Abstract
Purpose
The incidence of nonsuicidal self-injury (NSSI) behavior among adolescents increases year by year. Patients with a history of both depression and NSSI behaviors tend to have greater risk of suicide. At present, the mechanism of adolescent depressive disorder patients with NSSI behaviors is not clear, epigenetic mechanism may be involved. Proopiomelanocortin (POMC) gene may be associated with depressive disorder. The purpose of this study was to investigate DNA methylation of POMC gene promoter region of adolescent depressive disorder patients with NSSI behaviors.
Methods
Bisulfite Sequencing PCR (BSP) was used to test the methylation level of POMC promoter of 15 adolescent depressive disorder patients with NSSI behaviors and 15 healthy controls (HC). Self-made questionnaires were used to collect clinical data of the case group and control group. Hamilton depression scale-24 (HAMD-24), Hamilton anxiety scale (HAMA), Symptom Checklist-90 (SCL-90) were used to evaluate the characteristics and severity of depressive, anxiety and psychotic symptoms. Adolescent self-injury questionnaire was used to assess NSSI behaviors and its severity.
Results
BSP analysis found that the POMC methylation level of cytosine-guanine dinucleotide 1 (CpG1) site was higher in the case group than that of HC (P<0.05). The significance in POMC methylation at CpG1 between case group and HC was gender-independent, and CpG1 methylation level was higher in both male (P<0.05) and female (P<0.05) patients than that in HC. The CpG1 methylation level had a little correlation trends with family history of psychosis (P=0.05). We also found that POMC methylation level at CpG17 in female patients was significantly higher than that of the female HC (P<0.05).
Conclusion
There was abnormal methylation in the POMC promoter region of adolescent depressive disorder patients with NSSI behaviors, the methylation of CpG1 may act as epigenetic markers for those adolescents.
Keywords: adolescent, depressive disorder, POMC, methylation, nonsuicidal self-injury behavior
Introduction
Adolescent depressive disorder, which is a group of diseases mainly manifested by low mood, may be accompanied by varying degrees of cognitive and behavior changes, psychotic symptoms and suicidal self-injurious behaviors, etc,1–5 it was estimated that the prevalence of depressive disorders in adolescents (13–18 years) is 3–9%.6,7 Adolescent depressive disorder, which is a common social problem, deserves more and more attention.
The pathogenesis of depression is complex, studies have shown that epigenetics, especially DNA methylation, played an important role in the occurrence, development and progression of depressive disorders,8–10 such as the methylation of tachykinin receptors 2,11 glucocorticoid receptor gene, the mineralocorticoid receptor gene,12 brain-derived neurotrophic factor13 and catechol-O-methyltransferase.14 In addition, research on epigenetics proves that environmental factors are also closely related to the occurrence of diseases.15 Abnormal DNA methylation may be caused by various environmental and lifestyle exposures, these factors may affect the methylation status of CpG in the regulatory region by interfering with the specific binding between transcription factors and recognition sites.16,17 Adverse life experiences or stress reaction in childhood or adolescence would lead to the functional changes of hypothalamic-pituitary-adrenal (HPA) axis or disorders of adrenocorticotropic hormone (ACTH) in the body.18 Depression may be caused by a combination of the above genetic and environmental factors. Studies have shown that ACTH, which is derived from POMC is closely related to the occurrence of depressive disorders.19 Some studies that have found a correlation between POMC and depressive disorders, but all these studies focus on the relationship between POMC gene polymorphism and depressive disorders,20 however, there are no epigenetic studies of POMC in depressive disorder.
Adolescent patients with depressive disorder may have nonsuicidal self-injury (NSSI) behaviors, the relationship between depression and NSSI behaviors is complex and is still being explored. Some studies believed that depression was an important risk factor of NSSI behaviors,2,21 and anhedonia may be one of the motivations of NSSI behaviors.22 Elevated β-endorphin, as a analgesic medium, can reduce pain and increase pain tolerance so that patients cannot feel pain, thus reinforcing NSSI behaviors.23 Interestingly, both β-endorphin and ACTH come from POMC.
There have been studies showing that POMC is associated with mental illness. Primary study showed that the mice exhibited depression-like behavior when POMC neurons in the arcuate nucleus were inhibited.24 Studies have pointed out that heroin addicts treated with intravenous diacetylmorphine could cause an POMC promoter methylation and reduce the level of stress hormone in the patient’s body, thus playing a role in emotional regulation of treatment.25 In alcohol dependence, POMC promoter methylation is associated with craving.26 The degree of POMC promoter methylation in smokers is significantly higher than that in nonsmokers. Researchers believe that the change of POMC methylation in smokers indicates the adaptability of stress signals, thereby possibly serving as a marker related to addiction.27 Although the causality of adolescent patients with depressive disorder and NSSI behaviors cannot be clear, studies have shown that the patients who had a history of depression and NSSI behaviors at the same time may have a greater risk of suicide.28,29 Therefore, it is important for us to find biological markers of adolescent depressive disorder patients with NSSI behaviors for early detection, prevention and individualized treatment.30 We hypothesize that the epigenetics of the promoter region of the POMC gene has altered in adolescent depressive disorder patients with NSSI behaviors. The purpose of our study was to investigate whether the methylation of the POMC gene promoter is related to the NSSI behaviors of adolescent depression disorders.
