micro-grated polydimethylsiloxane for human mesenchymal stem cell culture. Bioactive materials, 2019. 4: p. 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xing Q, et al. , Highly Aligned Nanofibrous Scaffold Derived from Decellularized Human Fibroblasts. Advanced Functional Materials, 2014. 24(20): p. 3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qian Z, et al. , Engineering stem cell cardiac patch with microvascular features representative of native myocardium. Theranostics, 2019. 9(8): p. 2143–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chan YC, et al. , Electrical stimulation promotes maturation of cardiomyocytes derived from human embryonic stem cells. J Cardiovasc Transl Res, 2013. 6(6): p. 989–99. [DOI] [PubMed] [Google Scholar]
- 81.Hern, et al. , Electrical Stimulation Promotes Cardiac Differentiation of Human Induced Pluripotent Stem Cells %J Stem Cells International. 2016. 2016: p. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stoppel WL, Kaplan DL, and Black LD 3rd, Electrical and mechanical stimulation of cardiac cells and tissue constructs. Advanced drug delivery reviews, 2016. 96: p. 135–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Herron Todd J, et al. , Extracellular Matrix–Mediated Maturation of Human Pluripotent Stem Cell–Derived Cardiac Monolayer Structure and Electrophysiological Function. Circulation: Arrhythmia and Electrophysiology, 2016. 9(4): p. e003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ye L, et al. , Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell, 2014. 15(6): p. 750–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iyer RK, et al. , Vascular endothelial growth factor secretion by nonmyocytes modulates Connexin-43 levels in cardiac organoids. Tissue Eng Part A, 2012. 18(17–18): p. 1771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shiba Y, et al. , Electrical Integration of Human Embryonic Stem Cell-Derived Cardiomyocytes in a Guinea Pig Chronic Infarct Model. J Cardiovasc Pharmacol Ther, 2014. 19(4): p. 368–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chong JJ, et al. , Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature, 2014. 510(7504): p. 273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sharma D, et al. , Upgrading prevascularization in tissue engineering: A review of strategies for promoting highly organized microvascular network formation. Acta Biomater, 2019. [DOI] [PubMed] [Google Scholar]
- 89.Dvir T, et al. , Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proceedings of the National Academy of Sciences of the United States of America, 2009. 106(35): p. 14990–14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noguchi R, et al. , Development of a three-dimensional pre-vascularized scaffold-free contractile cardiac patch for treating heart disease. J Heart Lung Transplant, 2016. 35(1): p. 137–45. [DOI] [PubMed] [Google Scholar]
- 91.Anker SD, et al. , A prospective comparison of alginate-hydrogel with standard medical therapy to determine impact on functional capacity and clinical outcomes in patients with advanced heart failure (AUGMENT-HF trial). Eur Heart J, 2015. 36(34): p. 2297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]