Materials and Methods
Subject Recruitment
The study sample included 15 adolescent depressive disorder patients with NSSI behaviors (five males, 10 females) and 15 healthy controls (HCs) (six males, nine females). For the case group, the inclusion criteria were as follows: each participant suffered from depressive disorder as defined by the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5); the diagnosis was made by at least two experienced psychiatrists according to the structured clinical interview for DSM-5 (SCID); between the ages of 10 and 19 years old. The exclusion criteria were as follows: severe somatic or brain disease, such as cerebral ischemia, cerebral hemorrhage, epilepsy, cardiovascular and renal diseases; depressive disorder due to other psychoactive drug substance abuse (legal or illegal substances); pregnant women or patients with acquired immunodeficiency syndrome; the score of HAMD-24 third item (suicide) ≧2 points; bipolar or related disorders, disruptive mood disorders, persistent mood disorders, other and unspecified mood disorders, abuse of alcohol or other psychoactive substances; patients who have received electroconvulsive therapy, transcranial magnetic stimulation or systematic psychotherapy in the last three months; other mental disorders defined in DSM-5. For the control group, the inclusion criteria were as follows: aged from 10 to 19 years old; who were not concomitant psychiatric disorders, such as schizophrenia spectrum, bipolar or related disorders, depression, anxiety, compulsion, separation, somatic symptoms or other related disorders defined in DSM-5. The exclusion criteria were as follows: first-degree relatives have psychiatric and neurological disorders or previous NSSI behaviors.
Molecular Analysis
Participants’ peripheral blood (5 mL) was extracted and placed in anticoagulation tubes containing EDTA. Then it was centrifuged to extract the leukocyte layer. The online software CpG Island Searcher was used to analyze the gene promoter region CpG island, ranging from 2000 bp upstream to 500 bp downstream of the transcription start point, to obtain sequence information. Sequenom EpiDesigner software was used for analyzing and designing methylated primers at both ends of CpG island. The extraction of DNA was performed using the DNA Kit (GENERAY, GK0122). DNA samples were bisulfite-converted using the Bisulfite Kit (QIAGEN, cat; 59824). DNA amplification was conducted using polymerase chain reaction (PCR). After purification of the PCR product using kit (Generay, cat; GK2043), each product was visualized on a standard agarose gel. T/A cloning was performed and positive clones were selected from each clone for sequencing. Finally, comparing with the measured sequence and the original sequence to determine the methylation sites and number, and analyze the degree of methylation.
The primers used in the experiment were as follows: B474-S1-F, TAAATGYGAATTAGGTAGATGT; B474-S1-R, AACACAAAAAACAACTCCC; the fragment length is 312 bp. BSP-F (control), AGGGAGGTGAAGTAGTGGTG; BSP-R (control), ACTCCTCCACTCTTCTCCTCC; the fragment length is 247 bp.
Scales Assessment
The characteristics and severity of depressive symptoms in patients were assessed with HAMD-24, which consisted of seven factors (anxiety/somatization, weight, cognitive disorder, circadian changes, hysteresis, dyssomnia, feeling of despair), each item adopted the five degree scoring of 0–4 points, representing no, slight, moderate, severe, very severe, respectively. The characteristics and severity of anxiety symptoms in patients were assessed with HAMA, which consisting of two factors (somatic anxiety, psychic anxiety), each item adopted the five degree scoring of 0–4 points, representing no, slight, moderate, severe, very severe, respectively. The psychiatrists (all authors) were simultaneously trained in the use of the HAMD-24, HAMA, SCL-90 before this study was initiated. The mental health of the patients was assessed with SCL-90, which consisting of 10 factors (somatization, obsession, interpersonal sensitivity, depressed, anxious, hostile, terror, paranoia, psychosis, average), each item adopted the five degree scoring of 0–4 points, representing no, mild, moderate, a little severe, and severe, respectively. We separately calculated the factor scores of the above scales for each case group member. All these scales are the Chinese version and have good reliability and validity among the Chinese population.31–33
Statistical Analysis
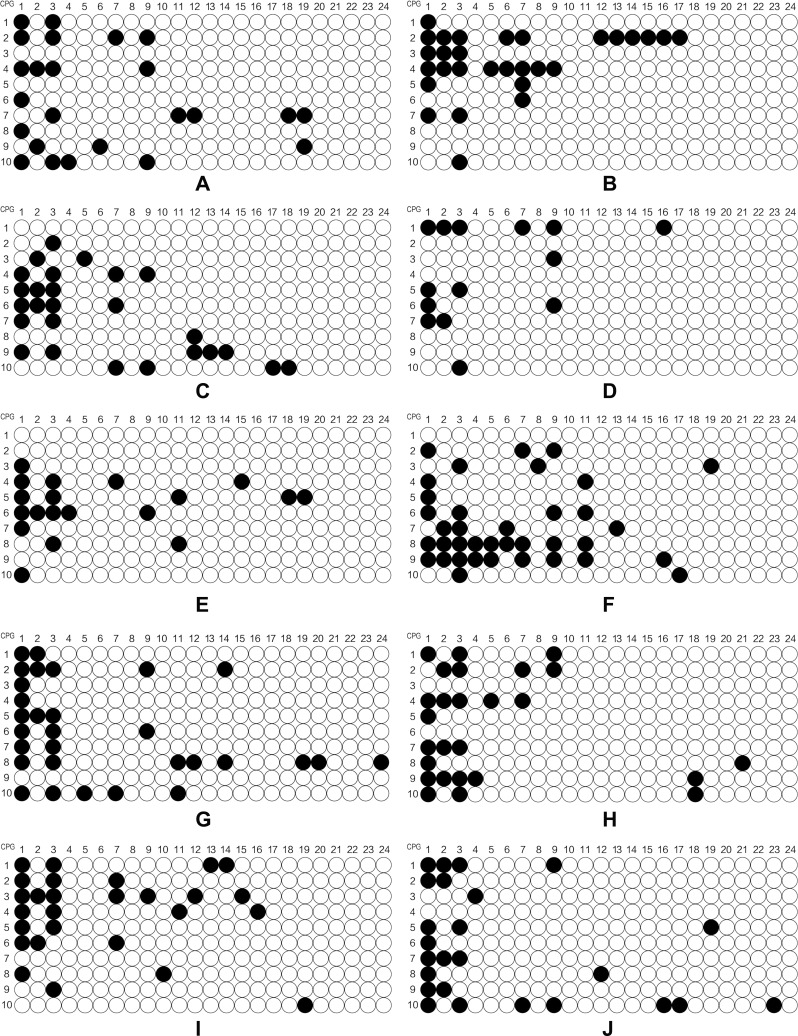
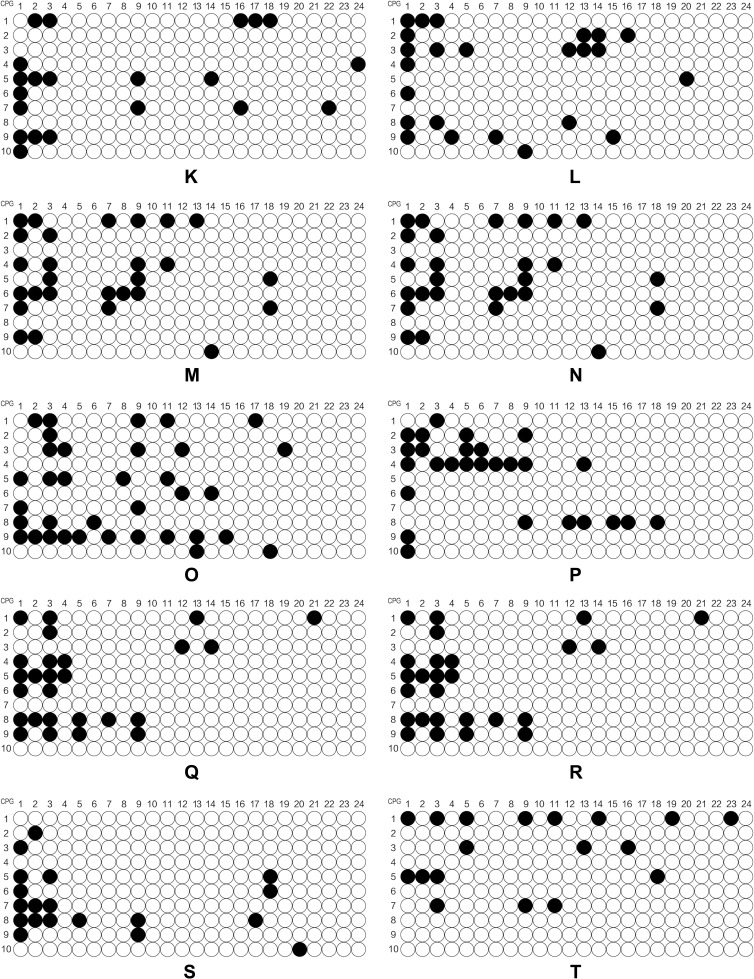
SPSS 21.0 software was used to establish the database and all data were expressed as mean ±standard deviation. The independent samples t-test and one-way analysis of variance was used to compare the continuous variables between groups. The chi-squared test was used to compare the categorical variables between groups. The independent samples t-test was used to analyze the difference between methylation level of CpG1 site of patients with/without family history of psychosis. Spearman correlation analysis was used to assess the correlation of CpG1 DNA methylation level of patients and related factor scores of SCL-90, HAMD and HAMA. The online software MSR Calcalate was used to draw the lollipop model of the methylation state. Each line represents the methylated state of a positive clone after sequencing and comparison, white circles represents unmethylated CpG sites and black circles represents the methylated CpG sites. All statistical tests were considered statistically significant at P<0.05.
Results
Data from 15 adolescent depressive disorder patients with NSSI behaviors and 15 HC were analyzed. The results showed that there was no statistical difference in gender (t = 0.435, P = 0.670) and age (t = −2.016, P = 0.063) between the two groups. In our experimental results, the mean of each scale is as follows: total scores of HAMD (32.867 ± 1.367), total scores of HAMA (19.733 ± 1.270), total scores of SCL-90 (305.467 ± 94.396).
Lollipop Model of the Methylation State
The online software Methylation Share Rate (MSR) Calcalate was used to draw the lollipop model of the methylation state. Each line represents the methylated state of a positive clone after sequencing and comparison, white circles represents unmethylated CpG sites and black circles represented the methylated CpG sites.
Figure 1a.
Continued.
Figure 1b.
Continued.
Figure 1c.
The level of methylation of the POMC gene in different sites.
Notes: (A-O) represented the level of methylation of 15 adolescent depressive disorder patients with NSSI behaviors; (P-DD) represented the level of methylation of 15 HCs.
DNA Methylation Level at 24 CpG Sites in Case and Control Groups and Difference Between Gender
In this study, we explored the level of DNA methylation at 24 POMC promoter region CpG sites. We could clearly find that the mean level of CpG1 methylation was higher in adolescent depressive disorder patients with NSSI behaviors than that of HC (P<0.05). What is more, four sites (CpG1, CpG2, CpG3, and CpG9) among 24 CpG sites of the POMC gene were expressed in adolescent depressive disorder patients with NSSI behaviors and HCs. (Table 1). We found that POMC methylation level at 24 CpG sites between case and control groups was gender-independent (data not shown), but our further analysis found that CpG1 methylation level was higher in male patients (P<0.05) and female (P<0.05) patients of case group than that of HCs. In addition, we concluded that POMC methylation at CpG17 sites in female patients was higher than that of female HCs, and the difference was statistically significant (P<0.05). (Table 2)
Table 1.
Methylation Level of Different CpG Sites Between Case and Control Groups
| (A) | |||||
|---|---|---|---|---|---|
| Group | CpG1 | CpG2 | CpG3 | CpG4 | CpG5 |
| Case group | 6.13±1.36 | 2.67±0.98 | 4.80±1.21 | 0.67±0.90 | 0.60±0.63 |
| Control group | 4.07±1.22 | 2.13±0.99 | 4.60±1.56 | 0.73±1.16 | 0.93±1.10 |
| t | 4.384 | 1.486 | 0.387 | −0.176 | −1.018 |
| P | 0.000* | 0.149 | 0.701 | 0.862 | 0.320 |
| Notes: P<0.05 represented the statistical significance between case and control group. *P<0.001. | |||||
| (B) | |||||
| Group | CpG6 | CpG7 | CpG8 | CpG9 | CpG10 |
| Case group | 0.40±0.74 | 1.80±1.15 | 0.27±0.46 | 2.33±1.11 | 0.07±0.26 |
| Control group | 0.47±0.92 | 1.47±1.30 | 0.60±1.06 | 1.93±0.88 | 0.20±0.56 |
| t | −0.220 | 0.744 | −1.122 | 1.090 | −0.837 |
| P | 0.828 | 0.463 | 0.271 | 0.285 | 0.410 |
| (C) | |||||
| Group | CpG11 | CpG12 | CpG13 | CpG14 | CpG15 |
| Case group | 1.00±1.31 | 0.80±0.78 | 0.60±0.74 | 0.67±0.72 | 0.33±0.49 |
| Control group | 0.80±0.94 | 0.87±0.83 | 0.67±0.72 | 0.40±0.51 | 0.33±0.49 |
| t | 0.480 | −0.227 | −0.250 | 1.169 | 0.000 |
| P | 0.635 | 0.822 | 0.804 | 0.252 | 1.000 |
| (D) | |||||
| Group | CpG16 | CpG17 | CpG18 | CpG19 | CpG20 |
| Case group | 0.53±0.64 | 0.47±0.52 | 0.73±0.80 | 0.53±0.64 | 0.20±0.41 |
| Control group | 0.47±0.64 | 0.27±0.46 | 0.60±0.91 | 0.27±0.46 | 0.27±0.59 |
| t | 0.285 | 1.122 | 0.426 | 1.313 | −0.357 |
| P | 0.778 | 0.271 | 0.673 | 0.201 | 0.724 |
| (E) | |||||
| Group | CpG21 | CpG22 | CpG23 | CpG24 | |
| Case group | 0.07±0.26 | 0.13±0.35 | 0.07±0.26 | 0.13±0.35 | |
| Control group | 0.07±0.26 | 0.07±0.26 | 0.13±0.35 | 0.07±0.26 | |
| t | 0.000 | 0.592 | −0.592 | 0.592 | |
| P | 1.000 | 0.559 | 0.559 | 0.559 | |
Table 2.
Comparison of Methylation Level in Male and Female Patients at Different Sites in Case and Control Groups
| (A) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gender | Group | CpG1 | CpG2 | CpG3 | CpG4 | |||
| Male | Case group | 6.80±0.84 | 3.00±1.00 | 4.80±1.30 | 0.60±0.55 | |||
| Control group | 4.17±1.33 | 2.17±1.17 | 4.17±1.47 | 0.33±0.52 | ||||
| t | 3.825 | 1.254 | 0.747 | 0.830 | ||||
| P | 0.004** | 0.241 | 0.474 | 0.428 | ||||
| Female | Case group | 5.80±1.48 | 2.50±0.97 | 4.80±1.23 | 0.70±1.06 | |||
| Control group | 4.00±1.23 | 2.11±0.93 | 4.89±1.69 | 1.00±1.41 | ||||
| t | 2.873 | 0.890 | −0.132 | −0.527 | ||||
| P | 0.011* | 0.386 | 0.896 | 0.605 | ||||
| Notes: P<0.05 represented the statistical significance at CpG1 site in male and female patients of case and control groups. *P<0.05; **P<0.01. | ||||||||
| (B) | ||||||||
| Gender | Group | CpG5 | CpG6 | CpG7 | CpG8 | |||
| Male | Case group | 0.20±0.45 | 0.20±0.45 | 1.40±1.14 | 0.00±0.00 | |||
| Control group | 1.00±1.27 | 0.50±0.84 | 1.00±0.89 | 0.33±0.52 | ||||
| t | −1.336 | −0.717 | 0.653 | −1.581 | ||||
| P | 0.214 | 0.492 | 0.530 | 0.175 | ||||
| Female | Case group | 0.80±0.63 | 0.50±0.85 | 2.00±1.16 | 0.40±0.52 | |||
| Control group | 0.89±1.05 | 0.44±1.01 | 1.78±1.48 | 0.78±1.30 | ||||
| t | −0.220 | 0.130 | 0.367 | −0.849 | ||||
| P | 0.829 | 0.898 | 0.718 | 0.408 | ||||
| (C) | ||||||||
| Gender | Group | CpG9 | CpG10 | CpG11 | CpG12 | |||
| Male | Case group | 2.00±0.71 | 0.20±0.45 | 0.40±0.55 | 0.60±0.55 | |||
| Control group | 1.83±0.75 | 0.00±0.00 | 0.50±0.84 | 1.00±1.10 | ||||
| t | 0.376 | 1.000 | −0.229 | −0.739 | ||||
| P | 0.716 | 0.374 | 0.824 | 0.479 | ||||
| Female | Case group | 2.50±1.27 | 0.00±0.00 | 1.30±1.49 | 0.90±0.88 | |||
| Control group | 2.00±1.00 | 0.33±0.71 | 1.00±1.00 | 0.78±0.67 | ||||
| t | 0.946 | −1.414 | 0.519 | 0.339 | ||||
| P | 0.357 | 0.195 | 0.611 | 0.739 | ||||
| (D) | ||||||||
| Gender | Group | CpG13 | CpG14 | CpG15 | CpG16 | |||
| Male | Case group | 0.20±0.45 | 0.40±0.55 | 0.20±0.45 | 0.80±0.84 | |||
| Control group | 0.83±0.98 | 0.33±0.52 | 0.17±0.41 | 0.67±0.82 | ||||
| t | −1.412 | 0.208 | 0.129 | 0.267 | ||||
| P | 0.199 | 0.840 | 0.900 | 0.796 | ||||
| Female | Case group | 0.80±0.79 | 0.80±0.79 | 0.40±0.52 | 0.40±0.52 | |||
| Control group | 0.56±0.53 | 0.44±0.53 | 0.44±0.53 | 0.33±0.50 | ||||
| t | 0.784 | 1.141 | −0.186 | 0.285 | ||||
| P | 0.444 | 0.270 | 0.855 | 0.779 | ||||
| (E) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gender | Group | CpG17 | CpG18 | CpG19 | CpG20 | |||
| Male | Case group | 0.40±0.55 | 0.80±0.84 | 0.80±0.84 | 0.00±0.00 | |||
| Control group | 0.67±0.52 | 0.67±0.82 | 0.17±0.41 | 0.17±0.41 | ||||
| t | −0.830 | 0.267 | 1.646 | −1.000 | ||||
| P | 0.428 | 0.796 | 0.134 | 0.363 | ||||
| Female | Case group | 0.50±0.53 | 0.70±0.82 | 0.40±0.52 | 0.30±0.48 | |||
| Control group | 0.00±0.00 | 0.56±1.01 | 0.33±0.50 | 0.33±0.71 | ||||
| t | 2.838 | 0.343 | 0.285 | −0.121 | ||||
| P | 0.011* | 0.736 | 0.779 | 0.905 | ||||
| Notes: P<0.05 represented the statistical significance at CpG17 site in male and female patients of case and control groups.*P<0.05. | ||||||||
| (F) | ||||||||
| Gender | Group | CpG21 | CpG22 | CpG23 | CpG24 | |||
| Male | Case group | 0.20±0.45 | 0.20±0.45 | 0.20±0.45 | 0.20±0.45 | |||
| Control group | 0.00±0.00 | 0.17±0.41 | 0.00±0.00 | 0.17±0.41 | ||||
| t | 1.000 | 0.129 | 1.000 | 0.129 | ||||
| P | 0.374 | 0.900 | 0.374 | 0.900 | ||||
| Female | Case group | 0.00±0.00 | 0.10±0.32 | 0.00±0.00 | 0.10±0.32 | |||
| Control group | 0.11±0.33 | 0.00±0.00 | 0.22±0.44 | 0.00±0.00 | ||||
| t | −1.058 | 1.000 | −1.512 | 1.000 | ||||
| P | 0.305 | 0.343 | 0.169 | 0.343 | ||||
Difference Between Methylation Level of CpG1 Site of Patients with/without Family History of Psychosis
The independent samples t-test was used to analyze the difference between methylation level of CpG1 site of patients with/without family history of psychosis. The methylation level of CpG1 site of patients with family history of psychosis was 5.73±1.19, and that of patients without family history of psychosis was 7.25±1.26, the results showed that the CpG1 methylation level had a little statistical correlation trends with the family history of psychosis (P=0.05).
The Relationship Between Methylation Level of CpG1 Site and SCL-90, HAMD, HAMA Scales
Spearman correlation analysis was used to analyze the relationship between methylation level of CpG1 site and the factor scores of SCL-90, HAMD and HAMA scales, and the results showed that the CpG1 methylation level had no correlation with these scales.
Discussion
At present, studies have shown that the POMC gene may be related to the pathological process of depression. However, there are no epigenetic studies to investigate whether this gene is related to the NSSI behavior of adolescent depression patients. As we know, this is the first case–control study investigating the epigenetic alterations of POMC promoter methylation in adolescent depressive disorder patients with NSSI behaviors.
POMC methylation level of CpG1 site was higher in adolescent depressive disorder patients with NSSI behaviors than that of HCs. One possibility is that adolescent depressive disorder patients carried an elevated POMC promoter methylation before having NSSI behaviors. The alteration of methylation level could be the onset of a multigenerational effect established by environmental alterations regarding a NSSI behaviors’ mother during pregnancy,34 and our research results showed that abnormal methylation level at the CpG1 site showed little correlation trend with the family history of psychosis. In this assumption, we consider that the abnormal methylation of the parent’s POMC promoter may be passed on to the child and initiate the development of NSSI behaviors. We presume that high methylation level in adolescent depressive disorder patients with NSSI behaviors, compared to HCs, leads to a lowered level of POMC and its derivatives.35 That condition may be restored through the epigenetic feedback regulation mechanism of HPA axis. But because of lacking some related data (NSSI behaviors conditions of patients’ parents) and biological material from their ancestors, we were not able to deliver further evidence for this assumption. So we need to do more animal studies under controlled prerequisites to provide evidence for the existence of a multigenerational effect caused by some negative environmental conditions in early life on epigenetic patterns of adolescent depression patients.
Another possibility is that abnormal POMC methylation is the result of NSSI behaviors and the adaptation of the HPA axis to maintain the steady state of POMC and its derivatives. Studies have shown that adolescent patients with depressive disorder suffer from anhedonia due to disorders of the HPA axis or certain substances in the body.18 Anhedonia may be one of the motivations of NSSI behaviors, which promotes NSSI behaviors in adolescents with depressive disorder. NSSI behaviors, such as a stress or pain mediator, activated POMC neurons to interfere with the HPA axis by stimulating the production of β-endorphin and ACTH in body of adolescent patients with depressive disorder, resulting in the dysfunction of HPA. On the one hand, adolescent patients with depressive disorder stimulate the body to produce β-endorphin,35 ACTH and other substances through NSSI behaviors to activate POMC neurons to interfere with the HPA axis, so that the body reaches a steady state. On the other hand, some studies also suggested that NSSI individuals may have emotional management disorders with the inability to express emotions, and NSSI behaviors can be an emotional regulation strategy for patients with depressive disorder to involve in regulating emotions.36–38 What is more, while the significance in POMC methylation at CpG1 sites between adolescent depressive disorder patients with NSSI behaviors and HCs was gender-independent from our research, this effect was more pronounced in male patients. Studies have found that male students were earlier and more prone to NSSI behaviors than female students. Relatively speaking, when dealing with adverse events, most male students were more likely to adopt a negative coping style than female, preferring to compare themselves with peers who were not doing well in everything, and reluctant to talk and confide.39
There are limitations of our work. Although we concluded that there was abnormal methylation in the POMC promoter region, considering the small sample size of the preliminary experiment, our study has not been divided into so many groups (such as, adolescent depressive disorder patients without NSSI behaviors) for research and analysis, so it is temporarily impossible to determine whether the abnormal methylation in the POMC promoter region is caused by depressive disorder or NSSI behaviors. Therefore, the results may be biased, so whether this anomaly could be used as a possible marker needs to be further verified by expanding the sample size, this is also the focus of our future research. In addition, the missing data of these patients’ parents and their ancestors, makes it difficult for us to further investigate whether the abnormal methylation of CpG1 site is related to the mother’s NSSI or other adverse stimuli during pregnancy. For future studies, we will take ample samples and more clinical data to verify the possible epigenetic feedback regulation mechanism and strive for the early detection of biological markers applied in clinical work.
Conclusion
We concluded that there was abnormal methylation in the POMC promoter region, POMC CpG1 methylation level in adolescent depressive disorder patients with NSSI behaviors was higher than that of HCs, which may be a possible epigenetic marker of the POMC gene. Meanwhile, the abnormality of CpG1 methylation may also be a feedback regulatory mechanism. Our research shows that in adolescent depressive disorder patients with NSSI behaviors, the methylation of CpG1 may act as epigenetic markers for those adolescents.
Acknowledgments
We thank all participant for their support in this study.
Doudou Zheng and Xiaojiao Bi contributed equally to this study.
Funding Statement
This study was supported by Shandong Natural Science Foundation (No. ZR2017MH094). The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Abbreviations
ACTH, adrenocorticotropic hormone; BSP, bisulfite sequencing PCR; CpG1, cytosine-guanine dinucleotide 1; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, 5th edition; HAMA, Hamilton anxiety scale; HAMD-24, Hamilton depression scale-24; HCs, healthy controls; HPA axis, hypothalamic-pituitary-adrenal axis; MSR, methylation share rate; NSSI, nonsuicidal self-injury; POMC, proopiomelanocortin; SCL-90, Symptom Checklist-90; SCID, structured clinical interview for DSM-5.
Data Sharing Statement
Researchers interested in the study may contact corresponding author to obtain relevant data via email: liulf521@163.com.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of Shandong Mental Health Center, reference number: (2020) Ethics Review (R11). All participants and their legal guardians signed the informed consent before participating in the study.
Consent for Publication
Not applicable.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Guerry JD, Prinstein MJ. Longitudinal prediction of adolescent nonsuicidal self-injury: examination of a cognitive vulnerability-stress model. J Clin Child Adolesc Psychol. 2010;39(1):77–89. doi: 10.1080/15374410903401195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin L, Abela, Hankin JRZ, et al. Nonsuicidal self-injury in adolescence: prospective rates and risk factors in a 2½ year longitudinal study. Psychiatry Res. 2011;186(1):65–70. doi: 10.1016/j.psychres.2010.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall SK, Tilton-Weaver LC, Stattin H. Non-suicidal self-injury and depressive symptoms during middle adolescence: a longitudinal analysis. J Youth Adolesc. 2013;42(8):1234–1242. doi: 10.1007/s10964-013-9919-3 [DOI] [PubMed] [Google Scholar]
- 4.Muehlenkamp JJ, Gutierrez PM. Risk for suicide attempts among adolescents who engage in non-suicidal self-injury. Arch Suicide Res. 2007;11(1):69–82. doi: 10.1080/13811110600992902 [DOI] [PubMed] [Google Scholar]
- 5.You J, Leung F. The role of depressive symptoms, family invalidation and behavioral impulsivity in the occurrence and repetition of non-suicidal self-injury in Chinese adolescents: a 2-year follow-up study. J Adolesc. 2012;35(2):389–395. doi: 10.1016/j.adolescence.2011.07.020 [DOI] [PubMed] [Google Scholar]
- 6.Jane CE, Erkanli A, Angold A. Is there an epidemic of child or adolescent depression. J Child Psychol Psychiatry. 2006;47:1263–1271. [DOI] [PubMed] [Google Scholar]
- 7.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62(6):593. doi: 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- 8.Menke A, Binder EB. Epigenetic alterations in depression and antidepressant treatment. Dialogues Clin Neurosci. 2014;16(3):395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Numata S, Ishii K, Tajima A, et al. Blood diagnostic biomarkers for major depressive disorder using multiplex DNA methylation profiles: discovery and validation. Epigenetics. 2015;10(2):135–141. doi: 10.1080/15592294.2014.1003743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muehlenkamp JJ, Claes L. International prevalence of adolescent non-suicidal self-injury and deliberate self-harm. Child Adolescent psychiatry Mental Health. 2012;6(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiang D, Xiao J, Fu L, et al. DNA methylation of the Tacr2 gene in a CUMS model of depression. Behav Brain Res. 2019;3(365):103–109. [DOI] [PubMed] [Google Scholar]
- 12.Stonawski V, Frey S, Golub Y, et al. Associations of prenatal depressive symptoms with DNA methylation of HPA axis-related genes and diurnal cortisol profiles in primary school-aged children. Dev Psychopathol. 2019;31(2):419–431. doi: 10.1017/S0954579418000056 [DOI] [PubMed] [Google Scholar]
- 13.Li M, D’Arcy C, Li X, Zhang T, Joober R, Meng X. What do DNA methylation studies tell us about depression? A systematic review. Transl Psychiatry. 2019;9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Na KS, Won E, Kang J, Kim A, Ham BJ. Differential effect of COMT gene methylation on the prefrontal connectivity in subjects with depression versus healthy subjects. Neuropharmacology. 2018;137:59–70. doi: 10.1016/j.neuropharm.2018.04.030 [DOI] [PubMed] [Google Scholar]
- 15.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2011;12(7):620–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godfrey KM, Sheppard A, Gluckman PD, Lillycrop KA, Hanson MA. Epigenetic gene promoter methylation at birth is associated with childrens later adiposity. Diabetes. 2011;60(5):1528–1534. doi: 10.2337/db10-0979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson GP, Gao X, Wright FA, et al. Aberrant CpG-island methylation has non-random and tumour-type–specific patterns. Nat Genet. 2000;24(2):132–138. doi: 10.1038/72785 [DOI] [PubMed] [Google Scholar]
- 18.Lu S, Gao W, Huang M, Li L, Xu Y. In search of the HPA axis activity in unipolar depression patients with childhood trauma: combined cortisol awakening response and dexamethasone suppression test. J Psychiatr Res. 2016;78:24–30. doi: 10.1016/j.jpsychires.2016.03.009 [DOI] [PubMed] [Google Scholar]
- 19.Sandman CA, Hetrick W, Talyor D, Marion S, Chicz-DeMet A. Uncoupling of proopiomelanocortin (POMC) fragments is related to self-injury. Peptides. 2000;21(6):785–791. doi: 10.1016/S0196-9781(00)00209-6 [DOI] [PubMed] [Google Scholar]
- 20.Chang HS, Won ES, Lee HY, Ham BJ, Kim YG, Lee MS. The association of proopiomelanocortin polymorphisms with the risk of major depressive disorder and the response to antidepressants via interactions with stressful life events. J Neural Transm (Vienna). 2015;122(1):59–68. doi: 10.1007/s00702-014-1333-9 [DOI] [PubMed] [Google Scholar]
- 21.Giletta M, Scholte RHJ, Engels RCME, Ciairano S, Prinstein MJ. Adolescent non-suicidal self-injury: a cross-national study of community samples from Italy, the Netherlands and the United States. Psychiatry Res. 2012;197(1–2):66–72. doi: 10.1016/j.psychres.2012.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zielinski MJ, Veilleux JC, Winer ES, Nadorff MR. A short-term longitudinal examination of the relations between depression, anhedonia, and self-injurious thoughts and behaviors in adults with a history of self-injury. Compr Psychiatry. 2017;73:187–195. doi: 10.1016/j.comppsych.2016.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smyth DG. 60 YEARS OF POMC: lipotropin and beta-endorphin: a perspective. J Mol Endocrinol. 2016;56(4):T13–25. doi: 10.1530/JME-16-0033 [DOI] [PubMed] [Google Scholar]
- 24.Qu N, He Y, Wang C, et al. A POMC-originated circuit regulates stress-induced hypophagia, depression, and anhedonia. Mol Psychiatry. 2020;25(5):1006–1021. doi: 10.1038/s41380-019-0506-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groh A, Rhein M, Buchholz V, Burkert A, Walter M. Epigenetic effects of intravenous diacetylmorphine on the methylation of POMC and NR3C1. Neuropsychobiology. 2018;75(4):193–199. doi: 10.1159/000486973 [DOI] [PubMed] [Google Scholar]
- 26.Muschler MAN, Lenz B, Hillemacher T, Kraus C, Bleich S. CAGn repeat of the androgen receptor is linked to proopiomelanocortin promoter methylation - Relevance for craving of male alcohol-dependent patients. Psychopharmacology (Berl). 2013;231(10):2059–2066. doi: 10.1007/s00213-013-3349-5 [DOI] [PubMed] [Google Scholar]
- 27.Muschler M, Rhein, Ritter A, et al. Epigenetic alterations of the POMC promoter in tobacco dependence. Eur Neuropsychopharmacol. 2018;5(4):924–925. [DOI] [PubMed] [Google Scholar]
- 28.Yuan N, Kwok, Kwok KHR, et al. Treatment engagement in specific psychological treatment vs. treatment as usual for adolescents with self-harm: systematic review and meta-analysis. Front Psychol. 2019;10:104. doi: 10.3389/fpsyg.2019.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knorr AC, Tull MT, Anestis MD, Dixon-Gordon KL, Bennett MF, Gratz KL. The interactive effect of major depression and nonsuicidal self-injury on current suicide risk and lifetime suicide attempts. Arch Suicide Res. 2016;20(4):539–552. doi: 10.1080/13811118.2016.1158679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanford S, Jones MP, Hudson JL. Appreciating complexity in adolescent self-harm risk factors: psychological profiling in a longitudinal community sample. J Youth Adolesc. 2018;47(5):916–931. doi: 10.1007/s10964-017-0721-5 [DOI] [PubMed] [Google Scholar]
- 31.Chen S, Li L. Re-testing reliability, validity and norm applicatility of SCL-90. Chin J Nerv Ment Dis. 2003;29(5):323–327. [Google Scholar]
- 32.Zhao J, Zheng Y. The reliability, validity of HAMD. Chin Ment Health J. 1992;5:214–216. [Google Scholar]
- 33.Wang X, et al. A Handbook On Psychological Hygiene. Chin Ment Health J. 1999;253–254. [Google Scholar]
- 34.Yohn NL, Bartolomei MS, Blendy JA. Multigenerational and transgenerational inheritance of drug exposure: the effects of alcohol, opiates, cocaine, marijuana, and nicotine. Prog Biophys Mol Biol. 2015;118(1–2):21–33. doi: 10.1016/j.pbiomolbio.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandman CA, Touchette P. beta-Endorphin and ACTH are dissociated after self-injury in adults with developmental disabilities. Am J Ment Retard. 2003;108(6):414–424. doi: [DOI] [PubMed] [Google Scholar]
- 36.Wioletta R, Magdalena L. Deliberate self-injury functions and their clinical correlates among adolescent psychiatric inpatients. Psychiatr Pol. 2017;51(2):303–322. doi: 10.12740/PP/63802 [DOI] [PubMed] [Google Scholar]
- 37.Klonsky ED. The functions of deliberate self-injury: a review of the evidence. Clin Psychol Rev. 2007;27(2):226–239. doi: 10.1016/j.cpr.2006.08.002 [DOI] [PubMed] [Google Scholar]
- 38.Zetterqvist M, Lundh L, Svedin CG. A cross-sectional study of adolescent non-suicidal self-injury: support for a specific distress-function relationship. Child Adolesc Psychiatry Ment Health. 2014;8(1):23. doi: 10.1186/1753-2000-8-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plener PL, Fegert JM, Kaess M, et al. Nonsuicidal self-injury in adolescence: a clinical guideline for diagnostics and therapy. Z Kinder Jugend Psychiatr Psychother. 2017;45(6):463–474. [DOI] [PubMed] [Google Scholar]